Submitted:
12 December 2023
Posted:
13 December 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
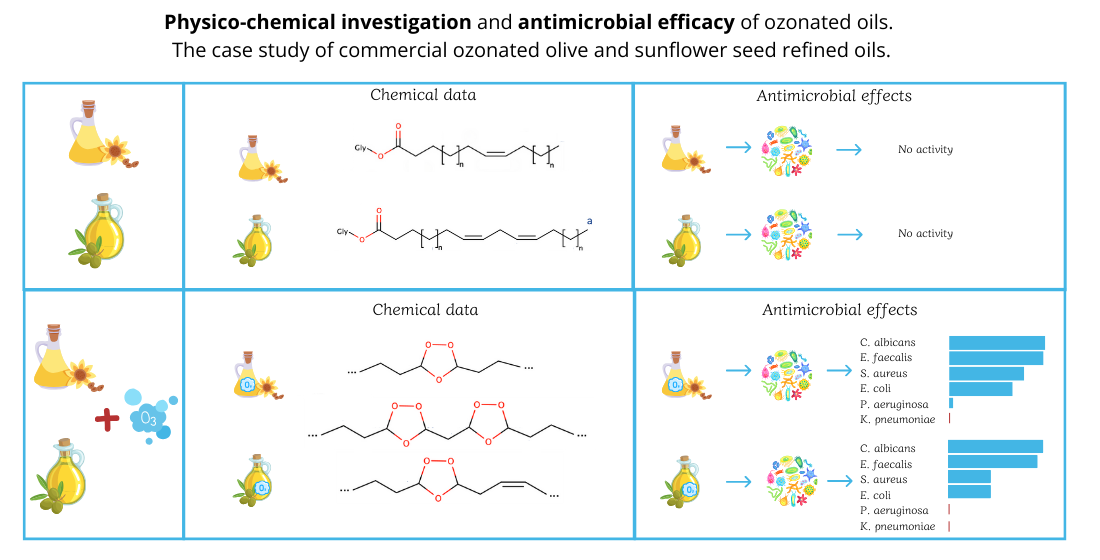
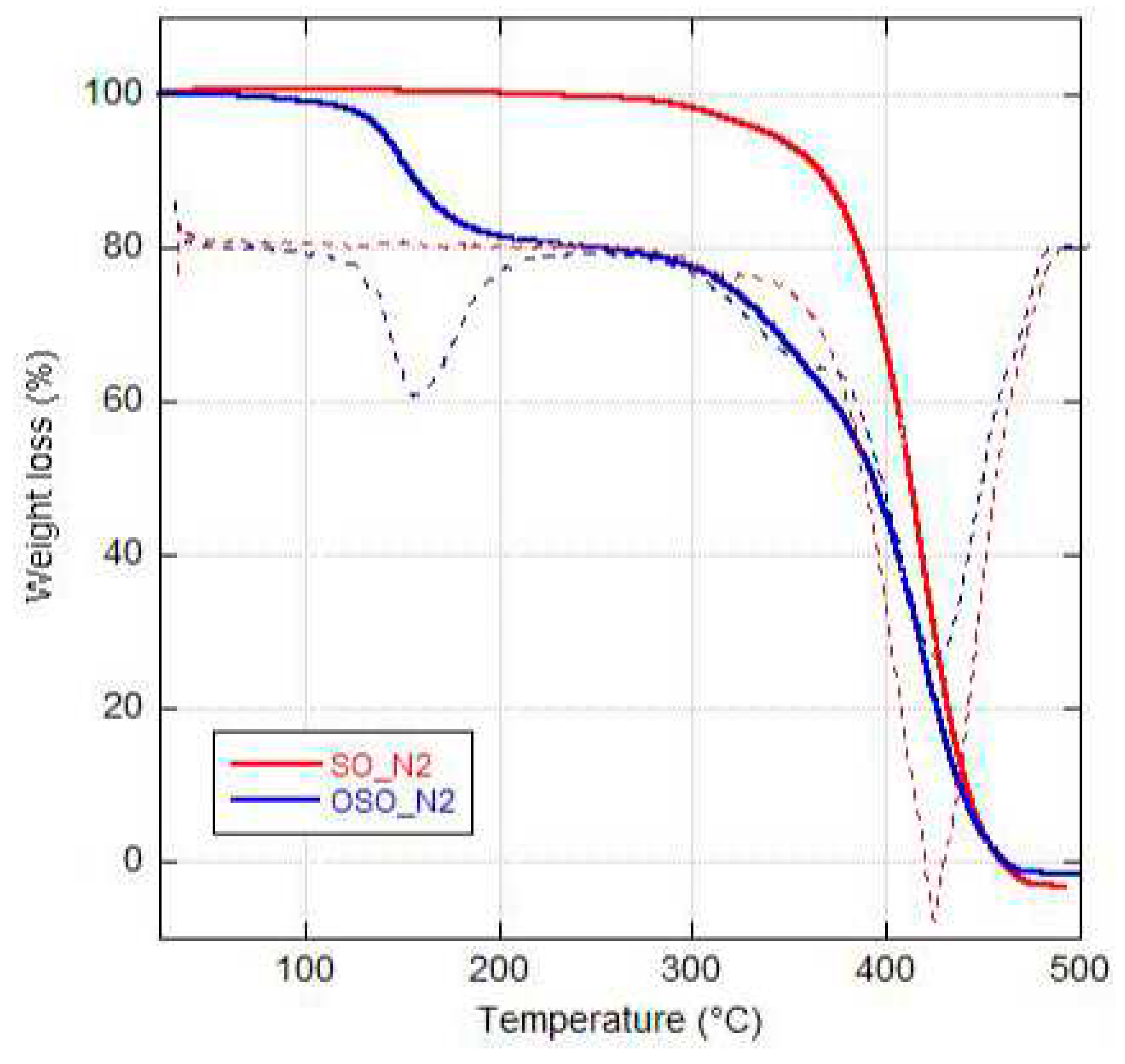
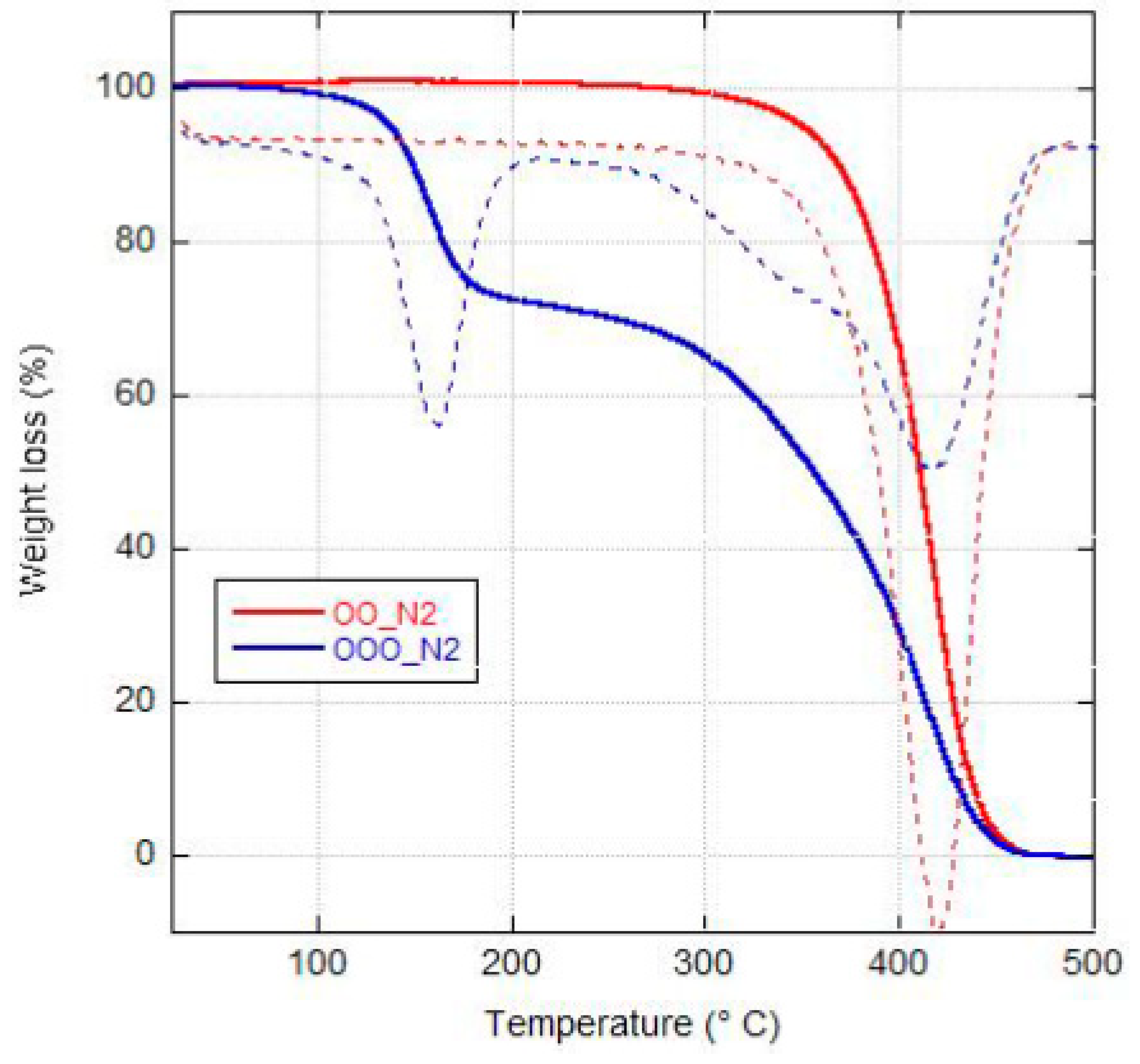
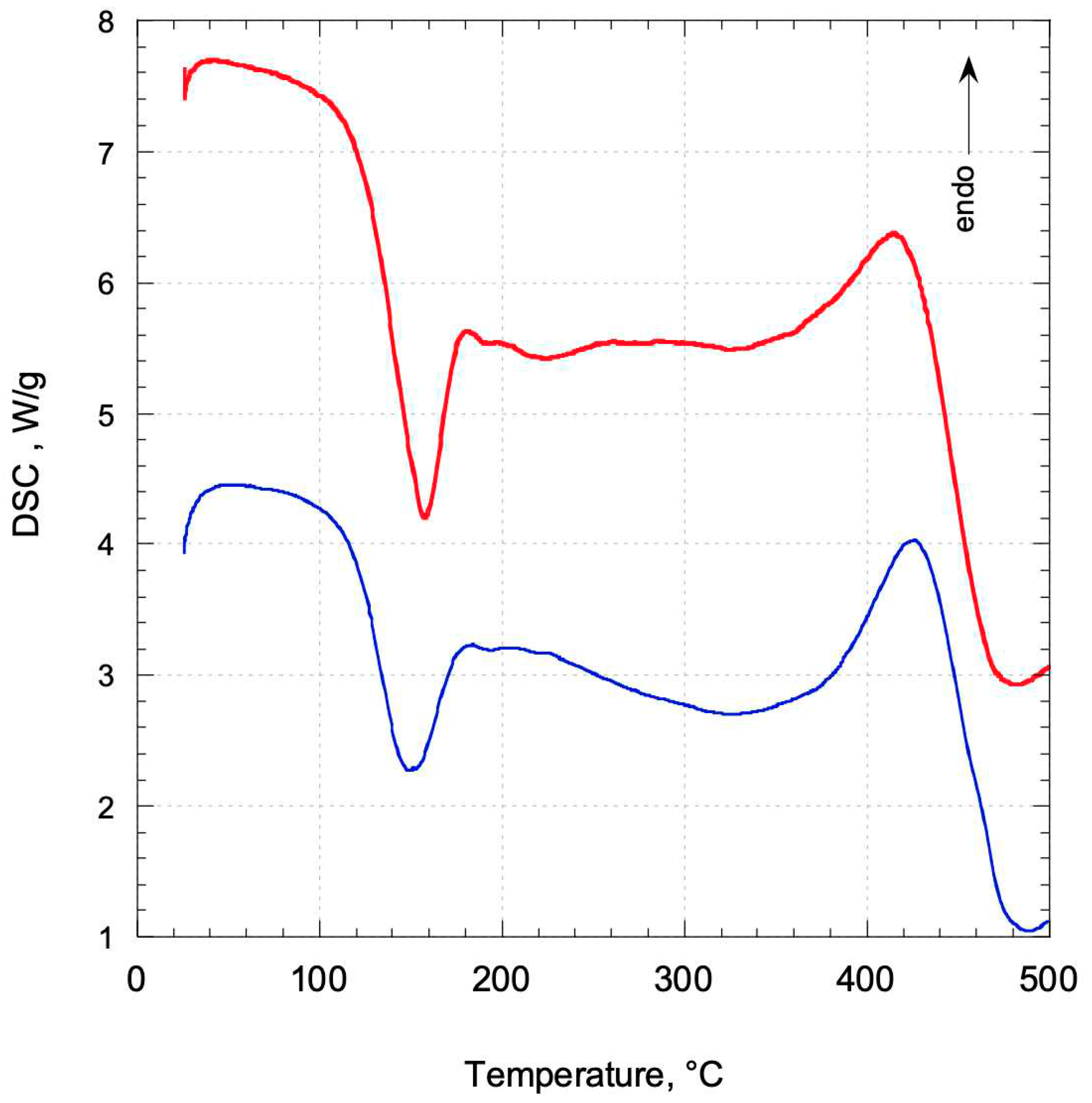
2.1. Thermogravimetric analysis and Differential Scanning Calorimetry.
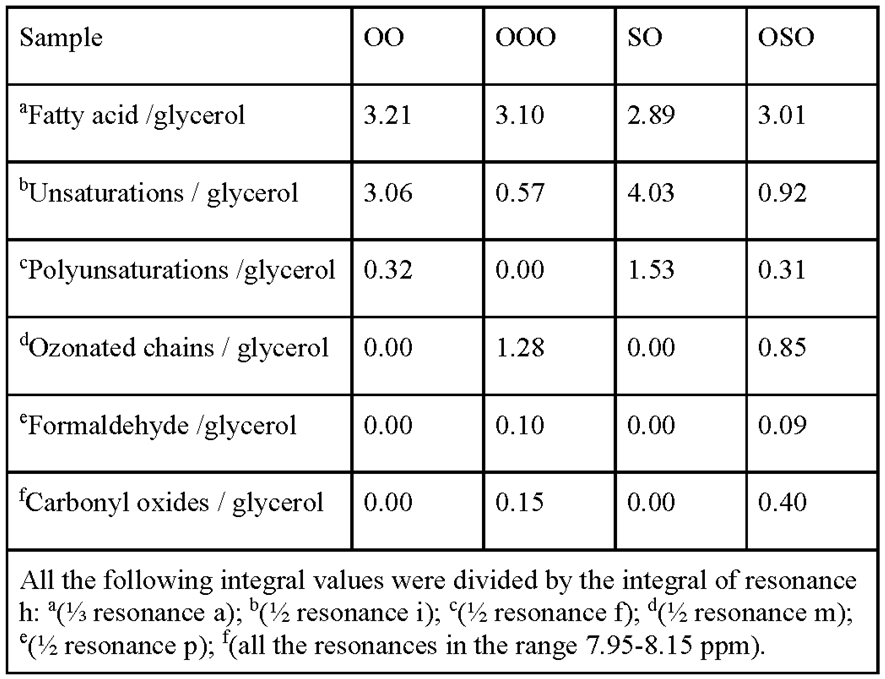
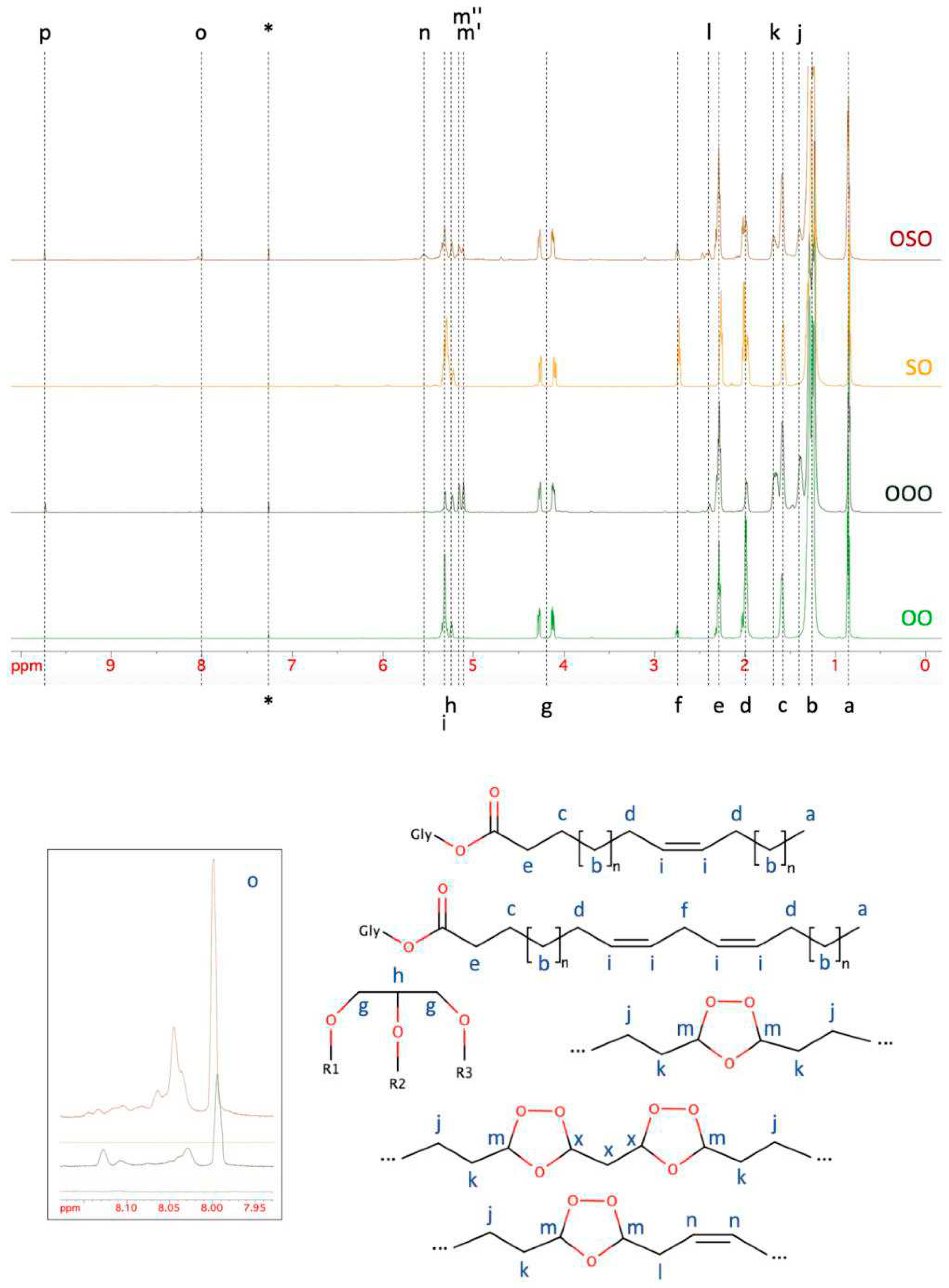
2.2. Nuclear Magnetic Resonance Spectroscopy
 |
 |
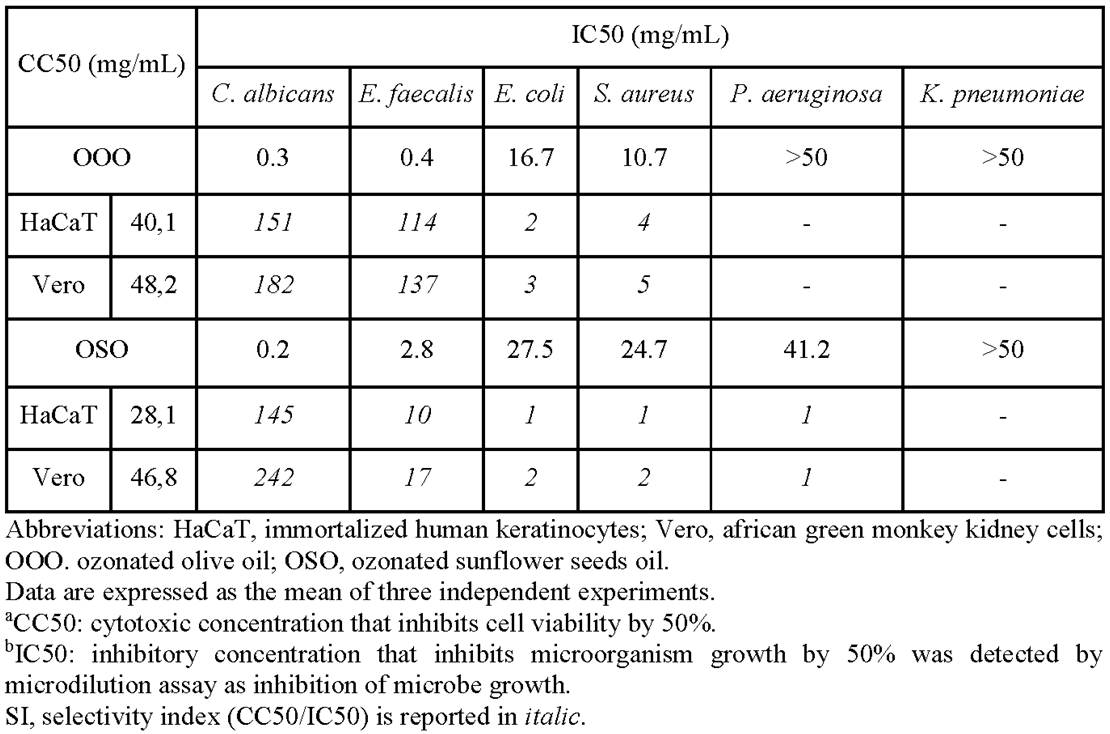
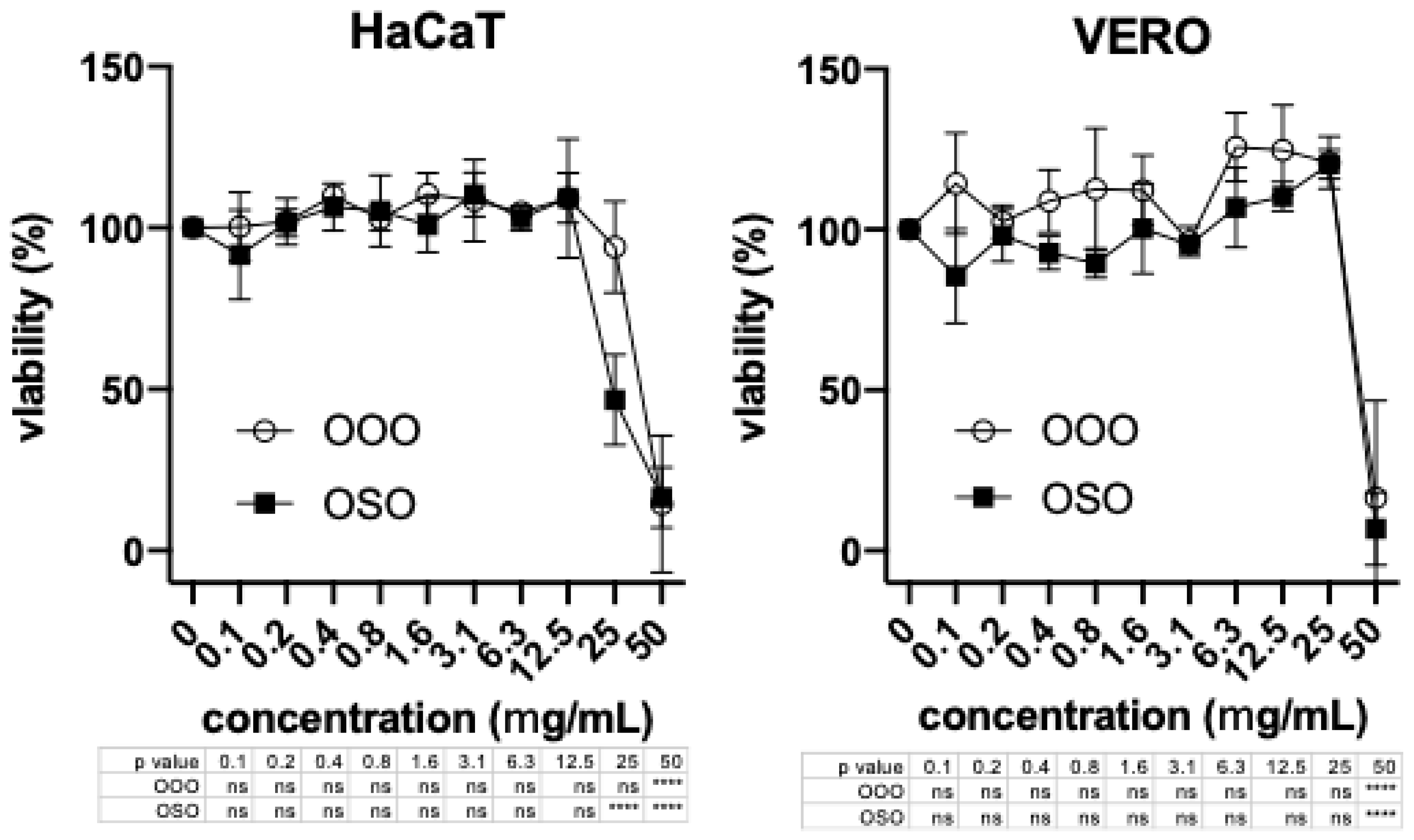
2.3. Cytotoxicity
 |
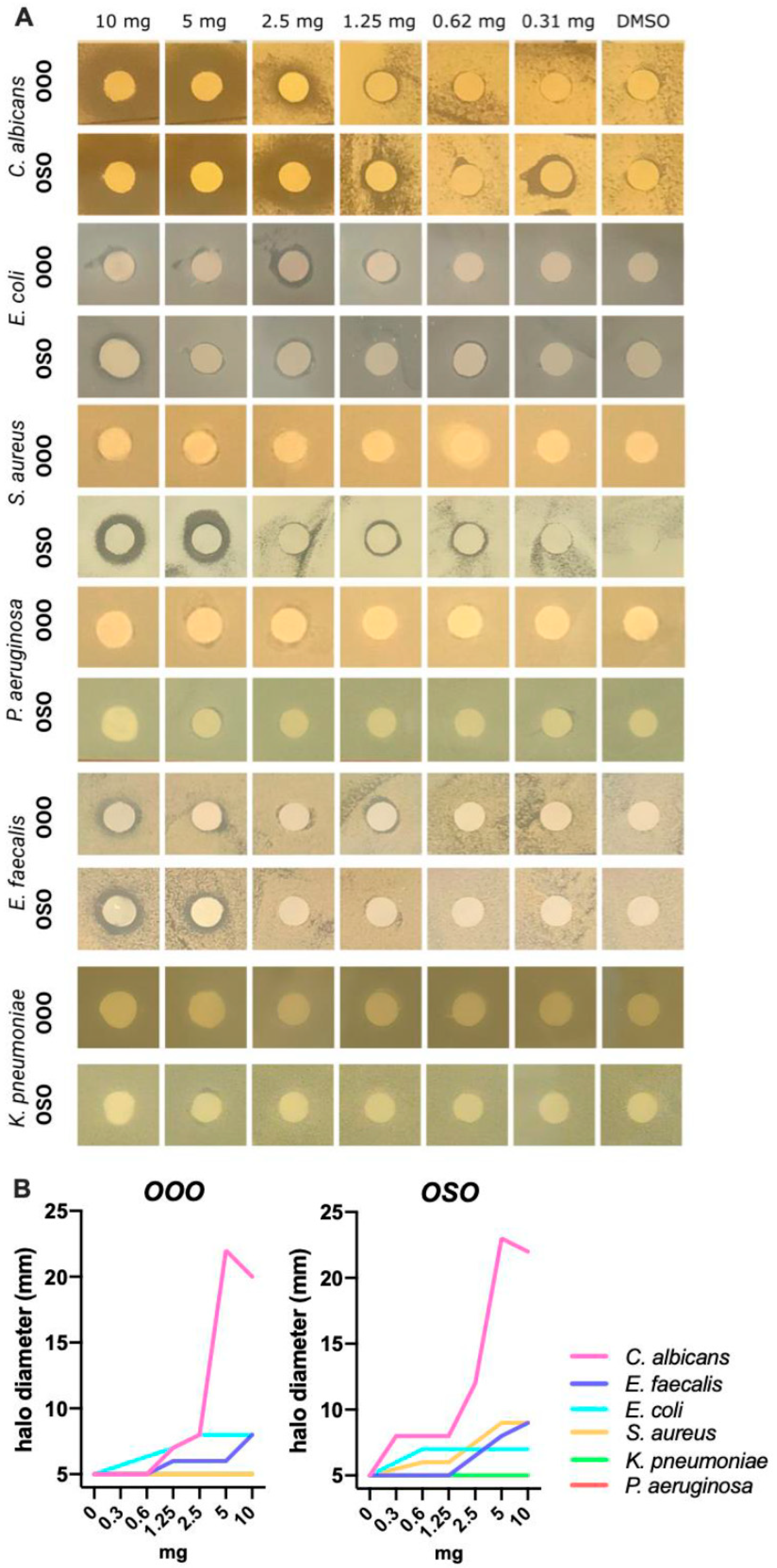
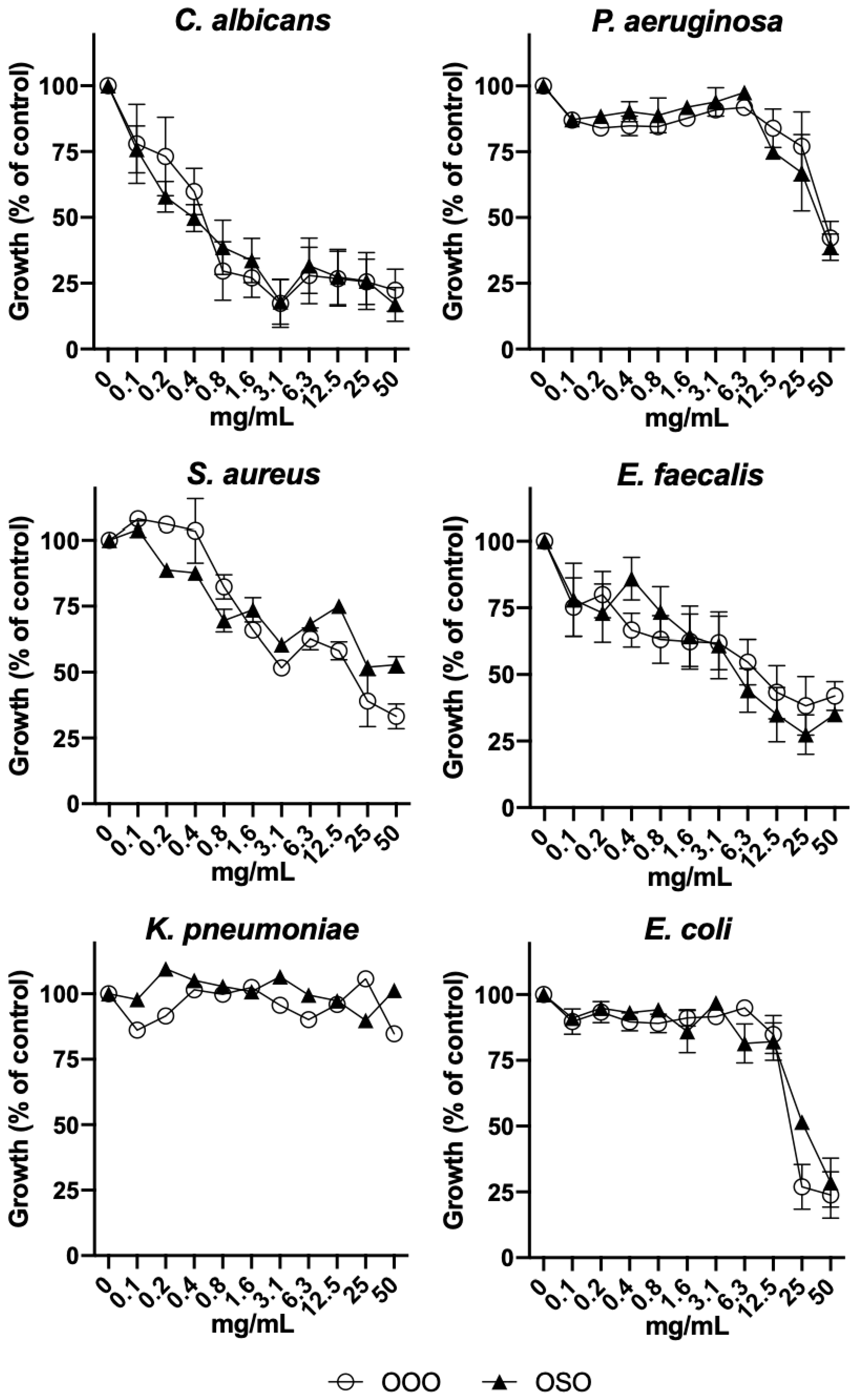
2.4. Antimicrobial effect
3. Discussion
4. Materials and Methods
4.1. Materials
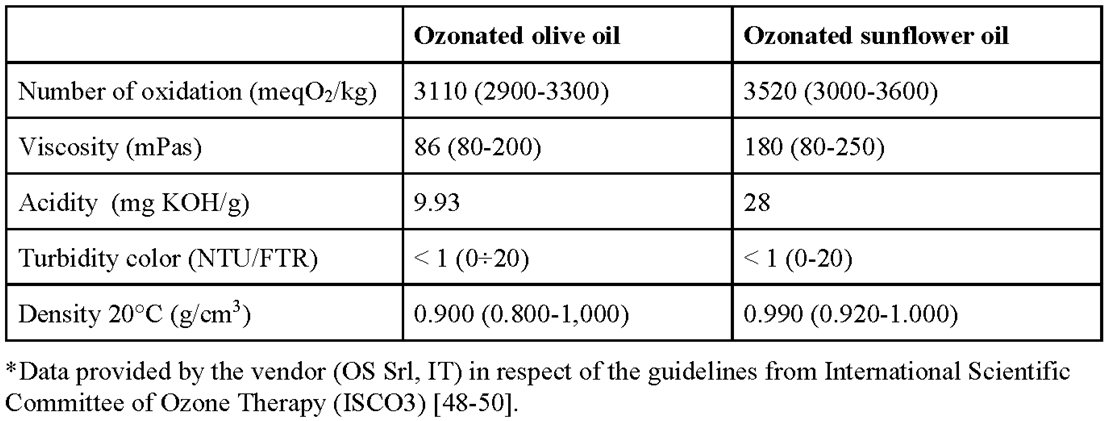
4.2. Physico-chemical characterization of Ozonated Oils
4.2.1. Thermal Characterization and Differential Scanning Calorimetry
4.2.2. Nuclear Magnetic Resonance Spectroscopy (NMR)
4.3. Cell viability assay
4.4. Microbial strains and culture conditions
4.5. Antimicrobial activity
4.6. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- WHO. Coronavirus (COVID-19) Dashboard. Availabe online: https://covid19.who.int.
