Submitted:
15 December 2023
Posted:
18 December 2023
You are already at the latest version
Abstract
Keywords:
1. The Search for High Quality Oocytes
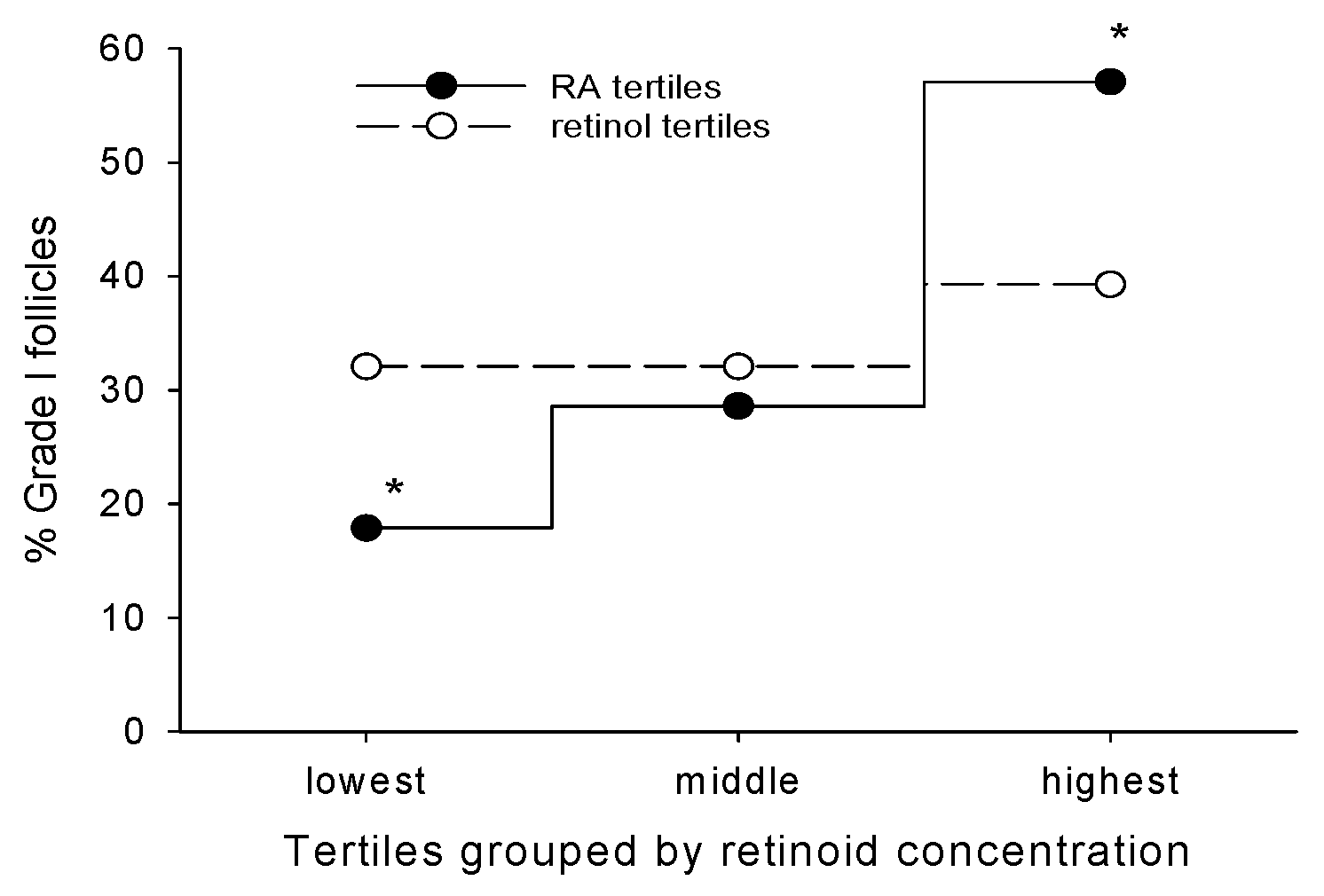
2. Retinoic Acid Levels in Cumulus Cells as a Non-Invasive Predictor of Oocyte Quality
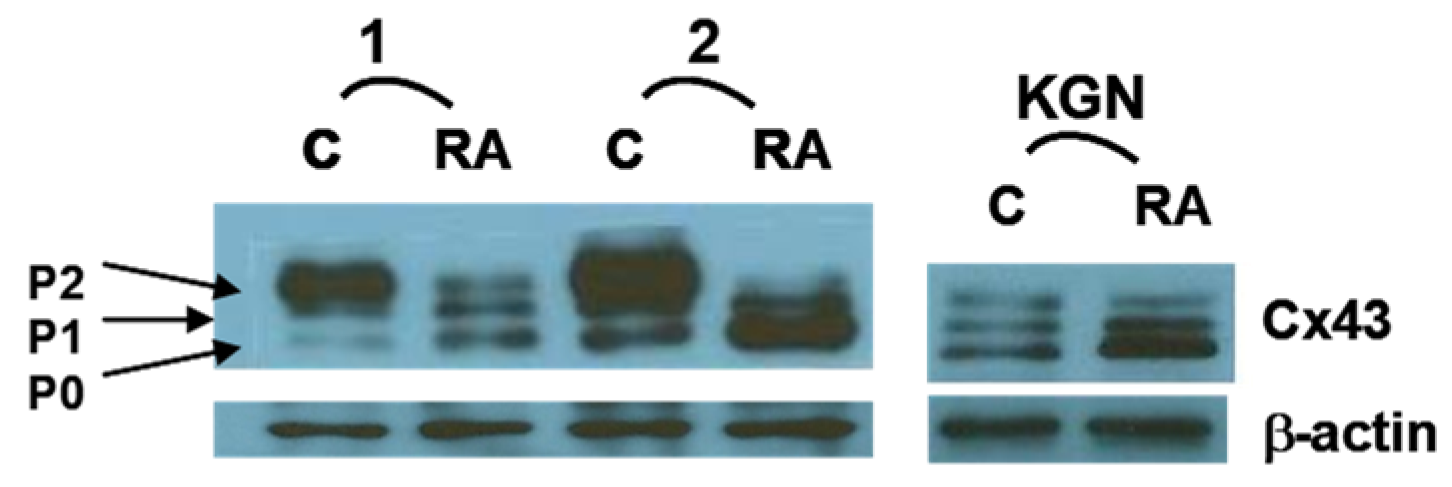
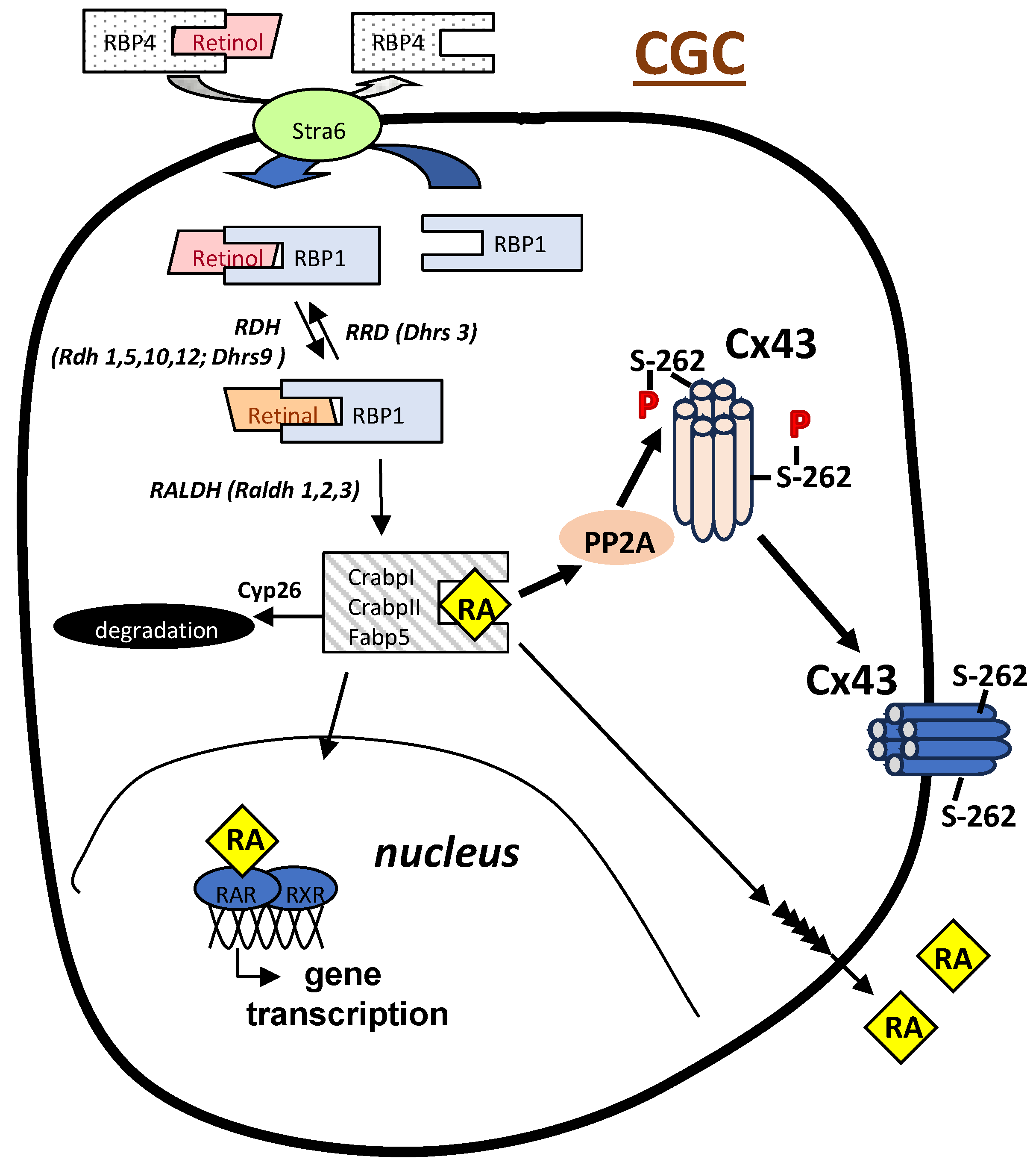
3. RA Can Promote Oocyte Competency Via Regulation of Cx43 in CGC
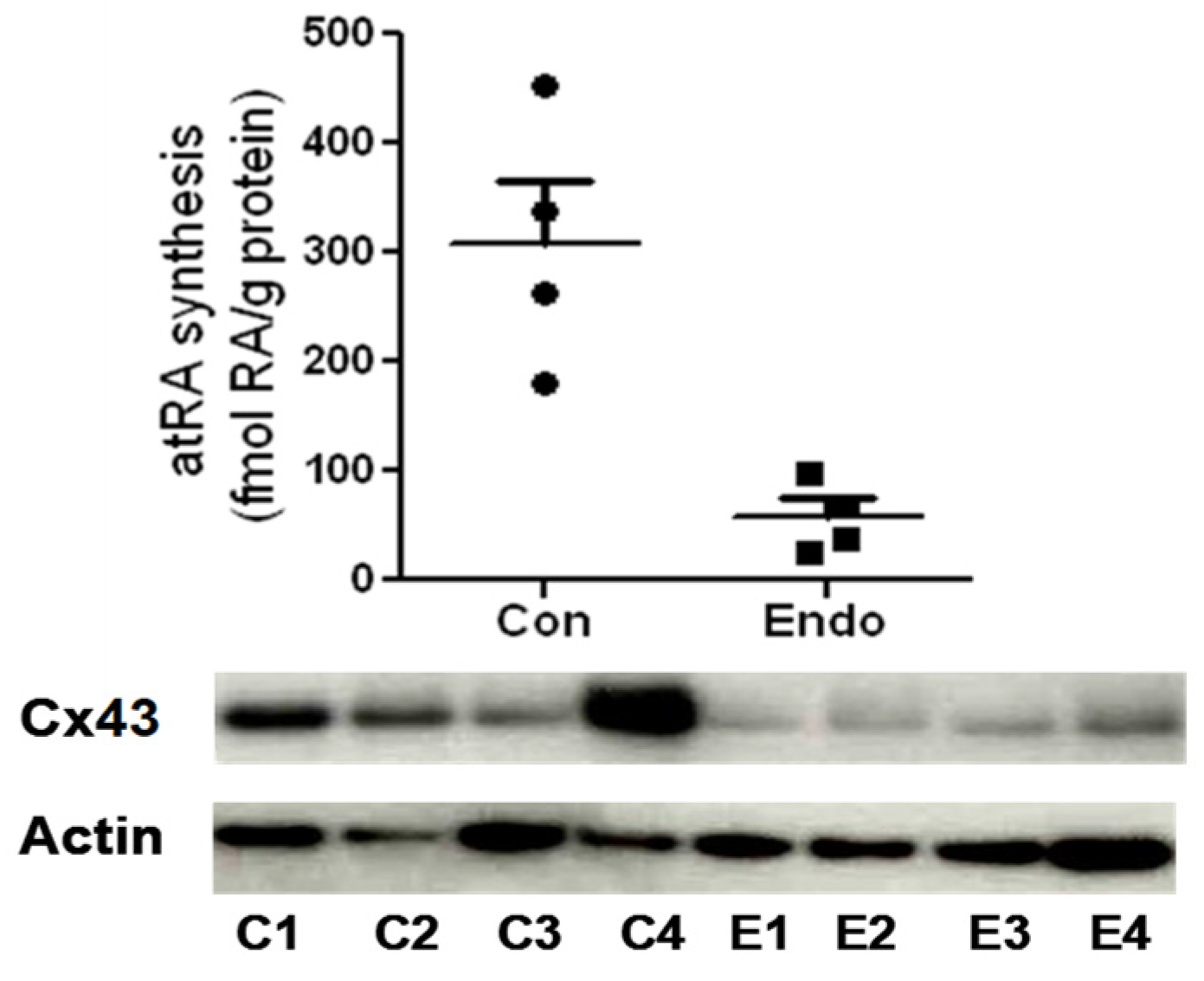
4. A Role for RA in Endometriosis-Associated Infertility Via Action on Cx43
5. Concluding Remarks
References
- Del Collado, M.; G. M. Andrade, N. J. N. Goncalves, S. Fortini, F. Perecin and M. M. Carriero. "The embryo non-invasive pre-implantation diagnosis era: How far are we?" Anim Reprod 20 (2023): e20230069. https://www.ncbi.nlm.nih.gov/pubmed/37720726. [CrossRef]
- Neal, S. A., S. J. Morin, J. M. Franasiak, L. R. Goodman, C. R. Juneau, E. J. Forman, M. D. Werner and R. T. Scott, Jr. "Preimplantation genetic testing for aneuploidy is cost-effective, shortens treatment time, and reduces the risk of failed embryo transfer and clinical miscarriage." Fertil Steril 110 (2018): 896-904. https://www.ncbi.nlm.nih.gov/pubmed/30316435. [CrossRef]
- Paulson, R. J. "Preimplantation genetic screening: What is the clinical efficiency?" Fertil Steril 108 (2017): 228-30. https://www.ncbi.nlm.nih.gov/pubmed/28697911. [CrossRef]
- Assou, S., D. Haouzi, J. De Vos and S. Hamamah. "Human cumulus cells as biomarkers for embryo and pregnancy outcomes." Mol Hum Reprod 16 (2010): 531-8. https://www.ncbi.nlm.nih.gov/pubmed/20435608. [CrossRef]
