Submitted:
15 December 2023
Posted:
15 December 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Test Organism
2.2. Organisms Sampling
2.3. Toxicity Bioassay
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Santos, J.V.N.; Martins, R.; Fontes, M.K.; Campos, B.G.; Silva, M.B.M.P.; Maia, F.; Abessa, D.M.S.; Perina, F.C. Can encapsulation of the biocide DCOIT affect the anti-fouling efficacy and toxicity on tropical bivalves? Appl. Sci. 2020, 10(23), 8579. [Google Scholar] [CrossRef]
- Gabe, H.B.; Guerreiro, A.D.S.; Sandrini, J.Z. Molecular and biochemical effects of the antifouling DCOIT in the mussel Perna perna. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2021, 239, 108870. [Google Scholar] [CrossRef] [PubMed]
- Abreu, F.E.L.; Silva, J.N.L.; Castro, Í.B.; Fillmann, G. Are antifouling residues a matter of concern in the largest South American port? J. Hazard. Mater. 2020, 398, 122937. [Google Scholar] [CrossRef] [PubMed]
- Jesus, É.P.S.; Figueirêdo, L.P.; Maia, F.; Martins, R.; Nilin, J. Acute and chronic effects of innovative antifouling nanostructured biocides on a tropical marine microcrustacean. Mar. Pollut. Bull. 2021, 164, 111970. [Google Scholar] [CrossRef] [PubMed]
- Creed, J.C.; Fenner, D.; Sammarco, P.; Cairns, S.; Capel., K.; Junqueira, A.O.R.; Cruz, I.; Miranda, R.J.; Carlos-Junior, L.; Mantelatto, M.C.; Oigman-Pszczol, S. The invasion of the azooxanthellate coral Tubastraea (Scleractinia: Dendrophylliidae) throughout the world: history, pathways and vectors. Biol. Invasions 2017, 19, 283–305. [Google Scholar] [CrossRef]
- Fonseca, V.B.; Guerreiro, A.S.; Vargas, M.A.; Sandrini, J.Z. Effects of DCOIT (4,5-dichloro-2-octyl-4-isothiazolin-3-one) to the haemocytes of mussels Perna perna. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2020, 232, 108737. [Google Scholar] [CrossRef] [PubMed]
- Castro, Í.B.; Westfal, E.; Fillmann, G. Third generation antifouling paints: new biocides in the aquatic environment. Quím. Nova 2011, 34(6), 1021-1031. [CrossRef]
- Fernandez, M.A.; Pinheiro, F.M. New approaches for monitoring the marine environment: the case of antifouling paints. Int. J. Environ. Res. Public Health. 2007, 1(3), 427–448. [Google Scholar] [CrossRef]
- Omae, I. General aspects of natural products antifoulants in the environment. In: Antifouling Paint Biocides, Konstantinou, I.K. (ed). Springer, Berlin, Germany, 2005. Volume 50, pp. 227- 262. [CrossRef]
- Campos, B.G.; Figueiredo, J.; Perina, F.C.; Abessa, D.M.S.; Loureiro, S.; Martins, R. Occurrence, effects and environmental risk of antifouling biocides (EU PT21): Are marine ecosystems threatened? Crit. Rev. Environ. Sci. Technol. 2022a, 51. [Google Scholar] [CrossRef]
- Great Britain, Parliament. International Convention on the control of harmful anti-fouling system on ships. Great Britain Parliament. The Stationery Office: London, U.K., 2012. (Treaty Series, v. 13). https://assets.publishing.service.gov.uk/media/5a7cabd5e5274a2f304ef5f5/8284.pdf.
