Submitted:
12 December 2023
Posted:
14 December 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
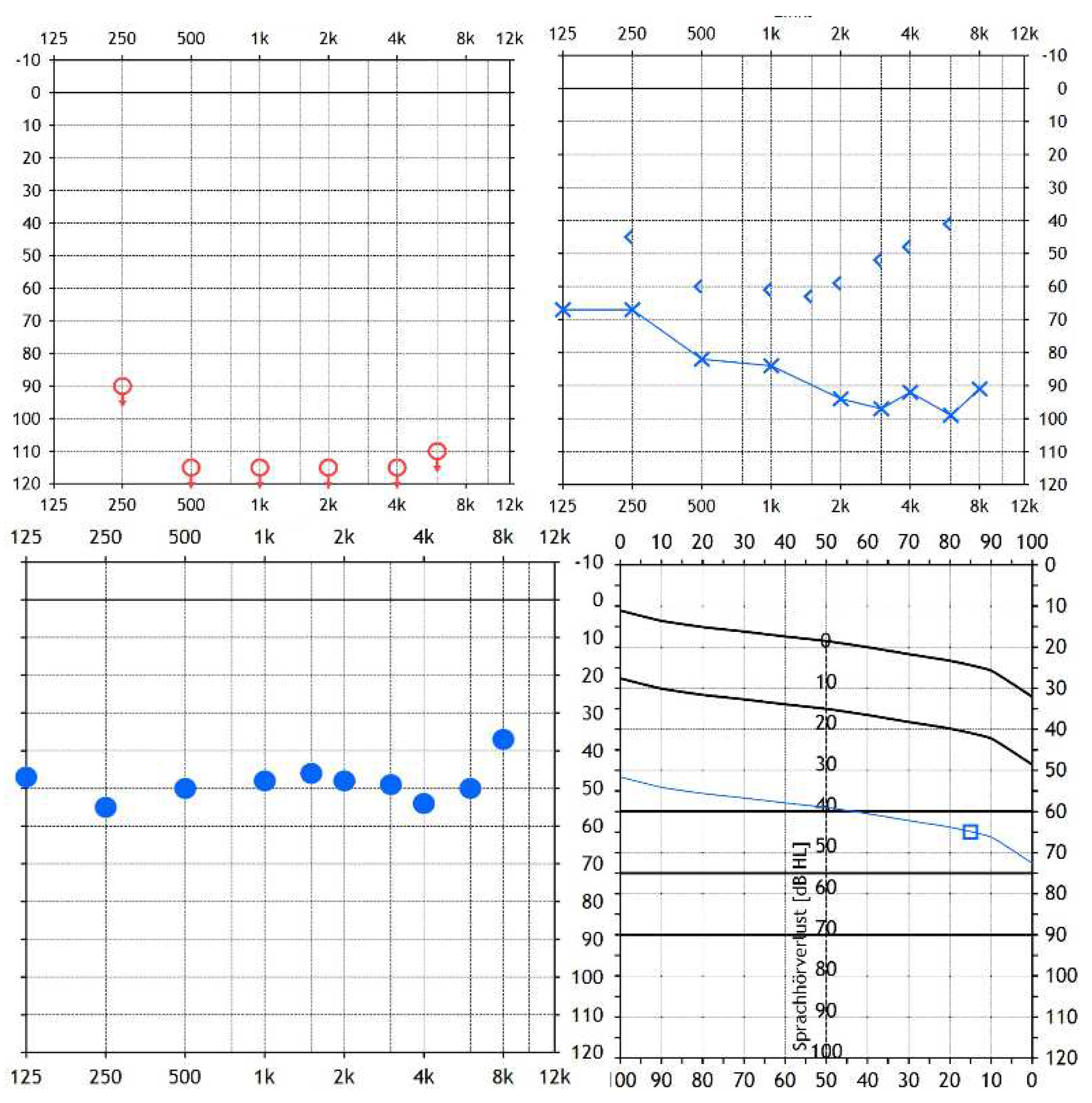
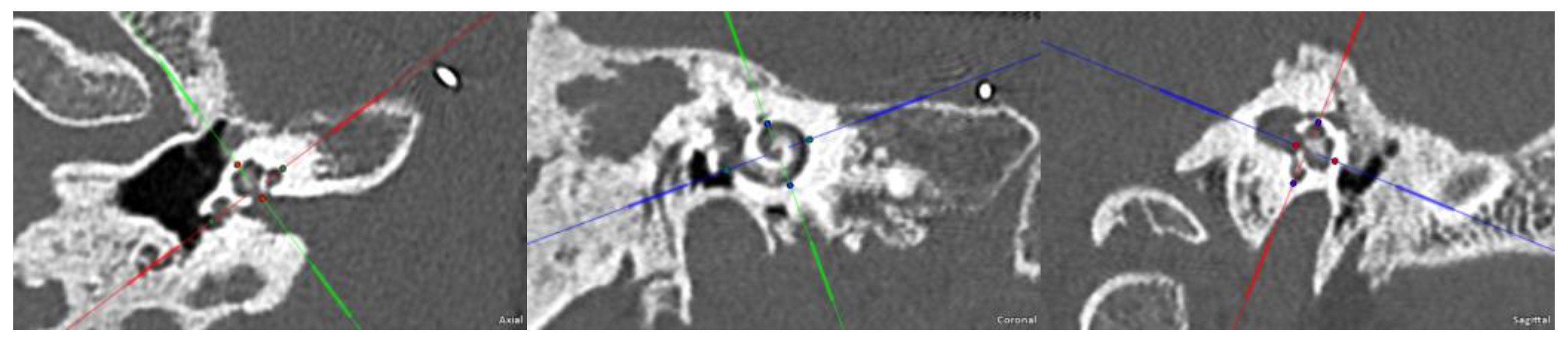
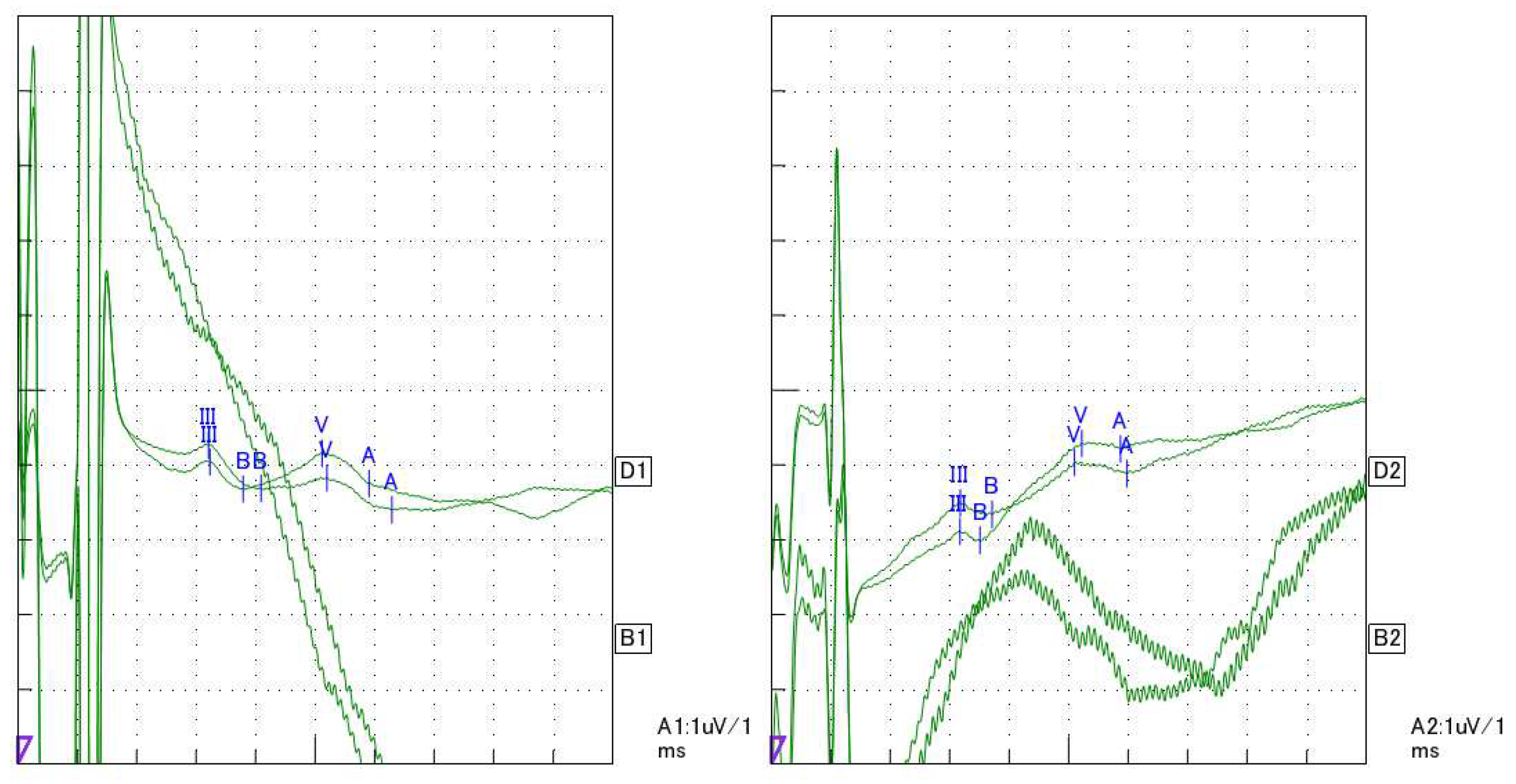
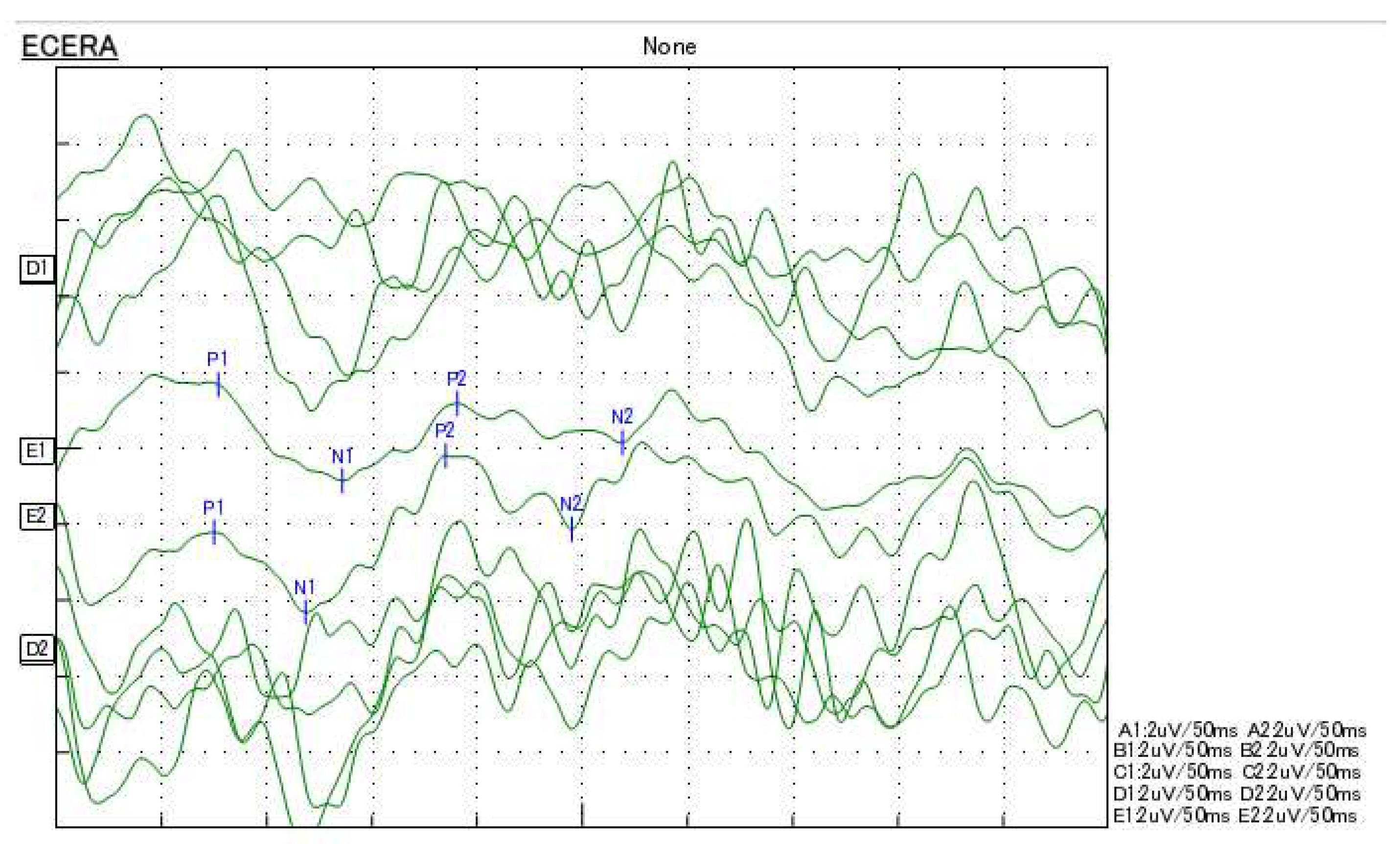
2. Case Presentation
3. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hochmair, I., Nopp, P., Jolly, C., Schmidt, M., Schößer, H., Garnham, C., & Anderson, I. (2006). MED-EL cochlear implants: state of the art and a glimpse into the future. Trends in amplification, 10(4), 201-219. [CrossRef]
- WHO (1991). Report of the Informal Working Group on Prevention of Deafness and Hearing Impairment Programme Planning, Geneva, 18-21 June 1991. URL: https://apps.who.int/iris/handle/10665/58839.
