1. Introduction
According to historical data, the first high-energy materials (HEM) were introduced to Europe in the 13th century for military purposes, and since the 17th century, they have been used in mining and construction industries [
1]. The development and investigation of these materials are focused on their practical usage: the energetic compound should be powerful, stable, insensitive to mechanical stimuli, and provide large quantities of energy release during intentional detonation [
2,
3,
4]. Generally, in high-energy materials, energy, and stability are intrinsically competing against each other, and it is difficult to mediate these two major requirements for HEMs. Thus the development of high-stability and performance materials along with the ecological requirements are the focus of the research and it seems never-ending. Existing HEMs cannot fully satisfy the user’s demand for improved performance and reduced vulnerability. Additionally, costs leading to changes in the processes for producing the new high-energy materials must be considered.
Based on structural analysis and synthetic routes, there are found general approaches for the enlargement of the thermal stability of high-energy materials [
5,
6]. These approaches are the following: ‘Salt Formation’, ‘Introduction of Amino Groups’, ‘Introduction of Conjugation’, and ‘Condensation with a Triazole ring’. There are plenty of research results published on increasing stability due to the introduction of amino groups and conjugation and condensing with Triazole rings [
7,
8]. However, only a little research has been done to achieve stability of HEMS through the ‘Salt Formation’ approach. Thus only meager examples have been published yet.
Recently, we performed an investigation of the influence of the number of extra substituents on the thermal and chemical stabilities as well as the explosive performance of a series of molecules based on N-(3,5-dimethyl-2,4,6-trinitrophenyl)-1
H-1,2,4-triazol-3′-amine [
9]. The obtained results coincide well with the above described because they prove that the amination, conjugation, and condensation with the triazole ring leads to higher stability, insensitivity to impact, and worsening of the explosive properties of parent compounds, although, they still could be classified as high-brisance materials. Hence, the question arises if the stability of the compound will increase, and whether their energetic properties remain the same or even will be improved due to salt formation. For further study, we chose 3-amino-5-[(2,4,6-trinitrophenyl)amino]-1
H-1,2,4-triazole (APATO). This material is a thermostable high-energy material, one of the energetic derivatives of 1,2,4- triazole [
10,
11]. The molecule consists of additional amino groups, triazole ring, and conjugation to exhibit the influence of the various energetic salt formations/ on the stability and explosive properties of 3-amino-5-[(2,4,6-trinitrophenyl)amino]-1
H-1,2,4-triazole (APATO) [
11,
12].
Energetic salts are a numerous group of high-energy materials possessing high-density, insensitive munition components, high thermostability, and propellants. Some popular representatives of known energetic salts are Ammonium picrate (Dunnite) [
13,
14,
15], Ammonium perchlorate [
16,
17], Hydrazine nitroformate (HNF) [
18,
19,
20], Ammonium dinitramide (ADN) [
21,
22,
23,
24,
25], anionic salts [
26,
27,
28], and TKX-50 (Dihydroxylammonium–5,5′-bitetrazole-1,1′-dioxide) [
29,
30,
31,
32,
33] structures. Salt is a chemical compound consisting of an ionic aggregation of positively charged cations and negatively charged anions. More strictly, an energetic salt is an assembly of negative or positive energetic ionic components, but a better case occurs when both ionic components are energetic and in pairs build a new stable energetic compound with improved characteristics. We selected the last method for the theoretical investigation of a new perspective on ionic compounds where both ions in the assembly are energetic. Probably the most known energetic salt in the world is Ammonium nitrate (NH
4NO
3). It found a wide application in mixtures used for mining and construction industries, but due to its high hygroscopicity is rarely applied in military energetic compositions. Another popular representative of known energetic salts is Ammonium perchlorate. Previously four different salts of APATO (3-amino-5-(picrylamino)-1,2,4-triazole) with potassium, guanidinium, aminoguanidinium, and triethylammonium cations were investigated by Chioato et al., 2016 in Klapotke lab [
13]. The crystalline structure of these materials is also described by them.