- WHO. WHO publishes list of bacteria for which new antibiotics are urgently needed. Availabe online: https://www.who.int/news/item/27-02-2017-who-publishes-list-of-bacteria-for-which-new-antibiotics-are-urgently-needed.
- de Kraker, M.E.; Stewardson, A.J.; Harbarth, S. Will 10 Million People Die a Year due to Antimicrobial Resistance by 2050? PLoS Med 2016, 13, e1002184. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, Z.H.; Agarwal, K.; Sarkar, R. Hand Dermatitis: A Comprehensive Review with Special Emphasis on COVID-19 Pandemic. Indian J Dermatol 2021, 66, 508–519. [Google Scholar] [CrossRef]
- Rundle, C.W.; Presley, C.L.; Militello, M.; Barber, C.; Powell, D.L.; Jacob, S.E.; Atwater, A.R.; Watsky, K.L.; Yu, J.; Dunnick, C.A. Hand hygiene during COVID-19: Recommendations from the American Contact Dermatitis Society. J Am Acad Dermatol 2020, 83, 1730–1737. [Google Scholar] [CrossRef] [PubMed]
- Tasar, R.; Wiegand, C.; Elsner, P. How irritant are n-propanol and isopropanol? - A systematic review. Contact Dermatitis 2021, 84, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; von Gunten, U. Oxidative transformation of micropollutants during municipal wastewater treatment: comparison of kinetic aspects of selective (chlorine, chlorine dioxide, ferrate VI, and ozone) and non-selective oxidants (hydroxyl radical). Water Res 2010, 44, 555–566. [Google Scholar] [CrossRef]
- Dodd, M.C. Potential impacts of disinfection processes on elimination and deactivation of antibiotic resistance genes during water and wastewater treatment. J Environ Monit 2012, 14, 1754–1771. [Google Scholar] [CrossRef] [PubMed]
- Hollender, J.; Zimmermann, S.G.; Koepke, S.; Krauss, M.; McArdell, C.S.; Ort, C.; Singer, H.; von Gunten, U.; Siegrist, H. Elimination of organic micropollutants in a municipal wastewater treatment plant upgraded with a full-scale post-ozonation followed by sand filtration. Environ Sci Technol 2009, 43, 7862–7869. [Google Scholar] [CrossRef] [PubMed]
- Luddeke, F.; Hess, S.; Gallert, C.; Winter, J.; Gude, H.; Loffler, H. Removal of total and antibiotic resistant bacteria in advanced wastewater treatment by ozonation in combination with different filtering techniques. Water Res 2015, 69, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Y.; Ren, H.; Geng, J.; Zhang, Y.; Zhang, Y.; Ding, L.; Xu, K. Inactivation of antibiotic resistance genes in municipal wastewater by chlorination, ultraviolet, and ozonation disinfection. Environ Sci Pollut Res Int 2015, 22, 7037–7044. [Google Scholar] [CrossRef]
