Submitted:
12 December 2023
Posted:
13 December 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction to Plasma Technology
2. Plasma Medicine
2.1. Medical Applications of Plasma
2.1.1. Cold Atmospheric Plasma (CAP)
2.1.2. Thermal Plasma
3. CAP in Dermatology
3.1. Physical Characteristics and Classification of CAP:
3.2. Direct and Indirect Plasma
3.3. Penetration and Mechanism of Action of Active Components of CAP in the Skin
4. CAP for Therapeutic Purposes in Dermatology
4.1. CAP's Suppressive Effect on Microbial Infection
4.1.1. Infectious Skin Diseases
4.1.2. Effects of CAP on Bacteria
4.1.3. Effects of CAP on Fungi
4.1.4. Effects of CAP on Viruses and Parasites
4.2. CAP Promotes Tissue Proliferation and Wound Healing
4.3. CAP for the Treatment of Inflammatory Skin Diseases
4.3.1. Psoriasis
4.3.2. Atopic Dermatitis
4.3.3. Vitiligo
4.4. CAP Suppresses Tumor Cell Proliferation and Migration
4.5. Cosmetic and Skincare Applications of CAP
4.6. CAP's Role in Treating Immune-Mediated Skin Diseases
5. Safety and Tolerance of CAP Applications
6. Challenges and Solutions in CAP Applications for Skin Disease Treatment and Skincare:
7. Conclusion and Future Prospects of CAP in Dermatology
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gan L, Jiang J, Duan JW, Wu XJZ, Zhang S, Duan XR, Song JQ, Chen HX. Cold atmospheric plasma applications in dermatology: A systematic review. J Biophotonics. 2021 Mar;14(3):e202000415. Epub 2020 Dec 21. PMID: 33231354. [CrossRef]
- Braný, D., Dvorská, D., Halašová, E., & Škovierová, H. (2020). Cold Atmospheric Plasma: A Powerful Tool for Modern Medicine. International journal of molecular sciences, 21(8), 2932. [CrossRef]
- Laroussi, M. (2020). Cold plasma in medicine and healthcare: The new frontier in low-temperature plasma applications. Frontiers in Physics, 8, 74. [CrossRef]
- Chen, Z., Garcia, G., Arumugaswami, V., & Wirz, R. E. (2020). Cold atmospheric plasma for SARS-CoV-2 inactivation. Physics of Fluids, 32(11).
- Chen, Z., Chen, G., Obenchain, R., Zhang, R., Bai, F., Fang, T., ... & Gu, Z. (2022). Cold atmospheric plasma delivery for biomedical applications. Materials Today, 54, 153-188. [CrossRef]
- Duarte, S., & Panariello, B. H. D. (2020). Comprehensive biomedical applications of low temperature plasmas. Archives of biochemistry and biophysics, 693, 108560. [CrossRef]
- Bekeschus, S., von Woedtke, T., Emmert, S., & Schmidt, A. (2021). Medical gas plasma-stimulated wound healing: Evidence and mechanisms. Redox biology, 46, 102116. [CrossRef]
- Khanikar, R. R., & Bailung, H. (2022). Cold atmospheric pressure plasma technology for biomedical application. Plasma science and technology.
- Khalaf, A. T., Sun, Y., Wang, F., Sheng, M., Li, Y., & Liu, X. (2020). Photodynamic therapy using HMME for port-wine stains: clinical effectiveness and sonographic appearance. BioMed Research International, 2020. [CrossRef]
- Khanikar, R. R., Bailung, H., & Sankaranarayanan, K. (2022). Review of the cold atmospheric plasma technology application in food, disinfection, and textiles: A way forward for achieving circular economy. Frontiers in Physics, 10, 942952. [CrossRef]
- Von Woedtke, T., Reuter, S., Masur, K., & Weltmann, K. D. (2013). Plasmas for medicine. Physics Reports, 530(4), 291-320.
- Laroussi, M. Plasma Medicine: A Brief Introduction. Plasma 2018, 1, 47-60. [CrossRef]
- Sakudo, A., Yagyu, Y., & Onodera, T. (2019). Disinfection and sterilization using plasma technology: Fundamentals and future perspectives for biological applications. International journal of molecular sciences, 20(20), 5216. [CrossRef]
- Liu, D., Szili, E. J., & Ostrikov, K. K. (2020). Plasma medicine: Opportunities for nanotechnology in a digital age. Plasma processes and polymers (Print), 17(10), 2000097. [CrossRef]
- Alizadeh, E., & Ptasińska, S. (2021). Recent advances in plasma-based cancer treatments: approaching clinical translation through an intracellular view. Biophysica, 1(1), 48-72. [CrossRef]
- Ercan, U. K., Özdemir, G. D., Özdemir, M. A., & Güren, O. (2023). Plasma medicine: The era of artificial intelligence. Plasma Processes and Polymers, e2300066. [CrossRef]
- Choi, E. H., Uhm, H. S., & Kaushik, N. K. (2021). Plasma bioscience and its application to medicine. AAPPS Bulletin, 31, 1-38. [CrossRef]
- Privat-Maldonado, A., Schmidt, A., Lin, A., Weltmann, K. D., Wende, K., Bogaerts, A., & Bekeschus, S. (2019). ROS from physical plasmas: Redox chemistry for biomedical therapy. Oxidative medicine and cellular longevity, 2019. [CrossRef]
- Busco, G., Robert, E., Chettouh-Hammas, N., Pouvesle, J. M., & Grillon, C. (2020). The emerging potential of cold atmospheric plasma in skin biology. Free Radical Biology and Medicine, 161, 290-304. [CrossRef]
- Koga-Ito, C. Y., Kostov, K. G., Miranda, F. S., Milhan, N. V. M., Azevedo Neto, N. F., Nascimento, F., & Pessoa, R. S. (2023). Cold Atmospheric Plasma as a Therapeutic Tool in Medicine and Dentistry. Plasma Chemistry and Plasma Processing, 1-37. [CrossRef]
- von Woedtke, T., Emmert, S., Metelmann, H. R., Rupf, S., & Weltmann, K. D. (2020). Perspectives on cold atmospheric plasma (CAP) applications in medicine. Physics of Plasmas, 27(7). [CrossRef]
- Bharti, B., Li, H., Ren, Z., Zhu, R., & Zhu, Z. (2022). Recent advances in sterilization and disinfection technology: A review. Chemosphere, 136404. [CrossRef]
- Deng, L. Z., Mujumdar, A. S., Pan, Z., Vidyarthi, S. K., Xu, J., Zielinska, M., & Xiao, H. W. (2020). Emerging chemical and physical disinfection technologies of fruits and vegetables: a comprehensive review. Critical reviews in food science and nutrition, 60(15), 2481-2508. [CrossRef]
- von Woedtke, T., Schmidt, A., Bekeschus, S., Wende, K., & Weltmann, K. D. (2019). Plasma medicine: A field of applied redox biology. In vivo, 33(4), 1011-1026. [CrossRef]
- Azzariti, A., Iacobazzi, R. M., Di Fonte, R., Porcelli, L., Gristina, R., Favia, P., ... & Sardella, E. (2019). Plasma-activated medium triggers cell death and the presentation of immune activating danger signals in melanoma and pancreatic cancer cells. Scientific reports, 9(1), 4099. [CrossRef]
- Mitra, S., Nguyen, L. N., Akter, M., Park, G., Choi, E. H., & Kaushik, N. K. (2019). Impact of ROS generated by chemical, physical, and plasma techniques on cancer attenuation. Cancers, 11(7), 1030. [CrossRef]
- Tanaka, H., Bekeschus, S., Yan, D., Hori, M., Keidar, M., & Laroussi, M. (2021). Plasma-treated solutions (PTS) in cancer therapy. Cancers, 13(7), 1737. [CrossRef]
- Canady, J., Murthy, S. R., Zhuang, T., Gitelis, S., Nissan, A., Ly, L., ... & Basadonna, G. (2023). The first cold atmospheric plasma phase I clinical trial for the treatment of advanced solid tumors: A novel treatment arm for cancer. Cancers, 15(14), 3688. [CrossRef]
- Zhao, L. X., Zhao, H. X., Chen, H., Hu, C., Zhang, Y., & Li, H. P. (2023). Characteristics of Portable Air Floating-Electrode Dielectric-Barrier-Discharge Plasmas Used for Biomedicine. Plasma Chemistry and Plasma Processing, 1-19. [CrossRef]
- Milhan, N. V. M., Chiappim, W., Sampaio, A. D. G., Vegian, M. R. D. C., Pessoa, R. S., & Koga-Ito, C. Y. (2022). Applications of plasma-activated water in dentistry: A review. International Journal of Molecular Sciences, 23(8), 4131. [CrossRef]
- Nguyen, L., Lu, P., Boehm, D., Bourke, P., Gilmore, B. F., Hickok, N. J., & Freeman, T. A. (2018). Cold atmospheric plasma is a viable solution for treating orthopedic infection: a review. Biological chemistry, 400(1), 77-86. [CrossRef]
- Moszczyńska, J., Roszek, K., & Wiśniewski, M. (2023). Non-thermal plasma application in medicine—focus on reactive species involvement. International Journal of Molecular Sciences, 24(16), 12667. [CrossRef]
- Bhatt, P., Kumar, V., Subramaniyan, V., Nagarajan, K., Sekar, M., Chinni, S. V., & Ramachawolran, G. (2023). Plasma modification techniques for natural polymer-based drug delivery systems. Pharmaceutics, 15(8), 2066. [CrossRef]
- Thanigaivel, S., Priya, A. K., Balakrishnan, D., Dutta, K., Rajendran, S., & Soto-Moscoso, M. (2022). Insight on recent development in metallic biomaterials: Strategies involving synthesis, types and surface modification for advanced therapeutic and biomedical applications. Biochemical Engineering Journal, 187, 108522. [CrossRef]
- Kolimi, P., Narala, S., Nyavanandi, D., Youssef, A. A. A., & Dudhipala, N. (2022). Innovative treatment strategies to accelerate wound healing: trajectory and recent advancements. Cells, 11(15), 2439. [CrossRef]
- Haertel, B., Von Woedtke, T., Weltmann, K. D., & Lindequist, U. (2014). Non-thermal atmospheric-pressure plasma possible application in wound healing. Biomolecules & therapeutics, 22(6), 477. [CrossRef]
- Joseph, A., Rane, R., & Vaid, A. (2021). Atmospheric Pressure Plasma Therapy for Wound Healing and Disinfection: A Review. Wound Healing Research: Current Trends and Future Directions, 621-641.
