Submitted:
13 December 2023
Posted:
14 December 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Overview of Viral Infections
3. A Brief History of the Use of Medicinal Plants Against Viral Infections
4. Mechanism of Actions of Antiviral Secondary Metabolites in Medicinal Plants
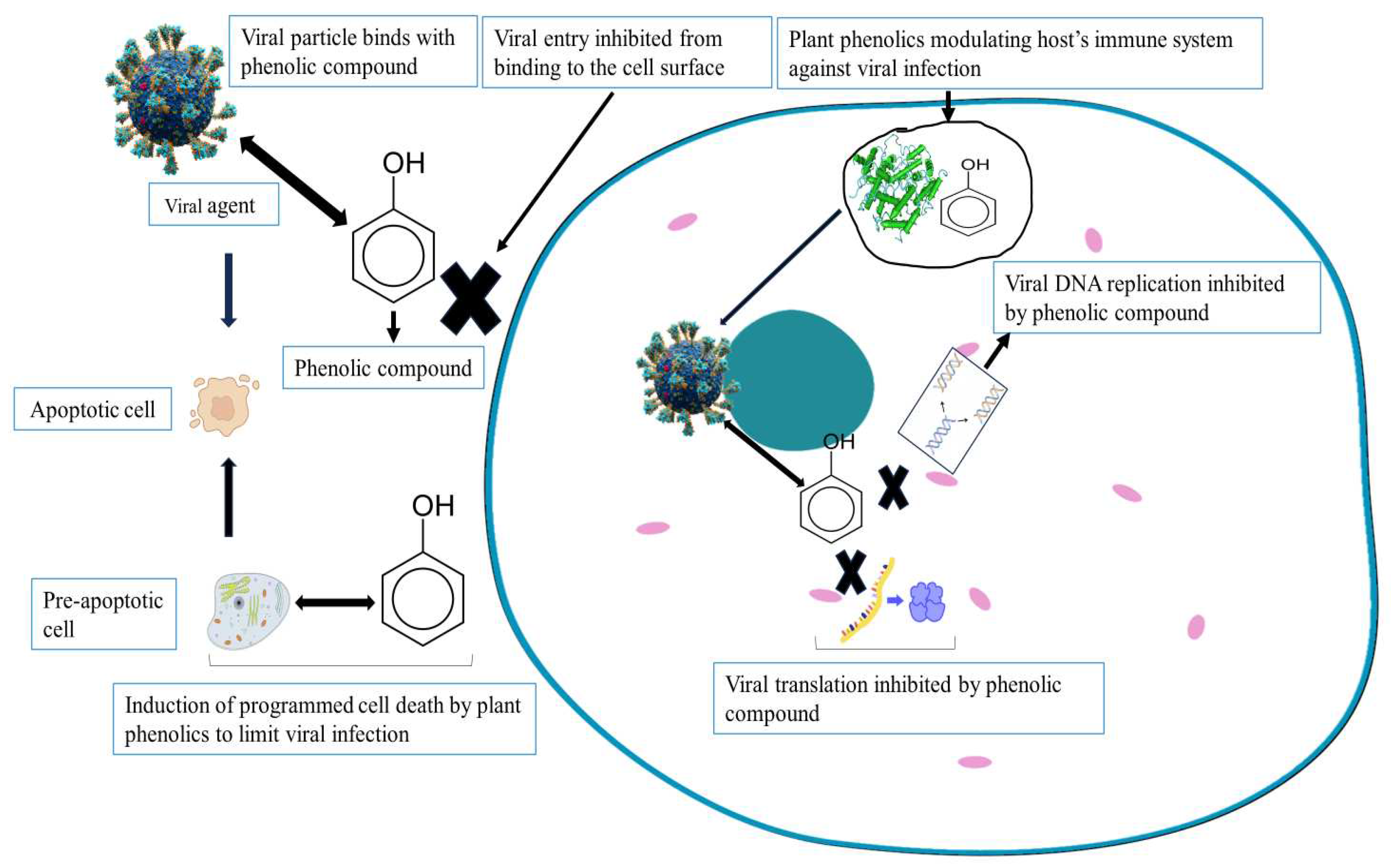
4.1. Phenolics
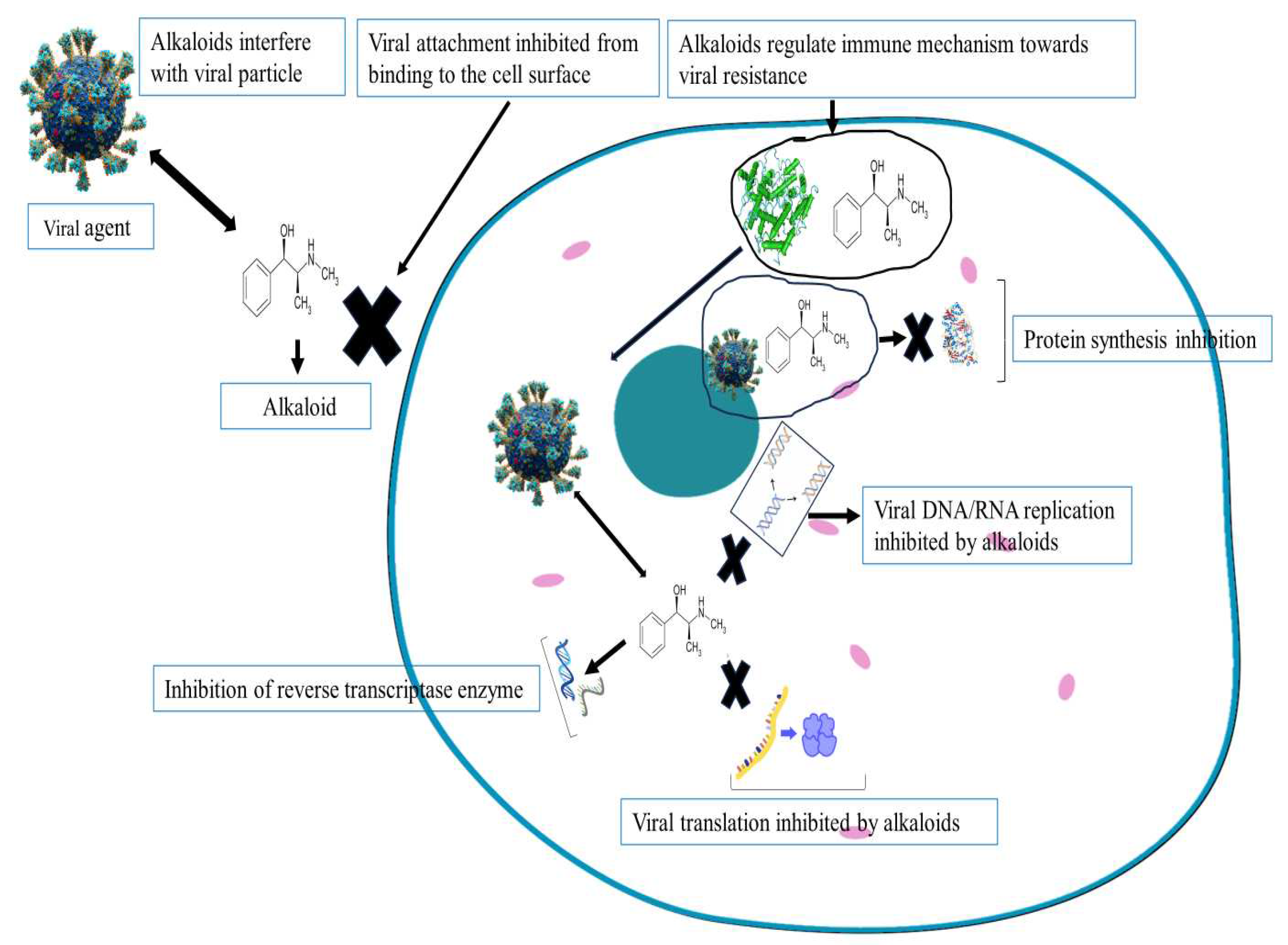
4.2. Alkaloids
4.3. Terpenoids
5. Applications of Metabolomics to Plant Antiviral Research
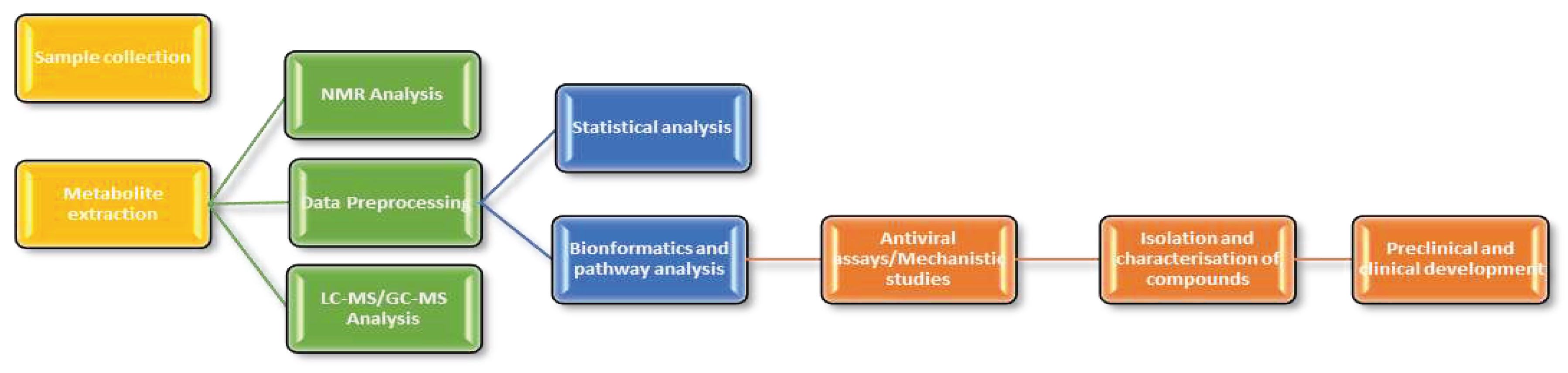
6. Steps for the Discovery of Antiviral Compounds in Plant Metabolomics Studies
7. Future Directions for the Application of Metabolomics towards the Development of Antiviral Therapies from Plants Sources
References
- Baker, R.E.; Mahmud, A.S.; Miller, I.F.; Rajeev, M.; Rasambainarivo, F.; Rice, B.L.; Takahashi, S.; Tatem, A.J.; Wagner, C.E.; Wang, L.F.; et al. Infectious Disease in an Era of Global Change. Nat Rev Microbiol 2022, 20, 193–205. [Google Scholar] [CrossRef]
- Strasfeld, L.; Chou, S. Antiviral Drug Resistance: Mechanisms and Clinical Implications. Infect Dis Clin North Am 2010, 24, 413–437. [Google Scholar] [CrossRef] [PubMed]
- Chitalia, V.C.; Munawar, A.H. A Painful Lesson from the COVID-19 Pandemic: The Need for Broad-Spectrum, Host-Directed Antivirals. J Transl Med 2020, 18. [Google Scholar] [CrossRef]
- Fleßa, S.; Marschall, P. Socio-Economic Impact of Antiviral Intervention; 2009.
- Morris, D.J. Adverse Effects and Drug Interactions of Clinical Importance with Antiviral Drugs; 1994; Vol. 10. [CrossRef]
- Ntie-Kang, F.; Svozil, D. An Enumeration of Natural Products from Microbial, Marine and Terrestrial Sources. Physical Sciences Reviews 2020, 5. [Google Scholar] [CrossRef]
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; Orhan, I.E.; Banach, M.; Rollinger, J.M.; Barreca, D.; Weckwerth, W.; Bauer, R.; Bayer, E.A.; et al. Natural Products in Drug Discovery: Advances and Opportunities. Nat Rev Drug Discov 2021, 20, 200–216. [Google Scholar] [CrossRef] [PubMed]
- Ji, H.-F.; Li, X.-J.; Zhang, H.-Y. Natural Products and Drug Discovery Can Thousands of Years of Ancient Medical Knowledge Lead Us to New and Powerful Drug Combinations in the Fight against Cancer and Dementia?; 2009; Vol. 10. [CrossRef]
- Dias, D.A.; Urban, S.; Roessner, U. A Historical Overview of Natural Products in Drug Discovery. Metabolites 2012, 2, 303–336. [Google Scholar] [CrossRef]
