1. Introduction
Due to its low production cost, durability and flexibility, demand for plastic has remained high since its original manufacture. In 2022, over four hundred million tons of plastic were produced globally [28], much of which will enter mismanaged waste streams or be littered in the environment [16]. Once in the environment, plastic’s durability is no longer desirable and ensures it persists in the environment indefinitely, negatively impacting ecosystem services [19] and biota [27]. In the environment, litter fragments, generating microplastics (MPs) which are smaller than 5 mm. Due to their small size, MPs are readily ingested by various marine species [18] and can impact development, feeding, breathing, and reproduction [42] and can even be fatal. Concerns regarding MPs, however, expand beyond ingestion since they can act as transport vectors for other contaminants, such as persistent organic compounds and heavy metals [12,44] and pathogens [8]. MPs can also penetrate the food chain by consuming infested fish and food products, causing harm to human health. [32,46]. Furthermore, numerous investigations have demonstrated that MPs generate extracellular and intracellular reactive oxygen species (ROS) in aquatic organisms. [38,40,48]
Commercial species, such fish and shellfish, are vulnerable to direct and indirect ingestion of MPs. MPs can be ingested with targeted predation, with evidence of color preferences in some visual predators [24,25], as well as passively through intake of contaminated water [17]. Since most MPs are often found in the gastrointestinal system, gutting the fish will reduce human exposure compared to eating it whole. However, small shrimps and bivalves like clams, mussels, and oysters are exempt from this rule. Crustaceans are particularly susceptible to consuming microplastics due to their varied eating habits, which include scavenger, deposit feeder, suspension feeder, and predator. It is frequent practice to ingest edible meat combined with a piece of the digestive tract, raising the chance of a health risk. Because microplastics are in the same size range as the prey or meals of shrimp, smaller commercial seafood, such as shrimp or decapod crustaceans, are more likely to be affected by them than larger fish [23]. While the effects of MPs on human cells and tissues are still mostly unknown, [35,46] some authors have pointed out that MPs can enter the respiratory, digestive, and circulatory systems of humans. They can also function as physical and chemical stressors to these systems, and they have been linked to a number of diseases, including diabetes, obesity, endocrine disruption, cancer, cardiovascular disease, problems with reproduction, and developmental issues. These diseases pose a serious risk to human health [1,21,26,34,43]
The sea has traditionally been essential to Sri Lanka's people's economic and nutritional security. The fact that Sri Lanka is an island nation in the Indian Ocean gives it a distinct advantage when it comes to being a destination for procuring seafood [36] The Negombo lagoon is a coastal lagoon located in the south-west of Sri Lanka, which has a diverse ecosystem, including mangroves and seagrass beds. The lagoon is also an important breeding ground for shrimp, which is a valuable fishery resource for the local community. However, the Negombo lagoon is also subject to anthropogenic pressures, such as untreated sewage discharge and solid waste dumping [4], which can contribute to the presence of microplastics in the lagoon ecosystem. Additionally, a fire aboard the cargo ship MV X-Press Pearl, which was anchored with a nitric acid leak, nine nautical miles northwest of Colombo in the Sri Lankan Sea, released ca. 1,680 tons of nurdles [10]. This is the worst chemical and plastic-based marine disaster from a single vessel in Sri Lanka's maritime history. The main environmental concerns this event has raised are: a massive distant black smoke plume emanating from the fire, Potential release into the ocean of 15 dangerous goods from the ship, including 25 metric tons of nitric acid, Large amounts of plastic pellets, cargo, and other debris from the ship have been washing ashore along Sri Lanka's west coast, most notably in Negombo.[31]
To date, no studies have investigated the presence of microplastics in shrimps harvested from the Negombo lagoon. As shrimps are an important aquatic organism consumed by humans in the region, it is essential to understand the extent of microplastic contamination in this organism to assess the potential risks to human health. This study aimed to fill this knowledge gap and investigate any effect to the shrimp in Negombo lagoon from X-Press Pearl incident.
2. Materials and Methods
2.1. Sample collection and processing
Fresh samples of two species of shrimps, Penaeus monodon (n=25) and Penaeus indicus (n=95), were collected from ten locations (12 samples from each site) in the Negombo lagoon, western province of Sri Lanka on 12th September 2022. The collected samples were placed in an icebox and transferred to the Laboratory of Environmental studies division of the National Aquatic Resources Research and Development Agency (NARA) and preserved at -20 °C until further processing and analysis. The specimens of preserved shrimp were defrosted in a metal tray and rinsed with Milli-Q water (Merck, Millipore). Body measurements were recorded, including total body weight, total length, ocular length, and carapace length. Shrimps were deshelled and dissected with a metal scalpel and forceps; then, the gastrointestinal tract (GT) and gills (GI) were isolated and weighed.
2.2. Tissue digestion and filtration.
Separated tissues were carefully transferred into separate glass bottles, and 20 mL of 10% (w/v) KOH was added to each of the glass bottles and stoppered, which after that, were kept in an oven (GALLENKAMP) at 60 °C for 24 hrs. Then bottles were kept in an orbital shaker (Scilogex SK-0330-Pro) at 150 rpm for 24 hrs. After which, they were allowed to cool to room temperature. Digested samples were vacuum filtered using nitrocellulose membrane filters with 0.45 μm pore size and 47 mm diameter. Filter papers, whilst still in the filtration system, were covered with a small volume (0.5 -1 mL) of 0.01 mg mL-1 Nile red dye prepared in reagent grade ethanol and left to stand for 5 min. Then Milli-Q water (Merck, Millipore) was passed to eliminate the unbound dye [29,30] and filters were allowed to dry out. Each filter paper was stored after filtration in separate, clean Petri dishes with lids for subsequent microscopic analysis.
2.3. Identification and characterization of microplastics
Filter papers were placed on the stage plate of the stereomicroscope (Euromex StereoBlue SB.1902-P) with fixed microscope adapter (DC 1355 F050) and observed under blue light (470 nm) with an orange lens filter to identify the possible microplastics. Microplastics were identified using ImageFocus Plus v2.2.0 software, and photographs were taken according to their physical characteristics. MPs were identified and categorized based on their size, color, type, and composition. Filters were checked visually using a Leica MZ10F microscope with a GXCAM-U3PRO-20 camera adapter. Suspect polymers were transferred to a Whatman anodiscs (VWR, UK) with a 25 mm diameter and 0.2 m porosity for FTIR examination. The anodics were dried in a drying cabinet (100 L S/S, LTE, UK) at 40°C for at least 24 hours. A Lumos II FTIR (Bruker, UK) was used to identify polymers. A subset of particles was chosen and analyzed with ATR-FTIR and a liquid nitrogen-cooled MCT detector. In reflectance mode, 32 scans were acquired with a resolution of 4 cm-1 in the range of 4000 - 500 cm-1 [20]. The polymer composition of the MP fragments with a size >1 mm was identified using ATR-FTIR (Bruker ALPHA) in the range 500–4000 cm−1 with 32 scans and resolutions of 4 cm−1 [5]. The percentage of the matching score against polymer databases was used to confirm polymer identification. (ATR-FTIR-library complete, vol. 1-4; Bruker Optics ATR-Polymer Library; IR-Spectra of Polymers, Diamond-ATR, Geranium-AT & IR-Spectra of Additives, Diamond-ATR) Only matches greater than 60% probability were chosen for positive microplastic confirmation and polymer identification.