- Gibson, P., & Suslov, N. (2012). The design of the PlasmaJet® thermal plasma system and its application in surgery. Plasma Medicine, 2(1-3). [CrossRef]
- Tang, L., & Huang, H. (2005). Biomass gasification using capacitively coupled RF plasma technology. Fuel, 84(16), 2055-2063. [CrossRef]
- Von Woedtke, T., Laroussi, M., & Gherardi, M. (2022). Foundations of plasmas for medical applications. Plasma Sources Science and Technology, 31(5), 054002. [CrossRef]
- Heinlin, J., Isbary, G., Stolz, W., Morfill, G., Landthaler, M., Shimizu, T., ... & Karrer, S. (2011). Plasma applications in medicine with a special focus on dermatology. Journal of the European Academy of Dermatology and Venereology, 25(1), 1-11. [CrossRef]
- Charles, C. (2014). Grand challenges in low-temperature plasma physics. Frontiers in Physics, 2, 39. [CrossRef]
- Bernhardt, T., Semmler, M. L., Schäfer, M., Bekeschus, S., Emmert, S., & Boeckmann, L. (2019). Plasma medicine: Applications of cold atmospheric pressure plasma in dermatology. Oxidative medicine and cellular longevity, 2019. [CrossRef]
- Ghadirian, F., Abbasi, H., Bavi, O., & Naeimabadi, A. (2023). How living cells are affected during the cold atmospheric pressure plasma treatment. Free Radical Biology and Medicine. [CrossRef]
- Soliman, H. M. (2014). Design and Experimental Investigations of Electrical Breakdown in a Plasma Jet Device and Applications (Doctoral dissertation, Benha University).
- Ngo, A. D. (2023). Investigation of Cold Atmospheric Plasma for Wound Care (Doctoral dissertation, Oklahoma State University).
- Seong, M. J., Ha, Y. J., Park, G. H., Kim, S. J., Joh, H. M., & Chung, T. H. (2023). Effects of operational parameters on plasma characteristics and liquid treatment of a DBD-based unipolar microsecond-pulsed helium atmospheric pressure plasma jet. Physics of Plasmas, 30(11). [CrossRef]
- Mumtaz, S., Khan, R., Rana, J. N., Javed, R., Iqbal, M., Choi, E. H., & Han, I. (2023). Review on the Biomedical and Environmental Applications of Nonthermal Plasma. Catalysts, 13(4), 685. [CrossRef]
- Tsoukou, E. (2021). Understanding the Potential of Plasma Activated Liquids for Biomedical Applications.
- Zhou, R., Zhou, R., Wang, P., Xian, Y., Mai-Prochnow, A., Lu, X., ... & Bazaka, K. (2020). Plasma-activated water: Generation, origin of reactive species and biological applications. Journal of Physics D: Applied Physics, 53(30), 303001. [CrossRef]
- Al-Shehri, S. S. (2021). Reactive oxygen and nitrogen species and innate immune response. Biochimie, 181, 52-64. [CrossRef]
- Edge, R., & Truscott, T. G. (2021). The reactive oxygen species singlet oxygen, hydroxy radicals, and the superoxide radical anion—examples of their roles in biology and medicine. Oxygen, 1(2), 77-95. [CrossRef]
- Jirešová, J., Scholtz, V., Julák, J., & Šerá, B. (2022). Comparison of the effect of plasma-activated water and artificially prepared plasma-activated water on wheat grain properties. Plants, 11(11), 1471. [CrossRef]
- Chen, J., Wang, Z., Sun, J., Zhou, R., Guo, L., Zhang, H., ... & Ostrikov, K. (2023). Plasma-Activated Hydrogels for Microbial Disinfection. Advanced Science, 10(14), 2207407. [CrossRef]
- Chen, Z., Chen, G., Obenchain, R., Zhang, R., Bai, F., Fang, T., ... & Gu, Z. (2022). Cold atmospheric plasma delivery for biomedical applications. Materials Today, 54, 153-188. [CrossRef]
- Banga, A. K., & Panus, P. C. (2017). lontophoretic devices: clinical applications and rehabilitation medicine. Critical Reviews™ in Physical and Rehabilitation Medicine, 29(1-4). [CrossRef]
- Wen, X., Xin, Y., Hamblin, M. R., & Jiang, X. (2021). Applications of cold atmospheric plasma for transdermal drug delivery: A review. Drug Delivery and Translational Research, 11, 741-747. [CrossRef]
- Chen, C., Liu, D. X., Liu, Z. C., Yang, A. J., Chen, H. L., Shama, G., & Kong, M. G. (2014). A model of plasma-biofilm and plasma-tissue interactions at ambient pressure. Plasma Chemistry and Plasma Processing, 34, 403-441. [CrossRef]
- Mai-Prochnow, A., Zhou, R., Zhang, T., Ostrikov, K., Mugunthan, S., Rice, S. A., & Cullen, P. J. (2021). Interactions of plasma-activated water with biofilms: Inactivation, dispersal effects and mechanisms of action. npj Biofilms and Microbiomes, 7(1), 11. [CrossRef]
- Jang, J. Y., Hong, Y. J., Lim, J., Choi, J. S., Choi, E. H., Kang, S., & Rhim, H. (2018). Cold atmospheric plasma (CAP), a novel physicochemical source, induces neural differentiation through cross-talk between the specific RONS cascade and Trk/Ras/ERK signaling pathway. Biomaterials, 156, 258-273. [CrossRef]
- Arndt, S., Unger, P., Wacker, E., Shimizu, T., Heinlin, J., Li, Y. F., ... & Karrer, S. (2013). Cold atmospheric plasma (CAP) changes gene expression of key molecules of the wound healing machinery and improves wound healing in vitro and in vivo. PloS one, 8(11), e79325. [CrossRef]
- Frescaline, N., Duchesne, C., Favier, M., Onifarasoaniaina, R., Guilbert, T., Uzan, G., ... & Lataillade, J. J. (2020). Physical plasma therapy accelerates wound re-epithelialisation and enhances extracellular matrix formation in cutaneous skin grafts. The Journal of Pathology, 252(4), 451-464. [CrossRef]
- Daeschlein, G. (2018). Antimicrobial activity of plasma. Comprehensive Clinical Plasma Medicine: Cold Physical Plasma for Medical Application, 113-125.