- Zimmermann, S.G.; Wittenwiler, M.; Hollender, J.; Krauss, M.; Ort, C.; Siegrist, H.; von Gunten, U. Kinetic assessment and modeling of an ozonation step for full-scale municipal wastewater treatment: micropollutant oxidation, by-product formation and disinfection. Water Res 2011, 45, 605–617. [Google Scholar] [CrossRef]
- Al-Saadi, H.; Potapova, I.; Rochford, E.T.; Moriarty, T.F.; Messmer, P. Ozonated saline shows activity against planktonic and biofilm growing Staphylococcus aureus in vitro: a potential irrigant for infected wounds. Int Wound J 2016, 13, 936–942. [Google Scholar] [CrossRef] [PubMed]
- Ibanez-Cervantes, G.; Cruz-Cruz, C.; Duran-Manuel, E.M.; Loyola-Cruz, M.A.; Cureno-Diaz, M.A.; Castro-Escarpulli, G.; Lugo-Zamudio, G.E.; Rojo-Gutierrez, M.I.; Razo-Blanco Hernandez, D.M.; Lopez-Ornelas, A.; et al. Disinfection efficacy of ozone on ESKAPE bacteria biofilms: Potential use in difficult-to-access medical devices. Am J Infect Control 2023, 51, 11–17. [Google Scholar] [CrossRef]
