1. Introduction
Soil was always considered as fundamental part to every aspect of life on Earth, especially when it comes to humans. The cultivation of land was a key factor in the survival of early humans, which in turn, changed the whole evolutionary path of our species. And yet, modern human societies are still dependent, probably even more than ever before, on products and functional services from the soil [
1]. As the population of modern human is constantly growing, the projected needs for food and fuel in order to achieve and sustain humanity’s food and energy security is going to place increased pressure on the Earth’s soil. Even though the revolutionary advances in technology in the last two centuries have boosted the productivity in agriculture, the urbanization of modern societies, especially in the developed countries, is requiring more and more land for urban growth at the expense of arable land. Thus, modern agriculture is facing the challenge to produce even more products but with less land available. And the challenge is becoming even harder, since a major percentage of cultivable land nowadays is strongly degraded [
2,
3].
Soil degradation is a multifactorial process, which is defined by the transition of a stable soil ecosystem to a more unsteady and fragile state, usually as a result of unfavorable environmental conditions and more often, due to extensive and improper human exploitation [
2,
4,
5]. Some of the typical forms of soil degradation are the loss of organic matter, deterioration of soil structure, decreased fertility and acidification, all of which negatively affect crop production and threaten the environment, in general. Soil acidification, in particular, is becoming an emerging threat to the sustainability of global agricultural food production, with several studies estimating that over 30% of the global ice-free land area is composed of acidic soils (topsoil pH < 5.5) [
4,
6,
7,
8,
9]. Even though soil acidification is a natural process that can occur after periods of long rainfall and the leaching of basic cations in the topsoil (e.g., Ca
2+ or Mg
2+), current rates of soil acidification have been accelerated by human activities, mostly industrial emissions of sulphur and nitrogen oxides [
10], along with the vast increase of nitrogen inputs through fertilizers [
6,
7,
8]. Apart from the loss of nutrients due to the decrease in the availability of basic cations [
11], low pH conditions in the soil can facilitate the release of toxic aluminum (Al) and manganese (Mn), which can damage plant roots, thus reducing growth and potential crop yield [
12].
The most common practice for soil acidity adjustment is considered to be the use of materials which contain calcium carbonate such as marl, chalk, limestone, hydrated lime (calcium and magnesium hydroxide) [
9,
13,
14]. The problem with this method is that aluminum and manganese toxicity on an acidic soil can be mitigated only by using large amounts (e.g., 5-20 mg/ha) of limed materials. For example, in South Africa 4.5 million mg limed materials are used, annually, for acidity improvement. Therefore, the application of a calcification material is not considered to be effective and economically feasible, especially for the resource-poor small - scale farmers [
15,
16].
For the above reasons, in the last decades, research efforts have been made so that alternative ways, which are able to improve acidic soils, can be found. A first approach concerns the exploitation of industrial activity waste products and mainly from the aquaculture sector [
17]. Every year a vast amount of aquaculture by-products is disposed in landfills. This disposal, frequently, leads to foul odors which caused from the decomposition of the salts into gases and into amines. This procedure negatively affects the quality of people’s lives in these areas and, also, environmental pollution problems are caused [
17,
18]. For example, in China where the world’s largest quantities of shellfish are produced, every year about 10 million ton end up in landfills. Of these, only a small portion are reused for other purposes such as industrial fertilizers [
19].
The use of mussels by – products is considered to be a promising soil acidic amendment. The composition of their shells mainly consists of calcium carbonate and a small portion of other elements, such as nitrogen, phosphorus, potassium, magnesium and sodium. These by –products have the ability to be used as biological materials with low cost, are environmental friendly and have excellent environmental remediation capabilities [
20]. The aim of the current study was to evaluate the potential use of mussel shells as alternative liming materials in acidic soil and to determine the efficiency of different proportions of processed mussel shells and their effect on soil pH, on organic matter and on soil nutrients (Nitrogen (N), Phosphorus (P), Potassium (K)).
2. Materials and Methods
2.1. Sampling process and soil attributes
Samplings of soils took place within the wider rural area of the city of Almiros (
Figure 1). According to local landowners and farmers, the area is known for its acidic soils, so preliminary surveys were conducted in order to determine the soil with the lowest pH. Soil samples were collected from 30 different fields, from 3 randomly selected plots within the field, at depth range between 0 – 15 cm. The pH values of the samples were initially tested with a portable pH meter (Consort C520 Multi Parameter Analyses) and then taken to the lab for a more precise evaluation with chemical analyses and from the field with the most acidic soil, 20 samples were taken from random locations within the field, adding to a total amount of 100 kg of collected soil.
