Submitted:
06 December 2023
Posted:
13 December 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
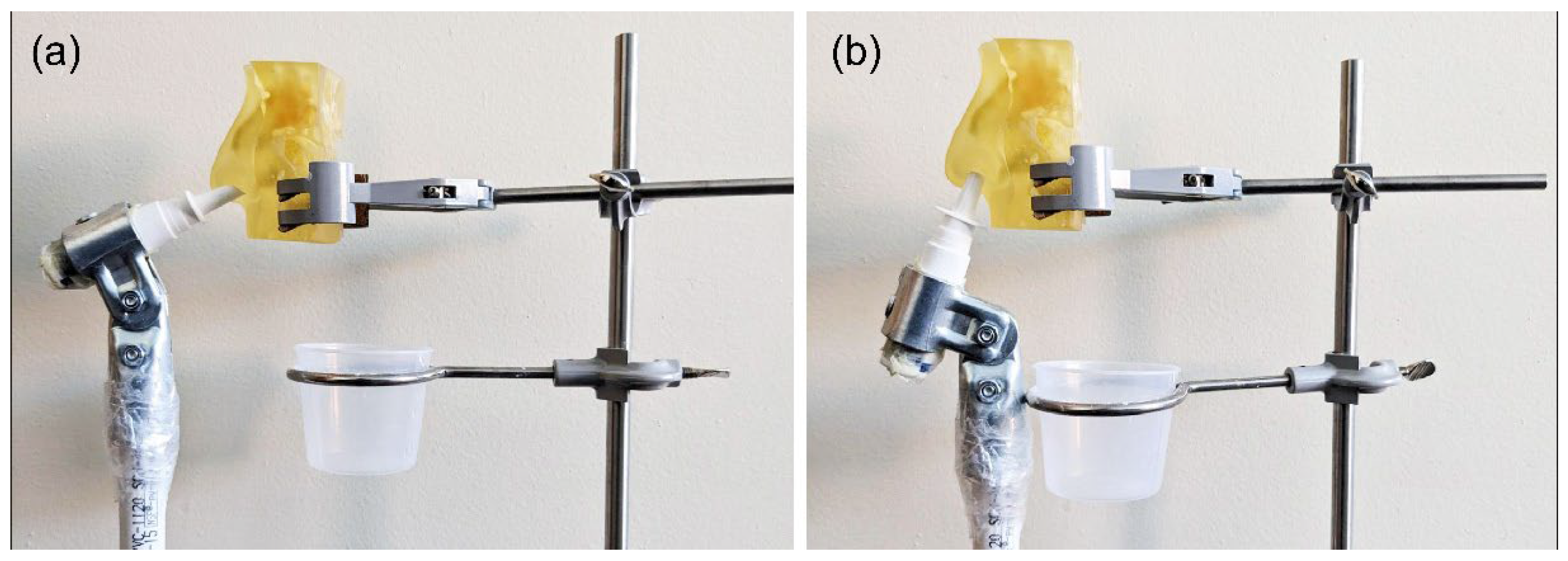
2.1. Experimental Setup for Physical Spray Tests
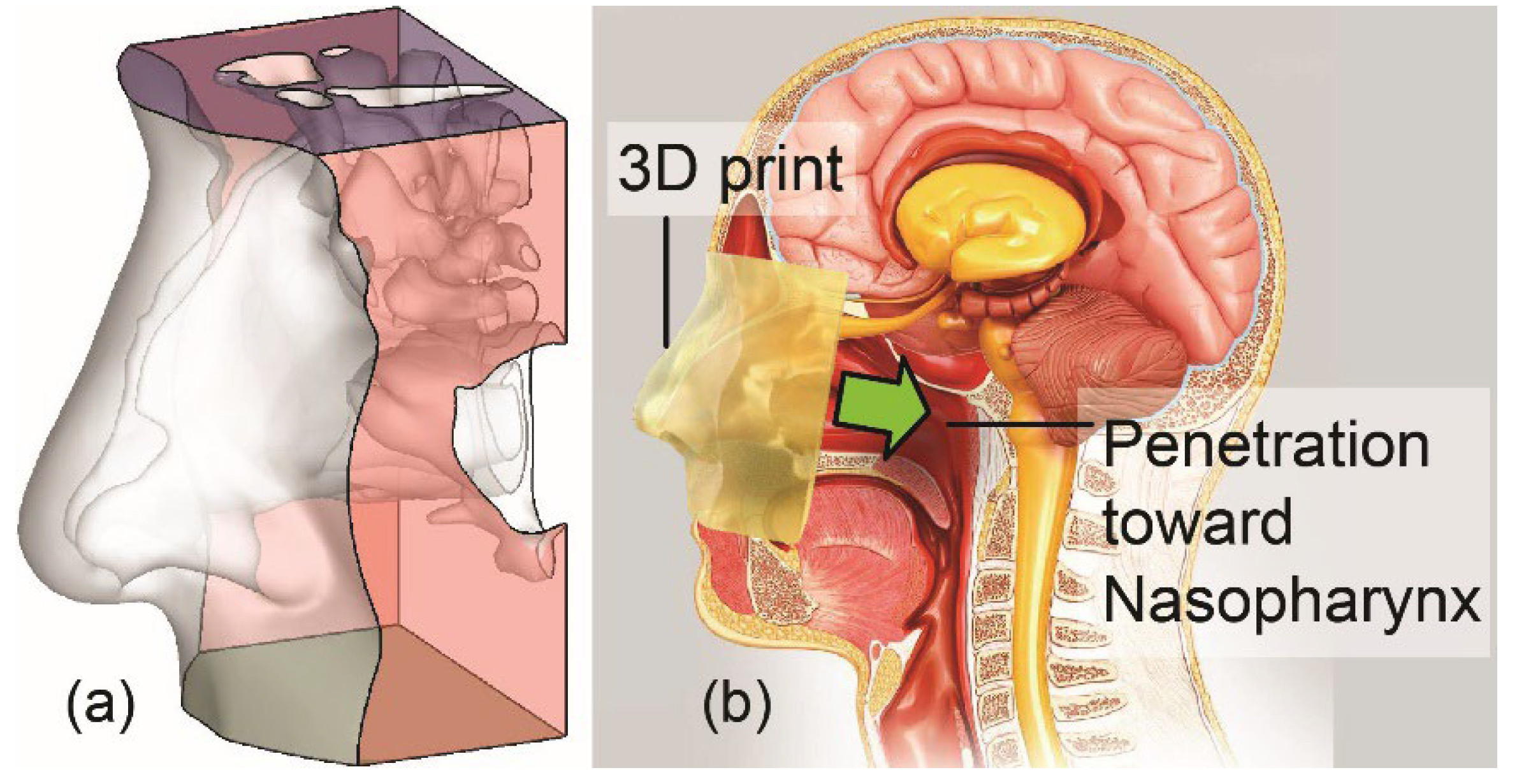
2.2. Human Factors Usability Study Procedure
2.4. Human Factors: Task Analysis
2.5. Knowledge Task
2.6. Human Factors Study Participants
- • Simulation group: This group included 50 participants (38 females, average age 53.40 ± 2.58 years), with 40 having experience with a nasal spray device. In the simulation group, participants did not apply the nasal spray to themselves. Instead, they simulated the action of administering the nasal spray on a mannequin. This approach is common in studies to assess user interaction with a device without the need for actual physical usage, focusing on technique, handling, and potential errors in a controlled setting.
- • Real-use group: This group consisted of 141 participants (63 females, with an average age of 53.86 ± 18.7 years), a significant number of whom (121 participants) had prior experience using a nasal spray device. The "real-use" aspect indicates that these participants actually used the nasal spray device on themselves, in a realistic manner. This means they were applying the nasal spray as they would in a normal, everyday setting, thus providing practical insights into the usability and effectiveness of the nasal spray device under real-life conditions.
