Submitted:
14 December 2023
Posted:
15 December 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
1.1. Current gaps and problems
1.2. Materials and Methods - Inclusion criteria for proposed therapies
- very promising effectiveness, based on favorable randomized controlled trials (RCTs) of sufficient validity,* complemented by consistent observational studies, available on the major databases of biomedical literature. Even if the evidence is not definitive, attending physicians and informed patients can consider them to the extent that they are at the same time also:
- safe (primum non nocere!), to the state of knowledge
- affordable, with a very favorable opportunity cost, allowing resources to be saved for other valuable healthcare uses
- biologically plausible (or at least not clearly implausible)
- accessible (or capable of quickly granting accessibility)
- without major commercial sponsors in the trials promoting them, and realized by researchers without major conflicts of interests.
(but also by closing the list with a negative didactic example, of an active ingredient among the most used and recommended worldwide, although it does not meet five of the six aforementioned golden criteria).
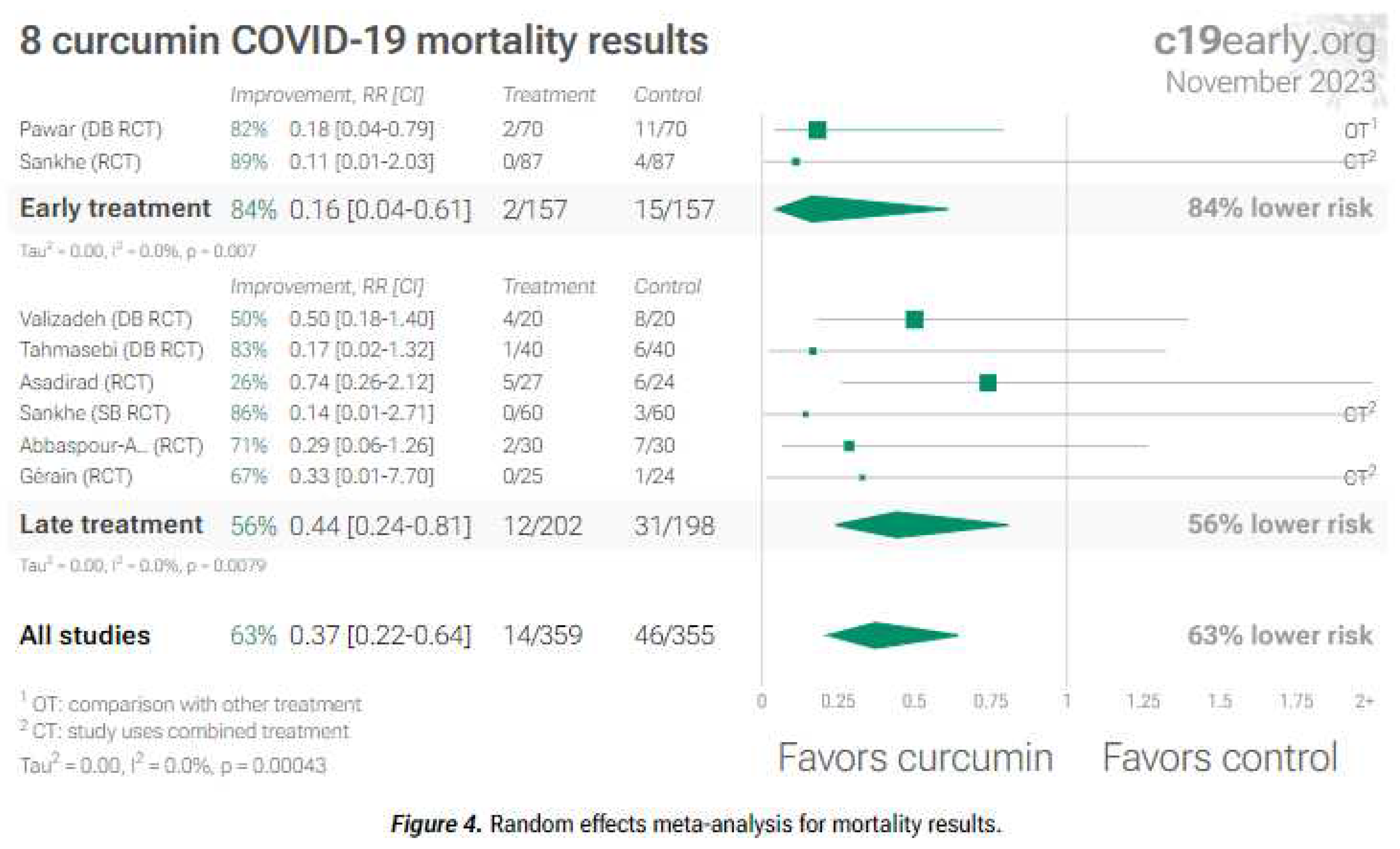
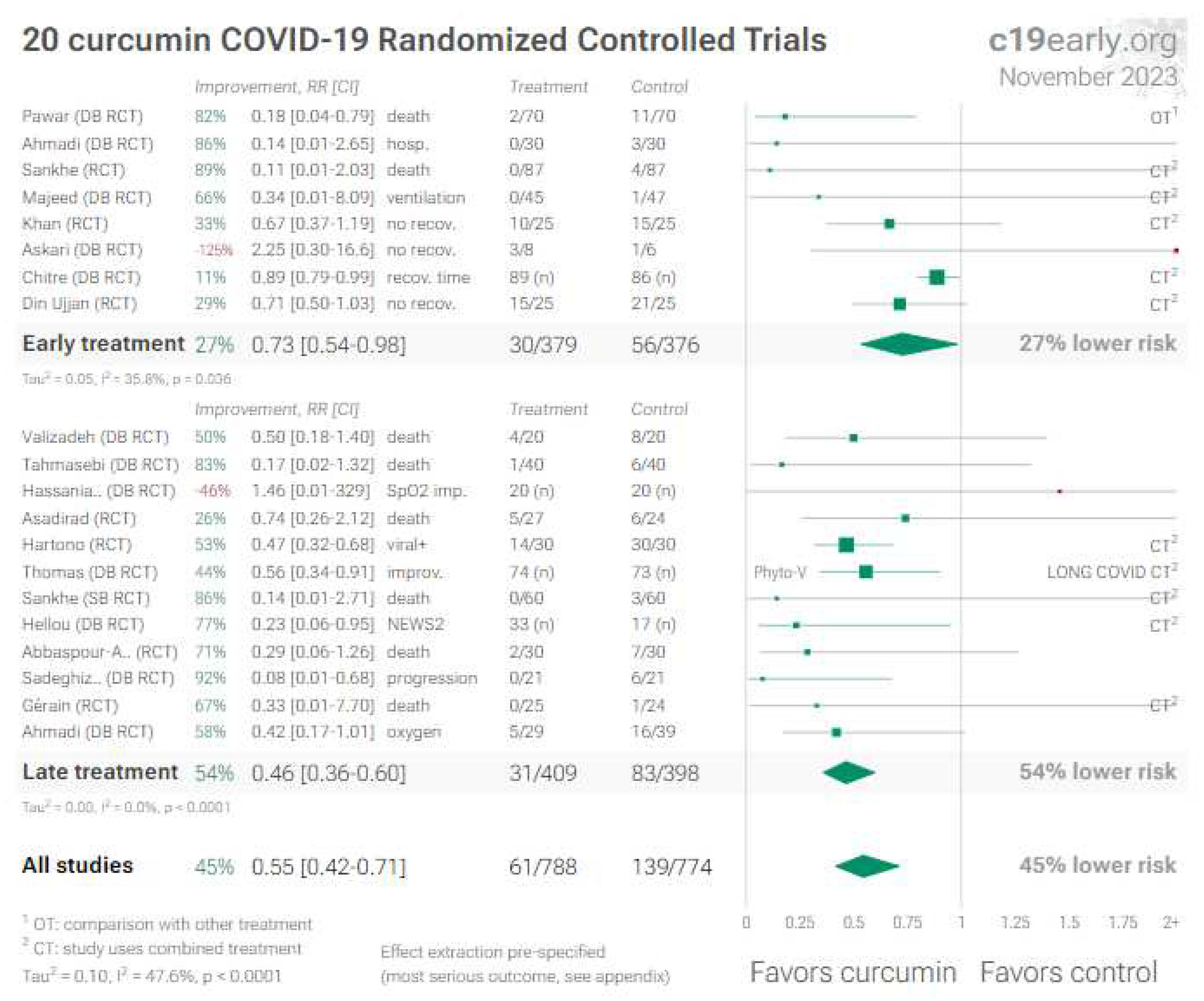
2. Curcumin
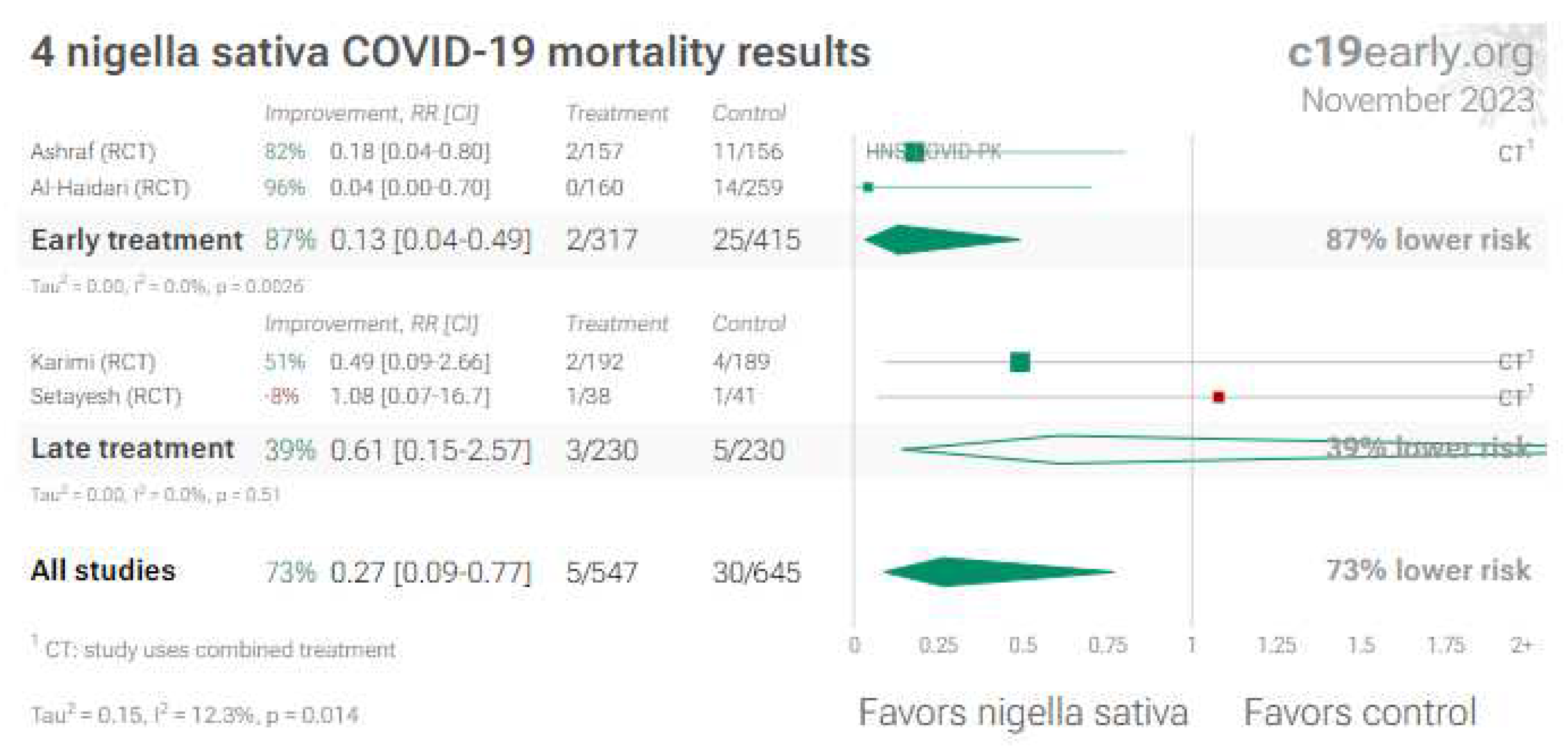
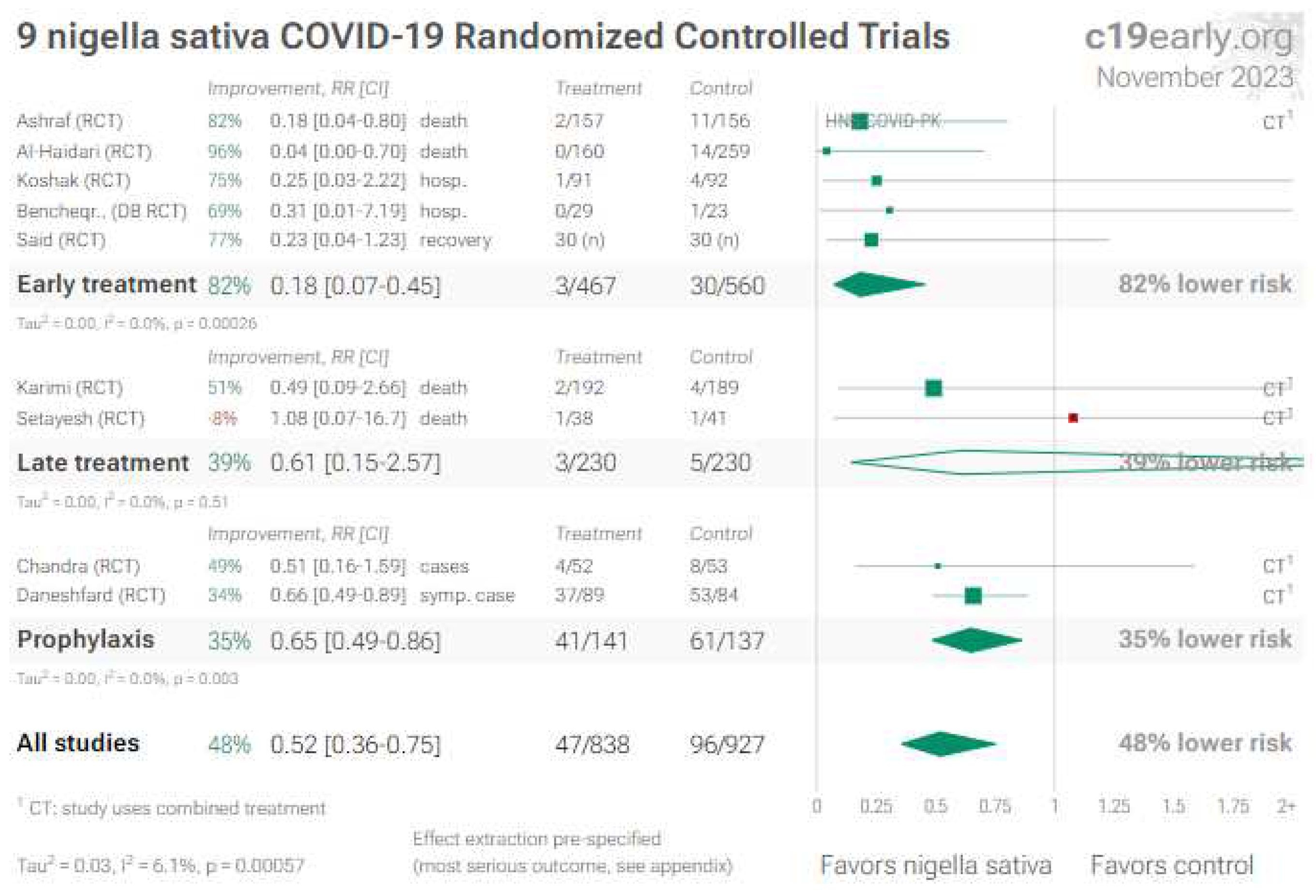