- Lin, L.T.; Hsu, W.C.; Lin, C.C. Antiviral Natural Products and Herbal Medicines. J Tradit Complement Med 2014, 4, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Kumar Shakya, A.; Arvind Kumar Shakya, C. Medicinal Plants: Future Source of New Drugs. Int J Herb Med 2016, 4, 59–64. [Google Scholar] [CrossRef]
- Guerriero, G.; Berni, R.; Muñoz-Sanchez, J.A.; Apone, F.; Abdel-Salam, E.M.; Qahtan, A.A.; Alatar, A.A.; Cantini, C.; Cai, G.; Hausman, J.F.; et al. Production of Plant Secondary Metabolites: Examples, Tips and Suggestions for Biotechnologists. Genes (Basel) 2018, 9. [Google Scholar] [CrossRef]
- Isah, T. Stress and Defense Responses in Plant Secondary Metabolites Production. Biol Res 2019, 52, 39. [Google Scholar] [CrossRef]
- Ramakrishna, A.; Ravishankar, G.A. Influence of Abiotic Stress Signals on Secondary Metabolites in Plants. Plant Signal Behav 2011, 6, 1720–1731. [Google Scholar] [CrossRef] [PubMed]
- Mark R., TePaske; James, B. Gloer Tubingensin A: An Antiviral Carbazole Alkaloid from the Sclerotia of Aspergillus Tubingensis; 1989; Vol. 54. [CrossRef]
- Rob Verpoorte Exploration of Nature’s: The Role of secondary Metabolites as leads in Drug Development; 1998. [CrossRef]
- Parveen, A.; Parveen, B.; Parveen, R.; Ahmad, S. Challenges and Guidelines for Clinical Trial of Herbal Drugs. In Proceedings of the Journal of Pharmacy and Bioallied Sciences; Wolters Kluwer Medknow Publications, October 1 2015; Vol. 7; pp. 329–333. [Google Scholar] [CrossRef]
- Owen, L.; Laird, K.; Shivkumar, M. Antiviral Plant-Derived Natural Products to Combat RNA Viruses: Targets throughout the Viral Life Cycle. Lett Appl Microbiol 2022, 75, 476–499. [Google Scholar] [CrossRef]
- Ghildiyal, R.; Prakash, V.; Chaudhary, V.K.; Gupta, V.; Gabrani, R. Phytochemicals as Antiviral Agents: Recent Updates. In Plant-derived Bioactives: Production, Properties and Therapeutic Applications; Springer Singapore, 2020; pp. 279–295 ISBN 9789811517617. [CrossRef]
- Yeshi, K.; Crayn, D.; Ritmejerytė, E.; Wangchuk, P. Plant Secondary Metabolites Produced in Response to Abiotic Stresses Has Potential Application in Pharmaceutical Product Development. Molecules 2022, 27. [Google Scholar] [CrossRef]
- A. Hussein, R.; A. El-Anssary, A. Plants Secondary Metabolites: The Key Drivers of the Pharmacological Actions of Medicinal Plants. In Herbal Medicine; IntechOpen, 2019. [CrossRef]
- Louten, J. Virus Structure and Classification. In Essential Human Virology; Elsevier, 2016; pp. 19–29. [CrossRef]
- Gelderblom, H.R. Structure and Classification of Viruses; 1996.