2.7. Use Environments
2.8. Interventions
2.9. Study Outcomes
2.10. Data Analysis
2.11. Use-related risk analysis
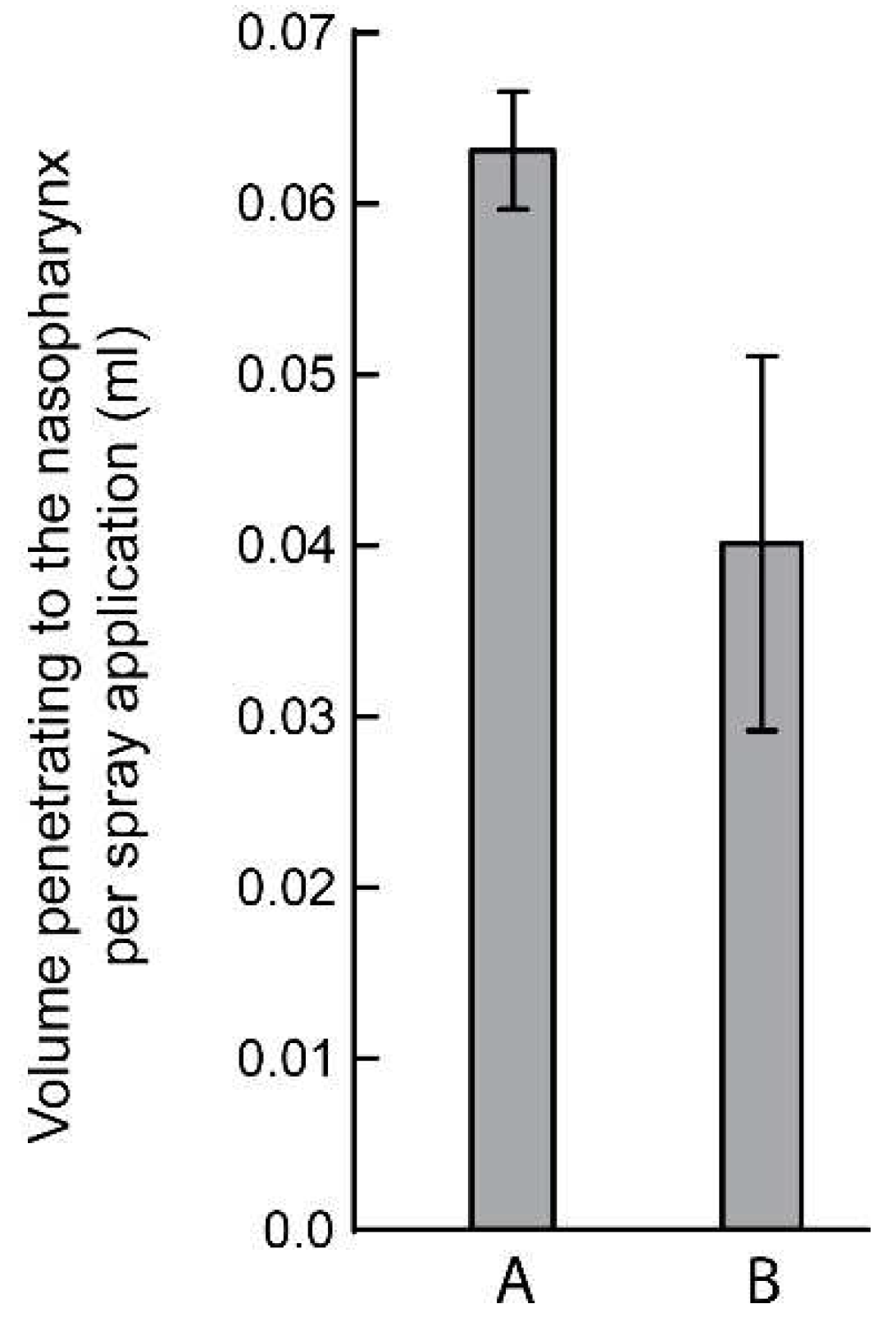
3. Results
| Trial No. | Number of spray pumping action | Mean volume penetration to the nasopharynx per spray application (ml) | ||||
|---|---|---|---|---|---|---|
| 1 | 80 | 0.0625 | ||||
| 2 | 77 | 0.0649 | ||||
| 3 | 81 | 0.0617 | ||||
| 4 | 75 | 0.0666 | ||||
| 5 | 72 | 0.0694 | ||||
| M 77.0 |
SD ± 3.6742 |
CV % 4.77 |
M 0.0650 |
SD ± 0.0031 |
CV 4.76% |
|
| Trial No. | Number of spray pumping action | Mean volume penetration to the nasopharynx per spray application (ml) | ||||
|---|---|---|---|---|---|---|
| 1 | 83 | 0.0625 | ||||
| 2 | 96 | 0.0649 | ||||
| 3 | 94 | 0.0617 | ||||
| 4 | 85 | 0.0588 | ||||
| 5 | 86 | 0.0581 | ||||
| M 88.8 |
SD ± 5.8052 |
CV 6.54 % |
M 0.0612 |
SD ± 0.0028 |
CV 4.58 % |
|
| Trial No. | Number of spray pumping action | Mean volume penetration to the nasopharynx per spray application (ml) | ||||
|---|---|---|---|---|---|---|
| 1 | 160 | 0.0312 | ||||
| 2 | 157 | 0.0318 | ||||
| 3 | 175 | 0.0285 | ||||
| 4 | 174 | 0.0287 | ||||
| 5 | 169 | 0.0295 | ||||
| M 167.0 |
SD ± 8.1548 |
CV 4.88 % |
M 0.0299 |
SD 0.0015 |
CV 5.02 % |
|
| Trial No. | Number of spray pumping action | Mean volume penetration to the nasopharynx per spray application (ml) | ||||
|---|---|---|---|---|---|---|
| 1 | 95 | 0.0526 | ||||
| 2 | 96 | 0.0520 | ||||
| 3 | 110 | 0.0454 | ||||
| 4 | 98 | 0.0510 | ||||
| 5 | 99 | 0.0505 | ||||
| M 99.6 |
SD 6.0249 |
CV 6.05 % |
M 0.0503 |
SD 0.0029 |
CV 5.77 % |
|
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hicke, A.J. Pharmaceutical Inhalation Aerosol Technology, 2nd Ed Marcel Dekker, Inc: NewYork, 2004.
