Submitted:
13 December 2023
Posted:
14 December 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Raw materials
2.2. Mix design and sample preparation
2.3. Methods
2.3.1. Setting time and consistency
2.3.2. Slump flow, V-funnel and L-box
2.3.3. Yield stress
2.3.4. Compressive strength
2.3.5. Bulk density
3. Results and discussion
3.1. Characterization of aloe vera mucilage
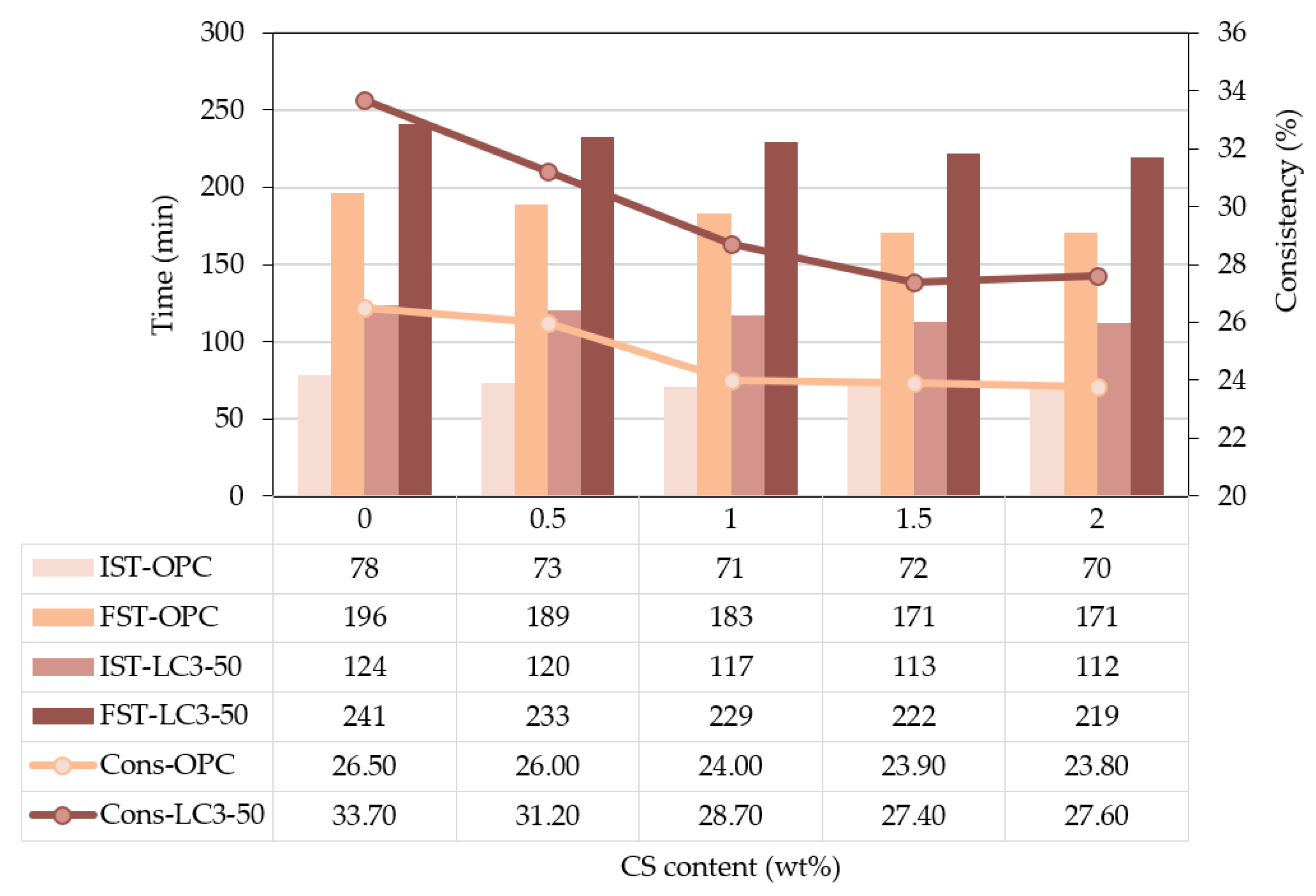
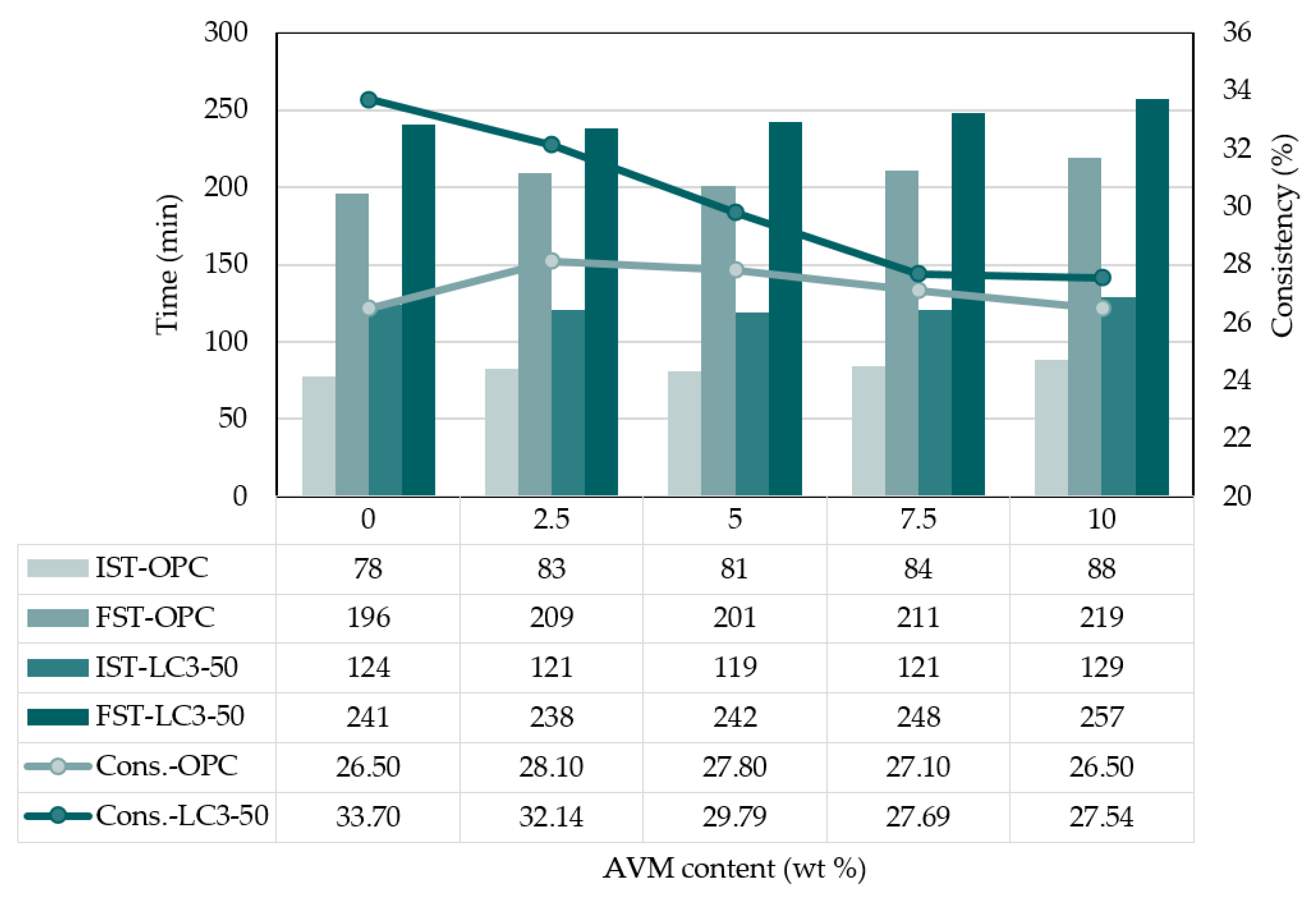
3.2. Setting time and consistency
3.3. Slump flow test, V-funnel test, and L-box test
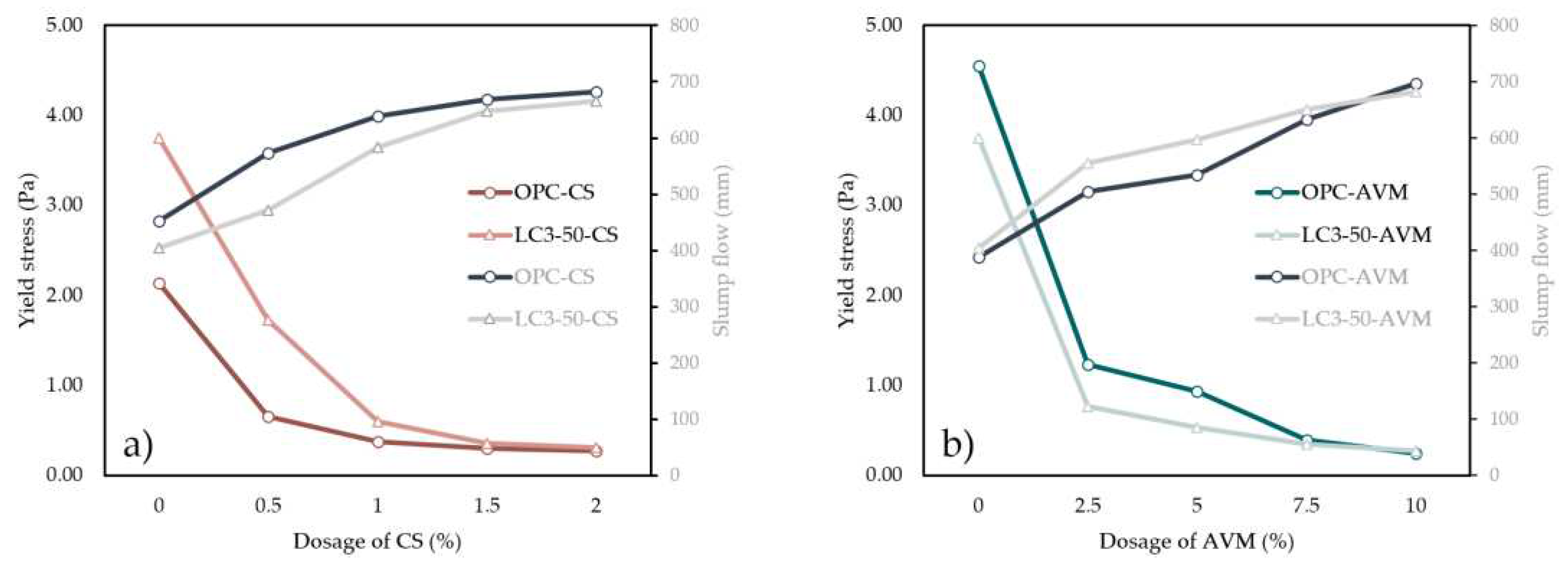
3.4. Yield stress
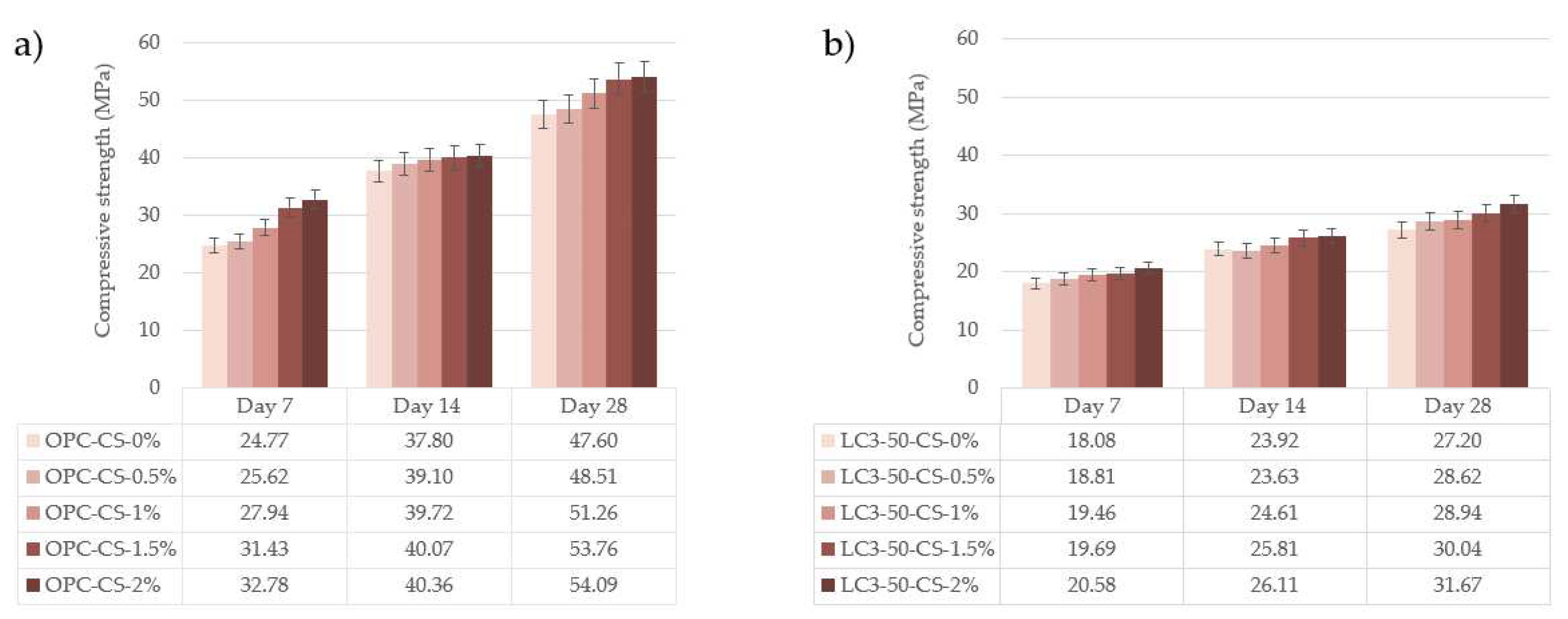
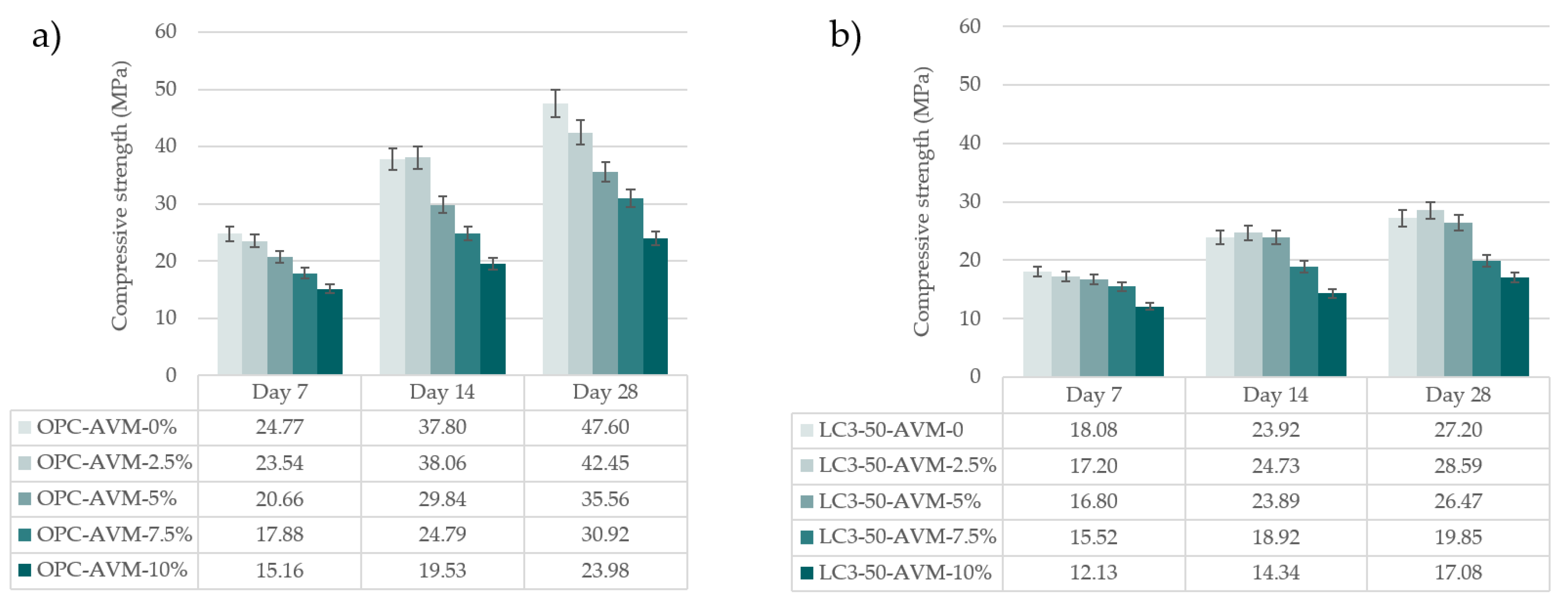
3.5. Compressive strength
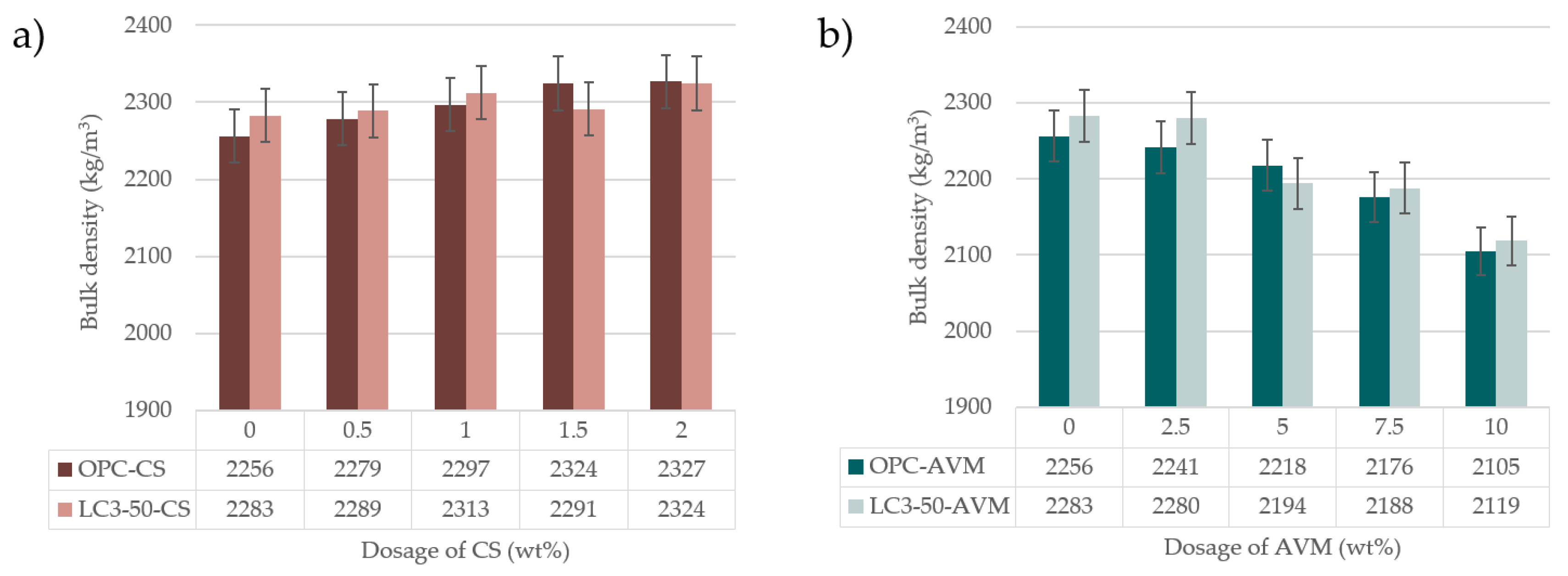