- Janeway, C.A.; Medzhitov, R. Innate Immune Recognition. Annu Rev Immunol 2002, 20, 197–216. [Google Scholar] [CrossRef] [PubMed]
- Cohen, F.S. How Viruses Invade Cells. Biophys J 2016, 110, 1028–1032. [Google Scholar] [CrossRef]
- Sudhan, S.S.; Sharma, P. Human Viruses: Emergence and Evolution. In Emerging and Reemerging Viral Pathogens: Volume 1: Fundamental and Basic Virology Aspects of Human, Animal and Plant Pathogens; Elsevier, 2019; pp. 53–68 ISBN 9780128194003. [CrossRef]
- Siegel, R.D. Classification of Human Viruses. In Principles and Practice of Pediatric Infectious Diseases; Elsevier Inc., 2017; pp. 1044–1048 ISBN 9780323401814. [CrossRef]
- Mathekga, A.D.M.; Meyer, J.J.M.; Horn, M.M.; Drewes, S.E. An Acylated Phloroglucinol with Antimicrobial Properties from Helichrysum Caespititium. Phytochemistry 2000, 53, 93–96. [Google Scholar] [CrossRef] [PubMed]
- Siegel, D.; Hui, H.C.; Doerffler, E.; Clarke, M.O.; Chun, K.; Zhang, L.; Neville, S.; Carra, E.; Lew, W.; Ross, B.; et al. Discovery and Synthesis of a Phosphoramidate Prodrug of a Pyrrolo[2,1-f][Triazin-4-Amino] Adenine C-Nucleoside (GS-5734) for the Treatment of Ebola and Emerging Viruses. J Med Chem 2017, 60, 1648–1661. [Google Scholar] [CrossRef]
- Boncristiani, H.F. Respiratory Viruses Defining Statement Introduction Human Respiratory Syncytial Virus Human Parainfluenza Viruses Human Metapneumovirus Rhinovirus Respiratory Adenoviruses Human Coronaviruses Unrelated to SARS SARS Coronavirus Human Bocavirus Further Reading; 2009.
- Hannah Ritchie, F.S. and M.R. Causes of Death.
- WHO WHO Launches New Global Influenza Strategy; 2019.
- WHO Methods for Estimating the Excess Mortality Associated with the COVID-19 Pandemic; 2023.
- Pawlotsky, J.M. Pathophysiology of Hepatitis C Virus Infection and Related Liver Disease. Trends Microbiol 2004, 12, 96–102. [Google Scholar] [CrossRef]
- Plummer, M.; de Martel, C.; Vignat, J.; Ferlay, J.; Bray, F.; Franceschi, S. Global Burden of Cancers Attributable to Infections in 2012: A Synthetic Analysis. Lancet Glob Health 2016, 4, e609–e616. [Google Scholar] [CrossRef]
- Maurice Green Oncogenic Viruses. Annu Rev Biochem 1970, 39, 701–756. [CrossRef]
- MacIntyre, C.R.; Chughtai, A.A.; Barnes, M.; Ridda, I.; Seale, H.; Toms, R.; Heywood, A. The Role of Pneumonia and Secondary Bacterial Infection in Fatal and Serious Outcomes of Pandemic Influenza a(H1N1)Pdm09. BMC Infect Dis 2018, 18. [Google Scholar] [CrossRef] [PubMed]
- Morris, D.E.; Cleary, D.W.; Clarke, S.C. Secondary Bacterial Infections Associated with Influenza Pandemics. Front Microbiol 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Dunn, C.; Brunetto, M.; Reynolds, G.; Christophides, T.; Kennedy, P.T.; Lampertico, P.; Das, A.; Lopes, A.R.; Borrow, P.; Williams, K.; et al. Cytokines Induced during Chronic Hepatitis B Virus Infection Promote a Pathway for NK Cell-Mediated Liver Damage. Journal of Experimental Medicine 2007, 204, 667–680. [Google Scholar] [CrossRef] [PubMed]
- Kawana, R. Viral and Other Myocarditis Cardiovascular Diseases Due to Viruses; 1985. [CrossRef]
- Collins, S.D. Excess Mortality from Causes Other than Influenza and Pneumonia during Influenza Epidemics; 1932; Vol. 47. [CrossRef]
- Bekkering, S.; Burgner, D. Viruses and Cardiovascular Disease: From Bad to Worse. Nature Cardiovascular Research 2022, 1, 601–602. [Google Scholar] [CrossRef]
- Schultheiss, H.P.; Baumeier, C.; Pietsch, H.; Bock, C.T.; Poller, W.; Escher, F. Cardiovascular Consequences of Viral Infections: From COVID to Other Viral Diseases. Cardiovasc Res 2021, 117, 2610–2623. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.P.; Viswanathan, S.; Wang, M.; Sun, L.Q.; Clark, G.C.; D'elia, R. V. Current and Future Developments in the Treatment of Virus-Induced Hypercytokinemia. Future Med Chem 2017, 9, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Rouse, B.T.; Lukacher, A.E. Some Unmet Challenges in the Immunology of Viral Infections; 2010.
- H Chantrill, B.B.; Coulthard, C.E.; Dickinson, L.; Inkley, G.W.; Morris, W.; Pyle, A.H. The Action of Plant Extracts on a Bacteriophage of Pseudornonas Pyocyanea and on Influenza A Virus; 1952; Vol. 6. [CrossRef]
- Thomas, E.; Stewart, L.E.; Darley, B.A.; Pham, A.M.; Esteban, I.; Panda, S.S. Plant-Based Natural Products and Extracts: Potential Source to Develop New Antiviral Drug Candidates. Molecules 2021, 26. [Google Scholar] [CrossRef]
- Welz, A.N.; Emberger-Klein, A.; Menrad, K. Why People Use Herbal Medicine: Insights from a Focus-Group Study in Germany. BMC Complement Altern Med 2018, 18. [Google Scholar] [CrossRef] [PubMed]
- Chattopadhyay, D.; Mukherjee, H. ; Paromita Bag, •; Ghosh, S.; Amalesh Samanta, •; Chakrabarti, • Sekhar Ethnomedicines in Antiviral Drug Discovery; 2009.
- Mukhtar, M.; Arshad, M.; Ahmad, M.; Pomerantz, R.J.; Wigdahl, B.; Parveen, Z. Antiviral Potentials of Medicinal Plants. Virus Res 2008, 131, 111–120. [Google Scholar] [CrossRef]
- Behl, T.; Rocchetti, G.; Chadha, S.; Zengin, G.; Bungau, S.; Kumar, A.; Mehta, V.; Uddin, M.S.; Khullar, G.; Setia, D.; et al. Phytochemicals from Plant Foods as Potential Source of Antiviral Agents: An Overview. Pharmaceuticals 2021, 14. [Google Scholar] [CrossRef]
- Bachar, S.C.; Mazumder, K.; Bachar, R.; Aktar, A.; Al Mahtab, M. A Review of Medicinal Plants with Antiviral Activity Available in Bangladesh and Mechanistic Insight Into Their Bioactive Metabolites on SARS-CoV-2, HIV and HBV. Front Pharmacol 2021, 12. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, A.; Banerjee, M.; Mandal, V.; Shukla, A.C.; Mandal, S.C. Modernization of Ayurveda: A Brief Overview of Indian Initiatives; 2013. [CrossRef]
- Stiefel, M.; Shaner, A.; Schaefer, S.D. The Edwin Smith Papyrus: The Birth of Analytical Thinking in Medicine and Otolaryngology. Laryngoscope 2006, 116, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Chung, V.C.H.; Ma, P.H.X.; Lau, C.H.; Wong, S.Y.S.; Yeoh, E.K.; Griffiths, S.M. Views on Traditional Chinese Medicine amongst Chinese Population: A Systematic Review of Qualitative and Quantitative Studies. Health Expectations 2014, 17, 622–636. [Google Scholar] [CrossRef] [PubMed]
- Reid, D.P. The Shambhala Guide to Traditional Chinese Medicine; 1st ed.; Shambhala, 1996.
- Bensky, D.; Clavey, S.; Stõger, E.; Lai, L. Materia Medica 3rd Edition Compiled and Translated By; 2004.
- Fashner, J.; Ericson, K.; Werner, S.; Joseph, S. Treatment of the Common Cold in Children and Adults; 2012; Vol. 86.
- Song, J.M.; Seong, B.L. Tea Catechins as a Potential Alternative Anti-Infectious Agent. Expert Rev Anti Infect Ther 2007, 5, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Wang, J.; Deng, F.; Hu, Z.; Wang, H. Green Tea Extract and Its Major Component Epigallocatechin Gallate Inhibits Hepatitis B Virus in Vitro. Antiviral Res 2008, 78, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Tran, J. Green Tea: A Potential Alternative Anti-Infectious Agent Catechins and Viral Infections. Advances in Anthropology 2013, 03, 198–202. [Google Scholar] [CrossRef]
- Aboelsoud, N.H. Herbal Medicine in Ancient Egypt. Journal of Medicinal Plants Research 2010, 4, 82–086. [Google Scholar]
- Rivlin, R.S. Recent Advances on the Nutritional Effects Associated with the Use of Garlic as a Supplement Historical Perspective on the Use of Garlic 1,2; 2001.