2.2. Mussel shells collection and processing
The mussel shells (Mytilus galloprovincialis) were provided by a local sea food processing company. The mussels were pre-boiled and their shells were removed, in order for the edible part to be packed and frozen for consumer use. The shells are considered a by-product and, normally, they would get disposed to a local landfill. For the experimental purposes, we visited the processing factory on 10 different days and collected approximately 2-3 kg of discarded mussel shells, adding to a total amount of 25 kg.
The shells were rinsed with distilled water, air -dried in an oven at 40°C for 24 h and then grounded with mortar and pestle. Finally, the powder from the grounded shells was sieved through a 2 mm and a 1 mm stainless sieve, in order to acquire the two different powders for the experiments, in terms of grain size: Fine (< 1 mm) and Coarse (1 – 2 mm).
2.3. Chemical analyses
Soil samples were analyzed for pH (1:2.5 d. H
2O, particle size distribution (Bouyoucos hydrometer), organic matter (Walkley – Black wet oxidation with 0.17N K
2Cr
2O
7 back – titrated with 0.5M FeSO
4), total nitrogen (Kjeldahl method), Available P (Olsen method, analyzed with ammonium vanadomolybdate / ascorbic blue and measured in a UV spectrophotometer at 882 nm) and Exchangeable Κ (1:10 at 1M CH
3COONH
4 pH 7, analyzed in a flame photometer) according to [
21]. Ground mussel shells were analyzed for pH, organic matter, total nitrogen, with the same methods used for soil samples. Furthermore, mussel shells were also analyzed for calcium carbonate (CaCO
3) content using a typical Schreiber calcimeter (Lita Analytical).
2.4. Experimental design
The experiment was carried out within the campus premises of the Department of Ichthyology and Aquatic Environment, using square plastic pots (40 x 40 x 20 cm). For each type of ground mussel shells grain size (Coarse and Fine) 6 different ratios were used as treatments. Specifically, 4 kg of soil were mixed with 4, 12, 20, 40, 120 and 240 g of ground mussels (i.e., 0.1, 0.3, 0.5, 1, 3 and 6% ratio). Also, an untreated pot with 4 kg soil was used as the “Control”. Each treatment was replicated in 10 pots, resulting to a total of 130 pots.
The pots were placed in a storage room, with an ambient temperature between 22 - 25°C and were watered every three days, making sure that soil moisture was maintained at approximately 65%. Also, every two weeks, the soil was mixed with a small garden shovel to ensure that no lumps or crust was created. For the pH measurements, a small sample was taken from every pot at 30, 60, 90, 120, 150 and 180 days after treatment.
2.5. Response Surface Methodology- Central Composite Design
Response Surface Methodology (RSM) is a statistical and mathematical technique used in experimental design to optimize and analyze complex processes [
22,
23]. It is a particularly valuable tool in situations where multiple variables interact, making it challenging to understand their individual and combined effects. Central Composite Design (CCD) is a specific experimental design with RSM that explores the response surface by combining factorial points with additional axial and center points, allowing quadratic model fitting and enabling the estimation of curvature in the response surface [
24]. RSM was employed in this study in order to predict maximum pH values as a function of the different mussel seashell ratios and days of incubation. Fine (< 1 mm) and Coarse (1 – 2 mm) powder, as well as the six mussel shell ratios (4, 12, 20, 40, 120 and 240 g, per 4 kg of soil) were used as the control variables and maximum pH was the predicted response variable. A second order polynomial was fitted to the experiment data and the following equation (Eq. 1) was used to corelate the effect of the two variables (Days of incubation, mussel shell ratio) with the maximum pH.
where: β0, is constant; β
1, β
2, β
12, β
11, β
22, are regression coefficients, X
1, Days of incubation and X
2, mussel shell ratios.
All statistical analyses were performed by the “STATGRAPHICS Centurion” software package (v.18.1.01, Statgraphics Technologies, Inc., The Plains, VA, USA) and values of p < 0.05 were considered significant.
3. Results
3.1. Physicochemical properties of soil and mussel shells
According to the chemical analyses the lowest pH was 4.26, which was eventually the soil used for the experiments and the maximum pH was 5.76. The physicochemical properties of the used soil in the experiment are illustrated in
Table 1.
