Submitted:
11 December 2023
Posted:
13 December 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
1.1. Angiogenesis
1.2. Integrins
1.3. Snake Venom Metalloproteinases and Snake Venom Disintegrins
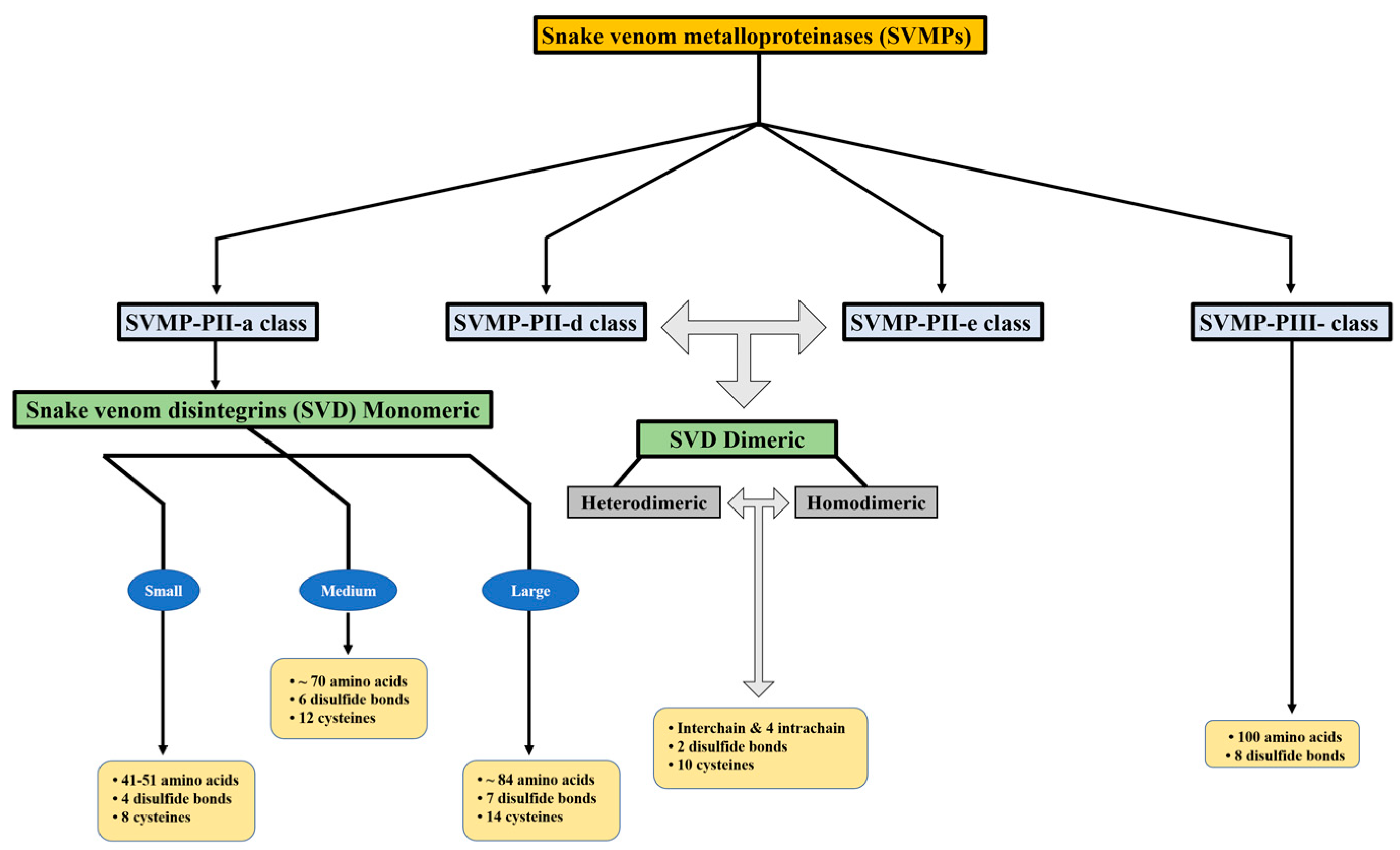
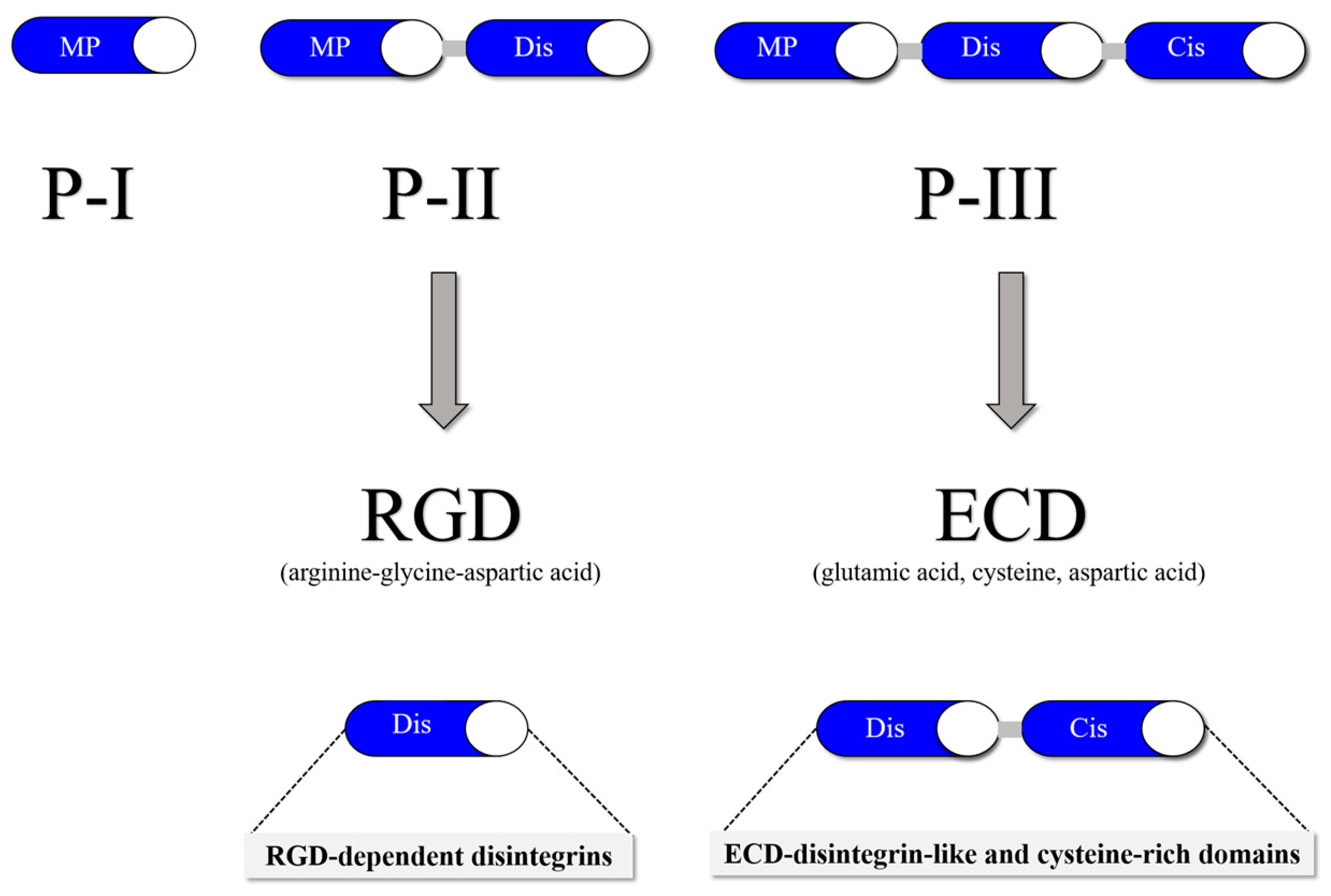
1.4. The role and properties of SVDs in angiogenesis
| Name (Source) | Recognizing Motif | Physiological Target | Angiogenic factors | References |
|---|---|---|---|---|
| Jararhagin-C | ECD-disintegrin-like/cysteine-rich domains | Interferes with α2β1 integrin functions | Pro-angiogenic | [8] |
| Alternagina-C | Exhibits both pro- and anti-angiogenic effects | [30,51,52] | ||
| Leberagin-C | Disintegrin-like | Interferes with αvβ3, αvβ6, and α5β1 integrins | Anti-angiogenesis | [53](63) |
| Echistatin | RGD-dependent disintegrins | Binds to integrin αIIbβ3, GPIIb/IIIa and interacts with αvβ3 integrin | Anti-angiogenesis | [14,33,54] |
| Rhodostomin | [50] | |||
| Contortrostatin | Interacts with the integrins αvβ3 and α5β1 | [38] | ||
| Vicrostatin | antagonize the function of the αIIbβ3, αvβ3, αvβ5 and α5β1 integrins | [23] | ||
| DisBa-01 | Binds to integrin αvβ3 | [55] | ||
| Aggretin | Binds to integrin α2β1 | Pro-angiogenic | [56] | |
| Trigramin | Binds to αIIbβ3, α8β1, αvβ3, αvβ5and/or α5β1 integrins | [57] | ||
| Obtustatin | KTS-disintegrin | Selectively inhibit α1β1-integrin | Anti-angiogenesis | [47] |
| Viperistatin | Inhibitory activity against collagen receptors, α1β1 and α2β1-integrins | [48] | ||
| Lebestatin | Inhibis binding of α1β1 integrin to type IV and type I collagen | [43] | ||