- Chen, L.; Lam, J.C.W. SeaNine 211 as antifouling biocide: A coastal pollutant of emerging concern. J. Environ. Sci. (China) 2017, 61, 68–79. [Google Scholar] [CrossRef]
- Cima, F.; Ferrari, G.; Ferreira, N.G.; Rocha, R.J.; Serôdio, J.; Loureiro, S.; Calado, R. Preliminary evaluation of the toxic effects of the antifouling biocide Sea-Nine211TM in the soft coral Sarcophyton cf. glaucum (Octocorallia, Alcyonacea) based on PAM fluorometry and biomarkers. Mar. Environ. Res. 2013; 83, 16–22. [Google Scholar] [CrossRef]
- Jacobson, A.H.; Willingham, G.L. Sea-nine antifoulant: an environmentally acceptable alternative to organotin antifoulants. Sci. Total Environ. 2020, 258, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Shade, W.D.; Hurt, S.S.; Jacobson, A.H.; Reinert, K.H. Shade, W.D.; Hurt, S.S.; Jacobson, A.H.; Reinert, K.H. Ecological risk assessment of a novel marine antifoulant. Am. Soc. Test. Mater. 1993, 2, 381-408. https://www.astm.org/stp13169s.html. Am. Soc. Test. Mater. 1993, 2, 381–408. [Google Scholar]
- Sinha, S.; Kumar, R.; Anand, J.; Gupta, R.; Gupta, A.; Pant, K.; Dohare, S.; Tiwari, P.; Kesari, K.K.; Krishnan, S.; Gupta, P.K. Nanotechnology-based solutions for antibiofouling applications: an overview. ACS Appl. Nano Mater. 2023, 6(14), 12828–12848. [Google Scholar] [CrossRef]
- Figueiredo, J.; Oliveira, T.; Ferreira, V.; Sushkova, A.; Silva, S.; Carneiro, D.; Cardoso, D.N.; Gonçalves, S.F.; Maia, F.; Rocha, C.; Tedim, J.; Loureiro, S.; Martins, R. Toxicity of innovative anti-fouling nano-based solutions to marine species. Environ. Sci. Nano 2019, 6(5), 1418–1429. [Google Scholar] [CrossRef]
- Figueiredo, J.; Loureiro, S.; Martins, R. Hazard of novel anti-fouling nanomaterials and biocides DCOIT and silver to marine organisms. Environ. Sci. Nano 2020, 7(6), 1670–1680. [Google Scholar] [CrossRef]
- Duprey, N.N.; Yasuhara, M.; Baker, D.M. Reefs of tomorrow: eutrophication reduces coral biodiversity in an urbanized seascape. Glob. Change Biol. 2016, 22(11), 3550–3565. [Google Scholar] [CrossRef] [PubMed]
- Fukushima, L.M.; Capel, K.C.C.; Abessa, D.M.S. Sun Corals - a resistant invader: assessment of the acute toxicity of contaminants associated with petroleum and petrochemical activities in the species Tubastraea coccinea. Proceedings of the 24th AMOP Technical Seminar on Environmental Contamination and Response, Environment and Climate Change Canada, Ottawa, ON, Canada, pp. 626-638. 2022. Available at: http://hdl.handle.net/11449/241533.