- Komanapalli, I.R.; Lau, B.H. Inactivation of bacteriophage lambda, Escherichia coli, and Candida albicans by ozone. Appl Microbiol Biotechnol 1998, 49, 766–769. [Google Scholar] [CrossRef] [PubMed]
- Monzillo, V.; Lallitto, F.; Russo, A.; Poggio, C.; Scribante, A.; Arciola, C.R.; Bertuccio, F.R.; Colombo, M. Ozonized Gel Against Four Candida Species: A Pilot Study and Clinical Perspectives. Materials (Basel) 2020, 13. [Google Scholar] [CrossRef]
- Piletic, K.; Kovac, B.; Percic, M.; Zigon, J.; Broznic, D.; Karleusa, L.; Lucic Blagojevic, S.; Oder, M.; Gobin, I. Disinfecting Action of Gaseous Ozone on OXA-48-Producing Klebsiella pneumoniae Biofilm In Vitro. Int J Environ Res Public Health 2022, 19. [Google Scholar] [CrossRef]
- Skorup, P.; Fransson, A.; Gustavsson, J.; Sjoholm, J.; Rundgren, H.; Ozenci, V.; Wong, A.Y.W.; Karlsson, T.; Svensen, C.; Gunther, M. Evaluation of an extracorporeal ozone-based bactericide system for the treatment of Escherichia coli sepsis. Intensive Care Med Exp 2022, 10, 14. [Google Scholar] [CrossRef] [PubMed]
- Zanardi, I.; Borrelli, E.; Valacchi, G.; Travagli, V.; Bocci, V. Ozone: A Multifaceted Molecule with Unexpected Therapeutic Activity. Curr Med Chem 2016, 23, 304–314. [Google Scholar] [CrossRef] [PubMed]