| Jerdostatin | RTS-disintegrin | Antagonizes the function of the α1β1 integrin | Anti-angiogenesis | [49] |
| Agkistin-s | InteractS with GPIB | Anti-angiogenesis | [58] |
1.4.1. Interference of SVD with VEGF
1.5. Anti-angiogenic effects of SVDs
2. Potential applications of SVD in angiogenesis-related diseases
2.1. Cancer
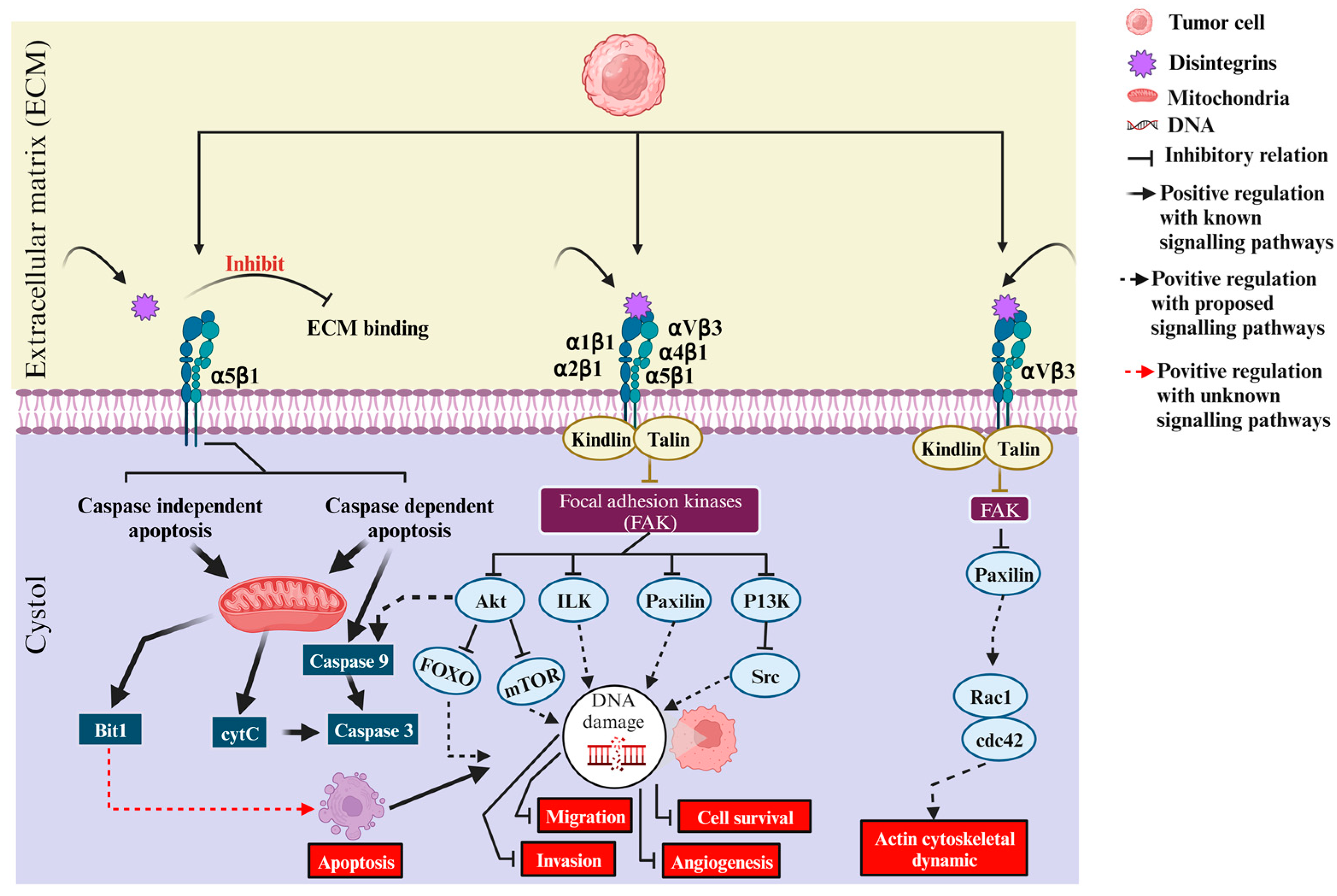
2.2. Ocular Diseases, e.g., Diabetic Retinopathy
3. Utilization of SVD as Pro-Angiogenic Agents
3.1. Tissue regeneration and wound healing
3.2. Cardiovascular Diseases
4. Conclusion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Carmeliet, P.; Jain, R.K. Angiogenesis in cancer and other diseases. Nature 2000, 407, 249-257. [CrossRef]
- Folkman, J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nature medicine 1995, 1, 27-31. [CrossRef]
- Potente, M.; Gerhardt, H.; Carmeliet, P. Basic and therapeutic aspects of angiogenesis. Cell 2011, 146, 873-887. [CrossRef]
- Matsumoto, T.; Claesson-Welsh, L. VEGF receptor signal transduction. Science's STKE : signal transduction knowledge environment 2001, 2001, re21. [CrossRef]
- Zhang, F.; Tang, Z.; Hou, X.; Lennartsson, J.; Li, Y.; Koch, A.W.; Scotney, P.; Lee, C.; Arjunan, P.; Dong, L.; et al. VEGF-B is dispensable for blood vessel growth but critical for their survival, and VEGF-B targeting inhibits pathological angiogenesis. Proceedings of the National Academy of Sciences of the United States of America 2009, 106, 6152-6157. [CrossRef]