- Carpes, R.M.; Alves, M.A.; Creed, J.C.; Da Silva, C.A.; Hamerski, L.; Garden, S.J.; Fleury, B.G.; Felzenszwalb, I. Mutagenic, genotoxic and cytotoxic studies of invasive corals Tubastraea coccinea and Tubastraea tagusensis. J. Appl. Toxicol. 2020, 40(3), 373–387. [Google Scholar] [CrossRef] [PubMed]
- Capel, K.C.C.; Creed, J.; Kitahara, M.V.; Chen, C.A.; Zilberberg, C. Multiple introductions and secondary dispersion of Tubastraea spp. in the Southwestern Atlantic. Sci. Rep. 2019, 9, 13978 (2019). [CrossRef]
- Santos, L.A.H.; Ribeiro, F.V.; Creed, J.C. Antagonism between invasive pest corals Tubastraea spp. and the native reef-builder Mussismilia hispida in the southwest Atlantic. J. Exp. Mar. Biol. Ecol. 2013; 449, 69–76. [Google Scholar] [CrossRef]
- Precht, W.F.; Hickerson, E.L.; Schmahl, G.P.; Aronson, R.B. The Invasive coral Tubastraea coccinea (lesson, 1829): Implications for natural habitats in the Gulf of Mexico and the Florida Keys. Gulf Mex. Sci. 2014, 32(1–2), 55–59, https://aquila.usm.edu/goms/vol32/iss1/5. [Google Scholar] [CrossRef]
- Castro, C.B.; Pires, D.O. Castro, C.B.; Pires, D.O. Brazilian Coral Reefs: What we already know and what is still missing. Bull. Mar. Sci. 2001, 69, 357-371. https://www.ingentaconnect.com/contentone/umrsmas/bullmar/2001/00000069/00000002/art00013. Bull. Mar. Sci. 2001, 69, 357–371. [Google Scholar]
- De Paula, A.F.; Creed, J.C. De Paula, A.F.; Creed, J.C. Two species of the coral Tubastraea (Cnidaria, Scleractinia) in Brazil: a case of accidental introduction. Bull. Mar. Sci. 2004, 74(1). 175-183. https://www.ingentaconnect.com/content/umrsmas/bullmar/2004/00000074/00000001/art00014#. Bull. Mar. Sci. 2004, 74(1), 175–183. [Google Scholar]
- Braga, M.D.A.; Paiva, S.V.; Gurjão, L.M.; Teixeira, C.E.P.; Gurgel, A.L.A.R.; Pereira, P.H.C.; Soares, M.O. Retirement risks: Invasive coral on old oil platform on the Brazilian equatorial continental shelf. Mar. Pollut. Bull. 2021, 165, 112156. [Google Scholar] [CrossRef]
- De Paula, A.F.; Pires, D.O.; Creed, J.C. Reproductive strategies of two invasive sun corals (Tubastraea spp.) in the southwestern Atlantic. J. Mar. Biolog. Assoc. U.K. 2014, 94(3), 481–492. [Google Scholar] [CrossRef]
