1. Introduction
Sheep milk and dairy products represent a significant part of agrarian economies in Middle east and Mediterranean Sea countries, and, in others, they are considered a delicacy and a functional food [
1,
2]. Indeed, they are part of a new market niche that seeks consumer recognition, satisfaction, and acceptance gaining more and more market size [
1,
2,
3]. For this reason, dairy products and fermented milks are considered important because they are a source of energy and they contain essential nutrients including carbohydrate, lipids, proteins, minerals, vitamins, and they are the main source of bioactive peptides [
4,
5].
As far as protein content is concerned, sheep milk shows higher quantity than cow [
1]. Proteins molecular forms and amino acid sequences have high nutritional quality, as well as positive impact on digestibility, and thermostability; this milk contains the best composition of exogenous amino acids which fully cover the requirement for all essential amino acids [
3]. Furthermore, αs1- and αs2- casein differ markedly from those of other species and there is a low percentage of αs1-casein suggesting a lower allergic sensitization [
3,
6]. Moreover, these proteins, due to enzymatic hydrolysis, can release bioactive peptides that are able to exert specific biological activities on human health, such as antioxidant, antihypertensive, antimicrobial, opioid, antioxidant, immunomodulatory, or mineral-binding. These compounds are released during gastrointestinal digestion and/or during food processing depending on the technology and microbiological aspects [
1,
2,
7].
Furthermore, sheep milk fat content is higher and globules size is lower than those of cow milk. This is advantageous for digestibility and a more efficient metabolism compared to cow milk fat [
1,
3]. Moreover, sheep milk fat is highly saturated containing more caproic (C6:0), caprylic (C8:0), and capric (C10:0) acids than cow’s milk which are supposed to reduce body weight and body fat, they are easy to be digested, they may contribute to lower circulating cholesterol, and they are responsible to the characteristic flavours of these products. However, the most represented fatty acids are oleic acid (C18: 1n9), palmitic acid (C16:0) and myristic acid (C14:0). The presence of high amount of oleic acid are reported to decrease the level of LDL cholesterol. Besides, sheep milk has higher CLA content if compared to goat and cow milk which is known to own anticarcinogenic and antiatherogenic properties [
1,
2].
In addition, fermented milks are also considered functional foods with prebiotics and probiotics. Lactic acid bacteria used during the fermentation process are important not only for quality, sensory and safety of the product, but also to help intestinal function, stabilize gut microbiota and other health benefits [
8]. To exert these positive effects, they must reach unaltered and in an adequate concentration the gastrointestinal tract. This is possible thanks to the protection given by the high content in protein and fat present in sheep yogurts which confer denser matrices [
9]. All these positive aspects can be increased considering the use of LABs which have different proteolytic activity during fermentation which can produce more bioactive peptides [
10].
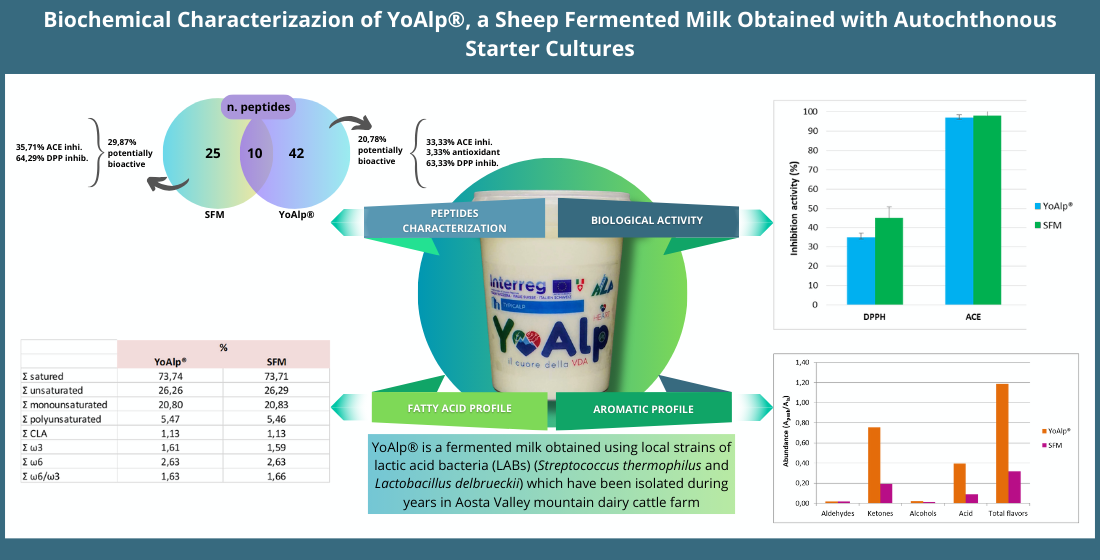
Aim of this study has been to perform a biochemical characterization and a comparison between sheep fermented milk (SFM) made with commercial starter cultures and sheep milk YoAlp®, a fermented milk obtained using local strains of lactic acid bacteria (LABs) (Streptococcus thermophilus and Lactobacillus delbrueckii) which have been isolated during years in Aosta Valley mountain dairy cattle farm.