The respective properties of the different sizes of ground mussel shells are presented in
Table 2.
3.2. Effects and Optimizatiojn of amendments on soil pH
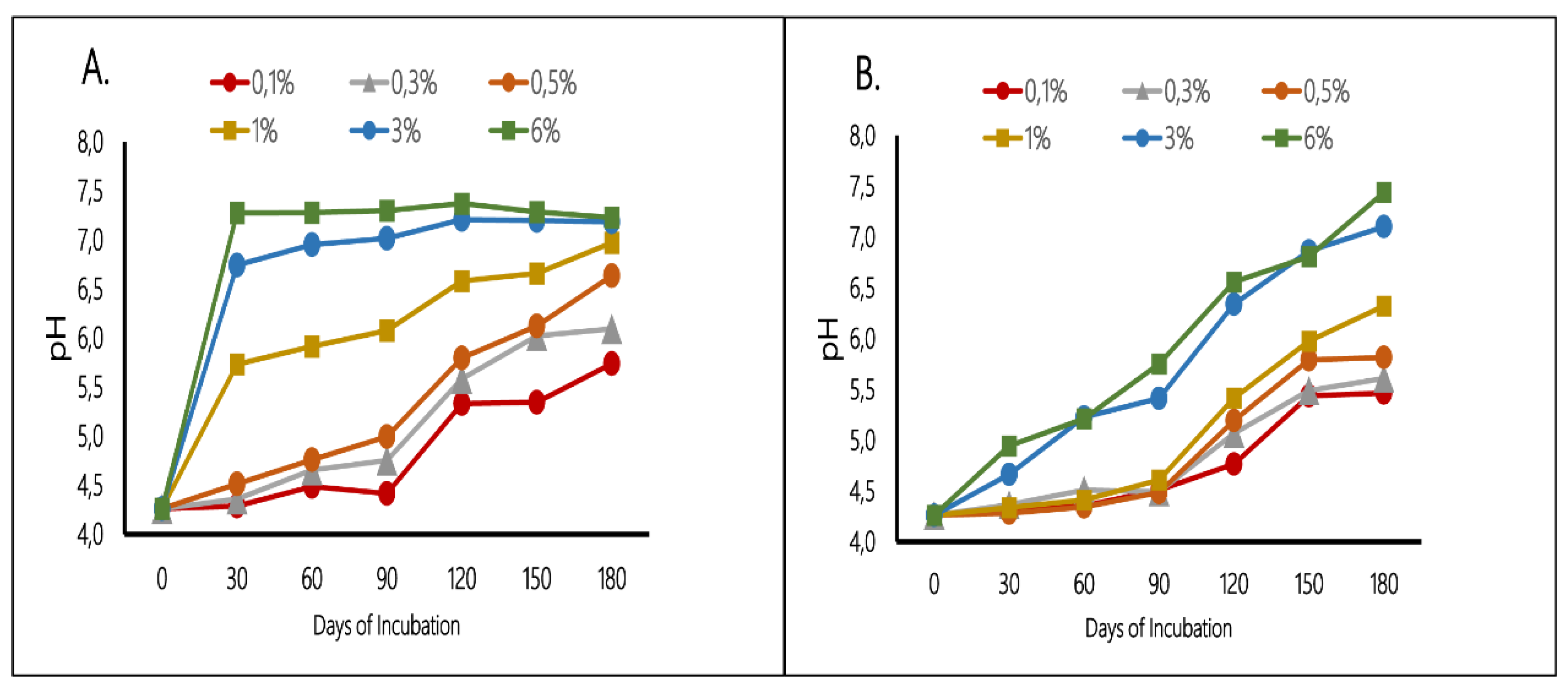
The pH values of the soils at the different proportions during the experiment are shown in
Figure 2.
As expected all the different ratios of mussel shell caused an overall increase of soil pH. Among the different treatments, the highest pH values were observed in plots treated with 3% and 6% soil – mussel shell mixture.
Soil pH increased significantly and quickly after the addition of 1% FP, 3% FP, 6% FP (
Figure 2 A) and the pH of amended soils using the formentioned ratios increased by 1.47, 2.48 and 3.02 units compared to control (pH 4.26), respectively.