- Anadón, A.; Martínez-Larrañaga, M.R.; Ares, I.; Martínez, M.A. Interactions between Nutraceuticals/Nutrients and Therapeutic Drugs. In Nutraceuticals: Efficacy, Safety and Toxicity; Elsevier Inc., 2016; pp. 855–874 ISBN 9780128021477. [CrossRef]
- Hudson, J.; Vimalanathan, S. Echinacea-A Source of Potent Antivirals for Respiratory Virus Infections. Pharmaceuticals 2011, 4, 1019–1031. [Google Scholar] [CrossRef]
- Singh, D.; Singh, B.; Goel, R.K. Traditional Uses, Phytochemistry and Pharmacology of Ficus Religiosa: A Review. J Ethnopharmacol 2011, 134, 565–583. [Google Scholar] [CrossRef]
- Alzohairy, M.A. Therapeutics Role of Azadirachta Indica (Neem) and Their Active Constituents in Diseases Prevention and Treatment. Evidence-based Complementary and Alternative Medicine 2016, 2016. [CrossRef]
- Manandhar, B.; Paudel, K.R.; Sharma, B.; Karki, R. Phytochemical Profile and Pharmacological Activity of Aegle Marmelos Linn. J Integr Med 2018, 16, 153–163. [Google Scholar] [CrossRef]
- Cecil, G. Helman Feed a Cold, Starve a Fever — Folk Models of Infection in an English Suburban Community, and Their Relation to Medical Treatment. Culture, Medicine and Psychiatry volume 1978. [CrossRef]
- Harnett, S.M.; Oosthuizen, V.; Van De Venter, M. Anti-HIV Activities of Organic and Aqueous Extracts of Sutherlandia Frutescens and Lobostemon Trigonus. J Ethnopharmacol 2005, 96, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.Q.; Van der Kooy, F.; Verpoorte, R. Artemisia Afra: A Potential Flagship for African Medicinal Plants? South African Journal of Botany 2009, 75, 185–195. [Google Scholar] [CrossRef]
- Sagaya Jansi, R.; Khusro, A.; Agastian, P.; Alfarhan, A.; Al-Dhabi, N.A.; Arasu, M.V.; Rajagopal, R.; Barcelo, D.; Al-Tamimi, A. Emerging Paradigms of Viral Diseases and Paramount Role of Natural Resources as Antiviral Agents. Science of the Total Environment 2021, 759. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Dong, T.; Qi, G.; Qu, L.; Liang, W.; Qi, B.; Zhang, Z.; Shang, L.; Gao, H.; Du, X.; et al. Prevalence of Common Respiratory Viral Infections and Identification of Adenovirus in Hospitalized Adults in Harbin, China 2014 to 2017. Front Microbiol 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Strasfeld, L.; Chou, S. Antiviral Drug Resistance: Mechanisms and Clinical Implications. Infect Dis Clin North Am 2010, 24, 413–437. [Google Scholar] [CrossRef] [PubMed]
- Mera, I.F.G.; Falconí, D.E.G.; Córdova, V.M. Secondary Metabolites in Plants: Main Classes, Phytochemical Analysis and Pharmacological Activities. Bionatura 2019, 4. [Google Scholar] [CrossRef]
- Ávalos García Elena Pérez-Urria Carril, A. Metabolismo Secundario de Plantas. Reduca (Biología). Serie Fisiología Vegetal 2009, 2, 119–145. [Google Scholar]
- Ebenezer, K.S.; Manivannan, R.; Punniyamoorthy, A.; Tamilselvan, C. Plant Secondary Metabolites of Antiviral Properties a Rich Medicinal Source for Drug Discovery: A Mini Review. Journal of Drug Delivery and Therapeutics 2019, 9, 161–167. [Google Scholar] [CrossRef]
- Helfer, M.; Koppensteiner, H.; Schneider, M.; Rebensburg, S.; Forcisi, S.; Müller, C.; Schmitt-Kopplin, P.; Schindler, M.; Brack-Werner, R. The Root Extract of the Medicinal Plant Pelargonium Sidoides Is a Potent HIV-1 Attachment Inhibitor. PLoS One 2014, 9. [Google Scholar] [CrossRef]
- Derksen, A.; Kühn, J.; Hafezi, W.; Sendker, J.; Ehrhardt, C.; Ludwig, S.; Hensel, A. Antiviral Activity of Hydroalcoholic Extract from Eupatorium Perfoliatum L. Against the Attachment of Influenza A Virus. J Ethnopharmacol 2016, 188, 144–152. [Google Scholar] [CrossRef]
- Ebenezer, K.S.; Manivannan, R.; Punniyamoorthy, A.; Tamilselvan, C. Plant Secondary Metabolites of Antiviral Properties a Rich Medicinal Source for Drug Discovery: A Mini Review. Journal of Drug Delivery and Therapeutics 2019, 9, 161–167. [Google Scholar] [CrossRef]
- Ghildiyal, R.; Prakash, V.; Chaudhary, V.K.; Gupta, V.; Gabrani, R. Phytochemicals as Antiviral Agents: Recent Updates. In Plant-derived Bioactives: Production, Properties and Therapeutic Applications; Springer Singapore, 2020; pp. 279–295 ISBN 9789811517617. [CrossRef]
- Mansouri, S.; Kutky, M.; Hudak, K.A. Pokeweed Antiviral Protein Increases HIV-1 Particle Infectivity by Activating the Cellular Mitogen Activated Protein Kinase Pathway. PLoS One 2012, 7. [Google Scholar] [CrossRef] [PubMed]
- Oloche, J.J.; Oluremi, B.B.; Aruwa, C.E.; Sabiu, S. Molecular Modeling Identification of Key Secondary Metabolites from Xylopia Aethiopica as Promising Therapeutics Targeting Essential Measles Viral Proteins. Evidence-based Complementary and Alternative Medicine 2023, 2023. [CrossRef]
- Wink, M. Potential of DNA Intercalating Alkaloids and Other Plant Secondary Metabolites against SARS-CoV-2 Causing COVID-19. Diversity (Basel) 2020, 12. [Google Scholar] [CrossRef]
- Alhazmi, H.A.; Najmi, A.; Javed, S.A.; Sultana, S.; Al Bratty, M.; Makeen, H.A.; Meraya, A.M.; Ahsan, W.; Mohan, S.; Taha, M.M.E.; et al. Medicinal Plants and Isolated Molecules Demonstrating Immunomodulation Activity as Potential Alternative Therapies for Viral Diseases Including COVID-19. Front Immunol 2021, 12. [Google Scholar] [CrossRef] [PubMed]
- Bhuiyan, F.R.; Howlader, S.; Raihan, T.; Hasan, M. Plants Metabolites: Possibility of Natural Therapeutics Against the COVID-19 Pandemic. Front Med (Lausanne) 2020, 7. [Google Scholar] [CrossRef] [PubMed]
- Perez, G.R.M. Antiviral Activity of Compounds Isolated from Plants. Pharm Biol 2003, 41, 107–157. [Google Scholar] [CrossRef]
- Lin, D.; Xiao, M.; Zhao, J.; Li, Z.; Xing, B.; Li, X.; Kong, M.; Li, L.; Zhang, Q.; Liu, Y.; et al. An Overview of Plant Phenolic Compounds and Their Importance in Human Nutrition and Management of Type 2 Diabetes. Molecules 2016, 21. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.; Debnath, P.; Singh, S.; Kumar, N. An Overview of Plant Phenolics and Their Involvement in Abiotic Stress Tolerance. Stresses 2023, 3, 570–585. [Google Scholar] [CrossRef]
- Dai, J.; Mumper, R.J. Plant Phenolics: Extraction, Analysis and Their Antioxidant and Anticancer Properties. Molecules 2010, 15, 7313–7352. [Google Scholar] [CrossRef]
- Uchida, S.; Ozaki, M.; Akashi, T.; Yamashita, K.; Niwat, M.; Taniyamat, K. EFFECTS OF (-)-EPIGALLOCATECHIN-3-O-GALLATE (GREEN TEA TANNIN) ON THE LIFE SPAN OF STROKE-PRONE SPONTANEOUSLY HYPERTENSIVE RATS; 1993.