2.4 Quality control
The samples were collected, prepared, and observed using glass and metallic materials. Prior to use, all of the materials were rinsed with Milli-Q water (Merck, Millipore), then dried in an oven at 70 °C. Throughout all processes, cotton lab coats and apparel were worn, and 70% ethanol was utilized to clean the workspaces. To account for potential contamination during the entire procedure, procedural blank samples (n = 4 for each batch) free of tissues were created for each batch of samples [47]. To check for potential contamination, atmospheric blanks (two GF/F filters in clear, glass Petri dishes) were also utilized at each stage of the procedure. The average contamination recorded in the procedural blanks was removed from each sample prior to statistical analysis.
2.5. Data Analysis
Percentages of the detected MPs in the samples by type, shape, color, and size; the average number of MPs per gram GT+GI weight; and the average number of MPs (items/g) per individual (according to the weight of the shrimp) were calculated. Since the data from this study were not normally distributed, a Kruskal-Wallis Test was used to evaluate differences in MPs distribution between sampling sites. Spearman correlation was performed to know the associations between MPs abundance and GT+GI/ body weight. The association between the size of MPs and shrimp species was studied using the Chi-square test. All statistical tests and chart designs were conducted using the -SPSS (IBM Corp. Released 2021. IBM SPSS Statistics for Windows, Version 28.0. Armonk, NY: IBM Corp.) Map diagrams were created using Esri-ArcGIS.
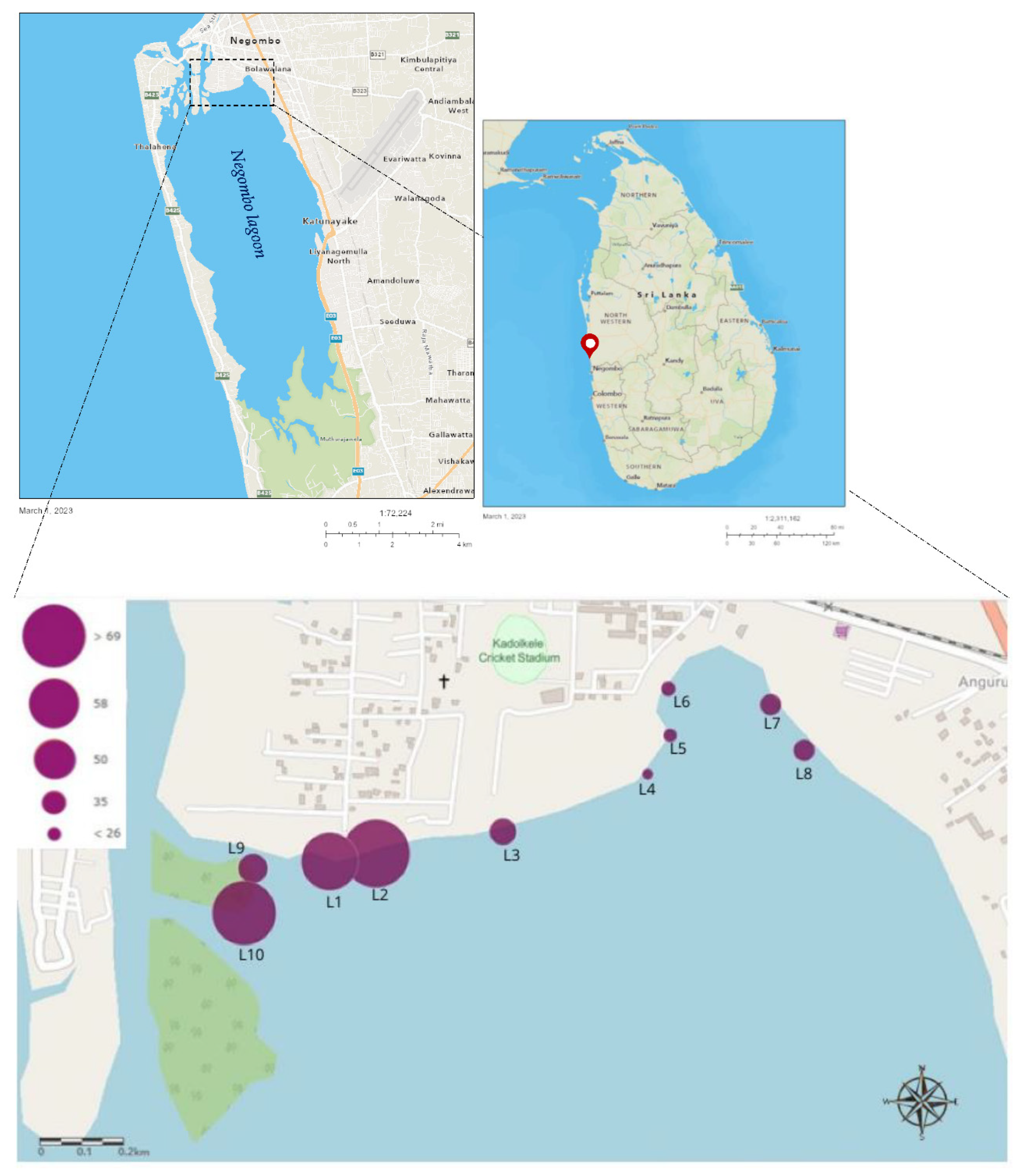
Figure 1.
Maps showing the location of the Negombo lagoon and the spatial occurrence of microplastic abundance in shrimps in selected sampling sites. (The size of the circles indicates the number of identified MPs in shrimp tissues).
Figure 1.
Maps showing the location of the Negombo lagoon and the spatial occurrence of microplastic abundance in shrimps in selected sampling sites. (The size of the circles indicates the number of identified MPs in shrimp tissues).
3. Results and Discussion
3.1. Abundance of microplastics
Major morphometric parameters, which are total body weight and total/extended length for P. monodon, were 5.80 ± 2.82 g (mean ± SD) and 85 ± 19 mm, respectively; those for the P. indicus were 4.91 ± 2.31 g and 81 ± 23 mm. From the total of 120 samples, 415 MPs were detected in this study. The average number of MPs per individual for P. monodon was 4.72 ± 2.72, and that for P. indicus was 3.13 ± 2.04. Furthermore, the average number of MPs per gram of GT and GI weight for P. monodon was 8.29 ± 4.63 items per gram, and that for P. indicus was 5.52 ± 3.78 items per gram.