After 180 days, the decreasing order of pH values using mussel shell as Fine powder (<1mm) was: 7.23 (6% FP) > 7.19 (3% FP) > 6.97 (1% FP) > 6.64(0.5% FP) > 6.09 (0.3% FP) > 5.74 (0.1% FP). Moreover, using mussel shell as Coarse powder (1-2mm), resulted to pH values: 7.44 (6% CP) > 7.10 (3% CP) > 6.32 (1% CP) > 5.81 (0.5% CP) > 5.61 (0.3% CP) > 5.46 (0.1% CP).
Furthermore,
Figure 2 B indicated that Coarse powder mussel shell caused a slower pH raise compared to Fine powder use. At the end of the experinment (180 days), the plots mixed with 6% Coarse powder of ground mussel shells (grain size 1 – 2 mm) resulted to a higher pH value (7.44) in comparison to 6% Fine powder treatment (7.23).
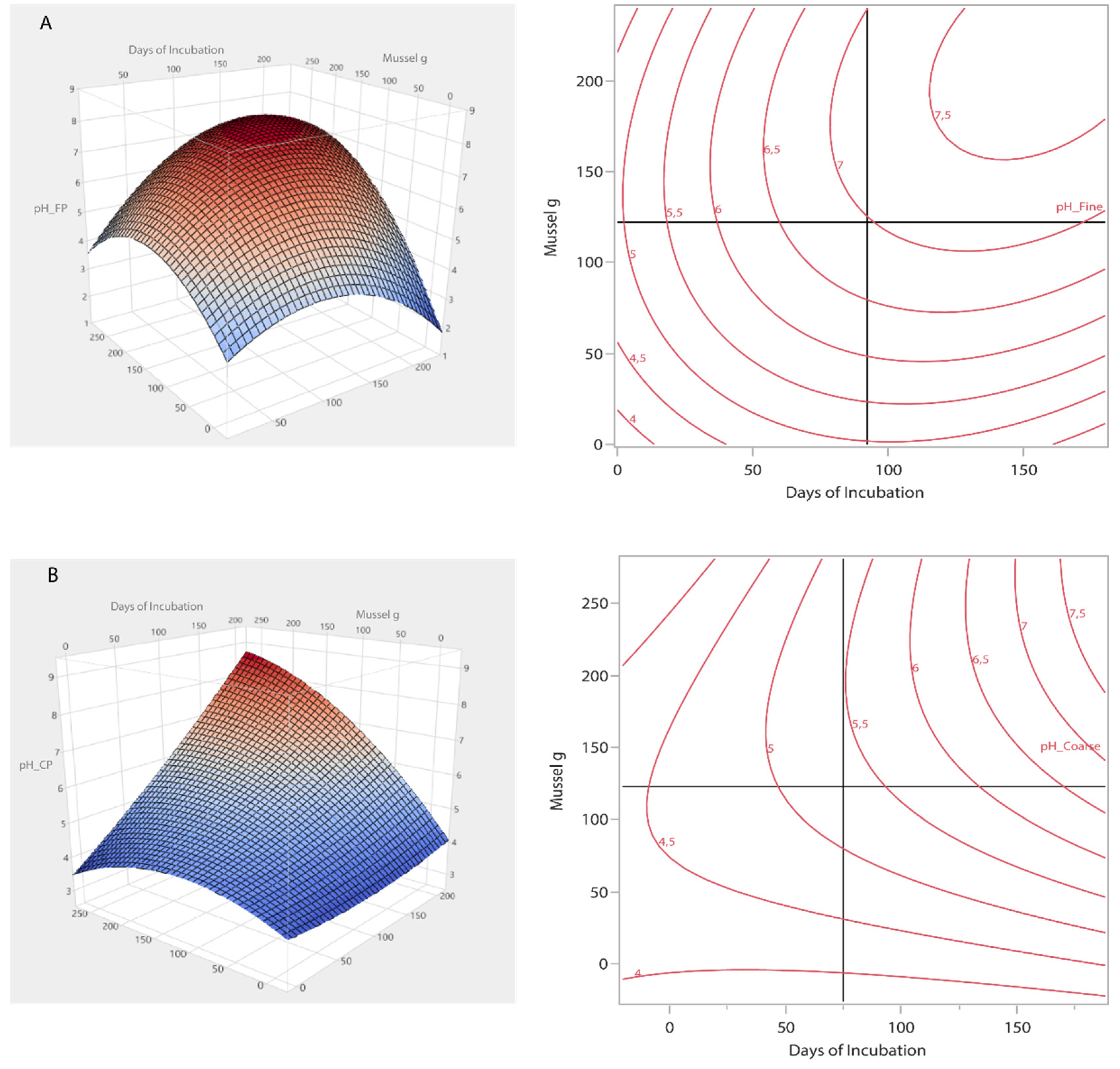
Two models were constructed utilizing Response Surface Analysis for the optimization of pH using the Fine and Coarse powder. Both models yielded a p value less than 0.01 indicating that they were significant. Regarding Fine powder model, the independent variables had a higly significant effect (p < 0.001) on pH for both linear and quadratic interactions. The model yielded an R2= 0.94 with a Root Mean Square Error = 0.29. For the Coarse powder model, the indepedent variable quadratic term of Days of incubation was not significant (p>0.05), while all the other interactions were again higly significant (p < 0.001). The R2 value of the model was 0.96 with a Root Mean Square Error = 0.22.