3. Nigella Sativa (Black Seed)
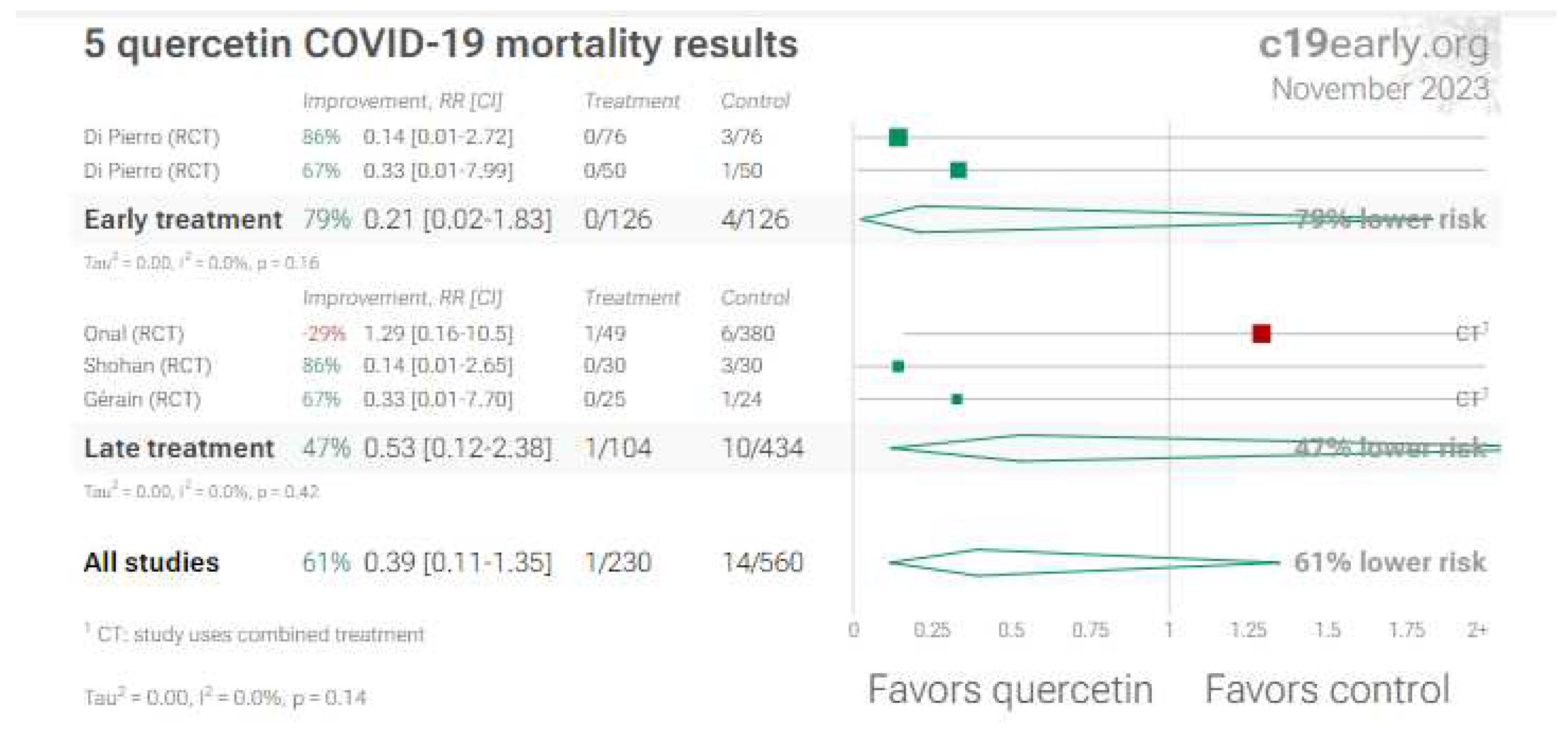
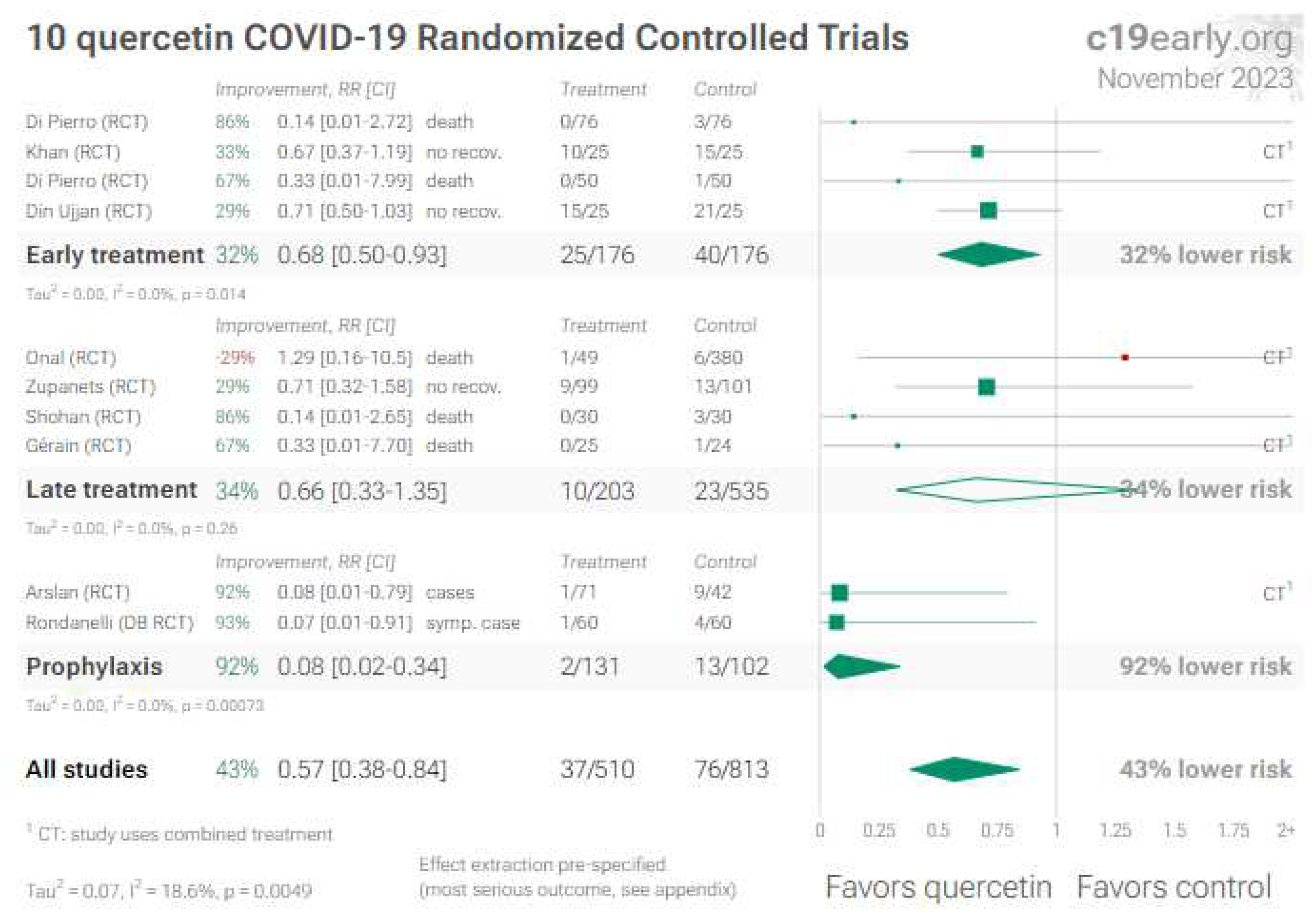
4. Quercetin
5. L-arginine
5.1. Long-COVID treatment
- it is true that the RCT [91] is not double blind, has only 4 weeks of follow-up and it is very small, with the possibility that a larger RCT would not confirm its excellent results, however
- one does not see valid reasons to speak of “uncertainty about potential harms”, which the trial’s results do not allow to hypothesize, even more so given that the active ingredients are introduced daily even with a healthy diet, albeit at doses lower than those effectively administered in the RCT. Moreover, the review [93] states “Adverse events and Severe adverse events: No information”, but this is not true, because the Authors [91] report that “L-arginine plus vitamin C supplementation was safe and well tolerated, and no adverse events were recorded”; even more so because the retention was complete in both groups
- the problem of Long-COVID is widespread and socially very relevant, and currently lacks therapeutic options confirmed by valid research
- the product used in the RCT [91] still satisfies all the “Inclusion criteria for proposed therapies”, and, although awaiting more definitive research, there are good reasons to inform doctors and to offer it to patients with persistent COVID-19 sequelae.
