2. Materials and Methods
The sports/medical product is aimed at an audience that requires a precise dosage of the protein substitutes administered. With my product I want to develop a compact, mobile and precise dosing solution for this substitute. The product aims to revolutionize the way substitutes are administered by providing a convenient and accessible delivery solution where beneficiaries are not limited by the circumstances of administration.
2.1. The background of the dispenser design
We have explored a number of concepts of these models, some of which are presented below.
The first dispenser examined was: for the preparation of beverages, mixtures by selectively introducing a predefined amount of an edible preparation and then mixing it in a closed housing, in a portable device with a predefined volume for drinkable liquid and which has a metering device for the introduction of the basic drinkable preparation. The system of the invention also provides a brewing element and a portable heating unit, wherein the heating unit fits into the beverage container with its heating element to heat the liquid [1].
The second was a smart medicine container that allows access to large quantities of pills, which are stored and then dispensed. The medication container has a control unit, a memory, a communication interface unit, a housing configured to provide large quantities of pills, a means for locking the supply of pills to block access to the housing supply, a dispensing assembly for dispensing pills from the large supply of pills and a dispensing device for already dispensed pills, a housing for receiving and storing the pills, a pill locking means for blocking access to the housing of dispensed pills. [2]
Another dispenser examined is a beverage dispenser that includes a control unit and a touchscreen interface for calibrating the amount of beverage dispensed. Inputs are made on the touchscreen to dispense drinks and make system settings. The control unit controls a variety of beverage dispensing fixtures based on the data entered on the touchscreen [3]
The last, a portable beverage dispenser, consists of two assemblies, one for dispensing and the other for storage.
2.2. The customer requirements
The most important requirements of the interviewees are listed in
Table 1.
2.3. The concepts innovated.
With these requirements in mind, we have designed this device for dosing protein powders and created several concepts, which we present below:
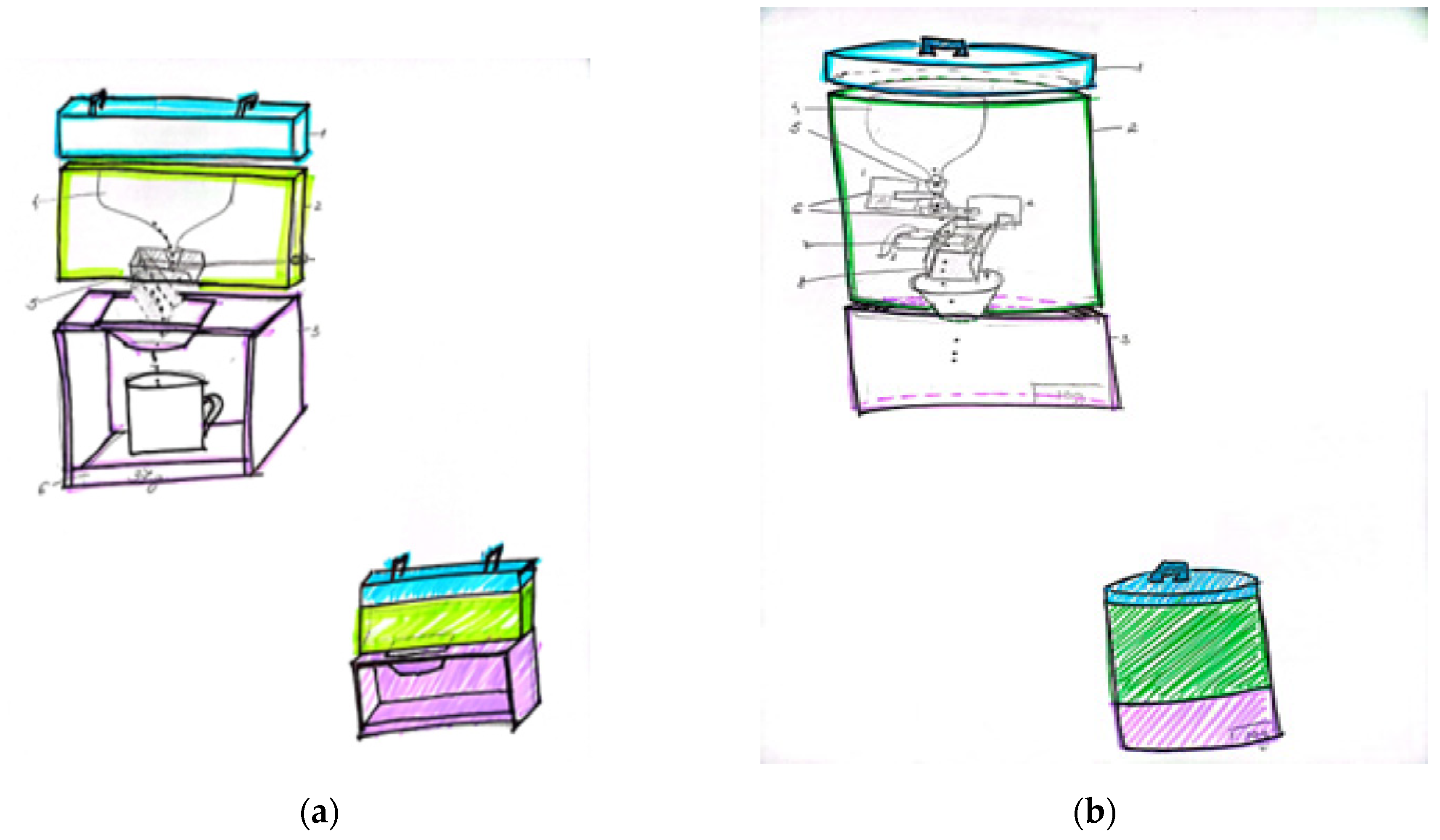
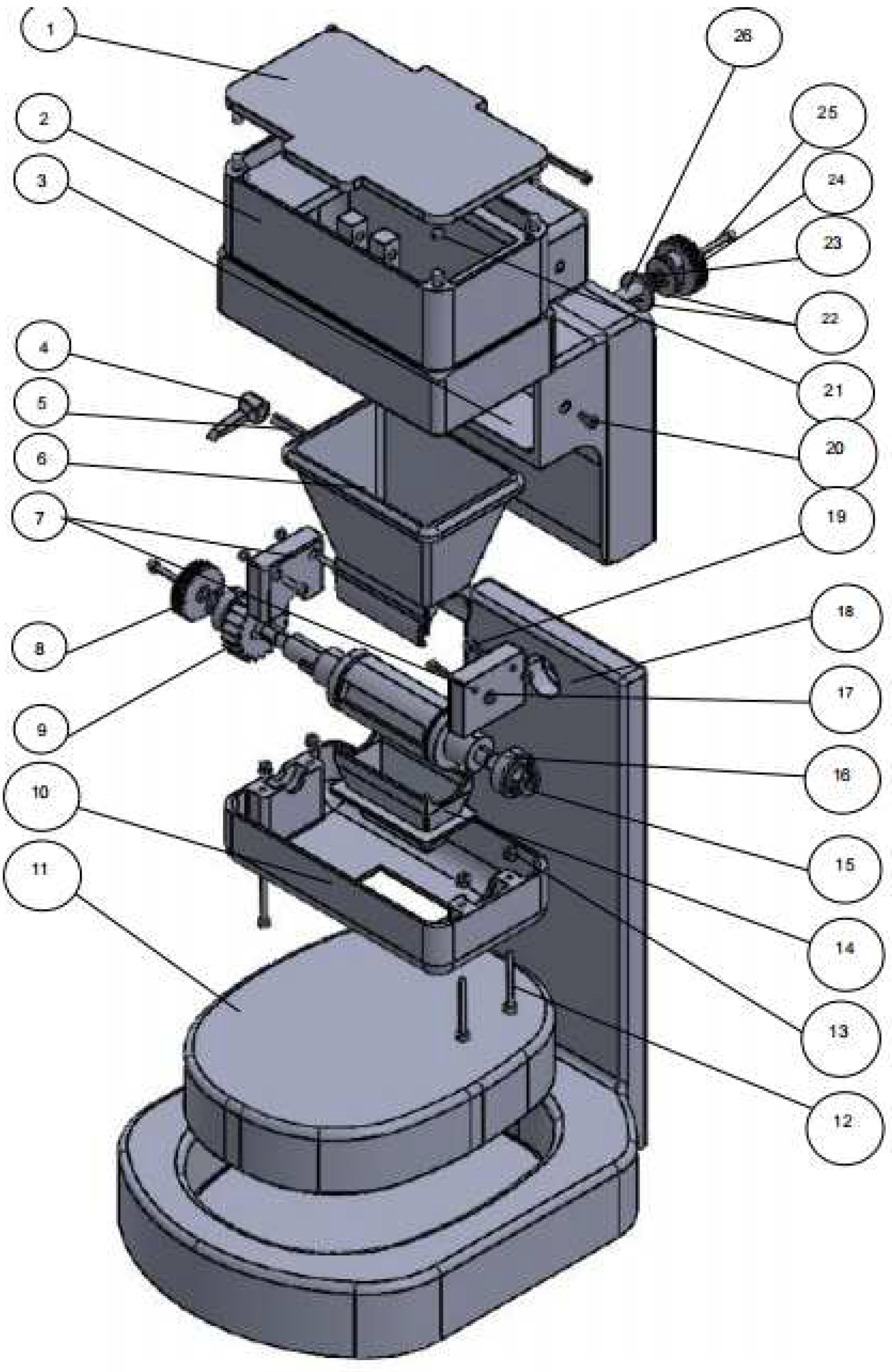
In
Figure 1a, the device consists of 3 parts:
1-lid, 2. upper housing, 4 into which 150 grams of protein powder is poured, 5 folding boxes into which 10 grams are dispensed, 7 button to lock/unlock the flow of protein powder, 3. lower housing, 6 a built-in scale.