Figure 3 displays the Response Surface Analysis of pH.
3.3. Effects of Amendments on Organic Matter, Available P, Exchangable K and Total N
The effect of the different treatments compared to unamended control on Organic matter, Available P, Exchangable K and Total N at the end of the experiment are presented in
Figure 4.
Organic matter in all the applied doses raised with the application of mussel shell. The higher values of OM achieved in 6% ration (2.27% in Fine powder pots and 2.09% in Coarse powder treatment). Organic matter was higher in all Fine powder pots than in Coarse powder treatments. Organic matter in 6% FP was 18% higher than 6% CP pots.
The mean concentration of P varied between 23 mg kg-1 (control pots) and 46.2 mg kg-1 (6% FP pots). Statistically differences were noticed between the treated and control pots (p<0.0001), except from the 0.1% CP treatment. The highest exchangeable K concentration was noticed in treated plots with 6% FP of ground mussel shells (190.1 mg kg-1) and the lowest in 0.3% CP pots (128.7 mg kg-1). According to the statistic analysis, statistically differences occurred among mussel shell treatments and unamended control (p<0.0001). Content of Available P, Exchangable K were slightly higher but statistically significant in the amended treatments with Fine powder compared to Coarse powder.
The mean Total N content ranged between 0.11 (1%, 0.5% and 1% CP) and 0.14% (3% and 6% FP) in treated pots and it was 0.11% in control treatment. No statistically significant differences were observed among the different treatments or between the treatments and control pots. Concerning Total N concentration, the results revealed a slightly increasing trend within the Fine powder mussel shell treatments at all ratios, but not significantly different from the Coarse powder treatments. The different grain size of the used mussel shell affected the concentration of οrganic matter, Available P and Exchangable K.
4. Discussion
Soil acidity is one of the most significant problems that can affect the crop productivity, nutrient availability and soil fertility [
25].
In this study, we evaluated the use of mussel shell as an alternative liming material in an acidic soil.
4.1. Effects and Optimizatiojn of amendments on soil pH
The application of mussel shell as amendment at different rates increased the soil pH, either using Fine powder grain size or Coarse powder. The highest increases were noticed at 3% and 6% ratios, regardless of the grain size. Specifically, 30g and 60g of mussel seashells per 1 kg of soil have achieved to increase soil pH above 7.0, regardless of the grain size of the used material. According to the response surface analysis, at the end of the experiment (180 days) 64.6 g of Fine powder and 76.6 g Coarse powder mussel shells needed to neutralize pH on the acidic soil, respectively. In contrast, Leiva-Vega et al. [
26], who carried out an experiment using as liming material the mussel seashell
Mytilus chilensis, succeeded to raise soil pH close to 7.0 using 190 g kg
-1, amount 84% and 68% higher compared to our results. Various studies have mentioned the positive impacts of seashells in soil pH increase [
27,
28,
29].
It is remarkable that soil pH was significantly raised after 30 days from the addition of 1%, 3% and 6% Fine powder mussel shell. That increase is due to the fact that calcium carbonate content (CaCO
3) of Fine powder was higher than in Coarse powder material [
30]. The use of calcium carbonate (CaCO
3) have positive effect to the increase of exchangeable calcium (Ca) and that lead to raise of soil pH in values near to neutral [
31,
32].