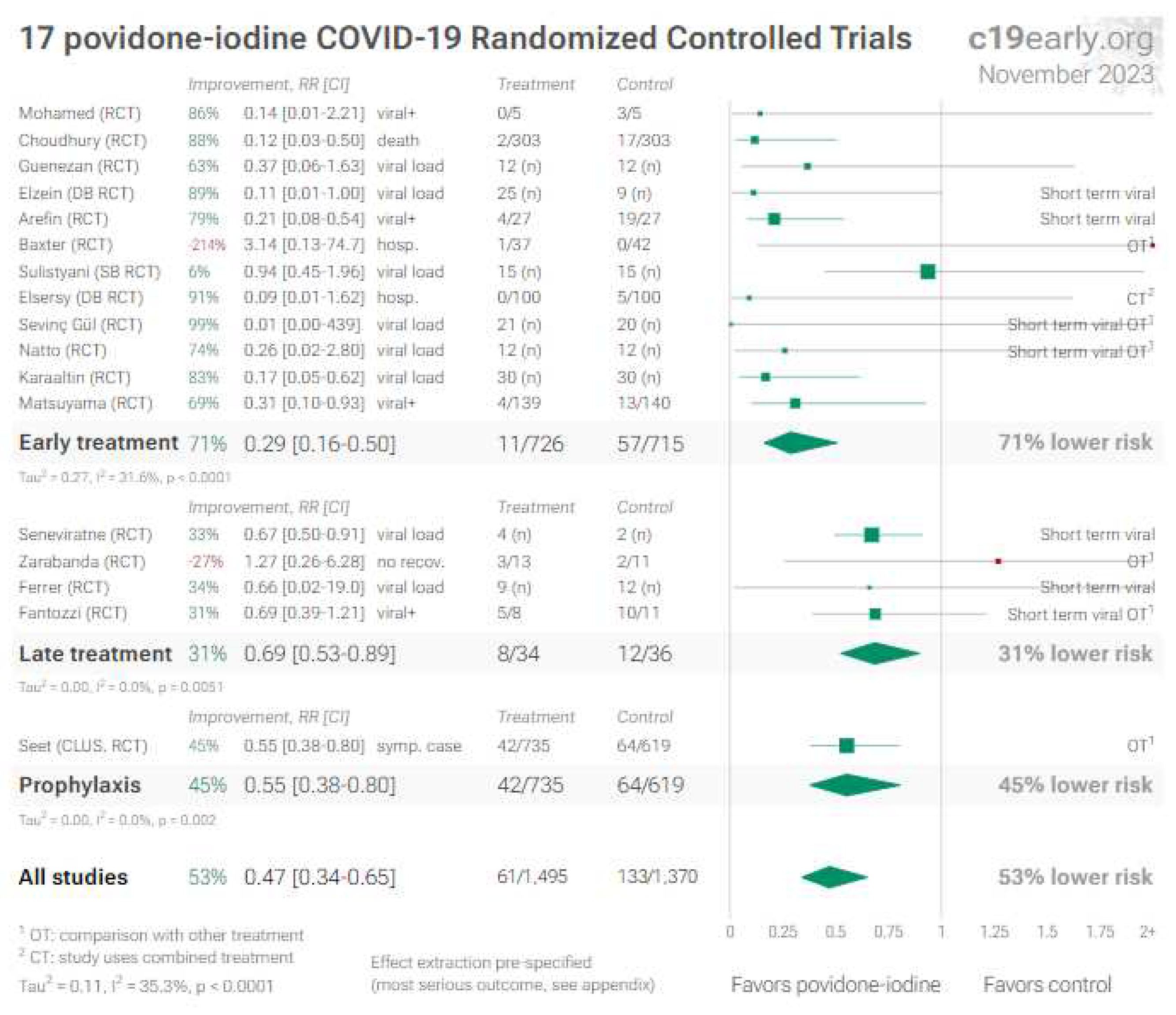
6. Povidone-iodine (and other antiseptics for local use)
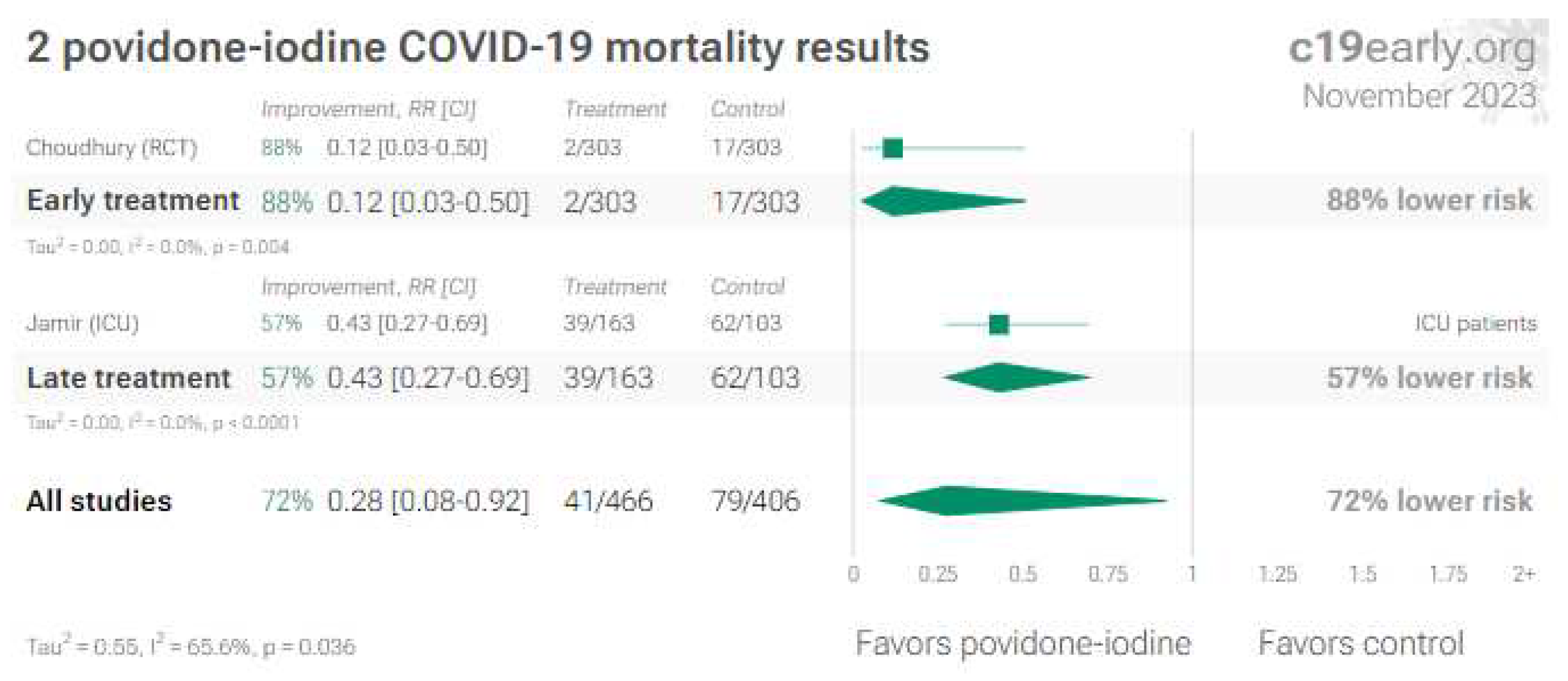
6.1. Nose/oropharyngeal treatments
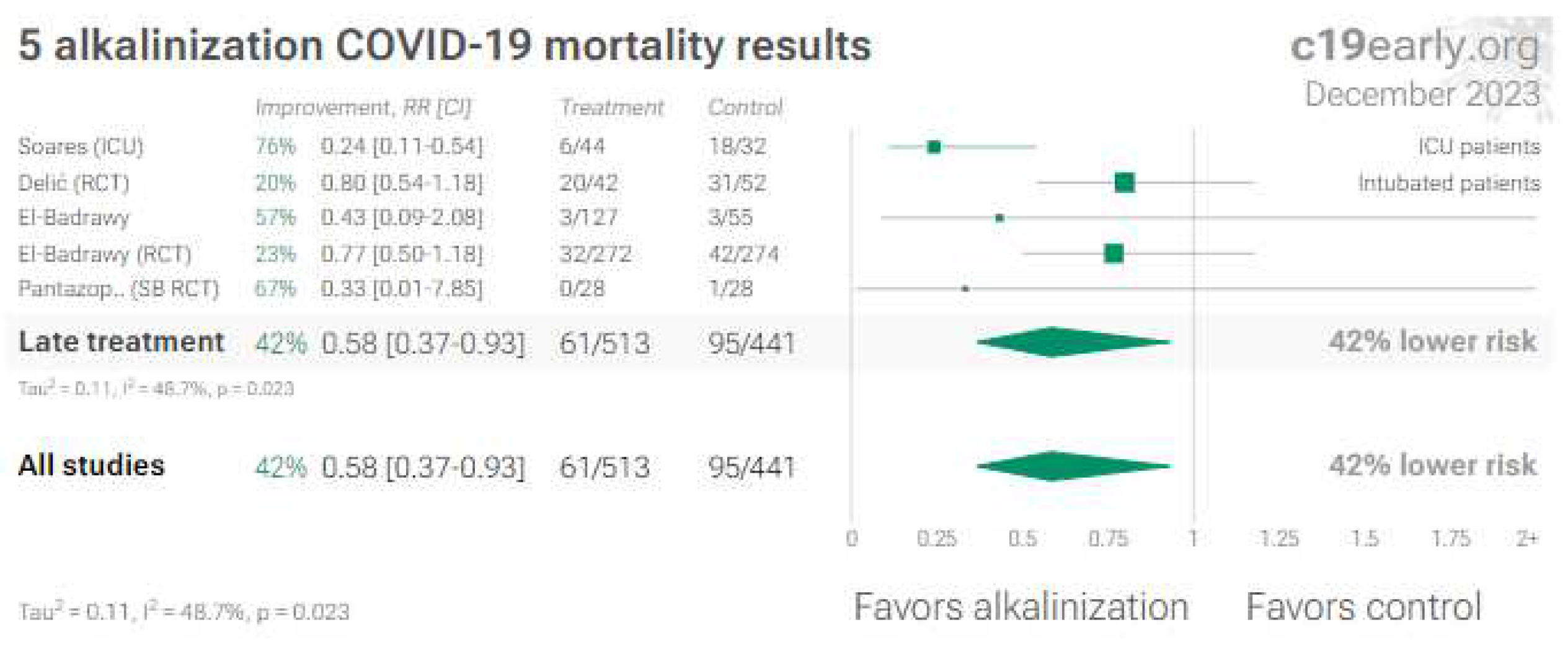
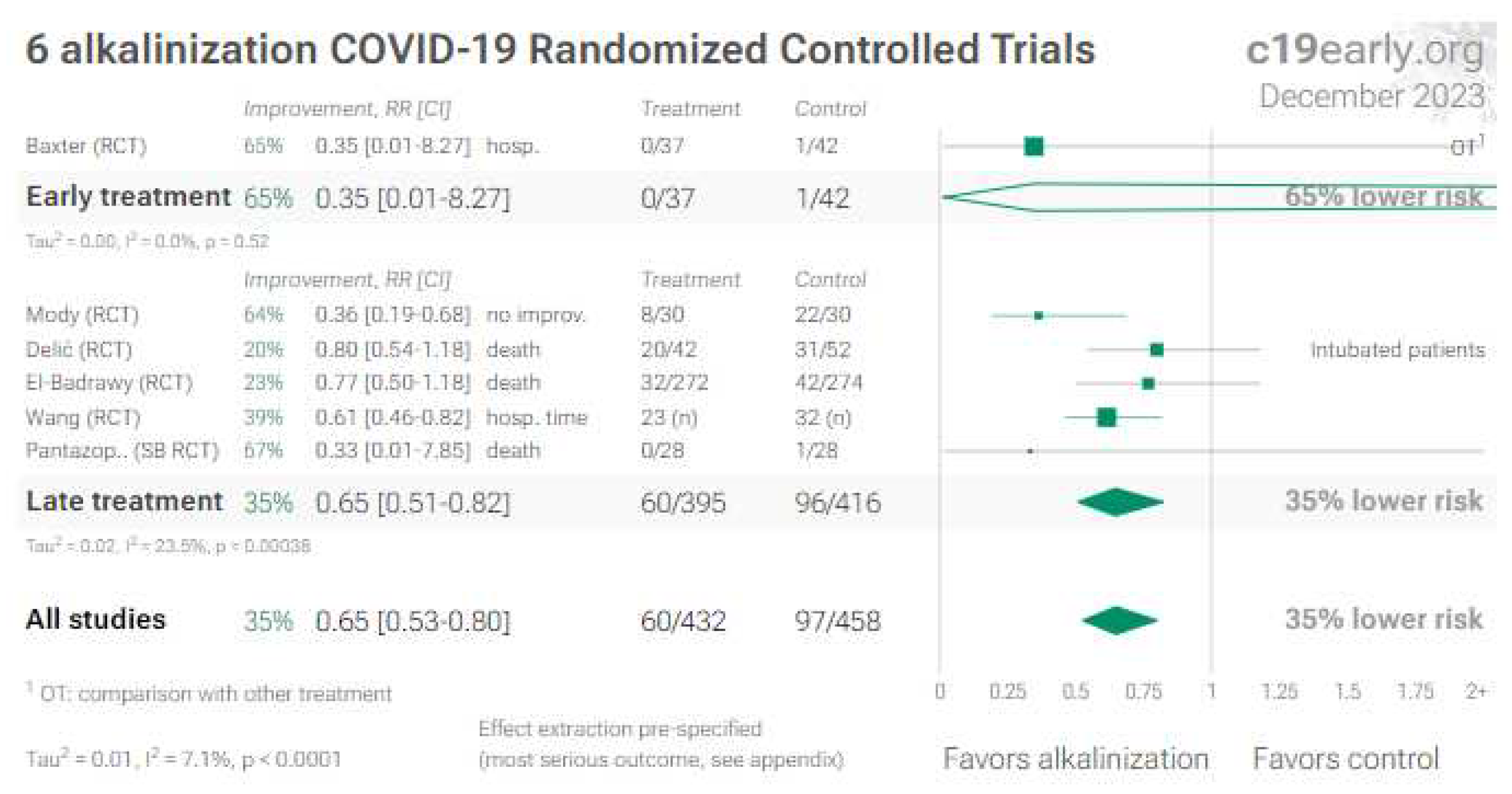
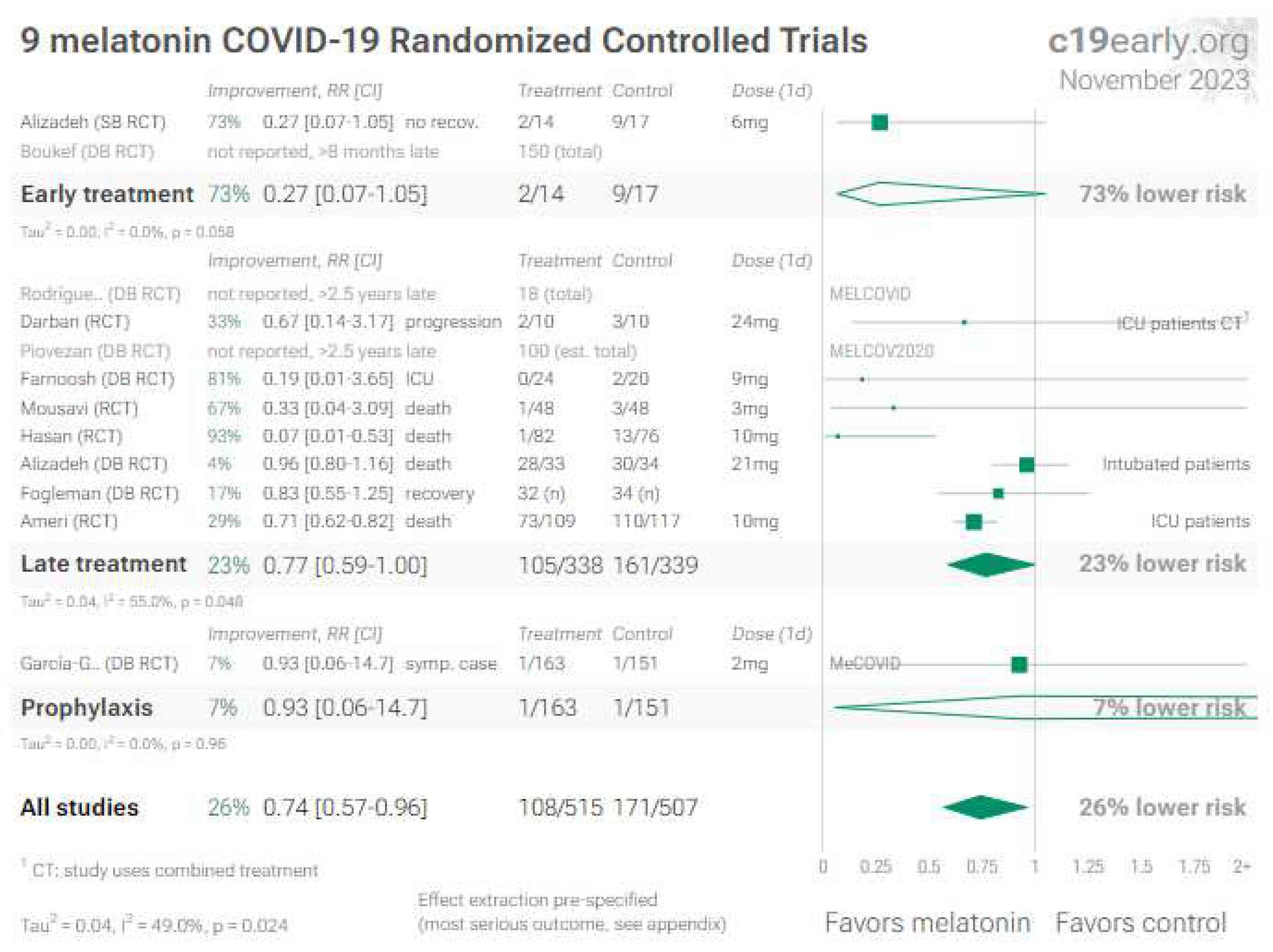
7. Melatonin
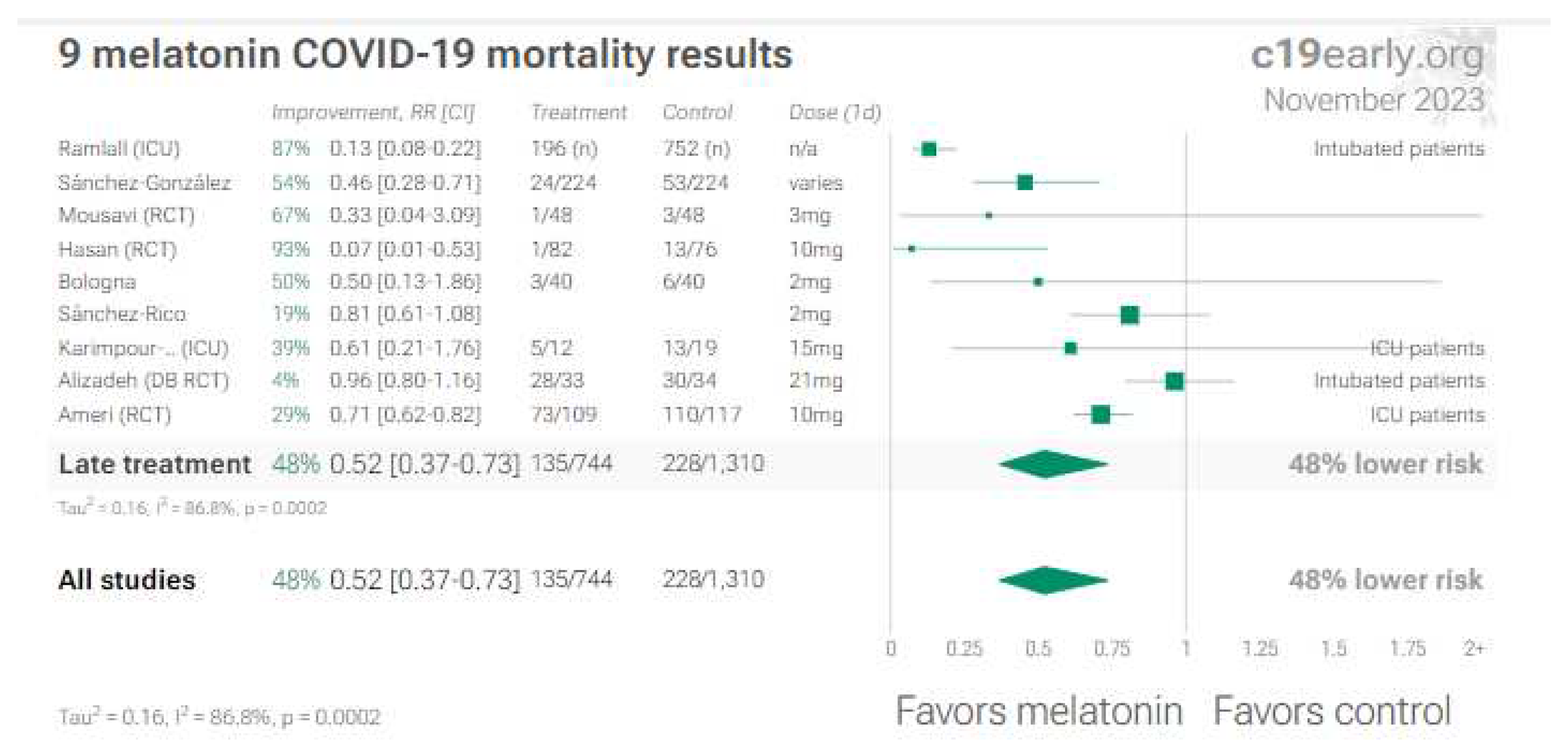
8. Discussion
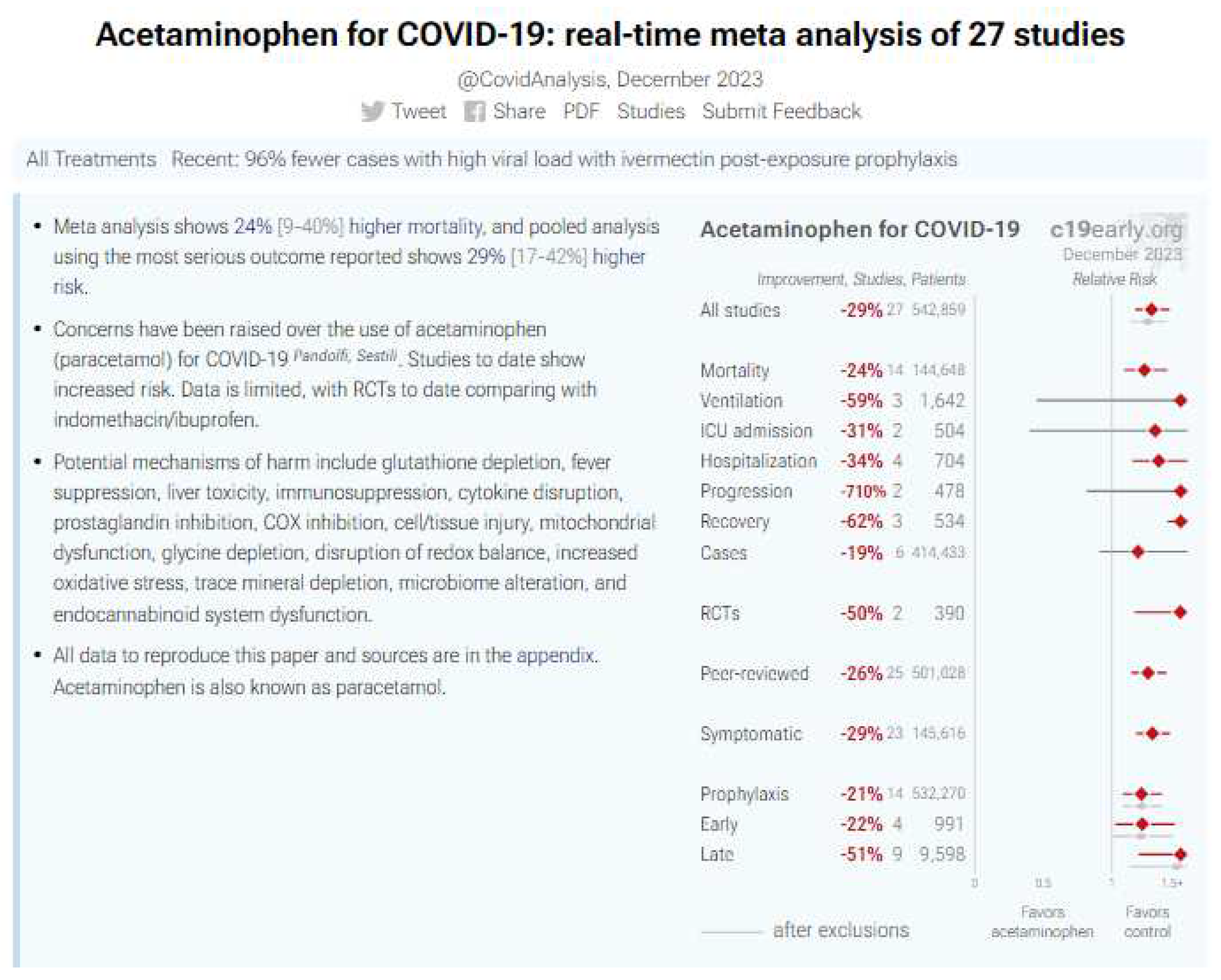
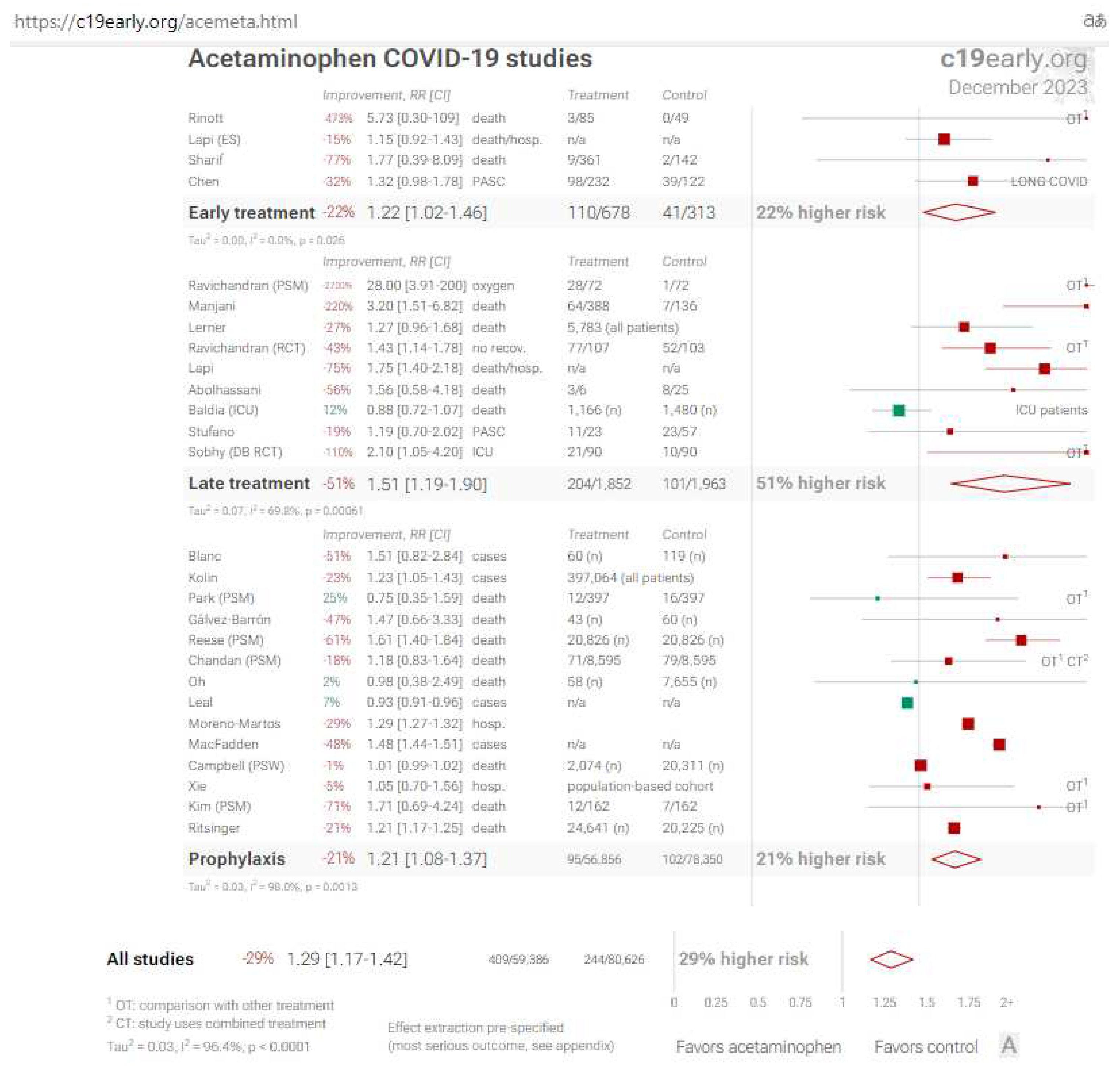
8.1. Acetaminophen (Paracetamol): a negative didactic example
9. Conclusions
10. Future Directions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Agarwal, A.; Hunt, B.; Stegemann, M.; et al. A living WHO guideline on drugs for covid-19. BMJ. 2020 Sep 4;370:m3379. Update in: BMJ. 2020 Nov 19;371:m4475. Update in: BMJ. 2021 Mar 31;372:n860. Update in: BMJ. 2021 Jul 6;374:n1703. Update in: BMJ. 2021 Sep 23;374:n2219. Erratum in: BMJ. 2022 Apr 25;377:o1045. [CrossRef]
- Qaseem, A.; Yost, J.; Miller, M.C.; et al. Outpatient Treatment of Confirmed COVID-19: Living, Rapid Practice Points From the American College of Physicians (Version 1). Ann Intern Med. 2023 Jan;176(1):115-124. Epub 2022 Nov 29. Erratum in: Ann Intern Med. 2023 May;176(5):735-736. [CrossRef]
- Lagevrio: Withdrawn application | European Medicines Agency (europa.eu).