In our previous work, we found that one of our newly synthesized inorganic salts, APATO perchlorate, possessed a perspective property as thermostable high-density energetic material (HDEM) [
34]. However, it is currently well known, that perchlorate anion is established as hazardous for human health and the environment [
35,
36]. Scientists, working in energetic materials area are trying to avoid the use of perchlorates in HEMs and stepwise exchange them with other oxidizing salts [
37,
40]. It was an attractive idea to study a group of new energetic salts based on the APATO (abbreviation in paper for better communication is HE-I) cation and some selected perspective oxidizable anions:
- a)
chlorate (ClO3-), ( HE-II)
- b)
dinitramide [(NO2)2N- (HE-III)
- c)
nitroformate [(NO2)3C-] (another name: trinitromethanide (HE-IV)
- d)
bromate (BrO3-), (HE-V)
- e)
iodate(IO3-), (HE-VI)
- f)
periodate (IO4-), (HE-VII)
- g)
perbromate (BrO4-),(HE-VIII)
- h)
picrate [(NO2)3C6H2O-). ((HE-IX)
- i)
NH3 (HE-X).-
The application of chlorate, bromate, and picrate energetic ions is comparatively well-known in special literature, another two oxidizable ions, jodates, and perjodates are rarely mentioned. Surprisingly, concerning potential energetic perbromates (BrO4-) to the best of our knowledge no one study is available at present.
For this reason, it looks rational to introduce more information about this kind of anion in a short but detailed mode. Important to note, that the listed anions (a-h) were selected by us rationally for their high oxidizing properties and positive impact on oxygen balance [
37,
38], and, also, because of their potential to increase the density of final energetic salts and stabilize the overall system [
39,
40].
2. Materials and Methods
We remind you that the study was performed aiming to exhibit the influence of various salts on the stability and explosive properties of the aminated compound possessing a triazole ring. The used methodology in the research is described in detail in [
4]. First, we performed an investigation of the 3-amino-5-[(2, 4, 6-trinitrophenyl) amino]-1
H-1, 2, 4-triazole salt to establish the placement of the amino group. For this study, we selected a compound with the lowest total energy when an additional amino group is substituted for a carbon atom of the triazole ring (see
Figure 1). Second, we designed at least two different compounds with this molecule and different salt to obtain the most relevant position of them in respect each other. The Berny optimization without any symmetry constraints (all bond lengths, angles, and dihedral angles are changed) was applied to find an equilibrium point. The vibration frequencies analysis was performed to be sure that the energy minima were reached, and the structure of the most stable conformer was found. Becke’s three-parameter hybrid functional approach with non-local correlation provided by Lee, Yang, and Parr (B3LYP) and the cc-pVTZ basis set for C, O, N, H atoms and 6-311G one for Br and I implemented in a GAUSSIAN package were applied in our studies [
42,
43,
44]. This approach described well the geometric and electronic structure of various molecules and their derivatives [
45,
46,
47,
48,
49,
50,
51,
52,
53]. Then, the total energies of the conformers under study were compared. For further study of the stability and explosive properties, we choose compounds whose energy is significantly lower. In the case when the total energies of the compounds were different by less than 0.02 eV, which coincides with the energy of thermal movement, both compounds were under research.
Thermal stability was established based on the binding energy per atom calculation results. This energy indicates the amount of energy required to separate an atom from a system of particles and is calculated as follows:
Where E
t and E
i are the total energy of the compound and atoms consisting of it respectively, and N is the number of atoms in the molecule. A larger value of binding energy per atom shows higher thermal stability. HOMO-LUMO gap, and chemical hardness, were calculated and analyzed to predict chemical properties (chemical stability) and aging of the compounds investigated. Generally, compounds with larger HOMO-LUMO gaps and chemical hardness are more resistant to undergoing a chemical reaction or to being transformed by an external perturbation, such as an applied electric field. The chemical softness was evaluated, too. A low chemical softness value denotes a high tendency of the molecule to degrade [
54].
The compounds under study could be divided into two groups. Some of them could be described as CaHbNcOd despite the formation of the salts, while others consist of Cl, I, and Br. The energetic properties of the CaHbNcOd under study were obtained by using a low computational demand required approach that reliability was checked earlier by the comparison of the detonation velocity of Tetryl and the theoretical results comparison with experimental ones. In short, the detonation velocity calculated by applying the above approach coincides well with that of 7.59-7.7 km/s presented by other researchers. Additionally, it is an experimentally confirmed conclusion followed by the above approach results, that tetryl energetic properties are worse than that of N-(2,4,6-trinitrophenyl)-1
H-1,2,4-triazol-3-amine, and their ones are worse than TNT [
9,
10,
11,
12,
13,
34,
55,
56]. However, this approach is not acceptable for the rest of the compounds, i.e. compounds consisting of halogens. Thus another approach suitable for these compounds was used to evaluate their and CaHbNcOd compound energetic properties. The comparison of the parameters of CaHbNcOd obtained by different approaches applied by us allows one to predict the reliability of the results obtained and avoid unexpected errors.
