Submitted:
12 December 2023
Posted:
13 December 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. HER2 Discovery
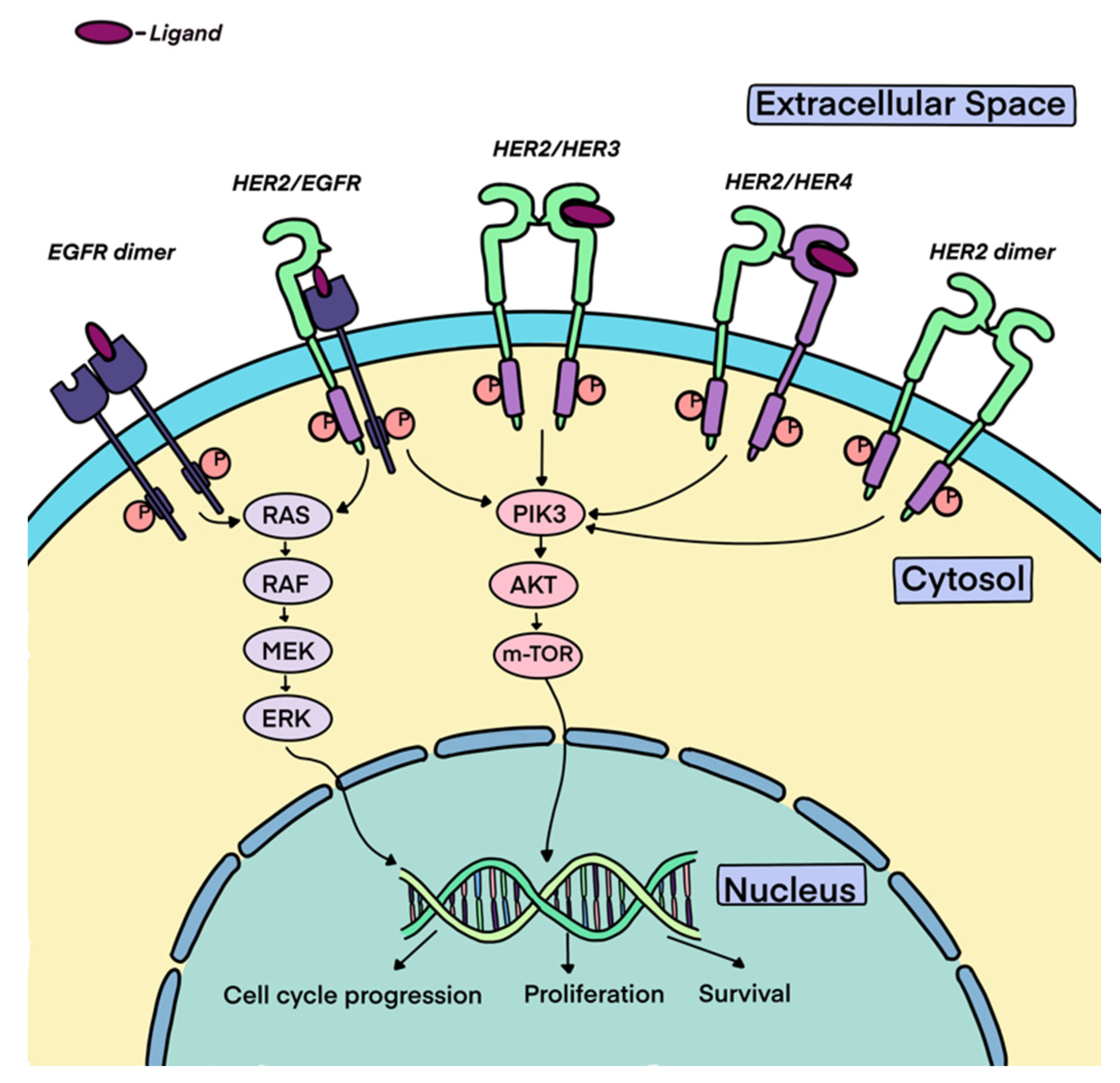
3. HER2 Biology
4. HER2 as an Oncogene and Overexpression in Cancers
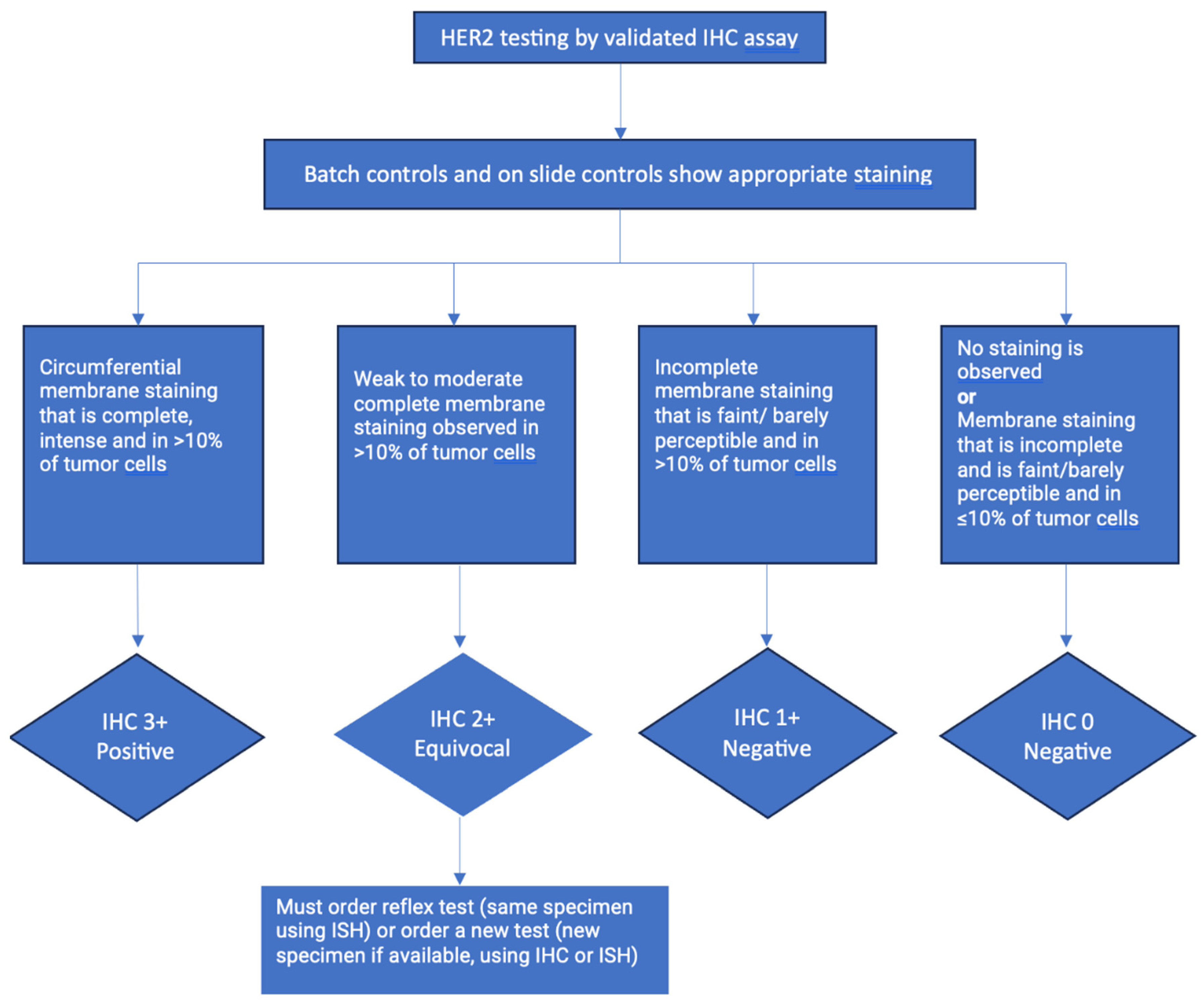
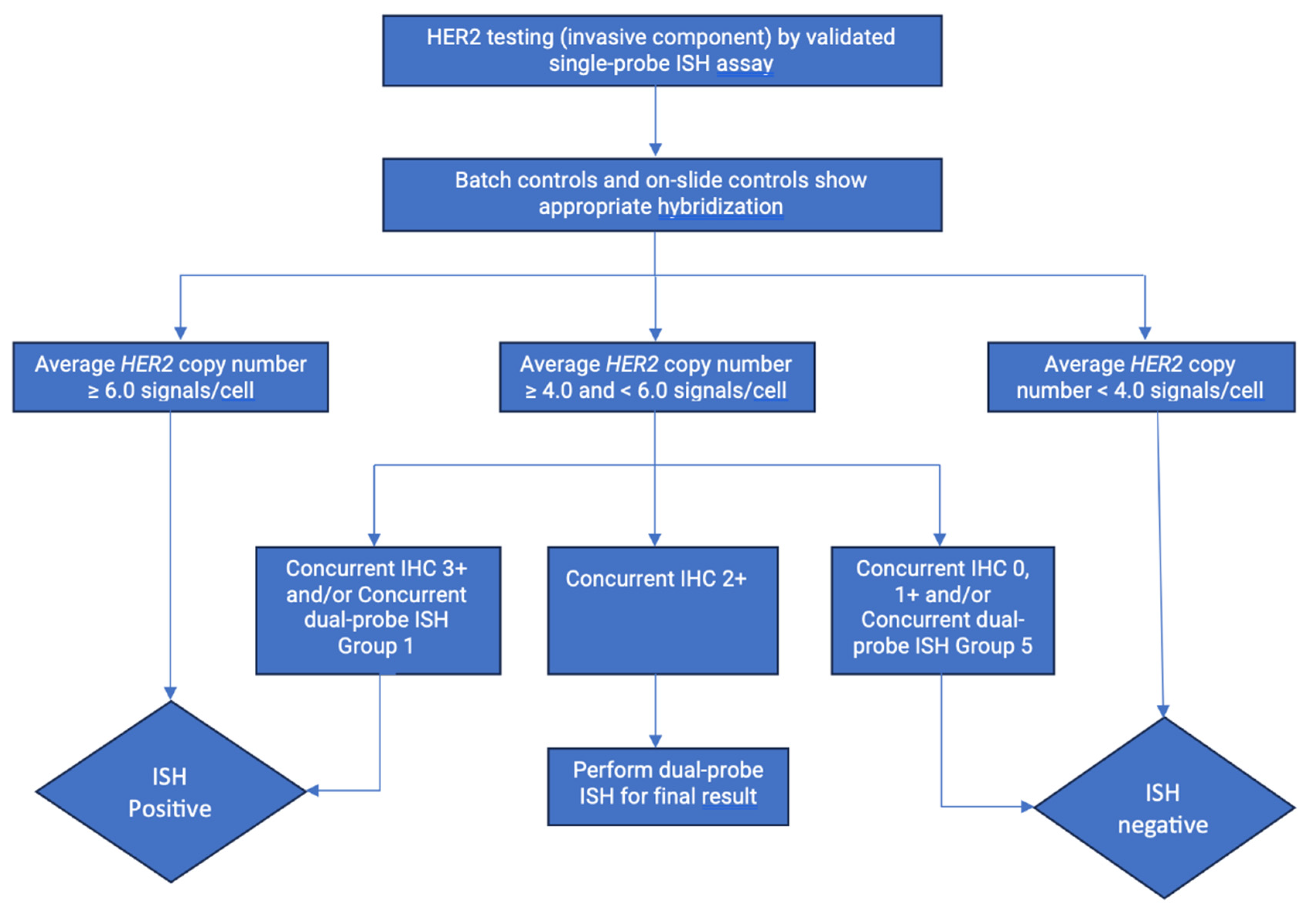
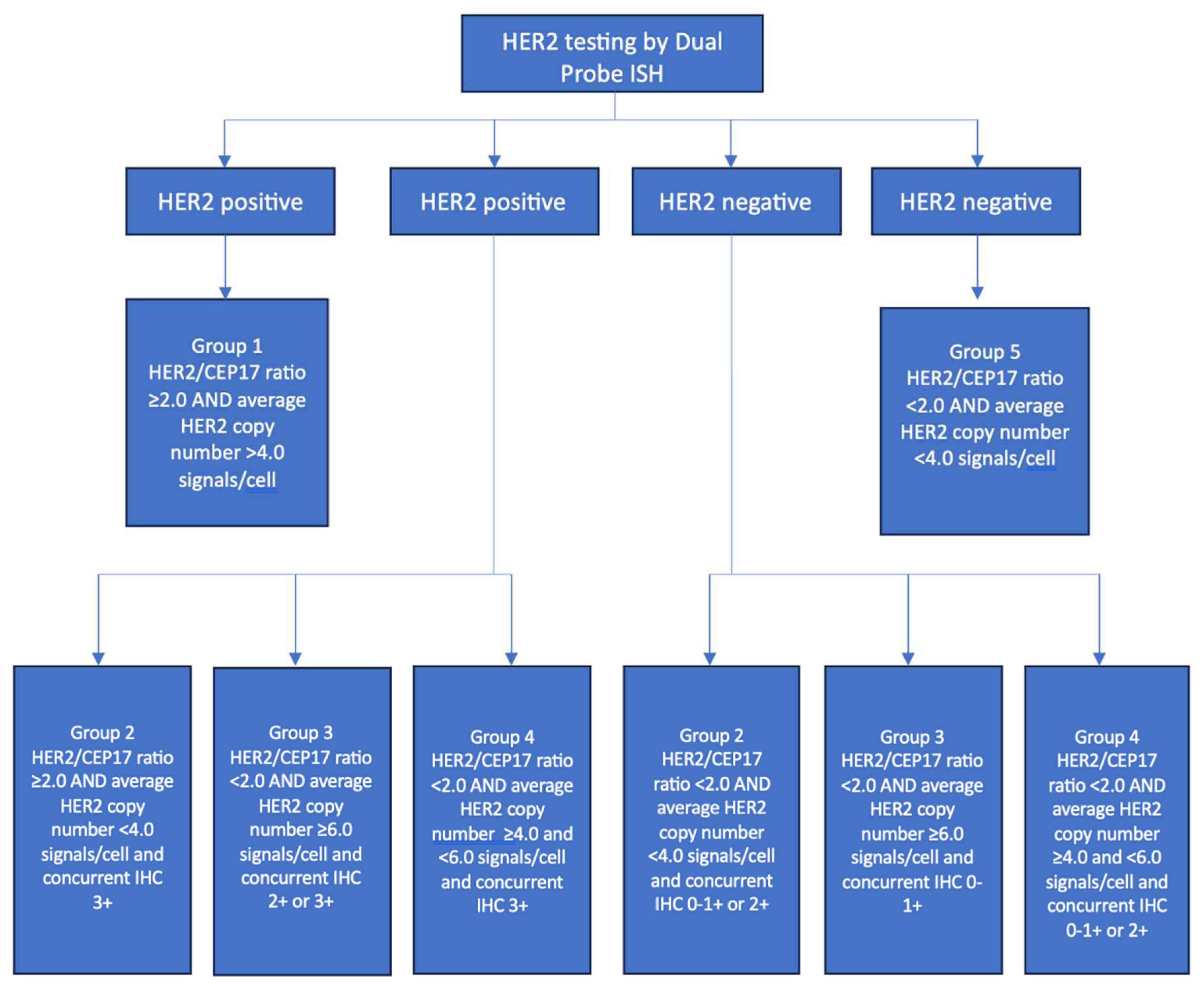
5. TESTING FOR HER2
5.1. Immunohistochemistry
5.2. In Situ Hybridization
5.3. Current challenges with IHC testing and “HER2-low” in breast cancer.
5.4. HER2 testing in other malignancies:
5.5. The Future: De-escalating Therapy with the HER2DX Genomic Tool:
- Immunoglobulin (IGG) signature (14 genes)
- Tumor cell proliferation signature (4 genes)
- Luminal differentiation signature (5 genes)
- HER2 amplicon signature (4 genes)
- HER2Dx risk score - based on the IGG, the luminal and the proliferation signatures.
- HER2DX pCR likelihood score - based on HER2, IGG, luminal and proliferation signatures.
- HER2DX ERBB2 score - based on the ERBB2 mRNA levels
6. HER2 as a prognostic and predictive biomarker:
7. Anti-HER2 Therapies
7.1. Monoclonal Antibodies
- HER2 downregulation
- Anti-HER2 mAbs reduce the amount of her HER2 protein expressed on cell surface; this is due to accelerated endocytic degradation of the overexpressed HER2 homo/heterodimer. When the mAb binds to HER2, it has also been shown to inhibit tyrosine auto-phosphorylation of the receptor. The presence of decreased amounts of HER2 on the surface of the cell reduces HER2 homodimerization and stimulatory activity, reversing the transformed phenotype of HER2 overexpressing cells. [56]
- Prevention of heterodimer formation
- Formation of heterodimers between HER2 and other members of the HER family is important for the complex control of intracellular signaling and cell growth. HER3 and HER4 preferentially heterodimerize with HER2, and their activity is impaired when HER2 is not present in a cell. Anti-HER2 mAbs interfere with the stability of HER2-HER3 and HER2-HER4 heterodimers, which leads to accelerated ligand dissociation. This leads to decreased cell growth signaling. [56]
- Initiation of G1 cell cycle arrest and induction of p27 tumor suppressor
- Anti-HER2 mAbs have anti-proliferative activity and their effect is cytostatic rather than Anti-HER2 mAbs have anti-proliferative activity, and this effect is cytostatic rather than cytotoxic. This results in an increase in the percentage of cells in G0/G1 phase, accompanied by a decrease in the percentage of cells in the S phase. Known inhibitors of the cell cycle, such as p27 and p130, are induced when HER2-overexpressing cells are exposed to mAbs. Furthermore, mAbs have cytotoxic effects by sensitizing HER2 overexpressing cells to tumor necrosis factor-alpha (TNF-alpha), part of the host defense mechanism against tumors. [56]
- Prevention of HER2 cleavage
- It has been demonstrated that HER2 extracellular domain levels (ECDL) correlate with poor prognosis and decreased responsiveness to hormone therapy and chemotherapy. [56] ] MAbs appear to inhibit HER2 cleavage from HER2 overexpressing cells, which leads to decreased HER2 ECDL. Maintenance of the intact form of HER2 on the cell surface decreases constitutive receptor activation and signal transduction, thereby inhibiting cell growth. [56]
- Inhibition of angiogenesis
- Studies have demonstrated that treating HER2 overexpressing breast cancer cells with mAbs can inhibit vascular endothelial growth factor (VEGF) production. [56] VEGF stimulates angiogenesis through intracellular signaling after binding to endothelial cells. This process is controlled by the balance of other signals from angiogenesis inhibitors. In normal homeostasis, there is a balance of both of these signals so that new vessels are created only when they are in need, such as during recovery or growth. Cancer cells can produce pro-angiogenic signals due to an increased demand in blood supply and nutrients. This further allows the cancer cells to grow and invade surrounding tissue, potentially leading to distant metastases.
- Induction of host immune response
- Evidence indicates that mAbs efficiently induce antibody-dependent cellular cytotoxicity against HER2 positive cancer cells but not against cells that do not overexpress HER2. [56]. The fragment C (Fc) portion of mAbs such as trastuzumab can bind to polymorphic receptors on immune cells (natural-killer cells, lymphocytes, macrophages and neutrophils), activating them and enhancing their cytotoxic antitumor activity. [57]
- Anti-Her2 MAbs and chemotherapeutic agents
- Various anti-HER2 mAbs have been shown to have synergistic anti-tumor effects when used in combination with chemotherapy. These effects are specific to HER2 overexpressing cells. [56] The higher response rates of trastuzumab when used in combination with chemotherapy (60%) vs. monotherapy (11%) show the importance of the two treatment synergistically working together. [58] This synergistic approach occurs due to DNA damage caused by chemotherapy, while mAbs help to block DNA repair in HER2+ overexpressing cells. [16] It has also been shown that when paclitaxel is given prior to trastuzumab, antibody-dependent cellular toxicity is significantly enhanced with rapid recruitment of natural killer cells. [59]
7.2. Tyrosine kinases
7.3. Antibody Drug Conjugates
8. Anti-HER2 Therapy - Landmark Trials in Breast Cancer
8.1. Monoclonal Antibodies
8.2. Antibody Drug Conjugates
8.3. Tyrosine Kinase Inhibitors
9. Targeting HER2 across cancers other than breast
9.1. Gastric and gastroesophageal junction cancers
9.2. Colorectal cancer
9.3. Non-small cell lung cancer
9.4. Ovarian cancer
9.5. Endometrial cancer
9.6. Urothelial cancer
9.7. Salivary gland tumor
9.8. Biliary tract cancer
| Study | Phase | Study population | Number of patients (subgroup) | Intervention /subgroup | PFS (months) | OS (months) |
ORR (%) |
DOR (months) |
FDA approval |
|---|---|---|---|---|---|---|---|---|---|
| Bang et al. TOGA trial 2010 [96] |
III | Locally advanced/metastatic HER2 positive gastric or GE junction cancer, as first line treatment | 294 | trastuzumab + chemotherapy | 6.7 | 13.8 | 47 | 6.9 | October 20, 2010 |
| 290 | chemotherapy (cisplatin + 5FU or capecitabine) |
5.5 | 11.1 | 35 | 4.8 | ||||
| Thuss-Patience et al. GATSBY trial 2017 [102] |
III | Locally advanced/metastatic HER2 positive gastric or GE junction cancer, progressed on first line | 153 | T-DM1 | 2.7 | 7.9 | 20.6 | 4.3 | |
| 80 | taxanes | 2.9 | 8.6 | 19.6 | 3.7 | ||||
| Satoh et al. TyTAN trial 2014 [105] |
III | Metastatic HER2 positive gastric cancer, progressed on first line treatment | 132 | lapatinib | 5.5 | 11.0 | 27 | 7.4 | |
| 129 | paclitaxel | 4.4 | 8.9 | 9 | 5.1 | ||||
| Hecht et al. TRIO-013/LOGiC 2017 [106] |
III | Locally advanced or metastatic HER2 positive gastric or GE adenocarcinomas, as first line | 249 | lapatinib + chemotherapy | 6 | 12.2 | 53 | 7.3 | |
| 238 | Chemotherapy (capecitabine and oxaliplatin) | 5.4 | 10.5 | 39 | 5.6 | ||||