- Liverpool COVID-19 Interactions (covid19-druginteractions.org).
- Arbel, R.; Wolff Sagy, Y.; Hoshen, M.; et al. Nirmatrelvir Use and Severe Covid-19 Outcomes during the Omicron Surge. New Engl J Med 2022; 387:790-798. [CrossRef]
- Edelstein, G.E.; Boucau, J.; Uddin, R.; et al. SARS-CoV-2 Virologic Rebound With Nirmatrelvir–Ritonavir Therapy: An Observational Study. Ann Intern Med. [Epub 14 November 2023]. [CrossRef]
- Cohen, M.S.; Brown, E.R. Rebound of COVID-19 With Nirmatrelvir–Ritonavir Antiviral Therapy. Ann Intern Med. [Epub 14 November 2023]. [CrossRef]
- Ioannou, G.N.; Berry, K.; Rajeevan, N.; Li, Y.; Mutalik, P.; Yan, L.; Bui, D; Cunningham, F.; Hynes, D.M.; Rowneki, M.; et al. Effectiveness of Nirmatrelvir–Ritonavir Against the Development of Post–COVID-19 Conditions Among U.S. Veterans. Ann Intern Med. 2023;176(11):1486-1497. [CrossRef]
- Gøtzsche, P.C. Why some of us no longer want to publish in prestigious medical journals. Nov 14, 2023. 2023-Gotsche-Why-some-of-us-no-longer-want-to-publish-in-prestigious-medical-journals.docx (live.com).
- Prasad, S.; Tyagi, A.K.; Aggarwal, B.B. Recent Developments in Delivery, Bioavailability, Absorption and Metabolism of Curcumin: The Golden Pigment from Golden Spice. Cancer Res. Treat. 2014, 46, 2–18. [Google Scholar] [CrossRef]
- Kunnumakkara, A.B.; Bordoloi, D.; Padmavathi, G.; Monisha, J.; Roy, N.K.; Prasad, S.; Aggarwal, B.B. Curcumin, the golden nutraceutical: Multitargeting for multiple chronic diseases. Br. J. Pharmacol 2017, 174, 1325–1348. [Google Scholar] [CrossRef]
- Praditya D, Kirchhoff L, Brüning J, Rachmawati H, Steinmann J, Steinmann E: Anti-infective properties of 2023 Hulscher et al. Cureus 15(11): e49204. DOI 10.7759/cureus.49204 11 of 12 the golden spice curcumin. Front Microbiol. 2019, 10:912. [CrossRef]
- Karampela, I.; Kokoris, S.; Dalamaga, M. The combination of bromelain and curcumin as an immune-boosting nutraceutical in the prevention of severe COVID-19. Metabol Open. 2020 Dec;8:100066. [CrossRef]
- Dehzad, M.J.; Ghalandari, H.; Nouri, M.; Askarpour, M. Antioxidant and anti-inflammatory effects of curcumin/turmeric supplementation in adults: A GRADE-assessed systematic review and dose–response meta-analysis of randomized controlled trials. Cytokine 2023, 164, 156144. [Google Scholar] [CrossRef]
- Naghsh, N.; Musazadeh, V.; Nikpayam, O.; Kavyani, Z.; Moridpour, A.H.; Golandam, F.; Faghfouri, A.H.; Ostadrahimi, A. Profiling Inflammatory Biomarkers following Curcumin Supplementation: An Umbrella Meta-Analysis of Randomized Clinical Trials. Evid.-Based Complement. Altern. Med 2023, 2023, 4875636. [Google Scholar] [CrossRef]
- Anand, P.; Kunnumakkara, A.B.; Newman, R.A.; Aggarwal, B.B. Bioavailability of curcumin: Problems and promises. Mol. Pharm 2007, 4, 807–818. [Google Scholar] [CrossRef]
- Pawar K.S., Mastud R.N., Pawar S.K., et al. Oral Curcumin With Piperine as Adjuvant Therapy for the Treatment of COVID-19: A Randomized Clinical Trial. Frontiers in Pharmacology. 2021. https://www.frontiersin.org/articles/10.3389/fphar.2021.669362/full. [CrossRef]
- Thimmulappa, R.K.; Mudnakudu-Nagaraju, K.K.; Shivamallu, C.; Subramaniam, K.; Radhakrishnan, A.; Bhojraj, S.; Kuppusamy, G. Antiviral and immunomodulatory activity of curcumin: A case for prophylactic therapy for COVID-19. Heliyon 2021, 7, e06350. [Google Scholar] [CrossRef]
- Abdelazeem, B.; Ahmed, K. Awad, A.K.; Elbadawy, M.A.; Manasrah, N.; Malik, B.; Yousaf, A.; Alqasem, S.; Banour, S.; Abdelmohsen, S.M. The effects of curcumin as dietary supplement for patients with COVID-19: A systematic review of randomized clinical trials. Drug Discoveries & Therapeutics. 2022; 16(1):14-22. [CrossRef]
- Hegde M; Girisa, S.; BharathwajChetty, B.; Vishwa, R.; and B. Kunnumakkara, A.B. Curcumin formulations for better bioavailability: what we learned from clinical trials thus far?. ACS Omega. 2023, 8:10713-46. [CrossRef]
- COVID-19 early treatment: real-time analysis of 3,492 studies. COVID-19 early treatment: real-time analysis of 3,492 studies (c19early.org).
- Sankhe A.P., Memane N.S., Gawali V.P. et al., A prospective, multi center, single blind, randomized controlled study evaluating “AyurCoro3” as an adjuvant in the treatment of mild to moderate COVID, Journal of Ayurveda and Integrated Medical Sciences, 2021. https://jaims.in/jaims/article/view/1386/1425. [CrossRef]
- Valizadeh H., Abdolmohammadi-Vahid S., Danshina S., et al., Nano-curcumin therapy, a promising method in modulating inflammatory cytokines in COVID-19 patients, Int. Immunopharmacol. 2020. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7574843/. [CrossRef]
- Tahmasebi S., Saeed B.Q., Temirgalieva E. et al., Nanocurcumin improves Treg cell responses in patients with mild and severe SARS-CoV2, Life Sciences, 2021. https://www.sciencedirect.com/scie../article/abs/pii/S0024320521004227. [CrossRef]
- Asadirad A., Nashibi R., Khodadadi A., et al., Antiinflammatory potential of nano-curcumin as an alternative therapeutic agent for the treatment of mild-to-moderate hospitalized COVID-19 patients in a placebo-controlled clinical trial, Phytotherapy Research, 2023. https://onlinelibrary.wiley.com/doi/10.1002/ptr.7375. [CrossRef]
- Sankhe (B) A.P., Memane N.S., Gawali V.P. et al., A Randomized, Controlled, Blinded, Parallel Group, Clinical Trial to study the role of Ayurcov (AyurCoro3), one day regimen as an adjuvant therapy for COVID-19 disease management, at dedicated Covid Hospital (DCH) in India, Complementary Therapies in Medicine, 2022. https://www.sciencedirect.com/science/article/pii/S0965229922000267. [CrossRef]
- Abbaspour-Aghdam S., Hazrati A., Abdolmohammadi-Vahid S., et al., Immunomodulatory role of Nanocurcumin in COVID-19 patients with dropped natural killer cells frequency and function, European Journal of Pharmacology, 2022. https://www.sciencedirect.com/science/article/pii/S0014299922005283. [CrossRef]
- Gérain J., Uebelhoer M., Costes B. et al., NASAFYTOL® supplementation in adults hospitalized with COVID-19 infection: results from an exploratory open-label randomized controlled trial, Frontiers in Nutrition, 2023 . https://www.frontiersin.org/articles/10.3389/fnut.2023.1137407/full. [CrossRef]
- Ahmadi R., Salari S., Sharifi M.D. et al., Oral nano-curcumin formulation efficacy in the management of mild to moderate outpatient COVID-19: A randomized triple-blind placebo-controlled clinical trial, Food Science and Nutrition, 2021. https://onlinelibrary.wiley.com/doi/full/10.1002/fsn3.2226. [CrossRef]
- Majeed M., Nagabhushanam K., Shah K. et al., A Randomized, Double-Blind, Placebo-Controlled Study to Assess the Efficacy and Safety of a Nutritional Supplement (ImmuActive) for COVID-19 Patients, Evidence-Based Complementary and Alternative Medicine, 2021. https://www.hindawi.com/journals/ecam/2021/8447545/. [CrossRef]
- Khan A., Iqtadar S., Mumtaz S.U., et al., Oral Co-Supplementation of Curcumin, Quercetin, and Vitamin D3 as an Adjuvant Therapy for Mild to Moderate Symptoms of COVID-19—Results From a Pilot Open-Label, Randomized Controlled Trial, Frontiers in Pharmacology, 2022. https://www.frontiersin.org/articles/10.3389/fphar.2022.898062/full. [CrossRef]
- Askari G., Sahebkar A., Soleimani D. et al., The efficacy of curcumin-piperine co-supplementation on clinical symptoms, duration, severity, and inflammatory factors in COVID-19 outpatients: a randomized double-blind, placebo-controlled trial, Trials, 2022 . https://trialsjournal.biomedcentra..rticles/10.1186/s13063-022-06375-w. [CrossRef]
- Chitre D., Nadkarni S., Jagtap N et al., Phase III randomized clinical trial of BV-4051, an Ayurvedic polyherbal formulation in moderate SARS-CoV-2 infections and its impact on inflammatory biomarkers, Phytotherapy Research, 2023. https://onlinelibrary.wiley.com/doi/10.1002/ptr.7683. [CrossRef]
- Ujjan I.D., Khan S., Nigar R. et al., The possible therapeutic role of curcumin and quercetin in the early-stage of COVID-19—Results from a pragmatic randomized clinical trial, Frontiers in Nutrition, 2022. https://www.frontiersin.org/articles/10.3389/fnut.2022.1023997/full. [CrossRef]
- Hassaniazad M., Eftekhar E., Inchehsablagh B.R. et al., A triple-blind, placebo-controlled, randomized clinical trial to evaluate the effect of curcumin-containing nanomicelles on cellular immune responses subtypes and clinical outcome in COVID-19 patients, Phytotherapy Research, 2021. https://onlinelibrary.wiley.com/doi/full/10.1002/ptr.7294. [CrossRef]
- Hartono B., Suryawati Y. Sari A. et al., The Effect of Curcumin and Virgin Coconut Oil Towards Cytokines Levels in COVID-19 Patients at Universitas Sebelas Maret Hospital, Surakarta, Indonesia, Pharmacognosy Journal, 2022. https://phcogj.com/article/1755. [CrossRef]
- Thomas R., Williams M., Aldous J., et al., A Randomised, Double-Blind, Placebo-Controlled Trial Evaluating Concentrated Phytochemical-Rich Nutritional Capsule in Addition to a Probiotic Capsule on Clinical Outcomes among Individuals with COVID-19—The UK Phyto-V Study, COVID, 2022. https://www.mdpi.com/2673-8112/2/4/31. [CrossRef]
- Hellou E., Mohsin J., Elemy A., et al., Effect of ArtemiC in patients with COVID-19: A Phase II prospective study, Journal of Cellular and Molecular Medicine, 2022 . https://onlinelibrary.wiley.com/doi/10.1111/jcmm.17337. [CrossRef]
- Sadeghizadeh M., Asadollahi E., Jahangiri B. et al., Promising clinical outcomes of nano-curcumin treatment as an adjunct therapy in hospitalized COVID-19 patients: A randomized, double-blinded, placebo-controlled trial, Phytotherapy Research, 2023 . https://onlinelibrary.wiley.com/doi/10.1002/ptr.7844. [CrossRef]
- Ahmadi (B) S., Mehrabi Z., Zare M. et al., Efficacy of Nanocurcumin as an Add-On Treatment for Patients Hospitalized with COVID-19: A Double-Blind, Randomized Clinical Trial, International Journal of Clinical Practice, 2023 hindawi.com, doi.org.
- Fessler, S.N.; Chang, Y.; Liu, L.; Johnston, C.S. Curcumin Confers Anti-Inflammatory Effects in Adults Who Recovered from COVID-19 and Were Subsequently Vaccinated: A Randomized Controlled Trial. Nutrients 2023, 15, 1548. [Google Scholar] [CrossRef]
- Koshak A, Wei L, Koshak E, et al. Nigella sativa supplementation improves asthma control and biomarkers: a randomized, double-blind, placebo-controlled trial. Phyther Res. 2017;(January). [CrossRef]
- Razmpoosh E, Safi S, Abdollahi N, et al. The effect of Nigella sativa on the measures of liver and kidney parameters: a systematic review and meta-analysis of randomized-controlled trials. Pharmacol Res. 2020;156:104767. [CrossRef]
- Molla S, Abul M, Azad K, et al. A review on antiviral effects of Nigella Sativa L. Pharmacologyonline. 2019;2:47–53 (Accessed 18 March 2020). http://pharmacologyo nline.silae.it.