- Deepak, G. D. (2022). Review on recent advances in cold plasma technology. The European Physical Journal Applied Physics, 97, 39. [CrossRef]
- Murillo, D., Huergo, C., Gallego, B., Rodríguez, R., & Tornín, J. (2023). Exploring the Use of Cold Atmospheric Plasma to Overcome Drug Resistance in Cancer. Biomedicines, 11(1), 208. [CrossRef]
- Rao, Y., Shang, W., Yang, Y., Zhou, R., & Rao, X. (2020). Fighting mixed-species microbial biofilms with cold atmospheric plasma. Frontiers in Microbiology, 11, 1000. [CrossRef]
- Maisch, T., Shimizu, T., Mitra, A., Heinlin, J., Karrer, S., Li, Y. F., ... & Zimmermann, J. L. (2012). Contact-free cold atmospheric plasma treatment of Deinococcus radiodurans. Journal of industrial microbiology and biotechnology, 39(9), 1367-1375. [CrossRef]
- Mai-Prochnow, A., Alam, D., Zhou, R., Zhang, T., Ostrikov, K., & Cullen, P. J. (2021). Microbial decontamination of chicken using atmospheric plasma bubbles. Plasma Processes and Polymers, 18(1), 2000052. [CrossRef]
- Dezest, M., Bulteau, A. L., Quinton, D., Chavatte, L., Le Bechec, M., Cambus, J. P., ... & Cousty, S. (2017). Oxidative modification and electrochemical inactivation of Escherichia coli upon cold atmospheric pressure plasma exposure. PLoS One, 12(3), e0173618. [CrossRef]
- Ziuzina, D., Boehm, D., Patil, S., Cullen, P. J., & Bourke, P. (2015). Cold plasma inactivation of bacterial biofilms and reduction of quorum sensing regulated virulence factors. PLoS One, 10(9), e0138209. [CrossRef]
- Brun, P., Bernabè, G., Marchiori, C., Scarpa, M., Zuin, M., Cavazzana, R., ... & Martines, E. (2018). Antibacterial efficacy and mechanisms of action of low power atmospheric pressure cold plasma: membrane permeability, biofilm penetration and antimicrobial sensitization. Journal of applied microbiology, 125(2), 398-408. [CrossRef]
- Mirpour, S., Fathollah, S., Mansouri, P., Larijani, B., Ghoranneviss, M., Mohajeri Tehrani, M., & Amini, M. R. (2020). Cold atmospheric plasma as an effective method to treat diabetic foot ulcers: A randomized clinical trial. Scientific Reports, 10(1), 10440. [CrossRef]
- Hiller, J., Stratmann, B., Timm, J., Costea, T. C., & Tschoepe, D. (2022). Enhanced growth factor expression in chronic diabetic wounds treated by cold atmospheric plasma. Diabetic Medicine, 39(6), e14787. [CrossRef]
- Daeschlein, G., Napp, M., Lutze, S., Arnold, A., von Podewils, S., Guembel, D., & Jünger, M. (2015). Skin and wound decontamination of multidrug-resistant bacteria by cold atmospheric plasma coagulation. JDDG: Journal der Deutschen Dermatologischen Gesellschaft, 13(2), 143-149. [CrossRef]
- Gu, X., Huang, D., Chen, J., Li, X., Zhou, Y., Huang, M., ... & Yu, P. (2022). Bacterial inactivation and biofilm disruption through indigenous prophage activation using low-intensity cold atmospheric plasma. Environmental Science & Technology, 56(12), 8920-8931. [CrossRef]
- O'connor, N., Cahill, O., Daniels, S., Galvin, S., & Humphreys, H. (2014). Cold atmospheric pressure plasma and decontamination. Can it contribute to preventing hospital-acquired infections?. Journal of hospital infection, 88(2), 59-65. [CrossRef]
- Liu, J., Yang, C., Cheng, C., Zhang, C., Zhao, J., & Fu, C. (2021). In vitro antimicrobial effect and mechanism of action of plasma-activated liquid on planktonic Neisseria gonorrhoeae. Bioengineered, 12(1), 4605-4619. [CrossRef]
- Xu, H., Liu, D., Wang, W., Liu, Z., Guo, L., Rong, M., & Kong, M. G. (2018). Investigation on the RONS and bactericidal effects induced by He+ O2 cold plasma jets: In open air and in an airtight chamber. Physics of Plasmas, 25(11). [CrossRef]
- Das, S., Gajula, V. P., Mohapatra, S., Singh, G., & Kar, S. (2022). Role of cold atmospheric plasma in microbial inactivation and the factors affecting its efficacy. Health Sciences Review, 100037. [CrossRef]
- Yang, L., Niyazi, G., Qi, Y., Yao, Z., Huang, L., Wang, Z., ... & Liu, D. (2021). Plasma-activated saline promotes antibiotic treatment of systemic methicillin-resistant Staphylococcus aureus infection. Antibiotics, 10(8), 1018. [CrossRef]
- Cooper, R. A., Bjarnsholt, T., & Alhede, M. (2014). Biofilms in wounds: a review of present knowledge. Journal of wound care, 23(11), 570-582. [CrossRef]
- Shabani, H., Dezhpour, A., Jafari, S., Moghaddam, M. M., & Nilkar, M. (2023). Antimicrobial activity of cold atmospheric-pressure argon plasma combined with chicory (Cichorium intybus L.) extract against P. aeruginosa and E. coli biofilms. Scientific Reports, 13(1), 9441. [CrossRef]
- Guo, J., Wang, J., Xie, H., Jiang, J., Li, C., Li, W., ... & Lin, F. (2022). Inactivation effects of plasma-activated water on Fusarium graminearum. Food Control, 134, 108683. [CrossRef]
- Khalaf, A. T., Wei, Y., Wan, J., Zhu, J., Peng, Y., Abdul Kadir, S. Y., ... & Shi, Z. (2022). Bone tissue engineering through 3D bioprinting of bioceramic scaffolds: a review and update. Life, 12(6), 903. Zhai, S. Y., Kong, M. G., & Xia, Y. M. (2022). Cold atmospheric plasma ameliorates skin diseases involving reactive oxygen/nitrogen species-mediated functions. Frontiers in Immunology, 13, 868386.
- Sharma, N. K., Misra, S., Varun, V., & Pal, U. N. (2020). Experimental and simulation analysis of dielectric barrier discharge based pulsed cold atmospheric pressure plasma jet. Physics of Plasmas, 27(11). [CrossRef]
- Siadati, S., Pet’ková, M., Kenari, A. J., Kyzek, S., Gálová, E., & Zahoranová, A. (2020). Effect of a non-thermal atmospheric pressure plasma jet on four different yeasts. Journal of physics D: Applied physics, 54(2), 025204.
- Al-Ghanmi, A. R., Fuliful, F. K., Shukur, A. S., Alsultany, F. H., & Wadi, I. A. Cold Atmospheric Pressure Plasma Apparatus for Use in Treatment of Fungal Skin Infections.
- Fuliful, F. K., Shukur, A. S., Al-Ghanmi, A. R., & Wadi, I. (2022). Cold Atmospheric Pressure Plasma Apparatus for Use in Treatment of Fungal Skin Infections. Available at SSRN 4201870.
- Lux, J., Dobiáš, R., Kuklová, I., Litvik, R., Scholtz, V., Soušková, H., ... & Julák, J. (2020). Inactivation of dermatophytes causing onychomycosis and its therapy using non-thermal plasma. Journal of Fungi, 6(4), 214. [CrossRef]
- Liu, Z., Zheng, Y., Dang, J., Zhang, J., Dong, F., Wang, K., & Zhang, J. (2019). A novel antifungal plasma-activated hydrogel. ACS applied materials & interfaces, 11(26), 22941-22949. [CrossRef]
- Filipić, A., Gutierrez-Aguirre, I., Primc, G., Mozetič, M., & Dobnik, D. (2020). Cold plasma, a new hope in the field of virus inactivation. Trends in Biotechnology, 38(11), 1278-1291. [CrossRef]
- Friedman, P. C., Miller, V., Fridman, G., Lin, A., & Fridman, A. (2021). U.S. Patent Application No. 17/255,853.
- Friedman, P. C., Fridman, G., & Fridman, A. (2020). Using cold plasma to treat warts in children: A case series. Pediatric Dermatology, 37(4), 706-709. [CrossRef]
- Bunz, O., Mese, K., Funk, C., Wulf, M., Bailer, S. M., Piwowarczyk, A., & Ehrhardt, A. (2020). Cold atmospheric plasma as antiviral therapy–effect on human herpes simplex virus type 1. The Journal of general virology, 101(2), 208. [CrossRef]
- Malik, S., Gill, M., Fridman, G., Fridman, A., & Friedman, P. C. (2022). Cold atmospheric plasma reduces demodex count on the face comparably to topical ivermectin, as measured by reflectance confocal microscopy. [CrossRef]
- Ten Bosch, L., Habedank, B., Siebert, D., Mrotzek, J., & Viöl, W. (2019). Cold atmospheric pressure plasma comb—a physical approach for pediculosis treatment. International Journal of Environmental Research and Public Health, 16(1), 19. [CrossRef]
- Gao, J., Wang, L., Xia, C., Yang, X., Cao, Z., Zheng, L., ... & Cheng, C. (2019). Cold atmospheric plasma promotes different types of superficial skin erosion wounds healing. International Wound Journal, 16(5), 1103-1111. [CrossRef]
- Arndt, S., Unger, P., Berneburg, M., Bosserhoff, A. K., & Karrer, S. (2018). Cold atmospheric plasma (CAP) activates angiogenesis-related molecules in skin keratinocytes, fibroblasts and endothelial cells and improves wound angiogenesis in an autocrine and paracrine mode. Journal of dermatological science, 89(2), 181-190. [CrossRef]
- Hasse, S., Duong Tran, T., Hahn, O., Kindler, S., Metelmann, H. R., von Woedtke, T., & Masur, K. (2016). Induction of proliferation of basal epidermal keratinocytes by cold atmospheric-pressure plasma. Clinical and experimental dermatology, 41(2), 202-209. Hasse, S., Duong Tran, T., Hahn, O., Kindler, S., Metelmann, H. R., von Woedtke, T., & Masur, K. (2016). Induction of proliferation of basal epidermal keratinocytes by cold atmospheric-pressure plasma. Clinical and experimental dermatology, 41(2), 202-209. [CrossRef]
- Arndt, S., Landthaler, M., Zimmermann, J. L., Unger, P., Wacker, E., Shimizu, T., ... & Karrer, S. (2015). Effects of cold atmospheric plasma (CAP) on ß-defensins, inflammatory cytokines, and apoptosis-related molecules in keratinocytes in vitro and in vivo. PLoS one, 10(3), e0120041. [CrossRef]
- Tan, F., Rui, X., Xiang, X., Yu, Z., & Al-Rubeai, M. (2021). Multimodal treatment combining cold atmospheric plasma and acidic fibroblast growth factor for multi-tissue regeneration. The FASEB Journal, 35(5), e21442. [CrossRef]
- Bolgeo, T., Maconi, A., Gardalini, M., Gatti, D., Di Matteo, R., Lapidari, M., Longhitano, Y., Savioli, G., Piccioni, A., & Zanza, C. (2023). The Role of Cold Atmospheric Plasma in Wound Healing Processes in Critically Ill Patients. Journal of personalized medicine, 13(5), 736. [CrossRef]
- Dubey, S. K., Parab, S., Alexander, A., Agrawal, M., Achalla, V. P. K., Pal, U. N., ... & Kesharwani, P. (2022). Cold atmospheric plasma therapy in wound healing. Process Biochemistry, 112, 112-123. [CrossRef]
- Guo, J., Huang, Y., Xu, B., & Yang, J. (2022). Efficacy of Cold Atmospheric Plasma Therapy on Chronic Wounds: An Updated Systematic Review and Meta-Analysis of RCTs. Computational and mathematical methods in medicine, 2022, 5798857. [CrossRef]
- Stratmann, B., Costea, T. C., Nolte, C., Hiller, J., Schmidt, J., Reindel, J., ... & Tschoepe, D. (2020). Effect of cold atmospheric plasma therapy vs standard therapy placebo on wound healing in patients with diabetic foot ulcers: a randomized clinical trial. JAMA network open, 3(7), e2010411-e2010411.
- Li, M., Gao, J., Wang, L., Liu, J., Fu, C., Yang, X., ... & Yang, C. (2023). Basic research and clinical exploration of cold atmospheric plasma for skin wounds. Bioengineering & Translational Medicine, e10550.