We did not opt for this concept for the following reasons: High percentage of human error as the button has to be pulled 7, low accuracy due to errors, problems with folding the box 5. The box loaded with protein powder folds as soon as it is loaded with 10g. The biggest error is the loss of dust when folding the box.
Dosing the weight can only be done in 10 gram increments, so the dosed amount is always a multiple of 10 (e.g.: 10-20-30-40...). So if someone wants to dose 37 grams of protein powder, this is impossible.
The second concept (
Figure 1, (b)) consists of 3 parts: 1. the lid, 2. the housing of the dispenser
Where: 4 is the container for filling 100 grams of protein powder, 5 is a clamp-shaped funnel for a slower powder flow, 6 is the actuator1 for blocking/releasing the flow, 7 is the actuator2 for filling the powder from 5 to 5 grams, 8 is the loading cell, 9 is the guide drawer, 3. the dispenser housing. The container into which the desired amount is dosed 10 is a display to show the weight
This concept was not chosen for the following reasons:
- -
After dosing the amount of powder, the housing of the dispenser 2 is unscrewed from the container 3 together with its lid 1. As a result, the load cell is incorrectly calibrated each time it is unscrewed, which leads to precision errors.
- -
since the actuator 2 has 3 dust filling slots of 5 grams each, the dosing quantity will always be a multiple of the number 5 (e.g.: 5-10-15-20-25...). So if someone wants to dose 37 grams of protein powder, this is impossible.
- -
Loss of dust, resulting in low accuracy and incorrect dosing.
- -
The impossibility of dosing in a cup/glass/shaker.
The results
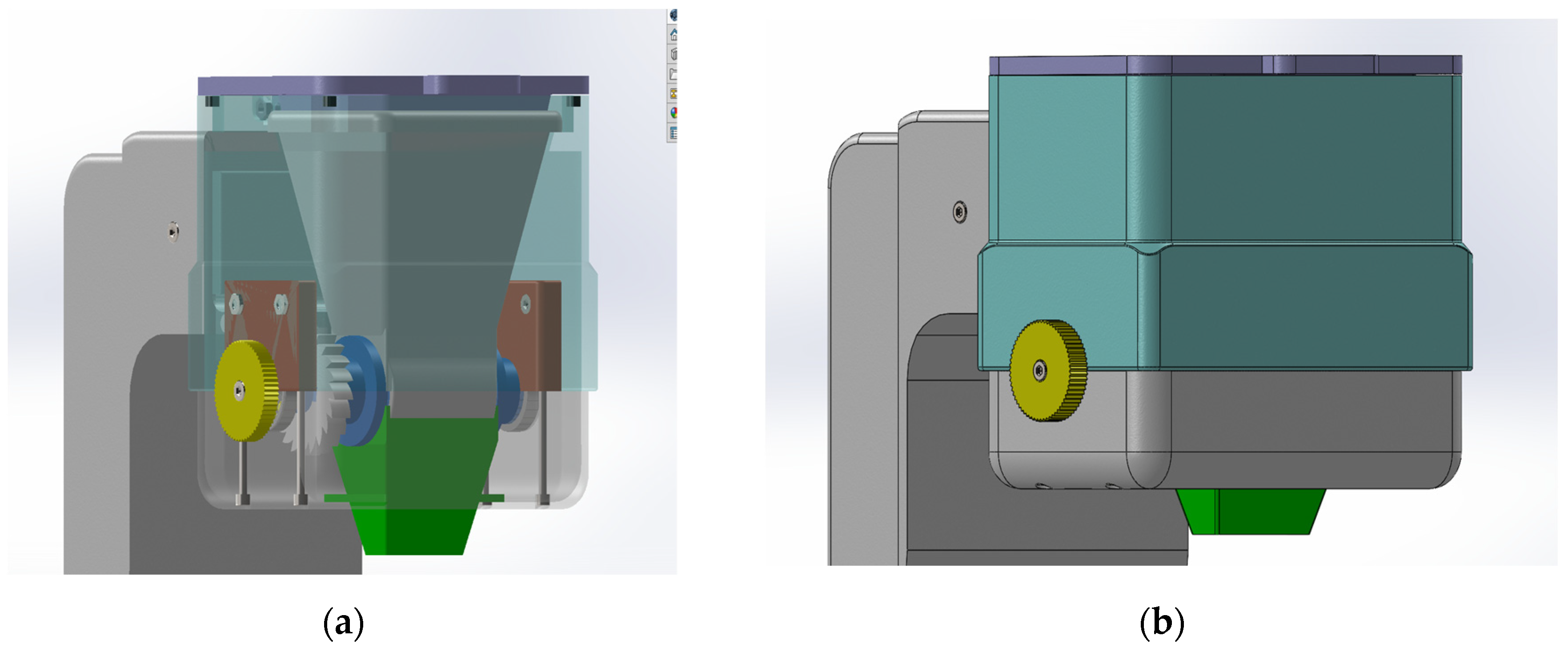
In
Figure 2 you can see the final concept that was developed to solve the problems discussed in the context of Concept 1 and Concept 2. The following problems were solved: human error; the probability of error has decreased significantly even though human error exists.