2. Materials and Methods
2.1. Sampling and Fermented Milks Production
Sheep milk samples from Sarda and Lacaune breeds have been collected from “Azienda Agricola Morzenti” (Aymavilles – AO – Italy) twice in March 2022. 100 mL of raw milk have been frozen and 1 L of milk has been immediately transformed into fermented milk. YoAlp® has been realized using sheep milk and local isolated strains of lactic acid bacteria belonging to species Streptococcus thermophilus and Lactobacillus delbrueckii. In addition, another fermented milk made with the same sheep milk has been produced using commercial strains of lactic acid bacteria to perform a biochemical comparison. Analyses on fermented milks have been performed at 48 hours from the production.
2.2. Fatty Acids Profile
Fatty acid composition analysis has been carried out on milk matrix and on sheep fermented milks by performing the lipid component extraction with a mixture of solvents according to the method reported by Jiang et al. [
11] followed by trans-methylation in a basic environment according to FIL-IDF 182:1999. After that, the identification of the methyl ester of the extracted fatty acids has been carried out by GC/MS analysis (Trace 1300/ TSQ9000 Thermoscientific) while their quantification has been performed with GC/FID analysis (Trace 1300, Thermoscientific,); in both cases a capillary column SP 2560 (100 m x 0.25 mm x 0.2 μm fixed phase non-bonded biscyanopropylpolysiloxane) has been used maintaining the same chromatographic conditions reported by Cadiddu et al. [
12].
2.3. Aromatic Profile
The qualitative and semi-quantitative evaluation of the profile of the volatile organic compounds responsible for the organoleptic properties of sheep’s raw milk and fermented milks has been performed using chromatographic technique SPME-GC/MS with two dif-ferent analytical methods. As far as raw milk is concerned, the analysis was carried out ac-cording to Bergamaschi and Bittante [
13] with some modifications. A 2 cm SPME fibre, 50/30 μm DVB/CAR/ PDMS, StableFlexTM, Supelco has been used. Then this fibre has de-sorbed into the GC/MS split/splitless injector (Trace 1300/TSQ 9000 Thermoscientific) at 250°C. Separation of the extracted volatile compounds was carried out with a DB-WAX J&W column (length 60 m, inner diameter 0,32 mm, film thickness 0,5 μm). Considering fermented milk, the method described by Pan et al. [
14] has been followed with some modifications. For the extraction of volatile organic compounds, an 85 μm SPME Carbox-en/PDMS StableFlexTM fibre has been used, then desorbed into the split/spiless injector of the GC/MS at 250°C. Separation of extracted VOCs was carried out with DB-WAX J&W column (length 30 m, inner diameter 0,25 mm, film thickness 0,25 μm). For both raw milk and fermented milks, the identification of the compounds has been made comparing the mass spectra obtained with those present in the library and comparing their retention times with those described in the literature.