- Chen, A. A., L. Tan, V. Suraj, R. Reijo Pera and S. Shen. "Biomarkers identified with time-lapse imaging: Discovery, validation, and practical application." Fertil Steril 99 (2013): 1035-43. https://www.ncbi.nlm.nih.gov/pubmed/23499001. [CrossRef]
- Brown, J. A., D. M. Eberhardt, F. N. Schrick, M. P. Roberts and J. D. Godkin. "Expression of retinol-binding protein and cellular retinol-binding protein in the bovine ovary." Mol Reprod Dev 64 (2003): 261-9. https://www.ncbi.nlm.nih.gov/pubmed/12548658. [CrossRef]
- Ikeda, S., M. Kitagawa, H. Imai and M. Yamada. "The roles of vitamin a for cytoplasmic maturation of bovine oocytes." J Reprod Dev 51 (2005): 23-35. https://www.ncbi.nlm.nih.gov/pubmed/15750294. [CrossRef]
- Livera, G., V. Rouiller-Fabre, J. Valla and R. Habert. "Effects of retinoids on the meiosis in the fetal rat ovary in culture." Mol Cell Endocrinol 165 (2000): 225-31. https://www.ncbi.nlm.nih.gov/pubmed/10940501. [CrossRef]
- Zheng, W. L., R. A. Bucco, E. Sierra-Rievera, K. G. Osteen, M. H. Melner and D. E. Ong. "Synthesis of retinoic acid by rat ovarian cells that express cellular retinoic acid-binding protein-ii." Biol Reprod 60 (1999): 110-4. https://www.ncbi.nlm.nih.gov/pubmed/9858493. [CrossRef]
- Eberhardt, D. M., W. A. Will and J. D. Godkin. "Retinol administration to superovulated ewes improves in vitro embryonic viability." Biol Reprod 60 (1999): 1483-7. https://www.ncbi.nlm.nih.gov/pubmed/10330109. [CrossRef]
- Whaley, S. L., V. S. Hedgpeth, C. E. Farin, N. S. Martus, F. C. Jayes and J. H. Britt. "Influence of vitamin a injection before mating on oocyte development, follicular hormones, and ovulation in gilts fed high-energy diets." J Anim Sci 78 (2000): 1598-607. https://www.ncbi.nlm.nih.gov/pubmed/10875644. [CrossRef]