- Koshak AE, Koshak EA. Nigella sativa L as a potential phytotherapy for coronavirus disease 2019: a mini review of in silico studies. Curr Ther Res - Clin Exp. 2020;93: 100602. [CrossRef]
- Majdalawieh AF, Fayyad MW. Immunomodulatory and anti-inflammatory action of Nigella sativa and thymoquinone: a comprehensive review. Int Immunopharmacol. 2015;28(1):295–304. [CrossRef]
- Al-Ghamdi MS. The anti-inflammatory, analgesic and antipyretic activity of Nigella sativa. J Ethnopharmacol. 2001;76(1):45–48. [CrossRef]
- Ashraf S., Ashraf S., Ashraf M. et al., Honey and Nigella sativa against COVID-19 in Pakistan (HNS-COVID-PK): A multi-center placebo-controlled randomized clinical trial, Phytotherapy Research, 2023 (preprint 11/3/2020). https://onlinelibrary.wiley.com/doi/10.1002/ptr.7640. [CrossRef]
- Al-Haidari K.A., Faiq T.N., Ghareeb O. A. et al., Clinical Trial of Black Seeds Against COVID – 19 in Kirkuk City / Iraq, Indian Journal of Forensic Medicine & Toxicology, 15:3 (preprint 1/2021). https://www.researchgate.net/publi..inst_COVID_-19_in_Kirkuk_City_Iraq.
- Karimi M., Zarei A., Soleymani S. et al., Efficacy of Persian medicine herbal formulations (capsules and decoction) compared to standard care in patients with COVID-19, a multicenter open-labeled, randomized, controlled clinical trial, Phytotherapy Research, 2021 . https://onlinelibrary.wiley.com/doi/10.1002/ptr.7277. [CrossRef]
- Setayesh M., Karimi M., Zargaran A. et al., Efficacy of a Persian herbal medicine compound on coronavirus disease 2019 (COVID-19): a randomized clinical trial, Integrative Medicine Research, 2022. https://www.sciencedirect.com/science/article/pii/S2213422022000373. [CrossRef]
- Koshak A.E., Koshak E.A., Mobeireek A.F. et al., Nigella sativa for the treatment of COVID-19: An open-label randomized controlled clinical trial, Complementary Therapies in Medicine, 2021 . https://www.sciencedirect.com/science/article/pii/S0965229921001102. [CrossRef]
- Bencheqroun H., Ahmed Y., Kocak M. et al., A Randomized, Double-Blind, Placebo-Controlled, Multicenter Study to Evaluate the Safety and Efficacy of ThymoQuinone Formula (TQF) for Treating Outpatient SARS-CoV-2, Pathogens, 2022. https://www.mdpi.com/2076-0817/11/5/551. [CrossRef]
- Said S.A., Abdulbaset A., El-Kholy A.A. et al., The effect of Nigella sativa and vitamin D3 supplementation on the clinical outcome in COVID-19 patients: A randomized controlled clinical trial, Frontiers in Pharmacology, 2022 . https://www.frontiersin.org/articles/10.3389/fphar.2022.1011522/full. [CrossRef]
- Chandra K., Das A.K., Banday S., et al., Efficacy of polyherbal formulations for prevention of COVID-19 infection in high-risk subjects: A randomized open-label controlled clinical trial, Phytotherapy Research, 2022. https://onlinelibrary.wiley.com/doi/10.1002/ptr.7531. [CrossRef]
- Daneshfard B., Aghanouri R., Kazemnejad A. et al., Effect of Sinamaz nasal drop on asymptomatic family members of COVID 19 patients: An open-label randomized controlled trial, Phytotherapy Research, 2023. https://onlinelibrary.wiley.com/doi/epdf/10.1002/ptr.7915. [CrossRef]
- Bhaskar S., Kumar K. S., Krishnan K., Antony H. (2013). Quercetin alleviates hypercholesterolemic diet induced inflammation during progression and regression of atherosclerosis in rabbits. Nutrition (Burbank, Los Angeles County, Calif.), 29(1), 219–229. [CrossRef]
- Khan, H., Ullah, H., Aschner, M., Cheang, W. S., & Akkol, E. K. (2019). Neuroprotective effects of quercetin in Alzheimer’s disease. Biomolecules, 10(1), 59. [CrossRef]
- Batiha GE, Beshbishy AM, Ikram M, et al. (2020). The pharmacological activity, biochemical properties, and pharmacokinetics of the major natural polyphenolic flavonoid: Quercetin. Foods (Basel, Switzerland), 9(3), 374. [CrossRef]
- Xu Z, Shi L, Wang Y, et al. (2020). Pathological findings of COVID-19 associated with acute respiratory distress syndrome. The Lancet Respiratory Medicine, 8(4), 420–422. [CrossRef]
- Oei S, Millar CL, Nguyen Lily TN, et al. Higher intake of dietary flavonols, specifically dietary quercetin, is associated with lower odds of frailty onset over 12 years of follow-up among adults in the Framingham Heart Study. Am J Clin Nutr. 2023 Jul;118(1):27-33. [CrossRef]
- Brito J, Lima WG, Cordeiro L, da Cruz Nizer WS. (2021). Effectiveness of supplementation with quercetin-type flavonols for treatment of viral lower respiratory tract infections: Systematic review and metaanalysis of preclinical studies. Phytotherapy Research: PTR, 35(9), 4930–4942. [CrossRef]
- Mehrbod P, Abdalla MA, Fotouhi F, et al. Immunomodulatory properties of quercetin-3-O-α-L-rhamnopyranoside from Rapanea melanophloeos against influenza a virus. BMC Complementary and Alternative Medicine 2018, 18, 184. [Google Scholar] [CrossRef]
- Derosa G, Maffioli P, D’Angelo A, Di Pierro F (2021). A role for quercetin in coronavirus disease 2019 (COVID-19). Phytotherapy Research: PTR, 35(3), 1230–1236. [CrossRef]
- Di Pierro, F, Derosa, G, Maffioli, P, et al. (2021). Possible therapeutic effects of adjuvant quercetin supplementation against early-stage COVID-19 infection: A prospective, randomized, controlled, and open-label study. International Journal of General Medicine, 14, 2359–2366. [CrossRef]
- Di Pierro F., Khan A., Iqtadar S., et al., Quercetin as a possible complementary agent for early-stage COVID-19: Concluding results of a randomized clinical trial, Frontiers in Pharmacology, 2022. https://www.frontiersin.org/articles/10.3389/fphar.2022.1096853/full. [CrossRef]
- Di Pierro F, Iqtadar S, Khan A, et al. (2021). Potential clinical benefits of quercetin in the early stage of COVID-19: Results of a second, pilot, randomized, controlled and open-label clinical trial. International Journal of General Medicine, 14, 2807–2816. [CrossRef]
- Shohan M, Nashibi R, Mahmoudian-Sani MR, et al. (2022). The therapeutic efficacy of quercetin in combination with antiviral drugs in hospitalized COVID-19 patients: A randomized controlled trial. European Journal of Pharmacology, 914, 174615. [CrossRef]
- Cheema HA, Sohail A, Fatima A, et al. Quercetin for the treatment of COVID-19 patients: A systematic review and meta-analysis. Rev Med Virol. 2023 Mar;33(2):e2427. Epub 2023 Feb 13. [CrossRef] [PubMed]
- Onal et al., Treatment of COVID-19 patients with quercetin: a prospective, single center, randomized, controlled trial, Turk. J. Biol., 45:518-529 (preprint 1/19/2021). https://journals.tubitak.gov.tr/biology/vol45/iss7/13/.
- Gérain J., Uebelhoer M., Costes B. et al., NASAFYTOL® supplementation in adults hospitalized with COVID-19 infection: results from an exploratory open-label randomized controlled trial, Frontiers in Nutrition, 2023 . https://www.frontiersin.org/articles/10.3389/fnut.2023.1137407/full. [CrossRef]
- Zupanets I.A., Holubovska О.А., Tarasenko O.O. et al., Quercetin effectiveness in patients with COVID-19 associated pneumonia, Zaporozhye Med. J., 2021. http://zmj.zsmu.edu.ua/article/view/231714. [CrossRef]
- Ujjan I.D., Khan S., Nigar R. et al., The possible therapeutic role of curcumin and quercetin in the early-stage of COVID-19—Results from a pragmatic randomized clinical trial, Frontiers in Nutrition, 2022 . https://www.frontiersin.org/articles/10.3389/fnut.2022.1023997/full. [CrossRef]
- Khan A., Iqtadar S. , Sami Ullah Mumtaz S.U. et al., Oral Co-Supplementation of Curcumin, Quercetin, and Vitamin D3 as an Adjuvant Therapy for Mild to Moderate Symptoms of COVID-19—Results From a Pilot Open-Label, Randomized Controlled Trial, Frontiers in Pharmacology, 2022 . https://www.frontiersin.org/articles/10.3389/fphar.2022.898062/full. [CrossRef]
- Rondanelli M., Perna S., Gasparri C., et al., Promising Effects of 3-Month Period of Quercetin Phytosome® Supplementation in the Prevention of Symptomatic COVID-19 Disease in Healthcare Workers: A Pilot Study, Life, 2022 . https://www.mdpi.com/2075-1729/12/1/66/htm. [CrossRef]
- Arslan B., Ergun N.U., Topuz S. et al., et al., Synergistic Effect of Quercetin and Vitamin C Against COVID-19: Is a Possible Guard for Front Liners, SSRN, 2020 . https://europepmc.org/article/ppr/ppr239932. [CrossRef]
- Wu G., Meininger C.J., McNeal C.J., Bazer F.W., Rhoads J.M. Role of L-Arginine in nitric oxide synthesis and health in humans, Adv. Exp. Med. Biol. 1332 (2021) 167–187. [CrossRef]
- Gambardella J, Khondkar W, Morelli MB, Wang X, Santulli G, Trimarco V. Arginine and endothelial function. Biomedicines 2020;8(8):277. [CrossRef]
- Wijnands K.A., Castermans T.M., Hommen M.P., Meesters D.M., Poeze M. Arginine and citrulline and the immune response in sepsis, Nutrients 7 (2015) 1426–1463. [CrossRef]
- Rees CA, Rostad CA, Mantus G, et al. Altered amino acid profile in patients with SARS-CoV-2 infection. Proc Natl Acad Sci USA 2021, 118, e2101708118. [Google Scholar]
- Reizine F, Lesouhaitier M, Gregoire M, et al. SARS-CoV-2-induced ARDS associates with MDSC expansion, lymphocyte dysfunction, and arginine shortage. J Clin Immunol 2021, 41, 515–25. [Google Scholar] [CrossRef]
- Fiorentino G, Coppola A, Izzo R, et al. Effects of adding L-arginine orally to standard therapy in patients with COVID-19: A randomized, double-blind, placebo-controlled, parallel-group trial. Results of the first interim analysis. EClinicalMedicine. 2021 Oct;40:101125. [CrossRef]
- Gambardella J, Fiordelisi A, Spigno L, et al. Effects of chronic supplementation of Larginine on physical fitness in water polo players. Oxid Med Cell Longev 2021, 2021, 6684568. [Google Scholar]
- Lucas R, Czikora I, Sridhar S, et al. Arginase 1: an unexpected mediator of pulmonary capillary barrier dysfunction in models of acute lung injury. Front Immunol 2013;4:228. [CrossRef]
- WHO. A clinical case definition of post COVID-19 condition by a Delphi consensus. 6 October, 2021. Available from: https://apps.who.int/iris/rest/bitstreams/1376291/retrieve 85′. WHO. A clinical case definition for post COVID-19 condition in children and adolescents by expert consensus (16 February 2023). Available from. https://www.who.int/publications/i/item/WHO-2019-nCoV-Post-COVID-19-condition-CA-Clinical-case-definition-2023-1.
- Izzo R, Trimarco V, Mone P, et al. Combining L-Arginine with vitamin C improves long-COVID symptoms: The LINCOLN Survey. Pharmacol Res. 2022 Sep;183:106360. [CrossRef]
- Oikonomou E., Souvaliotis N., Lampsas S., et al., Endothelial dysfunction in acute and long standing COVID- 19: a prospective cohort study, Vasc. Pharm. 144 (2022), 106975. [CrossRef]
- Guervilly C., et al., Circulating endothelial cells as a marker of endothelial injury in severe COVID -19, J. Infect. Dis. 222 (2020) 1789–1793. [CrossRef]
- Charfeddine S, Ibn Hadj Amor H., Jdidi J., et al., Long COVID 19 syndrome: is it related to microcirculation and endothelial dysfunction? Insights from TUN-EndCOV study, Front Cardiovasc. Med. 8 (2021), 745758. [CrossRef]
- Sacchi A., Grassi G., Notari S., et al., Expansion of myeloid derived suppressor cells contributes to platelet activation by L-Arginine deprivation during SARS-CoV-2 infection, Cells 10 (2021). [CrossRef]
- Tosato M, Calvani R, Picca A, et al. Effects of l-Arginine Plus Vitamin C Supplementation on Physical Performance, Endothelial Function, and Persistent Fatigue in Adults with Long COVID: A Single-Blind Randomized Controlled Trial. Nutrients. 2022 Nov 23;14(23):4984. [CrossRef]
- Peiris S, Izcovich A, Ordunez P, et al. Challenges to delivering evidence-based management for long COVID. BMJ Evidence-Based Medicine 2023, 28, 295–298. [Google Scholar] [CrossRef]
- PAHO/IMS/EIH/COVID-19/23-0024. Living Systematic Review of Therapeutic Options for Post Acute or Post COVID-19 Condition, 25 August 2023. https://iris.paho.org/bitstream/handle/10665.2/57278/PAHOIMSEIHCOVID19230024_eng.pdf?sequence=18&isAllowed=y.