The detonation velocity and pressure were calculated to estimate the energetic properties of the compound investigated. The detonation velocity of the CaHbNcOd compounds was calculated followingly:
where N is the number of -NO
2 groups in the molecule, E is total energy, a.u., M is molar mass, g/mol. These equations are given in [
57]. The detonation velocities calculated by these Equations coincide well with those obtained by the very well-known Kamlet-Jacobs equation [
41,
55,
60,
61].
When the detonation velocity is known, the detonation pressure is evaluated followingly:
where D is detonation velocity and ρ is the density of the compounds.
The results of these calculations were used to check the relevance of the approach suggested by Zohari et al. [
62]. These authors propose the equation to evaluate detonation pressure the following:
where P is the detonation pressure of the compound in GPa, nN, nC, nH and nO are the number of nitrogen, carbon, hydrogen, and oxygen atoms, respectively. Mw is the molecular weight in g·mol–1 of the compound. The equation is named reliable for predicting the detonation pressure of new energetic co-crystals without any remarks on restriction. In any case, the reliability of the approach was checked by comparison of detonation pressure and velocity obtained by using different approaches.
When the detonation pressure is known, the detonation velocity could be evaluated followingly:
where P (kbar) is detonation pressure, and is the density of the explosive in g/cm3. This equation follows from Kamlet -Jakob equations for detonation pressure and velocity calculation.
We obtained the detonation pressure P
1 and P
2 as well as detonation velocity D
1 and D
2 for the compounds HE-I, HE-III, and HE-IV, i.e. for the cases where both described approaches are possibly applied. The results obtained are presented in
Table 1. These results exhibit a strong correlation between values of detonation pressures as well as velocities obtained by using different approaches. Considering this good coincidence between the results obtained by the experimentally confirmed approach and that first used, we may state that both approaches mentioned above are reliable for our study, and results obtained by them could be used for the prediction of practical usage of the materials under study.
To predict the sensitivity of the compounds investigated, we estimated oxygen balance. Some compounds under study consist of only one halogen atom, whose rate in comparison to N or O is insignificant, so their influence on the energetic properties of the materials