4.2. Effects of Amendments on Organic Matter, Available P, Exchangable K and Total N
Our results have shown that organic matter values were positively influenced by the use of mussel seashell at all ratios. In amended pots with 6% FP, organic matter increased 47% compared to control. The decomposition of organic matter reduces and the activity of the soil microorganisms stops under acidification conditions [
33].
As for the elements of Available P and Exchangeable K the amended treatments with Fine powder grain size at all rates had positive effect in their concentration at the end of the experiment. Our results are in agreement with other comparable studies [
27,
34]. Soil pH is below 6.0, deficiencies of phosphorus (P) and potassium (K) can be caused due to their low bioavailability [
35]. The availability of Available P and Exchangeable K are less affected at pH values from 6.0 to 7.5 [
17,
36]. Fageria and Nascente [
25] have mentioned that the availability of Phosphorus (P) become higher as soil pH increases.
As for the Available P, Alvarez et al. [
29] reported decrease of phosphorus (P) content when ground mussel shell used as amendment compared to control, in contrary to our findings.
Furthermore, results of Paz-Ferreiro et al. [
20] noticed no effect of the mussel shell application to the concentration of phosphorus (P) and potassium (K).
On the other hand, the Total N content was not statistically affected by the application of mussel shell in all the amended treatments. Our results are in accordance with the Paz-Ferreiro et al. [
20] experiments where the Total N among treated mussel shell had no statistically significant differences with the control.
5. Conclusions
The results of our investigation showed that mussel shell application not only increased the soil pH but also ameliorated the organic matter and the content of Available P, Exchangeable K and Total N.
There is a widespread consensus that mussel shells, due to the fact that contain a significant amount of calcium carbonate can cause a significant raise in soil pH and improve the soil fertility. They can used as a natural, inexpensive and eco-friendly material in organic agriculture.
We discern that this investigation could give useful knowledge of the use of mussel shell as soil pH and soil fertility improvement. It is recommended that more investigation should be carried out to assess the application of mussel shell as an amendment in acidic soil under field conditions.
Author Contributions
Conceptualization, A.L. and A.M.; methodology, A.L., A.M., K.G., C.A. and A.P.; validation, A.L., A.M., K.G., C.A. and A.P.; software, A.L. and K.G.; investigation, A.L., A.Μ, K.G. and A.P.; data curation, A.L, A.M., K.G., C.A., A.P., K.S. and D.V.; writing—original draft preparation, A.L, K.M., K.G., C.A., A.P., K.S. and D.V; writing—review and editing, A.L, A.M., K.G., A.P., A.P., K.S. and D.V; supervision, A.L., A.M. and K.G.; project administration, A.L., A.M., and K.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- FAO. Status of the World’s Soil Resources. 2015.
- Karlen, D.L.; Rice, C.W. Soil Degradation: Will Humankind Ever Learn? Sustainability 2015, 7, 12490–12501. [Google Scholar] [CrossRef]
- Bindraban, P.S.; van der Velde, M.; Ye, L.; van den Berg, M.; Materechera, S.; Kiba, D.I.; Tamene, L.; Ragnarsdóttir, K.V.; Jongschaap, R.; Hoogmoed, M.; et al. Assessing the impact of soil degradation on food production. Current Opinion in Environmental Sustainability 2012, 4, 478–488. [Google Scholar] [CrossRef]
- Goulding, K.W.T. Soil acidification and the importance of liming agricultural soils with particular reference to the United Kingdom. Soil Use and Management 2016, 32, 390–399. [Google Scholar] [CrossRef] [PubMed]