- Blanchard, E.L.; Lawrence, J.D.; Noble, J.A.; Xu, M.; Joo, T.; Ng, N.L.; Schmidt, B.E.; Santangelo, P.J.; Finn, M.G. Enveloped Virus Inactivation on Personal Protective Equipment by Exposure to Ozone. medRxiv 2020, 10.1101/2020.05.23.20111435. [CrossRef]
- Cattel, F.; Giordano, S.; Bertiond, C.; Lupia, T.; Corcione, S.; Scaldaferri, M.; Angelone, L.; De Rosa, F.G. Ozone therapy in COVID-19: A narrative review. Virus Res 2021, 291, 198207. [Google Scholar] [CrossRef]
- Clavo, B.; Santana-Rodriguez, N.; Llontop, P.; Gutierrez, D.; Suarez, G.; Lopez, L.; Rovira, G.; Martinez-Sanchez, G.; Gonzalez, E.; Jorge, I.J.; et al. Ozone Therapy as Adjuvant for Cancer Treatment: Is Further Research Warranted? Evid Based Complement Alternat Med 2018, 2018, 7931849. [Google Scholar] [CrossRef]
- de Sire, A.; Agostini, F.; Lippi, L.; Mangone, M.; Marchese, S.; Cisari, C.; Bernetti, A.; Invernizzi, M. Oxygen-Ozone Therapy in the Rehabilitation Field: State of the Art on Mechanisms of Action, Safety and Effectiveness in Patients with Musculoskeletal Disorders. Biomolecules 2021, 11. [Google Scholar] [CrossRef]