| Tabernero et al. JACOB trial 2018 [98] |
III | Metastatic HER2 positive gastric or GEJ cancer, as first line treatment | 388 | pertuzumab + trastuzumab + chemotherapy | 8.5 | 17.5 | 56.7 | 10.2 | |
| 392 | trastuzumab + chemotherapy | 7.0 | 14.2 | 48.3 | 8.4 | ||||
| Shitara et al. DESTINY Gastric 01 2020 [103] |
III | Advanced HER2 positive gastric or GE junction cancer, progressed on 2 prior lines including trastuzumab | 126 | T-DXd | 5.6 | 12.5 | 51 | 11.3 | January 15, 2021 |
| 62 | Chemotherapy (paclitaxel or irinotecan) | 3.5 | 8.4 | 14 | 3.9 | ||||
| Janjigian et al. KEYNOTE 811 2021 [100] |
III | Metastatic HER2 positive gastric or GE junction cancers, as first line treatment | 217 | pembrolizumab + trastuzumab + chemotherapy | 74.4 | 10.6 | May 5, 2021 | ||
| 216 | trastuzumab + chemotherapy (5FU + cisplatin or capecitabine + oxaliplatin) | 51.9 | 9.5 | ||||||
| Sartore-Bianchi at al. HERACLES 2016 [119] |
II | HER2 positive KRAS wild-type metastatic CRC, previously treated | 27 | lapatinib + trastuzumab | 21w | 46w | 30 | 38w | |
| Meric-Bernstam et al. MyPathwayCRC 2019 [109] |
II | Metastatic HER2 positive CRC, previously treated | 57 | trastuzumab and pertuzumab | 2.9 | 11.5 | 32 | 5.9 | |
| Sartore-Bianchi et al. HERACLES-B 2020 [115] |
II | RAS/BRAF wild-type HER2 positive metastatic CRC, previously treated | 31 | pertuzumab + TDM1 | 4.9 | 9.7 | |||
| Gupta et al. TAPUR 2022 [113] |
II | HER2 amplified metastatic CRC, previously treated | 28 | trastuzumab and pertuzumab | 17.2 w | 60 w | 25 | ||
| Yoshino et al. DESTINY-CRC01 2022 [117] |
II | HER2 positive RAS wild type metastatic CRC, previously treated | 53 Cohort A |
T-DXd | 6.9 | 15.5 | 45.3 | 7 | |
| Strickler et al. MOUNTAINEER trial 2023 [108] |
II | HER 2 positive RAS wild-type unresectable or metastatic CRC, previously treated | 84 | tucatinib + trastuzumab | 8.2 | 24.1 | 38.1 | 12.4 | January 19, 2023 |
| 31 | tucatinib | 3.3 | |||||||
| Bob et al. DESTINY-Lung01 2022 [123] |
II | HER2-mutant NSCLC refractory to standard treatment | 91 | T-DXd | 8.2 | 17.8 | 55 | 9.3 | August 11, 2022 |
| Bookman et al. 2003 [131] |
II | HER2 positive epithelial ovarian or primary peritoneal carcinoma, previously treated | 41 | trastuzumab | 2 | 7.3 | 8w | ||
| Fader et al. 2018/2020 [139,140] |
II | HER2 positive advanced or recurrent endometrial cancer, either as primary treatment or for recurrent disease | 30 | trastuzumab + carboplatin and paclitaxel | 12.9 | 29.6 | |||
| 28 | carboplatin and paclitaxel | 8 | 24.4 | ||||||
| Hussein et al. 2007 [138] |
II | HER2 positive metastatic urothelial cancer as first line treatment | 44 | trastuzumab, paclitaxel, carboplatin, and gemcitabine | 9.3 | 14.1 | 70 | 7.1 | |
| Kurzrock et al. MyPathway 2020 [152] |
II | Cohort of HER2 positive advanced salivary gland carcinoma, prior 0-3 lines | 15 | trastuzumab + pertuzumab | 8.6 | 20.4 | 60 | 9.2 | |
| Javle et al. MyPathway 2021 [157] |
II | Cohort of HER2 positive, biliary tract cancer, previously treated | 39 | trastuzumab + pertuzumab | 4 | 10.9 | 23 | 10.8 |
| Level | HER2 alterations | Cancer types | Drugs |
|---|---|---|---|
| 1 | ERBB2 amplification | Breast cancer | Ado-Trastuzumab Emtansine |
| 1 | ERBB2 amplification | Breast cancer | Lapatinib + Capecitabine, Lapatinib + Letrozole |
| 1 | ERBB2 amplification | Breast cancer | Margetuximab + Chemotherapy |
| 1 | ERBB2 amplification | Breast cancer | Trastuzumab + Pertuzumab + Chemotherapy |
| 1 | ERBB2 amplification | Breast cancer | Trastuzumab + Tucatinib + Capecitabine |
| 1 | ERBB2 amplification | Breast cancer | Neratinib, Neratinib + Capecitabine |
| 1 | ERBB2 amplification | Breast cancer | Trastuzumab Deruxtecan |
| 1 | ERBB2 amplification | Breast cancer | Trastuzumab, Trastuzumab + Chemotherapy |
| 1 | ERBB2 amplification | Colorectal Cancer | Tucatinib + Trastuzumab |
| 1 | ERBB2 amplification | Esophagogastric Cancer | Pembrolizumab + Trastuzumab + Chemotherapy |
| 1 | ERBB2 amplification | Esophagogastric Cancer | Trastuzumab + Chemotherapy |
| 1 | ERBB2 amplification | Esophagogastric Cancer | Trastuzumab Deruxtecan |
| 1 | ERBB2 oncogenic mutations | Non-Small Cell Lung Cancer | Trastuzumab Deruxtecan |
| 2 | ERBB2 amplification | Biliary tract cancer, NOS | Trastuzumab + Pertuzumab |
| 2 | ERBB2 amplification | Colorectal cancer | Lapatinib + Trastuzumab |
| 2 | ERBB2 amplification | Colorectal cancer | Trastuzumab + Pertuzumab |
| 2 | ERBB2 amplification | Colorectal cancer | Trastuzumab Deruxtecan |
| 2 | ERBB2 amplification | Uterine Serous Carcinoma/Uterine Papillary Serous Carcinoma | Trastuzumab + Carboplatin-Taxol Regimen |
| 2 | ERBB2 oncogenic mutations | Non-Small Cell Lung Cancer | Ado-Trastuzumab Emtansine |
| 3 | ERBB2 oncogenic mutations | Breast cancer | Neratinib |
| 3 | ERBB2 oncogenic mutations | Non-Small Cell Lung Cancer | Neratinib |
| 3 | ERBB2 oncogenic mutations | Non-Small Cell Lung Cancer | Trastuzumab + Pertuzumab + Docetaxel |
| Agent | MOA | U.S. Food and Drug Administration Indication | |
|---|---|---|---|
| Type of Cancer | Setting / Line of Treatment (year of approval) |
||
| Trastuzumab | mAb | Breast cancer | Adjuvant (2006) or metastatic / 1L (1998) |
| Gastric cancer | Metastatic / 1L (2010) | ||
| Colorectal cancer* | Metastatic / 2L (2023) | ||
| Pertuzumab | mAb | Breast cancer | Neoadjuvant, adjuvant or metastatic / 1L (2012) |
| Margetuximab | mAb | Breast cancer | Metastatic / 3L or later (2020) |
| Ado-trastuzumab emtansine (T-DM1) | ADC | Breast cancer | Adjuvant (2019) or metastatic / 2L (2013) |
| Fam-trastuzumab deruxtecan-nxki (T-DXd) |
ADC | Breast cancer | Unresectable or metastatic or neoadjuvant/adjuvant** / 2L (2019) Unresectable or metastatic, HER2-low (2022) |
| Gastric cancer | Locally advanced or metastatic / 2L (2021) | ||
| Non-small cell lung cancer | Unresectable or metastatic, HER2-mutant (2022) | ||
| Tucatinib | TKI | Breast cancer | Advanced unresectable or metastatic (2020) |
| Colorectal cancer | Unresectable or metastatic, RAS wild-type (2023) | ||
| Neratinib | TKI | Breast cancer | Extended adjuvant treatment (2017)Advanced or metastatic / 3L or later (2020) |
| Lapatinib | TKI | Breast cancer | Advanced or metastatic / 2L (2007) |
10. Mechanisms of Resistance to ERBB inhibitors
11. Mechanism and management of the most relevant toxicities
12. Look into the future: Novel anti- HER2 therapy in development
12.1. Antibody Drug Conjugates
12.2. Tyrosine Kinase Inhibitors
12.3. CAR T-Cell Therapy
12.4. Targeted Protein Degraders
12.5. Checkpoint Inhibitors
12.6. Bispecific Antibodies
12.7. Anti-HER2 Cancer Vaccines
Supplementary Materials
References
- team, T.A.C.S.m.a.e.c. Breast Cancer HER2 Status. Available online: https://www.cancer.org/cancer/types/breast-cancer/understanding-a-breast-cancer-diagnosis/breast-cancer-her2-status.html#:~:text=About%2015%25%20to%2020%25%20of,called%20HER2%2Dpositive%20breast%20cancers (accessed on November 4).
