Submitted:
13 December 2023
Posted:
14 December 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Histological methods
2.3. An immunohistochemical method for visualization of choline acetyltransferase and tryptase reactive structures
2.4. Statistical analysis
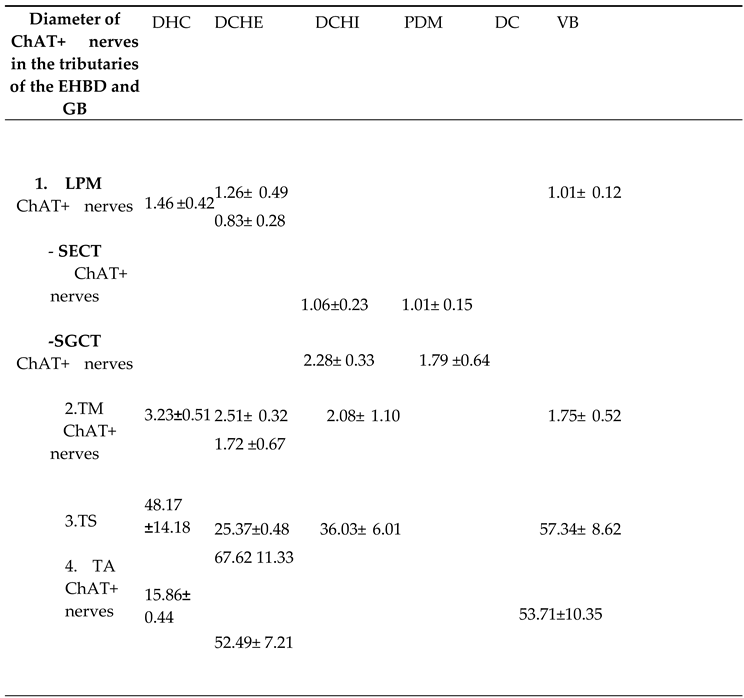
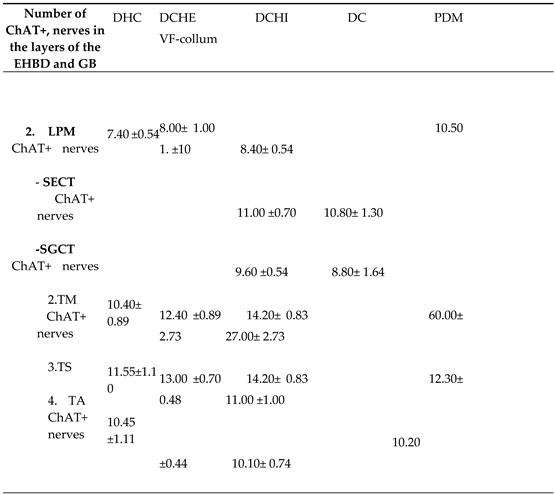
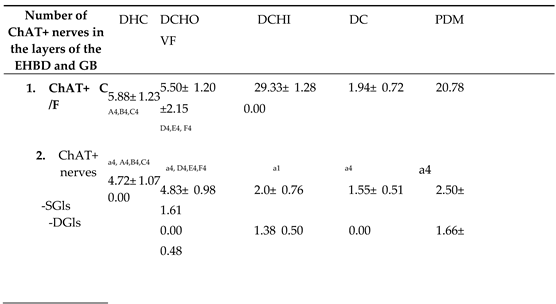
3. Results
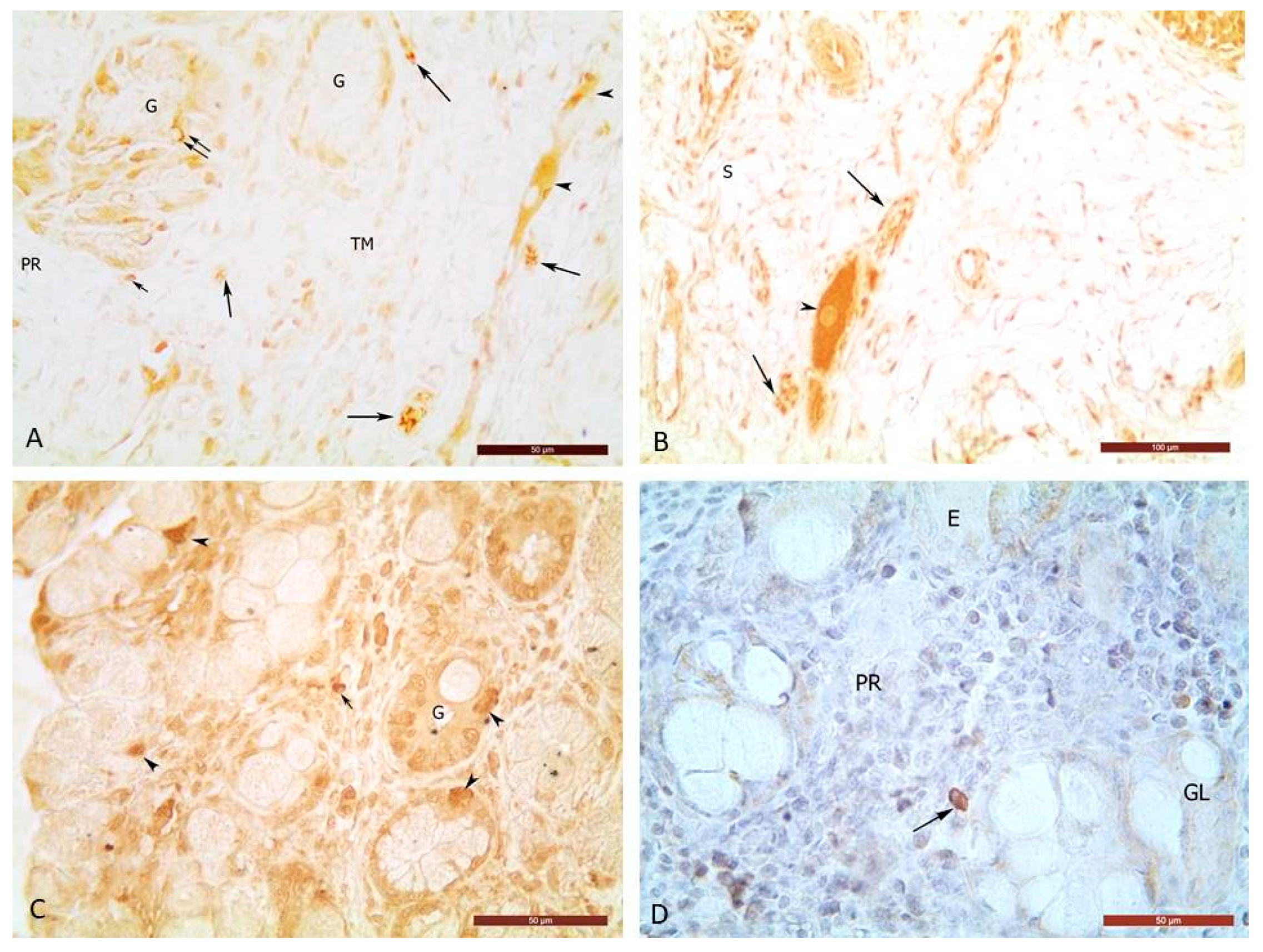
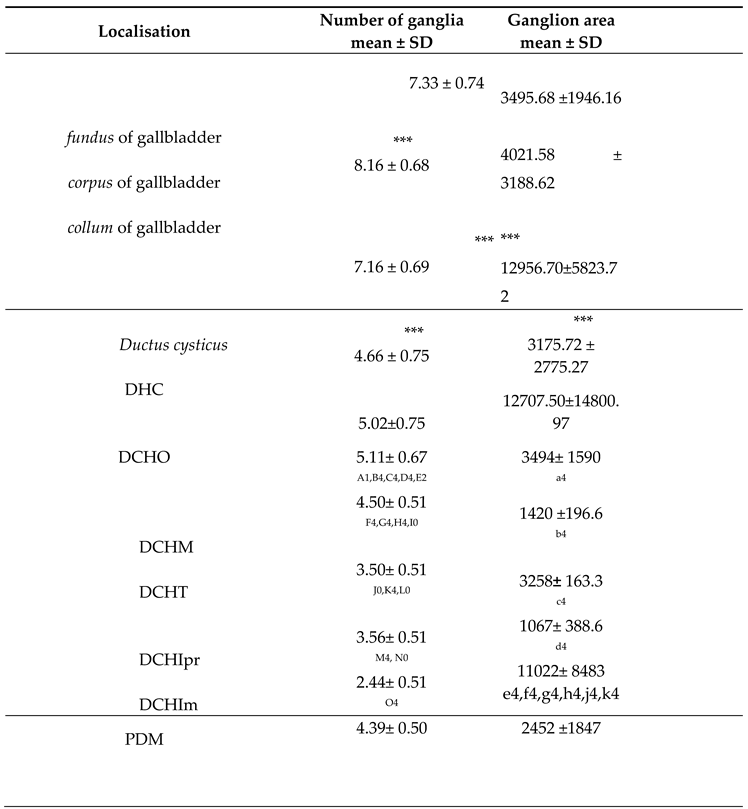
3.1. Choline acetyltransferase positive nerve structures
3.2. Choline acetyltransferase positive glandular cells (ChAT+Cs)
3.3. Immunohistochemical localization of ChAT+ mast cells in GB, EHBD and PDM
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kurzen, H.; Wessler, I.; Kirkpatrick, C.J.; Kawashima, K.; Grando, S.A. The non-neuronal cholinergic system of human skin. Horm Metab Res 2007, 39, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Lips, K.S.; Luhrmann, A.; Tscherni, T.; Stoeger, T.; Alessandrini, F.; Grau, V.; et al. Down-regulation of the non-neuronal acetylcholine synthesis and release machinery in acute allergic airway inflammation of rat and mouse. Life Sci 2007, 80, 2263–2269. [Google Scholar] [CrossRef] [PubMed]
- Wessler, I.; Kirkpatrick, C.J. Acetylcholine beyond neurons: the non-neuronal cholinergic system in humans. Br J Pharmacol 2008, 154, 1558–1571. [Google Scholar] [CrossRef]
- Wessler, I.; Panter, H.; Bittinger, F.; Kriegsmann, J.; Kirkpatrick, C.J.; Kawashima, K.; et al. Subcellular location of choline acetyltransferase (ChAT) and acetylcholine (ACh) in human placenta. Naunyn Schmiedebergs Arch Pharmacol (Suppl) 2001, 363, R23. [Google Scholar]
- Talmage, E.K.; Pouliot, W.A.; Schemann, M.; Mawe, G.M. Structure and chemical coding of human, canine and opossum gallbladder ganglia. Cell Tissue Res 1996, 284, 289–302. [Google Scholar] [CrossRef] [PubMed]
- Mawe, G.M.; Ellis, L.M. Chemical coding of intrinsic and extrinsic nerves in the guinea pig gallbladder: distributions of PACAP and orphanin FQ. Anat Rec 2001, 262, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Mawe, G.M. Neurobiology of the gallbladder and sphincter of Oddi. In Neurogastroenterology: from the basics to the clinics.H.J. Krammer. M.V. Singer, (eds). New York: Kluwer Academic Publishers and Falk Foundation. 2000; pp. 288–302.