- Hansen KS, Mogensen TH, Agergaard J, et al. High-dose coenzyme Q10 therapy versus placebo in patients with post COVID-19 condition: a randomized, phase 2, crossover trial. Lancet Reg Health Eur. 2023 Jan;24:100539. [CrossRef]
- Mateos-Moreno MV, Mira A, Ausina-Marquez V, and Ferrer MD. Oral antiseptics against coronavirus: In-vitro and clinical evidence. J. Hosp. Infect. 2021, 113, 30–43. [Google Scholar] [CrossRef]
- Kariwa H, Fujii N, Takashima I. Inactivation of SARS coronavirus by means of povidone-iodine, physical conditions, and chemical reagents. Jpn. J. Vet. Res. 2004, 52, 105–112. [Google Scholar]
- Eggers M, Eickmann M, Zorn J. Rapid and effective virucidal activity of povidone-iodine products against Middle East respiratory syndrome coronavirus (MERS-CoV) and modified vaccinia virus Ankara (MVA). Infect. Dis. Ther 2015, 4, 491–501. [Google Scholar] [CrossRef]
- Anderson DE, et al. Povidone-iodine demonstrates rapid in vitro virucidal activity against SARS-CoV-2, the virus causing COVID-19 disease. Infect. Dis. Ther. 2020, 9, 669–675. [Google Scholar] [CrossRef]
- Martinez Lamas, L. et al. Is povidone iodine mouthwash effective against SARS-CoV-2? First in vivo tests. Oral Dis. 2020. [Google Scholar] [CrossRef]
- (Observational) Jamir L., Tripathi M., Shankar S. et al. Determinants of Outcome Among Critically Ill Police Personnel With COVID-19: A Retrospective Observational Study From Andhra Pradesh, India. Cureus cureus.com, doi.org. 2021. [Google Scholar]
- Choudhury I. M., Shabnam, N., Ahsan, T. et al. Effect of 1% Povidone Iodine Mouthwash/Gargle, Nasal and Eye Drop in COVID-19 patient. Bioresearch Communications banglajol.info, doi.org. 2021. [Google Scholar]
- Mohamed N.A., Baharom N., Sulaiman W.S. et al. Early viral clearance among COVID-19 patients when gargling with povidone-iodine and essential oils: a pilot clinical trial. medRxiv, medrxiv.org, doi.org. 2020. [Google Scholar]
- Guenezan J., Garcia M., Strasters D. et al. Povidone Iodine Mouthwash, Gargle, and Nasal Spray to Reduce Nasopharyngeal Viral Load in Patients With COVID-19: A Randomized Clinical Trial. JAMA Otolaryngol Head Neck Surg jamanetwork.com, doi.org. 2021. [Google Scholar]
- Elzein R., Abdel-Sater F., Fakhreddine S. et al. In vivo evaluation of the virucidal efficacy of chlorhexidine and povidone-iodine mouthwashes against salivary SARS-CoV-2. A randomized-controlled clinical trial. Journal of Evidence Based Dental Practice sciencedirect.com, doi.org. 2021. [Google Scholar]
- Arefin M.K., Rumi S.K.N.F., Uddin A.K.M.N. et al. Virucidal effect of povidone iodine on COVID-19 in the nasopharynx: an open-label randomized clinical trial. Indian Journal of Otolaryngology and Head & Neck Surgery springer.com, doi.org. 2022. [Google Scholar]
- Baxter A.L., Schwartz K.R., Johnson R.W. et al. Rapid initiation of nasal saline irrigation to reduce severity in high-risk COVID+ outpatients. Ear, Nose & Throat Journal sagepub.com, doi.org. 2022. [Google Scholar]
- Sulistyani L.D., Julia V., Soeprapto A., et al. The effects of mouth rinsing and gargling with mouthwash containing povidone-iodine and hydrogen peroxide on the cycle threshold value of Severe Acute Respiratory Syndrome Coronavirus 2: A randomized controlled trial of asymptomatic and mildly symptomatic patients, F1000Research f1000research.com, doi.org. 2022. [Google Scholar]
- Elsersy H.E., Zahran M.A.H., Elbakry A.E. et al. Combined Nasal, Oropharyngeal Povidone Iodine Plus Glycyrrhizic Acid Sprays, Accelerate Clinical and Laboratory Recovery and Reduces Household Transmission of SARS-CoV-2: A Randomized Placebo-Controlled Clinical Trial. Frontiers in Medicine frontiersin.org, doi.org. 2022. [Google Scholar]
- Sevinç Gül S.N., Dilsiz A., Sağlık İ., et al. Effect of oral antiseptics on the viral load of SARS-CoV-2: A randomized controlled trial. Dental and Medical Problems edu.pl, doi.org. 2022. [Google Scholar]
- Natto Z.S., Bakhrebah M.A., Afeef M. et al. The short-term effect of different chlorhexidine forms versus povidone iodine mouth rinse in minimizing the oral SARS-CoV-2 viral load: An open label randomized controlled clinical trial study. Medicine, lww.com, doi.org. 2022. [Google Scholar]
- Batioglu-Karaaltin A., Yigit O., Cakan D. et al. Effect of the povidone iodine, hypertonic alkaline solution and saline nasal lavage on nasopharyngeal viral load in COVID-19. Authorea, Inc authorea.com, doi.org. 2022. [Google Scholar]
- Matsuyama A., Okura H., Hashimoto S. et al., A prospective, randomized, open-label trial of early versus late povidone-iodine gargling in patients with COVID-19, Scientific Reports, 2022 nature.com. [CrossRef]
- Seneviratne C.J., Balan P., Ko KKK. et al. Efficacy of commercial mouth-rinses on SARS-CoV-2 viral load in saliva: randomized control trial in Singapore. Infection springer.com, doi.org. 2021. [Google Scholar]
- Zarabanda D., Vukkadala N., Phillips K.M., et al. The Effect of Povidone-Iodine Nasal Spray on COVID-19 Nasopharyngeal Viral Load in Patients: A Randomized Control Trial, Laryngoscope wiley.com, doi.org. 2022. [Google Scholar]
- Ferrer M.D., Barrueco, Á.S., Martinez-Beneyto, Y. et al. Clinical evaluation of antiseptic mouth rinses to reduce salivary load of SARS-CoV-2. Scientific Reports nature.com, doi.org. 2021. [Google Scholar]
- Fantozzi P.J., Pampena E., Pierangeli A., et al. Efficacy of antiseptic mouthrinses against SARS-CoV-2: A prospective randomized placebo-controlled pilot study. American Journal of Otolaryngology sciencedirect.com, doi.org. 2022. [Google Scholar]
- Seet R.C.S., Quek A.M.L., Ooi D.S.Q. et al. Positive impact of oral hydroxychloroquine and povidone-iodine throat spray for COVID-19 prophylaxis: an open-label randomized trial. International Journal of Infectious Diseases ijidonline.com, doi.org. 2021. [Google Scholar]
- Hasan F, Chiu HY, Salamanca E, et al. Effects of Chlorhexidine and Povidone-Iodine on the SARS-CoV-2 Load: A Systematic Review and Meta-analysis. Eur J Dent. 2023 Jul;17(3):587-601. [CrossRef]
- Baxter A.L., Schwartz K.R., Johnson R.W. et al. Rapid initiation of nasal saline irrigation to reduce severity in high-risk COVID+ outpatients. Ear, Nose & Throat Journal. 2022;0(0). [CrossRef]
- Mody, K., et al. Effect of 8.4% Soda-Bicarbonate Steam Inhalation on the Course of Disease in Mild to Moderate Cases of Covid-19, Acta Scientific Orthopaedics, 4:4, actascientific.com.
- Delić N., Matetic A., Domjanović J. et al. Effects of Different Inhalation Therapy on Ventilator-Associated Pneumonia in Ventilated COVID-19 Patients: A Randomized Controlled Trial. Microorganisms. 2022; 10(6):1118. [CrossRef]
- El-Badrawy M., Elmorsey R., Shehta M. et al. A randomized controlled trial of adjuvant inhalable sodium bicarbonate role in treatment of COVID-19, 18 November 2022, PREPRINT (Version 1) available at Research Square. [CrossRef]
- Wang T., Zhang Y., Zhang R. et al. Efficacy of nasal irrigation and oral rinse with sodium bicarbonate solution on virus clearance for COVID-19 patients. Front Public Health. 2023 Mar 15;11:1145669. [CrossRef]
- Pantazopoulos I., Chalkias A., Miziou A.,et al. A Hypertonic Seawater Nasal Irrigation Solution Containing Algal and Herbal Natural Ingredients Reduces Viral Load and SARS-CoV-2 Detection Time in the Nasal Cavity. Journal of Personalized Medicine. 2023; 13(7):1093. [CrossRef]
- Soares, C. P., da Silva, S. A. F., Soares, F. F.,et al. Preliminary observation of the use of sodium bicarbonate solution as an adjunct in the treatment of coronavirus 2019 disease (COVID-19): prognosis improvement in patients requiring intensive care / Observação preliminar do uso de solução de bicarbonato de sódio como coadjuvante no tratamento da doença coronavírus 2019 (COVID-19): melhora do prognóstico na necessidade de terapia intensiva. Brazilian Journal of Development, 7(12), 110698–110708. [CrossRef]
- El-Badrawy M. K., Saleh A. M., El-Badrawy A. et al. Role of Sodium Bicarbonate as Adjuvant Treatment of Nonsevere Computed Tomography-identified COVID-19 Pneumonia: A Preliminary Report. Indian Journal of Respiratory Care ; 10(3):318-325, 2022.
- Farrell NF, Klatt-Cromwell C, Schneider JS. Benefits and Safety of Nasal Saline Irrigations in a Pandemic-Washing COVID-19 Away. JAMA Otolaryngol Head Neck Surg. 2020;146(9):787-788. [CrossRef]
- Kanjanawasee D, Seresirikachorn K, Chitsuthipakorn W, Snidvongs K. Hypertonic Saline Versus Isotonic Saline Nasal Irrigation: Systematic Review and Meta-analysis. Am J Rhinol Allergy. 2018 Jul;32(4):269-279. [CrossRef]
- Ramalingam S., Graham C., Dove J., et al. A pilot, open labelled, randomised controlled trial of hypertonic saline nasal irrigation and gargling for the common cold. Sci Rep. 2019 Jan 31;9(1):1015. [CrossRef]
- Vlachou, M., Siamidi, A., Dedeloudi, A., Konstantinidou, S.K., Papanastasiou, I.P., 2021. Pineal hormone melatonin as an adjuvant treatment for COVID-19 (Review). Int. J. Mol. Med. 47 (4). [CrossRef]
- Bahrampour Juybari, K., Pourhanifeh, M.H., Hosseinzadeh, A., Hemati, K., Mehrzadi, S. Melatonin potentials against viral infections including COVID-19: Current evidence and new findings. Virus Res 2020, 287, 198108. [Google Scholar] [CrossRef]
- Camp, O.G.; Bai, D.; Gonullu, D.C.; Nayak, N.; Abu-Soud, H.M. Melatonin interferes with COVID-19 at several distinct ROS-related steps. J. Inorg. Biochem 2021, 223, 111546. [Google Scholar] [CrossRef]
- Faridzadeh A, Tabashiri A, Miri HH, Mahmoudi M. The role of melatonin as an adjuvant in the treatment of COVID-19: A systematic review. Heliyon. 2022 Oct;8(10):e10906. [CrossRef]
- Hasan Z.T., Atrakji D.M.Q.Y.M.A.A., Mehuaiden D.A.K., The Effect of Melatonin on Thrombosis, Sepsis and Mortality Rate in COVID-19 Patients, International Journal of Infectious Diseases, 2021 . https://www.sciencedirect.com/science/article/pii/S1201971221007980. [CrossRef]
- Bologna C., Madonna P., Pone E. et al., Efficacy of Prolonged-Release Melatonin 2 mg (PRM 2 mg) Prescribed for Insomnia in Hospitalized Patients for COVID-19: A Retrospective Observational Study, Journal of Clinical Medicine, 2021 . https://www.mdpi.com/2077-0383/10/24/5857. [CrossRef]
- Vijendra Ramlall V., Zucker J., Tatonetti N., Melatonin is significantly associated with survival of intubated COVID-19 patients, medRxiv, 020. https://www.medrxiv.org/content/10.1101/2020.10.15.20213546v1. [CrossRef]
- Sánchez-González M., Mahıllo-Fernandez I., Villar-Alvarez F., et al., What if melatonin could help COVID-19 severe patients?, 2022 . https://jcsm.aasm.org/doi/10.5664/jcsm.9554.
- Mousavi S.A., Heydari K., Mehravaran H., et al., Melatonin effects on sleep quality and outcomes of COVID-19 patients: An open-label, randomized, controlled trial, Journal of Medical Virology, 2022 . https://onlinelibrary.wiley.com/doi/full/10.1002/jmv.27312. [CrossRef]
- Sánchez-Rico M., de la Muela P., Herrera-Morueco J.J. et al., Melatonin does not reduce mortality in adult hospitalized patients with COVID-19: a multicenter retrospective observational study, Journal of Travel Medicine, 2022 . https://academic.oup.com/jtm/advan..le/doi/10.1093/jtm/taab195/6523003. [CrossRef]
- Karimpour-razkenari E., Naderi-Behdani F., Salahshoor A., et al., Melatonin as adjunctive therapy in patients admitted to the Covid-19, Annals of Medicine and Surgery, 2022 . https://www.sciencedirect.com/science/article/pii/S2049080122002527. [CrossRef]
- Alizadeh Z., Keyhanian N., Ghaderkhani S., et al., A Pilot Study on Controlling Coronavirus Disease 2019 (COVID-19) Inflammation Using Melatonin Supplement, Iranian Journal of Allergy, Asthma and Immunology, 2021 . https://ijaai.tums.ac.ir/index.php/ijaai/article/view/3086.