- Schmidt, Kang, S. U., Choi, J. W., Chang, J. W., Kim, K. I., Kim, Y. S., Park, J. K., ... & Kim, C. H. (2017). N2 non-thermal atmospheric pressure plasma promotes wound healing in vitro and in vivo: Potential modulation of adhesion molecules and matrix metalloproteinase-9. Experimental dermatology, 26(2), 163-170. [CrossRef]
- A., Bekeschus, S., Wende, K., Vollmar, B., & von Woedtke, T. (2017). A cold plasma jet accelerates wound healing in a murine model of full-thickness skin wounds. Experimental dermatology, 26(2), 156-162.
- Schmidt, A., Liebelt, G., Nießner, F., von Woedtke, T., & Bekeschus, S. (2021). Gas plasma-spurred wound healing is accompanied by regulation of focal adhesion, matrix remodeling, and tissue oxygenation. Redox biology, 38, 101809. [CrossRef]
- Ma, Y., Ha, C. S., Hwang, S. W., Lee, H. J., Kim, G. C., Lee, K. W., & Song, K. (2014). Non-thermal atmospheric pressure plasma preferentially induces apoptosis in p53-mutated cancer cells by activating ROS stress-response pathways. PloS one, 9(4), e91947. [CrossRef]
- Laroussi, M., Mohades, S., & Barekzi, N. (2015). Killing adherent and nonadherent cancer cells with the plasma pencil. Biointerphases, 10(2). [CrossRef]
- Laroussi, M., Lu, X., & Keidar, M. (2017). Perspective: The physics, diagnostics, and applications of atmospheric pressure low temperature plasma sources used in plasma medicine. Journal of Applied Physics, 122(2). [CrossRef]
- Lertpatipanpong, P., Sillapachaiyaporn, C., Oh, G., Kang, Y. H., Hwang, C. Y., & Baek, S. J. (2023). Effect of cold atmospheric microwave plasma (CAMP) on wound healing in canine keratinocytes. Frontiers in Cell and Developmental Biology, 11, 1105692. [CrossRef]
- Barton, A., Wende, K., Bundscherer, L., Hasse, S., Schmidt, A., Bekeschus, S., ... & Masur, K. (2013). Nonthermal plasma increases expression of wound healing related genes in a keratinocyte cell line. Plasma Medicine, 3(1-2). [CrossRef]
- Dezest, M., Chavatte, L., Bourdens, M., Quinton, D., Camus, M., Garrigues, L., ... & Bulteau, A. L. (2017). Mechanistic insights into the impact of cold atmospheric pressure plasma on human epithelial cell lines. Scientific reports, 7(1), 41163. [CrossRef]
- Brun, P., Brun, P., Vono, M., Venier, P., Tarricone, E., Deligianni, V., ... & Leonardi, A. (2012). Disinfection of ocular cells and tissues by atmospheric-pressure cold plasma. PloS one, 7(3), e33245. [CrossRef]
- Okazaki, Y., Ito, N., Tanaka, H., Hori, M., & Toyokuni, S. (2022). Non-thermal plasma elicits ferrous chloride-catalyzed DMPO-OH. Free Radical Research, 56(9-10), 595-606. [CrossRef]
- Lee, H. R., Kang, S. U., Kim, H. J., Ji, E. J., Yun, J. H., Kim, S., ... & Kim, C. H. (2023). Liquid plasma as a treatment for cutaneous wound healing through regulation of redox metabolism. Cell Death & Disease, 14(2), 119. [CrossRef]
- Zou, X., Xu, M., Pan, S., Gan, L., Zhang, S., Chen, H., ... & Ostrikov, K. K. (2019). Plasma activated oil: Fast production, reactivity, stability, and wound healing application. ACS Biomaterials Science & Engineering, 5(3), 1611-1622.
- Lee, H. R., Lee, H. Y., Heo, J., Jang, J. Y., Shin, Y. S., & Kim, C. H. (2021). Liquid-type nonthermal atmospheric plasma enhanced regenerative potential of silk–fibrin composite gel in radiation-induced wound failure. Materials Science and Engineering: C, 128, 112304. [CrossRef]
- Xu, D., Wang, S., Li, B., Qi, M., Feng, R., Li, Q., ... & Kong, M. G. (2020). Effects of plasma-activated water on skin wound healing in mice. Microorganisms, 8(7), 1091. [CrossRef]
- Lee, H. R., Kang, S. U., Kim, H. J., Ji, E. J., Yun, J. H., Kim, S., ... & Kim, C. H. (2023). Liquid plasma as a treatment for cutaneous wound healing through regulation of redox metabolism. Cell Death & Disease, 14(2), 119. [CrossRef]
- Zou, X., Xu, M., Pan, S., Gan, L., Zhang, S., Chen, H., ... & Ostrikov, K. K. (2019). Plasma activated oil: Fast production, reactivity, stability, and wound healing application. ACS Biomaterials Science & Engineering, 5(3), 1611-1622.
- Lee, H. R., Lee, H. Y., Heo, J., Jang, J. Y., Shin, Y. S., & Kim, C. H. (2021). Liquid-type nonthermal atmospheric plasma enhanced regenerative potential of silk–fibrin composite gel in radiation-induced wound failure. Materials Science and Engineering: C, 128, 112304. [CrossRef]
- Boo Y. C. (2020). Natural Nrf2 Modulators for Skin Protection. Antioxidants (Basel, Switzerland), 9(9), 812. [CrossRef]
- Ishitsuka, Y., Ogawa, T., & Roop, D. (2020). The KEAP1/NRF2 Signaling Pathway in Keratinization. Antioxidants (Basel, Switzerland), 9(8), 751. [CrossRef]
- Zhai, S. Y., Kong, M. G., & Xia, Y. M. (2022). Cold atmospheric plasma ameliorates skin diseases involving reactive oxygen/nitrogen species-mediated functions. Frontiers in Immunology, 13, 868386. [CrossRef]
- Scharf, C., Eymann, C., Emicke, P., Bernhardt, J., Wilhelm, M., Görries, F., ... & Beule, A. (2019). Improved wound healing of airway epithelial cells is mediated by cold atmospheric plasma: A time course-related proteome analysis. Oxidative Medicine and Cellular Longevity, 2019. [CrossRef]
- Bourdens, M., Jeanson, Y., Taurand, M., Juin, N., Carrière, A., Clément, F., ... & Planat-Bénard, V. (2019). Short exposure to cold atmospheric plasma induces senescence in human skin fibroblasts and adipose mesenchymal stromal cells. Scientific reports, 9(1), 8671. [CrossRef]
- Gan, L., Duan, J., Zhang, S., Liu, X., Poorun, D., Liu, X., ... & Chen, H. (2019). Cold atmospheric plasma ameliorates imiquimod-induced psoriasiform dermatitis in mice by mediating antiproliferative effects. Free radical research, 53(3), 269-280. [CrossRef]
- Lee, Y. S., Lee, M. H., Kim, H. J., Won, H. R., & Kim, C. H. (2017). Non-thermal atmospheric plasma ameliorates imiquimod-induced psoriasis-like skin inflammation in mice through inhibition of immune responses and up-regulation of PD-L1 expression. Scientific reports, 7(1), 15564. [CrossRef]
- Zhong, S. Y., Dong, Y. Y., Liu, D. X., Xu, D. H., Xiao, S. X., Chen, H. L., & Kong, M. G. (2016). Surface air plasma-induced cell death and cytokine release of human keratinocytes in the context of psoriasis. British Journal of Dermatology, 174(3), 542-552. [CrossRef]
- Zheng, L., Gao, J., Cao, Y., Yang, X., Wang, N., Cheng, C., & Yang, C. (2020). Two case reports of inverse psoriasis treated with cold atmospheric plasma. Dermatologic Therapy, 33(6), e14257. [CrossRef]
- Gareri, C., Bennardo, L., & De Masi, G. (2020). Use of a new cold plasma tool for psoriasis treatment: A case report. SAGE Open Medical Case Reports, 8, 2050313X20922709. [CrossRef]
- Kim, N., Lee, S., Lee, S., Kang, J., Choi, Y. A., Park, J., ... & Kim, S. H. (2022). Portable Cold Atmospheric Plasma Patch-Mediated Skin Anti-Inflammatory Therapy. Advanced Science, 9(34), 2202800.