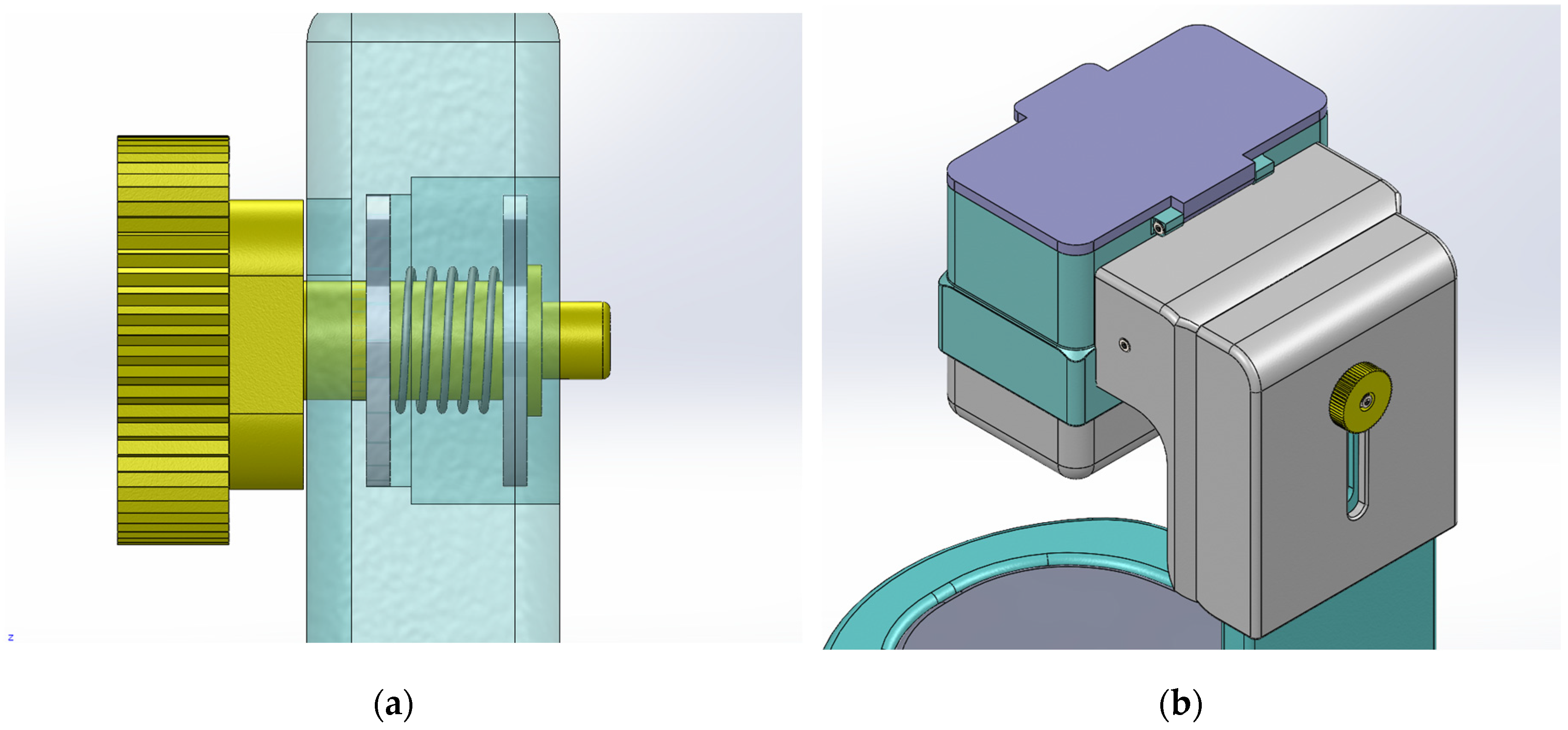
The solution to this problem was the development of a ratchet mechanism. The dosing of the customized amount is done by the user turning the wheel to the right; this movement of the wheel engages the shaft with the dosing system.