- Grieb, B.C.; Agarwal, R. HER2-Directed Therapy in Advanced Gastric and Gastroesophageal Adenocarcinoma: Triumphs and Troubles. Curr Treat Options Oncol 2021, 22, 88. [Google Scholar] [CrossRef]
- Koopman, T.; van der Vegt, B.; Dijkstra, M.; Bart, J.; Duiker, E.; Wisman, G.B.A.; de Bock, G.H.; Hollema, H. HER2 immunohistochemistry in endometrial and ovarian clear cell carcinoma: Discordance between antibodies and with in-situ hybridisation. Histopathology 2018, 73, 852–863. [Google Scholar] [CrossRef]
- Sanguedolce, F.; Zanelli, M.; Palicelli, A.; Bisagni, A.; Zizzo, M.; Ascani, S.; Pedicillo, M.C.; Cormio, A.; Falagario, U.G.; Carrieri, G.; et al. HER2 Expression in Bladder Cancer: A Focused View on Its Diagnostic, Prognostic, and Predictive Role. Int J Mol Sci 2023, 24. [Google Scholar] [CrossRef]
- Abu Al Karsaneh, O.; Al Anber, A.; Alqudah, M.; Al-Mustafa, S.; AlMa’aitah, H.; Sughayer, M. Prevalence and clinicopathological associations of HER2 expression in non-small cell lung cancer: A retrospective study in Jordanian patients. Diagnostic Pathology 2023, 18, 75. [Google Scholar] [CrossRef]
- Djaballah, S.A.; Daniel, F.; Milani, A.; Ricagno, G.; Lonardi, S. HER2 in Colorectal Cancer: The Long and Winding Road From Negative Predictive Factor to Positive Actionable Target. American Society of Clinical Oncology Educational Book 2022, 219–232. [Google Scholar] [CrossRef]
- Pollock, N.I.; Grandis, J.R. HER2 as a therapeutic target in head and neck squamous cell carcinoma. Clin Cancer Res 2015, 21, 526–533. [Google Scholar] [CrossRef]
- Rubin, I.; Yarden, Y. The basic biology of HER2. Ann Oncol 2001, 12 (Suppl 1), S3–S8. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, C.; Schiff, R. HER2: Biology, detection, and clinical implications. Arch Pathol Lab Med 2011, 135, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.; Unni, N.; Peng, Y. The Changing Paradigm for the Treatment of HER2-Positive Breast Cancer. Cancers 2020, 12. [Google Scholar] [CrossRef] [PubMed]
- Slamon, D.J.; Leyland-Jones, B.; Shak, S.; Fuchs, H.; Paton, V.; Bajamonde, A.; Fleming, T.; Eiermann, W.; Wolter, J.; Pegram, M.; et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med 2001, 344, 783–792. [Google Scholar] [CrossRef]
- Iqbal, N.; Iqbal, N. Human Epidermal Growth Factor Receptor 2 (HER2) in Cancers: Overexpression and Therapeutic Implications. Molecular Biology International 2014, 2014, 852748. [Google Scholar] [CrossRef] [PubMed]
- van der Geer, P.; Hunter, T.; Lindberg, R.A. Receptor protein-tyrosine kinases and their signal transduction pathways. Annu Rev Cell Biol 1994, 10, 251–337. [Google Scholar] [CrossRef] [PubMed]
- Burgess, A.W.; Cho, H.S.; Eigenbrot, C.; Ferguson, K.M.; Garrett, T.P.; Leahy, D.J.; Lemmon, M.A.; Sliwkowski, M.X.; Ward, C.W.; Yokoyama, S. An open-and-shut case? Recent insights into the activation of EGF/ErbB receptors. Mol Cell 2003, 12, 541–552. [Google Scholar] [CrossRef] [PubMed]
- Garrett, T.P.; McKern, N.M.; Lou, M.; Elleman, T.C.; Adams, T.E.; Lovrecz, G.O.; Kofler, M.; Jorissen, R.N.; Nice, E.C.; Burgess, A.W.; et al. The crystal structure of a truncated ErbB2 ectodomain reveals an active conformation, poised to interact with other ErbB receptors. Mol Cell 2003, 11, 495–505. [Google Scholar] [CrossRef] [PubMed]
- Sliwkowski, M.X. Ready to partner. Nat Struct Biol 2003, 10, 158–159. [Google Scholar] [CrossRef] [PubMed]
- Moasser, M.M. The oncogene HER2: Its signaling and transforming functions and its role in human cancer pathogenesis. Oncogene 2007, 26, 6469–6487. [Google Scholar] [CrossRef] [PubMed]
- Citri, A.; Yarden, Y. EGF-ERBB signalling: Towards the systems level. Nat Rev Mol Cell Biol 2006, 7, 505–516. [Google Scholar] [CrossRef] [PubMed]
- Tzahar, E.; Waterman, H.; Chen, X.; Levkowitz, G.; Karunagaran, D.; Lavi, S.; Ratzkin, B.J.; Yarden, Y. A hierarchical network of interreceptor interactions determines signal transduction by Neu differentiation factor/neuregulin and epidermal growth factor. Mol Cell Biol 1996, 16, 5276–5287. [Google Scholar] [CrossRef]
- Sajjadi, E.; Venetis, K.; Ivanova, M.; Fusco, N. Improving HER2 testing reproducibility in HER2-low breast cancer. Cancer Drug Resist 2022, 5, 882–888. [Google Scholar] [CrossRef]
- Wolff, A.C.; Somerfield, M.R.; Dowsett, M.; Hammond, M.E.H.; Hayes, D.F.; McShane, L.M.; Saphner, T.J.; Spears, P.A.; Allison, K.H. Human Epidermal Growth Factor Receptor 2 Testing in Breast Cancer. Arch Pathol Lab Med 2023, 147, 993–1000. [Google Scholar] [CrossRef]
- Modi, S.; Jacot, W.; Yamashita, T.; Sohn, J.; Vidal, M.; Tokunaga, E.; Tsurutani, J.; Ueno, N.T.; Prat, A.; Chae, Y.S.; et al. Trastuzumab Deruxtecan in Previously Treated HER2-Low Advanced Breast Cancer. N Engl J Med 2022, 387, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Gampenrieder, S.P.; Rinnerthaler, G.; Tinchon, C.; Petzer, A.; Balic, M.; Heibl, S.; Schmitt, C.; Zabernigg, A.F.; Egle, D.; Sandholzer, M.; et al. Landscape of HER2-low metastatic breast cancer (MBC): Results from the Austrian AGMT_MBC-Registry. Breast Cancer Res 2021, 23, 112. [Google Scholar] [CrossRef]
- de Calbiac, O.; Lusque, A.; Mailliez, A.; Bachelot, T.; Uwer, L.; Mouret-Reynier, M.A.; Emile, G.; Jouannaud, C.; Gonçalves, A.; Patsouris, A.; et al. Comparison of Management and Outcomes in ERBB2-Low vs ERBB2-Zero Metastatic Breast Cancer in France. JAMA Netw Open 2022, 5, e2231170. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Karakas, C.; Tyburski, H.; Turner, B.M.; Peng, Y.; Wang, X.; Katerji, H.; Schiffhauer, L.; Hicks, D.G. HER2-low breast cancers: Current insights and future directions. Semin Diagn Pathol 2022, 39, 305–312. [Google Scholar] [CrossRef]
- Tarantino, P.; Gandini, S.; Nicolò, E.; Trillo, P.; Giugliano, F.; Zagami, P.; Vivanet, G.; Bellerba, F.; Trapani, D.; Marra, A.; et al. Evolution of low HER2 expression between early and advanced-stage breast cancer. Eur J Cancer 2022, 163, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Prat, A.; Perou, C.M. Deconstructing the molecular portraits of breast cancer. Mol Oncol 2011, 5, 5–23. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, S.; Caini, S.; Paglierani, M.; Saieva, C.; Vezzosi, V.; Baroni, G.; Simoni, A.; Palli, D. Accuracy and Reproducibility of HER2 Status in Breast Cancer Using Immunohistochemistry: A Quality Control Study in Tuscany Evaluating the Impact of Updated 2013 ASCO/CAP Recommendations. Pathol Oncol Res 2015, 21, 477–485. [Google Scholar] [CrossRef]
- Jensen, S.G.; Thomas, P.E.; Christensen, I.J.; Balslev, E.; Hansen, A.; Høgdall, E. Evaluation of analytical accuracy of HER2 status in patients with breast cancer: Comparison of HER2 GPA with HER2 IHC and HER2 FISH. Apmis 2020, 128, 573–582. [Google Scholar] [CrossRef]
- Wu, S.; Yue, M.; Zhang, J.; Li, X.; Li, Z.; Zhang, H.; Wang, X.; Han, X.; Cai, L.; Shang, J.; et al. The Role of Artificial Intelligence in Accurate Interpretation of HER2 Immunohistochemical Scores 0 and 1+ in Breast Cancer. Mod Pathol 2023, 36, 100054. [Google Scholar] [CrossRef]
- Fernandez, A.I.; Liu, M.; Bellizzi, A.; Brock, J.; Fadare, O.; Hanley, K.; Harigopal, M.; Jorns, J.M.; Kuba, M.G.; Ly, A.; et al. Examination of Low ERBB2 Protein Expression in Breast Cancer Tissue. JAMA Oncol 2022, 8, 1–4. [Google Scholar] [CrossRef]
- Prat, A.; Modi, S.; Tsurutani, J.; Cameron, D.; Harbeck, N.; Garrido, C.; Karnoub, M.; Hsu, C.; Feng, W.; Yung, L.; et al. Abstract HER2-18: HER2-18 Determination of HER2-low status in tumors of patients with unresectable and/or metastatic breast cancer in DESTINY-Breast04. Cancer Research 2023, 83, HER2-18. [Google Scholar] [CrossRef]
- Viale, G.; Basik, M.; Niikura, N.; Tokunaga, E.; Brucker, S.; Penault-Llorca, F.; Hayashi, N.; Sohn, J.; Teixeira de Sousa, R.; Brufsky, A.M.; et al. Retrospective study to estimate the prevalence and describe the clinicopathological characteristics, treatments received, and outcomes of HER2-low breast cancer. ESMO Open 2023, 8, 101615. [Google Scholar] [CrossRef] [PubMed]
- Moutafi, M.; Robbins, C.J.; Yaghoobi, V.; Fernandez, A.I.; Martinez-Morilla, S.; Xirou, V.; Bai, Y.; Song, Y.; Gaule, P.; Krueger, J.; et al. Quantitative measurement of HER2 expression to subclassify ERBB2 unamplified breast cancer. Lab Invest 2022, 102, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
- Bartley, A.N.; Washington, M.K.; Colasacco, C.; Ventura, C.B.; Ismaila, N.; Benson, A.B., 3rd; Carrato, A.; Gulley, M.L.; Jain, D.; Kakar, S.; et al. HER2 Testing and Clinical Decision Making in Gastroesophageal Adenocarcinoma: Guideline From the College of American Pathologists, American Society for Clinical Pathology, and the American Society of Clinical Oncology. J Clin Oncol 2017, 35, 446–464. [Google Scholar] [CrossRef] [PubMed]
- Valtorta, E.; Martino, C.; Sartore-Bianchi, A.; Penaullt-Llorca, F.; Viale, G.; Risio, M.; Rugge, M.; Grigioni, W.; Bencardino, K.; Lonardi, S.; et al. Assessment of a HER2 scoring system for colorectal cancer: Results from a validation study. Mod Pathol 2015, 28, 1481–1491. [Google Scholar] [CrossRef] [PubMed]
- Ahcene Djaballah, S.; Daniel, F.; Milani, A.; Ricagno, G.; Lonardi, S. HER2 in Colorectal Cancer: The Long and Winding Road From Negative Predictive Factor to Positive Actionable Target. Am Soc Clin Oncol Educ Book 2022, 42, 1–14. [Google Scholar] [CrossRef]
- Schrock, A.B.; Pavlick, D.; Klempner, S.J.; Chung, J.H.; Forcier, B.; Welsh, A.; Young, L.; Leyland-Jones, B.; Bordoni, R.; Carvajal, R.D.; et al. Hybrid Capture-Based Genomic Profiling of Circulating Tumor DNA from Patients with Advanced Cancers of the Gastrointestinal Tract or Anus. Clin Cancer Res 2018, 24, 1881–1890. [Google Scholar] [CrossRef] [PubMed]