3.6. Bulk density
4. Conclusions
- In the study, the setting time of OPC and LC3-50 was increased with the percentage dosage of AVM, indicating that AVM could play the role of a potential set retarder.
- In terms of workability (slump flow, V-funnel, and L-Box), the results at 10 wt.% AVM dosage are well comparable with 2 wt.% of CS dosage in OPC and LC3-50 systems. AVM recorded a slump flow of 672.5 ± 23.25 mm and 656.5 ± 9 mm compared with the control for both OPC and LC3-50 cement systems.
- From the yield stress results, it can be concluded that the percentage dosage of AVM relatively reduced the yield stress. This implies that AVM acts as a plasticizer and can be used to improve the workability and rheology of concrete systems.
- In the case of the compressive strength, AVM only improved this properly at very small dosages of 2.5 wt.%, by 42.45 ± 1.04 MPa of OPC and 28.59 ± 1.39 MPa of LC3-50 at 28 days. A further increase in dosage reduces overall compressive strength for both systems. The findings suggest that AVM is not a set accelerator in concrete systems when used if added in large volumes. However, at 7.5 wt.%, the dosage of AVM to concrete recorded allowable structural concrete strength [28] of 30.92 ± 1.55 MPa MPa and 19.85 ± 0.99 MPa MPa for OPC and LC3-50 systems after water curing for 28 days, respectively.
- The density of SCC concrete prepared using 2.5 wt.% of AVM resulted in a bulk density comparable to conventional structural concrete, but it reduced with an increase in AVM contents.
- The findings overall suggest that AVM is a potential admixture for making SCC at 7.5 wt.% addition to concrete as the mix achieved a flow of 633.5 ± 32.25 mm and 667 ± 15.75 mm, V-funnel of 9 and 8 seconds and L-box blocking ratio of 0.81 and 0.82 for OPC and LC3-50 systems, and provided allowable structural concrete strength. Nevertheless, the long-term durability properties of such SCC should be evaluated.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kiran, K.; Naresh, J. Determination of fresh and hardened state properties of Self Compacting Concrete using GGBFS and RHA. IJIRSET 2018. [Google Scholar] [CrossRef]
- Siddique, R. Self-compacting concrete: materials, properties and applications; Woodhead Publishing: 2019.