could not be crucial. In any case, each halogen’s behavior is similar under the same conditions. Thus we predict that for the halogens-containing high-energy materials, the corrected oxygen-fluorine balance is acceptable because the introduction of another similar to fluorine oxidant element takes place [
63].
To obtain the density of the materials under investigation, we computed their molar volume by the approach implemented in the Gaussian program. When the molar volume is known, the density of the materials is molar divided by molar volume. The obtained densities were compared with those of such kinds of materials to be sure that they fit the range of the densities published elsewhere to ensure that random errors are avoided [
64,
65].
3. Results
The view of the most stable compound obtained by us is depicted in
Figure 1 and the coordinates of the obtained structures could be sent under the request of the readers.
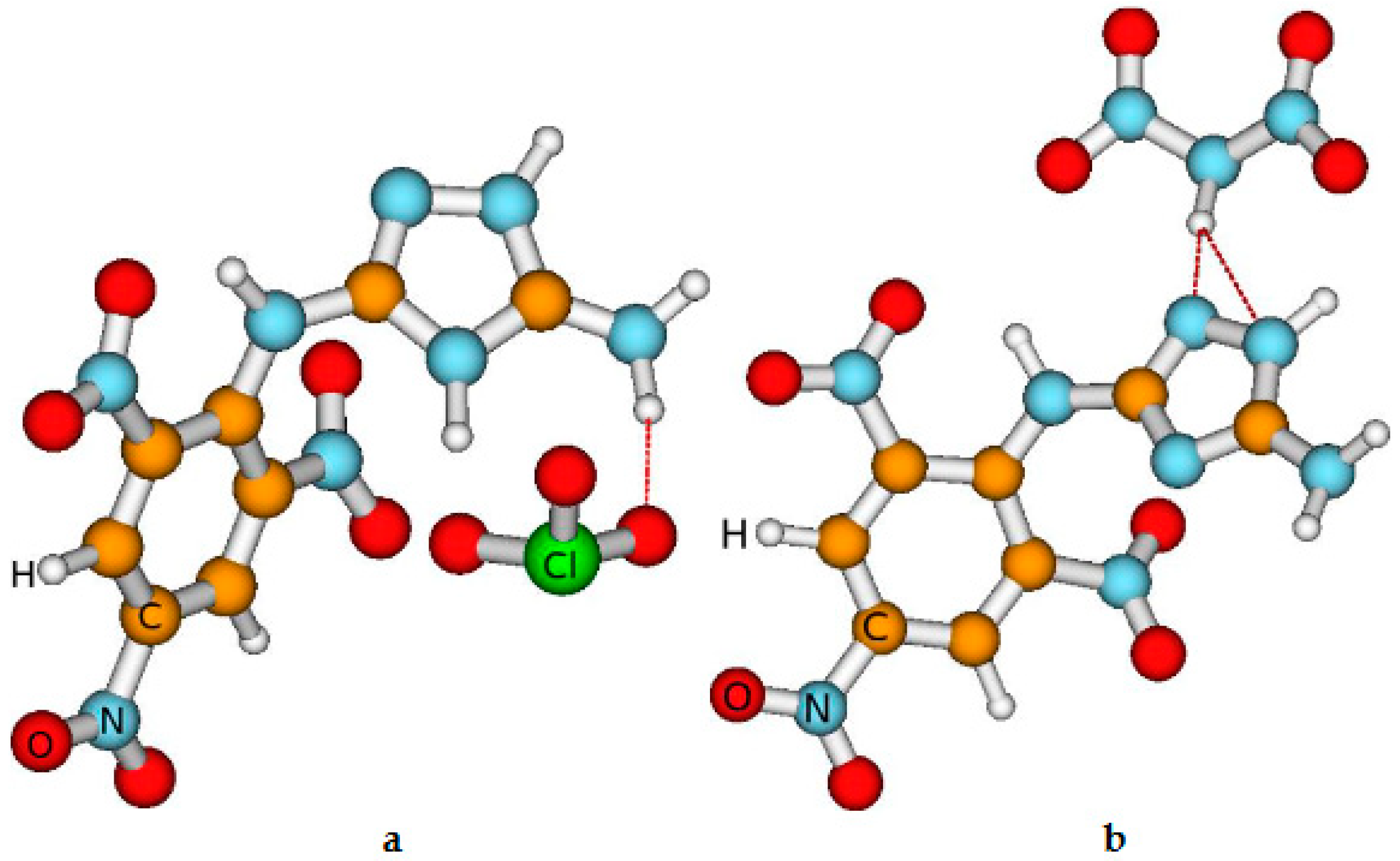
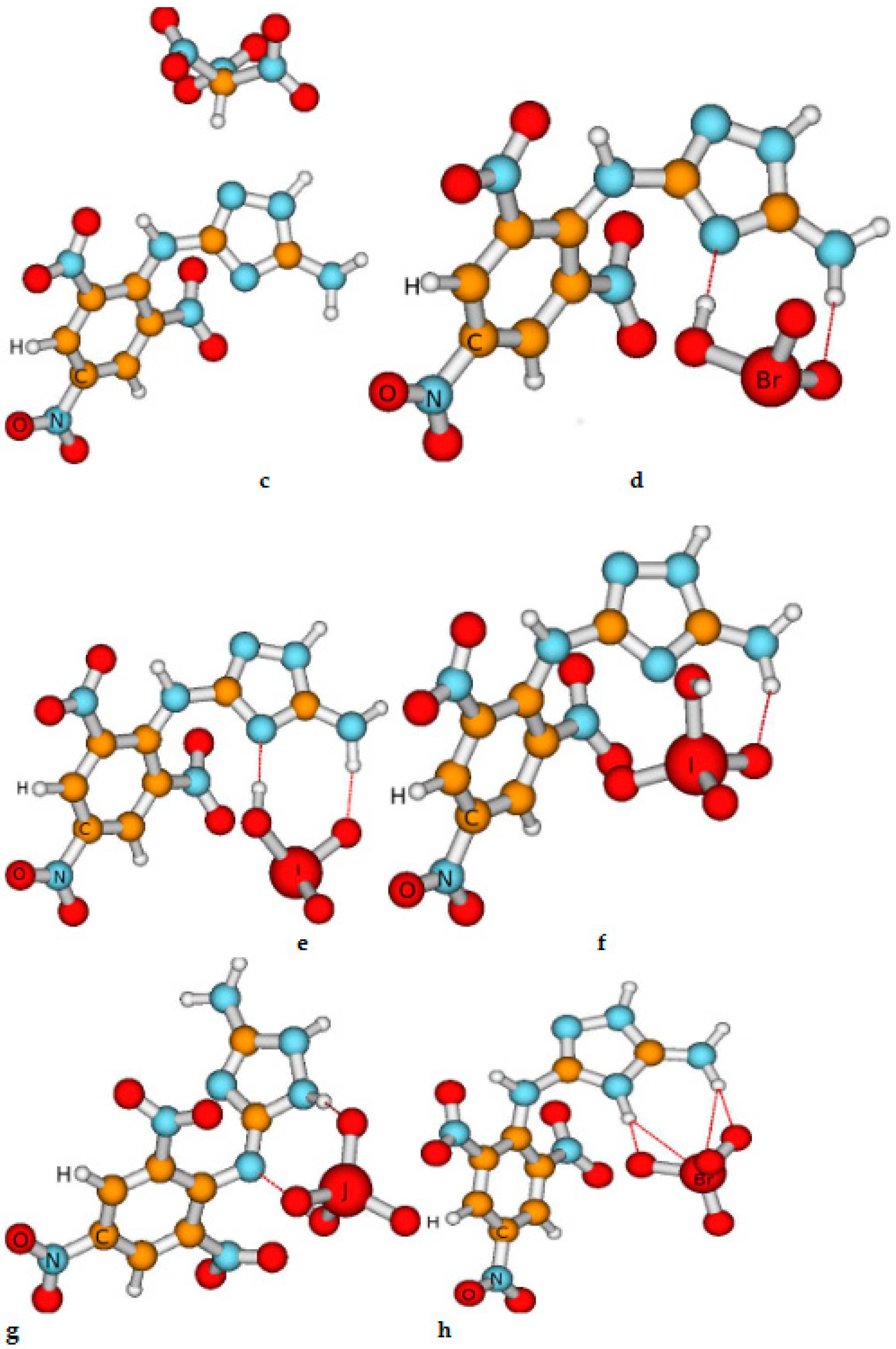
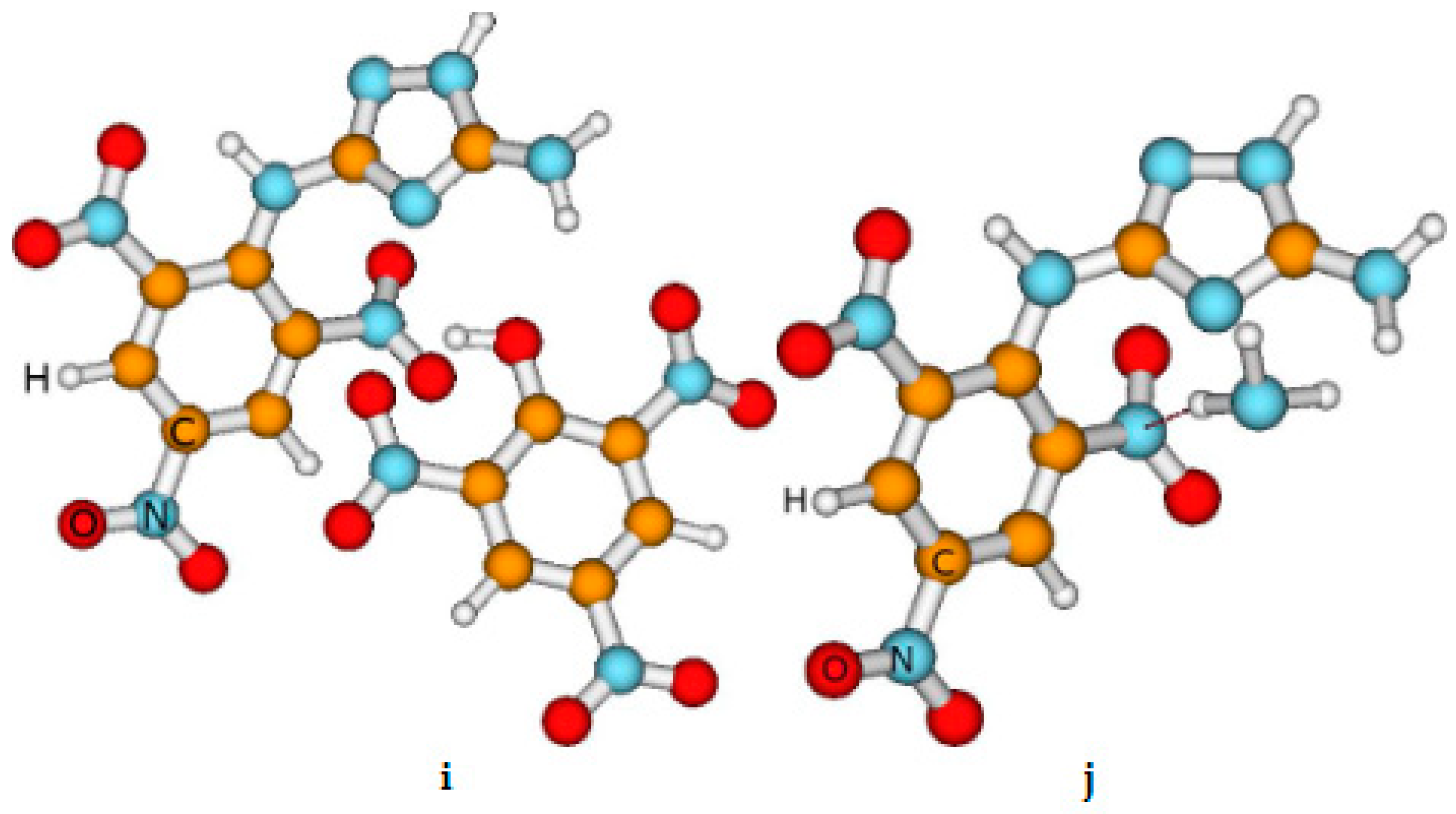
Figure 1.
The view of the most stable compounds obtained by us. The red dashed line indicates the presence of the Van der Waals bond occurrence. This presence is established based on the distance between the atoms Here, a is chlorate (ClO3-), ( HE-II); b