- Izadi, M.; Cegolon, L.; Javanbakht, M.; Sarafzadeh, A.; Abolghasemi, H.; Alishiri, G.; Zhao, S.; Einollahi, B.; Kashaki, M.; Jonaidi-Jafari, N.; et al. Ozone therapy for the treatment of COVID-19 pneumonia: A scoping review. Int Immunopharmacol 2021, 92, 107307. [Google Scholar] [CrossRef] [PubMed]
- Juchniewicz, H.; Lubkowska, A. Oxygen-Ozone (O(2)-O(3)) Therapy in Peripheral Arterial Disease (PAD): A Review Study. Ther Clin Risk Manag 2020, 16, 579–594. [Google Scholar] [CrossRef] [PubMed]
- Muto, M.; Giurazza, F.; Silva, R.P.; Guarnieri, G. Rational approach, technique and selection criteria treating lumbar disk herniations by oxygen-ozone therapy. Interv Neuroradiol 2016, 22, 736–740. [Google Scholar] [CrossRef] [PubMed]
- Rowen, R.J.; Robins, H. Ozone Therapy for Complex Regional Pain Syndrome: Review and Case Report. Curr Pain Headache Rep 2019, 23, 41. [Google Scholar] [CrossRef] [PubMed]
- Scassellati, C.; Galoforo, A.C.; Bonvicini, C.; Esposito, C.; Ricevuti, G. Ozone: a natural bioactive molecule with antioxidant property as potential new strategy in aging and in neurodegenerative disorders. Ageing Res Rev 2020, 63, 101138. [Google Scholar] [CrossRef] [PubMed]
- CDC. Ozone Availabe online: [WHO, 2021 #7] (accessed on.