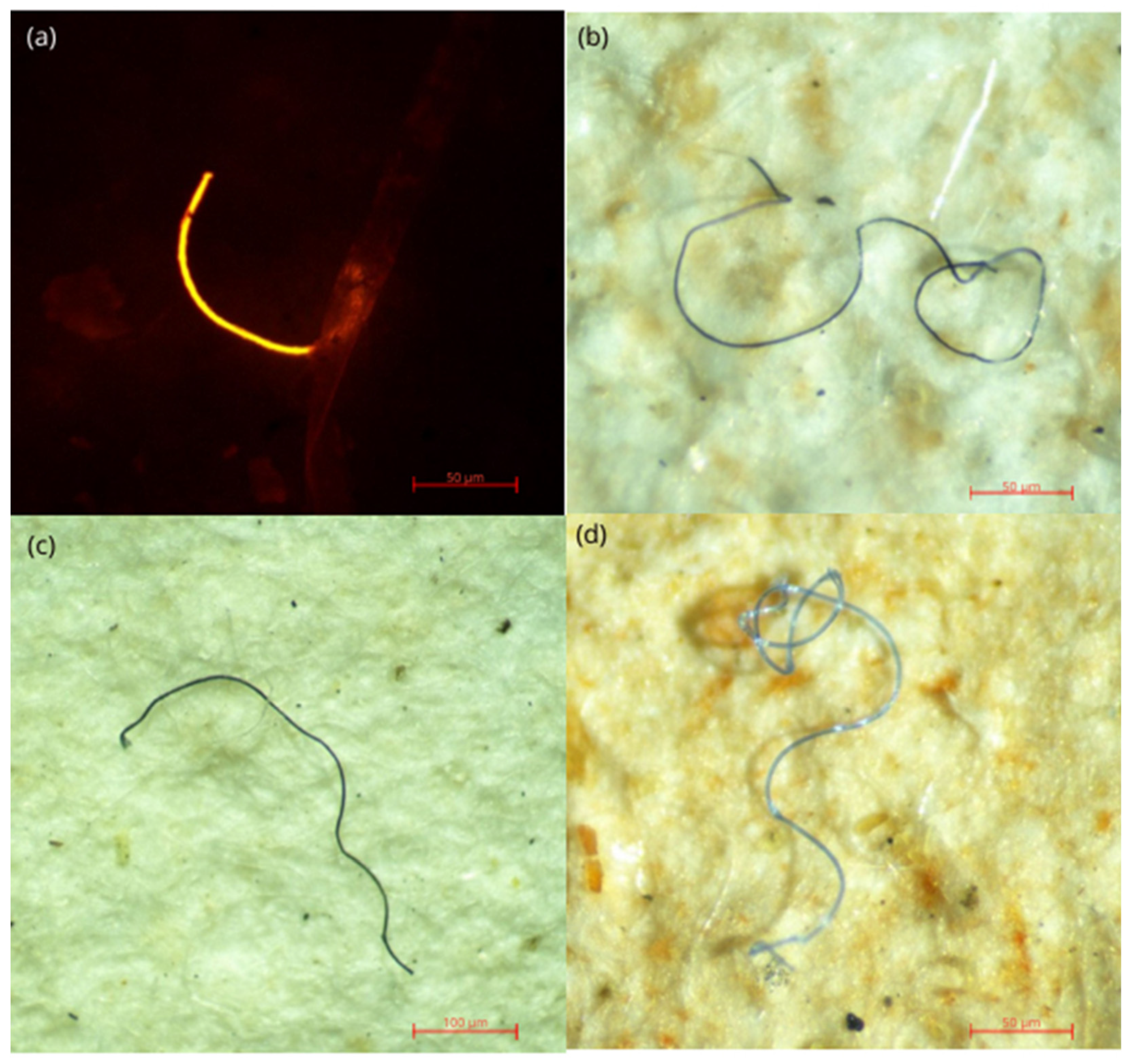
Figure 2.
Some of the microplastics identified from the gastrointestinal tract and gills of the shrimps. (a) Under blue light with Nile red fluorescence (b), (c), (d) under white light.
Figure 2.
Some of the microplastics identified from the gastrointestinal tract and gills of the shrimps. (a) Under blue light with Nile red fluorescence (b), (c), (d) under white light.
Table 1.
Average number of MPs per gram of GT and GI weight identified based on the location of sampling sites.
Table 1.
Average number of MPs per gram of GT and GI weight identified based on the location of sampling sites.
| Sampling site |
MPs per gram of GT and GI weight / g-1
|
| L1 |
9.54 ± 4.61 |
| L2 |
10.42 ± 3.93 |
| L3 |
5.71 ± 3.45 |
| L4 |
3.94 ± 2.88 |
| L5 |
4.17 ± 1.80 |
| L6 |
4.34 ± 2.63 |
| L7 |
5.12 ± 1.44 |
| L8 |
5.09 ± 2.95 |
| L9 |
5.90 ± 3.93 |
| L10 |
8.12 ± 5.32 |
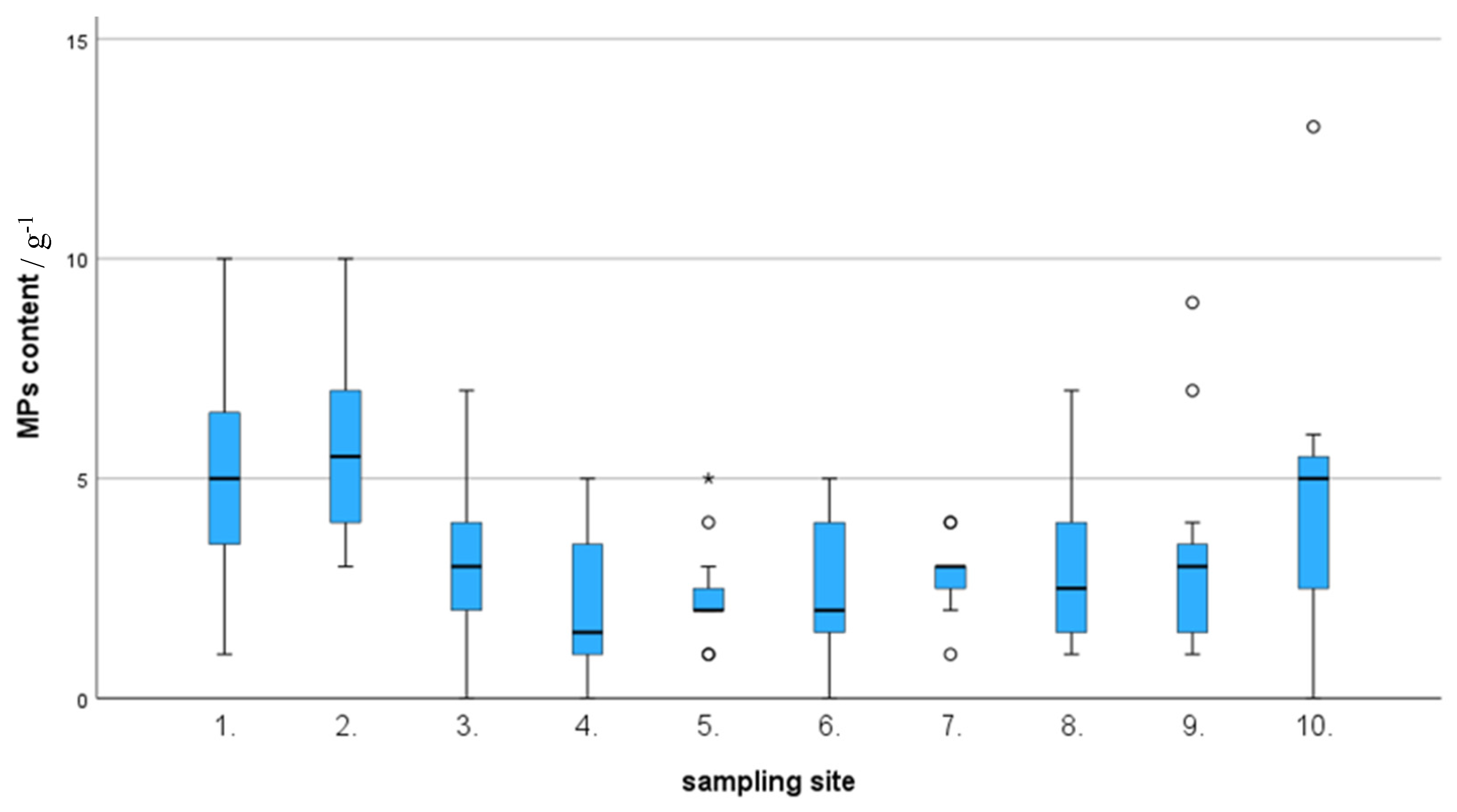
Results revealed that the highest MPs abundance was examined at the sampling site L2, whereas the lowest abundance was noticed at site L4. Asymptotic significance (df = 9; p < 0.001; test statistic = 32.779) is displayed in the hypothesis testing of the independent samples Kruskal-Wallis test, indicating at least two groups/sites are significantly different in terms of MPs abundance. Box and Whisker plots generated using significance test results indicate that groups 5, 7, 9, and 10 have potential outliers that could cause significant differences between the groups. (
Figure 3) Furthermore, a pairwise comparison of sample sites performed under the Kruskal-Walli’s test (significance values have been adjusted by the Bonferroni correction for multiple tests) revealed that there is a significant difference in MPs abundance between sites L4 and L1 (p=0.04), L4 and L2 (p=0.004), L5 and L2 (p=0.009) and L6 and L2 (p=0.019). It is interesting to note that sampling sites L1, L2, and L10 which shows highest levels of MPs contamination, located closer to the heart of Negombo city that contributes greatest anthropogenic influence on the lagoon.