is dinitramide [(NO2)2N], (HE-III); c is nitroformate [(NO2)3C-] (another name: trinitromethanide), (HE-IV); d is bromate (BrO3-, (HE-V); e is iodate(IO3-), (HE-VI); f is periodate (IO4), (HE-VIIb); g is periodate (IO4), HE-VIIa; h is perbromate (BrO4-),(HE-VIII), i is picrate [(NO2)3C6H2O-). (HE-IX); and j is NH3 (HE-X).
Figure 1.
The view of the most stable compounds obtained by us. The red dashed line indicates the presence of the Van der Waals bond occurrence. This presence is established based on the distance between the atoms Here, a is chlorate (ClO3-), ( HE-II); b is dinitramide [(NO2)2N], (HE-III); c is nitroformate [(NO2)3C-] (another name: trinitromethanide), (HE-IV); d is bromate (BrO3-, (HE-V); e is iodate(IO3-), (HE-VI); f is periodate (IO4), (HE-VIIb); g is periodate (IO4), HE-VIIa; h is perbromate (BrO4-),(HE-VIII), i is picrate [(NO2)3C6H2O-). (HE-IX); and j is NH3 (HE-X).
As mentioned previously, we use the abbreviation of the compounds given in the caption of
Figure 1 for convenience. These abbreviations are also presented in
Table 2. In this table, the calculated binding energy per atom, HOMO-LUMO gap, chemical hardness, and softness along with the harness index describing the thermal and chemical stability of the compounds under study are presented.