- Alminana, C., M. A. Gil, C. Cuello, I. Caballero, J. Roca, J. M. Vazquez, E. Gomez and E. A. Martinez. "In vitro maturation of porcine oocytes with retinoids improves embryonic development." Reprod Fertil Dev 20 (2008): 483-9. https://www.ncbi.nlm.nih.gov/pubmed/18462610. [CrossRef]
- Nasiri, E., R. Mahmoudi, M. H. Bahadori and I. Amiri. "The effect of retinoic acid on in vitro maturation and fertilization rate of mouse germinal vesicle stage oocytes." Cell J 13 (2011): 19-24. https://www.ncbi.nlm.nih.gov/pubmed/23671823.
- Tahaei, L. S., H. Eimani, P. E. Yazdi, B. Ebrahimi and R. Fathi. "Effects of retinoic acid on maturation of immature mouse oocytes in the presence and absence of a granulosa cell co-culture system." J Assist Reprod Genet 28 (2011): 553-8. https://www.ncbi.nlm.nih.gov/pubmed/21681498. [CrossRef]
- Goldberg, G. S., V. Valiunas and P. R. Brink. "Selective permeability of gap junction channels." Biochim Biophys Acta 1662 (2004): 96-101. https://www.ncbi.nlm.nih.gov/pubmed/15033581. [CrossRef]
- Wang, H. X., D. Tong, F. El-Gehani, F. R. Tekpetey and G. M. Kidder. "Connexin expression and gap junctional coupling in human cumulus cells: Contribution to embryo quality." J Cell Mol Med 13 (2009): 972-84. https://www.ncbi.nlm.nih.gov/pubmed/18505471. [CrossRef]
- Best, M. W., J. Wu, S. A. Pauli, M. A. Kane, K. Pierzchalski, D. R. Session, D. C. Woods, W. Shang, R. N. Taylor and N. Sidell. "A role for retinoids in human oocyte fertilization: Regulation of connexin 43 by retinoic acid in cumulus granulosa cells." Mol Hum Reprod 21 (2015): 527-34. https://www.ncbi.nlm.nih.gov/pubmed/25877907. [CrossRef]