- Karakurt, C.; Dumangöz, M.J.M. Rheological and durability properties of self-compacting concrete produced using marble dust and blast furnace slag. Materials 2022, 15, 1795. [Google Scholar] [CrossRef]
- Dey, S.; Kumar, V.V.P.; Goud, K.R.; Basha, S.K.J. State of art review on self compacting concrete using mineral admixtures. J. Build. Pathol. Rehabil. 2021, 6, 18. [Google Scholar] [CrossRef]
- Shi, C.; Wu, Z.; Lv, K.; Wu, L.J.C.; Materials, B. A review on mixture design methods for self-compacting concrete. Constr. Build. Mater. 2015, 84, 387–398. [Google Scholar] [CrossRef]
- Okamura, H.; Ouchi, M. Self-compacting concrete. J. Adv. Concr. Technol. 2003, 1, 5–15. [Google Scholar] [CrossRef]
- Tejaswini, G.L.S.; Rao, A.V. A detailed report on various behavioral aspects of self-compacting concrete. Mater. Today: Proc. 2020, 33, 839–844. [Google Scholar]
- Kumar, M.A.; Magesh, R.; Selvapraveen, S.; Vignesh, M. Assessment on fresh properties and hardened properties of self compacting concrete. AIP Conf. Proc. 2020. [Google Scholar]
- Meko, B.; Ighalo, J.O.; Ofuyatan, O.M. Enhancement of self-compactability of fresh self-compacting concrete: A review. Clean. Mater. 2021, 1, 100019. [Google Scholar] [CrossRef]
- Kalaimani, R.; Subha, C.; Reymond, D.J. Investigation on Strength Characteristics of Self Compacting Concrete incorporated with AR Glass Fibers. E3S Web Conf. 2023, 03005. [Google Scholar] [CrossRef]
- Mahajan, G. experimental investigation on properties of self compacting concrete using mineral admixtures. ir.aiktclibrary.org 2016. [Google Scholar]
- Scrivener, K.; Martirena, F.; Bishnoi, S.; Maity, S. Calcined clay limestone cements (LC3). Cem. Concr. Res. 2018, 114, 49–56. [Google Scholar] [CrossRef]
- Kafodya, I.; Basuroy, D.; Marangu, J.M.; Kululanga, G.; Maddalena, R.; Novelli, V.I. Mechanical performance and physico-chemical properties of limestone calcined clay cement (LC3) in Malawi. Buildings 2023, 13, 740. [Google Scholar] [CrossRef]
- Marangu, J.M. Physico-chemical properties of Kenyan made calcined Clay-Limestone cement (LC3). Case Stud. Constr. Mater. 2020, 12, e00333. [Google Scholar] [CrossRef]
- Marangu, J.M.; Riding, K.; Alaibani, A.; Zayed, A.; Thiong’o, J.K.; Wachira, J.M. Potential for selected kenyan clay in production of limestone calcined clay cement. In Proceedings of the Calcined Clays for Sustainable Concrete: Proceedings of the 3rd International Conference on Calcined Clays for Sustainable Concrete, 2020; pp. 19–25.
- Odhiambo, V.O.; Scheinherrová, L.; Abuodha, S.O.; Mwero, J.N.; Marangu, J.M. Effects of Alternate Wet and Dry Conditions on the Mechanical and Physical Performance of Limestone Calcined Clay Cement Mortars Immersed in Sodium Sulfate Media. Materials 2022, 15, 8935. [Google Scholar] [CrossRef]
- Muzenda, T.R.; Hou, P.; Kawashima, S.; Sui, T.; Cheng, X. The role of limestone and calcined clay on the rheological properties of LC3. Cem. Concr. Compos. 2020, 107, 103516. [Google Scholar] [CrossRef]
- Canbek, O.; Xu, Q.; Mei, Y.; Washburn, N.R.; Kurtis, K.E. Predicting the rheology of limestone calcined clay cements (LC3): Linking composition and hydration kinetics to yield stress through Machine Learning. Cem. Concr. Res. 2022, 160, 106925. [Google Scholar] [CrossRef]
- Athman, C.M.; Abuodha, S.O.; Nyomboi, T. USE of GUM Arabic as a Superplasticizer in Self-Compacting Concrete. IJISME 2018. [Google Scholar]
- Isik, I.E.; Ozkul, M.H. Materials, B. Utilization of polysaccharides as viscosity modifying agent in self-compacting concrete. Constr. Build. Mater. 2014, 72, 239–247. [Google Scholar] [CrossRef]
- Oni, D.; Mwero, J.; Kabubo, C. The effect of cassava starch on the durability characteristics of concrete. Open J. Civ. Eng. 2020, 14. [Google Scholar] [CrossRef]
- Surjushe, A.; Vasani, R.; Saple, D.J. Aloe vera: a short review. Indian J. Dermatol. 2008, 53, 163. [Google Scholar] [CrossRef]
- Pegu, A.J.; Sharma, M.A. Review on Aloe vera. Int. J. Trend. Sci. Res. Dev. 2019, 3, 35–40. [Google Scholar] [CrossRef]
- Aburto-Moreno, Z.; Alvarado-Quintana, H.; Vásquez-Alfaro, I. Influencia del aloe-vera sobre la resistencia a la compresión, infiltración, absorción capilar, tiempo de fraguado y asentamiento en un concreto estructural. SCIENDO 2018, 21, 105–118. [Google Scholar] [CrossRef]
- Herrera-Hernandez, H.; Franco-Tronco, M.I.; Miranda-Hernandez, J.G.; Hernandez-Sanchez, E.; Espinoza-Vazquez, A.; Fajardo, G. Aloe-vera gel as potential corrosion inhibitor for concrete steel reinforcement. Avances en Ciencias e Ingeniería 2015, 6, 9–23. [Google Scholar]
- Oggu, A.; Madupu, L.N.K.S. Study on properties of porous concrete incorporating aloevera and marble waste powder as a partial cement replacement. Mater. Today: Proc. 2022, 52, 1946–1951. [Google Scholar] [CrossRef]
- ASTM. Standard Specification for Concrete Aggregates (ASTM C33/C33M-18). West Conshohocken: American Society for Testing and Materials; 2023.