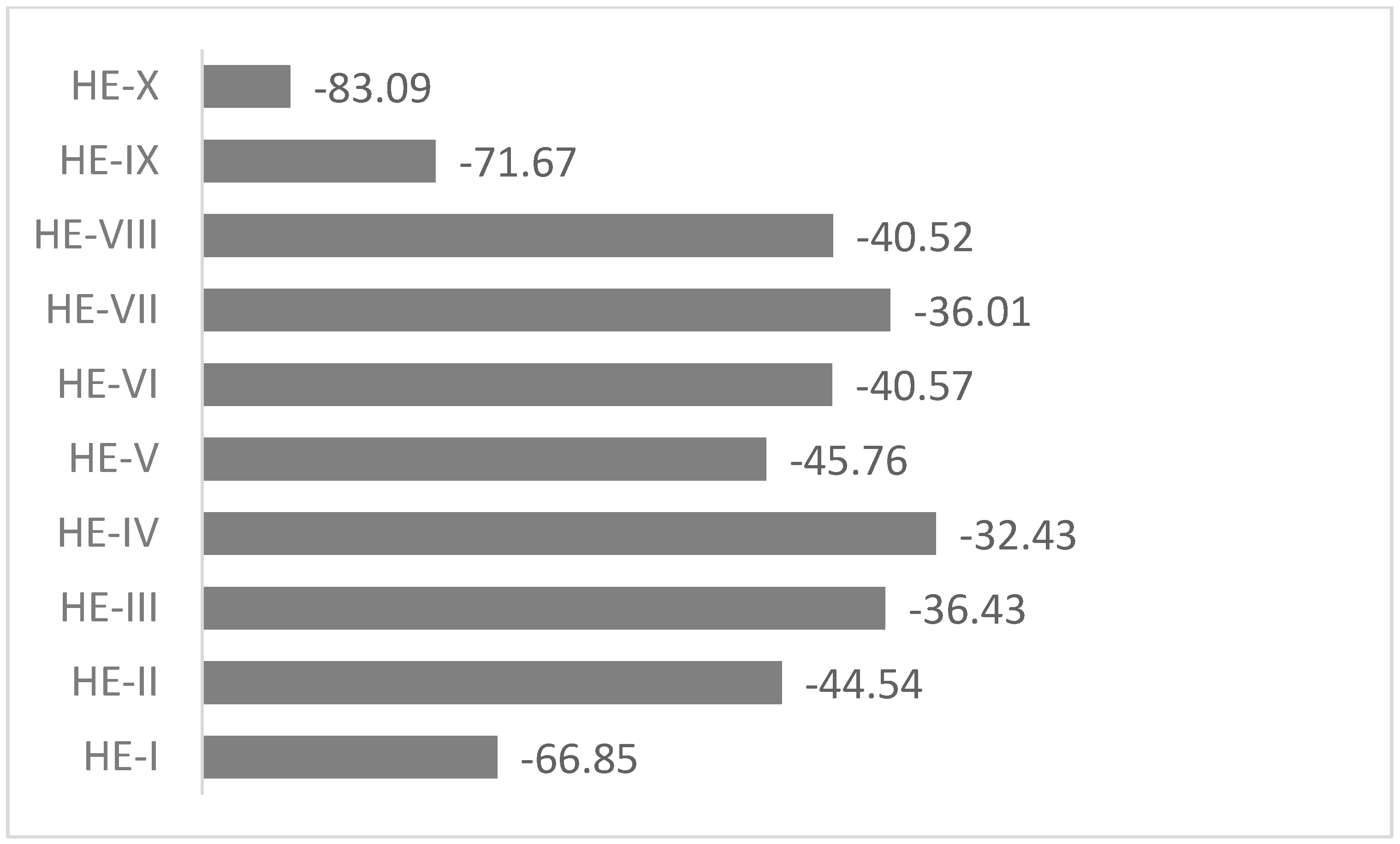
The sensitivity to the impact stimuli represented by oxygen balance is exhibited in
Figure 2.
In
Table 3 there are given parameters describing the energetic properties of the compounds.
4. Discussion
We remind you that we investigated approximately 30 compounds, i.e. various conformers. However, we present the results of the investigations of energetic properties and stability of the most stable (selected) compounds. It is emphasized, that in these selected conformers the Van der Walls bonds take place among HE-I and salt molecules which exhibit that agglomeration due to the Van der Walls bond increases the stability of the high-energy compounds [
63,
64]. It is obvious. that the size of the salt molecules and their chemical compositions are the main factors for their positions in the compounds with 3-amino-5-[(2, 4, 6-trinitrophenyl) amino]-1
H-1, 2, 4-triazole. The relatively large salt molecules such as HN
3O
4, and CHN
3O
4, could not be placed among the loophole formed by Phenyl and Triazole rings due to steric hindrance. So they displace the above triazole ring where the formation of Van der Walls bonds is possible. The relatively small salt molecule could both fill this loophole and form Van der Walls bonds. So they placed in this loophole. The presence of two HE-VI conformers confirms the findings concerning the size of the salt molecule and the possibility of the Van der Wals bond formation. It is necessary to emphasize that in the case of the HE-II HE-VIIb, and HE-VIII formation, the H transfer from salt molecules to HE-I 3-amino-5-[(2, 4, 6-trinitrophenyl) amino]-1
H-1, 2, 4-triazole process could take place. It is confirmed by the results of experimental measurement and theoretical studies [
65,
66,
67]. We cannot give any statements concerning the influence of this transfer on the energetic properties of the compounds yet because the approaches used do not include the influence of the chemical bonding on these properties. However, the comparison of the parameters describing the stability of the HE-VIIa and HE-VIIb indicates that H transfer does not influence the thermal and chemical stability of these compounds.