- Read, C. C. and P. W. Dyce. "All-trans retinoic acid exposure increases connexin 43 expression in cumulus cells and improves embryo development in bovine oocytes." Mol Reprod Dev 86 (2019): 1865-73. https://www.ncbi.nlm.nih.gov/pubmed/31544318. [CrossRef]
- Tanmahasamut, P. and N. Sidell. "Up-regulation of gap junctional intercellular communication and connexin43 expression by retinoic acid in human endometrial stromal cells." J Clin Endocrinol Metab 90 (2005): 4151-6. https://www.ncbi.nlm.nih.gov/pubmed/15811935. [CrossRef]
- Wu, J., R. N. Taylor and N. Sidell. "Retinoic acid regulates gap junction intercellular communication in human endometrial stromal cells through modulation of the phosphorylation status of connexin 43." J Cell Physiol 228 (2013): 903-10. https://www.ncbi.nlm.nih.gov/pubmed/23042455. [CrossRef]
- Haliloglu, S., N. Baspinar, B. Serpek, H. Erdem and Z. Bulut. "Vitamin a and beta-carotene levels in plasma, corpus luteum and follicular fluid of cyclic and pregnant cattle." Reprod Domest Anim 37 (2002): 96-9. https://www.ncbi.nlm.nih.gov/pubmed/11975747. [CrossRef]
- Schweigert, F. J., B. Steinhagen, J. Raila, A. Siemann, D. Peet and U. Buscher. "Concentrations of carotenoids, retinol and alpha-tocopherol in plasma and follicular fluid of women undergoing ivf." Hum Reprod 18 (2003): 1259-64. https://www.ncbi.nlm.nih.gov/pubmed/12773456. [CrossRef]
- Schweigert, F. J. and H. Zucker. "Concentrations of vitamin a, beta-carotene and vitamin e in individual bovine follicles of different quality." J Reprod Fertil 82 (1988): 575-9. https://www.ncbi.nlm.nih.gov/pubmed/3361492. [CrossRef]
- Pauli, S. A., D. R. Session, W. Shang, K. Easley, F. Wieser, R. N. Taylor, K. Pierzchalski, J. L. Napoli, M. A. Kane and N. Sidell. "Analysis of follicular fluid retinoids in women undergoing in vitro fertilization: Retinoic acid influences embryo quality and is reduced in women with endometriosis." Reprod Sci 20 (2013): 1116-24. https://www.ncbi.nlm.nih.gov/pubmed/23427183. [CrossRef]