- Talmage, E.K.; Pouliot, W.A.; Cornbrooks, E.B.; Mawe, G.M. Transmitter diversity in ganglion cells of the guinea pig gallbladder: an immunohistochemical study. J Comp Neurol. 1992, 317, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Talmage, E.K.; Mawe, G.M. NADPH-diaphorase and VIP are colocalized in neurons of gallbladder ganglia. J Auton Nerv Syst 1993, 4, 83–99. [Google Scholar] [CrossRef] [PubMed]
- Mawe, G.; Gershon, MD. Structure, afferent innervation, and transmitter content of ganglia of the guinea pig gallbladder: Relationship to the enteric nervous system. J Comp Neurol 1989, 283, 374–390. [Google Scholar] [CrossRef] [PubMed]
- Wells, D.G.; Talmage, E.K.; Mawe, G.M. Immunohistochemical identification of neurons in ganglia of the guinea pig sphincter of Oddi. J Comp Neurol. 1995, 352, 106–116. [Google Scholar] [CrossRef]
- O’Donnell AM, Ellis LM, Riedl MS, Elde RP, Mawe, GM. Distribution and chemical coding of orphanin FQ/nociceptin-immunoreactive neurons in the myenteric plexus of guinea pig intestines and sphincter of Oddi. J Comp Neurol 2001, 430, 1–11. [CrossRef]
- Dahlstrand, C.; Theodorsson, E.; Dahlstfm .;, Ahlman, H. VIP antisera inhibit the relaxatory motor responses of the feline sphincter of Oddi and gall-bladder induced by VIP or vagal nerve stimulation. Acta Physiol Scand 1989, 137, 375–378. [CrossRef] [PubMed]
- Boyden, E.A. The sphincter of Oddi in man and certain representative mammals. Surgery 1937, 1, 25–37. [Google Scholar] [CrossRef]
- Ritter, U. Autonomous nervous system and bile ducts. Leber Magen Darm 1979, 9, 81–4. [Google Scholar] [PubMed]
- Puzikov, A.M; Lychkova, A.E. Nervous regulation of biliary tract motility. Eksp Klin Gastroenterol 2016, (7), 62–5. [Google Scholar]
- Mizuno, K.; Ueno, Y. Autonomic nervous system and the liver. Hepatol Res. 2017, 47, 160–16. [Google Scholar] [CrossRef] [PubMed]