- Kawai, K.; Tsuno, N.H.; Kitayama, J.; Okaji, Y.; Yazawa, K.; Asakage, M.; Hori, N.; Watanabe, T.; Takahashi, K.; Nagawa, H. Basic and Clinical Immunology Basic and Clinical Immunology Epigallocatechin Gallate, the Main Component of Tea Polyphenol, Binds to CD4 and Interferes with Gp120 Binding. 2003. [CrossRef]
- Hamza, A.; Zhan, C.G. How Can (-)-Epigallocatechin Gallate from Green Tea Prevent HIV-1 Infection? Mechanistic Insights from Computational Modeling and the Implication for Rational Design of Anti-HIV-1 Entry Inhibitors. Journal of Physical Chemistry B 2006, 110, 2910–2917. [Google Scholar] [CrossRef]
- Chen, T.Y.; Chen, D.Y.; Wen, H.W.; Ou, J.L.; Chiou, S.S.; Chen, J.M.; Wong, M.L.; Hsu, W.L. Inhibition of Enveloped Viruses Infectivity by Curcumin. PLoS One 2013, 8. [Google Scholar] [CrossRef] [PubMed]
- Orfali, R.; Rateb, M.E.; Hassan, H.M.; Alonazi, M.; Gomaa, M.R.; Mahrous, N.; Gaballah, M.; Kandeil, A.; Perveen, S.; Abdelmohsen, U.R.; et al. Antibiotics Sinapic Acid Suppresses SARS CoV-2 Replication by Targeting Its Envelope Protein. Antibiotics 2021. [Google Scholar] [CrossRef] [PubMed]
- Goc, A.; Sumera, W.; Rath, M.; Niedzwiecki, A. Phenolic Compounds Disrupt Spike-Mediated Receptor-Binding and Entry of SARS-CoV-2 Pseudo-Virions. PLoS One 2021, 16. [Google Scholar] [CrossRef] [PubMed]
- Yamada, K.; Ogawa, H.; Hara, A.; Yoshida, Y.; Yonezawa, Y.; Karibe, K.; Nghia, V.B.; Yoshimura, H.; Yamamoto, Y.; Yamada, M.; et al. Mechanism of the Antiviral Effect of Hydroxytyrosol on Influenza Virus Appears to Involve Morphological Change of the Virus. Antiviral Res 2009, 83, 35–44. [Google Scholar] [CrossRef]
- Lin, C.W.; Tsai, F.J.; Tsai, C.H.; Lai, C.C.; Wan, L.; Ho, T.Y.; Hsieh, C.C.; Chao, P.D.L. Anti-SARS Coronavirus 3C-like Protease Effects of Isatis Indigotica Root and Plant-Derived Phenolic Compounds. Antiviral Res 2005, 68, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Montenegro-Landívar, M.F.; Tapia-Quirós, P.; Vecino, X.; Reig, M.; Valderrama, C.; Granados, M.; Cortina, J.L.; Saurina, J. Polyphenols and Their Potential Role to Fight Viral Diseases: An Overview. Science of the Total Environment 2021, 801. [Google Scholar] [CrossRef]
- Tirado-Kulieva, V.A.; Hernández-Martínez, E.; Choque-Rivera, T.J. Phenolic Compounds versus SARS-CoV-2: An Update on the Main Findings against COVID-19. Heliyon 2022, 8. [Google Scholar] [CrossRef] [PubMed]
- Steinmann, J.; Buer, J.; Pietschmann, T.; Steinmann, E. Anti-Infective Properties of Epigallocatechin-3-Gallate (EGCG), a Component of Green Tea. Br J Pharmacol 2013, 168, 1059–1073. [Google Scholar] [CrossRef]
- Wu, Y.; Crich, D.; Pegan, S.D.; Lou, L.; Hansen, M.C.; Booth, C.; Desrochers, E.; Mullininx, L.N.; Starling, E.B.; Chang, K.Y.; et al. Polyphenols as Potential Inhibitors of Sars-Cov-2 Rna Dependent Rna Polymerase (Rdrp). Molecules 2021, 26. [Google Scholar] [CrossRef]
- Zhu, J.; Ou, L.; Zhou, Y.; Yang, Z.; Bie, M. (-)-Epigallocatechin-3-Gallate Induces Interferon-Λ2 Expression to Anti-Influenza A Virus in Human Bronchial Epithelial Cells (BEAS-2B) through P38 MAPK Signaling Pathway. J Thorac Dis 2020, 12, 989–997. [Google Scholar] [CrossRef]
- ONO, K.; NAKANE, H.; FUKUSHIMA, M.; CHERMANN, J.-C; BARRÉ-SINOUSSI, F. Differential Inhibitory Effects of Various Flavonoids on the Activities of Reverse Transcriptase and Cellular DNA and RNA Polymerases. Eur J Biochem 1990, 190, 469–476. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Ping, S.; Huang, S.; Hu, L.; Xuan, H.; Zhang, C.; Hu, F. Molecular Mechanisms Underlying the in Vitro Anti-Inflammatory Effects of a Flavonoid-Rich Ethanol Extract from Chinese Propolis (Poplar Type). Evidence-based Complementary and Alternative Medicine 2013, 2013. [CrossRef]
- Giovinazzo, G.; Gerardi, C.; Uberti-Foppa, C.; Lopalco, L. Can Natural Polyphenols Help in Reducing Cytokine Storm in COVID-19 Patients? Molecules 2020, 25. [Google Scholar] [CrossRef]
- Upton, J.W.; Chan, F.K.M. Staying Alive: Cell Death in Antiviral Immunity. Mol Cell 2014, 54, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Dejani, N.N.; Elshabrawy, H.A.; Bezerra Filho, C. da S.M.; de Sousa, D.P. Anticoronavirus and Immunomodulatory Phenolic Compounds: Opportunities and Pharmacotherapeutic Perspectives. Biomolecules 2021, 11. [CrossRef]
- Wang, B.; Ding, Y.; Zhao, P.; Li, W.; Li, M.; Zhu, J.; Ye, S. Systems Pharmacology-Based Drug Discovery and Active Mechanism of Natural Products for Coronavirus Pneumonia (COVID-19): An Example Using Flavonoids. Comput Biol Med 2022, 143. [Google Scholar] [CrossRef] [PubMed]
- Moradi, M.T.; Karimi, A.; Lorigooini, Z. Alkaloids as the Natural Anti-Influenza Virus Agents: A Systematic Review. Toxin Rev 2018, 37, 11–18. [Google Scholar] [CrossRef]
- Abookleesh, F.L.; Al-Anzi, B.S.; Ullah, A. Potential Antiviral Action of Alkaloids. Molecules 2022, 27. [Google Scholar] [CrossRef] [PubMed]
- Gürü, M.; Gürü, S.; Yılmaz Aydın, D. Effect of Alkaloids on SARS-CoV-2. NATURENGS MTU Journal of Engineering and Natural Sciences, Malatya Turgut Ozal University 2020, Special Issue. 10–18. [CrossRef]
- Peng, J.; Hu, J.F.; Kazi, A.B.; Li, Z.; Avery, M.; Peraud, O.; Hill, R.T.; Franzblau, S.G.; Zhang, F.; Schinazi, R.F.; et al. Manadomanzamines A and B: A Novel Alkaloid Ring System with Potent Activity against Mycobacteria and HIV-1. J Am Chem Soc 2003, 125, 13382–13386. [Google Scholar] [CrossRef] [PubMed]