- Pierzchalski, K., R. N. Taylor, C. Nezhat, J. W. Jones, J. L. Napoli, G. Yang, M. A. Kane and N. Sidell. "Retinoic acid biosynthesis is impaired in human and murine endometriosis." Biol Reprod 91 (2014): 84. https://www.ncbi.nlm.nih.gov/pubmed/25143356. [CrossRef]
- Pierzchalski, K., J. Yu, V. Norman and M. A. Kane. "Crbpi regulates mammary retinoic acid homeostasis and the mammary microenvironment." FASEB J 27 (2013): 1904-16. https://www.ncbi.nlm.nih.gov/pubmed/23362116. [CrossRef]
- Zheng, W. L. and D. E. Ong. "Spatial and temporal patterns of expression of cellular retinol-binding protein and cellular retinoic acid-binding proteins in rat uterus during early pregnancy." Biol Reprod 58 (1998): 963-70. https://www.ncbi.nlm.nih.gov/pubmed/9546726. [CrossRef]
- Wang, Y. A., K. Shen, Y. Wang and S. C. Brooks. "Retinoic acid signaling is required for proper morphogenesis of mammary gland." Dev Dyn 234 (2005): 892-9. https://www.ncbi.nlm.nih.gov/pubmed/16217742. [CrossRef]
- Gittens, J. E., A. A. Mhawi, D. Lidington, Y. Ouellette and G. M. Kidder. "Functional analysis of gap junctions in ovarian granulosa cells: Distinct role for connexin43 in early stages of folliculogenesis." Am J Physiol Cell Physiol 284 (2003): C880-7. https://www.ncbi.nlm.nih.gov/pubmed/12620892. [CrossRef]
- Tong, D., J. E. Gittens, G. M. Kidder and D. Bai. "Patch-clamp study reveals that the importance of connexin43-mediated gap junctional communication for ovarian folliculogenesis is strain specific in the mouse." Am J Physiol Cell Physiol 290 (2006): C290-7. https://www.ncbi.nlm.nih.gov/pubmed/16135542. [CrossRef]