- Illum, L. Nasal drug delivery-possibilities, problems and solutions. J. Control Release 2003, 87, 187–198. [Google Scholar] [CrossRef] [PubMed]
- Ugwoke, M.I.; Verbek, N.; Kinget, R. The biopharmaceutical aspects of nasal mucoadhesion drug delivery. J. Pharm. Pharmacol. 2001, 59, 3–22. [Google Scholar]
- Arora, P. Sharma; Gary, S. Permeability issues in nasal drug delivery. Drug Discov. Today 2002, 7, 967–975. [Google Scholar] [CrossRef] [PubMed]
- Marttin, E.; Nicolaas, G.M.; Schipper, J.; Coos, V.; Frans, W.H. Nasal mucociliary clearance as a factor in nasal drug delivery. Adv. Drug Deliv. Rev. 1997, 29, 13–38. [Google Scholar] [CrossRef] [PubMed]
- Alabsi, W.; Eedara, B.B.; Encinas-Basurto, D.; Polt, R.; Mansour, H.M. Nose-to-Brain Delivery of Therapeutic Peptides as Nasal Aerosols. Pharmaceutics 2022, 14, 1870. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.R.; Liu, M.; Khan, M.W.; Zhai, G. Progress in brain targeting drug delivery system by nasal route. J. Control. Release 2017, 268, 364–389. [Google Scholar] [CrossRef] [PubMed]
- Formica, M.L.; Real, D.A.; Picchio, M.L.; Catlin, E.; Donnelly, R.F.; Paredes, A.J. On a highway to the brain: A review on nose-to-brain drug delivery using nanoparticles. Appl. Mater. Today 2022, 29, 101631. [Google Scholar] [CrossRef]
- Illum, L. Nasal drug delivery: new developments and strategies. Drug Discov. Today 2002, 7, 1184–1189. [Google Scholar] [CrossRef] [PubMed]
- Graff, L.C.; Pollock, G.M. Nasal drug administration: potential for targeted central nervous system delivery. J. Pharm. Sci. 2005, 94, 1187–1195. [Google Scholar] [CrossRef]
- Malakar, A.; Borojeni, A.; Basu, S. Parametric numerical analysis of targeted drug delivery for intranasal sprays inside the upper respiratory airspace. Bull. Am. Phys. Soc. 2023. [Google Scholar]
- Bruno, B.J.; Miller, G.D.; Lim, C.S. Basics and recent advances in peptide and protein drug delivery. Ther. Deliv. 2013, 4, 1443–1467. [Google Scholar] [CrossRef]
- Pontiroli, A.E.; Pozza, G. Intranasal administration of peptide hormones: Factors affecting transmucosal absorption. Diabet. Med. 1990, 7, 770–774. [Google Scholar] [CrossRef] [PubMed]
- Lipworth, B.J.; Jackson, C.M. Safety of inhaled and intranasal corticosteroids: lessons for the new millennium. Drug Saf. 2000, 23, 11–33. [Google Scholar] [CrossRef] [PubMed]
- Wiseman, J.W. Steroid hormone enhancement of gene delivery to a human airway epithelial cell line in vitro and mouse airways in vivo. Gene Ther. 2001, 8, 1562–1571. [Google Scholar] [CrossRef] [PubMed]
- Krishnamoorthy, R.; Mitra, N.K. Prodrugs for nasal drug delivery. Adv. Drug Deliv. Rev. 1998, 29, 135–146. [Google Scholar] [CrossRef] [PubMed]
- Duquesnoy, C.; Mamet, J.P.; Sumner, D.; Fuseau, E. Comparative clinical pharmacokinetics of single doses of sumatriptan following subcutaneous, oral, rectal and intranasal administration. Eur. J. Pharm. Sci. 1998, 6, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Mittal, R.; Ni, R.; Seo, J.H. The flow physics of COVID-19. J. Fluid Mech. 2020, 894, F2. [Google Scholar] [CrossRef]
- Williams, G.; Suman, J.D. In vitro anatomical models for nasal drug delivery. Pharmaceutics 2022, 14, 1353. [Google Scholar] [CrossRef] [PubMed]
- Akash, M.M.; Mituniewicz, A.; Lao, Y.; Balivada, P.; Ato, P.; Ka, N.; Silfen, Z.; Chakravarty, A.; Joseph-McCarthy, D.; Basu, S. A better way to spray?-a model-based optimization of nasal spray use protocols. APS Division of Fluid Dynamics Meeting Abstracts. 2021; pp. Q14-007).