2.4. Peptides Characterization
Samples preparation and analysis has been performed according to the method of Sforza et al. [
15] and Rizzello et al. [
16]. Briefly, fermented milks have been centrifugated at 3800 rpm for 30 minutes at 4°C, then filtered with Whatman N° 2 Paper filters. Filtered samples were again centrifuged a 3000 rpm for 1,30 hours with a Cut-off 10 kDa with Amicon Ultra – 2 or 4 – UltraCel 10 K. After that, 5 ml of each sample was aliquoted and evaporated in Centrivap overnight. Evaporated samples were dissolved in 500 µL of mobile phase A and vortexed in a Thermomixer for 90 minutes. Samples were filtered again at 0,45 µm and injected (10 µl) into the column (Jupiter 4u Proteo 90A, 250´4.6 mm). Solvent used during the analysis were mobile phase A (0,2% CH3CN e 0,1% HCOOH in H2O v/v) and mobile phase B (0,2% H2O e 0,1% HCOOH in CH3CN). These samples have been analysed by Reverse-phase high performance liquid chromatography coupled with a mass spectrometer (RP-HPLC-ESI (+)/MS). RP-HPLC was performed with ACCELA LC system (Thermofisher Scientific, Milan, Italy) equipped with an auto sampler maintained at 15°C. The output was directly interfaced with an ESI-Ion Trap mass spectrometry LTQ XL (Thermofisher). The positive ion mode has been used, and the mass scan was acquired in a range of 150-1900 m/z. Cone voltage and capillary voltage has been set at 30 V 3,2 kV. This analysis has been replicated three times per sample. Finally, peptide identification has been performed using an internal database based on literature data and online databases (ESI PROT or BIOPEP). Each peptide has been also associated to a possible bioactive effect and the Peptide Ranker online software gave an indication on the probability of each sequence to exert the supposed bioactive effect.
2.5. Biological Activity
2.5.1. Sample Preparation
Fermented milk has been prepared for analysis by homogenization in Ultraturrax. Then, samples have been centrifuged at 5000 x g, 4°C and 30 min. Supernatant has undergone to a filtration in centrifuge to obtain the 10 kDa fraction. All treated samples have been analysed with following bioactivity microassay. Raw milk samples have been analysed without this preparation.
2.5.2. Antioxidant Activity
Potential antioxidant activity has been evaluated using the method developed by Prieto [
17] with some modifications. DPPH solution 0,2 mM have been prepared pure DPPH powder dissolved in EtOH. This solution must be kept in the fridge wrapped in foil when not in use, to reduce its degradation.
The analysis has been performed in microplate reader using a 96-well microplate in which has been added 100 µL of sample and 100 µL DPPH 0,2 mM. Serial dilutions (1:2) of samples can be done using EtOH. As standard has been used Vitamin C in different dilution. In addition, for each sample and standard, a blank has been pre-pared by adding only 100 µL EtOH instead of DPPH solution. Moreover, the maxi-mum signal of the radical has been obtained by reacting 100 µL DPPH solution with 100 µL EtOH.
Then the plate has been covered with the lid to minimize evaporation and it has been incubated for 30 minutes. The absorbance (517 nm) has been read in a microplate reader. The percentage of radical scavenging can be obtained using the following expression (1):
2.5.3. Antioxidant Activity
Angiotensin I Converting Enzyme (ACE) inhibition activity has been evaluated using the method developed by Jimsheena and Gowda [
18] with some modifications.
Working solution of ACE from rabbit lung has been prepared at the concentration of 100 mU/mL in sodium borate (SB) buffer 100 mM, pH 8,3 containing 300 mM NaCl. 8 serial dilutions 1:2 of the sample (I1-8) have been prepared in SB buffer. 50 µL of each sample has been pre-incubated at 37°C for 5 min with 25 µL of ACE and 25 µL of buffer. Then, 100 µL of the enzymatic substrate has been added (HHL hippuryl-histidyl-leucine 5 mM) and the reaction has been stopped after 30 min by the addiction of 200 µL of HCl 1 M. The maximum activity (R) of the enzyme has been obtained by reacting the enzyme and its substrate at the same condition in absence of the inhibitor. As a standard has been used Captopril 0,1 µM (C1-8). For each sample, a blank has been prepared in which the enzyme has been previously inactivated with HCl (IB1-8, CB1-8, RB1-8). Finally, the extraction of the reaction product HA (hippuric acid) has been performed in ice by adding 400 µL of pyridine and 200 µL of BSC (benzenesulfonyl-chloride). The signal obtained from the microplate reader at 410 nM has been related at the HA calibration curve ranging from 0 to 100 µM in SB buffer. The percentage of ACE inhibitory activity has been obtained using the following expression (2):
It has been calculated the IC50 from the enzyme inhibition x sample concentration plot using GraphPad Prism 9 (GraphPad Prism software, 225 Franklin Street. Fl. 26, Boston, MA 02110) and the related Captopril equivalent in µM.