- De Los Reyes, M., J. Palomino, C. Gallegos, R. Espinoza, P. Dettleff, O. A. Peralta, V. H. Parraguez and G. Ramirez. "Gene and protein expression of connexins 37 and 43 in cumulus-oocytes complexes throughout the canine oestrous cycle." Reprod Fertil Dev 32 (2020): 976-87. https://www.ncbi.nlm.nih.gov/pubmed/32693910. [CrossRef]
- Veitch, G. I., J. E. Gittens, Q. Shao, D. W. Laird and G. M. Kidder. "Selective assembly of connexin37 into heterocellular gap junctions at the oocyte/granulosa cell interface." J Cell Sci 117 (2004): 2699-707. https://www.ncbi.nlm.nih.gov/pubmed/15138288. [CrossRef]
- Read, C. C., G. Willhelm and P. W. Dyce. "Connexin 43 coupling in bovine cumulus cells, during the follicular growth phase, and its relationship to in vitro embryo outcomes." Mol Reprod Dev 85 (2018): 579-89. https://www.ncbi.nlm.nih.gov/pubmed/29697878. [CrossRef]
- Norris, R. P., M. Freudzon, L. M. Mehlmann, A. E. Cowan, A. M. Simon, D. L. Paul, P. D. Lampe and L. A. Jaffe. "Luteinizing hormone causes map kinase-dependent phosphorylation and closure of connexin 43 gap junctions in mouse ovarian follicles: One of two paths to meiotic resumption." Development 135 (2008): 3229-38. https://www.ncbi.nlm.nih.gov/pubmed/18776144. [CrossRef]
- Sela-Abramovich, S., E. Chorev, D. Galiani and N. Dekel. "Mitogen-activated protein kinase mediates luteinizing hormone-induced breakdown of communication and oocyte maturation in rat ovarian follicles." Endocrinology 146 (2005): 1236-44. https://www.ncbi.nlm.nih.gov/pubmed/15576461. [CrossRef]
- Dekel, N. "Cellular, biochemical and molecular mechanisms regulating oocyte maturation." Mol Cell Endocrinol 234 (2005): 19-25. https://www.ncbi.nlm.nih.gov/pubmed/15836949. [CrossRef]