- Dvorak, H.F. Vascular permeability factor/vascular endothelial growth factor: a critical cytokine in tumor angiogenesis and a potential target for diagnosis and therapy. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2002, 20, 4368-4380. [CrossRef]
- Swenson, S.; Ramu, S.; Markland, F.S. Anti-angiogenesis and RGD-containing snake venom disintegrins. Current pharmaceutical design 2007, 13, 2860-2871. [CrossRef]
- Usami, Y.; Fujimura, Y.; Miura, S.; Shima, H.; Yoshida, E.; Yoshioka, A.; Hirano, K.; Suzuki, M.; Titani, K. A 28 kDa-protein with disintegrin-like structure (jararhagin-C) purified from Bothrops jararaca venom inhibits collagen- and ADP-induced platelet aggregation. Biochemical and biophysical research communications 1994, 201, 331-339. [CrossRef]
- Takada, Y.; Ye, X.; Simon, S. The integrins. Genome biology 2007, 8, 215. [CrossRef]
- Rose, D.M.; Alon, R.; Ginsberg, M.H. Integrin modulation and signaling in leukocyte adhesion and migration. Immunological reviews 2007, 218, 126-134. [CrossRef]
- Hynes, R.O. Integrins: bidirectional, allosteric signaling machines. Cell 2002, 110, 673-687. [CrossRef]
- Ruoslahti, E.; Pierschbacher, M.D. New perspectives in cell adhesion: RGD and integrins. Science 1987, 238, 491-497. [CrossRef]
- Lu, X.; Lu, D.; Scully, M.F.; Kakkar, V.V. Integrins in drug targeting-RGD templates in toxins. Current pharmaceutical design 2006, 12, 2749-2769. [CrossRef]
- Marcinkiewicz, C.; Vijay-Kumar, S.; McLane, M.A.; Niewiarowski, S. Significance of RGD loop and C-terminal domain of echistatin for recognition of alphaIIb beta3 and alpha(v) beta3 integrins and expression of ligand-induced binding site. Blood 1997, 90, 1565-1575.