- (ISCO), I.S.C.o.O.T. Madrid Declaration on Ozone Therapy. Availabe online: https://ozonewithoutborders.ngo/wp-content/uploads/2021/04/2020-Madrid-Declaration.pdf.
- Kumar, A.; Bali, K.; Singh, S.; Naja, M.; Mishra, A.K. Estimates of reactive trace gases (NMVOCs, CO and NOx) and their ozone forming potentials during forest fire over Southern Himalayan region. Atmospheric Research 2019, 227, 41–51. [Google Scholar] [CrossRef]
- Moureu, F.; Violleau, F.; Ali Haimoud-Lekhal, D. Influence of Storage Temperature on the Composition and the Antibacterial Activity of Ozonized Sunflower Oil. Ozone: Science & Engineering 2016, 38, 143–149. [Google Scholar] [CrossRef]
- Santos, L.; Silva, E.S.D.; Oliveira, F.O.; Rodrigues, L.A.P.; Neves, P.R.F.; Meira, C.S.; Moreira, G.A.F.; Lobato, G.M.; Nascimento, C.; Gerhardt, M.; et al. Ozonized Water in Microbial Control: Analysis of the Stability, In Vitro Biocidal Potential, and Cytotoxicity. Biology (Basel) 2021, 10. [Google Scholar] [CrossRef]
- Rodrigues de Almeida Kogawa, N.; José de Arruda, E.; Micheletti, A.C.; de Fatima Cepa Matos, M.; Silva de Oliveira, L.C.; Pires de Lima, D.; Pereira Carvalho, N.C.; Dias de Oliveira, P.; de Castro Cunha, M.; Ojeda, M.; et al. Synthesis, characterization, thermal behavior, and biological activity of ozonides from vegetable oils. RSC Advances 2015, 5, 65427–65436. [Google Scholar] [CrossRef]
- Ugazio, E.; Tullio, V.; Binello, A.; Tagliapietra, S.; Dosio, F. Ozonated Oils as Antimicrobial Systems in Topical Applications. Their Characterization, Current Applications, and Advances in Improved Delivery Techniques. Molecules 2020, 25. [Google Scholar] [CrossRef]
- Frank D. Gunstone; John L. Harwood; Padley, F.B. The Lipid Handbook, Occurrence and characteristics of oils and fats, Second Edition ed.; Press, C., Ed.
- Cho, K.H.; Kang, D.J.; Nam, H.S.; Kim, J.H.; Kim, S.Y.; Lee, J.O.; Kim, B.J. Ozonated Sunflower Oil Exerted Protective Effect for Embryo and Cell Survival via Potent Reduction Power and Antioxidant Activity in HDL with Strong Antimicrobial Activity. Antioxidants (Basel) 2021, 10. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues de Almeida Kogawa, N.; Beatriz, A.; Micheletti, A.C.; José de Arruda, E. Ozonized vegetable oils and therapeutic properties: A review. Orbital - The Electronic Journal of Chemistry 2013, 4. [Google Scholar] [CrossRef]
- Sechi, L.A.; Lezcano, I.; Nunez, N.; Espim, M.; Dupre, I.; Pinna, A.; Molicotti, P.; Fadda, G.; Zanetti, S. Antibacterial activity of ozonized sunflower oil (Oleozon). J Appl Microbiol 2001, 90, 279–284. [Google Scholar] [CrossRef]
- Gharby, S. Refining Vegetable Oils: Chemical and Physical Refining. ScientificWorldJournal 2022, 2022, 6627013. [Google Scholar] [CrossRef]
- Silva, V.; Peirone, C.; Capita, R.; Alonso-Calleja, C.; Marques-Magallanes, J.A.; Pires, I.; Maltez, L.; Pereira, J.E.; Igrejas, G.; Poeta, P. Topical Application of Ozonated Oils for the Treatment of MRSA Skin Infection in an Animal Model of Infected Ulcer. Biology 2021, 10. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Zeng, Q.; Xiang, Y.; Gao, L.; Huang, J.; Huang, J.; Wu, K.; Lu, J. The antibacterial effect of topical ozone on the treatment of MRSA skin infection. Mol Med Rep 2018, 17, 2449–2455. [Google Scholar] [CrossRef] [PubMed]
- Grandi, G.; Cavallo, R.; Zanotto, E.; Cipriani, R.; Panico, C.; Protti, R.; Scapagnini, G.; Davinelli, S.; Costagliola, C. In vitro antimicrobial activity of ozonated oil in liposome eyedrop against multidrug-resistant bacteria. Open Med (Wars) 2022, 17, 1057–1063. [Google Scholar] [CrossRef]
- Angius, F.; Floris, A. Liposomes and MTT cell viability assay: an incompatible affair. Toxicol In Vitro 2015, 29, 314–319. [Google Scholar] [CrossRef] [PubMed]
- Barry, A.L.; Thornsberry, C.; Badal, R.E.; Baker, C.N.; Jones, R.N.; Gerlach, E.H. Piperacillin susceptibility tests by the single-disk agar diffusion technique. Antimicrob Agents Chemother 1979, 16, 378–385. [Google Scholar] [CrossRef]
- Montevecchi, M.; Dorigo, A.; Cricca, M.; Checchi, L. Comparison of the antibacterial activity of an ozonated oil with chlorhexidine digluconate and povidone-iodine. A disk diffusion test. New Microbiol 2013, 36, 289–302. [Google Scholar]
- Mushtaq, S.; Warner, M.; Johnson, A.P.; Livermore, D.M. Activity of dalbavancin against staphylococci and streptococci, assessed by BSAC and NCCLS agar dilution methods. J Antimicrob Chemother 2004, 54, 617–620. [Google Scholar] [CrossRef] [PubMed]
- (ISCO), I.S.C.o.O.T. ISCO3/LAB/00/04 Physico-chemical characterization of ozonized oil. Peroxide Value. Availabe online: https://ozonewithoutborders.ngo/wp-content/uploads/2021/04/2020-Madrid-Declaration.pdf.
- (ISCO), I.S.C.o.O.T. ISCO3/LAB/00/03 Physico-chemical characterization of ozonized oil. Iodine Value. Availabe online: https://isco3.org/wp-content/uploads/2017/03/ISCO3-LAB-00-03-IV-Iodine-Value-V1.pdf.
- (ISCO), I.S.C.o.O.T. ISCO3/LAB/00/02 Physico-chemical characterization of ozonized oil. Acid Values. Availabe online: https://isco3.org/wp-content/uploads/2017/03/ISCO3-LAB-00-02-Acid-Values-V1.pdf.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