- Takegawa, N.; Yonesaka, K.; Sakai, K.; Ueda, H.; Watanabe, S.; Nonagase, Y.; Okuno, T.; Takeda, M.; Maenishi, O.; Tsurutani, J.; et al. HER2 genomic amplification in circulating tumor DNA from patients with cetuximab-resistant colorectal cancer. Oncotarget 2016, 7, 3453–3460. [Google Scholar] [CrossRef]
- Ren, S.; Wang, J.; Ying, J.; Mitsudomi, T.; Lee, D.H.; Wang, Z.; Chu, Q.; Mack, P.C.; Cheng, Y.; Duan, J.; et al. Consensus for HER2 alterations testing in non-small-cell lung cancer. ESMO Open 2022, 7, 100395. [Google Scholar] [CrossRef]
- Guarneri, V.; Bras-Maristany, F.; Dieci, M.V.; Griguolo, G.; Par, L.; Mar Ín-Aguilera, M.; Miglietta, F.; Bottosso, M.; Giorgi, C.A.; Blasco, P.; et al. HER2DX genomic test in HER2-positive/hormone receptor-positive breast cancer treated with neoadjuvant trastuzumab and pertuzumab: A correlative analysis from the PerELISA trial. EBioMedicine 2022, 85, 104320. [Google Scholar] [CrossRef]
- Prat, A.; Guarneri, V.; Pascual, T.; Brasó-Maristany, F.; Sanfeliu, E.; Paré, L.; Schettini, F.; Martínez, D.; Jares, P.; Griguolo, G.; et al. Development and validation of the new HER2DX assay for predicting pathological response and survival outcome in early-stage HER2-positive breast cancer. EBioMedicine 2022, 75, 103801. [Google Scholar] [CrossRef]
- Waks, A.G.; Ogayo, E.R.; Paré, L.; Marín-Aguilera, M.; Brasó-Maristany, F.; Galván, P.; Castillo, O.; Martínez-Sáez, O.; Vivancos, A.; Villagrasa, P.; et al. Assessment of the HER2DX Assay in Patients With ERBB2-Positive Breast Cancer Treated With Neoadjuvant Paclitaxel, Trastuzumab, and Pertuzumab. JAMA Oncology 2023, 9, 835–840. [Google Scholar] [CrossRef]
- Burstein, H.J. The distinctive nature of HER2-positive breast cancers. N Engl J Med 2005, 353, 1652–1654. [Google Scholar] [CrossRef]
- Slamon, D.J.; Clark, G.M.; Wong, S.G.; Levin, W.J.; Ullrich, A.; McGuire, W.L. Human breast cancer: Correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science 1987, 235, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Press, M.F.; Pike, M.C.; Chazin, V.R.; Hung, G.; Udove, J.A.; Markowicz, M.; Danyluk, J.; Godolphin, W.; Sliwkowski, M.; Akita, R.; et al. Her-2/neu expression in node-negative breast cancer: Direct tissue quantitation by computerized image analysis and association of overexpression with increased risk of recurrent disease. Cancer Res 1993, 53, 4960–4970. [Google Scholar] [PubMed]
- Gabos, Z.; Sinha, R.; Hanson, J.; Chauhan, N.; Hugh, J.; Mackey, J.R.; Abdulkarim, B. Prognostic significance of human epidermal growth factor receptor positivity for the development of brain metastasis after newly diagnosed breast cancer. J Clin Oncol 2006, 24, 5658–5663. [Google Scholar] [CrossRef]
- Seshadri, R.; Firgaira, F.A.; Horsfall, D.J.; McCaul, K.; Setlur, V.; Kitchen, P. Clinical significance of HER-2/neu oncogene amplification in primary breast cancer. The South Australian Breast Cancer Study Group. J Clin Oncol 1993, 11, 1936–1942. [Google Scholar] [CrossRef]
- Yonemura, Y.; Ninomiya, I.; Yamaguchi, A.; Fushida, S.; Kimura, H.; Ohoyama, S.; Miyazaki, I.; Endou, Y.; Tanaka, M.; Sasaki, T. Evaluation of immunoreactivity for erbB-2 protein as a marker of poor short term prognosis in gastric cancer. Cancer Res 1991, 51, 1034–1038. [Google Scholar] [PubMed]
- Uchino, S.; Tsuda, H.; Maruyama, K.; Kinoshita, T.; Sasako, M.; Saito, T.; Kobayashi, M.; Hirohashi, S. Overexpression of c-erbB-2 protein in gastric cancer. Its correlation with long-term survival of patients. Cancer 1993, 72, 3179–3184. [Google Scholar] [CrossRef]
- Nakajima, M.; Sawada, H.; Yamada, Y.; Watanabe, A.; Tatsumi, M.; Yamashita, J.; Matsuda, M.; Sakaguchi, T.; Hirao, T.; Nakano, H. The prognostic significance of amplification and overexpression of c-met and c-erb B-2 in human gastric carcinomas. Cancer 1999, 85, 1894–1902. [Google Scholar] [CrossRef]
- Begnami, M.D.; Fukuda, E.; Fregnani, J.H.; Nonogaki, S.; Montagnini, A.L.; da Costa, W.L., Jr.; Soares, F.A. Prognostic implications of altered human epidermal growth factor receptors (HERs) in gastric carcinomas: HER2 and HER3 are predictors of poor outcome. J Clin Oncol 2011, 29, 3030–3036. [Google Scholar] [CrossRef]
- Yonesaka, K.; Zejnullahu, K.; Okamoto, I.; Satoh, T.; Cappuzzo, F.; Souglakos, J.; Ercan, D.; Rogers, A.; Roncalli, M.; Takeda, M.; et al. Activation of ERBB2 signaling causes resistance to the EGFR-directed therapeutic antibody cetuximab. Sci Transl Med 2011, 3, 99ra86. [Google Scholar] [CrossRef]
- Santin, A.D.; Bellone, S.; Van Stedum, S.; Bushen, W.; Palmieri, M.; Siegel, E.R.; De Las Casas, L.E.; Roman, J.J.; Burnett, A.; Pecorelli, S. Amplification of c-erbB2 oncogene: A major prognostic indicator in uterine serous papillary carcinoma. Cancer 2005, 104, 1391–1397. [Google Scholar] [CrossRef]
- Loibl, S.; Jassem, J.; Sonnenblick, A.; Parlier, D.; Winer, E.; Bergh, J.; Gelber, R.; Restuccia, E.; Im, Y.; Huang, C. VP6-2022: Adjuvant pertuzumab and trastuzumab in patients with early HER-2 positive breast cancer in APHINITY: 8.4 years’ follow-up. Annals of Oncology 2022, 33, 986–987. [Google Scholar] [CrossRef]
- Baselga, J.; Albanell, J. Mechanism of action of anti-HER2 monoclonal antibodies. Ann Oncol 2001, 12 (Suppl. 1), S35–S41. [Google Scholar] [CrossRef] [PubMed]
- Costa, R.L.B.; Czerniecki, B.J. Clinical development of immunotherapies for HER2+ breast cancer: A review of HER2-directed monoclonal antibodies and beyond. npj Breast Cancer 2020, 6, 10. [Google Scholar] [CrossRef] [PubMed]
- Pietras, R.J.; Poen, J.C.; Gallardo, D.; Wongvipat, P.N.; Lee, H.J.; Slamon, D.J. Monoclonal antibody to HER-2/neureceptor modulates repair of radiation-induced DNA damage and enhances radiosensitivity of human breast cancer cells overexpressing this oncogene. Cancer Res 1999, 59, 1347–1355. [Google Scholar] [PubMed]
- Miura, D.; Yoneyama, K.; Furuhata, Y.; Shimizu, K. Paclitaxel enhances antibody-dependent cell-mediated cytotoxicity of trastuzumab by rapid recruitment of natural killer cells in HER2-positive breast cancer. J Nippon Med Sch 2014, 81, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Junttila, T.T.; Akita, R.W.; Parsons, K.; Fields, C.; Lewis Phillips, G.D.; Friedman, L.S.; Sampath, D.; Sliwkowski, M.X. Ligand-independent HER2/HER3/PI3K complex is disrupted by trastuzumab and is effectively inhibited by the PI3K inhibitor GDC-0941. Cancer Cell 2009, 15, 429–440. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Jiang, Z.; Mortenson, E.D.; Deng, L.; Radkevich-Brown, O.; Yang, X.; Sattar, H.; Wang, Y.; Brown, N.K.; Greene, M.; et al. The therapeutic effect of anti-HER2/neu antibody depends on both innate and adaptive immunity. Cancer Cell 2010, 18, 160–170. [Google Scholar] [CrossRef] [PubMed]
- Ishii, K.; Morii, N.; Yamashiro, H. Pertuzumab in the treatment of HER2-positive breast cancer: An evidence-based review of its safety, efficacy, and place in therapy. Core Evid 2019, 14, 51–70. [Google Scholar] [CrossRef]
- Lee-Hoeflich, S.T., Crocker, L., Yao, E., Pham, T., Munroe, X., Hoeflich, K.P., Sliwkowski, M.X. and Stern, H.M. A Central Role for HER3 in HER2-Amplified Breast Cancer: Implications for Targeted Therapy. Cancer Research 2008, 68, 5878–5887. [Google Scholar] [CrossRef]
- Mamidi, S.; Cinci, M.; Hasmann, M.; Fehring, V.; Kirschfink, M. Lipoplex mediated silencing of membrane regulators (CD46, CD55 and CD59) enhances complement-dependent anti-tumor activity of trastuzumab and pertuzumab. Mol Oncol 2013, 7, 580–594. [Google Scholar] [CrossRef]
- Schroeder, R.L.; Stevens, C.L.; Sridhar, J. Small molecule tyrosine kinase inhibitors of ErbB2/HER2/Neu in the treatment of aggressive breast cancer. Molecules 2014, 19, 15196–15212. [Google Scholar] [CrossRef]
- Schlam, I.; Nunes, R.; Lynce, F. Profile of Margetuximab: Evidence to Date in the Targeted Treatment of Metastatic HER2-positive Breast Cancer. Onco Targets Ther 2022, 15, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Spector, N.; Xia, W.; El-Hariry, I.; Yarden, Y.; Bacus, S. HER2 therapy. Small molecule HER-2 tyrosine kinase inhibitors. Breast Cancer Research 2007, 9, 205. [Google Scholar] [CrossRef]
- Scaltriti, M.; Verma, C.; Guzman, M.; Jimenez, J.; Parra, J.L.; Pedersen, K.; Smith, D.J.; Landolfi, S.; Ramon y Cajal, S.; Arribas, J.; et al. Lapatinib, a HER2 tyrosine kinase inhibitor, induces stabilization and accumulation of HER2 and potentiates trastuzumab-dependent cell cytotoxicity. Oncogene 2009, 28, 803–814. [Google Scholar] [CrossRef] [PubMed]
- Roy, V.; Perez, E.A. Beyond Trastuzumab: Small Molecule Tyrosine Kinase Inhibitors in HER-2–Positive Breast Cancer. The Oncologist 2009, 14, 1061–1069. [Google Scholar] [CrossRef]
- Huang, L.; Jiang, S.; Shi, Y. Tyrosine kinase inhibitors for solid tumors in the past 20 years (2001–2020). Journal of Hematology & Oncology 2020, 13, 143. [Google Scholar] [CrossRef]
- Saura, C.; Oliveira, M.; Feng, Y.H.; Dai, M.S.; Chen, S.W.; Hurvitz, S.A.; Kim, S.B.; Moy, B.; Delaloge, S.; Gradishar, W.; et al. Neratinib Plus Capecitabine Versus Lapatinib Plus Capecitabine in HER2-Positive Metastatic Breast Cancer Previously Treated With ≥ 2 HER2-Directed Regimens: Phase III NALA Trial. J Clin Oncol 2020, 38, 3138–3149. [Google Scholar] [CrossRef] [PubMed]
- Lin, N.U.; Murthy, R.K.; Abramson, V.; Anders, C.; Bachelot, T.; Bedard, P.L.; Borges, V.; Cameron, D.; Carey, L.A.; Chien, A.J.; et al. Tucatinib vs Placebo, Both in Combination With Trastuzumab and Capecitabine, for Previously Treated ERBB2 (HER2)-Positive Metastatic Breast Cancer in Patients With Brain Metastases: Updated Exploratory Analysis of the HER2CLIMB Randomized Clinical Trial. JAMA Oncol 2023, 9, 197–205. [Google Scholar] [CrossRef]