- Ackert, C. L., J. E. Gittens, M. J. O'Brien, J. J. Eppig and G. M. Kidder. "Intercellular communication via connexin43 gap junctions is required for ovarian folliculogenesis in the mouse." Dev Biol 233 (2001): 258-70. https://www.ncbi.nlm.nih.gov/pubmed/11336494. [CrossRef]
- Solan, J. L. and P. D. Lampe. "Connexin43 phosphorylation: Structural changes and biological effects." Biochem J 419 (2009): 261-72. https://www.ncbi.nlm.nih.gov/pubmed/19309313. [CrossRef]
- Lampe, P. D. and A. F. Lau. "Regulation of gap junctions by phosphorylation of connexins." Arch Biochem Biophys 384 (2000): 205-15. https://www.ncbi.nlm.nih.gov/pubmed/11368307. [CrossRef]
- Lampe, P. D. and A. F. Lau. "The effects of connexin phosphorylation on gap junctional communication." Int J Biochem Cell Biol 36 (2004): 1171-86. https://www.ncbi.nlm.nih.gov/pubmed/15109565. [CrossRef]
- Pahujaa, M., M. Anikin and G. S. Goldberg. "Phosphorylation of connexin43 induced by src: Regulation of gap junctional communication between transformed cells." Exp Cell Res 313 (2007): 4083-90. https://www.ncbi.nlm.nih.gov/pubmed/17956757. [CrossRef]
- Weng, S., M. Lauven, T. Schaefer, L. Polontchouk, R. Grover and S. Dhein. "Pharmacological modification of gap junction coupling by an antiarrhythmic peptide via protein kinase c activation." FASEB J 16 (2002): 1114-6. https://www.ncbi.nlm.nih.gov/pubmed/12039852. [CrossRef]
- Imanaga, I., L. Hai, K. Ogawa, K. Matsumura and T. Mayama. "Phosphorylation of connexin in functional regulation of the cardiac gap junction." Exp Clin Cardiol 9 (2004): 161-4. https://www.ncbi.nlm.nih.gov/pubmed/19641718.