- Caires, E.F.; Haliski, A.; Bini, A.R.; Scharr, D.A. Surface liming and nitrogen fertilization for crop grain production under no-till management in Brazil. European Journal of Agronomy 2015, 66, 41–53. [Google Scholar] [CrossRef]
- Meng, C.; Tian, D.; Zeng, H.; Li, Z.; Yi, C.; Niu, S. Global soil acidification impacts on belowground processes. Environmental Research Letters 2019, 14, 074003. [Google Scholar] [CrossRef]
- Tian, D.; Niu, S. A global analysis of soil acidification caused by nitrogen addition. Environmental Research Letters 2015, 10, 024019. [Google Scholar] [CrossRef]
- Wright, R.F.; Alewell, C.; Cullen, J.M.; Evans, C.D.; Marchetto, A.; Moldan, F.; Prechtel, A.; Rogora, M. Trends in nitrogen deposition and leaching in acid-sensitive streams in Europe. Hydrol. Earth Syst. Sci. 2001, 5, 299–310. [Google Scholar] [CrossRef]
- von Uexküll, H.R.; Mutert, E. Global extent, development and economic impact of acid soils. Plant and Soil 1995, 171, 1–15. [Google Scholar] [CrossRef]
- Zhu, Q.; De Vries, W.; Liu, X.; Zeng, M.; Hao, T.; Du, E.; Zhang, F.; Shen, J. The contribution of atmospheric deposition and forest harvesting to forest soil acidification in China since 1980. Atmospheric Environment 2016, 146, 215–222. [Google Scholar] [CrossRef]
- Zhang, Y.; He, X.; Liang, H.; Zhao, J.; Zhang, Y.; Xu, C.; Shi, X. Long-term tobacco plantation induces soil acidification and soil base cation loss. Environ Sci Pollut Res Int 2016, 23, 5442–5450. [Google Scholar] [CrossRef] [PubMed]
- Hue, N.V.; Vega, S.; Silva, J.A. Manganese Toxicity in a Hawaiian Oxisol Affected by Soil pH and Organic Amendments. Soil Science Society of America Journal 2001, 65, 153–160. [Google Scholar] [CrossRef]
- Vogel, S.; Bönecke, E.; Kling, C.; Kramer, E.; Lück, K.; Philipp, G.; Rühlmann, J.; Schröter, I.; Gebbers, R. Direct prediction of site-specific lime requirement of arable fields using the base neutralizing capacity and a multi-sensor platform for on-the-go soil mapping. Precision Agriculture 2022, 23, 127–149. [Google Scholar] [CrossRef]
- Masud, M.M.; Baquy, M.A.-A.; Akhter, S.; Sen, R.; Barman, A.; Khatun, M.R. Liming effects of poultry litter derived biochar on soil acidity amelioration and maize growth. Ecotoxicology and Environmental Safety 2020, 202, 110865. [Google Scholar] [CrossRef]
- Holland, J.E.; Bennett, A.E.; Newton, A.C.; White, P.J.; McKenzie, B.M.; George, T.S.; Pakeman, R.J.; Bailey, J.S.; Fornara, D.A.; Hayes, R.C. Liming impacts on soils, crops and biodiversity in the UK: A review. Science of The Total Environment 2018, 610-611, 316–332. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, S.K.; Kurzman, A.L.; Arango, C.; Jin, L.; Robertson, G.P. Evidence for carbon sequestration by agricultural liming. Global Biogeochemical Cycles 2007, 21. [Google Scholar] [CrossRef]
- Jovic, M.; Mandic, M.; Sljivic-Ivanovic, M.; Smičiklas, I. Recent trends in application of shell waste from mariculture. 32, -62. [CrossRef]
- Lu, W.; Lu, S.; Jing, H.; Sun, J.; Ji, L.; Guo, J.; Wang, Y.; Cai, L.; Song, F.; Song, W. Hierarchical porous mussel shells as soil amendment for oil spill remediation. Environmental Technology 2022, 43, 3189–3197. [Google Scholar] [CrossRef]
- Mo, K.H.; Alengaram, U.J.; Jumaat, M.Z.; Lee, S.C.; Goh, W.I.; Yuen, C.W. Recycling of seashell waste in concrete: A review. Construction and Building Materials 2018, 162, 751–764. [Google Scholar] [CrossRef]
- Paz-Ferreiro, J.; Baez-Bernal, D.; Castro Insúa, J.; García Pomar, M.I. Effects of mussel shell addition on the chemical and biological properties of a Cambisol. Chemosphere 2012, 86, 1117–1121. [Google Scholar] [CrossRef]
- Dinnis, E.R. Soil science: Methods & applications D. L. Rowell, Longman Scientific & Technical, Longman Group UK Ltd., Harlow, Essex, UK (co-published in the USA with John Wiley & Sons Inc. New York), 1994,. 1994, 66, 573–574. [Google Scholar] [CrossRef]