- Amasheh, M.; Fromm, A.; Krug, S.M.; Amasheh, S.; Andres, S.; Zeitz, M.; Fromm, M.; Schulzke, J.D. TNFα-Induced and Berberine-Antagonized Tight Junction Barrier Impairment via Tyrosine Kinase, Akt and NFκB Signaling. J Cell Sci 2010, 123, 4145–4155. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, I.I.; Quax, W.J. A Glimpse into the Biosynthesis of Terpenoids. KnE Life Sciences 2017, 3, 81. [Google Scholar] [CrossRef]
- Rodriguez-Concepcion, M.; Avalos, J.; Bonet, M.L.; Boronat, A.; Gomez-Gomez, L.; Hornero-Mendez, D.; Limon, M.C.; Meléndez-Martínez, A.J.; Olmedilla-Alonso, B.; Palou, A.; et al. A Global Perspective on Carotenoids: Metabolism, Biotechnology, and Benefits for Nutrition and Health. Prog Lipid Res 2018, 70, 62–93. [Google Scholar] [CrossRef]
- Yazaki, K.; Arimura, G.I.; Ohnishi, T. "Hidden" Terpenoids in Plants: Their Biosynthesis, Localization and Ecological Roles. Plant Cell Physiol 2017, 58, 1615–1621. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Xie, F.J.; Cao, X.; Li, M.Y. Research Progress in Biosynthesis and Regulation of Plant Terpenoids. Biotechnology and Biotechnological Equipment 2021, 35, 1800–1809. [Google Scholar] [CrossRef]
- Ashour, M.; Wink, M.; Gershenzon, J. Biochemistry of Terpenoids: Monoterpenes, Sesquiterpenes and Diterpenes. In Biochemistry of Plant Secondary Metabolism: Second Edition; Wiley Blackwell, 2010; Vol. 40, pp. 258–303 ISBN 9781444320503. [CrossRef]
- Hassan, S.T.S.; Masarčíková, R.; Berchová, K. Bioactive Natural Products with Anti-Herpes Simplex Virus Properties. Journal of Pharmacy and Pharmacology 2015, 67, 1325–1336. [Google Scholar] [CrossRef] [PubMed]
- Rizzato, G.; Scalabrin, E.; Radaelli, M.; Capodaglio, G.; Piccolo, O. A New Exploration of Licorice Metabolome. Food Chem 2017, 221, 959–968. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yang, R.; Yuan, B.; Liu, Y.; Liu, C. The Antiviral and Antimicrobial Activities of Licorice, a Widely-Used Chinese Herb. Acta Pharm Sin B 2015, 5, 310–315. [Google Scholar] [CrossRef] [PubMed]
- Thyagarajan, S.P.; Jayaram, S.; Gopalakrishnan, V.; Hari, R.; And, J.; Sripathi, M.S. CONFERENCE PROCEEDINGS Herbal Medicines for Liver Diseases in India. J Gastroenterol Hepatol 2002, 17, 370–376. [Google Scholar] [CrossRef] [PubMed]
- Marisa, R.; Assessor, D.; Calapai, G.; Delbò, M. Assessment Report on Glycyrrhiza Glabra L. and/or Glycyrrhiza Inflata Bat. and/or Glycyrrhiza Uralensis Fisch., Radix; 2013.
- Fiore, C.; Eisenhut, M.; Krausse, R.; Ragazzi, E.; Pellati, D.; Armanini, D.; Bielenberg, J. ANTIVIRAL EFFECTS OF GLYCYRRHIZA SPECIES 141 Antiviral Effects of Glycyrrhiza Species. Phytother. Res 2008, 22, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Richard, S.A. Exploring the Pivotal Immunomodulatory and Anti-Inflammatory Potentials of Glycyrrhizic and Glycyrrhetinic Acids. Mediators Inflamm 2021, 2021. [CrossRef]
- Schmidt, R.; Enzinger, C.; Ropele, S.; Schmidt, H.; Fazekas, F.; Cinatl, J.; Morgenstern, B.; Bauer, G.; Chandra, P.; Rabenau, H.; et al. Glycyrrhizin, an Active Component of Liquorice Roots, and Replication of SARS-Associated Coronavirus; 1997; Vol. 71.
- Armaka, M.; Papanikolaou, E.; Sivropoulou, A.; Arsenakis, M. Antiviral Properties of Isoborneol, a Potent Inhibitor of Herpes Simplex Virus Type 1; 1999; Vol. 43. [CrossRef]
- Astani, A.; Schnitzler, P. Antiviral Activity of Monoterpenes Beta-Pinene and Limonene against Herpes Simplex Virus in Vitro; 2014.
- Yu, J.S.; Tseng, C.K.; Lin, C.K.; Hsu, Y.C.; Wu, Y.H.; Hsieh, C.L.; Lee, J.C. Celastrol Inhibits Dengue Virus Replication via Up-Regulating Type I Interferon and Downstream Interferon-Stimulated Responses. Antiviral Res 2017, 137, 49–57. [Google Scholar] [CrossRef]
- Narayan, V.; Ravindra, K.C.; Chiaro, C.; Cary, D.; Aggarwal, B.B.; Henderson, A.J.; Prabhu, K.S. Celastrol Inhibits Tat-Mediated Human Immunodeficiency Virus (HIV) Transcription and Replication. J Mol Biol 2011, 410, 972–983. [Google Scholar] [CrossRef]
- Tseng, C.K.; Hsu, S.P.; Lin, C.K.; Wu, Y.H.; Lee, J.C.; Young, K.C. Celastrol Inhibits Hepatitis C Virus Replication by Upregulating Heme Oxygenase-1 via the JNK MAPK/Nrf2 Pathway in Human Hepatoma Cells. Antiviral Res 2017, 146, 191–200. [Google Scholar] [CrossRef]
- Roberts, L.D.; Souza, A.L.; Gerszten, R.E.; Clish, C.B. Targeted Metabolomics. Curr Protoc Mol Biol 2012, 1. [Google Scholar] [CrossRef] [PubMed]
- Steinfath, M.; Strehmel, N.; Peters, R.; Schauer, N.; Groth, D.; Hummel, J.; Steup, M.; Selbig, J.; Kopka, J.; Geigenberger, P.; et al. Discovering Plant Metabolic Biomarkers for Phenotype Prediction Using an Untargeted Approach. Plant Biotechnol J 2010, 8, 900–911. [Google Scholar] [CrossRef] [PubMed]
- Nanusha, M.Y.; Krauss, M.; Schönsee, C.D.; Günthardt, B.F.; Bucheli, T.D.; Brack, W. Target Screening of Plant Secondary Metabolites in River Waters by Liquid Chromatography Coupled to High-Resolution Mass Spectrometry (LC-HRMS). Environ Sci Eur 2020, 32. [Google Scholar] [CrossRef]
- Ehrlich, P.R.; Raven, P.H. Butterflies and Plants: A Study in Coevolution; 1964; Vol. 18. [CrossRef]
- Jones, C.G.; Firn, R.D. On the Evolution of Plant Secondary Chemical Diversity. Philosophical Transactions - Royal Society of London, B 1991, 333, 273–280. [Google Scholar] [CrossRef]