- KS EAS 148-1, Cement — Test methods — Part 1: Determination of strength, Kenya Bureau of Standards, Nairobi, Kenya, 2017.
- ASTM. Standard Specification for Chemical Admixtures for Concrete (ASTM C494/C494M-17). West Conshohocken: American Society for Testing and Materials; 2017.
- EFNARC. The European Guidelines for Self-Compacting Concrete Specification, Production and Use. May, 2005.
- ASTM. Standard Test Method for Density (Unit Weight), Yield, and Air Content (Gravimetric) of Concrete (ASTM C138/C138M-17a). West Conshohocken: American Society for Testing and Materials; 2023.
- KS EAS 148-3, Cement-Test Methods Part 3: Determination of Setting Times and Soundness, Kenya Bureau of Standards, Nairobi, Kenya, 2017.
- ASTM. Standard Guide for Measurement of the Rheological Properties of Hydraulic Cementious Paste Using a Rotational Rheometer(ASTM C1749-17a). West Conshohocken: American Society for Testing and Materials; 2017.
- Pierre, A.; Lanos, C.; Estelle, P. Extension of spread-slump formulae for yield stress evaluation. Appl. Rheol. 2013, 23, 63849. [Google Scholar]
- Scheinherrová, L.; Pommer, V.; Vejmelková, E.; Černý, R. Comparison of water removal methods from cement paste at early age. In Proceedings of the AIP Conference Proceedings, 2021.
- Sebbar, N.; Bozzelli, J.W.; Bockhorn, H. Kinetic Study of Di-Tert-Butyl Peroxide: Thermal Decomposition and Product Reaction Pathways. Int. J. Chem. Kinet. 2015, 47, 133–161. [Google Scholar] [CrossRef]
- Moo-Young, M. Comprehensive biotechnology; Elsevier: 2019.
- Wakayama, T.; Ito, Y.; Sakai, K.; Miyake, M.; Shibata, E.; Ohno, H.; Kamijima, M. Comprehensive review of 2-ethyl-1-hexanol as an indoor air pollutant. J. Occup. Health 2019, 61, 19–35. [Google Scholar] [CrossRef] [PubMed]
- McGinty, D.; Scognamiglio, J.; Letizia, C.S.; Api, A.M. Fragrance material review on 2-ethyl-1-hexanol. FCT 2010, 48, S115–S129. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, W.A.; Yarmo, A.; Salih, N.; Derawi, M.D.; Yusop, M.R.; Salimon, J. Synthesis and lubricity properties analysis of branched dicarboxylate esters based lubricant. MJAS 2015, 19, 106–117. [Google Scholar]
- Bodaghi, A. An overview on the recent developments in reactive plasticizers in polymers. Polym. Adv. Technol. 2020, 31, 355–367. [Google Scholar] [CrossRef]
- Stamatelatou, K.; Pakou, C.; Lyberatos, G. Occurrence, toxicity, and biodegradation of selected emerging priority pollutants in municipal sewage sludge. Compr. Biotech. 2011, 6, 473–484. [Google Scholar]
- Chemicals, A. “2-Ethylhexanol,” Information Society Services Human Resources. Available online: https://www.atamanchemicals.com/2-ethylhexanol_u24548/ (accessed on 12.12.2023).
- Wang, Y.; Qian, H. Phthalates and their impacts on human health. In Proceedings of the Healthcare; 2021; p. 603. [Google Scholar]
- Fan, Z.; Lin, L. Exposure science: contaminant mixtures. Ency. Envir. Health 2011. [Google Scholar]
- Bhandari, I.; Kumar, R.; Sofi, A.; Nighot, N.S. A systematic study on sustainable low carbon cement–Superplasticizer interaction: Fresh, mechanical, microstructural and durability characteristics. Heliyon 2023. [Google Scholar] [CrossRef] [PubMed]
- Flatt, R.J. Interparticle forces and superplasticizers in cement suspensions; Thesis. EPFL: 1999.