- Ameri A., Frouz Asadi, M., Ziaei et al., Efficacy and safety of oral melatonin in patients with severe COVID-19: a randomized controlled trial, Inflammopharmacology, 2023 . https://link.springer.com/10.1007/s10787-022-01096-7. [CrossRef]
- Darban M., Malek F., Memarian M., et al., Efficacy of High Dose Vitamin C, Melatonin and Zinc in Iranian Patients with Acute Respiratory Syndrome due to Coronavirus Infection: A Pilot Randomized Trial, Journal of Cellular & Molecular Anesthesia, 2021 . https://journals.sbmu.ac.ir/jcma/article/view/32182. [CrossRef]
- Farnoosh G., Akbariqomi M., Badriet T. et al., Efficacy of a Low Dose of Melatonin as an Adjunctive Therapy in Hospitalized Patients with COVID-19: A Randomized, Double-blind Clinical Trial, Archives of Medical Research, 2021 . https://www.sciencedirect.com/science/article/pii/S0188440921001417. [CrossRef]
- Fogleman C., Cohen D., Mercier A. et al., A Pilot of a Randomized Control Trial of Melatonin and Vitamin C for Mild-to-Moderate COVID-19, The Journal of the American Board of Family Medicine, 2022 . http://www.jabfm.org/lookup/doi/10.3122/jabfm.2022.04.210529. [CrossRef]
- García-García I.; Seco-Meseguer, E.; Ruiz-Seco, P. et al., Melatonin in the Prophylaxis of SARS-CoV-2 Infection in Healthcare Workers (MeCOVID): A Randomised Clinical Trial, Journal of Clinical Medicine, 2022 . https://www.mdpi.com/2077-0383/11/4/1139. [CrossRef]
- WHO updates guidelines on treatments for COVID-19. WHO updates guidelines on treatments for COVID-19.
- European Medicine Agency, March 18, 2020. L’EMA fornisce indicazioni sull’uso degli antinfiammatori non steroidei per COVID-19 (aifa.gov.it).
- Italian Drug Agency/AIFA. AIFA recommendations on drugs for home management of COVID-19 Vers. 10 – Update 10/03/2023. https://www.aifa.gov.it/documents/20142/1616529/IT_Raccomandazioni_AIFA_gestione_domiciliare_COVID-19_Vers10_10.03.2023.pdf.
- Ministry of Health - Italy. Open Data - Data - Drugs, 50 Sop and Otc best sellers in the 2nd half of the year 2022 - www.dati.salute.gov.it.
- Rinott E., Kozer E., Shapira Y., et al. Ibuprofen use and clinical outcomes in COVID-19 patients. Clin Microbiol Infect. 2020 Sep;26(9):1259.e5-1259.e7. [CrossRef]
- Lapi F., Marconi E., Grattagliano I., et al. To clarify the safety profile of paracetamol for home-care patients with COVID-19: a real-world cohort study, with nested case-control analysis, in primary care. Intern Emerg Med. 2022 Nov;17(8):2237-2244. [CrossRef]
- Sharif N., Opu R.R., Khan A., et al. Impact of Zinc, Vitamins C and D on Disease Prognosis among Patients with COVID-19 in Bangladesh: A Cross-Sectional Study. Nutrients. 2022 Nov 26;14(23):5029. [CrossRef]
- Chen C., Parthasarathy S., Leung J.M., et al. Distinct temporal trajectories and risk factors for Post-acute sequelae of SARS-CoV-2 infection. Front Med (Lausanne). 2023 Oct 16;10:1227883. [CrossRef]
- Ravichandran R., Mohan S.K., Sukumaran S.K., et al. An open label randomized clinical trial of Indomethacin for mild and moderate hospitalised Covid-19 patients. Sci Rep. 2022 Apr 19;12(1):6413. Erratum in: Sci Rep. 2022 Jun 20;12(1):10389. [CrossRef]
- Manjani l., Desai n., Kohli A.,et al. Effects of acetaminophen on outcomes in patients hospitalized with covid-19. Chest. 2021 Oct;160(4):A1072. [CrossRef]
- Lerner I., Serret-Larmande A., Rance B., et al. Mining Electronic Health Records for Drugs Associated With 28-day Mortality in COVID-19: Pharmacopoeia-wide Association Study (PharmWAS). JMIR Med Inform. 2022 Mar 30;10(3):e35190. Erratum in: JMIR Med Inform. 2022 Apr 12;10(4):e38505. [CrossRef]
- Ravichandran R., Mohan S.K., Sukumaran S.K., et al. An open label randomized clinical trial of Indomethacin for mild and moderate hospitalised Covid-19 patients. Sci Rep. 2022 Apr 19;12(1):6413. Erratum in: Sci Rep. 2022 Jun 20;12(1):10389. [CrossRef]
- Stufano A., Isgrò C., Palese L.L., et al. Oxidative Damage and Post-COVID Syndrome: A Cross-Sectional Study in a Cohort of Italian Workers. Int J Mol Sci. 2023 Apr 18;24(8):7445. [CrossRef]
- Abolhassani H., Delavari S., Landegren N., et al. Genetic and immunologic evaluation of children with inborn errors of immunity and severe or critical COVID-19. J Allergy Clin Immunol. 2022 Nov;150(5):1059-1073. [CrossRef]
- Baldia P.H., Wernly B., Flaatten H., et al. The association of prior paracetamol intake with outcome of very old intensive care patients with COVID-19: results from an international prospective multicentre trial. BMC Geriatr. 2022 Dec 27;22(1):1000. [CrossRef]
- Blanc F., Waechter C., Vogel T., et al. Interest of Proton Pump Inhibitors in Reducing the Occurrence of COVID-19: A Case-Control Study. Preprints 2020, 2020050016. [CrossRef]
- Sobhy A., Saleh L. A., Abdelatty M. E. L. Early Use of Ibuprofen in Moderate Cases of COVID-19 Might be a Promising Agent to Attenuate the Severity of Disease: A Randomized Controlled Trial. Open Anesthesia Journal; 17 (no pagination), 2023.
- Kolin D.A., Kulm S., Christos P.J.,et al. Clinical, regional, and genetic characteristics of Covid-19 patients from UK Biobank. PLoS One. 2020 Nov 17;15(11):e0241264. [CrossRef]
- Leal N.S., Yu Y., Chen Y., et al. Paracetamol Is Associated with a Lower Risk of COVID-19 Infection and Decreased ACE2 Protein Expression: A Retrospective Analysis. COVID. 2021; 1(1):218-229. [CrossRef]
- Park J., Lee S.H., You S.C., et al. Non-steroidal anti-inflammatory agent use may not be associated with mortality of coronavirus disease 19. Sci Rep. 2021 Mar 3;11(1):5087. [CrossRef]
- Gálvez-Barrón C., Arroyo-Huidobro M., Miňarro A., et al. COVID-19: Clinical Presentation and Prognostic Factors of Severe Disease and Mortality in the Oldest-Old Population: A Cohort Study. Gerontology. 2022;68(1):30-43. [CrossRef]
- Reese J.T., Coleman B., Chan L., et al. Cyclooxygenase inhibitor use is associated with increased COVID-19 severity. medRxiv 2021.04.13.21255438. [CrossRef]
- Chandan J.S., Zemedikun D.T., Thayakaran R., et al. . Nonsteroidal Antiinflammatory Drugs and Susceptibility to COVID-19. Arthritis Rheumatol. 2021 May;73(5):731-739. [CrossRef]
- Oh T.K., Song I.A., Lee J., et al. Musculoskeletal Disorders, Pain Medication, and in-Hospital Mortality among Patients with COVID-19 in South Korea: A Population-Based Cohort Study. Int J Environ Res Public Health. 2021 Jun 24;18(13):6804. [CrossRef]
- Moreno-Martos D., Verhamme K., Ostropolets A., et al. Characteristics and outcomes of COVID-19 patients with COPD from the United States, South Korea, and Europe. Wellcome Open Res. 2022 Mar 24;7:22. [CrossRef]
- MacFadden D.R., Brown K., Buchan S.A., et al. Screening Large Population Health Databases for Potential Coronavirus Disease 2019 Therapeutics: A Pharmacopeia-Wide Association Study of Commonly Prescribed Medications. Open Forum Infect Dis. 2022 Mar 29;9(5):ofac156. [CrossRef]
- Kim J.W., Yoon S., Lee J., et al.Serious Clinical Outcomes of COVID-19 Related to Acetaminophen or NSAIDs from a Nationwide Population-Based Cohort Study. Int J Environ Res Public Health. 2023 Feb 21;20(5):3832. [CrossRef]
- Campbell H.M., Murata A.E., Conner T.A., et al. Chronic use of non-steroidal anti-inflammatory drugs (NSAIDs) or acetaminophen and relationship with mortality among United States Veterans after testing positive for COVID-19. PLoS One. 2022 May 5;17(5):e0267462. [CrossRef]
- Xie J., Brash J.T., Turkmen C., et al. Risk of COVID-19 Diagnosis and Hospitalisation in Patients with Osteoarthritis or Back Pain Treated with Ibuprofen Compared to Other NSAIDs or Paracetamol: A Network Cohort Study. Drugs. 2023 Feb;83(3):249-263. [CrossRef]
- Kim J.W., Yoon S, Lee J., Lee S. Serious Clinical Outcomes of COVID-19 Related to Acetaminophen or NSAIDs from a Nationwide Population-Based Cohort Study. Int J Environ Res Public Health. 2023 Feb 21;20(5):3832. [CrossRef]
- Ritsinger V., Bodegård J., Kristofi R., et al. A. History of heart failure and chronic kidney disease and risk of all-cause death after COVID-19 during the first three waves of the pandemic in comparison with influenza outbreaks in Sweden: a registry-based, retrospective, case-control study. BMJ Open. 2023 Apr 28;13(4):e069037. [CrossRef]
- Sestili P., Fimognari C. Paracetamol-Induced Glutathione Consumption: Is There a Link With Severe COVID-19 Illness? Front Pharmacol. 2020 Oct 7;11:579944. [CrossRef]
- Haddad F., Soliman A.M., Wong M.E., et al. Fever integrates antimicrobial defences, inflammation control, and tissue repair in a cold-blooded vertebrate. Elife. 2023 Mar 14;12:e83644. [CrossRef]
- Muramoto Y., Takahashi S., Halfmann P.J., et al. Replicative capacity of SARS-CoV-2 omicron variants BA.5 and BQ.1.1 at elevated temperatures. Lancet Microbe. 2023 Jul;4(7):e486. [CrossRef]
- Roberts E., Delgado Nunes V., Buckner S., et al.Paracetamol: not as safe as we thought? A systematic literature review of observational studies. Ann Rheum Dis. 2016 Mar;75(3):552-9. [CrossRef]
- Doherty M., Hawkey C., Goulder M.,et al. A randomised controlled trial of ibuprofen, paracetamol or a combination tablet of ibuprofen/paracetamol in community-derived people with knee pain. Ann Rheum Dis. 2011 Sep;70(9):1534-41. [CrossRef]
- Baker B.H., Lugo-Candelas C., Wu H., et al. Association of Prenatal Acetaminophen Exposure Measured in Meconium With Risk of Attention-Deficit/Hyperactivity Disorder Mediated by Frontoparietal Network Brain Connectivity. JAMA Pediatr. 2020 Nov 1;174(11):1073-1081. [CrossRef]
| Outcome | Alternative Treatment (N=521) | L-Arginine + Vitamin C (N=869) | p | |
|---|---|---|---|---|
| Asthenia | absent (%) | 0,4 | 94,9 | |
| mild (%) | 5,2 | 4,0 | <0.0001 | |
| severe (%) | 94,4 | 1,1 | ||
| Dyspnea | absent (%) | 5,4 | 74,2 | |
| mild (%) | 55,1 | 25,4 | <0.0001 | |
| severe (%) | 39,5 | 0,4 | ||
| Chest tightness | absent (%) | 26,3 | 86,1 | |
| mild (%) | 50,9 | 13,4 | <0.0001 | |
| severe (%) | 22,8 | 0,5 | ||
| Dizziness | absent (%) | 66,6 | 87,3 | |
| mild (%) | 25,9 | 11,6 | <0.0001 | |
| severe (%) | 7,5 | 1,1 | ||
| Gastrointestinal disorders | absent (%) | 63,3 | 87,7 | |
| mild (%) | 26,7 | 11,7 | <0.0001 | |
| severe (%) | 10,0 | 0,6 | ||
| Headache | absent (%) | 39,2 | 81,8 | |
| mild (%) | 44,1 | 16,8 | <0.0001 | |
| severe (%) | 16,7 | 1,4 | ||
| Anosmia | absent (%) | 52,0 | 87,2 | |
| mild (%) | 34,0 | 11,0 | <0.0001 | |
| severe (%) | 14,0 | 1,8 | ||
| Concentration difficulty | absent (%) | 32,8 | 79,1 | |
| mild (%)(%) | 46,8 | 19,4 | <0.0001 | |
| severe (%) | 20,4 | 1,5 | ||
| Sleeplessness | absent (%) | 39,5 | 80,7 | |
| mild (%) | 42,6 | 17,5 | <0.0001 | |
| severe (%) | 17,9 | 1,8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