- Fava, G.; Marzioni, M.; Francis, H.; Glaser, S.; Demorrrow, S.; Ueno, Y.; et al. Novel interaction of bile acid and neural signaling in the regulation of cholangiocyte function. Hepatol Res 2007, 37 (Supp. 1), S420–429. [Google Scholar] [CrossRef] [PubMed]
- LeSage, G.; Glaser S, Alpini G. Regulatory mechanisms of ductal bile secretion. Dig Liver Dis 2000, 32, 563–566. [CrossRef] [PubMed]
- Acevedo, M. Effect of acetylcholine on ion transport in sheep tracheal epithelium. Pflugers Arch. 1994, 427, 543–546. [Google Scholar] [CrossRef] [PubMed]
- Hirota, C.L.; McKay, D.M. Cholinergic regulation of epithelial ion transport in the mammalian intestine. Br J Pharmacol. 2006, 149, 463–479. [Google Scholar] [CrossRef]
- Kawashima, K.; Yoshikawa, K.; Fujii, Y.X.; Moriwaki, Y.; Misawa, H. Expression and function of genes encoding cholinergic components in murine immune cells. Life Sci 2007, 80, 2314–2319. [Google Scholar] [CrossRef]
- Borovikova, L.V.; Ivanova, S.; Zhang, M.; Yang, H.; Botchkina, G.I.; Watkins, L.R.; et al. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature 2000, 405, 458–462. [Google Scholar] [CrossRef] [PubMed]
- Miao, F.J.; Ja¨nig, W.; Levine, J.D. Vagal branches involved in inhibition of bradykinin-induced synovial plasma extravasation by intrathecal nicotine and noxious stimulation in the rat. J Physiol 1997, 498, 473–481. [Google Scholar] [CrossRef] [PubMed]
- Hagforsen, E.; Einarsson, A.; Aronsson, F.; Nordlind, K.; Michaelsson, G. The distribution of choline acetyltransferase- and acetylcholinester-ase-like immunoreactivity in the palmar skin of patients with palmoplantar pustulosis. Br J Dermatol 2000, 142, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Nechushtan H, Soreq H, Kuperstein V, Tshori S, Razin E. Murine and human mast cell express acetylcholinesterase. FEBS Lett 1996, 379, 1–6. [CrossRef] [PubMed]
- Sudheer, P.S.; Hall, J.E;, Donev, R., Rea; G., Rowbottom, A.; Williams, P.E. Nicotinic acetylcholine receptors on basophils and mast cells. Anaesthesia 2006, 61, 1170–1174. [CrossRef] [PubMed]