2.6. Statistical Analysis
All samples have been analysed in three technical replicates. Results are expressed as the mean (n = 3) ± SD. All the statistical analyses have been performed using Jamovi software (The Jamovi project (2023). Jamovi Version 2.3, Sidney, Australia) and confirmed by JASP software (JASP Team (2023). JASP (Version 0.17.3), Amsterdam, The Netherlands). Data have been tested for normality using Shapiro-Wilk normality test and for homogeneity using the Levene’s Test for Homogeneity of Variance with default parameters. Since data resulted parametric, the analysis of variance test (ANOVA), followed by Tuckey’s post-hoc test (p < 0.05) has been performed to evaluate the differences among compared group.
3. Results and Discussion
3.1. Fatty Acids Profile
Analysis of fatty acid composition has been carried out in GC/MS. 68 compounds have been found and they can be grouped in different classes depending on the length of the fatty acid chain, the presence and the number of unsaturations, the presence of chain ramifications and essential fatty acids, and the geometric and positional isomerism of double bonds. Their quantification has been performed by GC/FID and data have been expressed as percentage of total fatty acids identified (g/100g of fat) to allow a comparison between analysed samples (
Table 1).
Results have highlighted that there are not statistically significant differences between sheep raw milk, SFM and YoAlp® considering each fatty acid class. So, in this study seems that the fermentation process and the use of different LABs have not affected the fatty acid profile of raw milk. Anyway, these analyses put in evidence that YoAlp® is an excellent source of essential fatty acids and it has a good content in CLA, like SFM and sheep raw milk. In particular, high levels of CLA 9c, 11t (up to 90% of total CLA), known to possess anticancer activities, have been found in both fermented milks. Furthermore, a good ω6/ω3 ratio has been observed which confer to these products a high nutritional value.
3.2. Aromatic Profile
The aromatic profile has been obtained through an SPME-GC/MS analysis, and the semi-quantification has been performed using 4-methyl-2-pentanone as internal standard which has allowed to obtain measures of relative average abundance based on the normalized area of chromatographic peaks.
30 and 22 different volatile organic compounds (VOCs) have been identified, respectively, in raw milk and fermented milks. They have been grouped in five classes: aldehydes, ketones, acids, esters and alcohols. In raw milk, ketones and alcohols are the most represented, while aldehydes and esters are in lower percentage (
Table 2). However, the presence of 1-octen-3-ol, pentanal, hexanal, eptanal and butanoic acid ethyl ester are supposed to influence the aromatic profile. Considering fermented milks, most of the typical compounds found belong, as expected [
19], to ketones and acids, while acetaldehydes are the only compound present in aldehydes class, which is essential for the final aroma. Furthermore, ketones and acids are also the most abundant classes if compared to the total identified VOCs (
Table 2).
YoAlp® has been shown to have a good content in volatile components, mainly ketones and acids, higher than SFM. However, this difference is not statistically significative. Moreover, considering the most important aromatic compounds that characterize the typical aroma of fermented milks, it has been shown that the autochthonous starters seem to have a better aptitude than commercial ones in diacetyl, acetoin, acetone and 2-butanone production.
3.3. Peptides Characterization
Peptides analysis by LC-MS ESI+ on the fraction below 10 kDa of sheep milk YoAlp® and SFM detected the presence of 77 different peptides. Thanks to literature research and online database, these peptides have been linked to one or more identification. Each m/z can be related to one or more aminoacidic sequences, so a total of 108 possible aminoacidic sequences has been found. Those peptides were originated from all milk proteins: αs1-casein (αs1-CN), αs2-casein (αs2-CN), k-casein (k-CN), β-casein (β-CN), α-lactalbumin (LALBA) and from β-lactoglobulin (LGB).
Comparing these two fermented milks, YoAlp® has higher number of peptides (52) than SFM (35) and only the 12,99% are in common. A different number can be due to a potential higher proteolytic activity in YoAlp® thanks to local strain of LABs.
In addition, among these peptides, the 71,43% has been identified in SFM, while only the 38,46% for YoAlp®. This can be explained considering the different lactic acid bacteria used. Commercial strains are widely used and most of the proteomic characterization studies on fermented milks present in literature have been done with these strains. On the contrary, the effect of Aosta Valley strains has not been evaluated yet.