- Kamata, T.; Handa, M.; Sato, Y.; Ikeda, Y.; Aiso, S. Membrane-proximal alpha/beta stalk interactions differentially regulate integrin activation. The Journal of biological chemistry 2005, 280, 24775-24783. [CrossRef]
- Calvete, J.J. Snake venomics: from the inventory of toxins to biology. Toxicon : official journal of the International Society on Toxinology 2013, 75, 44-62. [CrossRef]
- Arruda Macedo, J.K.; Fox, J.W.; de Souza Castro, M. Disintegrins from snake venoms and their applications in cancer research and therapy. Current protein & peptide science 2015, 16, 532-548. [CrossRef]
- Marcinkiewicz, C. Applications of snake venom components to modulate integrin activities in cell-matrix interactions. The international journal of biochemistry & cell biology 2013, 45, 1974-1986. [CrossRef]
- Tasoulis, T.; Isbister, G.K. A Review and Database of Snake Venom Proteomes. Toxins 2017, 9. [CrossRef]
- Fox, J.W.; Serrano, S.M. Timeline of key events in snake venom metalloproteinase research. Journal of proteomics 2009, 72, 200-209. [CrossRef]
- Fox, J.W.; Serrano, S.M. Insights into and speculations about snake venom metalloproteinase (SVMP) synthesis, folding and disulfide bond formation and their contribution to venom complexity. The FEBS journal 2008, 275, 3016-3030. [CrossRef]
- Calvete, J.J.; Marcinkiewicz, C.; Monleon, D.; Esteve, V.; Celda, B.; Juarez, P.; Sanz, L. Snake venom disintegrins: evolution of structure and function. Toxicon : official journal of the International Society on Toxinology 2005, 45, 1063-1074. [CrossRef]
- Schonthal, A.H.; Swenson, S.D.; Chen, T.C.; Markland, F.S. Preclinical studies of a novel snake venom-derived recombinant disintegrin with antitumor activity: A review. Biochemical pharmacology 2020, 181, 114149. [CrossRef]
- Calvete, J.J.; Moreno-Murciano, M.P.; Theakston, R.D.; Kisiel, D.G.; Marcinkiewicz, C. Snake venom disintegrins: novel dimeric disintegrins and structural diversification by disulphide bond engineering. The Biochemical journal 2003, 372, 725-734. [CrossRef]
- Marcinkiewicz, C. Functional characteristic of snake venom disintegrins: potential therapeutic implication. Current pharmaceutical design 2005, 11, 815-827. [CrossRef]
- !!! INVALID CITATION !!!
- Clissa, P.B.; Lopes-Ferreira, M.; Della-Casa, M.S.; Farsky, S.H.; Moura-da-Silva, A.M. Importance of jararhagin disintegrin-like and cysteine-rich domains in the early events of local inflammatory response. Toxicon : official journal of the International Society on Toxinology 2006, 47, 591-596. [CrossRef]
- Zychar, B.C.; Clissa, P.B.; Carvalho, E.; Baldo, C.; Goncalves, L.R.C. Leukocyte recruitment induced by snake venom metalloproteinases: Role of the catalytic domain. Biochemical and biophysical research communications 2020, 521, 402-407. [CrossRef]
- Zychar, B.C.; Clissa, P.B.; Carvalho, E.; Alves, A.S.; Baldo, C.; Faquim-Mauro, E.L.; Goncalves, L.R.C. Modulation of Adhesion Molecules Expression by Different Metalloproteases Isolated from Bothrops Snakes. Toxins 2021, 13. [CrossRef]
- Souza, D.H.; Iemma, M.R.; Ferreira, L.L.; Faria, J.P.; Oliva, M.L.; Zingali, R.B.; Niewiarowski, S.; Selistre-de-Araujo, H.S. The disintegrin-like domain of the snake venom metalloprotease alternagin inhibits alpha2beta1 integrin-mediated cell adhesion. Archives of biochemistry and biophysics 2000, 384, 341-350. [CrossRef]
- Mebs, D. Myotoxic activity of phospholipases A2 isolated from cobra venoms: neutralization by polyvalent antivenoms. Toxicon : official journal of the International Society on Toxinology 1986, 24, 1001-1008. [CrossRef]
- Della-Casa, M.S.; Junqueira-de-Azevedo, I.; Butera, D.; Clissa, P.B.; Lopes, D.S.; Serrano, S.M.; Pimenta, D.C.; Magalhaes, G.S.; Ho, P.L.; Moura-da-Silva, A.M. "Insularin, a disintegrin from Bothrops insularis venom: inhibition of platelet aggregation and endothelial cell adhesion by the native and recombinant GST-insularin proteins". Toxicon : official journal of the International Society on Toxinology 2011, 57, 125-133. [CrossRef]
- Gan, Z.R.; Gould, R.J.; Jacobs, J.W.; Friedman, P.A.; Polokoff, M.A. Echistatin. A potent platelet aggregation inhibitor from the venom of the viper, Echis carinatus. The Journal of biological chemistry 1988, 263, 19827-19832.