- Meilleur, M. A., C. D. Akpovi, R. M. Pelletier and M. L. Vitale. "Tumor necrosis factor-alpha-induced anterior pituitary folliculostellate ttt/gf cell uncoupling is mediated by connexin 43 dephosphorylation." Endocrinology 148 (2007): 5913-24. https://www.ncbi.nlm.nih.gov/pubmed/17872368. [CrossRef]
- Chang, H. K. and W. S. Hou. "Retinoic acid modulates interferon-gamma production by hepatic natural killer t cells via phosphatase 2a and the extracellular signal-regulated kinase pathway." J Interferon Cytokine Res 35 (2015): 200-12. https://www.ncbi.nlm.nih.gov/pubmed/25343668. [CrossRef]
- Park, J. H., D. H. Cho, J. Y. Lee, H. J. Lee, Y. Ha, J. H. Ahn and I. Jo. "B56delta subunit of protein phosphatase 2a decreases phosphorylation of endothelial nitric oxide synthase at serine 116: Mechanism underlying aphidicolin-stimulated no production." Nitric Oxide 50 (2015): 46-51. https://www.ncbi.nlm.nih.gov/pubmed/26255574. [CrossRef]
- Sirnes, S., A. Kjenseth, E. Leithe and E. Rivedal. "Interplay between pkc and the map kinase pathway in connexin43 phosphorylation and inhibition of gap junction intercellular communication." Biochem Biophys Res Commun 382 (2009): 41-5. https://www.ncbi.nlm.nih.gov/pubmed/19258009. [CrossRef]
- Bulun, S. E. "Endometriosis." N Engl J Med 360 (2009): 268-79. https://www.ncbi.nlm.nih.gov/pubmed/19144942. [CrossRef]
- Nezhat, C., E. D. Littman, R. B. Lathi, B. Berker, L. M. Westphal, L. C. Giudice and A. A. Milki. "The dilemma of endometriosis: Is consensus possible with an enigma?" Fertil Steril 84 (2005): 1587-8. https://www.ncbi.nlm.nih.gov/pubmed/16359950. [CrossRef]
- Al-Fadhli, R., S. M. Kelly, T. Tulandi and S. Lin Tan. "Effects of different stages of endometriosis on the outcome of in vitro fertilization." J Obstet Gynaecol Can 28 (2006): 888-91. https://www.ncbi.nlm.nih.gov/pubmed/17140505. [CrossRef]
- Garrido, N., A. Pellicer, J. Remohi and C. Simon. "Uterine and ovarian function in endometriosis." Semin Reprod Med 21 (2003): 183-92. https://www.ncbi.nlm.nih.gov/pubmed/12917788. [CrossRef]
- Kumbak, B., S. Kahraman, G. Karlikaya, S. Lacin and A. Guney. "In vitro fertilization in normoresponder patients with endometriomas: Comparison with basal simple ovarian cysts." Gynecol Obstet Invest 65 (2008): 212-6. https://www.ncbi.nlm.nih.gov/pubmed/18073487. [CrossRef]
- Hull, M. G., J. A. Williams, B. Ray, E. A. McLaughlin, V. A. Akande and W. C. Ford. "The contribution of subtle oocyte or sperm dysfunction affecting fertilization in endometriosis-associated or unexplained infertility: A controlled comparison with tubal infertility and use of donor spermatozoa." Hum Reprod 13 (1998): 1825-30. https://www.ncbi.nlm.nih.gov/pubmed/9740433. [CrossRef]
- Navarro, J., N. Garrido, J. Remohi and A. Pellicer. "How does endometriosis affect infertility?" Obstet Gynecol Clin North Am 30 (2003): 181-92. https://www.ncbi.nlm.nih.gov/pubmed/12699265. [CrossRef]
- Napoli, J. L. "Physiological insights into all-trans-retinoic acid biosynthesis." Biochim Biophys Acta 1821 (2012): 152-67. https://www.ncbi.nlm.nih.gov/pubmed/21621639. [CrossRef]
- lomhoff, R. "Transport and metabolism of vitamin a." Nutr Rev 52 (1994): S13-23. https://www.ncbi.nlm.nih.gov/pubmed/8202278. [CrossRef]
- Kayser, S., R. F. Schlenk and U. Platzbecker. "Management of patients with acute promyelocytic leukemia." Leukemia 32 (2018): 1277-94. https://www.ncbi.nlm.nih.gov/pubmed/29743722. [CrossRef]
- Ortiz, N. E., R. I. Nijhawan and J. M. Weinberg. "Acitretin." Dermatol Ther 26 (2013): 390-9. https://www.ncbi.nlm.nih.gov/pubmed/24099069. [CrossRef]
- Vallerand, I. A., R. T. Lewinson, M. S. Farris, C. D. Sibley, M. L. Ramien, A. G. M. Bulloch and S. B. Patten. "Efficacy and adverse events of oral isotretinoin for acne: A systematic review." Br J Dermatol 178 (2018): 76-85. https://www.ncbi.nlm.nih.gov/pubmed/28542914. [CrossRef]
- Adamson, P. C. "Clinical and pharmacokinetic studies of all-trans-retinoic acid in pediatric patients with cancer." Leukemia 8 Suppl 3 (1994): S22-5. https://www.ncbi.nlm.nih.gov/pubmed/7808020.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).