Furthermore, it has been possible to correlate the identified peptides to a specific potential bioactive effect and to predict the probability of each sequence to exert the supposed bioactivity. Among the 108 aminoacidic sequences, 52 have been related to a possible bioactive effect. Most of them are considered DPP-IV (Dipeptidyl Peptidase IV) and ACE (Angiotensin-converting enzyme) inhibitors, the others are supposed to be antioxidant, immunomodulator, and anticancer. Peptide Ranker analysis shows that 50,93% of peptides have a low probability to exert a bioactive effect (probability lower than 50%) and 13,89% has a high probability to exert a bioactive effect (probability higher than 75%). Considering SFM, the 29,87% has been suggested to have possible health benefits. On the contrary, only the 20,78% of aminoacidic sequences of YoAlp® is supposed to be bioactive. In particular, the 35,71% are ACE inhibitory and the 64,29% are DPP-IV inhibitory peptides for SFM, while the 33,33% are ACE inhibitory, the 3,33% are antioxidant and the 63,33% are DPP-IV inhibitory peptides for YoAlp®. The most interesting bioactive peptides are shown in
Table 3.
3.4. Biological Activity
3.4.1. ACE Inhibitory Activity
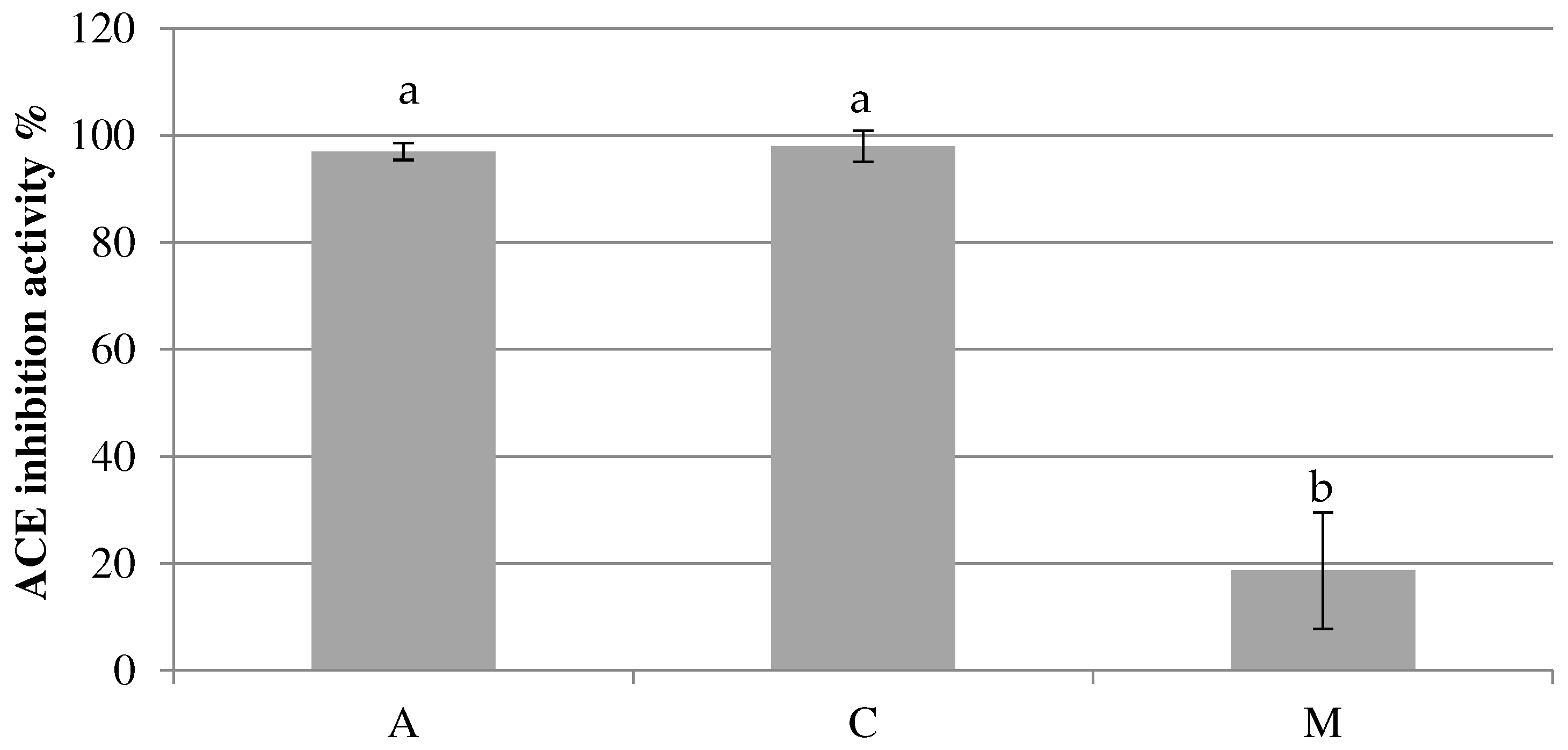
As far as bioactivity is concerned, as expected by peptide profile analysis, ACE inhibitor activity is high for both samples (97,00 ± 1,57% and 97,95 ± 2,90% which correspond to 0,093 Captopril EQ µM and 0,096 Captopril EQ µM of ACE 0,1 U/mL), while it is low for raw milk sample (18,66 ± 10,87% which correspond to 0,010 Captopril EQ µM of ACE 0,1 U/mL) (
Figure 1). These differences have been resulted as no statistical significative between fermented milks and significative (
p < 0.001) between raw milk and fermented milks.