According to the Spearman correlations, there was a positive correlation observed between the GT + GI weight and MPs content (rs (118) = 0.249, p < 0.01), but there was no significant positive/negative correlation observed between body weight and MPs content. (rs (118) = -0.048, p < 0.01)
3.2. Sizes of microplastics
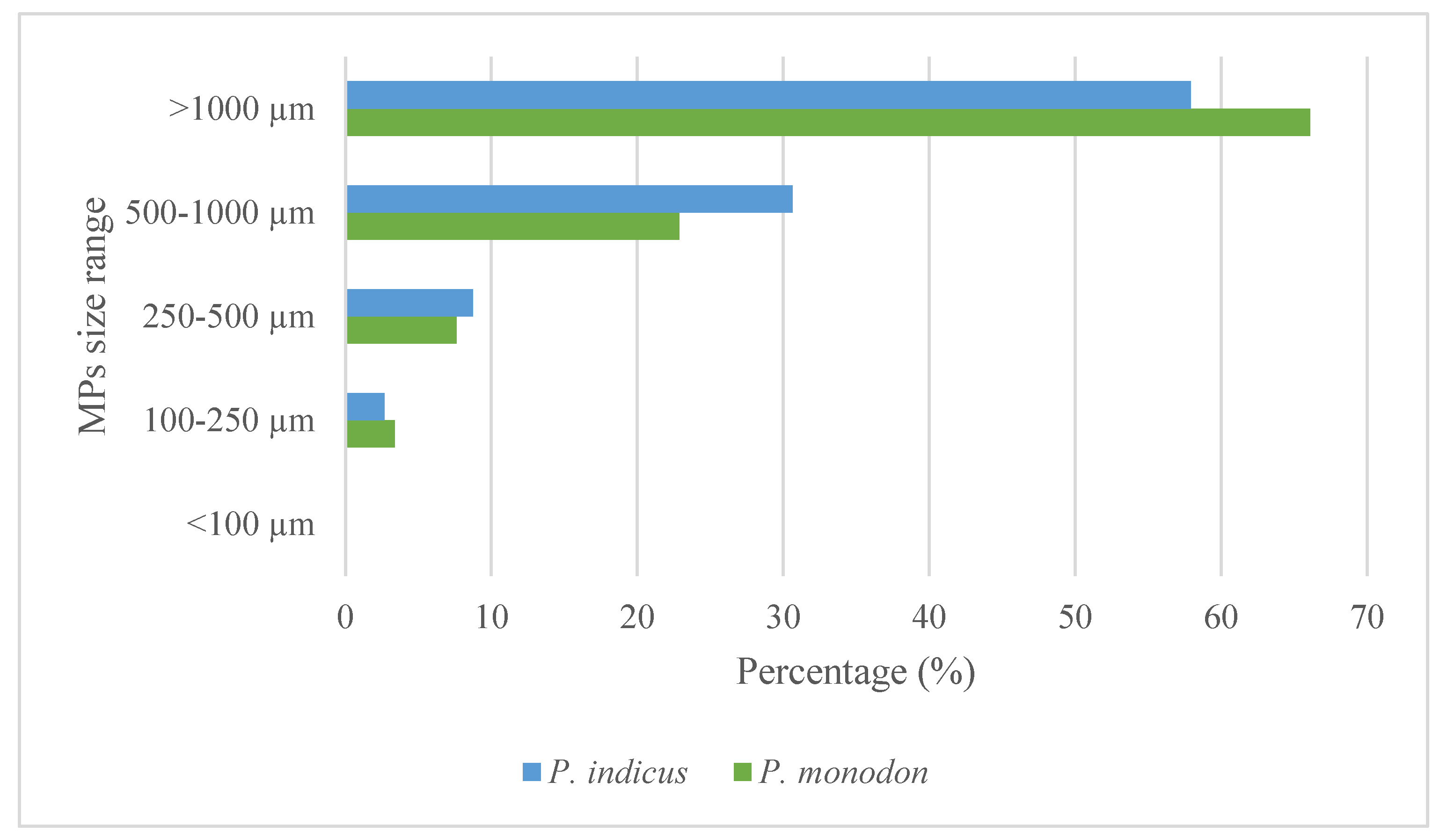
In this study, identified MPs were categorized into five groups, <100 μm, 100–250 μm, 250–500 μm, 500–1000 μm, and >1000 μm. (
Figure 4). Out of the five groups, the size of most of the MPs identified from both shrimp species was under the category of >1000 μm. The sizes recorded in the present study are in line with those of previous observations. In the Bay of Bengal, Bangladesh,
P. monodon were found to have mostly ingested MPs between 1 mm and 5 mm [15]. In the present study,
P. monodon contained 78 (66%) MPs larger 1000 μm, with 27 (23%) between 500-1000 µm, 9 (8%) between 250-500 µm, and 4 (4%) MPs in the size range of 100-250 µm. In contrast,
P. indicus shrimp had 172 (58%), 91 (31%), 26(9%), and 8 (3%) numbers of MPs under the size categories of >1000 μm, 500–1000 μm, 250–500 μm, and 100–250 μm, respectively. It is important to note that no MPs were identified with a size less than 100 μm. According to the Chi-square test, MP size proportion distribution was not significantly affected by the species investigated (χ 2 = 2.997; df = 3; p = 0.392).