- Gharibzadeh, F.; Kalantary, R.R.; Golshan, M. Optimization of Influencing Parameters on Phenanthrene Removal Efficiency in Soil Washing Process by Using Response Surface Methodology. Soil and Sediment Contamination: An International Journal 2018, 27, 46–59. [Google Scholar] [CrossRef]
- Ng, Y.-S.; Sen Gupta, B.; Hashim, M.A. Effects of operating parameters on the performance of washing–electrokinetic two stage process as soil remediation method for lead removal. Separation and Purification Technology 2015, 156, 403–413. [Google Scholar] [CrossRef]
- Sankha, B. Central Composite Design for Response Surface Methodology and Its Application in Pharmacy. In Response Surface Methodology in Engineering Science; Palanikumar, K., Ed.; IntechOpen: Rijeka, 2021. [Google Scholar] [CrossRef]
- Fageria, N.K.; Nascente, A.S. Chapter Six - Management of Soil Acidity of South American Soils for Sustainable Crop Production. In Advances in Agronomy; Sparks, D.L., Ed.; Academic Press, 2014; Volume 128, pp. 221–275. [Google Scholar]
- Leiva-Vega, J.; Shene, C.; Silva-Ferrer, D. OPTIMIZATION OF NEUTRALIZING POWER OF Mytilus chilensis SEASHELLS IN ACID ALLUVIAL SOIL OF ÑUBLE COAST. Chilean journal of agricultural & animal sciences 2023, 39, 210–216. [Google Scholar] [CrossRef]
- Egerić, M.; Smičiklas, I.; Dojčinović, B.; Sikirić, B.; Jović, M.; Šljivić-Ivanović, M.; Čakmak, D. Interactions of acidic soil near copper mining and smelting complex and waste-derived alkaline additives. Geoderma 2019, 352, 241–250. [Google Scholar] [CrossRef]
- Fernández-Calviño, D.; Garrido-Rodríguez, B.; Arias-Estévez, M.; Díaz-Raviña, M.; Álvarez-Rodríguez, E.; Fernández-Sanjurjo, M.J.; Nuñez-Delgado, A. Effect of crushed mussel shell addition on bacterial growth in acid polluted soils. Applied Soil Ecology 2015, 85, 65–68. [Google Scholar] [CrossRef]
- ÁLvarez, E.; FernÁNdez-Sanjurjo, M.J.; Seco, N.; NÚÑEz, A. Use of Mussel Shells as a Soil Amendment: Effects on Bulk and Rhizosphere Soil and Pasture Production. Pedosphere 2012, 22, 152–164. [Google Scholar] [CrossRef]
- Morris, J.P.; Backeljau, T.; Chapelle, G. Shells from aquaculture: a valuable biomaterial, not a nuisance waste product. Reviews in Aquaculture 2019, 11, 42–57. [Google Scholar] [CrossRef]
- Summa, D.; Lanzoni, M.; Castaldelli, G.; Fano, E.A.; Tamburini, E. Trends and Opportunities of Bivalve Shells’ Waste Valorization in a Prospect of Circular Blue Bioeconomy. Resources 2022, 11, 48. [Google Scholar] [CrossRef]
- Fernández-Calviño, D.; Cutillas-Barreiro, L.; Núñez-Delgado, A.; Fernández-Sanjurjo, M.J.; Álvarez-Rodriguez, E.; Nóvoa-Muñoz, J.C.; Arias-Estévez, M. Cu Immobilization and Lolium perenne Development in an Acid Vineyard Soil Amended with Crushed Mussel Shell. Land Degradation & Development 2017, 28, 762–772. [Google Scholar] [CrossRef]
- Jacoby, R.; Peukert, M.; Succurro, A.; Koprivova, A.; Kopriva, S. The Role of Soil Microorganisms in Plant Mineral Nutrition-Current Knowledge and Future Directions. Front Plant Sci 2017, 8, 1617. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Lee, D.K.; Ali, M.A.; Kim, P.J. Effects of oyster shell on soil chemical and biological properties and cabbage productivity as a liming materials. Waste Management 2008, 28, 2702–2708. [Google Scholar] [CrossRef] [PubMed]
- Yadav, D.S.; Jaiswal, B.; Gautam, M.; Agrawal, M. Soil Acidification and its Impact on Plants. In Plant Responses to Soil Pollution; Singh, P., Singh, S.K., Prasad, S.M., Eds.; Springer: Singapore, 2020; pp. 1–26. [Google Scholar] [CrossRef]
- Li, Y.; Cui, S.; Chang, S.X.; Zhang, Q. Liming effects on soil pH and crop yield depend on lime material type, application method and rate, and crop species: a global meta-analysis. Journal of Soils and Sediments 2019, 19, 1393–1406. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).