In both fermented milks there is an activity against ACE and these values are higher than those of non-fermented raw milks, so it is possible to suppose that this bioactivity is due to short aminoacidic sequences created by LABs during fermentation. Moreover, it is also important to highlight that autochthonous and commercial strains, which are responsible for the production of different peptides, are both increasing the antihypertensive activity of fermented milks.
3.4.2. Antioxidant Activity
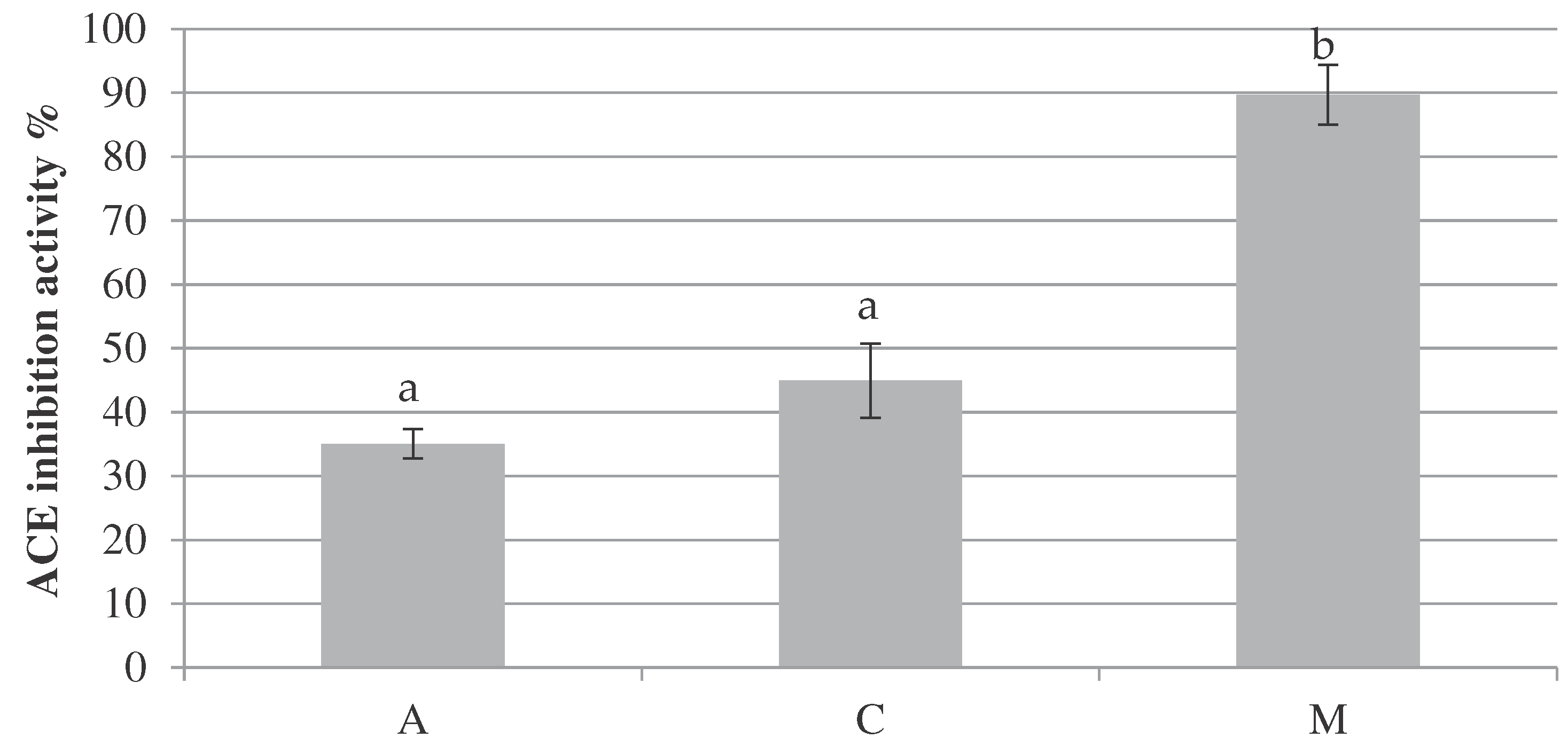
Considering antioxidant activity, it is possible highlight high values for raw milk (89,69 ± 4,68% - 11,46 CEAC µg/mL of DPPH 0,2 mM), while lower percentages of inhibition have been reached in both fermented milks. This result suggests that fermented milk production process, and in particular the heat treatment, has a negative impact on antioxidant compounds as reported by Stobiecka, Król and Brodziak [
20].