- Xu, B.; Yan, M.; Ma, F.; Hu, X.; Feng, J.; Ouyang, Q.; Tong, Z.; Li, H.; Zhang, Q.; Sun, T.; et al. Pyrotinib plus capecitabine versus lapatinib plus capecitabine for the treatment of HER2-positive metastatic breast cancer (PHOEBE): A multicentre, open-label, randomised, controlled, phase 3 trial. Lancet Oncol 2021, 22, 351–360. [Google Scholar] [CrossRef]
- Rassy, E.; Rached, L.; Pistilli, B. Antibody drug conjugates targeting HER2: Clinical development in metastatic breast cancer. Breast 2022, 66, 217–226. [Google Scholar] [CrossRef]
- Verma, S.; Miles, D.; Gianni, L.; Krop, I.E.; Welslau, M.; Baselga, J.; Pegram, M.; Oh, D.-Y.; Diéras, V.; Guardino, E.; et al. Trastuzumab Emtansine for HER2-Positive Advanced Breast Cancer. New England Journal of Medicine 2012, 367, 1783–1791. [Google Scholar] [CrossRef] [PubMed]
- Cortés, J.; Kim, S.-B.; Chung, W.-P.; Im, S.-A.; Park, Y.H.; Hegg, R.; Kim, M.H.; Tseng, L.-M.; Petry, V.; Chung, C.-F.; et al. Trastuzumab Deruxtecan versus Trastuzumab Emtansine for Breast Cancer. New England Journal of Medicine 2022, 386, 1143–1154. [Google Scholar] [CrossRef] [PubMed]
- Romond, E.H.; Perez, E.A.; Bryant, J.; Suman, V.J.; Geyer, C.E.; Davidson, N.E.; Tan-Chiu, E.; Martino, S.; Paik, S.; Kaufman, P.A.; et al. Trastuzumab plus Adjuvant Chemotherapy for Operable HER2-Positive Breast Cancer. New England Journal of Medicine 2005, 353, 1673–1684. [Google Scholar] [CrossRef] [PubMed]
- Slamon, D.; Eiermann, W.; Robert, N.; Pienkowski, T.; Martin, M.; Press, M.; Mackey, J.; Glaspy, J.; Chan, A.; Pawlicki, M.; et al. Adjuvant trastuzumab in HER2-positive breast cancer. N Engl J Med 2011, 365, 1273–1283. [Google Scholar] [CrossRef] [PubMed]
- Morganti, S.; Bianchini, G.; Giordano, A.; Giuliano, M.; Curigliano, G.; Criscitiello, C. How I treat HER2-positive early breast cancer: How long adjuvant trastuzumab is needed? ESMO Open 2022, 7, 100428. [Google Scholar] [CrossRef] [PubMed]
- Earl, H.; Hiller, L.; Vallier, A.L.; Loi, S.; McAdam, K.; Hughes-Davies, L.; Rea, D.; Howe, D.; Raynes, K.; Higgins, H.B.; et al. Six versus 12 months’ adjuvant trastuzumab in patients with HER2-positive early breast cancer: The PERSEPHONE non-inferiority RCT. Health Technol Assess 2020, 24, 1–190. [Google Scholar] [CrossRef] [PubMed]
- Swain, S.M.; Baselga, J.; Kim, S.B.; Ro, J.; Semiglazov, V.; Campone, M.; Ciruelos, E.; Ferrero, J.M.; Schneeweiss, A.; Heeson, S.; et al. Pertuzumab, trastuzumab, and docetaxel in HER2-positive metastatic breast cancer. N Engl J Med 2015, 372, 724–734. [Google Scholar] [CrossRef]
- Schneeweiss, A.; Chia, S.; Hickish, T.; Harvey, V.; Eniu, A.; Hegg, R.; Tausch, C.; Seo, J.H.; Tsai, Y.F.; Ratnayake, J.; et al. Pertuzumab plus trastuzumab in combination with standard neoadjuvant anthracycline-containing and anthracycline-free chemotherapy regimens in patients with HER2-positive early breast cancer: A randomized phase II cardiac safety study (TRYPHAENA). Ann Oncol 2013, 24, 2278–2284. [Google Scholar] [CrossRef]
- Loibl, S.; Jassem, J.; Sonnenblick, A.; Parlier, D.; Winer, E.; Bergh, J.; Gelber, R.D.; Restuccia, E.; Im, Y.H.; Huang, C.; et al. VP6-2022: Adjuvant pertuzumab and trastuzumab in patients with early HER-2 positive breast cancer in APHINITY: 8.4 years’ follow-up. Annals of Oncology 2022, 33, 986–987. [Google Scholar] [CrossRef]
- Tolaney, S.M.; Tarantino, P.; Graham, N.; Tayob, N.; Parè, L.; Villacampa, G.; Dang, C.T.; Yardley, D.A.; Moy, B.; Marcom, P.K.; et al. Adjuvant paclitaxel and trastuzumab for node-negative, HER2-positive breast cancer: Final 10-year analysis of the open-label, single-arm, phase 2 APT trial. The Lancet Oncology 2023, 24, 273–285. [Google Scholar] [CrossRef] [PubMed]
- Rugo, H.S.; Im, S.A.; Cardoso, F.; Cortés, J.; Curigliano, G.; Musolino, A.; Pegram, M.D.; Wright, G.S.; Saura, C.; Escrivá-de-Romaní, S.; et al. Efficacy of Margetuximab vs Trastuzumab in Patients With Pretreated ERBB2-Positive Advanced Breast Cancer: A Phase 3 Randomized Clinical Trial. JAMA Oncol 2021, 7, 573–584. [Google Scholar] [CrossRef] [PubMed]
- von Minckwitz, G.; Huang, C.-S.; Mano, M.S.; Loibl, S.; Mamounas, E.P.; Untch, M.; Wolmark, N.; Rastogi, P.; Schneeweiss, A.; Redondo, A.; et al. Trastuzumab Emtansine for Residual Invasive HER2-Positive Breast Cancer. New England Journal of Medicine 2018, 380, 617–628. [Google Scholar] [CrossRef] [PubMed]
- Modi, S.; Jacot, W.; Yamashita, T.; Sohn, J.; Vidal, M.; Tokunaga, E.; Tsurutani, J.; Ueno, N.T.; Prat, A.; Chae, Y.S.; et al. Trastuzumab Deruxtecan in Previously Treated HER2-Low Advanced Breast Cancer. New England Journal of Medicine 2022, 387, 9–20. [Google Scholar] [CrossRef]
- Geyer, C.E.; Forster, J.; Lindquist, D.; Chan, S.; Romieu, C.G.; Pienkowski, T.; Jagiello-Gruszfeld, A.; Crown, J.; Chan, A.; Kaufman, B.; et al. Lapatinib plus Capecitabine for HER2-Positive Advanced Breast Cancer. New England Journal of Medicine 2006, 355, 2733–2743. [Google Scholar] [CrossRef]
- Martin, M.; Holmes, F.A.; Ejlertsen, B.; Delaloge, S.; Moy, B.; Iwata, H.; von Minckwitz, G.; Chia, S.K.L.; Mansi, J.; Barrios, C.H.; et al. Neratinib after trastuzumab-based adjuvant therapy in HER2-positive breast cancer (ExteNET): 5-year analysis of a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 2017, 18, 1688–1700. [Google Scholar] [CrossRef]
- Chan, A.; Moy, B.; Mansi, J.; Ejlertsen, B.; Holmes, F.A.; Chia, S.; Iwata, H.; Gnant, M.; Loibl, S.; Barrios, C.H.; et al. Final Efficacy Results of Neratinib in HER2-positive Hormone Receptor-positive Early-stage Breast Cancer From the Phase III ExteNET Trial. Clin Breast Cancer 2021, 21, 80–91.e87. [Google Scholar] [CrossRef]
- Barcenas, C.H.; Hurvitz, S.A.; Di Palma, J.A.; Bose, R.; Chien, A.J.; Iannotti, N.; Marx, G.; Brufsky, A.; Litvak, A.; Ibrahim, E.; et al. Improved tolerability of neratinib in patients with HER2-positive early-stage breast cancer: The CONTROL trial. Annals of Oncology 2020, 31, 1223–1230. [Google Scholar] [CrossRef]
- Saura, C.; Oliveira, M.; Feng, Y.-H.; Dai, M.-S.; Chen, S.-W.; Hurvitz, S.A.; Kim, S.-B.; Moy, B.; Delaloge, S.; Gradishar, W.; et al. Neratinib Plus Capecitabine Versus Lapatinib Plus Capecitabine in HER2-Positive Metastatic Breast Cancer Previously Treated With ≥ 2 HER2-Directed Regimens: Phase III NALA Trial. Journal of Clinical Oncology 2020, 38, 3138–3149. [Google Scholar] [CrossRef]
- Murthy, R.K.; Loi, S.; Okines, A.; Paplomata, E.; Hamilton, E.; Hurvitz, S.A.; Lin, N.U.; Borges, V.; Abramson, V.; Anders, C.; et al. Tucatinib, Trastuzumab, and Capecitabine for HER2-Positive Metastatic Breast Cancer. New England Journal of Medicine 2019, 382, 597–609. [Google Scholar] [CrossRef]
- Kiss, B.; Wyatt, A.W.; Douglas, J.; Skuginna, V.; Mo, F.; Anderson, S.; Rotzer, D.; Fleischmann, A.; Genitsch, V.; Hayashi, T.; et al. Her2 alterations in muscle-invasive bladder cancer: Patient selection beyond protein expression for targeted therapy. Scientific Reports 2017, 7, 42713. [Google Scholar] [CrossRef]
- Van Cutsem, E.; Bang, Y.-J.; Feng-Yi, F.; Xu, J.M.; Lee, K.-W.; Jiao, S.-C.; Chong, J.L.; López-Sanchez, R.I.; Price, T.; Gladkov, O.; et al. HER2 screening data from ToGA: Targeting HER2 in gastric and gastroesophageal junction cancer. Gastric Cancer 2015, 18, 476–484. [Google Scholar] [CrossRef]
- Bang, Y.-J.P.; Van Cutsem, E.P.; Feyereislova, A.M.D.; Chung, H.C.P.; Shen, L.P.; Sawaki, A.M.D.; Lordick, F.M.D.; Ohtsu, A.M.D.; Omuro, Y.M.D.; Satoh, T.M.D.; et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): A phase 3, open-label, randomised controlled trial. The Lancet (British edition) 2010, 376, 687–697. [Google Scholar] [CrossRef]
- Drugs.com. FDA Approves Herceptin For HER2-Positive Metastatic Stomach Cancer. Available online: https://www.drugs.com/newdrugs/fda-approves-herceptin-her2-positive-metastatic-stomach-cancer-2375.html (accessed on 3 October 2023).
- Tabernero, J.; Hoff, P.M.; Shen, L.; Ohtsu, A.; Shah, M.A.; Cheng, K.; Song, C.; Wu, H.; Eng-Wong, J.; Kim, K.; et al. Pertuzumab plus trastuzumab and chemotherapy for HER2-positive metastatic gastric or gastro-oesophageal junction cancer (JACOB): Final analysis of a double-blind, randomised, placebo-controlled phase 3 study. The lancet oncology 2018, 19, 1372–1384. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.A.; Xu, R.-H.; Bang, Y.-J.; Hoff, P.M.; Liu, T.; Herráez-Baranda, L.A.; Xia, F.; Garg, A.; Shing, M.; Tabernero, J. HELOISE: Phase IIIb Randomized Multicenter Study Comparing Standard-of-Care and Higher-Dose Trastuzumab Regimens Combined With Chemotherapy as First-Line Therapy in Patients With Human Epidermal Growth Factor Receptor 2–Positive Metastatic Gastric or Gast. Journal of Clinical Oncology 2017, 35, 2558–2567. [Google Scholar] [CrossRef] [PubMed]
- Janjigian, Y.Y.; Kawazoe, A.; Yañez, P.; Li, N.; Lonardi, S.; Kolesnik, O.; Barajas, O.; Bai, Y.; Shen, L.; Tang, Y.; et al. The KEYNOTE-811 trial of dual PD-1 and HER2 blockade in HER2-positive gastric cancer. Nature 2021, 600, 727–730. [Google Scholar] [CrossRef] [PubMed]
- Drugs.com. FDA Approves Merck’s Keytruda (pembrolizumab) Combined With Trastuzumab and Chemotherapy as First-line Treatment in Locally Advanced Unresectable or Metastatic HER2-Positive Gastric or Gastroesophageal Junction Adenocarcinoma. Available online: https://www.drugs.com/newdrugs/fda-approves-merck-s-keytruda-pembrolizumab-combined-trastuzumab-chemotherapy-first-line-locally-5511.html (accessed on 3 October 2023).