- Masini, E.; Fantozzi, R.; Blandina, P.; Brunelleschi, S.; Mannaioni, P.F. Muscarinic cholinergic receptor binding in rat mast cells. Agents Actions 1983, 13, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Jones, S.V.P.; Choi, O.H.; Beaven, M.A. Carbachol induces secretion in a mast cell line (RBL-2H3) transfected with the ml muscarinic receptor gene. FEBS Lett. 1991, 289, 47–50. [Google Scholar] [CrossRef] [PubMed]
- Reinheimer, T.; Mohlig, T.; Zimmermann, S.; Hohle, K.D.; Wessler, I. Muscarinic control of histamine release from airways. Inhibitory M1-receptors in human bronchi but absence in rat trachea. Am J Respir Crit Care Med 2000, 162, 534–538. [Google Scholar] [CrossRef] [PubMed]
- Gulubova, M.V.; Vlaykova, T.I. Mast cells in human bile duct obstruction. J Mol Histol 2004, 35, 791–801. [Google Scholar] [CrossRef]
- Lindor, K.D.; Gershwin, M.E.; Poupon, R.; Kaplan, M.; Bergasa, N.V.; Heathcote, E.J. Primary biliary cirrhosis. Hepatology 2009, 50, 291–308. [Google Scholar] [CrossRef]
- Grizzi, F.; Caro, G.D.; Laghi, L.; Hermonat, P; Mazzola, P.; Nguyen, D.D. et al. Mast cells and the liver aging process. Immun Ageing 2013, 10, 9. [CrossRef]
- Glaser, S.S.; Gaudio, E.; Miller, T.; Alvaro, D.; Alpini, G. Cholangiocyte proliferation and liver fibrosis. Expert Rev Mol Med 2009, 11, e7. [Google Scholar] [CrossRef] [PubMed]
- Racanelli, V.; Rehermann, B. The liver as an immunological organ. Hepatology 2006, 43, S54–62. [Google Scholar] [CrossRef]
- Rau, B.; Friesen, C.A.; Daniel, J.F.; Qadeer, A.; You-Li, D.; Robert, C.C.; et al. Gallbladder wall inflammatory cells in pediatric patients with biliary dyskinesia and cholelithiasis: a pilot study. J Pediatr Surg 2006, 41, 1545–1548. [Google Scholar] [CrossRef]
- Jennings, L.J.; Salido, G.; Pozo, M.; Davison, J;, Sharkey, K.; Lea, R. et al. The source and action of histamine in the isolated guinea-pig gallbladder. Inflamm Res. 1995, 44, 447–453. [CrossRef] [PubMed]
- Seeger, J.; Stoffel, M.; Simoence, P. Nomina Histologica Veterinaria, submitted by The International Committee on Veterinary Histological Nomenclature (ICVHM) to the World Association of Veterinary Anatomists. ( 2017, submitted.