- Olusanya, B. O., Davis, A. C., & Hoffman, H. J. (2019). Hearing loss grades and the International classification of functioning, disability and health. Bulletin of the World Health Organization, 97(10), 725–728. [CrossRef]
- Zwolan, T. A., Kileny, P. R., Ashbaugh, C., & Telian, S. A. (1996). Patient performance with the Cochlear Corporation “20+ 2” implant: bipolar versus monopolar activation. Otology & Neurotology, 17(5), 717-723.
- Brown, Carolyn J.; Abbas, Paul J.; Gantz, Bruce J.. Preliminary Experience With Neural Response Telemetry in the Nucleus CI24M Cochlear Implant. The American Journal of Otology 19(3):p 320-327, May 1998.
- Lai, W, 1999. an NRT Cookbook: Guidelines for making 157 ™ measurements. Version 2.04.
- Zhu, Z., Tang, Q., Zeng, F. G., Guan, T., & Ye, D. (2012). Cochlear-implant spatial selectivity with monopolar, bipolar, and tripolar stimulation. Hearing research, 283(1-2), 45-58. [CrossRef]
- Mesnildrey, Q., & Macherey, O. (2015). Simulating the dual-peak excitation pattern produced by bipolar stimulation of a cochlear implant: effects on speech intelligibility. Hearing Research, 319, 32-47. [CrossRef]
- Wesarg, T., Arndt, S., Aschendorff, A., Laszig, R., & Zirn, S. (2014). Intraoperative audiological-technical diagnostics during cochlear implant surgery. HNO, 62(10), 725-734. [CrossRef]
- Parreño M, Di Lella FA, Fernandez F, Boccio CM and Ausili SA (2020) Toward Self-Measures in Cochlear Implants: Daily and “Homemade” Impedance Assessment. Front. Digit. Health 2:582562. [CrossRef]
- Weiss, B. G., Söchting, F., Bertlich, M., Busch, M., Blum, J., Ihler, F., & Canis, M. (2018). An objective method to determine the electrically evoked stapedius reflex threshold during cochlea implantation. Otology & Neurotology, 39(1), e5-e11. [CrossRef]
- Middlebrooks, J. C. (2015). Auditory system: Central pathways. [CrossRef]
- Spiegel, J. L., Polterauer, D., Hempel, J. M., Canis, M., Spiro, J. E., & Müller, J. (2022). Variation of the cochlear anatomy and cochlea duct length: analysis with a new tablet-based software. European Archives of Oto-rhino-laryngology, 279(4), 1851-1861. [CrossRef]
- Müller J. & Schön F. (1994). Category Scaling of Loudness in Cochlear Implant Patient Evaluation. Laryngorhinootologie 1994; 73(3): 128-131. [CrossRef]
- Cochlear Limited (2006). NIC V2 SOFTWARE INTERFACE SPECIFICATION. E11318RD Issue: 1.