- Moon, I. J., Yun, M. R., Yoon, H. K., Lee, K. H., Choi, S. Y., Lee, W. J., ... & Won, C. H. (2021). Treatment of atopic dermatitis using non-thermal atmospheric plasma in an animal model. Scientific Reports, 11(1), 16091. [CrossRef]
- Bai, F., Ran, Y., Zhai, S., & Xia, Y. (2023). Cold Atmospheric Plasma: A Promising and Safe Therapeutic Strategy for Atopic Dermatitis. International Archives of Allergy and Immunology, 1-14. [CrossRef]
- Sun, T., Zhang, X., Hou, C., Yu, S., Zhang, Y., Yu, Z., ... & Ni, G. (2022). Cold Plasma Irradiation Attenuates Atopic Dermatitis via Enhancing HIF-1α-Induced MANF Transcription Expression. Frontiers in Immunology, 13, 941219. [CrossRef]
- Moon, I. J., Yun, M. R., Yoon, H. K., Lee, K. H., Choi, S. Y., Lee, W. J., ... & Won, C. H. (2021). Treatment of atopic dermatitis using non-thermal atmospheric plasma in an animal model. Scientific Reports, 11(1), 16091. [CrossRef]
- Lee, M. H., Lee, Y. S., Kim, H. J., Han, C. H., Kang, S. U., & Kim, C. H. (2019). Non-thermal plasma inhibits mast cell activation and ameliorates allergic skin inflammatory diseases in NC/Nga mice. Scientific Reports, 9(1), 13510. [CrossRef]
- Choi, J. H., Song, Y. S., Lee, H. J., Hong, J. W., & Kim, G. C. (2016). Inhibition of inflammatory reactions in 2, 4-Dinitrochlorobenzene induced Nc/Nga atopic dermatitis mice by non-thermal plasma. Scientific Reports, 6(1), 27376. [CrossRef]
- Kim, Y. J., Lim, D. J., Lee, M. Y., Lee, W. J., Chang, S. E., & Won, C. H. (2021). Prospective, comparative clinical pilot study of cold atmospheric plasma device in the treatment of atopic dermatitis. Scientific Reports, 11(1), 14461. [CrossRef]
- Ahmed, M. M., Montaser, S. A., Elhadry, A. A., & El-Aragi, G. M. (2022). Study of the Possible Cytogenetic and Immunological Effects of Cold Atmospheric Pressure Plasma Jet on Whole Blood Cultures of Vitiligo Patients. Plasma Medicine, 12(4). [CrossRef]
- Zhai S, Xu M, Li Q, Guo K, Chen H, Kong MG, Xia Y. Successful Treatment of Vitiligo with Cold Atmospheric Plasma‒Activated Hydrogel. J Invest Dermatol. 2021 Nov;141(11):2710-2719.e6. Epub 2021 May 21. PMID: 34029575. [CrossRef]
- Dubuc, A., Monsarrat, P., Virard, F., Merbahi, N., Sarrette, J. P., Laurencin-Dalicieux, S., & Cousty, S. (2018). Use of cold-atmospheric plasma in oncology: A concise systematic review. Therapeutic advances in medical oncology, 10, 1758835918786475. [CrossRef]
- Limanowski, R., Yan, D., Li, L., & Keidar, M. (2022). Preclinical Cold Atmospheric Plasma Cancer Treatment. Cancers, 14(14), 3461. [CrossRef]
- Förster, S., Niu, Y., Eggers, B., Nokhbehsaim, M., Kramer, F. J., Bekeschus, S., ... & Stope, M. B. (2023). Modulation of the Tumor-Associated Immuno-Environment by Non-Invasive Physical Plasma. Cancers, 15(4), 1073. [CrossRef]
- Zimmermann, T., Staebler, S., Taudte, R. V., Ünüvar, S., Grösch, S., Arndt, S., ... & Bosserhoff, A. K. (2023). Cold Atmospheric Plasma Triggers Apoptosis via the Unfolded Protein Response in Melanoma Cells. Cancers, 15(4), 1064. [CrossRef]
- Faramarzi, F., Zafari, P., Alimohammadi, M., Moonesi, M., Rafiei, A., & Bekeschus, S. (2021). Cold physical plasma in cancer therapy: mechanisms, signaling, and immunity. Oxidative medicine and cellular longevity, 2021. [CrossRef]
- Alimohammadi, M., Golpour, M., Sohbatzadeh, F., Hadavi, S., Bekeschus, S., Niaki, H. A., ... & Rafiei, A. (2020). Cold atmospheric plasma is a potent tool to improve chemotherapy in melanoma in vitro and in vivo. Biomolecules, 10(7), 1011. [CrossRef]
- Golpour, M., Alimohammadi, M., Sohbatzadeh, F., Fattahi, S., Bekeschus, S., & Rafiei, A. (2022). Cold atmospheric pressure plasma treatment combined with starvation increases autophagy and apoptosis in melanoma in vitro and in vivo. Experimental Dermatology, 31(7), 1016-1028. [CrossRef]
- Yang, X., Chen, G., Yu, K. N., Yang, M., Peng, S., Ma, J., ... & Han, W. (2020). Cold atmospheric plasma induces GSDME-dependent pyroptotic signaling pathway via ROS generation in tumor cells. Cell death & disease, 11(4), 295. [CrossRef]
- Jo, A., Bae, J. H., Yoon, Y. J., Chung, T. H., Lee, E. W., Kim, Y. H., ... & Chung, J. W. (2022). Plasma-activated medium induces ferroptosis by depleting FSP1 in human lung cancer cells. Cell Death & Disease, 13(3), 212. [CrossRef]
- Lin, A., Sahun, M., Biscop, E., Verswyvel, H., De Waele, J., De Backer, J., ... & Bogaerts, A. (2023). Acquired non-thermal plasma resistance mediates a shift towards aerobic glycolysis and ferroptotic cell death in melanoma. Drug Resistance Updates, 67, 100914. [CrossRef]
- Xu, D., Xu, Y., Ning, N., Cui, Q., Liu, Z., Wang, X., ... & Kong, M. G. (2018). Alteration of metabolite profiling by cold atmospheric plasma treatment in human myeloma cells. Cancer cell international, 18, 1-11. [CrossRef]
- Keidar, M., Yan, D., Beilis, I. I., Trink, B., & Sherman, J. H. (2018). Plasmas for treating cancer: Opportunities for adaptive and self-adaptive approaches. Trends in biotechnology, 36(6), 586-593. [CrossRef]
- Bengtson, C., & Bogaerts, A. (2020). On the Anti-Cancer Effect of Cold Atmospheric Plasma and the Possible Role of Catalase-Dependent Apoptotic Pathways. Cells, 9(10), 2330. [CrossRef]
- Yadav, D. K., Adhikari, M., Kumar, S., Ghimire, B., Han, I., Kim, M. H., & Choi, E. H. (2020). Cold atmospheric plasma generated reactive species aided inhibitory effects on human melanoma cells: An in vitro and in silico study. Scientific reports, 10(1), 3396. [CrossRef]
- Alimohammadi, M., Golpour, M., Sohbatzadeh, F., Hadavi, S., Bekeschus, S., Niaki, H. A., ... & Rafiei, A. (2020). Cold atmospheric plasma is a potent tool to improve chemotherapy in melanoma in vitro and in vivo. Biomolecules, 10(7), 1011. [CrossRef]
- Arndt, S., Wacker, E., Li, Y. F., Shimizu, T., Thomas, H. M., Morfill, G. E., ... & Bosserhoff, A. K. (2013). Cold atmospheric plasma, a new strategy to induce senescence in melanoma cells. Experimental dermatology, 22(4), 284-289. [CrossRef]
- Xia, J., Zeng, W., Xia, Y., Wang, B., Xu, D., Liu, D., ... & Dong, Y. (2019). Cold atmospheric plasma induces apoptosis of melanoma cells via Sestrin2-mediated nitric oxide synthase signaling. Journal of biophotonics, 12(1), e201800046. [CrossRef]
- Pefani-Antimisiari, K., Athanasopoulos, D. K., Marazioti, A., Sklias, K., Rodi, M., De Lastic, A. L., ... & Antimisiaris, S. G. (2021). Synergistic effect of cold atmospheric pressure plasma and free or liposomal doxorubicin on melanoma cells. Scientific Reports, 11(1), 14788. [CrossRef]
- Chen, G., Chen, Z., Wang, Z., Obenchain, R., Wen, D., Li, H., ... & Gu, Z. (2021). Portable air-fed cold atmospheric plasma device for postsurgical cancer treatment. Science advances, 7(36), eabg5686. [CrossRef]
- Kim, S. J., Seong, M. J., Mun, J. J., Bae, J. H., Joh, H. M., & Chung, T. H. (2022). Differential Sensitivity of Melanoma Cells and Their Non-Cancerous Counterpart to Cold Atmospheric Plasma-Induced Reactive Oxygen and Nitrogen Species. International Journal of Molecular Sciences, 23(22), 14092. [CrossRef]
- Pereira, S., Pinto, E., Ribeiro, P. A., & Sério, S. (2019). Study of a Cold Atmospheric Pressure Plasma jet device for indirect treatment of Squamous Cell Carcinoma. Clinical Plasma Medicine, 13, 9-14. [CrossRef]
- Saadati, F., Moritz, J., Berner, J., Freund, E., Miebach, L., Helfrich, I., ... & Bekeschus, S. (2021). Patient-derived human basal and cutaneous squamous cell carcinoma tissues display apoptosis and immunomodulation following gas plasma exposure with a certified argon jet. International Journal of Molecular Sciences, 22(21), 11446. [CrossRef]
- Pasqual-Melo, G., Nascimento, T., Sanches, L. J., Blegniski, F. P., Bianchi, J. K., Sagwal, S. K., ... & Bekeschus, S. (2020). Plasma treatment limits cutaneous squamous cell carcinoma development in vitro and in vivo. Cancers, 12(7), 1993. [CrossRef]
- Dai, X., & Zhu, K. (2023). Cold atmospheric plasma: Novel opportunities for tumor microenvironment targeting. Cancer Medicine, 12(6), 7189-7206. [CrossRef]
- Mihai, C. T., Mihaila, I., Pasare, M. A., Pintilie, R. M., Ciorpac, M., & Topala, I. (2022). Cold atmospheric plasma-activated media improve paclitaxel efficacy on breast cancer cells in a combined treatment model. Current Issues in Molecular Biology, 44(5), 1995-2014. [CrossRef]
- Wang, L., Yang, X., Yang, C., Gao, J., Zhao, Y., Cheng, C., ... & Liu, S. (2019). The inhibition effect of cold atmospheric plasma-activated media in cutaneous squamous carcinoma cells. Future Oncology, 15(5), 495-505. [CrossRef]
- Yang, X., Yang, C., Wang, L., Cao, Z., Wang, Y., Cheng, C., ... & Zhao, Y. (2020). Inhibition of basal cell carcinoma cells by cold atmospheric plasma activated solution and differential gene expression analysis. International Journal of Oncology, 56(5), 1262-1273. [CrossRef]
- Szili, E. J., Hong, S. H., Oh, J. S., Gaur, N., & Short, R. D. (2018). Tracking the penetration of plasma reactive species in tissue models. Trends in biotechnology, 36(6), 594-602. [CrossRef]
- Živanić, M., Espona-Noguera, A., Lin, A., & Canal, C. (2023). Current State of Cold Atmospheric Plasma and Cancer-Immunity Cycle: Therapeutic Relevance and Overcoming Clinical Limitations Using Hydrogels. Advanced Science, 10(8), 2205803. [CrossRef]
- Zhang, H., Xu, S., Zhang, J., Wang, Z., Liu, D., Guo, L., ... & Chu, P. K. (2021). Plasma-activated thermosensitive biogel as an exogenous ROS carrier for post-surgical treatment of cancer. Biomaterials, 276, 121057. [CrossRef]
- Tan, F., Wang, Y., Zhang, S., Shui, R., & Chen, J. (2022). Plasma dermatology: Skin therapy using cold atmospheric plasma. Frontiers in Oncology, 12, 918484. [CrossRef]
- Mohammad Reza Lotfi, Mohammadreza Khani, Elahe Razaghiha et al. Immunometric study and examination of physical and chemical characteristics of spark plasma, 09 June 2023, PREPRINT (Version 1) available at Research Square. [CrossRef]
- Lan, T., Xiao, Y., Tang, L., Hamblin, M. R., & Yin, R. (2018). Treatment of atrophic acne scarring with fractional micro-plasma radio-frequency in Chinese patients: A prospective study. Lasers in surgery and medicine, 50(8), 844-850. [CrossRef]
- Lotfi, M., Khani, M., & Shokri, B. (2023). A review of cold atmospheric plasma applications in dermatology and aesthetics. Plasma Medicine, 13(1). [CrossRef]
- Metelmann, H. R., Böttger, K., & von Woedtke, T. (2022). Cold Plasma Treatment and Aesthetic Medicine. In Textbook of Good Clinical Practice in Cold Plasma Therapy (pp. 229-243). Cham: Springer International Publishing.