- Kamiguti, A.S.; Zuzel, M.; Theakston, R.D. Snake venom metalloproteinases and disintegrins: interactions with cells. Brazilian journal of medical and biological research = Revista brasileira de pesquisas medicas e biologicas 1998, 31, 853-862. [CrossRef]
- Tang, C.H.; Yang, R.S.; Liu, C.Z.; Huang, T.F.; Fu, W.M. Differential susceptibility of osteosarcoma cells and primary osteoblasts to cell detachment caused by snake venom metalloproteinase protein. Toxicon : official journal of the International Society on Toxinology 2004, 43, 11-20. [CrossRef]
- Brooks, S.A.; Lomax-Browne, H.J.; Carter, T.M.; Kinch, C.E.; Hall, D.M. Molecular interactions in cancer cell metastasis. Acta histochemica 2010, 112, 3-25. [CrossRef]
- Desgrosellier, J.S.; Cheresh, D.A. Integrins in cancer: biological implications and therapeutic opportunities. Nature reviews. Cancer 2010, 10, 9-22. [CrossRef]
- Golubkov, V.; Hawes, D.; Markland, F.S. Anti-angiogenic activity of contortrostatin, a disintegrin from Agkistrodon contortrix contortrix snake venom. Angiogenesis 2003, 6, 213-224. [CrossRef]
- Maiguel, D.; Faridi, M.H.; Wei, C.; Kuwano, Y.; Balla, K.M.; Hernandez, D.; Barth, C.J.; Lugo, G.; Donnelly, M.; Nayer, A.; et al. Small molecule-mediated activation of the integrin CD11b/CD18 reduces inflammatory disease. Science signaling 2011, 4, ra57. [CrossRef]
- Petpiroon, N.; Sritularak, B.; Chanvorachote, P. Correction: Phoyunnanin E inhibits migration of non-small cell lung cancer cells via suppression of epithelial-to-mesenchymal transition and integrin alphav and integrin beta3. BMC complementary medicine and therapies 2023, 23, 196. [CrossRef]
- Jahangiri, A.; Aghi, M.K.; Carbonell, W.S. beta1 integrin: Critical path to antiangiogenic therapy resistance and beyond. Cancer research 2014, 74, 3-7. [CrossRef]
- Liu, F.; Wu, Q.; Dong, Z.; Liu, K. Integrins in cancer: Emerging mechanisms and therapeutic opportunities. Pharmacology & therapeutics 2023, 247, 108458. [CrossRef]
- Olfa, K.Z.; Jose, L.; Salma, D.; Amine, B.; Najet, S.A.; Nicolas, A.; Maxime, L.; Raoudha, Z.; Kamel, M.; Jacques, M.; et al. Lebestatin, a disintegrin from Macrovipera venom, inhibits integrin-mediated cell adhesion, migration and angiogenesis. Laboratory investigation; a journal of technical methods and pathology 2005, 85, 1507-1516. [CrossRef]
- Yeh, C.H.; Peng, H.C.; Huang, T.F. Accutin, a new disintegrin, inhibits angiogenesis in vitro and in vivo by acting as integrin alphavbeta3 antagonist and inducing apoptosis. Blood 1998, 92, 3268-3276.
- Ferreira, B.A.; De Moura, F.B.R.; Tomiosso, T.C.; Correa, N.C.R.; Goulart, L.R.; Barcelos, L.S.; Clissa, P.B.; Araujo, F.A. Jararhagin-C, a disintegrin-like protein, improves wound healing in mice through stimulation of M2-like macrophage, angiogenesis and collagen deposition. International immunopharmacology 2021, 101, 108224. [CrossRef]
- Monteiro, D.A.; Kalinin, A.L.; Selistre-de-Araujo, H.S.; Nogueira, L.A.N.; Beletti, M.E.; Fernandes, M.N.; Rantin, F.T. Cardioprotective effects of alternagin-C (ALT-C), a disintegrin-like protein from Rhinocerophis alternatus snake venom, on hypoxia-reoxygenation-induced injury in fish. Comparative biochemistry and physiology. Toxicology & pharmacology : CBP 2019, 215, 67-75. [CrossRef]
- Marcinkiewicz, C.; Weinreb, P.H.; Calvete, J.J.; Kisiel, D.G.; Mousa, S.A.; Tuszynski, G.P.; Lobb, R.R. Obtustatin: a potent selective inhibitor of alpha1beta1 integrin in vitro and angiogenesis in vivo. Cancer research 2003, 63, 2020-2023.
- Staniszewska, I.; Walsh, E.M.; Rothman, V.L.; Gaathon, A.; Tuszynski, G.P.; Calvete, J.J.; Lazarovici, P.; Marcinkiewicz, C. Effect of VP12 and viperistatin on inhibition of collagen-receptor-dependent melanoma metastasis. Cancer biology & therapy 2009, 8, 1507-1516. [CrossRef]
- Sanz, L.; Chen, R.Q.; Perez, A.; Hilario, R.; Juarez, P.; Marcinkiewicz, C.; Monleon, D.; Celda, B.; Xiong, Y.L.; Perez-Paya, E.; et al. cDNA cloning and functional expression of jerdostatin, a novel RTS-disintegrin from Trimeresurus jerdonii and a specific antagonist of the alpha1beta1 integrin. The Journal of biological chemistry 2005, 280, 40714-40722. [CrossRef]
- Yeh, C.H.; Peng, H.C.; Yang, R.S.; Huang, T.F. Rhodostomin, a snake venom disintegrin, inhibits angiogenesis elicited by basic fibroblast growth factor and suppresses tumor growth by a selective alpha(v)beta(3) blockade of endothelial cells. Molecular pharmacology 2001, 59, 1333-1342. [CrossRef]
- Dos Santos, P.K.; Altei, W.F.; Danilucci, T.M.; Lino, R.L.B.; Pachane, B.C.; Nunes, A.C.C.; Selistre-de-Araujo, H.S. Alternagin-C (ALT-C), a disintegrin-like protein, attenuates alpha2beta1 integrin and VEGF receptor 2 signaling resulting in angiogenesis inhibition. Biochimie 2020, 174, 144-158. [CrossRef]