There is no risk of losing powder during dosing as the dosing cylinder is designed so that the protein powder container and the funnel form a closed space.
Containers with a maximum height of 20 cm can be used with the dispenser's height adjustment wheel.
Figure 4.
The upper part of the mechanism (a) The height adjustment mechanism (b) The upper part of the height adjustment system.
Figure 4.
The upper part of the mechanism (a) The height adjustment mechanism (b) The upper part of the height adjustment system.
Custom amounts can be dosed (eg 32, 47, 52) up to a maximum amount of 150 grams, as this is the capacity of the tank into which the powder is loaded.
The concept of the protein powder dosing device was designed in the SolidWorks 2022 program and the final assembly is composed of 63 elements, including nuts and bolts. The protein powder dosing device is ideal for both medical and sports use.Thanks to its innovative design, this high-quality device easily adapts to customer needs and offers numerous benefits.This dispenser is extremely versatile.
It fits perfectly with glasses of different sizes, as its height is adjustable.Thus, the beneficiaries no longer must worry that glasses of different sizes do not fit under the dispenser.The height of this dispenser adjusts according to the container used, which gives the user an easier handling of the device.The height of this device is adjusted to the current user's glass/bottle giving it a touch of comfort and practicality"
In the
Figure 5, can be seen all the device parts.
3.1. The mold size
Composite materials offer greater advantages than most other commonly used materials and continue to grow in importance each day. Composite materials are applicable across almost all domains, including the automotive, railway, aerospace, naval, electronics, medicinal, and civil construction industries. The widespread use of these materials generates a large amount of waste products from the technological processes of production or product removal. At present, the frequency of product removals has increased because of two main factors: faster development of new products and shorter product life cycles. Thus, it is critical to identify all possible recycling solutions for the waste management of composite-based products. At present, the main conventional recycling techniques for glass-fiber–reinforced polymer (GFRP) waste materials include incineration, thermal or chemical recycling, and mechanical recycling [8–10]. Mechanical recycling through grinding and milling processes is one of the most used techniques; additionally, due to the size reduction of the fibrous products, the recycling process itself does not contribute to atmospheric pollution and much simpler equipment is required compared with other methods. From this process, the resulting fibers can be incorporated into new composite materials [7].
Through the injection of the plastic material, a manufacturing process of the parts is obtained by melting the plastic materials in a matrix. Injection molding technology can be used to produce a wide range of plastic parts, from the largest, such as car bars and door panels, to the smallest.
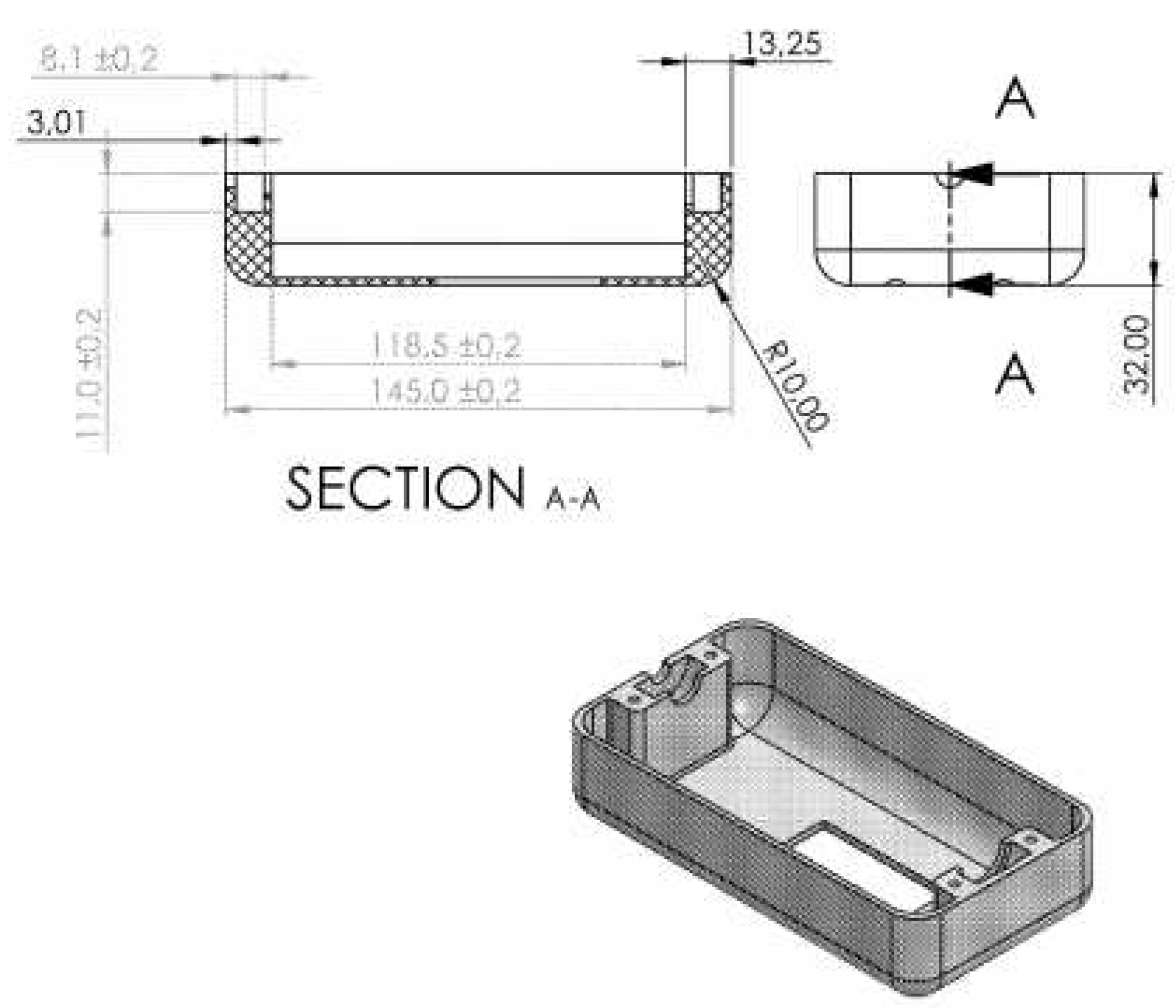
In the process of manufacturing each part by injection molding, calculations are made to determine the dimensions of the nest and mold core. These calculations are necessary to ensure the accurate assembly of components and to achieve quality results during the injection process. In this sense, we performed calculations for the dimension of the mold necessary for the manufacture of the dispenser.
The size is considered: 145 ± 0.2 mm.
where:
H is the size of the nest cavity
± Δ is the upper and lower nest size deviation.
h is the outer or inner dimension of the injection part
± 𝛿 is the upper- and lower-part size deviation.
Cmin=1% for PP
C max=2.8% for PP
C med=1.9%
It is obtained: 148 , from which. =2.2 [𝑚𝑚]
Sizing calculation number two:
The size is considered: 32±0.2
It is obtained: 33, din care =2.2 [𝑚𝑚]
Sizing calculation number three:
The size is considered: 8.1 mm
It is obtained: 8, din care = 0.54 [𝑚𝑚]
Sizing calculation number four:
The size is considered: 11 mm.
Se obține : 11, din care = 1.31 [𝑚𝑚]
The number of nests is calculated with the formula:
where:
-
G – The actual plasticizing capacity of the injection molding machine
[g/sec]
– The injection time [sec]
– the mass of the injected part or the correction factor from the table
The housing made of PP weighs 61.89 grams and the injection machine has the injection capacity of 122 g/sec. The injection time for a lower case is 2.702 seconds and the correction factor for case weight is 1.05.
Following calculation (13), n=1.40 → the mold will have only one nest.