- Lee, S.; Oh, D.G.; Singh, D.; Lee, J.S.; Lee, S.; Lee, C.H. Exploring the Metabolomic Diversity of Plant Species across Spatial (Leaf and Stem) Components and Phylogenic Groups. BMC Plant Biol 2020, 20. [Google Scholar] [CrossRef] [PubMed]
- Halouska, S.; Fenton, R.J.; Barletta, R.G.; Powers, R. Predicting the in Vivo Mechanism of Action for Drug Leads Using NMR Metabolomics. ACS Chem Biol 2012, 7, 166–171. [Google Scholar] [CrossRef]
- Shahid, M.; Singh, U.B.; Khan, M.S. Metabolomics-Based Mechanistic Insights into Revealing the Adverse Effects of Pesticides on Plants: An Interactive Review. Metabolites 2023, 13. [Google Scholar] [CrossRef]
- Hussein, M.; Han, M.-L.; Zhu, Y.; Zhou, Q.; Lin, Y.-W.; Hancock, R.E.W.; Hoyer, D.; Creek, D.J.; Li, J.; Velkov, T. Metabolomics Study of the Synergistic Killing of Polymyxin B in Combination with Amikacin against Polymyxin-Susceptible and-Resistant Pseudomonas Aeruginosa. 2019. [CrossRef]
- Liu, L.W.; Shi, Y.Y.; Li, Z.L.; Zuo, L.H.; Tang, M.; Jing, Z.W.; Zhao, H.Y.; Xue, P.; Zhou, L.; Du, Q.Z.; et al. Metabolomic Insights Into the Synergistic Effect of Biapenem in Combination With Xuebijing Injection Against Sepsis. Front Pharmacol 2020, 11. [Google Scholar] [CrossRef]
- Vidar, W.S.; Baumeister, T.U.H.; Caesar, L.K.; Kellogg, J.J.; Todd, D.A.; Linington, R.G.; M. Kvalheim, O.; Cech, N.B. Interaction Metabolomics to Discover Synergists in Natural Product Mixtures. J Nat Prod 2023, 86, 655–671. [Google Scholar] [CrossRef]
- Zhu, S.; Yue, J.; Wang, X.; Zhang, J.; Yu, M.; Zhan, Y.; Zhu, Y.; Sy, S.K.B.; Lv, Z. Metabolomics Revealed Mechanism for the Synergistic Effect of Sulbactam, Polymyxin-B and Amikacin Combination against Acinetobacter Baumannii. Front Microbiol 2023, 14. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Schillaci, M.; Roessner, U. Metabolomics as an Emerging Tool to Study Plant-Microbe Interactions. Emerg Top Life Sci 2022, 6, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Serag, A.; Salem, M.A.; Gong, S.; Wu, J.-L.; Farag, M.A. Decoding Metabolic Reprogramming in Plants under Pathogen Attacks, a Comprehensive Review of Emerging Metabolomics Technologies to Maximize Their Applications. Metabolites 2023, 13, 424. [Google Scholar] [CrossRef] [PubMed]
- Sampaio, B.L.; Edrada-Ebel, R.; Da Costa, F.B. Effect of the Environment on the Secondary Metabolic Profile of Tithonia Diversifolia: A Model for Environmental Metabolomics of Plants. Sci Rep 2016, 6. [Google Scholar] [CrossRef] [PubMed]
- Prinsloo, G.; Nogemane, N. The Effects of Season and Water Availability on Chemical Composition, Secondary Metabolites and Biological Activity in Plants. Phytochemistry Reviews 2018, 17. [Google Scholar] [CrossRef]
- Adeosun, W.B.; More, G.K.; Steenkamp, P.; Prinsloo, G. Influence of Seasonal and Geographic Variation on the Anti-HSV-1 Properties and Chlorogenic Acids Content of Helichrysum Aureonitens Sch. Bip. Front Mol Biosci 2022, 9. [Google Scholar] [CrossRef]
- Manchester, M.; Anand, A. Metabolomics: Strategies to Define the Role of Metabolism in Virus Infection and Pathogenesis. In Advances in Virus Research; Academic Press Inc., 2017; Vol. 98, pp. 57–81. [CrossRef]
- Ren, Z.; Fang, M.; Muhae-Ud-Din, G.; Gao, H.; Yang, Y.; Liu, T.; Chen, W.; Gao, L. Metabolomics Analysis of Grains of Wheat Infected and Noninfected with Tilletia Controversa Kühn. Sci Rep 2021, 11. [Google Scholar] [CrossRef] [PubMed]
- Nothias, L.F.; Boutet-Mercey, S.; Cachet, X.; De La Torre, E.; Laboureur, L.; Gallard, J.F.; Retailleau, P.; Brunelle, A.; Dorrestein, P.C.; Costa, J.; et al. Environmentally Friendly Procedure Based on Supercritical Fluid Chromatography and Tandem Mass Spectrometry Molecular Networking for the Discovery of Potent Antiviral Compounds from Euphorbia Semiperfoliata. J Nat Prod 2017, 80, 2620–2629. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, A.R.V.; Reis, J.D.E.; Gomes, P.W.P.; Ferraz, A.C.; Mardegan, H.A.; Menegatto, M.B. da S.; Souza Lima, R.L.; de Sarges, M.R. V.; Pamplona, S. das G.S.R.; Jeunon Gontijo, K.S.; et al. Untargeted-Based Metabolomics Analysis and in Vitro/in Silico Antiviral Activity of Extracts from Phyllanthus Brasiliensis (Aubl.) Poir. Phytochemical Analysis 2023. [CrossRef]
- Haggag, E.G.; Elshamy, A.M.; Rabeh, M.A.; Gabr, N.M.; Salem, M.; Youssif, K.A.; Samir, A.; Bin Muhsinah, A.; Alsayari, A.; Abdelmohsen, U.R. Antiviral Potential of Green Synthesized Silver Nanoparticles of Lampranthus Coccineus and Malephora Lutea. Int J Nanomedicine 2019, 14, 6217–6229. [Google Scholar] [CrossRef]
- Takeda, Y.; Okuyama, Y.; Nakano, H.; Yaoita, Y.; Machida, K.; Ogawa, H.; Imai, K. Antiviral Activities of Hibiscus Sabdariffa L. Tea Extract Against Human Influenza A Virus Rely Largely on Acidic PH but Partially on a Low-PH-Independent Mechanism. Food Environ Virol 2020, 12, 9–19. [Google Scholar] [CrossRef]
- More, G.K.; Vervoort, J.; Steenkamp, P.A.; Prinsloo, G. Metabolomic Profile of Medicinal Plants with Anti-RVFV Activity. Heliyon 2022, 8. [Google Scholar] [CrossRef]
- Aati, H.Y.; Ismail, A.; Rateb, M.E.; AboulMagd, A.M.; Hassan, H.M.; Hetta, M.H. Garcinia Cambogia Phenolics as Potent Anti-COVID-19 Agents: Phytochemical Profiling, Biological Activities, and Molecular Docking. Plants 2022, 11. [Google Scholar] [CrossRef] [PubMed]