- Selistre-de-Araujo, H.S.; Cominetti, M.R.; Terruggi, C.H.; Mariano-Oliveira, A.; De Freitas, M.S.; Crepin, M.; Figueiredo, C.C.; Morandi, V. Alternagin-C, a disintegrin-like protein from the venom of Bothrops alternatus, modulates alpha2beta1 integrin-mediated cell adhesion, migration and proliferation. Brazilian journal of medical and biological research = Revista brasileira de pesquisas medicas e biologicas 2005, 38, 1505-1511. [CrossRef]
- Limam, I.; Abdelkarim, M.; El Ayeb, M.; Crepin, M.; Marrakchi, N.; Di Benedetto, M. Disintegrin-like Protein Strategy to Inhibit Aggressive Triple-Negative Breast Cancer. International journal of molecular sciences 2023, 24. [CrossRef]
- Zhou, Q.; Nakada, M.T.; Arnold, C.; Shieh, K.Y.; Markland, F.S., Jr. Contortrostatin, a dimeric disintegrin from Agkistrodon contortrix contortrix, inhibits angiogenesis. Angiogenesis 1999, 3, 259-269. [CrossRef]
- Ramos, O.H.; Kauskot, A.; Cominetti, M.R.; Bechyne, I.; Salla Pontes, C.L.; Chareyre, F.; Manent, J.; Vassy, R.; Giovannini, M.; Legrand, C.; et al. A novel alpha(v)beta (3)-blocking disintegrin containing the RGD motive, DisBa-01, inhibits bFGF-induced angiogenesis and melanoma metastasis. Clinical & experimental metastasis 2008, 25, 53-64. [CrossRef]
- Chang, C.H.; Chung, C.H.; Hsu, C.C.; Peng, H.C.; Huang, T.F. Inhibitory effects of polypeptides derived from a snake venom C-type lectin, aggretin, on tumor cell-induced platelet aggregation. Journal of thrombosis and haemostasis : JTH 2014, 12, 540-549. [CrossRef]
- Huang, T.F.; Ouyang, C. Action mechanism of the potent platelet aggregation inhibitor from Trimeresurus gramineus snake venom. Thrombosis research 1984, 33, 125-138. [CrossRef]
- Ren, A.; Wang, S.; Cai, W.; Yang, G.; Zhu, Y.; Wu, X.; Zhang, Y. Agkistin-s, a disintegrin domain, inhibits angiogenesis and induces BAECs apoptosis. Journal of cellular biochemistry 2006, 99, 1517-1523. [CrossRef]
- Takahashi, H.; Hattori, S.; Iwamatsu, A.; Takizawa, H.; Shibuya, M. A novel snake venom vascular endothelial growth factor (VEGF) predominantly induces vascular permeability through preferential signaling via VEGF receptor-1. The Journal of biological chemistry 2004, 279, 46304-46314. [CrossRef]
- Yamazaki, Y.; Matsunaga, Y.; Tokunaga, Y.; Obayashi, S.; Saito, M.; Morita, T. Snake venom Vascular Endothelial Growth Factors (VEGF-Fs) exclusively vary their structures and functions among species. The Journal of biological chemistry 2009, 284, 9885-9891. [CrossRef]
- Ferrara, N. VEGF-A: a critical regulator of blood vessel growth. European cytokine network 2009, 20, 158-163. [CrossRef]
- Chakrabarty, D.; Chanda, C. Snake Venom Disintegrins. In Snake Venoms, Inagaki, H., Vogel, C.-W., Mukherjee, A.K., Rahmy, T.R., Gopalakrishnakone, P., Eds.; Springer Netherlands: Dordrecht, 2017; pp. 437-449.
- Swenson, S.; Costa, F.; Ernst, W.; Fujii, G.; Markland, F.S. Contortrostatin, a snake venom disintegrin with anti-angiogenic and anti-tumor activity. Pathophysiology of haemostasis and thrombosis 2005, 34, 169-176. [CrossRef]
- Cesar, P.H.S.; Braga, M.A.; Trento, M.V.C.; Menaldo, D.L.; Marcussi, S. Snake Venom Disintegrins: An Overview of their Interaction with Integrins. Current drug targets 2019, 20, 465-477. [CrossRef]
- Sanchez, E.E.; Rodriguez-Acosta, A.; Palomar, R.; Lucena, S.E.; Bashir, S.; Soto, J.G.; Perez, J.C. Colombistatin: a disintegrin isolated from the venom of the South American snake (Bothrops colombiensis) that effectively inhibits platelet aggregation and SK-Mel-28 cell adhesion. Archives of toxicology 2009, 83, 271-279. [CrossRef]
- Kumar, C.C.; Malkowski, M.; Yin, Z.; Tanghetti, E.; Yaremko, B.; Nechuta, T.; Varner, J.; Liu, M.; Smith, E.M.; Neustadt, B.; et al. Inhibition of angiogenesis and tumor growth by SCH221153, a dual alpha(v)beta3 and alpha(v)beta5 integrin receptor antagonist. Cancer research 2001, 61, 2232-2238.
- Al-Ostoot, F.H.; Salah, S.; Khamees, H.A.; Khanum, S.A. Tumor angiogenesis: Current challenges and therapeutic opportunities. Cancer treatment and research communications 2021, 28, 100422. [CrossRef]
- Weis, S.M.; Cheresh, D.A. Tumor angiogenesis: molecular pathways and therapeutic targets. Nature medicine 2011, 17, 1359-1370. [CrossRef]
- Akhtar, B.; Muhammad, F.; Sharif, A.; Anwar, M.I. Mechanistic insights of snake venom disintegrins in cancer treatment. European journal of pharmacology 2021, 899, 174022. [CrossRef]
- Kalita, B.; Saviola, A.J.; Mukherjee, A.K. From venom to drugs: a review and critical analysis of Indian snake venom toxins envisaged as anticancer drug prototypes. Drug discovery today 2021, 26, 993-1005. [CrossRef]
- Louis, D.N.; Ohgaki, H.; Wiestler, O.D.; Cavenee, W.K.; Burger, P.C.; Jouvet, A.; Scheithauer, B.W.; Kleihues, P. The 2007 WHO classification of tumours of the central nervous system. Acta neuropathologica 2007, 114, 97-109. [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: a cancer journal for clinicians 2018, 68, 394-424. [CrossRef]