- Braný, D., Dvorská, D., Halašová, E., & Škovierová, H. (2020). Cold atmospheric plasma: A powerful tool for modern medicine. International journal of molecular sciences, 21(8), 2932. [CrossRef]
- Emmert, S., Brehmer, F., Hänßle, H., Helmke, A., Mertens, N., Ahmed, R., ... & Viöl, W. (2013). Atmospheric pressure plasma in dermatology: Ulcus treatment and much more. Clinical Plasma Medicine, 1(1), 24-29. [CrossRef]
- Suwanchinda, A., & Nararatwanchai, T. (2022). The efficacy and safety of the innovative cold atmospheric-pressure plasma technology in the treatment of striae distensae: A randomized controlled trial. Journal of Cosmetic Dermatology, 21(12), 6805-6814. [CrossRef]
- Suwanchinda, A., & Nararatwanchai, T. (2022). Efficacy and safety of the innovative cold atmospheric-pressure plasma technology in the treatment of keloid: A randomized controlled trial. Journal of Cosmetic Dermatology, 21(12), 6788-6797. [CrossRef]
- Shakouri, R., Khani, M. R., Samsavar, S., Jezeh, M. A., Abdollahimajd, F., Hosseini, S. I., Dilmaghanian, A., Ghasemi, E., Alihoseini, M. R., & Shokri, B. (2021). In vivo study of the effects of a portable cold plasma device and vitamin C for skin rejuvenation. Scientific reports, 11(1), 21915. [CrossRef]
- Liu, Y., Wang, H., Taylor, M., Cook, C., Martínez-Berdeja, A., North, J. P., ... & Cheng, J. B. (2022). Classification of human chronic inflammatory skin disease based on single-cell immune profiling. Science immunology, 7(70), eabl9165. [CrossRef]
- Gan, L., Duan, J., Zhang, S., Liu, X., Poorun, D., Liu, X., ... & Chen, H. (2019). Cold atmospheric plasma ameliorates imiquimod-induced psoriasiform dermatitis in mice by mediating antiproliferative effects. Free radical research, 53(3), 269-280. [CrossRef]
- Zhang, S., Chen, B., Liu, D., & Chen, H. (2021). The Combination of Low-Temperature Plasma and Tripterygium wilfordii Hook F on Ameliorating Imiquimod-Induced Psoriasiform Dermatitis in Mice. Applied Sciences, 12(1), 356. [CrossRef]
- Lee, Y. S., Lee, M. H., Kim, H. J., Won, H. R., & Kim, C. H. (2017). Non-thermal atmospheric plasma ameliorates imiquimod-induced psoriasis-like skin inflammation in mice through inhibition of immune responses and up-regulation of PD-L1 expression. Scientific reports, 7(1), 15564. [CrossRef]
- Zhong, S. Y., Dong, Y. Y., Liu, D. X., Xu, D. H., Xiao, S. X., Chen, H. L., & Kong, M. G. (2016). Surface air plasma-induced cell death and cytokine release of human keratinocytes in the context of psoriasis. British Journal of Dermatology, 174(3), 542-552. [CrossRef]
- Kim, N., Lee, S., Lee, S., Kang, J., Choi, Y. A., Park, J., ... & Kim, S. H. (2022). Portable Cold Atmospheric Plasma Patch-Mediated Skin Anti-Inflammatory Therapy. Advanced Science, 9(34), 2202800.
- Zheng, L., Gao, J., Cao, Y., Yang, X., Wang, N., Cheng, C., & Yang, C. (2020). Two case reports of inverse psoriasis treated with cold atmospheric plasma. Dermatologic Therapy, 33(6), e14257. [CrossRef]
- Gareri, C., Bennardo, L., & De Masi, G. (2020). Use of a new cold plasma tool for psoriasis treatment: A case report. SAGE Open Medical Case Reports, 8, 2050313X20922709. [CrossRef]
- Sun, T., Zhang, X., Hou, C., Yu, S., Zhang, Y., Yu, Z., ... & Ni, G. (2022). Cold Plasma Irradiation Attenuates Atopic Dermatitis via Enhancing HIF-1α-Induced MANF Transcription Expression. Frontiers in Immunology, 13, 941219. [CrossRef]
- Moon, I. J., Yun, M. R., Yoon, H. K., Lee, K. H., Choi, S. Y., Lee, W. J., ... & Won, C. H. (2021). Treatment of atopic dermatitis using non-thermal atmospheric plasma in an animal model. Scientific Reports, 11(1), 16091. [CrossRef]
- Lee, M. H., Lee, Y. S., Kim, H. J., Han, C. H., Kang, S. U., & Kim, C. H. (2019). Non-thermal plasma inhibits mast cell activation and ameliorates allergic skin inflammatory diseases in NC/Nga mice. Scientific Reports, 9(1), 13510. [CrossRef]
- Choi, J. H., Song, Y. S., Lee, H. J., Hong, J. W., & Kim, G. C. (2016). Inhibition of inflammatory reactions in 2, 4-Dinitrochlorobenzene induced Nc/Nga atopic dermatitis mice by non-thermal plasma. Scientific Reports, 6(1), 27376. [CrossRef]
- Kim, Y. J., Lim, D. J., Lee, M. Y., Lee, W. J., Chang, S. E., & Won, C. H. (2021). Prospective, comparative clinical pilot study of cold atmospheric plasma device in the treatment of atopic dermatitis. Scientific Reports, 11(1), 14461. [CrossRef]
- Zhai, S., Xu, M., Li, Q., Guo, K., Chen, H., Kong, M. G., & Xia, Y. (2021). Successful treatment of vitiligo with cold atmospheric plasma‒activated hydrogel. Journal of Investigative Dermatology, 141(11), 2710-2719. [CrossRef]
- Kos, S., Blagus, T., Cemazar, M., Filipic, G., Sersa, G., & Cvelbar, U. (2017). Safety aspects of atmospheric pressure helium plasma jet operation on skin: In vivo study on mouse skin. PloS one, 12(4), e0174966. [CrossRef]
- Daeschlein, G., Scholz, S., Ahmed, R., von Woedtke, T., Haase, H., Niggemeier, M., ... & Juenger, M. (2012). Skin decontamination by low-temperature atmospheric pressure plasma jet and dielectric barrier discharge plasma. Journal of Hospital Infection, 81(3), 177-183. [CrossRef]
- Isbary, G., Heinlin, J., Shimizu, T., Zimmermann, J. L., Morfill, G., Schmidt, H. U., ... & Stolz, W. (2012). Successful and safe use of 2 min cold atmospheric argon plasma in chronic wounds: results of a randomized controlled trial. British Journal of Dermatology, 167(2), 404-410. [CrossRef]