- Capel, K.C.C.; Toonen, R.J.; Rachid, C.T.C.C.; Creed, J.C.; Kitahara, M.V.; Forsman, Z.; Zilberberg, C. Clone wars: Asexual reproduction dominates in the invasive range of Tubastraea spp. (Anthozoa: Scleractinia) in the South-Atlantic Ocean. PeerJ 2017, 5, e3873. [Google Scholar] [CrossRef] [PubMed]
- Glynn, P.W.; Colley, S.B.; Maté, J.L.; Cortés, J.; Guzman, H.M.; Bailey, R.L.; Feinglod, J.S.; Enochs, I.C. Reproductive ecology of the azooxanthellate coral Tubastraea coccinea in the Equatorial Eastern Pacific: Part V. Dendrophylliidae. Mar. Biol. 2008, 153(4), 529–544. [Google Scholar] [CrossRef]
- Costa, T.J.; Pinheiro, H.T.; Teixeira, J.B.; Mazzei, E.F.; Bueno, L.; Hora, M.S.; Joyeux, J.C.; Carvalho-Filho, A.; Amado-Filho, G.; Sampaio, C.L.; Rocha, L.A. Expansion of an invasive coral species over Abrolhos Bank, Southwestern Atlantic. Mar. Pollut. Bull. 2014, 85(1), 252–253. [Google Scholar] [CrossRef] [PubMed]
- Capel, K.C.C.; Migotto, A.E.; Zilberberg, C.; Kitahara, M.V. Another tool towards invasion? Polyp “bail-out” in Tubastraea coccinea. Coral Reefs 2014, 33, 1165. [Google Scholar] [CrossRef]
- Luz, B.L.P.; Capel, K.C.C.; Zilberberg, C. ; Flores. A.A.V.; Migotto, A.E.; Kitahara, M.V. A polyp from nothing: the extreme regeneration capacity of the Atlantic invasive sun-corals Tubastraea coccinea and T. tagusensis (Anthozoa, Scleractinia). J. Exp. Mar. Biol. Ecol. 2018, 503, 60–65. [Google Scholar] [CrossRef]
- ICMBIO. Pan Corais. Sumário Executivo do Plano de Ação Nacional para a Conservação dos Ambientes Coralíneos. Instituto Chico Mendes de Conservação da Biodiversidade. ICMBio/MMA. Brasília, DF, Brazil. 2016. Available at: https://www.icmbio.gov.br/portal/faunabrasileira/plano-de-acao-nacional-lista/3620-plano-de%02acao-nacional-paraconservacao-dos-recifes-de-corais. Accessed: 5 May 2022.
- Perina, F.; Ottoni, C.; Santos, J.; Santos, V.; Silva, M.; Campos, B.; Fontes, M.; Santana, D.; Maia, F.; Abessa, D.; Martins, R. Marine hazard assessment of soluble and nanostructured forms of the booster biocide DCOIT in tropical waters. Water 2023, 15, 1185. [Google Scholar] [CrossRef]
- Roepke, L.K.; Brefeld, D.; Soltmann, U.; Randall, C.J.; Negri, A.P.; Kunzmann, A. Antifouling coatings can reduce algal growth while preserving coral settlement. Sci. Rep. 2022a, 12, 15935. [Google Scholar] [CrossRef]
- Wendt, I.; Backhaus, T.; Blanck, H.; Arrhenius, Å. The toxicity of the three antifouling biocides DCOIT, TPBP and medetomidine to the marine pelagic copepod Acartia tonsa. Ecotoxicology 2016, 25, 871–879. [Google Scholar] [CrossRef]