- Thuss-Patience, P.C.; Shah, M.A.; Ohtsu, A.; Van Cutsem, E.; Ajani, J.A.; Castro, H.; Mansoor, W.; Chung, H.C.; Bodoky, G.; Shitara, K.; et al. Trastuzumab emtansine versus taxane use for previously treated HER2-positive locally advanced or metastatic gastric or gastro-oesophageal junction adenocarcinoma (GATSBY): An international randomised, open-label, adaptive, phase 2/3 study. Lancet Oncol 2017, 18, 640–653. [Google Scholar] [CrossRef] [PubMed]
- Shitara, K.; Bang, Y.-J.; Iwasa, S.; Sugimoto, N.; Ryu, M.-H.; Sakai, D.; Chung, H.-C.; Kawakami, H.; Yabusaki, H.; Lee, J.; et al. Trastuzumab Deruxtecan in Previously Treated HER2-Positive Gastric Cancer. New England Journal of Medicine 2020, 382, 2419–2430. [Google Scholar] [CrossRef] [PubMed]
- FDA.gov. FDA approves fam-trastuzumab deruxtecan-nxki for HER2-positive gastric adenocarcinomas. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-fam-trastuzumab-deruxtecan-nxki-her2-positive-gastric-adenocarcinomas (accessed on 3 October 2023).
- Satoh, T.; Xu, R.-H.; Chung, H.C.; Sun, G.-P.; Doi, T.; Xu, J.-M.; Tsuji, A.; Omuro, Y.; Li, J.; Wang, J.-W.; et al. Lapatinib Plus Paclitaxel Versus Paclitaxel Alone in the Second-Line Treatment of HER2-Amplified Advanced Gastric Cancer in Asian Populations: TyTAN—A Randomized, Phase III Study. Journal of Clinical Oncology 2014, 32, 2039–2049. [Google Scholar] [CrossRef]
- Hecht, J.R.; Bang, Y.-J.; Qin, S.K.; Chung, H.C.; Xu, J.M.; Park, J.O.; Jeziorski, K.; Shparyk, Y.; Hoff, P.M.; Sobrero, A.; et al. Lapatinib in Combination With Capecitabine Plus Oxaliplatin in Human Epidermal Growth Factor Receptor 2–Positive Advanced or Metastatic Gastric, Esophageal, or Gastroesophageal Adenocarcinoma: TRIO-013/LOGiC—A Randomized Phase III Trial. Journal of Clinical Oncology 2016, 34, 443–451. [Google Scholar] [CrossRef]
- Benson, A.B.; Venook, A.P.; Al-Hawary, M.M.; Arain, M.A.; Chen, Y.-J.; Ciombor, K.K.; Cohen, S.; Cooper, H.S.; Deming, D.; Farkas, L.; et al. Colon Cancer, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. Journal of the National Comprehensive Cancer Network 2021, 19, 329–359. [Google Scholar] [CrossRef]
- Strickler, J.H.; Cercek, A.; Siena, S.; André, T.; Ng, K.; Van Cutsem, E.; Wu, C.; Paulson, A.S.; Hubbard, J.M.; Coveler, A.L.; et al. Tucatinib plus trastuzumab for chemotherapy-refractory, HER2-positive, RAS wild-type unresectable or metastatic colorectal cancer (MOUNTAINEER): A multicentre, open-label, phase 2 study. The lancet oncology 2023, 24, 496–508. [Google Scholar] [CrossRef]
- Meric-Bernstam, F.; Hurwitz, H.; Kanwal Pratap Singh, R.; McWilliams, R.R.; Fakih, M.; VanderWalde, A.; Swanton, C.; Kurzrock, R.; Burris, H.; Sweeney, C.; et al. Pertuzumab and trastuzumab for HER2-amplified metastatic colorectal cancer: An updated report from MyPathway, a multicentre, open-label, phase 2a multiple basket study. The lancet oncology 2019, 20, 518–530. [Google Scholar] [CrossRef]
- NCCN. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) Colon Cancer, Version 3.2023 — September 21, 2023. 2023. Available online: https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1428 (accessed on 5 October 2023).
- Meric-Bernstam, F.; Hainsworth, J.; Bose, R.; III, H.A.B.; Friedman, C.F.; Kurzrock, R.; Swanton, C.; Wang, Y.; Levy, J.; Schulze, K.; et al. MyPathway HER2 basket study: Pertuzumab (P) + trastuzumab (H) treatment of a large, tissue-agnostic cohort of patients with HER2-positive advanced solid tumors. Journal of Clinical Oncology 2021, 39, 3004. [Google Scholar] [CrossRef]
- Hainsworth, J.D.; Meric-Bernstam, F.; Swanton, C.; Hurwitz, H.; Spigel, D.R.; Sweeney, C.; Burris, H.A.; Bose, R.; Yoo, B.; Stein, A.; et al. Targeted Therapy for Advanced Solid Tumors on the Basis of Molecular Profiles: Results From MyPathway, an Open-Label, Phase IIa Multiple Basket Study. Journal of Clinical Oncology 2018, 36, 536–542. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Meric-Bernstam, F.; Rothe, M.; Garrett-Mayer, E.; Mangat, P.K.; D’Andre, S.; Ahn, E.R.; O’Lone, R.; Halabi, S.; Grantham, G.N.; et al. Pertuzumab Plus Trastuzumab in Patients With Colorectal Cancer With ERBB2 Amplification or ERBB2/3 Mutations: Results From the TAPUR Study. JCO Precis Oncol 2022, 6, e2200306. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.; Okamoto, W.; Kato, T.; Esaki, T.; Kato, K.; Komatsu, Y.; Yuki, S.; Masuishi, T.; Nishina, T.; Ebi, H.; et al. Circulating tumor DNA-guided treatment with pertuzumab plus trastuzumab for HER2-amplified metastatic colorectal cancer: A phase 2 trial. Nature Medicine 2021, 27, 1899–1903. [Google Scholar] [CrossRef] [PubMed]
- Sartore-Bianchi, A.; Lonardi, S.; Martino, C.; Fenocchio, E.; Tosi, F.; Ghezzi, S.; Leone, F.; Bergamo, F.; Zagonel, V.; Ciardiello, F.; et al. Pertuzumab and trastuzumab emtansine in patients with HER2-amplified metastatic colorectal cancer: The phase II HERACLES-B trial. ESMO Open 2020, 5, e000911. [Google Scholar] [CrossRef] [PubMed]
- Siena, S.; Di Bartolomeo, M.; Raghav, K.; Masuishi, T.; Loupakis, F.; Kawakami, H.; Yamaguchi, K.; Nishina, T.; Fakih, M.; Elez, E.; et al. Trastuzumab deruxtecan (DS-8201) in patients with HER2-expressing metastatic colorectal cancer (DESTINY-CRC01): A multicentre, open-label, phase 2 trial. Lancet Oncol 2021, 22, 779–789. [Google Scholar] [CrossRef]
- Yoshino, T.; Bartolomeo, M.D.; Raghav, K.P.S.; Masuishi, T.; Kawakami, H.; Yamaguchi, K.; Nishina, T.; Wainberg, Z.A.; Elez, E.; Rodriguez, J.; et al. Trastuzumab deruxtecan (T-DXd; DS-8201) in patients (pts) with HER2-expressing metastatic colorectal cancer (mCRC): Final results from a phase 2, multicenter, open-label study (DESTINY-CRC01). Journal of Clinical Oncology 2022, 40, 119. [Google Scholar] [CrossRef]
- Raghav, K.P.S.; Yoshino, T.; Guimbaud, R.; Chau, I.; Eynde, M.V.D.; Maurel, J.; Tie, J.; Kim, T.W.; Yeh, K.-H.; Barrios, D.; et al. Trastuzumab deruxtecan in patients with HER2-overexpressing locally advanced, unresectable, or metastatic colorectal cancer (mCRC): A randomized, multicenter, phase 2 study (DESTINY-CRC02). Journal of Clinical Oncology 2021, 39, TPS3620. [Google Scholar] [CrossRef]
- Sartore-Bianchi, A.; Trusolino, L.; Martino, C.; Bencardino, K.; Lonardi, S.; Bergamo, F.; Zagonel, V.; Leone, F.; Depetris, I.; Martinelli, E.; et al. Dual-targeted therapy with trastuzumab and lapatinib in treatment-refractory, KRAS codon 12/13 wild-type, HER2-positive metastatic colorectal cancer (HERACLES): A proof-of-concept, multicentre, open-label, phase 2 trial. The Lancet Oncology 2016, 17, 738–746. [Google Scholar] [CrossRef]
- FDA.gov. FDA grants accelerated approval to tucatinib with trastuzumab for colorectal cancer. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-tucatinib-trastuzumab-colorectal-cancer (accessed on 4 October 2023).
- Imai, M.; Nakamura, Y.; Okamoto, W.; Kato, T.; Esaki, T.; Kato, K.; Komatsu, Y.; Yuki, S.; Masuishi, T.; Nishina, T.; et al. Artificial intelligence (AI)-powered HER2 quantification continuous score (QCS) and tumor microenvironment (TME) analysis in HER2-amplified metastatic colorectal cancer (mCRC) treated with pertuzumab plus trastuzumab. JCO Global Oncology 2023, 9, 34. [Google Scholar] [CrossRef]
- Li, B.T.; Shen, R.; Buonocore, D.; Olah, Z.T.; Ni, A.; Ginsberg, M.S.; Ulaner, G.A.; Offin, M.; Feldman, D.; Hembrough, T.; et al. Ado-Trastuzumab Emtansine for Patients With HER2-Mutant Lung Cancers: Results From a Phase II Basket Trial. Journal of Clinical Oncology 2018, 36, 2532–2537. [Google Scholar] [CrossRef] [PubMed]
- Li, B.T.; Smit, E.F.; Goto, Y.; Nakagawa, K.; Udagawa, H.; Mazières, J.; Nagasaka, M.; Bazhenova, L.; Saltos, A.N.; Felip, E.; et al. Trastuzumab Deruxtecan in HER2-Mutant Non–Small-Cell Lung Cancer. New England Journal of Medicine 2022, 386, 241–251. [Google Scholar] [CrossRef]
- Gatzemeier, U.; Groth, G.; Butts, C.; Van Zandwijk, N.; Shepherd, F.; Ardizzoni, A.; Barton, C.; Ghahramani, P.; Hirsh, V. Randomized phase II trial of gemcitabine-cisplatin with or without trastuzumab in HER2-positive non-small-cell lung cancer. Ann Oncol 2004, 15, 19–27. [Google Scholar] [CrossRef]
- Langer, C.J.; Stephenson, P.; Thor, A.; Vangel, M.; Johnson, D.H. Trastuzumab in the Treatment of Advanced Non-Small-Cell Lung Cancer: Is There a Role? Focus on Eastern Cooperative Oncology Group Study 2598. Journal of Clinical Oncology 2004, 22, 1180–1187. [Google Scholar] [CrossRef] [PubMed]
- Ricciardi, G.R.; Russo, A.; Franchina, T.; Ferraro, G.; Zanghì, M.; Picone, A.; Scimone, A.; Adamo, V. NSCLC and HER2: Between lights and shadows. J Thorac Oncol 2014, 9, 1750–1762. [Google Scholar] [CrossRef]
- NCCN. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) Non-Small Cell Lung Cancer, Version 3.2023 — April 13, 2023. Available online: https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1450 (accessed on 5 October 2023).
- Goto, K.; Goto, Y.; Kubo, T.; Ninomiya, K.; Kim, S.-W.; Planchard, D.; Ahn, M.-J.; Smit, E.F.; Langen, A.J.d.; Pérol, M.; et al. Trastuzumab Deruxtecan in Patients With HER2-Mutant Metastatic Non–Small-Cell Lung Cancer: Primary Results From the Randomized, Phase II DESTINY-Lung02 Trial. Journal of Clinical Oncology 2023, 41, 4852–4863. [Google Scholar] [CrossRef] [PubMed]
- FDA.gov. FDA grants accelerated approval to fam-trastuzumab deruxtecan-nxki for HER2-mutant non-small cell lung cancer. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-fam-trastuzumab-deruxtecan-nxki-her2-mutant-non-small-cell-lung (accessed on 24 September 2023).