- Tan, F., Wang, Y., Zhang, S., Shui, R., & Chen, J. (2022). Plasma dermatology: Skin therapy using cold atmospheric plasma. Frontiers in Oncology, 12, 918484. [CrossRef]
- Chen, Z., & Wirz, R. E. (2021). Cold atmospheric plasma (CAP) technology and applications. Morgan & Claypool Publishers. [CrossRef]
- Bonzanini, A. D., Shao, K., Stancampiano, A., Graves, D. B., & Mesbah, A. (2021). Perspectives on machine learning-assisted plasma medicine: Toward automated plasma treatment. IEEE Transactions on Radiation and Plasma Medical Sciences, 6(1), 16-32. [CrossRef]
- Chen, Z., Chen, G., Obenchain, R., Zhang, R., Bai, F., Fang, T., ... & Gu, Z. (2022). Cold atmospheric plasma delivery for biomedical applications. Materials Today, 54, 153-188. [CrossRef]
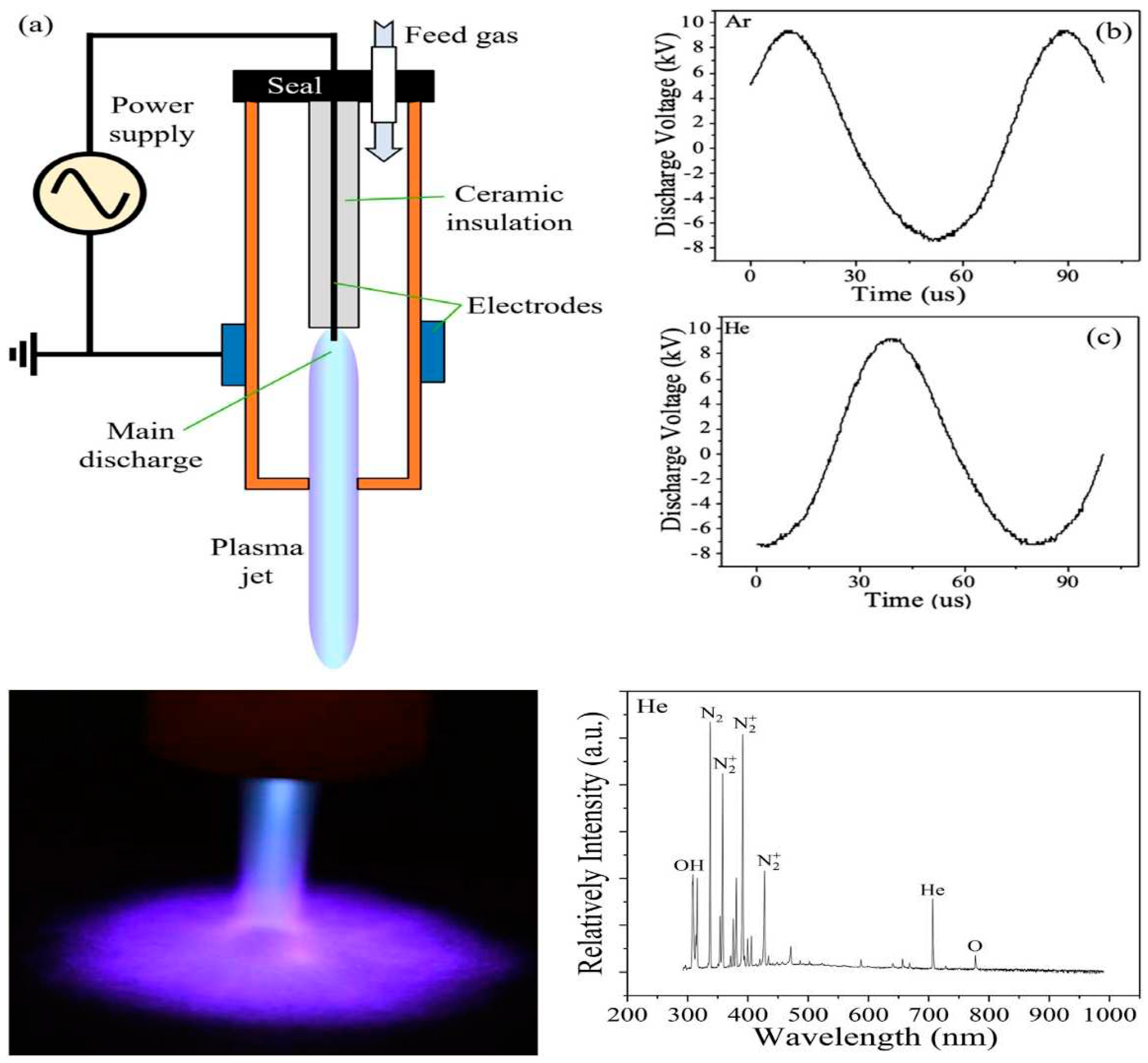
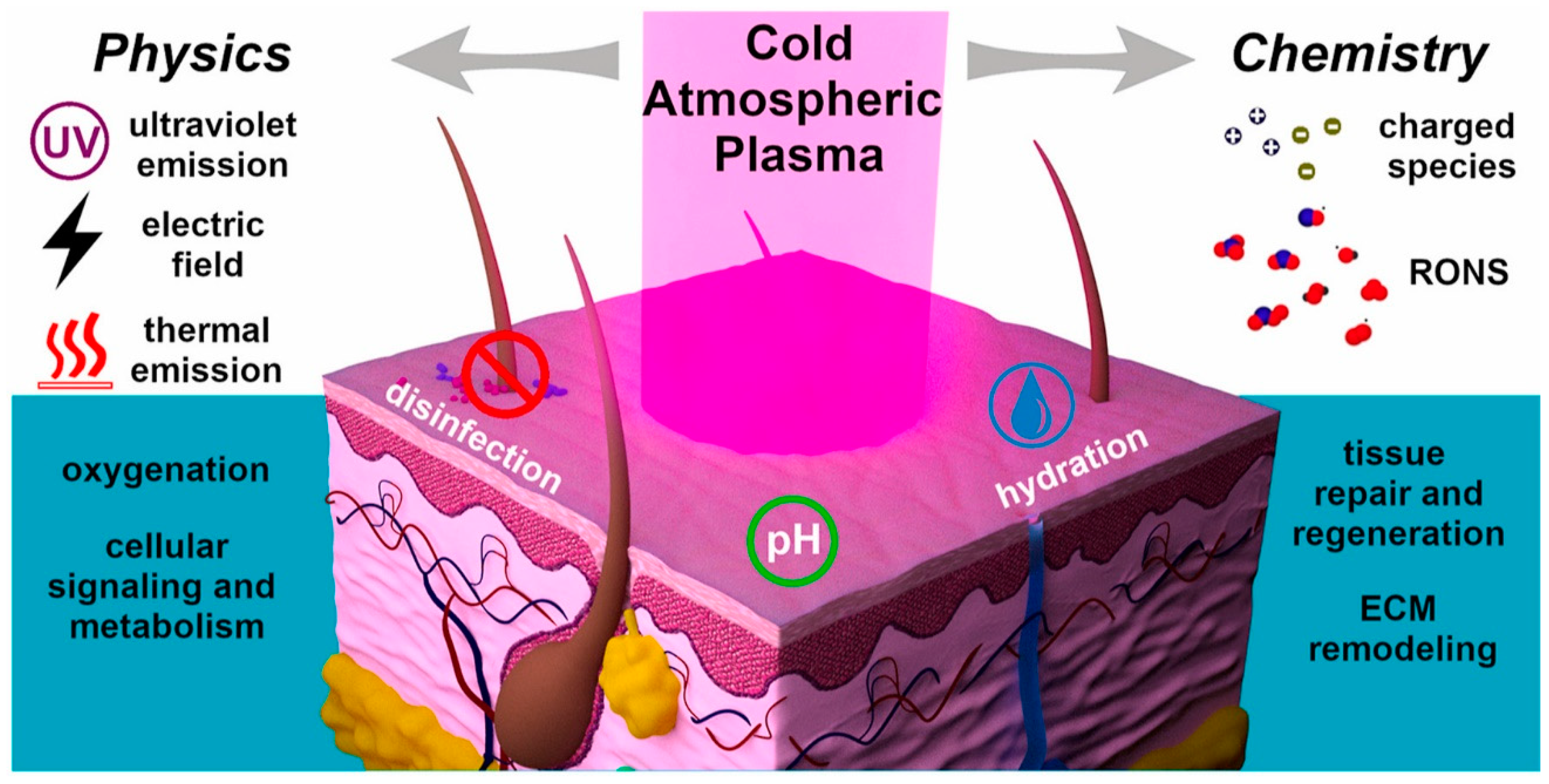
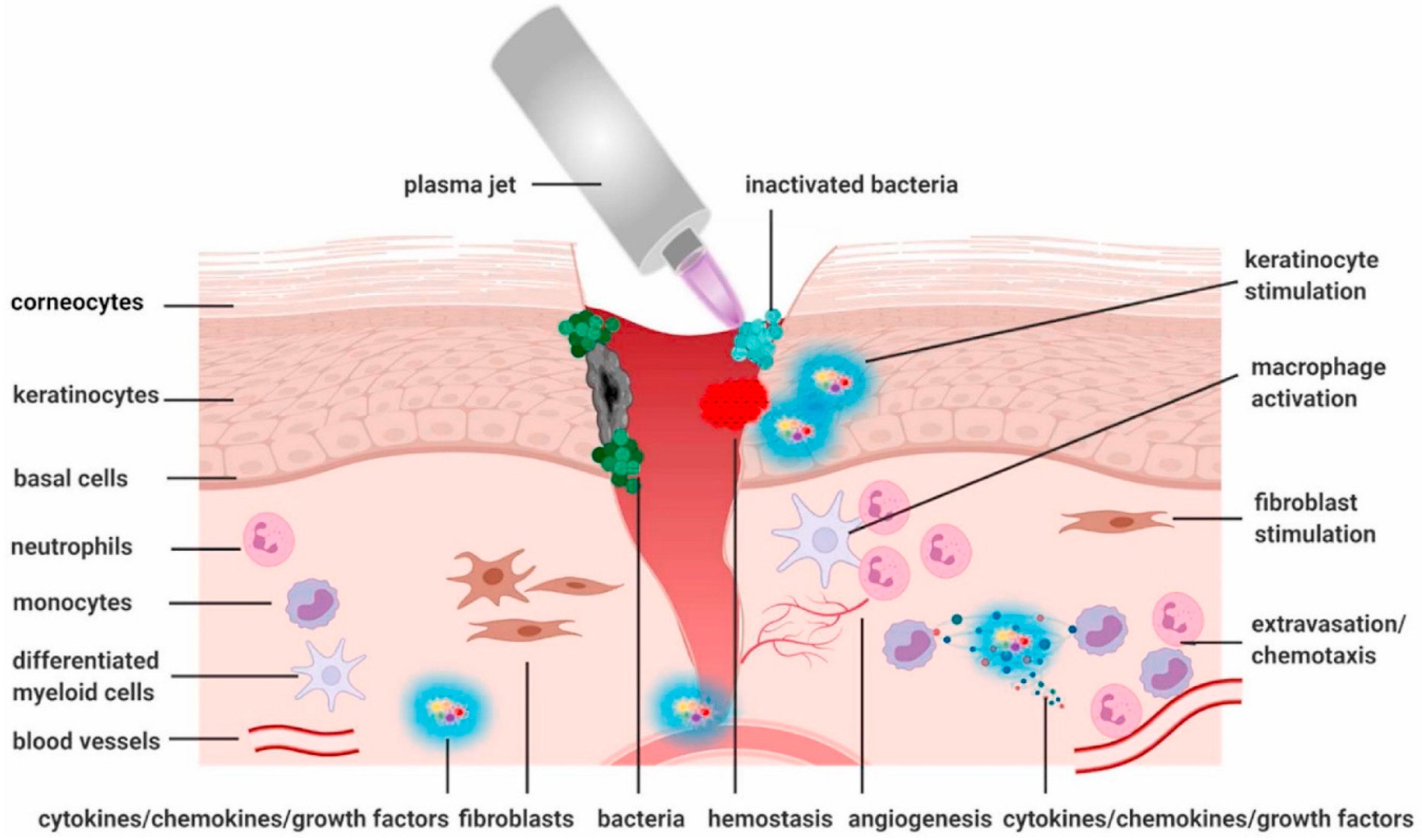
| Application | Description |
|---|---|
| Cold Atmospheric Plasma | An emerging multi-agent technology and multi-modal therapy with diverse applications across various biomedical fields [5]. |
| Oral Biofilm-Related Infections | Plasma medicine effectively addresses oral biofilm-related infections, showcasing its efficacy in promoting oral health [6]. |
| Therapeutic Use of Physical Plasma | Involves the direct application of physical plasma on or in the human body for therapeutic purposes [7]. |
| Plasma Technology for Biomedical Applications | There is growing interest in utilizing plasmas for biomedical applications, particularly in plasma medicine, focusing on therapeutic advancements [8]. |
| Biomedical Applications of Cold Atmospheric Plasma | Encompasses sterilization, wound healing, blood coagulation, oral/dental disease treatment, cancer therapy, and immunotherapy [5,7,9]. |
| Diverse Range of Applications | Demonstrates various applications and advancements, offering examples of ideas and applications in the medical and biomedical domains [10]. |
| Medical Applications | Description |
|---|---|
| Therapeutic Purposes | Direct application of Cold Atmospheric Plasma (CAP) for various medical treatments [20,21]. |
| Disinfection | Utilizes ionized gas (physical plasma) for disinfection, effectively inactivating various microorganisms, including viruses, resistant microbes, fungal cells, bacteria, spores, and biofilms created by microbes [13,22,23]. |
| Healing | Under study for its potential in healing, plasma medicine stimulates cell proliferation and angiogenesis with lower plasma treatment intensity, contributing to wound healing [17,21,24]. |
| Cancer Treatment | Exploration of plasma medicine for cancer treatment can inactivate cells and initiate cell death with higher plasma intensity [25,26,27,28]. |
| Blood Coagulation | Utilization of plasma for blood coagulation [28,29]. |
| Dental Applications | Application of ionized gas in plasma medicine for various dental purposes [20,30]. |
| Sterilization of Implants and Surgical Instruments | Plasma-generated active species are harnessed for sterilizing implants and surgical instruments [31,32]. |
| Modifying Biomaterial Surface Properties | Plasma medicine can modify biomaterial surface properties [33,34]. |
| Treatment of Skin Diseases and Wounds | Ongoing research on the potential of plasma medicine for treating skin diseases and wounds [17,21,24,35]. |
| Multidisciplinary Research | Intersection of scientific domains for diverse research in plasma medicine, combining plasma physics, life sciences, and clinical medicine [1,2,3,17]. |
| Application | Description |
|---|---|
| 1. CAP's Suppressive Effect on Microbial Skin Infection | With advantages over traditional antibiotics, CAP effectively inhibits infections on diverse surfaces. Studies demonstrate its safety and efficacy in sterilizing bacteria, disrupting biofilms, and reducing bacterial loads in diabetic foot ulcers. CAP's broad-spectrum mechanism challenges bacterial resistance, making it promising for combating infectious diseases. |
| 1.1 Effects of CAP on Bacteria | Research confirms CAP's efficacy against bacteria and biofilms, including common strains like Escherichia coli. Plasma-activated liquids, especially Plasma-Activated Water (PAW), show promise by generating reactive species, disrupting biofilms, and modulating inflammation, offering a novel approach to address biofilm-related infections. |
| 1.2 Effects of CAP on Fungi | CAP has shown efficacy in treating nail fungal infections, inhibiting the growth of causative agents in pathogen models and clinical trials. A pilot study and a cohort of 40 patients demonstrated overall clinical cure rates exceeding 70%, establishing atmospheric pressure cold plasma as a safe and promising alternative therapy for skin fungal infections. |
| 1.3 Effects of CAP on Viruses and Parasites | CAP shows potential antiviral effects, with positive outcomes reported in treating viral warts and lower but measurable results on HSV-1. It also demonstrates efficacy against parasites like Demodex mites and head lice, suggesting its possible use in dermatology pending further exploration and studies. |
| 2. CAP Promotes Tissue Proliferation and Wound Healing | CAP is effective in wound healing by promoting antiseptic properties, pro-angiogenic effects, tissue proliferation, and the expression of growth factors and chemokines. CAP accelerates wound healing by reducing bacterial load, stimulating cell proliferation, and inducing gene expression, offering a promising approach for improved wound treatment, including the use of plasma-activated liquids like Plasma-Activated Water (PAW) and Plasma-Activated Hydrogel (PAH). |