3.3. Color of microplastics
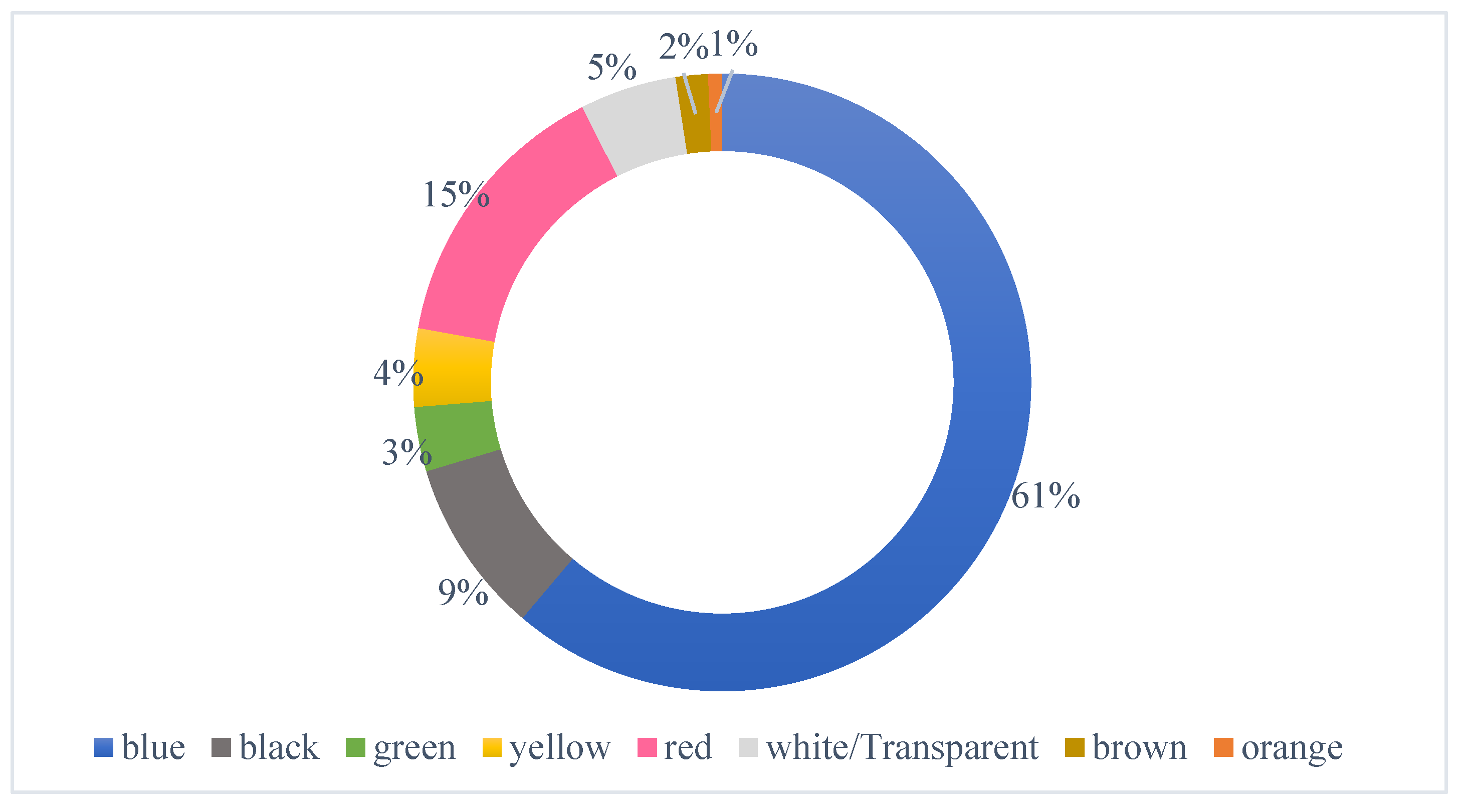
In this investigation, MPs under six color categories were observed, the most prevalent of which was blue (61%) MPs, followed by red (15%), black (9%), white/transparent (5%), yellow (4%), green (3%) brown (2%) and orange (1%). (
Figure 5) Substantial variations could be seen in the distribution of colors of observed MPs in examined sampling locations.
Blue was the most common color MP found at all sites, with over 75% of recovered MPs at sites 3, 4, and 7, being blue in color. Due to their morphological resemblance to natural food products, MP particles may be consumed directly or indirectly through the adherence of MPs to the food particles. Because plastic objects' colors visually resemble their native food or prey, they have an impact on their bioavailability. [25]
3.4. Types and shapes of MPs
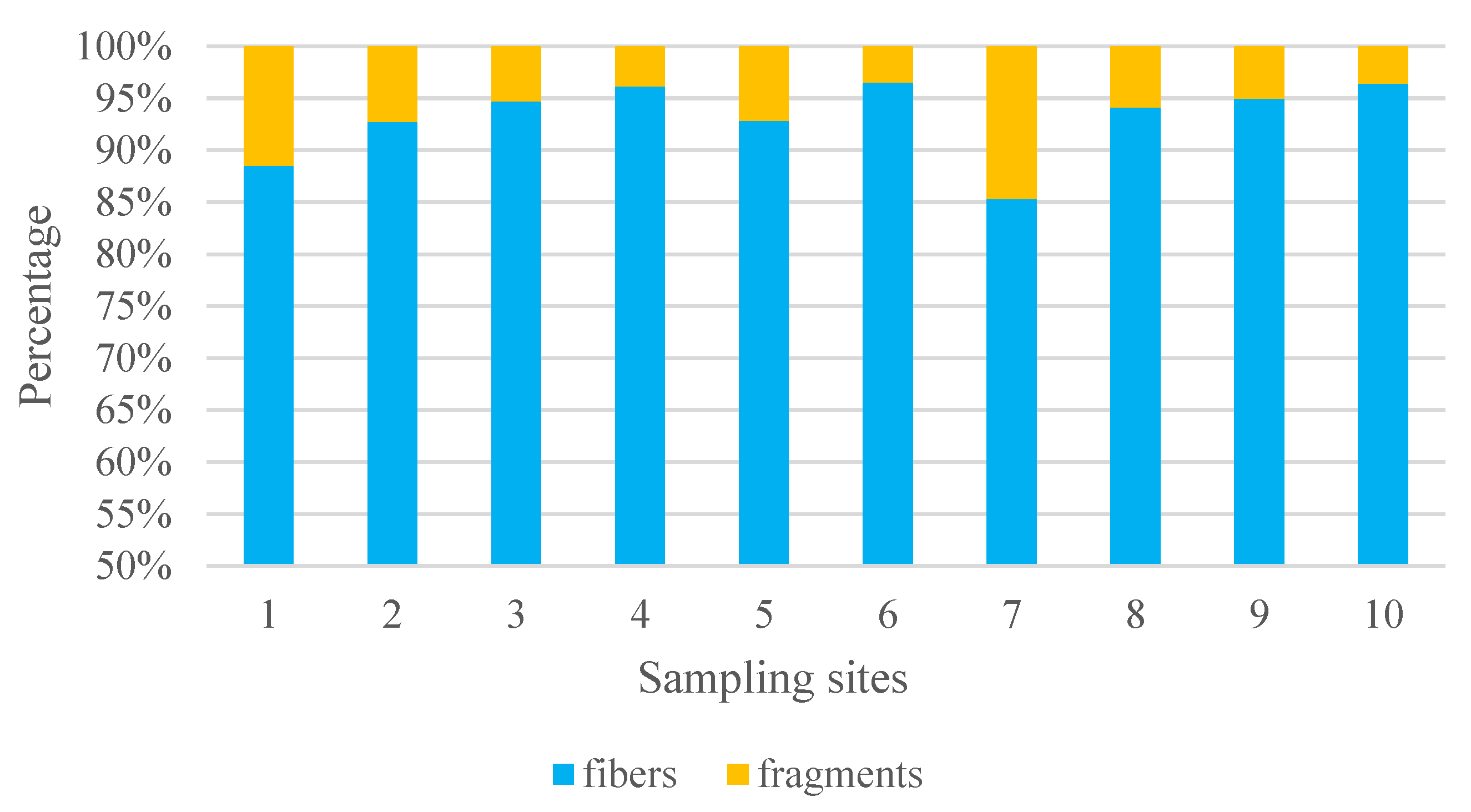
In this study, two main types of MPs were identified; fibers and fragments, but no MPs with the shape of films or spheres were identified. The predominant form of MPs was fiber (93.0%), followed by fragments (6.99%).
Figure 6.
Percentage variation of types of MPs identified from the gastrointestinal tract and gills of shrimps from different sampling sites of Negombo lagoon.
Figure 6.
Percentage variation of types of MPs identified from the gastrointestinal tract and gills of shrimps from different sampling sites of Negombo lagoon.
Up to 91% of the microplastics found in aquatic samples worldwide were made up of fibers. [7,14], which show great bioavailability and abundance. The relative abundance of fibers compared to other types of microplastics may be of special concern for the aquatic ecosystems' pollution. A substantial percentage of microplastics in shrimp species were found to be made of fiber, according to earlier studies. [9,15,22]
Table 2.
Comparative study on the presence of MPs in shrimp species from different regions. WB-whole body; GT-gastrointestinal tract; MU-Muscle tissue.