- Tuefferd, M.; Couturier, J.; Penault-Llorca, F.; Vincent-Salomon, A.; Broët, P.; Guastalla, J.-P.; Allouache, D.; Combe, M.; Weber, B.; Pujade-Lauraine, E.; et al. HER2 Status in Ovarian Carcinomas: A Multicenter GINECO Study of 320 Patients. PLoS ONE 2007, 2, e1138. [Google Scholar] [CrossRef] [PubMed]
- Bookman, M.A.; Darcy, K.M.; Clarke-Pearson, D.; Boothby, R.A.; Horowitz, I.R. Evaluation of Monoclonal Humanized Anti-HER2 Antibody, Trastuzumab, in Patients With Recurrent or Refractory Ovarian or Primary Peritoneal Carcinoma With Overexpression of HER2: A Phase II Trial of the Gynecologic Oncology Group. Journal of Clinical Oncology 2003, 21, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Li, B.T.; Makker, V.; Buonocore, D.J.; Offin, M.D.; Olah, Z.T.; Panora, E.; Shen, R.; Ho, A.L.; Yaeger, R.; Iyer, G.; et al. A multi-histology basket trial of ado-trastuzumab emtansine in patients with HER2 amplified cancers. Journal of Clinical Oncology 2018, 36, 2502. [Google Scholar] [CrossRef]
- Fleming, G.F.; Sill, M.W.; Darcy, K.M.; McMeekin, D.S.; Thigpen, J.T.; Adler, L.M.; Berek, J.S.; Chapman, J.A.; Disilvestro, P.A.; Horowitz, I.R.; et al. Phase II trial of trastuzumab in women with advanced or recurrent, HER2-positive endometrial carcinoma: A Gynecologic Oncology Group study. Gynecologic Oncology 2010, 116, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Grushko, T.A.; Filiaci, V.L.; Mundt, A.J.; Ridderstråle, K.; Olopade, O.I.; Fleming, G.F. An exploratory analysis of HER-2 amplification and overexpression in advanced endometrial carcinoma: A Gynecologic Oncology Group study. Gynecol Oncol 2008, 108, 3–9. [Google Scholar] [CrossRef]
- Diver, E.J.; Foster, R.; Rueda, B.R.; Growdon, W.B. The Therapeutic Challenge of Targeting HER2 in Endometrial Cancer. The Oncologist 2015, 20, 1058–1068. [Google Scholar] [CrossRef]
- Konecny, G.E.; Santos, L.; Winterhoff, B.; Hatmal, M.; Keeney, G.L.; Mariani, A.; Jones, M.; Neuper, C.; Thomas, B.; Muderspach, L.; et al. HER2 gene amplification and EGFR expression in a large cohort of surgically staged patients with nonendometrioid (type II) endometrial cancer. Br J Cancer 2009, 100, 89–95. [Google Scholar] [CrossRef]
- Morrison, C.; Zanagnolo, V.; Ramirez, N.; Cohn, D.E.; Kelbick, N.; Copeland, L.; Maxwell, L.G.; Fowler, J.M. HER-2 Is an Independent Prognostic Factor in Endometrial Cancer: Association With Outcome in a Large Cohort of Surgically Staged Patients. Journal of Clinical Oncology 2006, 24, 2376–2385. [Google Scholar] [CrossRef]
- Ahn, E.R.; Rothe, M.; Mangat, P.K.; Garrett-Mayer, E.; Ali-Ahmad, H.M.; Chan, J.; Maitland, M.L.; Patel, S.R.; Reese, Z.; Balmanoukian, A.S.; et al. Pertuzumab Plus Trastuzumab in Patients With Endometrial Cancer With ERBB2/3 Amplification, Overexpression, or Mutation: Results From the TAPUR Study. JCO Precis Oncol 2023, 7, e2200609. [Google Scholar] [CrossRef]
- Fader, A.N.; Roque, D.M.; Siegel, E.; Buza, N.; Hui, P.; Abdelghany, O.; Chambers, S.K.; Secord, A.A.; Havrilesky, L.; O’Malley, D.M.; et al. Randomized Phase II Trial of Carboplatin-Paclitaxel Versus Carboplatin-Paclitaxel-Trastuzumab in Uterine Serous Carcinomas That Overexpress Human Epidermal Growth Factor Receptor 2/neu. Journal of Clinical Oncology 2018, 36, 2044–2051. [Google Scholar] [CrossRef]
- Fader, A.N.; Roque, D.M.; Siegel, E.; Buza, N.; Hui, P.; Abdelghany, O.; Chambers, S.; Secord, A.A.; Havrilesky, L.; O’Malley, D.M.; et al. Randomized Phase II Trial of Carboplatin–Paclitaxel Compared with Carboplatin–Paclitaxel–Trastuzumab in Advanced (Stage III–IV) or Recurrent Uterine Serous Carcinomas that Overexpress Her2/Neu (NCT01367002): Updated Overall Survival Analysis. Clinical Cancer Research 2020, 26, 3928–3935. [Google Scholar] [CrossRef]
- Abu-Rustum, N.; Yashar, C.; Arend, R.; Barber, E.; Bradley, K.; Brooks, R.; Campos, S.M.; Chino, J.; Chon, H.S.; Chu, C.; et al. Uterine Neoplasms, Version 1.2023, NCCN Clinical Practice Guidelines in Oncology. Journal of the National Comprehensive Cancer Network 2023, 21, 181–209. [Google Scholar] [CrossRef]
- Liu, D.; Makker, V.; Buonocore, D.J.; Shen, R.; Yaeger, R.; Ginsberg, M.S.; Yeh, R.; Johnson, A.; Offin, M.; Solit, D.B.; et al. Final analysis of multi-histology basket trial expansion of ado-trastuzumab emtansine in patients with HER2 amplified cancers. Journal of Clinical Oncology 2023, 41, 3025. [Google Scholar] [CrossRef]
- Hussain, M.H.A.; Macvicar, G.R.; Petrylak, D.P.; Dunn, R.L.; Vaishampayan, U.; Lara, P.N.; Chatta, G.S.; Nanus, D.M.; Glode, L.M.; Trump, D.L.; et al. Trastuzumab, Paclitaxel, Carboplatin, and Gemcitabine in Advanced Human Epidermal Growth Factor Receptor-2/neu–Positive Urothelial Carcinoma: Results of a Multicenter Phase II National Cancer Institute Trial. Journal of Clinical Oncology 2007, 25, 2218–2224. [Google Scholar] [CrossRef]
- Albarrán, V.; Rosero, D.I.; Chamorro, J.; Pozas, J.; San Román, M.; Barrill, A.M.; Alía, V.; Sotoca, P.; Guerrero, P.; Calvo, J.C.; et al. Her-2 Targeted Therapy in Advanced Urothelial Cancer: From Monoclonal Antibodies to Antibody-Drug Conjugates. International Journal of Molecular Sciences 2022, 23, 12659. [Google Scholar] [CrossRef] [PubMed]
- Fleischmann, A.; Rotzer, D.; Seiler, R.; Studer, U.E.; Thalmann, G.N. Her2 Amplification is Significantly More Frequent in Lymph Node Metastases From Urothelial Bladder Cancer Than in the Primary Tumours. European Urology 2011, 60, 350–357. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Xu, W.; Zhang, Z.; Song, R.; Zeng, S.; Sun, Y.; Xu, C. Prognostic role of HER2 expression in bladder cancer: A systematic review and meta-analysis. Int Urol Nephrol 2015, 47, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Chae, H.K.; Nam, W.; Kim, H.G.; Lim, S.; Noh, B.J.; Kim, S.W.; Kang, G.H.; Park, J.Y.; Eom, D.W.; Kim, S.J. Identification of New Prognostic Markers and Therapeutic Targets for Non-Muscle Invasive Bladder Cancer: HER2 as a Potential Target Antigen. Front Immunol 2022, 13, 903297. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, H.; Tada, Y.; Saotome, T.; Akazawa, K.; Ojiri, H.; Fushimi, C.; Masubuchi, T.; Matsuki, T.; Tani, K.; Osamura, R.Y.; et al. Phase II Trial of Trastuzumab and Docetaxel in Patients With Human Epidermal Growth Factor Receptor 2–Positive Salivary Duct Carcinoma. Journal of Clinical Oncology 2019, 37, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Ross, J.S.; Gay, L.M.; Wang, K.; Vergilio, J.A.; Suh, J.; Ramkissoon, S.; Somerset, H.; Johnson, J.M.; Russell, J.; Ali, S.; et al. Comprehensive genomic profiles of metastatic and relapsed salivary gland carcinomas are associated with tumor type and reveal new routes to targeted therapies. Annals of Oncology 2017, 28, 2539–2546. [Google Scholar] [CrossRef]
- Thorpe, L.M.; Schrock, A.B.; Erlich, R.L.; Miller, V.A.; Knost, J.; Le-Lindqwister, N.; Jujjavarapu, S.; Ali, S.M.; Liu, J.J. Significant and durable clinical benefit from trastuzumab in 2 patients with HER2-amplified salivary gland cancer and a review of the literature. Head & Neck 2017, 39, E40–E44. [Google Scholar] [CrossRef]
- Lee, J.; Park, S.; Jung, H.A.; Lee, S.H.; Seo, S.; Kim, S.B.; Kim, J.W.; Lee, K.W.; Kang, E.J.; Kim, J.W.; et al. A phase 2 multicenter study of docetaxel-PM and trastuzumab-pkrb combination therapy in recurrent or metastatic salivary gland carcinomas. Cancer 2023. [Google Scholar] [CrossRef]
- Kurzrock, R.; Bowles, D.W.; Kang, H.; Meric-Bernstam, F.; Hainsworth, J.; Spigel, D.R.; Bose, R.; Burris, H.; Sweeney, C.J.; Beattie, M.S.; et al. Targeted therapy for advanced salivary gland carcinoma based on molecular profiling: Results from MyPathway, a phase IIa multiple basket study. Annals of Oncology 2020, 31, 412–421. [Google Scholar] [CrossRef]
- Jhaveri, K.L.; Wang, X.V.; Makker, V.; Luoh, S.W.; Mitchell, E.P.; Zwiebel, J.A.; Sharon, E.; Gray, R.J.; Li, S.; McShane, L.M.; et al. Ado-trastuzumab emtansine (T-DM1) in patients with HER2-amplified tumors excluding breast and gastric/gastroesophageal junction (GEJ) adenocarcinomas: Results from the NCI-MATCH trial (EAY131) subprotocol Q. Annals of Oncology 2019, 30, 1821–1830. [Google Scholar] [CrossRef]
- Li, B.T.; Shen, R.; Offin, M.; Buonocore, D.J.; Myers, M.L.; Venkatesh, A.; Razavi, P.; Ginsberg, M.S.; Ulaner, G.A.; Solit, D.B.; et al. Ado-trastuzumab emtansine in patients with HER2 amplified salivary gland cancers (SGCs): Results from a phase II basket trial. Journal of Clinical Oncology 2019, 37, 6001. [Google Scholar] [CrossRef]
- Bando, H.; Kinoshita, I.; Modi, S.; Tsurutani, J.; Bang, Y.-J.; Iwata, H.; Sato, Y.; Nakatani, S.; Lee, C.C.; Sugihara, M.; et al. Trastuzumab deruxtecan (T-DXd) in patients with human epidermal growth factor receptor 2 (HER2)-expressing salivary duct carcinoma: Subgroup analysis of two phase 1 studies. Journal of Clinical Oncology 2021, 39, 6079. [Google Scholar] [CrossRef]
- NCCN. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) Head and Neck Cancers, Version 2.2023 — May 15, 2023. Available online: https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1437 (accessed on 5 October 2023).
- Javle, M.; Borad, M.J.; Azad, N.S.; Kurzrock, R.; Abou-Alfa, G.K.; George, B.; Hainsworth, J.; Meric-Bernstam, F.; Swanton, C.; Sweeney, C.J.; et al. Pertuzumab and trastuzumab for HER2-positive, metastatic biliary tract cancer (MyPathway): A multicentre, open-label, phase 2a, multiple basket study. The lancet oncology 2021, 22, 1290–1300. [Google Scholar] [CrossRef] [PubMed]
- Ohba, A.; Morizane, C.; Kawamoto, Y.; Komatsu, Y.; Ueno, M.; Kobayashi, S.; Ikeda, M.; Sasaki, M.; Furuse, J.; Okano, N.; et al. Trastuzumab deruxtecan (T-DXd; DS-8201) in patients (pts) with HER2-expressing unresectable or recurrent biliary tract cancer (BTC): An investigator-initiated multicenter phase 2 study (HERB trial). Journal of Clinical Oncology 2022, 40, 4006. [Google Scholar] [CrossRef]
- Benson, A.B.; D’Angelica, M.I.; Abrams, T.; Abbott, D.E.; Ahmed, A.; Anaya, D.A.; Anders, R.; Are, C.; Bachini, M.; Binder, D.; et al. NCCN Guidelines® Insights: Biliary Tract Cancers, Version 2.2023: Featured Updates to the NCCN Guidelines. Journal of the National Comprehensive Cancer Network 2023, 21, 694–704. [Google Scholar] [CrossRef] [PubMed]
- Rexer, B.N.; Arteaga, C.L. Intrinsic and acquired resistance to HER2-targeted therapies in HER2 gene-amplified breast cancer: Mechanisms and clinical implications. Crit Rev Oncog 2012, 17, 1–16. [Google Scholar] [CrossRef]
- Swain, S.M.; Shastry, M.; Hamilton, E. Targeting HER2-positive breast cancer: Advances and future directions. Nat Rev Drug Discov 2023, 22, 101–126. [Google Scholar] [CrossRef]
- Li, J.; Xiao, Q.; Bao, Y.; Wang, W.; Goh, J.; Wang, P.; Yu, Q. HER2-L755S mutation induces hyperactive MAPK and PI3K-mTOR signaling, leading to resistance to HER2 tyrosine kinase inhibitor treatment. Cell Cycle 2019, 18, 1513–1522. [Google Scholar] [CrossRef]
- Zhang, Y. The root cause of drug resistance in HER2-positive breast cancer and the therapeutic approaches to overcoming the resistance. Pharmacol Ther 2021, 218, 107677. [Google Scholar] [CrossRef] [PubMed]
- Chung, W.P.; Huang, W.L.; Lee, C.H.; Hsu, H.P.; Huang, W.L.; Liu, Y.Y.; Su, W.C. PI3K inhibitors in trastuzumab-resistant HER2-positive breast cancer cells with PI3K pathway alterations. Am J Cancer Res 2022, 12, 3067–3082. [Google Scholar] [PubMed]
- Ocana, A.; Gil-Martin, M.; Antolín, S.; Atienza, M.; Montaño, Á.; Ribelles, N.; Urruticoechea, A.; Falcón, A.; Pernas, S.; Orlando, J.; et al. Efficacy and safety of dasatinib with trastuzumab and paclitaxel in first line HER2-positive metastatic breast cancer: Results from the phase II GEICAM/2010-04 study. Breast Cancer Res Treat 2019, 174, 693–701. [Google Scholar] [CrossRef] [PubMed]
- Tolaney, S.M.; Johnston, S.R.D.; Wei, R.; Andre, V.A.; Shahir, A.; Harbeck, N.; Martin, M. Adjuvant abemaciclib for high-risk early breast cancer (EBC): Factors increasing the rate of treatment discontinuations in monarchE. Journal of Clinical Oncology 2022, 40, 527. [Google Scholar] [CrossRef]
- Gámez-Chiachio, M.; Sarrió, D.; Moreno-Bueno, G. Novel Therapies and Strategies to Overcome Resistance to Anti-HER2-Targeted Drugs. Cancers 2022, 14. [Google Scholar] [CrossRef] [PubMed]
- Florido, R.; Smith, K.L.; Cuomo, K.K.; Russell, S.D. Cardiotoxicity From Human Epidermal Growth Factor Receptor-2 (HER2) Targeted Therapies. J Am Heart Assoc 2017, 6. [Google Scholar] [CrossRef]
- Chan, A.; Ruiz-Borrego, M.; Marx, G.; Chien, A.J.; Rugo, H.S.; Brufsky, A.; Thirlwell, M.; Trudeau, M.; Bose, R.; García-Sáenz, J.A.; et al. Final findings from the CONTROL trial: Strategies to reduce the incidence and severity of neratinib-associated diarrhea in patients with HER2-positive early-stage breast cancer. Breast 2023, 67, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Mandó, P.; Waisberg, F.; Pasquinelli, R.; Rivero, S.; Ostinelli, A.; Perazzo, F. HER2-Directed Therapy in Advanced Breast Cancer: Benefits and Risks. Onco Targets Ther 2023, 16, 115–132. [Google Scholar] [CrossRef] [PubMed]
- Liver Tox: Clinical and Research Information on Drug-Induced Liver Injury; 2020.