- Dehelean, C.A.; Marcovici, I.; Soica, C.; Mioc, M.; Coricovac, D.; Iurciuc, S.; Cretu, O.M.; Pinzaru, I. Plant-Derived Anticancer Compounds as New Perspectives in Drug Discovery and Alternative Therapy. Molecules 2021, 26. [CrossRef]
- Huang, M.; Lu, J.J.; Ding, J. Natural Products in Cancer Therapy: Past, Present and Future. Natural products and bioprospecting 2021, 11, 5-13. [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. Journal of natural products 2020, 83, 770-803. [CrossRef]
- Zhang, Q.Z.; Zhu, Y.P.; Rahat, M.A.; Kzhyshkowska, J. Editorial: Angiogenesis and tumor metastasis. Frontiers in oncology 2022, 12, 1129736. [CrossRef]
- Bielenberg, D.R.; Zetter, B.R. The Contribution of Angiogenesis to the Process of Metastasis. Cancer journal 2015, 21, 267-273. [CrossRef]
- Cross, M.J.; Claesson-Welsh, L. FGF and VEGF function in angiogenesis: signalling pathways, biological responses and therapeutic inhibition. Trends in pharmacological sciences 2001, 22, 201-207. [CrossRef]
- Laddha, A.P.; Kulkarni, Y.A. VEGF and FGF-2: Promising targets for the treatment of respiratory disorders. Respiratory medicine 2019, 156, 33-46. [CrossRef]
- Zhao, Y.; Adjei, A.A. Targeting Angiogenesis in Cancer Therapy: Moving Beyond Vascular Endothelial Growth Factor. The oncologist 2015, 20, 660-673. [CrossRef]
- Ansari, M.J.; Bokov, D.; Markov, A.; Jalil, A.T.; Shalaby, M.N.; Suksatan, W.; Chupradit, S.; Al-Ghamdi, H.S.; Shomali, N.; Zamani, A.; et al. Cancer combination therapies by angiogenesis inhibitors; a comprehensive review. Cell communication and signaling : CCS 2022, 20, 49. [CrossRef]
- Kolvekar, N.; Bhattacharya, N.; Sarkar, A.; Chakrabarty, D. How snake venom disintegrins affect platelet aggregation and cancer proliferation. Toxicon : official journal of the International Society on Toxinology 2023, 221, 106982. [CrossRef]
- Leasher, J.L.; Bourne, R.R.; Flaxman, S.R.; Jonas, J.B.; Keeffe, J.; Naidoo, K.; Pesudovs, K.; Price, H.; White, R.A.; Wong, T.Y.; et al. Global Estimates on the Number of People Blind or Visually Impaired by Diabetic Retinopathy: A Meta-analysis From 1990 to 2010. Diabetes care 2016, 39, 1643-1649. [CrossRef]
- Lee, R.; Wong, T.Y.; Sabanayagam, C. Epidemiology of diabetic retinopathy, diabetic macular edema and related vision loss. Eye and vision 2015, 2, 17. [CrossRef]
- Toh, H.; Smolentsev, A.; Bozadjian, R.V.; Keeley, P.W.; Lockwood, M.D.; Sadjadi, R.; Clegg, D.O.; Blodi, B.A.; Coffey, P.J.; Reese, B.E.; et al. Vascular changes in diabetic retinopathy-a longitudinal study in the Nile rat. Laboratory investigation; a journal of technical methods and pathology 2019, 99, 1547-1560. [CrossRef]
- Stitt, A.W.; Curtis, T.M.; Chen, M.; Medina, R.J.; McKay, G.J.; Jenkins, A.; Gardiner, T.A.; Lyons, T.J.; Hammes, H.P.; Simo, R.; et al. The progress in understanding and treatment of diabetic retinopathy. Progress in retinal and eye research 2016, 51, 156-186. [CrossRef]
- Gupta, N.; Mansoor, S.; Sharma, A.; Sapkal, A.; Sheth, J.; Falatoonzadeh, P.; Kuppermann, B.; Kenney, M. Diabetic retinopathy and VEGF. The open ophthalmology journal 2013, 7, 4-10. [CrossRef]
- Simo, R.; Sundstrom, J.M.; Antonetti, D.A. Ocular Anti-VEGF therapy for diabetic retinopathy: the role of VEGF in the pathogenesis of diabetic retinopathy. Diabetes care 2014, 37, 893-899. [CrossRef]
- Moore, S.W.; Bhat, V.K.; Flatt, P.R.; Gault, V.A.; McClean, S. Isolation and characterisation of insulin-releasing compounds from Crotalus adamanteus, Crotalus vegrandis and Bitis nasicornis venom. Toxicon : official journal of the International Society on Toxinology 2015, 101, 48-54. [CrossRef]
- Ramos, O.H.; Terruggi, C.H.; Ribeiro, J.U.; Cominetti, M.R.; Figueiredo, C.C.; Berard, M.; Crepin, M.; Morandi, V.; Selistre-de-Araujo, H.S. Modulation of in vitro and in vivo angiogenesis by alternagin-C, a disintegrin-like protein from Bothrops alternatus snake venom and by a peptide derived from its sequence. Archives of biochemistry and biophysics 2007, 461, 1-6. [CrossRef]
- Selistre-de-Araujo, H.S.; Pontes, C.L.; Montenegro, C.F.; Martin, A.C. Snake venom disintegrins and cell migration. Toxins 2010, 2, 2606-2621. [CrossRef]
- Gurtner, G.C.; Werner, S.; Barrandon, Y.; Longaker, M.T. Wound repair and regeneration. Nature 2008, 453, 314-321. [CrossRef]
- Zhao, R.; Liang, H.; Clarke, E.; Jackson, C.; Xue, M. Inflammation in Chronic Wounds. International journal of molecular sciences 2016, 17. [CrossRef]
- Schnittert, J.; Bansal, R.; Storm, G.; Prakash, J. Integrins in wound healing, fibrosis and tumor stroma: High potential targets for therapeutics and drug delivery. Advanced drug delivery reviews 2018, 129, 37-53. [CrossRef]