- Basu, S.; Holbrook, L.T.; Kudlaty, K.; Fasanmade, O.; Wu, J.; Burke, A.; Langworthy, B.W.; Mamdani, M.; Farzal, Z.; Bennett, W.D.; et al. Numerical evaluation of spray position for improved nasal drug delivery. Sci. Rep. 2020, 10, 10568. [Google Scholar] [CrossRef] [PubMed]
- Basu, S.; Khawaja, U.A.; Rizvi, S.A.A.; Sanchez-Gonzalez, M.A.; Ferrer, G. Evaluation of Patient Experience for a Computationally-Guided Intranasal Spray Protocol to Augment Therapeutic Penetration: Implications for Effective Treatments for COVID-19, Rhinitis, and Sinusitis. Med. Res. Arch. 2022, 10. [Google Scholar] [CrossRef]
- Treat, S.; Ebert Jr, C.S.; Farzal, Z.; Basu, S.; Kimbell, J.S.; Senior, B.A.; Shockley, W.W.; Madison Clark, J. Intranasal corticosteroids: patient administration angles and impact of education. Rhinology Online. 2020;3:160.
- Akash, M.M.; Lao, Y.; Balivada, P.A.; Ato, P.; Ka, N.K.; Mituniewicz, A.; Silfen, Z.; Suman, J.D.; Chakravarty, A.; Joseph-McCarthy, D.; et al. On a model-based approach to improve intranasal spray targeting for respiratory viral infections. Frontiers in Drug Delivery, Sec. Respir. Drug Deliv. 2023, 3, 1164671. [Google Scholar]
- Basu, S. Computational characterization of inhaled droplet transport to the nasopharynx. Sci. Rep. 2021, 11, 6652. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.J.; Okuda, K.; Edwards, C.E.; Martinez, D.R.; Asakura, T.; Dinnon, K.H.; Kato, T.; Lee, R.E.; Yount, B.L.; Mascenik, T.M.; et al. SARS-CoV-2 reverse genetics reveals a variable infection gradient in the respiratory tract. Cell 2020, 182, 429–446. [Google Scholar] [CrossRef] [PubMed]
- Matheson, N.J.; Lehner, P.J. How does SARS-CoV-2 cause COVID-19? Science 2020, 369, 510–1. [Google Scholar] [CrossRef] [PubMed]