- Pan, J.; Feng, K.; Wang, P.; Chen, H.; Yang, W. Retardation and compressive strength enhancement effect of upcycling waste carrot as bio-admixture for cement mortars. J. Build. Eng. 2022, 62, 105402. [Google Scholar] [CrossRef]
- Singh, N.B.; Singh, S.P.; Sarvehi, R. Effect of phenols on the hydration of Portland cement. Adv. Cem. Res. 1989, 2, 43–47. [Google Scholar] [CrossRef]
- Zhu, W.; Feng, Q.; Luo, Q.; Bai, X.; Lin, X.; Zhang, Z. Effects of pce on the dispersion of cement particles and initial hydration. Materials 2021, 14, 3195. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Mezhov, A.; Schmidt, W. Effect of Polycarboxylate Superplasticizer in Ordinary Portland Cement and Sulfate Resistant Cement. J. Mater. Civ. Eng. 2023, 35, 04023113. [Google Scholar] [CrossRef]
- Yu, J.; Wu, H.-L.; Mishra, D.K.; Li, G.; Leung, C.K.Y. Compressive strength and environmental impact of sustainable blended cement with high-dosage Limestone and Calcined Clay (LC2). J. Clean. Prod. 2021, 278, 123616. [Google Scholar] [CrossRef]
- Bullard, J.W.; Jennings, H.M.; Livingston, R.A.; Nonat, A.; Scherer, G.W.; Schweitzer, J.S.; Scrivener, K.L.; Thomas, J. J Mechanisms of cement hydration. Cem. Concr. Res. 2011, 41, 1208–1223. [Google Scholar] [CrossRef]
- Scrivener, K.; Avet, F.; Maraghechi, H.; Zunino, F.; Ston, J.; Hanpongpun, W.; Favier, A. Impacting factors and properties of limestone calcined clay cements (LC3). Green Mater. 2018, 7, 3–14. [Google Scholar] [CrossRef]
- Chang, J.; Jiang, T.; Cui, K. Influence on compressive strength and CO2 capture after accelerated carbonation of combination β-C2S with γ-C2S. Constr. Build. Mater. 2021, 312, 125359. [Google Scholar] [CrossRef]
- Bederina, M.; Makhloufi, Z.; Bouziani, T. Effect of limestone fillers the physic-mechanical properties of limestone concrete. Phys. Procedia 2011, 21, 28–34. [Google Scholar] [CrossRef]
- Dhandapani, Y.; Santhanam, M.; Gettu, R.; Pillai, R. Perspectives on blended cementitious systems with calcined clay-limestone combination for sustainable low carbon cement transition. Indian Concr. J. 2020, 94, 31–45. [Google Scholar]
- Nalet, C.; Nonat, A. Effects of functionality and stereochemistry of small organic molecules on the hydration of tricalcium silicate. Cem. Concr. Res. 2016, 87, 97–104. [Google Scholar] [CrossRef]
- Opara, H.E.; Eziefula, U.G.; Eziefula, B.I. Comparison of physical and mechanical properties of river sand concrete with quarry dust concrete. SSP - J. Civ. Eng. 2018, 13, 127–134. [Google Scholar] [CrossRef]
- Hospodarova, V.; Junak, J.; Stevulova, N. Color pigments in concrete and their properties. Pollack Periodica 2015, 10, 143–151. [Google Scholar] [CrossRef]
| Raw material | SO3 | Al2O3 | Fe2O3 | CaO | SiO2 | MgO | LOI |
|---|---|---|---|---|---|---|---|
| OPC | 1.14 | 5.43 | 3.68 | 64.83 | 21.64 | 2.5 | 0.78 |
| LC3-50 | 2.54 | 11.99 | 3.98 | 44.53 | 30.14 | 1.31 | 5.51 |
| L | 0.33 | 0.47 | 0.42 | 90.68 | 1.42 | 0.59 | 6.09 |
| Description | Property |
|---|---|
| Appearance | Whitish to light brown clear to cloudy liquid |