| 3. CAP for the Treatment of Inflammatory Skin Diseases | CAP regulates the formation of stratum corneum cells by modulating the redox balance and activating the NRF2 pathway. Clinical studies show that CAP can alleviate skin inflammation, restore normal cell differentiation, and may represent a novel therapeutic approach for inflammatory skin disorders by regulating cell viability, proliferation, migration, and inflammatory responses. |
| 3.1 Psoriasis | CAP demonstrated therapeutic effects on psoriasis in cellular and animal studies, reducing epidermal hyperplasia and improving symptoms by inducing apoptosis and regulating reactive species. Atmospheric pressure cold plasma patches also showed promise in treating psoriatic skin lesions by mitigating electric field effects and inducing calcium ion channel opening in keratinocytes. |
| 3.2 Atopic Dermatitis | CAP has shown positive effects in alleviating atopic dermatitis (AD) in mouse models by reducing skin inflammation, oxidative stress, and dermatitis severity. Clinical studies on AD patients revealed improvements in skin lesions, itching, and a reduction in Staphylococcus aureus proportion after plasma treatment. |
| 3.3 Vitiligo | CAP) shows promise in treating Vitiligo by stimulating repigmentation in affected areas. Studies, including one by Zhai et al., demonstrate CAP's efficacy and safety in enhancing melanocyte activity, reducing inflammation, and achieving partial or complete pigmentation in vitiligo skin lesions without adverse events. |
| 4. CAP Suppresses Tumor Cell Proliferation and Migration | CAP demonstrated therapeutic efficacy in tumor treatment, inducing apoptosis in melanoma and skin cancer cells, with potential applications in various cancers. Plasma-activated hydrogel (PAH) is a carrier for CAP, enhancing treatment response, and a novel plasma-activated biological hydrogel demonstrates efficacy in eliminating residual tumor tissue without systemic toxicity after surgical resection. |
| 5. Cosmetic and Skincare Applications of CAP | Microplasma radiofrequency treats scars, while CAP benefits beauty. Suwanchinda's studies show improvements in striae and hypertrophic scars with high satisfaction. Plasma's reactive oxygen species offer a novel approach to skin barrier regulation. Combining cold plasma with vitamin C boosts collagen and skin elasticity, promoting skin beauty. |
| 6. CAP's Role in Treating Immune-Mediated Skin Diseases | CAP has demonstrated therapeutic effects on immune skin diseases. It showed positive results in reducing inflammation and oxidative stress and improving skin lesions. Additionally, CAP, delivered through a plasma-activated hydrogel, proved effective and safe in mouse models and vitiligo patients, enhancing melanin distribution and reducing inflammatory factors. Overall, CAP presents a promising and safe approach for managing immune skin diseases, supported by laboratory and clinical evidence. |
| Challenge | Elaboration | Possible Solutions | References |
|---|---|---|---|
| 1. Lack of Standardization | The absence of standardized protocols in CAP applications for skin diseases hinders reproducibility and efficacy assessment. | Establish standardized treatment guidelines and parameters for CAP applications, considering variations in devices and conditions. | [2][6] |
| 2. Safety Concerns | Safety aspects, including potential risks and adverse effects, require thorough examination for wider clinical adoption. | Conduct comprehensive safety analyses, identify potential risks, and propose mitigation measures to ensure the safe application of CAP. | [1][5] |
| 3. Variable Patient Response | Variability in individual patient responses to CAP treatment challenges personalized medicine approaches. | Implement personalized treatment plans by considering patient-specific factors, such as skin type, medical history, and genetic factors. | [2][6] |
| 4. Optimizing Treatment Duration | Determining the optimal duration of CAP treatment to balance effectiveness and patient comfort is challenging. | Conduct controlled trials to identify the most effective treatment durations for specific skin diseases while minimizing potential discomfort. | [3][5] |
| 5. Understanding Mechanisms of Action | Limited understanding of the precise mechanisms underlying CAP's therapeutic effects impedes optimization. | Invest in comprehensive research to elucidate CAP action's molecular and cellular mechanisms in skin disease treatment. | [4][6] |
| 6. Integration with Standard Therapies | Integrating CAP into existing standard skincare and dermatological therapies poses integration challenges. | Explore synergistic effects with traditional therapies and optimize combination treatment strategies for enhanced efficacy. | [2][6] |
| 7. Cost of Treatment | The potential high cost of CAP devices and treatments may limit accessibility for some patients. | Foster research and development to create cost-effective CAP devices and treatment modalities without compromising efficacy. | [3][5] |
| 8. Long-Term Effects and Sustainability | Understanding the long-term effects of repeated CAP treatments and ensuring sustainability is crucial. | Conduct longitudinal studies to assess the long-term effects and establish sustainable practices in CAP therapy. | [1][6] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