- Cock, I.E.; Matthews, B. Metabolomic Profiling of Antiviral Scaevola Spinescens Extracts by High Resolution Tandem Mass Spectrometry. In Proceedings of the Acta Horticulturae; International Society for Horticultural Science, October 25 2016; Vol. 1125; pp. 1–18. [Google Scholar] [CrossRef]
- Nagai, T.; Shimizu, Y.; Shirahata, T.; Sunazuka, T.; Kiyohara, H.; Omura, S.; Yamada, H. Oral Adjuvant Activity for Nasal Influenza Vaccines Caused by Combination of Two Trihydroxy Fatty Acid Stereoisomers from the Tuber of Pinellia Ternata. Int Immunopharmacol 2010, 10, 655–661. [Google Scholar] [CrossRef] [PubMed]
- Mei, J.; Zhou, Y.; Yang, X.; Zhang, F.; Liu, X.; Yu, B. Active Components in Ephedra Sinica Stapf Disrupt the Interaction between ACE2 and SARS-CoV-2 RBD: Potent COVID-19 Therapeutic Agents. J Ethnopharmacol 2021, 278. [Google Scholar] [CrossRef] [PubMed]
- Ngoc, T.M.; Phuong, N.T.T.; Khoi, N.M.; Park, S.J.; Kwak, H.J.; Nhiem, N.X.; Trang, B.T.T.; Tai, B.H.; Song, J.H.; Ko, H.J.; et al. A New Naphthoquinone Analogue and Antiviral Constituents from the Root of Rhinacanthus Nasutus. Nat Prod Res 2019, 33, 360–366. [Google Scholar] [CrossRef]
- Chung, C.Y.; Liu, C.H.; Burnouf, T.; Wang, G.H.; Chang, S.P.; Jassey, A.; Tai, C.J.; Tai, C.J.; Huang, C.J.; Richardson, C.D.; et al. Activity-Based and Fraction-Guided Analysis of Phyllanthus Urinaria Identifies Loliolide as a Potent Inhibitor of Hepatitis C Virus Entry. Antiviral Res 2016, 130, 58–68. [Google Scholar] [CrossRef]
- Wu, S.F.; Lin, C.K.; Chuang, Y.S.; Chang, F.R.; Tseng, C.K.; Wu, Y.C.; Lee, J.C. Anti-Hepatitis C Virus Activity of 3-Hydroxy Caruilignan C from Swietenia Macrophylla Stems. J Viral Hepat 2012, 19, 364–370. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Calderón, C.; Mesa-Castro, C.; Robledo, S.; Gómez, S.; Bolivar-Avila, S.; Diaz-Castillo, F.; Martínez-Gutierrez, M. Antiviral Effect of Compounds Derived from the Seeds of Mammea Americana and Tabernaemontana Cymosa on Dengue and Chikungunya Virus Infections. BMC Complement Altern Med 2017, 17. [Google Scholar] [CrossRef] [PubMed]
- Herrera-Ruiz, M.; Román-Ramos, R.; Zamilpa, A.; Tortoriello, J.; Jiménez-Ferrer, J.E. Flavonoids from Tilia Americana with Anxiolytic Activity in Plus-Maze Test. J Ethnopharmacol 2008, 118, 312–317. [Google Scholar] [CrossRef]
- Goulas, V.; Manganaris, G.A. Towards an Efficient Protocol for the Determination of Triterpenic Acids in Olive Fruit: A Comparative Study of Drying and Extraction Methods. Phytochemical Analysis 2012, 23, 444–449. [Google Scholar] [CrossRef]
- Kim, K.H.; Moon, E.; Kim, S.Y.; Choi, S.U.; Lee, K.R. Lignan Constituents of Tilia Amurensis and Their Biological Evaluation on Antitumor and Anti-Inflammatory Activities. Food and Chemical Toxicology 2012, 50, 3680–3686. [Google Scholar] [CrossRef] [PubMed]
- Oniszczuk, A.; Podgórski, R.; Oniszczuk, T.; Zukiewicz-Sobczak, W.; Nowak, R.; Waksmundzka-Hajnos, M. Extraction Methods for the Determination of Phenolic Compounds from Equisetum Arvense L. Herb. Ind Crops Prod 2014, 61, 377–381. [Google Scholar] [CrossRef]
- Noguerón-Merino, M.C.; Jiménez-Ferrer, E.; Román-Ramos, R.; Zamilpa, A.; Tortoriello, J.; Herrera-Ruiz, M. Interactions of a Standardized Flavonoid Fraction from Tilia Americana with Serotoninergic Drugs in Elevated plus Maze. J Ethnopharmacol 2015, 164, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Sun, H.; Wang, X. Emerging Role and Recent Applications of Metabolomics Biomarkers in Obesity Disease Research. RSC Adv 2017, 7, 14966–14973. [Google Scholar] [CrossRef]
- Adeosun, W.B.; Bodede, O.; Prinsloo, G. Effect of Different Climatic Regions and Seasonal Variation on the Antibacterial and Antifungal Activity, and Chemical Profile of Helichrysum Aureonitens Sch. Bip. Metabolites 2022, 12. [Google Scholar] [CrossRef]
- Kell, D.B.; Oliver, S.G. The Metabolome 18 Years on: A Concept Comes of Age. Metabolomics 2016, 12. [Google Scholar] [CrossRef]
- Thompson, K.D. Lead Molecules from Natural Products: Discovery and New Trends; Khan, M.T.H., Ather, A., Eds.; First.; Elsevier: Amsterdam, 2006; Vol. Vol. 2. [Google Scholar]
- Bang, S.; Quy Ha, T.K.; Lee, C.; Li, W.; Oh, W.K.; Shim, S.H. Antiviral Activities of Compounds from Aerial Parts of Salvia Plebeia R. Br. J Ethnopharmacol 2016, 192, 398–405. [Google Scholar] [CrossRef] [PubMed]
- Yadav, A.K.; Banerjee, S.K.; Das, B.; Chaudhary, K. Editorial: Systems Biology and Omics Approaches for Understanding Complex Disease Biology. Front Genet 2022, 13. [Google Scholar] [CrossRef] [PubMed]
- Melini, F.; Luziatelli, F.; Bonini, P.; Ficca, A.G.; Melini, V.; Ruzzi, M. Optimization of the Growth Conditions through Response Surface Methodology and Metabolomics for Maximizing the Auxin Production by Pantoea Agglomerans C1. Front Microbiol 2023, 14. [Google Scholar] [CrossRef]
- Ferrell, J.E. Q&A: Systems Biology. J Biol 2009, 8. [Google Scholar] [CrossRef]
- Greene, C.S.; Troyanskaya, O.G. Integrative Systems Biology for Data-Driven Knowledge Discovery. Semin Nephrol 2010, 30, 443–454. [Google Scholar] [CrossRef] [PubMed]
- Debnath, M.; Prasad, G.B.K.S.; Bisen, P.S. Omics Technology. In Molecular Diagnostics: Promises and Possibilities; Springer Netherlands, 2010; pp. 11–31. [CrossRef]

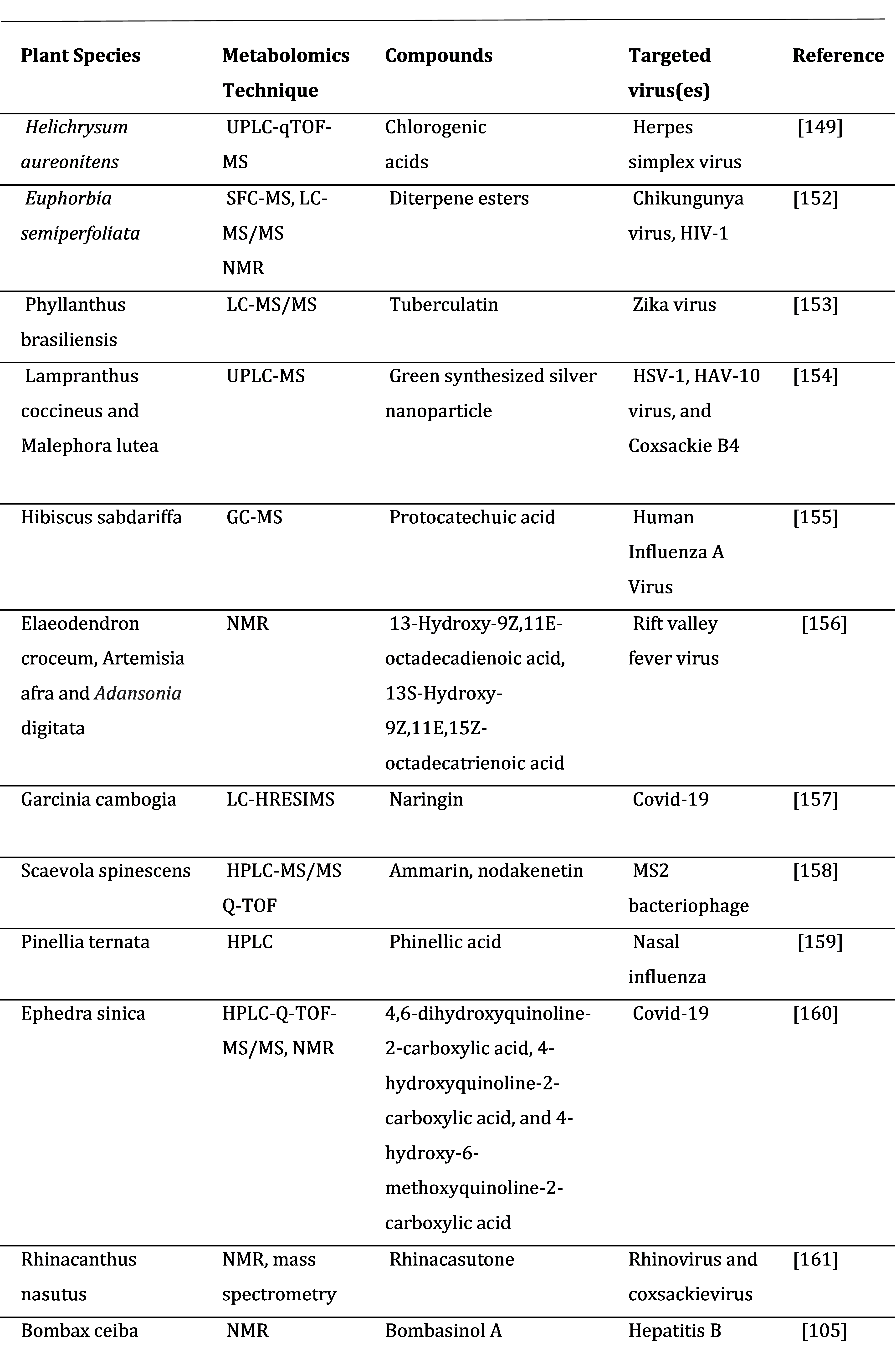

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