- Meedeniya, A.C.; Schloithe, A.C.; Toouli, J.; Saccon, G.T. Characterization of the intrinsic and extrinsic innervation of the gall bladder epithelium in the Australian brush-tailed possum (Trichosurus vulpecula). Neurogastroenterol Motil. 2003, 15, 383–392. [Google Scholar] [CrossRef]
- Alexander, W.F. The innervation of the biliary system. J Comp Neurol 1940, 72, 357–370. [Google Scholar] [CrossRef]
- Powley, T.L.; Baronowsky, E.A.; Gilbert, J.M.; Hudson, C.N.; Martin, F.N.; Mason, J.K. , et al. Vagal afferent innervation of the lower esophageal sphincter. Auton Neurosci 2013, 177, 129–142. [Google Scholar] [CrossRef]
- Cai, W.; Gabella, G. Innervation of the gallbladder and biliary pathways in the guinea pig. J Anat 1983, 136, 97–109. [Google Scholar]
- Baumgarten, H.G.; Lange, W. Extrinsic adrenergic innervation of the extrahepatic biliary system in guinea-pigs, cats and rhesus monkeys. Z Zellforsch Mik Ana 1969, 100, 606–615. [Google Scholar] [CrossRef] [PubMed]
- Mawe, G.M. Nerves and Hormones Interact to Control Gallbladder Function. News Physiol Sci 1998, 13, 84–90. [Google Scholar] [CrossRef]
- Gonda, T. , Akiyoshi, H., Ichihara, K. Scanning electron microscopic observations of nerves in the guinea-pig gallbladder after an acetylcholinesterase histochemistry. J Smooth Muscle Res, 1995, 31, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Zanchi, A.; Reidy, J.; Feldma, H.J.; Qualter, J.; Gouw, A.S.; Osbeck, J.; et al. Innervation of the proximal human biliary tree. Virchows Arch, 2020, 477, 385–392. [Google Scholar] [CrossRef]
- Gershon, MD. Nerves, reflexes, and the enteric nervous system: pathogenesis of the irritable bowel syndrome. J. Clin. Gastroenterol, 2005, 39, S184–S193. [Google Scholar] [CrossRef] [PubMed]
- Bioulac-Sage, P.; Lafon, M.E.; Saric, J.; Balabaud, C. Nerves and perisinusoidal cells in human liver. J Hepatol 1990, 10, 105–112. [Google Scholar] [CrossRef] [PubMed]
- McMurray, G; Chris, S.; Johnston, C.F.; Halton, D.W. Choline acetyltransferase (ChAT) immunoreactivity in a sub-population of mammalian intestinal endocrine cells. Comp. Biochem Physiol C Pharmacol Toxicol Endocrinol 1993, 106, 509–515. [CrossRef] [PubMed]
- Pearse, A,G,E. Histochemistry, 3rd edn, Edinburgh, London: Churchill Livingstone 1968, pp. 70–76.
- Furness, J.B.; Bornstein, J.C.; Smith, T.K.; Murphy, R.; Pompolo, S. Correlated functional and structural analysis of enteric neural circuits. Arch Histol Cytol 1989, 52S, 161. [Google Scholar] [CrossRef]
- Nakamura, A.; Yamazaki, K.; Suzuki, K.; Sato, S. Increased portal tract infiltration of mast cells and eosinophils in primary biliary cirrhosis. Am J Gastroenterol 1997, 92, 2245–2249. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