Anyway, even in this case, peptide profile bioactivity prediction has been confirmed by the assay showing a DPPH inhibition higher for SFM (44,92 ± 5,81% - 5,70 CEAC µg/mL of DPPH 0,2 mM) than for YoAlp® (35,02 ± 2,29% - 4,43 CEAC µg/mL of DPPH 0,2 mM) (
Figure 2). These differences have been resulted as no statistical significative between fermented milks and significative (
p < 0.001) between raw milk and fermented milks.
4. Conclusions
Local strains of lactic acid bacteria from Aosta Valley seems to work as well as commercial starters. Moreover, autochthonous starters contribute to preserve biodiversity and typicality. Further analyses are needed to identify more peptides generated by autochthonous LABs to understand microbial proteolytic activities, to confirm identified peptides biological effects and to investigate gastric digestion resistance of those which can have positive effects on human health.
Author Contributions
Conceptualization, S.V. and L.V.; methodology, L.T., T.F. and S.V.; validation, T.F., L.T., and S.V.; formal analysis, S.V.; investigation, T.F., M.M., L.T.; resources, S.V. and L.V.; data curation, T.F., M.M., R.P., S.Z., L.T. and S.V.; writing—original draft preparation, M.M. and S.V.; writing—review and editing, M.M. and S.V..; visualization, S.V.; supervision, S.V.; project administration, S.V.; funding acquisition, S.V. and L.V. All authors have read and agreed to the published version of the manuscript.
Funding
This experimental work was financially supported, through TYPICALP project, by European Union, European Regional Development Fund, Italian State, Swiss Confederation and Cantons co-financed operation, within the framework of the Interreg V-A Italy-Switzerland Cooperation Program.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Park, Y.W..; Juárez, M.; Ramos, M.; Hainlein, G.W.F. Physico-chemical characteristics of goat and sheep milk. Small Rumin. Res. 2007, 68, 88-113.
- Balthazar, C.F.; Pimentel, T.C.; Ferrão, L.L.; Almada, C.N. , Santillo, A.; Albenzio, M.; Mollakhalili, N.; Mortazavia, A.M.; Nascimento, J.S.; Silva, M.C.; Silva, M.C.; Freitas, M.Q.; Sant’Ana, A.S.; Granato, D.; Cruz, A.G. Sheep Milk: Physicochemical Characteristics and Relevance for Funcional Food Development. CRFSFS 2017, 00, 1–16. [Google Scholar]
- Mohapatra, A; Shinde, A.K.; Singh, R. Sheep milk: a pertinent functional food. Small Rumin. Res. 2019, 1-29.