- Campos, B.G.; Silva, M.B.M.P.; Avelelas, F.; Maia, F.; Loureiro, S.; Perina, F.; Abessa, D.M.S.; Martins, R. Toxicity of innovative antifouling additives on an early life stage of the oyster Crassostrea gigas: short and long-term exposure effects. Environ. Sci. Pollut. Res. 2022b, 29, 27534–27547. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, V.; Pavlaki, M.D.; Martins, R.; Monteiro, M.S.; Maia, F.; Tedim, J.; Soares, A.M.V.M.; Calado, R.; Loureiro, S. Effects of nanostructure antifouling biocides towards a coral species in the context of global changes. Sci. Total Environ. 2021, 799, 149324. [Google Scholar] [CrossRef] [PubMed]
- Roepke, L.K.; Brefeld, D.; Soltmann, U.; Randall, C.J.; Negri, A.P.; Kunzmann, A. Applying behavioral studies to the ecotoxicology of corals: A case study on Acropora millepora. Front. Mar. Sci. 2022b, 9. [CrossRef]
- Natálio, L.F.; Chernieski, D.; Tomida, L.; Capel, K.C.C. (2022) Alien corals in a Brazilian seaport and perspectives for improving marine bioinvasion detection and management in commercial ports. Ocean Coast. Manag. 2022, 218, 106021. [Google Scholar] [CrossRef]
- Tanasovici, R.M.; Kitahara, M.V.; Dias, G.M. Invasive coral Tubastraea spp. population growth in artificial habitats and its consequences to the diversity of benthic organisms. Mar. Biol. 2020, 167, 119. [Google Scholar] [CrossRef]
- Brockinton, E.E.; Peterson, M.R.; Wang, H.-H.; Grant, W.E. Importance of Anthropogenic Determinants of Tubastraea coccinea Invasion in the Northern Gulf of Mexico. Water 2022, 14, 1365. [Google Scholar] [CrossRef]

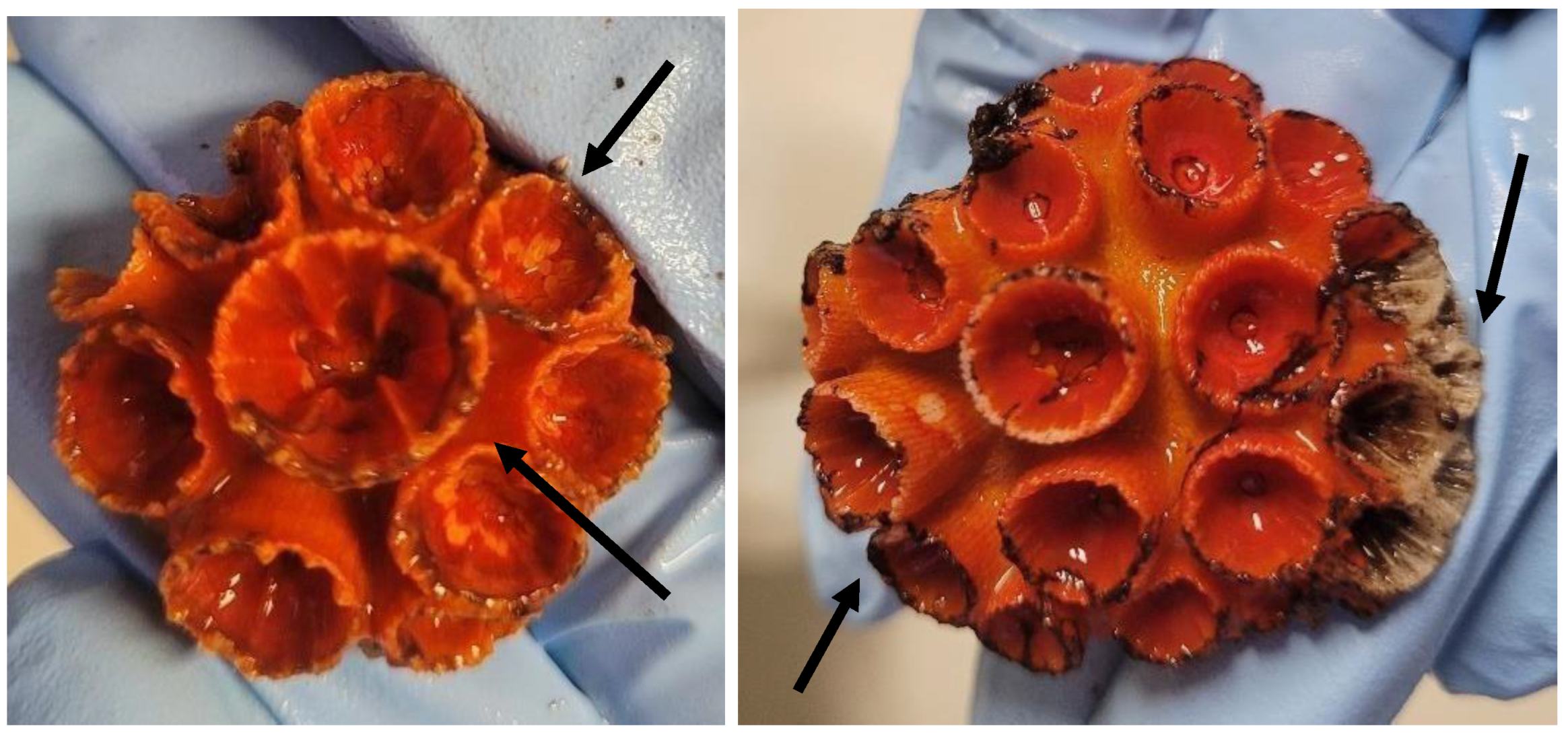
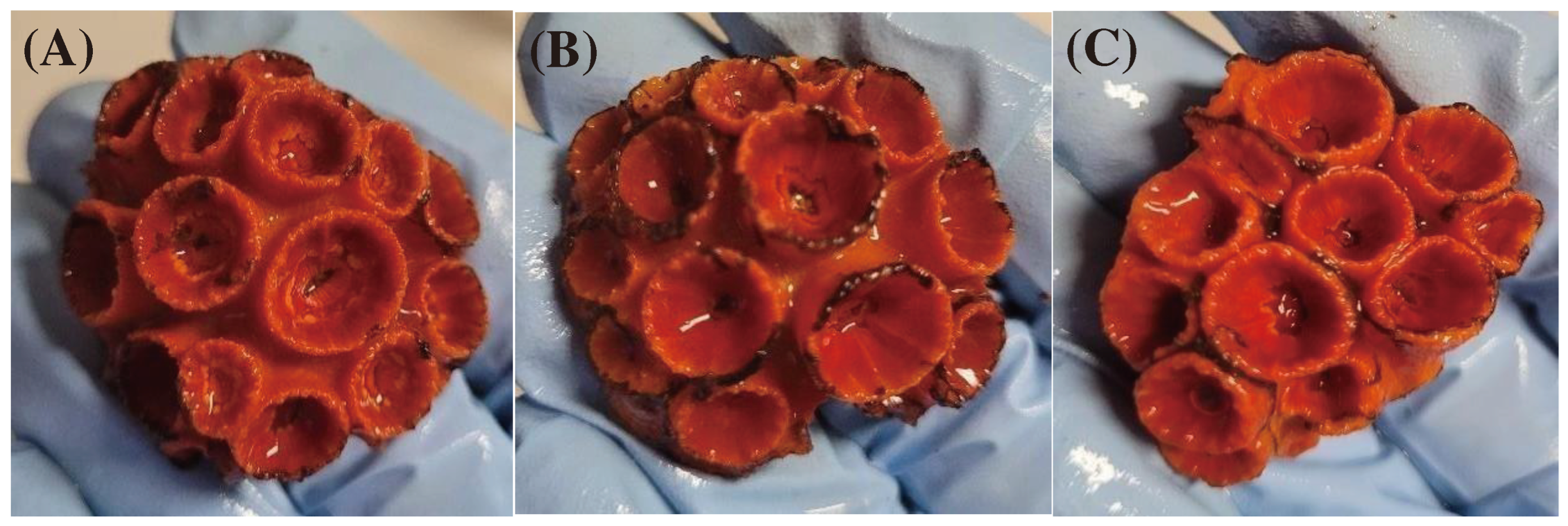
| Treatment | Concentration (µg L-1) | Tissue Necrosis (% of Total Colonies/Replicate) | Fragile Polyps (n.) | Dead Polyps (n.) |
|---|---|---|---|---|
| Control 1 | 0 | * | 0 | 0 |
| Control 2 | 0 | * | 0 | 0 |
| Control 3 | 0 | * | 0 | 0 |
| DCOIT | 3.33 | 100 | 0 | 0 |
| 10 | 66 | 0 | 0 | |
| 33 | 100 | 0 | 0 | |
| 100 | 66 | 2 | 0 | |
| SiNC | 500 | 66 | 0 | 0 |
| 1000 | 33 | 1 | 0 | |
| 2000 | 33 | 0 | 0 | |
| 4000 | 33 | 2 | 1 | |
| SiNC-DCOIT | 74.1 | 100 | 0 | 0 |
| 222.2 | 100 | 0 | 0 | |
| 666.7 | 33 | 3 | 0 | |
| 2000 | 33 | 0 | 0 | |
| SiNC-DCOIT-Ag | 74.1 | 100 | 0 | 0 |
| 222.2 | 66 | 1 | 0 | |
| 666.7 | 66 | 0 | 0 | |
| 2000 | 100 | 2 | 5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