- Ferreira, B.A.; Deconte, S.R.; de Moura, F.B.R.; Tomiosso, T.C.; Clissa, P.B.; Andrade, S.P.; Araujo, F.A. Inflammation, angiogenesis and fibrogenesis are differentially modulated by distinct domains of the snake venom metalloproteinase jararhagin. International journal of biological macromolecules 2018, 119, 1179-1187. [CrossRef]
- Sant'Ana, E.M.; Gouvea, C.M.; Durigan, J.L.; Cominetti, M.R.; Pimentel, E.R.; Selistre-de-Araujo, H.S. Rat skin wound healing induced by alternagin-C, a disintegrin-like, Cys-rich protein from Bothrops alternatus venom. International wound journal 2011, 8, 245-252. [CrossRef]
- Rabelo, L.F.G.; Ferreira, B.A.; Deconte, S.R.; Tomiosso, T.C.; Dos Santos, P.K.; Andrade, S.P.; Selistre de Araujo, H.S.; Araujo, F.A. Alternagin-C, a disintegrin-like protein from Bothrops alternatus venom, attenuates inflammation and angiogenesis and stimulates collagen deposition of sponge-induced fibrovascular tissue in mice. International journal of biological macromolecules 2019, 140, 653-660. [CrossRef]
- Turner, C.J.; Badu-Nkansah, K.; Crowley, D.; van der Flier, A.; Hynes, R.O. alpha5 and alphav integrins cooperate to regulate vascular smooth muscle and neural crest functions in vivo. Development 2015, 142, 797-808. [CrossRef]
- Topol, E.J.; Byzova, T.V.; Plow, E.F. Platelet GPIIb-IIIa blockers. Lancet 1999, 353, 227-231. [CrossRef]
- Hartman, G.D.; Egbertson, M.S.; Halczenko, W.; Laswell, W.L.; Duggan, M.E.; Smith, R.L.; Naylor, A.M.; Manno, P.D.; Lynch, R.J.; Zhang, G.; et al. Non-peptide fibrinogen receptor antagonists. 1. Discovery and design of exosite inhibitors. Journal of medicinal chemistry 1992, 35, 4640-4642. [CrossRef]
- Almeida, G.O.; de Oliveira, I.S.; Arantes, E.C.; Sampaio, S.V. Snake venom disintegrins update: insights about new findings. The journal of venomous animals and toxins including tropical diseases 2023, 29, e20230039. [CrossRef]
- Lang, S.H.; Manning, N.; Armstrong, N.; Misso, K.; Allen, A.; Di Nisio, M.; Kleijnen, J. Treatment with tirofiban for acute coronary syndrome (ACS): a systematic review and network analysis. Current medical research and opinion 2012, 28, 351-370. [CrossRef]
- Bordon, K.C.F.; Cologna, C.T.; Fornari-Baldo, E.C.; Pinheiro-Junior, E.L.; Cerni, F.A.; Amorim, F.G.; Anjolette, F.A.P.; Cordeiro, F.A.; Wiezel, G.A.; Cardoso, I.A.; et al. From Animal Poisons and Venoms to Medicines: Achievements, Challenges and Perspectives in Drug Discovery. Frontiers in pharmacology 2020, 11, 1132. [CrossRef]
- Scarborough, R.M.; Rose, J.W.; Hsu, M.A.; Phillips, D.R.; Fried, V.A.; Campbell, A.M.; Nannizzi, L.; Charo, I.F. Barbourin. A GPIIb-IIIa-specific integrin antagonist from the venom of Sistrurus m. barbouri. The Journal of biological chemistry 1991, 266, 9359-9362.
- Roth, G.A.; Huffman, M.D.; Moran, A.E.; Feigin, V.; Mensah, G.A.; Naghavi, M.; Murray, C.J. Global and regional patterns in cardiovascular mortality from 1990 to 2013. Circulation 2015, 132, 1667-1678. [CrossRef]
- Podrez, E.A.; Byzova, T.V.; Febbraio, M.; Salomon, R.G.; Ma, Y.; Valiyaveettil, M.; Poliakov, E.; Sun, M.; Finton, P.J.; Curtis, B.R.; et al. Platelet CD36 links hyperlipidemia, oxidant stress and a prothrombotic phenotype. Nature medicine 2007, 13, 1086-1095. [CrossRef]
- Venturini, W.; Olate-Briones, A.; Valenzuela, C.; Mendez, D.; Fuentes, E.; Cayo, A.; Mancilla, D.; Segovia, R.; Brown, N.E.; Moore-Carrasco, R. Platelet Activation Is Triggered by Factors Secreted by Senescent Endothelial HMEC-1 Cells In Vitro. International journal of molecular sciences 2020, 21. [CrossRef]
- Huilcaman, R.; Venturini, W.; Fuenzalida, L.; Cayo, A.; Segovia, R.; Valenzuela, C.; Brown, N.; Moore-Carrasco, R. Platelets, a Key Cell in Inflammation and Atherosclerosis Progression. Cells 2022, 11. [CrossRef]
- Bledzka, K.; Smyth, S.S.; Plow, E.F. Integrin alphaIIbbeta3: from discovery to efficacious therapeutic target. Circulation research 2013, 112, 1189-1200. [CrossRef]
- Rivas-Mercado, E.A.; Garza-Ocañas, L. Disintegrins obtained from snake venom and their pharmacological potential. Medicina Universitaria 2017, 19, 32-37. [CrossRef]
- Canas, C.A.; Castano-Valencia, S.; Castro-Herrera, F.; Canas, F.; Tobon, G.J. Biomedical applications of snake venom: from basic science to autoimmunity and rheumatology. Journal of translational autoimmunity 2021, 4, 100076. [CrossRef]
- Cook, J.J.; Huang, T.F.; Rucinski, B.; Strzyzewski, M.; Tuma, R.F.; Williams, J.A.; Niewiarowski, S. Inhibition of platelet hemostatic plug formation by trigramin, a novel RGD-peptide. The American journal of physiology 1989, 256, H1038-1043. [CrossRef]
- Monteiro, D.A.; Kalinin, A.L.; Selistre-de-Araujo, H.S.; Vasconcelos, E.S.; Rantin, F.T. Alternagin-C (ALT-C), a disintegrin-like protein from Rhinocerophis alternatus snake venom promotes positive inotropism and chronotropism in fish heart. Toxicon : official journal of the International Society on Toxinology 2016, 110, 1-11. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