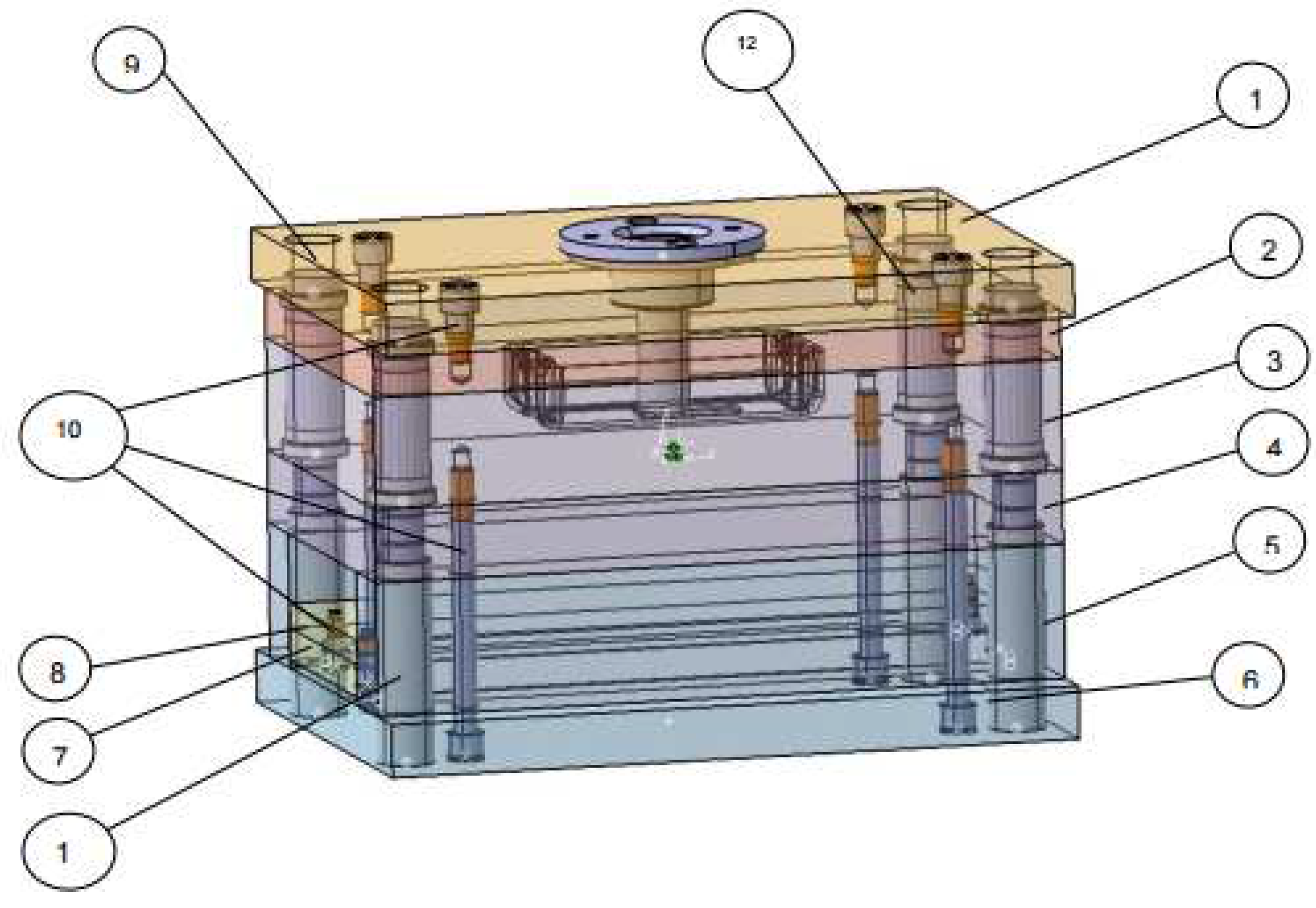
3.2. The mold design
The part mold, along with all its components including the core and nest, plays a critical role in the manufacturing process. The core shapes the features and structure within the mold, while the nest defines the outer shape and surface details. When the molten material is injected into the mold, it fills the space between the core and the nest, taking on the shape of the part model (see
Figure 6).
The mold consists of the following components: Fixing plate (1), Nest plate (2), Lower core package (3), Core support plate (4), Adjustment plate (6), Ejection plate (7), Ejection plate (8), along with the fasteners: Guide pins (9), Screws (10), Sleeve (11), Bushing (12). This arrangement is illustrated in
Figure 7 in lowercase.