- Polterauer, D., Mandruzzato, G., Neuling, M., Polak, M., Müller, J., & Hempel, J. M. (2022a). Evaluation of auditory pathway excitability using a pre-operative trans-tympanic electrically evoked auditory brainstem response under local anesthesia in cochlear implant candidates. International Journal of Audiology, 1-11. [CrossRef]
- Polterauer, D. Intraoperative and postoperative measurement of brainstem responses through electrical stimulation of the auditory nerve via implantable neurostimulators eABR by PATH MEDICAL SENTIERO ADVANCED. [CrossRef]
- Leake, P. A., Hradek, G. T., Bonham, B. H. & Snyder, R. L., 2008. Topography of auditory nerve projections to the cochlear nucleus in cats after neonatal deafness and electrical stimulation by a cochlear implant. Journal of the Association for Research in Otolaryngology, 9(3), pp. 349-372. [CrossRef]
- Lammers, M. J. et al., 2015. Delayed auditory brainstem responses in prelingually deaf and late implanted cochlear implant users. Journal of the Association for Research in Otolaryngology, Jul, Band 16, pp. 669-678. [CrossRef]
- Minami, S. B. et al., 2015. Usefulness of measuring electrically evoked auditory brainstem responses in children with inner ear malformations during cochlear implantation. Acta Oto-Laryngologica, 135(10), pp. 1007-1015. [CrossRef]
- Firszt, J. B., Chambers, R. D. & Kraus, N., 2002. Neurophysiology of cochlear implant users II: comparison among speech perception, dynamic range, and physiological measures. Ear Hear, 12, Band 23, pp. 516-531.
- Baljić, I., Müller, A., Fröhlich, L., Polterauer, D. & Dziemba, O. (2021). Elektrisch evozierte Potentiale des auditorischen Systems - Teil 2. Zeitschrift für Audiologie (Audiological Acoustics) 60(2):71-75.
- Polterauer, D., Mandruzzato, G., Neuling, M., Polak, M., Müller, J., & Hempel, J. M. (2022b). LA-TT-EALR/PromCERA: Comparison of preoperatively performed electrically evoked auditory potentials at the brainstem and cortical level during local anesthesia. Current Directions in Biomedical Engineering, 8(2), 233-236. [CrossRef]
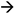 “Audio Processor”, licensed CC 4.0].
“Audio Processor”, licensed CC 4.0].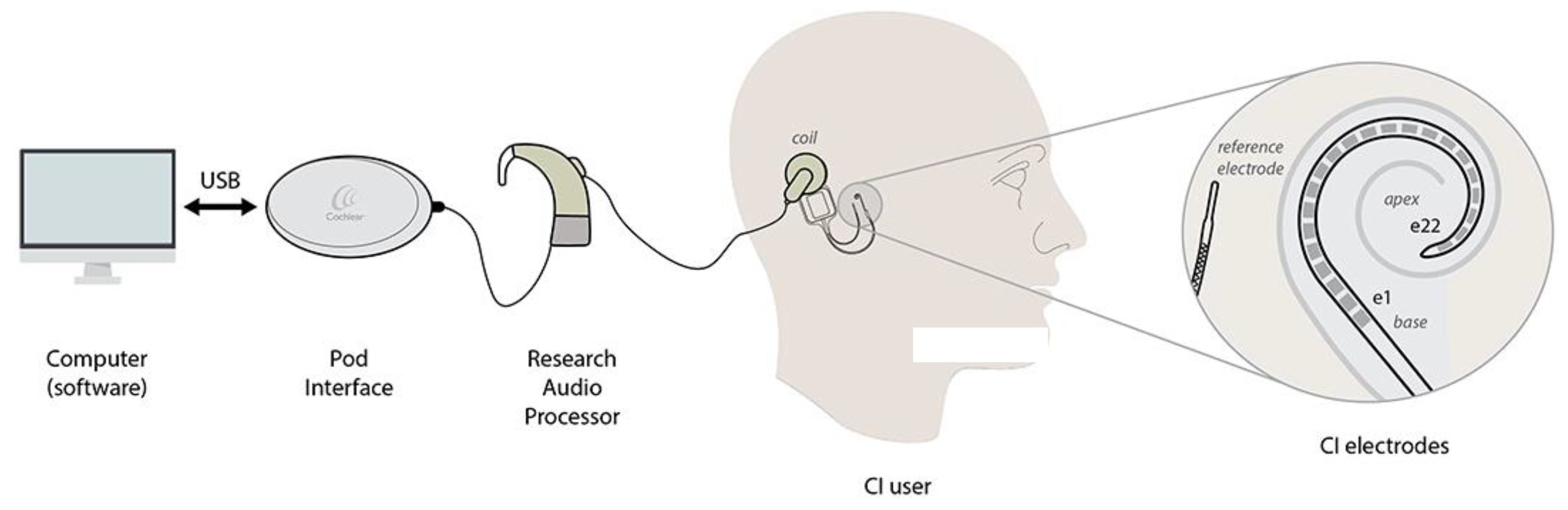
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