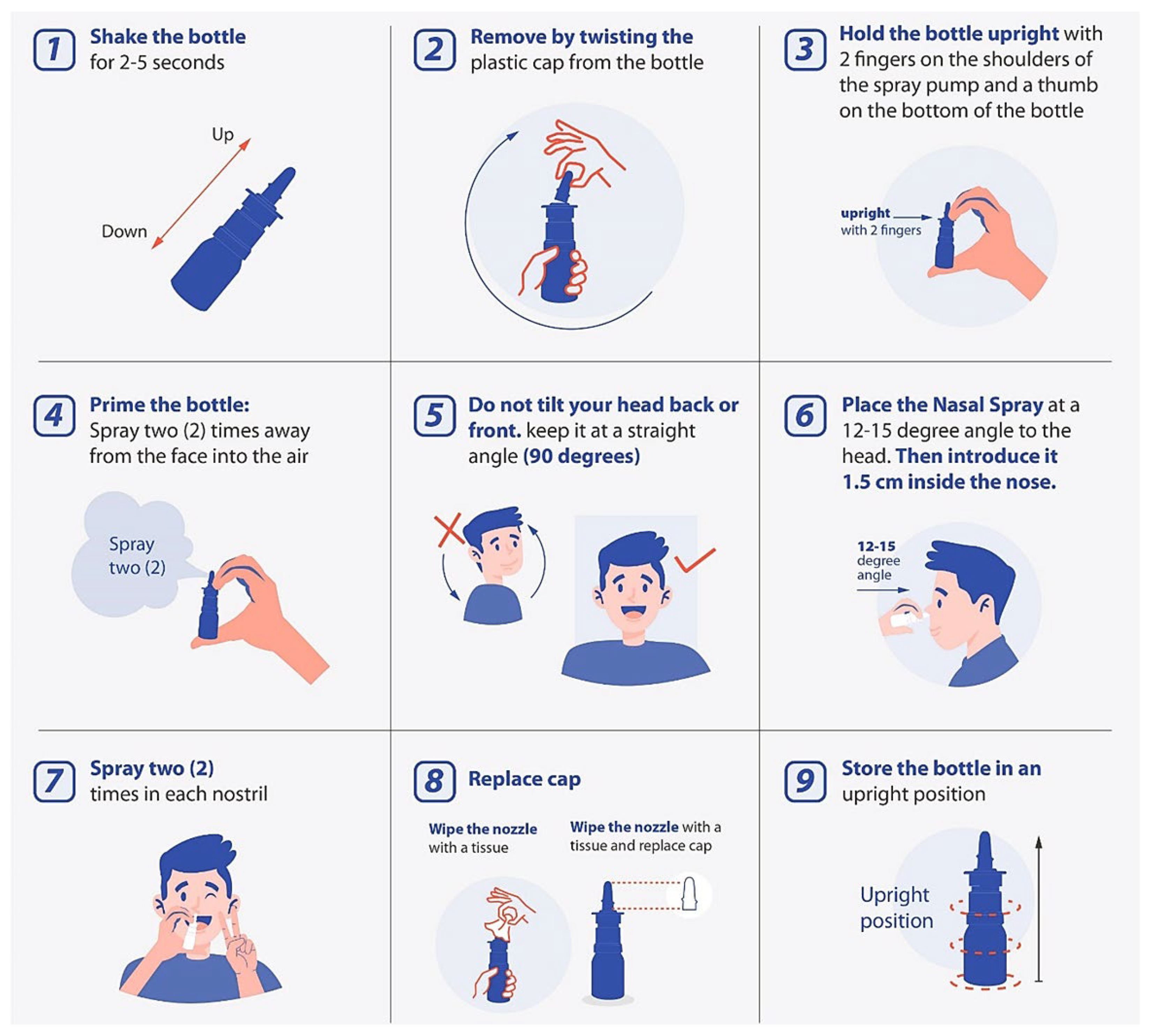
| Stages | Step | Instruction |
|---|---|---|
| Preparation | Step 1 | Shake spray bottle for 2-5 seconds. |
| Step 2 | Remove by twisting the plastic cap from the bottle. | |
| Step 3: Hand Position | Hold the bottle upright with 2 fingers on the shoulders of the spray pump and thumb on the bottom of the bottle. | |
| Step 4: Priming | Spray two times into the air away from the face. | |
| Device Positioning | Step 5: Head Position | Do not tilt your head back or front. |
| Step 6: Closing Nostril | Place the nasal spray at 12-15° angle to the head, as illustrated in the picture. | |
| Delivery | Step 7 | Spray two (2) sprays on each nostril. |
| After Use | Step 8 | Wipe the nozzle with a tissue and replace the cap. |
| Step 9 | Store the bottle in an upright position. |
| Step | Instruction Carried Out N=50 Simulation (%) |
Instruction Carried Out N=141 Real (%) |
Principal Root Causes of Failure if missed |
|---|---|---|---|
| 1 | 88.0 | 83.6 | > 90% of missing did not read the instructions. |
| 2 | 98.0 | 80.9 | > 80% of missing was associated with not reading the instructions or left-handed patients twisting in the wrong direction. |
| 3 | 98.0 | 92.2 | |
| 4 | 82.2 | 62.4 | The patient felt confident because of prior experience did not prime the spray. |
| 5 (essential) | 100 | 95.7 | |
| 6 (essential) | 81.0 | 85.1 | |
| 7 (essential) | 82.0 | 97.2 | |
| 8 | 88.0 | 81.0 | |
| 9 | 100.0 | 96.5 | |
| Mean ± SD | 90.8 ± 8.2% | 86.1 ± 11.1% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions, or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