Table 2.
Comparative study on the presence of MPs in shrimp species from different regions. WB-whole body; GT-gastrointestinal tract; MU-Muscle tissue.
| Species |
Study area |
Abundance (Items/g) |
Tissue examined |
Type of dominant MPs |
Source |
| Paratya australiensis |
Victoria, Australia |
24 ± 31 |
WB |
Fibers |
[22] |
| Litopenaeus vannamei |
Shrimp farm, Guangdong Province, China |
14.1±5.7 |
GT |
Fibers |
[9] |
| Metapenaeus monoceros |
Northern Bay of Bengal, Bangladesh |
3.87 ± 1.05 |
GT |
Fibers |
[15] |
| Penaeus monodon |
Northern Bay of Bengal,Bangladesh |
3.40 ± 1.23 |
GT |
Fibers |
[15] |
| Parapenaeopsis stylifera |
Northeastern Arabian Sea |
64.8±24.6 |
GT |
Fibers |
[13] |
| Metapenaeus monoceros |
Northeastern Arabian Sea |
78.5±48.4 |
GT |
Fibers |
[13] |
| Penaeus indicus |
Northeastern Arabian Sea |
47.5±38.0 |
GT |
Fibers |
[13] |
| Parapenaeopsis hardwickii |
Xiangshan Bay, China |
0.25 ± 0.08 |
GT, MU |
Fibers |
[47] |
3.5. Polymer characterization
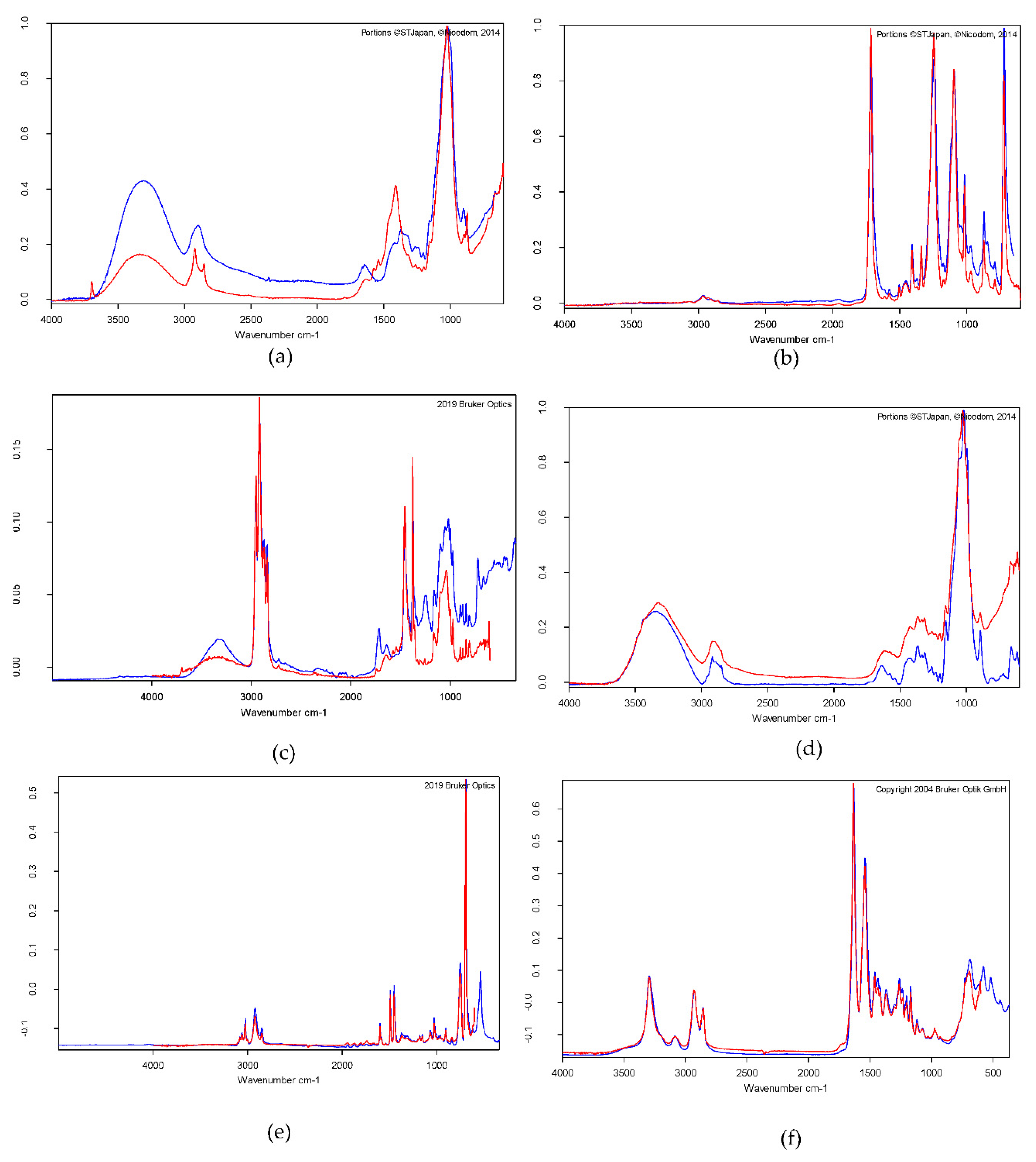
In the present study, the chemical composition of a set of samples that were randomly selected (n =26) was determined, and the MPs with the composition of polystyrene, polyamide, polyester, polypropylene, and rayons were identified. Results suggested that the common raw materials for ropes, fishing nets, floats, fish baskets, and coatings used in the fishing activities in Negombo lagoon and nearby ocean may be the main origin of the identified MPs in shrimps. The most MP fibers were identified as rayon which is a type of semi-synthetic fiber that also found in the deep sea. [45]. A similar study conducted on 11 sites of the Greater Melbourne Area (GMA) and the Goulburn River catchment in northern central Victoria on Paratya australiensis, indicated that rayon was found in shrimps, the most regularly (22.6%) and was found at all 10 locations. At nine sites, polyester was the second-most prevalent material (7.5%). [22]. In another study, the most representative polymer found in the tissues of commercial semi-intensive shrimps from the Gulf of California ecoregion was polyethylene (about 50%), followed by polyamide (about 20%). [41]
Figure 7.
FTIR spectra of different microplastics obtained from the shrimps from the Negombo lagoon: (a) Rayon, (b) Polyester, (c) Polypropylene, (d) Rayon 92% Elastan 8% (e) Polystyrene (f) Polyamide.
Figure 7.
FTIR spectra of different microplastics obtained from the shrimps from the Negombo lagoon: (a) Rayon, (b) Polyester, (c) Polypropylene, (d) Rayon 92% Elastan 8% (e) Polystyrene (f) Polyamide.
5. Conclusions
In this study researchers collected fresh shrimp samples from ten locations in Sri Lanka's Negombo lagoon and identified and characterized 415 microplastics in the samples, with an average of 8.29 ± 4.63 items per gram in P. monodon and 5.52 ± 3.78 items per gram in P. indicus. The most prevalent color was blue (61%), and the predominant form of MPs was fiber (93.0%). Chemical composition of most of the MPs identified in the study included polystyrene, polyamide, polyester, polypropylene, and rayons. By eliminating the intestine and gills entirely before readying it for consumption, MP contamination in the edible part of shrimp could be reduced. As a result, the health risks connected with microplastic-contaminated shrimps can be mitigated to some degree. Because MPs can accumulate and transfer throughout the food chain, a systematic program to reduce plastic waste is vital for avoiding broader issues from occurring in the future.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org.