| Specific gravity at 25 °C | 1.073 |
| pH value | 5.0 – 7.0 |
| Chloride content | “chloride-free” to EN 934-2 |
| Trials | CA (kg/m3) | FA (kg/m3) | OPC/LC3-50 (kg/m3) | L (kg/m3) | w/c | w/p | CS/AVM |
|---|---|---|---|---|---|---|---|
| TR1 | 1078.00 | 562.13 | 363.38 | 224.22 | 0.43 | 0.8 | 1.82 |
| TR2 | 1078.00 | 562.13 | 363.38 | 224.22 | 0.48 | 0.9 | 3.63 |
| TR3 | 970.20 | 644.57 | 331.41 | 204.49 | 0.48 | 0.9 | 4.97 |
| TR4 | 862.40 | 732.64 | 351.14 | 216.67 | 0.48 | 0.8 | 3.51 |
| TR5 | 862.40 | 732.64 | 351.14 | 216.67 | 0.48 | 0.9 | 7.02 |
| TR6 | 970.20 | 644.57 | 331.41 | 204.49 | 0.48 | 0.9 | 4.97 |
| TR7 | 970.20 | 644.57 | 331.41 | 204.49 | 0.48 | 0.9 | 6.62 |
| TR8 | 970.20 | 644.57 | 331.41 | 204.49 | 0.50 | 1.0 | 4.97 |
| TR9 | 970.20 | 644.57 | 331.41 | 204.49 | 0.48 | 0.9 | 6.62 |
| Trials | d (mm) | Relative Slump | H2/H1 (mm) | V-funnel (Sec) | Observations* | Further testing |
|---|---|---|---|---|---|---|
| TR1 | 398.5 | 0.99 | 0 | 15+ | S | No |
| TR2 | 432.5 | 1.16 | 0 | 15+ | S | No |
| TR3 | 615.0 | 2.07 | 0.68 | 8 | F; S-V | No |
| TR4 | 497.5 | 1.49 | 0 | 15 | S | No |
| TR5 | 762.5 | 2.81 | 0.88 | 6 | F; B | No |
| TR6 | 515.0 | 1.57 | 0.18 | 13 | H-V | No |
| TR7 | 672.5 | 2.36 | 0.81 | 8 | F | Yes |
| TR8 | 762.5 | 2.81 | 0.83 | 5 | F; B | No |
| TR9 | 659.0 | 2.30 | 0.20 | 6 | F; B | No |
| Peak Report TIC | ||||||
|---|---|---|---|---|---|---|
| Peak# | R.Time | Area | Area% | Height | Height % | Name |
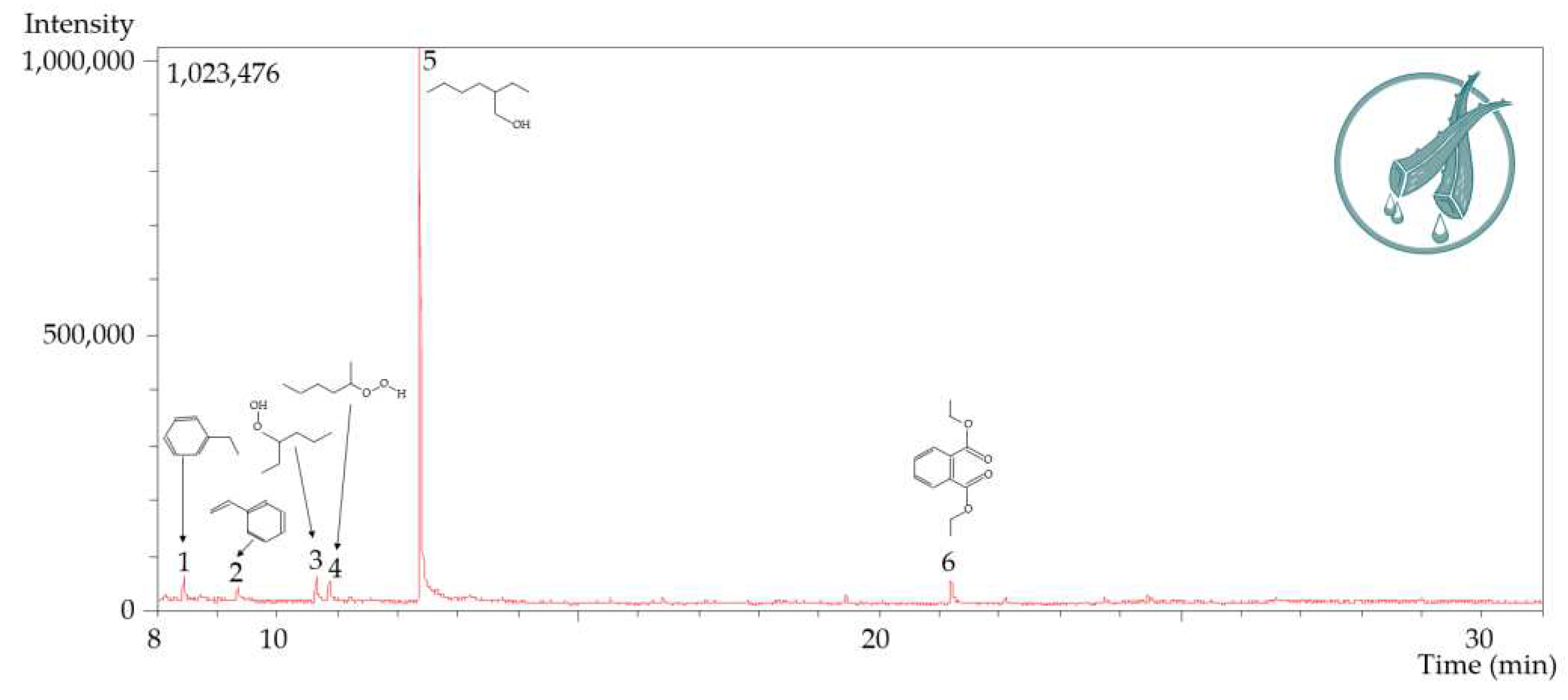
| 1 | 8.465 | 84270 | 4.12 | 42596 | 3.75 | Ethylbenzene |
| 2 | 9.356 | 73891 | 3.61 | 23475 | 2.07 | Styrene |
| 3 | 10.649 | 87366 | 4.27 | 44871 | 3.96 | Hydroperoxide, 1-ethylbutyl |
| 4 | 10.867 | 57727 | 2.82 | 30394 | 2.68 | Hydroperoxide, 1-methylpentyl |
| 5 | 12.376 | 1651585 | 80.74 | 956651 | 84.33 | 1-Hexanol, 2-ethyl- |
| 6 | 21.189 | 90686 | 4.43 | 36440 | 3.21 | Diethyl Phthalate |
| Mix type | % CS dosage |
Slump flow (mm) |
V-funnel (sec) |
L-Box (h2/h1) |
Observations |
|---|---|---|---|---|---|
| OPC-CS | 0 | 452.5 | No flow | 0 | No flow |
| 0.5 | 572.5 | High viscosity | 0 | No flow | |
| 1 | 639.5 | 11 | 0.46 | Flowing but viscous | |
| 1.5 | 668.5 | 9 | 0.88 | Flowing, no bleeding | |
| 2 | 681.5 | 8 | 0.94 | Flowing | |
| LC3-50-CS | 0 | 404.5 | No flow | 0 | No flow |
| 0.5 | 472.0 | High viscosity | 0 | No flow | |
| 1 | 583.0 | 13 | 0.43 | Flowing but viscous | |
| 1.5 | 647.5 | 10 | 0.60 | Flowing | |
| 2 | 666.0 | 9 | 0.88 | Flowing |
| Mix Type | % AVM dosage |
Slump Flow (mm) |
V-funnel (sec) |
L-Box (h2/h1) |
Observations |
|---|---|---|---|---|---|
| OPC-AVM | 0 | 389.0 | No flow | 0 | No flow |
| 2.5 | 505.0 | High viscosity | 0 | No flow | |
| 5 | 534.0 | 14 | 0 | Highly viscous | |
| 7.5 | 633.5 | 9 | 0.81 | Flowing | |
| 10 | 697.0 | 5 | 0.83 | Flowing | |
| LC3-50-AVM | 0 | 404.5 | No flow | 0 | No flow |
| 2.5 | 555.0 | High viscosity | 0 | No flow | |
| 5 | 596.5 | 14 | 0 | Highly viscous | |
| 7.5 | 651.0 | 8 | 0.82 | Flowing | |
| 10 | 682.5 | 5 | 0.94 | Flowing |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