- Jahan, N.; Rehman, S.; Khan, R.; Jones, C. Relative Risk of Peripheral Neuropathy With Ado-Trastuzumab Emtansine (T-DM1) Compared to Taxane-Based Regimens in Human Epidermal Growth Factor Receptor 2 (HER2)-Positive Cancers: A Systematic Review and Meta-Analysis. Cureus 2021, 13, e15282. [Google Scholar] [CrossRef]
- Manich, C.S.; O’Shaughnessy, J.; Aftimos, P.; Van Den Tweel, E.; Oesterholt, M.; Escrivá-de-Romaní, S.; Tueux, N.Q.; Tan, T.; Lim, J.; Ladoire, S. LBA15 Primary outcome of the phase III SYD985. 002/TULIP trial comparing [vic-] trastuzumab duocarmazine to physician’s choice treatment in patients with pre-treated HER2-positive locally advanced or metastatic breast cancer. Annals of Oncology 2021, 32, S1288. [Google Scholar] [CrossRef]
- Banerji, U.; van Herpen, C.M.L.; Saura, C.; Thistlethwaite, F.; Lord, S.; Moreno, V.; Macpherson, I.R.; Boni, V.; Rolfo, C.; de Vries, E.G.E.; et al. Trastuzumab duocarmazine in locally advanced and metastatic solid tumours and HER2-expressing breast cancer: A phase 1 dose-escalation and dose-expansion study. Lancet Oncol 2019, 20, 1124–1135. [Google Scholar] [CrossRef] [PubMed]
- Skidmore, L.; Sakamuri, S.; Knudsen, N.A.; Hewet, A.G.; Milutinovic, S.; Barkho, W.; Biroc, S.L.; Kirtley, J.; Marsden, R.; Storey, K. ARX788, a site-specific anti-HER2 antibody–drug conjugate, demonstrates potent and selective activity in HER2-low and T-DM1–resistant breast and gastric cancers. Molecular Cancer Therapeutics 2020, 19, 1833–1843. [Google Scholar] [CrossRef]
- Barok, M.; Le Joncour, V.; Martins, A.; Isola, J.; Salmikangas, M.; Laakkonen, P.; Joensuu, H. ARX788, a novel anti-HER2 antibody-drug conjugate, shows anti-tumor effects in preclinical models of trastuzumab emtansine-resistant HER2-positive breast cancer and gastric cancer. Cancer Letters 2020, 473, 156–163. [Google Scholar] [CrossRef]
- Hurvitz, S.A.; Park, H.; Frentzas, S.; Shannon, C.M.; Cuff, K.; Eek, R.W.; Budd, G.T.; McCartney, A.; O’Shaughnessy, J.; Lu, J.M. Safety and unique pharmacokinetic profile of ARX788, a site-specific ADC, in heavily pretreated patients with HER2-overexpresing solid tumors: Results from two phase 1 clinical trials. 2021. [Google Scholar] [CrossRef]
- Zhang, J.; Ji, D.; Shen, W.; Xiao, Q.; Gu, Y.; O’Shaughnessy, J.; Hu, X. Phase I trial of a novel anti-HER2 antibody–drug conjugate, ARX788, for the treatment of HER2-positive metastatic breast cancer. Clinical Cancer Research 2022, 28, 4212–4221. [Google Scholar] [CrossRef]
- Stanowicka-Grada, M.; Senkus, E. Anti-HER2 Drugs for the Treatment of Advanced HER2 Positive Breast Cancer. Current Treatment Options in Oncology 2023, 1–18. [Google Scholar] [CrossRef]
- Buckel, L.; Savariar, E.N.; Crisp, J.L.; Jones, K.A.; Hicks, A.M.; Scanderbeg, D.J.; Nguyen, Q.T.; Sicklick, J.K.; Lowy, A.M.; Tsien, R.Y. Tumor radiosensitization by monomethyl auristatin E: Mechanism of action and targeted delivery. Cancer research 2015, 75, 1376–1387. [Google Scholar] [CrossRef]
- Wang, J.; Liu, Y.; Zhang, Q.; Feng, J.; Fang, J.; Chen, X.; Han, Y.; Li, Q.; Zhang, P.; Yuan, P. RC48-ADC, a HER2-targeting antibody-drug conjugate, in patients with HER2-positive and HER2-low expressing advanced or metastatic breast cancer: A pooled analysis of two studies. 2021. [Google Scholar] [CrossRef]
- Tanaka, H.; Hirata, M.; Shinonome, S.; Wada, T.; Iguchi, M.; Dohi, K.; Inoue, M.; Ishioka, Y.; Hojo, K.; Yamada, T. Preclinical antitumor activity of S-222611, an oral reversible tyrosine kinase inhibitor of epidermal growth factor receptor and human epidermal growth factor receptor 2. Cancer Science 2014, 105, 1040–1048. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Hirata, M.; Shinonome, S.; Torii, M.; Nezasa, K.-i.; Tanaka, H. Distribution analysis of epertinib in brain metastasis of HER2-positive breast cancer by imaging mass spectrometry and prospect for antitumor activity. Scientific reports 2018, 8, 343. [Google Scholar] [CrossRef] [PubMed]
- Macpherson, I.R.; Spiliopoulou, P.; Rafii, S.; Saggese, M.; Baird, R.D.; Garcia-Corbacho, J.; Italiano, A.; Bonneterre, J.; Campone, M.; Cresti, N. A phase I/II study of epertinib plus trastuzumab with or without chemotherapy in patients with HER2-positive metastatic breast cancer. Breast Cancer Research 2020, 22, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; McAndrew, N.; Yu, W.; Pan, X.; Wang, M.; Hu, X. Abstract P2-13-43: Preclinical and early clinical safety and pharmacokinetics data of DZD1516, an BBB-penetrant selective HER2 inhibitor for the treatment of HER2 positive metastatic breast cancer. Cancer Research 2022, 82, P2-13-43. [Google Scholar] [CrossRef]
- Son, J.; Jang, J.; Beyett, T.S.; Eum, Y.; Haikala, H.M.; Verano, A.; Lin, M.; Hatcher, J.M.; Kwiatkowski, N.P.; Eser, P.Ö. A Novel HER2-Selective Kinase Inhibitor Is Effective in HER2 Mutant and Amplified Non–Small Cell Lung Cancer. Cancer Research 2022, 82, 1633–1645. [Google Scholar] [CrossRef] [PubMed]
- Reiss, K.; Yuan, Y.; Barton, D.; Ronczka, A.; Cushing, D.; Klichinsky, M.; Abramson, S.; Qureshi, R.; Condamine, T.; Dees, E. 951 a phase 1 first in human study of adenovirally transduced anti-HER2 CAR macrophages in subjects with HER2 overexpressing solid tumors: Preliminary safety, pharmacokinetics, and TME reprogramming data. 2021. [Google Scholar] [CrossRef]
- Hamza, B.; Nunez, A.; Marques, M.; Barandiaran, A.; Moreno, H.; Moore, F.; Walsh, M.; Choi, E.; Pradhan, K.; Daniel, K. CAT-179, an allogeneic NK cell product expressing HER2-CAR, IL-15 and TGFβ dominant negative receptor, durably regresses HER2-expressing xenograft tumors in mice. Cancer Research 2023, 83, 2904. [Google Scholar] [CrossRef]
- Békés, M.; Langley, D.R.; Crews, C.M. PROTAC targeted protein degraders: The past is prologue. Nature Reviews Drug Discovery 2022, 21, 181–200. [Google Scholar] [CrossRef] [PubMed]
- Maneiro, M.a.; Forte, N.; Shchepinova, M.M.; Kounde, C.S.; Chudasama, V.; Baker, J.R.; Tate, E.W. Antibody–PROTAC conjugates enable HER2-dependent targeted protein degradation of BRD4. ACS chemical biology 2020, 15, 1306–1312. [Google Scholar] [CrossRef]
- Loi, S.; Giobbie-Hurder, A.; Gombos, A.; Bachelot, T.; Hui, R.; Curigliano, G.; Campone, M.; Biganzoli, L.; Bonnefoi, H.; Jerusalem, G. Pembrolizumab plus trastuzumab in trastuzumab-resistant, advanced, HER2-positive breast cancer (PANACEA): A single-arm, multicentre, phase 1b–2 trial. The Lancet Oncology 2019, 20, 371–382. [Google Scholar] [CrossRef]
- Emens, L.A.; Esteva, F.J.; Beresford, M.; Saura, C.; De Laurentiis, M.; Kim, S.-B.; Im, S.-A.; Wang, Y.; Salgado, R.; Mani, A. Trastuzumab emtansine plus atezolizumab versus trastuzumab emtansine plus placebo in previously treated, HER2-positive advanced breast cancer (KATE2): A phase 2, multicentre, randomised, double-blind trial. The lancet oncology 2020, 21, 1283–1295. [Google Scholar] [CrossRef]
- Geurts, V.; Voorwerk, L.; Balduzzi, S.; Salgado, R.; Van de Vijver, K.; van Dongen, M.; Kemper, I.; Mandjes, I.; Heuver, M.; Sparreboom, W. Unleashing NK-and CD8 T cells by combining monalizumab and trastuzumab for metastatic HER2-positive breast cancer: Results of the MIMOSA trial. The Breast 2023, 70, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Li, J.Y.; Perry, S.R.; Muniz-Medina, V.; Wang, X.; Wetzel, L.K.; Rebelatto, M.C.; Hinrichs, M.J.M.; Bezabeh, B.Z.; Fleming, R.L.; Dimasi, N. A biparatopic HER2-targeting antibody-drug conjugate induces tumor regression in primary models refractory to or ineligible for HER2-targeted therapy. Cancer cell 2016, 29, 117–129. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Cai, H.; Jin, Y.; Wang, P.; Zhang, Q.; Lin, Y.; Wang, W.; Cheng, J.; Zeng, N.; Xu, T. Structural basis of a novel heterodimeric Fc for bispecific antibody production. Oncotarget 2017, 8, 51037. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Ji, D.; Cai, L.; Yao, H.; Yan, M.; Wang, X.; Shen, W.; Du, Y.; Pang, H.; Lai, X. First-in-human HER2-targeted bispecific antibody KN026 for the treatment of patients with HER2-positive metastatic breast cancer: Results from a phase I study. Clinical Cancer Research 2022, 28, 618–628. [Google Scholar] [CrossRef] [PubMed]
- Rudnik, M.; Grining, O.Y.; Hutter, S.; Greve, F.; Franyuti, D.O.; Matheis, R.; Breous-Nystrom, E.; Frey, O. Combined investigation of anti-tumor efficacy and liver safety of bispecific T cell engagers in immune-competent high-throughput co-culturing platform. Cancer Research 2023, 83, 2747. [Google Scholar] [CrossRef]
- Mittendorf, E.; Clifton, G.; Holmes, J.; Schneble, E.; Van Echo, D.; Ponniah, S.; Peoples, G. Final report of the phase I/II clinical trial of the E75 (nelipepimut-S) vaccine with booster inoculations to prevent disease recurrence in high-risk breast cancer patients. Annals of oncology 2014, 25, 1735–1742. [Google Scholar] [CrossRef] [PubMed]
- Mittendorf, E.A.; Lu, B.; Melisko, M.; Price Hiller, J.; Bondarenko, I.; Brunt, A.M.; Sergii, G.; Petrakova, K.; Peoples, G.E. Efficacy and safety analysis of nelipepimut-S vaccine to prevent breast cancer recurrence: A randomized, multicenter, phase III clinical trial. Clinical Cancer Research 2019, 25, 4248–4254. [Google Scholar] [CrossRef]
- Crosby, E.J.; Gwin, W.; Blackwell, K.; Marcom, P.K.; Chang, S.; Maecker, H.T.; Broadwater, G.; Hyslop, T.; Kim, S.; Rogatko, A. Vaccine-induced memory CD8+ T cells provide clinical benefit in HER2 expressing breast cancer: A mouse to human translational study. Clinical Cancer Research 2019, 25, 2725–2736. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