- Vargas-Bello-Pérez, E.; Márquez-Hernández, R.; Hernández-Castellano, E. Bioactive peptides from milk: animal determinantes and their implications in human health. J. Dairy Sci. 2019, 86, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Silva, E.O.O.; Nespolo, C.R.; Sehn, C.P.; Pinheiro, F.C.; Stefani, L.M. Lactic acid bacteria with antimicrobial, proteolytic and lypolitic ativities isolated from ovine dairy products. Food Sci. Technol. 2020, 40, 293–299. [Google Scholar] [CrossRef]
- Anagnostopoulos, A.K.; Katsafadou, A.I.; Pierros, V.; Kontopodis, E.; Fthenakis, G.C.; Arsenos, G.; Karkabounas, S.C.; Tzora, A.; Skoufos, I.; Tsangaris, G.T. Milk of Greek sheep and goat breeds; characterization by means of proteomics. J Proteomics. 2016, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Ha, M.; Sabherwal, M.; Duncan, E.; Stevens, S.; Stockwell, P.; McConnel, M.; El-Din Bekhit, A.; Carne, A. In Depth Characterization of Sheep (Ovis aries) Milk Whey Proteome and Comparison with Cow (Bos Taurus). PLOS one 2015, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.Y. , Yoon, K.S., 2022. Effect of probiotic lactic acid bacteria (LAB) on the quality and safety of Greek yogurt. Foods 2022, 11, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Wang, L.; Li, Q.; Long, Y.; Lin, Y.; Yin, J.; Zeng, Y.; Huang, L.; Yao, T.; Abbassi, M.N.; Yang, H.; Wang, Q.; Tang, C.; Khan, T.A.; Liu, Q.; Yin, J.; Tu, Q. , Yin, Y. Functional probiotics of lactic acid bacteria from Hu sheep milk. BMC Microbiology 2020, 1–12. [Google Scholar]
- Papadimitiou, C.G.; Vafopoulou-Mastrojiannaki, A.; Vieira Sila, S.; Gomes, A.M.; Malcata, F.X.; Alichanidis, E. Identification of peptides in traditional and probiotic sheep milk yoghurt with angiotensin I-converting enzyme (ACE)-inhibitory activity. Food Chem. 2007, 105, 647–656. [Google Scholar] [CrossRef]
- Jiang J., Bjoerck L., Fondén R. and Emanuelson M., 1996. Occurrence of Conjugated Cis-9, Trans-11-Octadecadienoic Acid in Bovine Milk: Effects of Feed and Dietary Regimen. Journal of Dairy Science. Vol. 79, Issue 3, pp. 438-445.
- Cadiddu; A.; Molle, G.; Decandia, M.; Spada, S.; Fiori, M.; Piredda, G.; Addis, M. Responses to condensed tannins of flowering sulla (Hedysarum coronarium L.) grazed by dairy sheep. Part 2: Effects on milk fatty acid profile. Lives. Sci. 2009, 123, 230-240.
- Bergamaschi, M.; Bittante, G. Detailed fatty acid profile of milk, cheese, ricotta and by products, from cows grazing summer highland pastures. J. Dairy Sci. 2017, 84, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Pan, D.D.; Wu, Z.; Peng, T.; Zeng, X.Q.; Li, H. Volatile organic compounds profile during milk fermentation by Lactobacillus pentosus and correlations between volatiles flavor and carbohydrate metabolism. J. Dairy Sci. 2014, 97, 624–631. [Google Scholar] [CrossRef] [PubMed]
- Sforza, S.; Cavatorta, V.; Lambertini, F.; Galaverna, G.; Dossena, A.; Marchelli, R. Cheese peptidomics: A detailed study on the evolution of the oligopeptide fraction in Parmigiano-Reggiano cheese from curd to 24 months of aging. J. Dairy Sci. 2012, 95, 3514–3526. [Google Scholar] [CrossRef] [PubMed]
- Rizzello, C.G.; Losito, I.; Gobbetti, M.; Carbonara, T.; De Bari, M.D.; Zambonin, P.G. Antibacterial Activities of Peptides from the Water-Soluble Extracts of Italian Cheese Varieties. J. Dairy Sci. 2005, 88, 2348–2360. [Google Scholar] [CrossRef] [PubMed]
- Prieto, J.M. Procedure: Preparation of DPPH Radical, and antioxidant scavenging assay. 2012.
- Jimsheena, V.K.; Gowda, L.R. Colorimetric, high-throughput assay for screening angiotensin I-Converting enzyme inhibitors. Anal. Chem. 2009, 81, 9388–9394. [Google Scholar] [CrossRef] [PubMed]
- Güler, Z.; Gürsoy-Balci, A.C. Evaluation of volatile compounds and free fatty acids in set types yogurts made of ewes’, goats’ milk and their mixture using two different commercial starter cultures during refrigerated storage. Food Chem. 2011, 127, 1065–1071. [Google Scholar] [CrossRef] [PubMed]
- Stobiecka, M.; Król, J.; Brodziak, A. Antioxidant Activity of Milk and Dairy Products. Animals 2022, 12, 1–27. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).