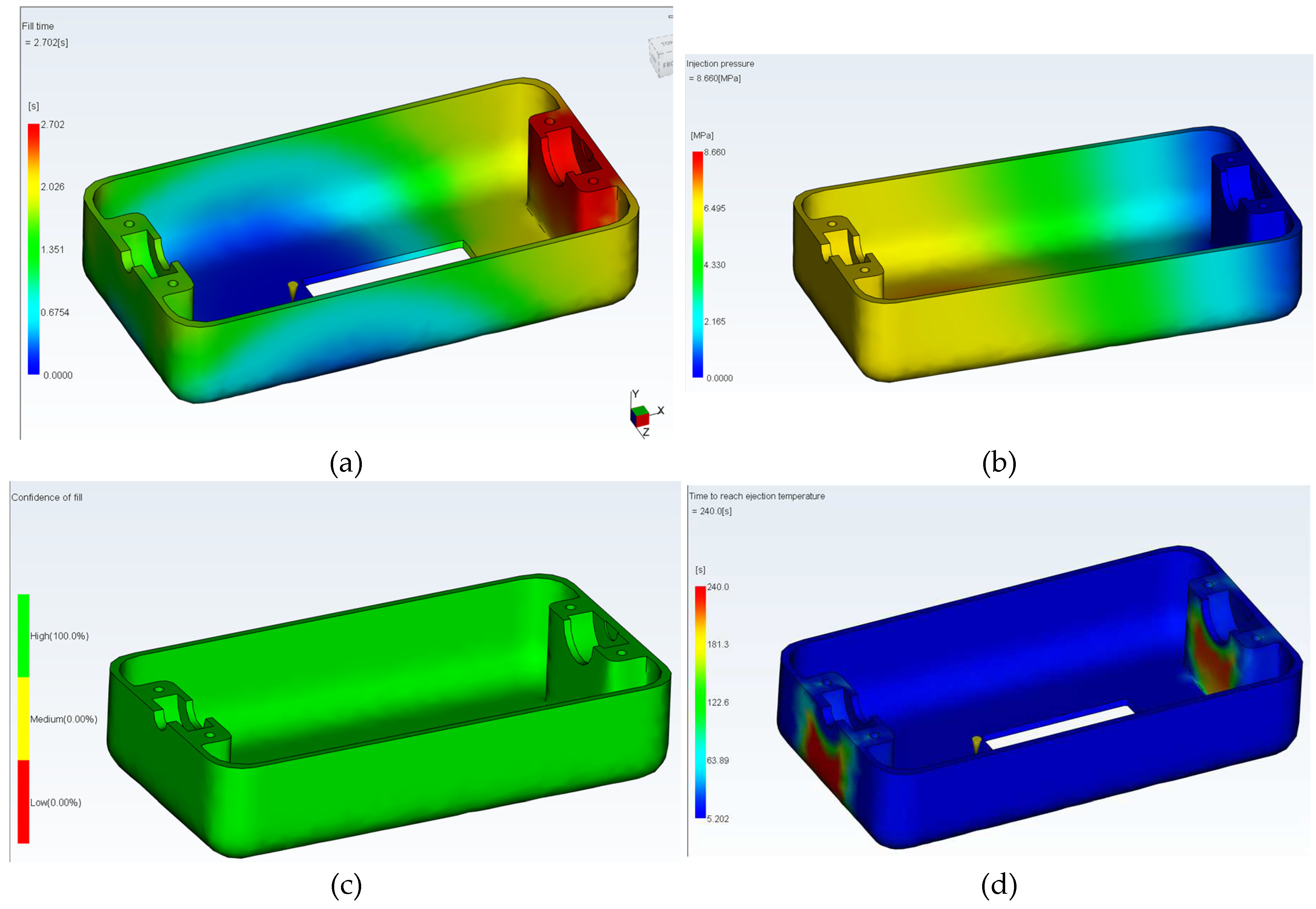
3.3. The simulation of mold filling
The filling of the mold in
Figure 4 was simulated using the MOLDFLOW program.
3.4. Final prototype in a different color: for use in medical environments and for use in sports environments.
Selecting the appropriate color for the product is crucial to significantly influence the recipient's perception. The sports/medical device targets a demographic that necessitates precise administration of protein substitutes. It is designed for patients with metabolic conditions that mandate dietary protein supplementation, enabling the accurate dispensing of additional grams.
Figure 9.
The simulation of the injection process includes: (a) Injection time (maximum 2.70 seconds) (b) Part injection pressure (maximum 8.65 MPa) (c) Quality of filling (d) Post-injection part cooling.
Figure 9.
The simulation of the injection process includes: (a) Injection time (maximum 2.70 seconds) (b) Part injection pressure (maximum 8.65 MPa) (c) Quality of filling (d) Post-injection part cooling.
Figure 10.
The ultimate model: (a) medical use dispenser (b) sports environment dispenser.
Figure 10.
The ultimate model: (a) medical use dispenser (b) sports environment dispenser.
The combination of blue and white colors is specific to the medical/pharmaceutical field because these colors have a strong contrast and products with these colors are easy to identify. White color is often identified with hygiene due to its appearance; Blue is associated with calmness, tranquility, and relaxation. This is very important for a recipient who is anxious or stressed, because blue can help create a more pleasant atmosphere. Together these colors not only provide a pleasant and calm contrast, but these colors can create a pleasant image of a clean and sterile environment. Often in training rooms, interior designers choose the dominant color green. Green is a vibrant and strong color, which stimulates energy. Apart from this, green is a color that helps concentration and is often compared to mental clarity, because it is often associated with nature, freshness, and balance. Black is the basic color when it comes to elegance. Its combination with green not only gives an air of elegance and energy, but also creates a sophisticated atmosphere stimulating a sense of professionalism and calmness. The combination of green and black is a strong and vibrant combination.