Author Contributions
Conceptualization, D. S. M. De Silva and A.A Deeptha Amarathunga; Data curation, P.L.M.J.H. Lawan and A. McGoran; Formal analysis, P.L.M.J.H. Lawan, A. McGoran and A. Bakir; Funding acquisition, D. S. M. De Silva; Investigation, P.L.M.J.H. Lawan; Methodology, P.L.M.J.H. Lawan, A. McGoran and A. Bakir; Project administration, D. S. M. De Silva, A.A Deeptha Amarathunga, D. B. Sivyer and C. Reeve; Resources, D. S. M. De Silva, A.A Deeptha Amarathunga, A. Bakir, D. B. Sivyer and C. Reeve; Software, P.L.M.J.H. Lawan and A. McGoran; Supervision, D. S. M. De Silva and A.A Deeptha Amarathunga; Validation, P.L.M.J.H. Lawan, A. McGoran and A. Bakir; Visualization, P.L.M.J.H. Lawan; Writing – original draft, P.L.M.J.H. Lawan; Writing – review & editing, D. S. M. De Silva, A.A Deeptha Amarathunga, A. McGoran, A. Bakir, D. B. Sivyer and C. Reeve.
Funding
This research was financially assisted by the University of Kelaniya (Grant number RP/03/02/06/02/2021) and the Centre for Environment, Fisheries and Aquaculture Science (Cefas), under the Ocean Country Partnership Programme (OCPP) of the Blue Planet Fund, UK.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, upon reasonable request.
Acknowledgments
The authors acknowledge the University of Kelaniya for financial assistance (Grant no-RP/03/02/06/02/2021) and the Centre for Environment, Fisheries and Aquaculture Science (Cefas), UK and the National Aquatic Resources Research and Development Agency (NARA), Sri Lanka for providing laboratory facilities and technical assistance.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Alharbi, OM, Khattab, RA, Ali, I. Health and environmental effects of persistent organic pollutants. J Mol Liq 2018;263:442–53. [CrossRef]
- Andrady, A. L. (2017). The plastic in microplastics: A review. In Marine Pollution Bulletin (Vol. 119, Issue 1, pp. 12–22). Pergamon. [CrossRef]
- Arthur C, Baker JE, Bamford HA. 2009. Proceedings of the international research workshop on the occurrence, effects, and fate of microplastic marine debris, September (2008) 9–11. Tacoma, WA, USA. University of Washington Tacoma. Available at https://repository.library.noaa.gov/view/noaa/2509/noaa_2509_DS1.pdf.
- Athukorala, D., Estoque, R. C., Murayama, Y., & Matsushita, B. (2021). Impacts of Urbanization on the Muthurajawela Marsh and Negombo Lagoon, Sri Lanka: Implications for Landscape Planning towards a Sustainable Urban Wetland Ecosystem. Remote Sensing 2021, Vol. 13, Page 316, 13(2), 316. [CrossRef]
- Atugoda, T., Wijesekara, H., Werellagama, D. R. I. B., Jinadasa, K. B. S. N., Bolan, N. S., & Vithanage, M. (2020). Adsorptive interaction of antibiotic ciprofloxacin on polyethylene microplastics: Implications for vector transport in water. Environmental Technology & Innovation, 19, 100971. [CrossRef]
- Barboza, L. G. A., Dick Vethaak, A., Lavorante, B. R. B. O., Lundebye, A. K., & Guilhermino, L. (2018). Marine microplastic debris: An emerging issue for food security, food safety and human health. Marine Pollution Bulletin, 133, 336–348. [CrossRef]
- Barrows, A. P. W., Cathey, S. E., & Petersen, C. W. (2018). Marine environment microfiber contamination: Global patterns and the diversity of microparticle origins. Environmental Pollution (Barking, Essex : 1987), 237, 275–284. [CrossRef]
- Bowley, J., Baker-Austin, C., Porter, A., Hartnell, R., Lewis, C. (2020). Oceanic hitchhikers – assessing pathogen risks from marine microplastic. Trends in Microbiology, 29, 107 – 116. [CrossRef]
- Curren, E., Leaw, C. P., Lim, P. T., & Leong, S. C. Y. (2020). Evidence of Marine Microplastics in Commercially Harvested Seafood. Frontiers in Bioengineering and Biotechnology, 8, 1390. [CrossRef]
- de Vos, A., Aluwihare, L., Youngs, S., DiBenedetto, M.H., Ward, C.P., Michel, A.P.M., Colson, B.C., Mazzotta, M.G., Walsh, A.N., Nelson, R.K., Reddy, C.M., James, B.D. (2022). The M/V X-Press Pearl nurdle spill: contamination of burnt plastic and unburnt nurdles along Sri Lanka’s beaches. ACS Environmental Au, 2, 128 – 135. [CrossRef]
- Gammanpila, M. (2013). Hydrography, nutrients and abundance and distribution of zooplankton in Negombo Lagoon, Sri Lanka. Sri Lanka Journal of Aquatic Sciences, 15(0), 13. [CrossRef]
- Godoy, V., Blázquez, G., Calero, M., Quesada, L., & Martín-Lara, M. A. (2019). The potential of microplastics as carriers of metals. Environmental Pollution (Barking, Essex : 1987), 255(Pt 3). [CrossRef]
- Gurjar, U. R., Xavier, M., Nayak, B. B., Ramteke, K., Deshmukhe, G., Jaiswar, A. K., & Shukla, S. P. (2021). Microplastics in shrimps: a study from the trawling grounds of north eastern part of Arabian Sea. Environmental Science and Pollution Research, 28(35), 48494–48504. [CrossRef]
- Güven, O., Gökdağ, K., Jovanović, B., & Kıdeyş, A. E. (2017). Microplastic litter composition of the Turkish territorial waters of the Mediterranean Sea, and its occurrence in the gastrointestinal tract of fish. Environmental Pollution (Barking, Essex : 1987), 223, 286–294. [CrossRef]
- Hossain, M. S., Rahman, M. S., Uddin, M. N., Sharifuzzaman, S. M., Chowdhury, S. R., Sarker, S., & Nawaz Chowdhury, M. S. (2020). Microplastic contamination in Penaeid shrimp from the Northern Bay of Bengal. Chemosphere, 238. [CrossRef]
- Jambeck, J.R., Geyer, R., Wilcox, C., Siegler, T.R., Perryman, M., Andrady, A., Narayan, R., Law, K.L. (2015). Plastic waste inputs from land into the ocean. Science 347, 768-771. [CrossRef]
- Kalaiselvan, K., Pandurangan, P., Velu, R., Robinson, J. (2022). Occurrence of microplastics in gastrointestinal tracts of planktivorous fish from the Thoothukudi region. Environmental Science and Pollution Research, 29, 44723–44731. [CrossRef]
- Kühn S., van Franeker J.A. (2020). Quantitative overview of marine debris ingested by marine megafauna. Marine Pollution Bulletin 151, 110858. [CrossRef]
- Kumar, P.S., Prasannamedha, G. (2021). Chapter two - Biological and chemical impacts on marine biology, in Kumar, P.S. (Ed.), Modern Treatment Strategies for Marine Pollution. Elsevier, pp. 11-27.
- Leistenschneider, C., Burkhardt-Holm, P., Mani, T., Primpke, S., Taubner, H., & Gerdts, G. (2021). Microplastics in the Weddell Sea (Antarctica): A Forensic Approach for Discrimination between Environmental and Vessel-Induced Microplastics. Environmental Science & Technology, 55(23), 15900–15911. 1591. [CrossRef]
- Mishra, S, charan Rath, C, Das, AP. Marine microfiber pollution: a review on present status and future challenges. Mar Pollut Bull 2019;140:188–97. [CrossRef]
- Nan, B., Su, L., Kellar, C., Craig, N. J., Keough, M. J., & Pettigrove, V. (2020). Identification of microplastics in surface water and Australian freshwater shrimp Paratya australiensis in Victoria, Australia. Environmental Pollution (Barking, Essex : 1987), 259. [CrossRef]
- Nimrat, S., Boonthai, T., & Vuthiphandchai, V. (2011). Effects of probiotic forms, compositions of and mode of probiotic administration on rearing of Pacific white shrimp (Litopenaeus vannamei) larvae and postlarvae. Animal Feed Science and Technology, 169(3–4), 244–258. [CrossRef]
- Ory N.C., Gallardo C., Lenz M., Thiel M. (2018). Capture, swallowing, and egestion of microplastics by a planktivorous juvenile fish. Environmental Pollution 240, 566–573. [CrossRef]
- Ory, N. C., Sobral, P., Ferreira, J. L., & Thiel, M. (2017). Amberstripe scad Decapterus muroadsi (Carangidae) fish ingest blue microplastics resembling their copepod prey along the coast of Rapa Nui (Easter Island) in the South Pacific subtropical gyre. The Science of the Total Environment, 586, 430–437. [CrossRef]
- Pal, D, Maiti, SK. Evaluation of potential human health risks from toxic metals via consumption of cultured fish species Labeo rohita: a case study from an urban aquaculture pond. Expos Health 2019;11:33–46. [CrossRef]
- Pirsaheb, M., Hossini, H., Makhdoumi, P. (2020). Review of microplastic occurrence and toxicological effects in marine environment: Experimental evidence of inflammation. Process Safety and Environmental Protection 142, 1-14. [CrossRef]
- PlasticsEurope (2023). Plastic – The Fast Facts [online]. Available at: https://plasticseurope.org/knowledge-hub/plastics-the-fast-facts-2023/ . [Last accessed 3rd November 2023].
- Prata, J. C., Alves, J. R., da Costa, J. P., Duarte, A. C., & Rocha-Santos, T. (2020). Major factors influencing the quantification of Nile Red stained microplastics and improved automatic quantification (MP-VAT 2.0). The Science of the Total Environment, 719. [CrossRef]
- Prata, J. C., Reis, V., Matos, J. T. V., da Costa, J. P., Duarte, A. C., & Rocha-Santos, T. (2019). A new approach for routine quantification of microplastics using Nile Red and automated software (MP-VAT). The Science of the Total Environment, 690, 1277–1283. [CrossRef]
- Programme, U. N. E., & Affairs, U. N. O. for the C. of H. (2021). X-Press Pearl Maritime Disaster: Sri Lanka - Report of the UN Environmental Advisory Mission. https://wedocs.unep.org/xmlui/handle/20.500.11822/36608.
- Rakib, M.R.J., Sarker, A., Ram, K., Uddin, M.G., Walker, T.R., Chowdry, T., Uddin, J., Khandaker, M.U., Rahman, M.M., Idris, A.M. (2023). Microplastic toxicity in aquatic organisms and aquatic ecosystems: a review. Water, Air & Soil Pollution, 234, 52. [CrossRef]
- Rocha-Santos, T.; Duarte, A.C. Characterization and analysis of Microplastics. Elsevier: Amsterdam, Netherlands, 2017; pp. 75–286. [Google Scholar]
- Ribeiro, F, O’Brien, JW, Galloway, T, Thomas, KV. Accumulation and fate of nano-and micro-plastics and associated contaminants in organisms. Trac Trends Anal Chem 2019;111:139–47. [CrossRef]
- Rubio, L, Marcos, R, Hernández, A. Potential adverse health effects of ingested micro-and nanoplastics on humans. Lessons learned from in vivo and in vitro mammalian models. J Toxicol Environ Health, Part B. 2020;23:51–68. [CrossRef]
- Seafood & Crustaceans from Sri Lanka. (2021, November 24). https://www.srilankabusiness.com/sea-food/sri-lankan-sea-food-products.html.
- Sharifinia, M., Penchah, M. M., Mahmoudifard, A., Gheibi, A., & Zare, R. (2015). Monthly variability of chlorophyll-α concentration in PersianGulf using remote sensing techniques. http://www.ukm.my/jsm/.
- Sussarellu, R., Suquet, M., Thomas, Y., Lambert, C., Fabioux, C., Eve, M., & Pernet, J. (2016). Oyster reproduction is affected by exposure to polystyrene microplastics. 113(9), 2430–2435. [CrossRef]
- Thompson, R. C., Olson, Y., Mitchell, R. P., Davis, A., Rowland, S. J., John, A. W. G., McGonigle, D., & Russell, A. E. (2004). Lost at sea: where is all the plastic? Science (New York, N.Y.), 304(5672), 838. [CrossRef]
- Umamaheswari, S., Priyadarshinee, S., Kadirvelu, K., & Ramesh, M. (2021). Polystyrene microplastics induce apoptosis via ROS-mediated p53 signaling pathway in zebrafish. Chemico-Biological Interactions, 345, 109550. [CrossRef]
- Valencia-Castañeda, G., Ruiz-Fernández, A. C., Frías-Espericueta, M. G., Rivera-Hernández, J. R., Green-Ruiz, C. R., & Páez-Osuna, F. (2022). Microplastics in the tissues of commercial semi-intensive shrimp pond-farmed Litopenaeus vannamei from the Gulf of California ecoregion. Chemosphere, 297, 134194. [CrossRef]
- Van Franeker, J. A. (1985). Plastic ingestion in the North Atlantic fulmar. Marine Pollution Bulletin, 16(9), 367–369. [CrossRef]
- Volschenk, C, Gerber, R, Mkhonto, M, Ikenaka, Y, Yohannes, Y, Nakayama, S, et al.. Bioaccumulation of persistent organic pollutants and their trophic transfer through the food web: human health risks to the rural communities reliant on fish from South Africa’s largest floodplain. Sci Total Environ 2019;685:1116–26. [CrossRef]
- Wang, Z., Chen, M., Zhang, L., Wang, K., Yu, X., Zheng, Z., & Zheng, R. (2018). Sorption behaviors of phenanthrene on the microplastics identified in a mariculture farm in Xiangshan Bay, southeastern China. The Science of the Total Environment, 628–629, 1617–1626. [CrossRef]
- Woodall, L. C., Sanchez-Vidal, A., Canals, M., Paterson, G. L. J., Coppock, R., Sleight, V., Calafat, A., Rogers, A. D., Narayanaswamy, B. E., & Thompson, R. C. (2014). The deep sea is a major sink for microplastic debris. Royal Society Open Science, 1(4). [CrossRef]
- Wright, S. L., & Kelly, F. J. (2017). Plastic and Human Health: A Micro Issue? Environmental Science and Technology, 51(12), 6634–6647. [CrossRef]
- Wu, F., Wang, Y., Leung, J. Y. S., Huang, W., Zeng, J., Tang, Y., Chen, J., Shi, A., Yu, X., Xu, X., Zhang, H., & Cao, L. (2020). Accumulation of microplastics in typical commercial aquatic species: A case study at a productive aquaculture site in China. Science of The Total Environment, 708, 135432. [CrossRef]
- Zhu, K., Jia, H., Sun, Y., Dai, Y., Zhang, C., Guo, X., Wang, T., & Zhu, L. (2020). Long-term phototransformation of microplastics under simulated sunlight irradiation in aquatic environments: Roles of reactive oxygen species. Water Research, 173, 115564. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).