The analysis of the binding energy per atom revealed that agglomeration with salts decreases HE-I thermal stability. It is not a surprise, because the decomposition of the covalent bonds requires more energy than that of the Van der Walls or/ electrostatic forces. Undoubtedly, the values of the binding energy per atom do not represent the stability of the compounds under study concerning other high-energy materials. They are used to reveal how the thermal stability of HE-I changes. So the hardness indexes were calculated to evaluate both the reactivity and thermal stability compounds under study. Let us remind you that a higher hardness index (0.8-1.0) indicates compounds that are less reactive and more thermally stable, as it requires a larger energy change to induce electron transitions. These indexes of 0.8 and above (
Table 1) indicate that generally, the compounds with salts are highly stable, although their thermal stability is lower than that of HE-I. The exception is HE-IX where the Van der Waals and strong ionic bonds are not formed. This conclusion is based on several research data. First, the bond length among the atoms of HE-I and Picrate is larger than 0.3-0.6 nm presented elsewhere. Second, the values of condensing to atom all electrons representing bond order between the above atoms equal to 0. Lastly, the total Mulliken charge of HE-I is only -0.01, which is approximately ten times smaller than that in other investigated compounds.
The HOMO-LUMO gaps of the salt compounds are similar to or larger than those of HE-I (
Table 1). A small HOMO-LUMO gap indicates that electrons can be easily promoted from the HOMO to the LUMO, allowing the molecule to participate in chemical reactions. So, compounds with smaller HOMO-LUMO gaps are generally more reactive because they require less energy to initiate chemical reactions. The HOMO-LUMO gaps of the HE-II, HE-II, HE-V, HE-VII, HE-VIII, and HE-X compounds are larger than that of HE-I. It implies that these compounds are chemically stabler than HE-I. The most stable among them is HE-VII. So, referring to our results obtained, we may state that only properly chosen salts could significantly increase the chemical stability of the aminated compound with a triazole ring.
The similar or higher resistance of salt compounds to undergo a chemical reaction or to be transformed by an external perturbation than that of HE-I indicates the values of chemical hardness presented in
Table 1. A significantly lower value of chemical softness of HE-VII in comparison to HE-I denotes a higher tendency of this salt compound to degrade, although in the rest cases, this tendency remains similar to the primary compound. So, in brief, again, we may state that the increasing chemical and thermal stability is salts dependent, i.e. only agglomeration with precise salts could lead to significant improvement of the stability of the specific high-energy materials. Moreover, the high chemical stability does not indicate high resistance to degradation due to environmental stimuli.
The need for the proper selection of salts for specific high-energy materials is confirmed by the results of the oxygen balance analysis (
Figure 2). It is clear that the Oxygen balance of the HE-II, HE-III, HE-IV, HE-V, HE-VI, and HE -VII is closer to zero than that of HE-I. It means that the materials could be more brisant, powerful, and sensitive. So, in the abovementioned cases, the aggregation with salts leads to an increase in the sensitivity to the impact of shock stimuli. The opposite result is received in the case of HE-VIII. The increase in the negative value of oxygen balance indicates that the agglomeration of HE-I within NH
3 decreases the above sensitivity.
The improving energetic properties of the HE-I due to agglomeration confirm the detonation velocity and pressure (
Table 2). The values of the detonation pressure and velocity of the salt compounds are higher than those of HE-I. Significant improvement is achieved when HE-I agglomeration with HClO
3 or HIO
3, and CH(NO
2)
3 is occurred. However, the improvement of the energetic properties of HE-I due to agglomeration within NH
3 could not be significant which is exhibited by the similar values of the detonation pressures and velocities of these compounds (
Table 2) although the above compounds possess better energetic properties than TNT, which experimentally obtained detonation velocity is 6.9 km/s [
56].
Hence, the results of our investigation exhibited that only agglomeration with specific salts could significantly improve the energetic properties of the aminated compound with a triazole ring and also remarkably decrease resistance to shock stimuli. Even thermal and chemical stability could be significantly improved only within the agglomeration of specific salts.
5. Conclusions
We studied a group of new energetic salts based on APATO aiming to reveal their stability and energetic properties. The results of our investigations confirm the findings of other researchers that the agglomeration due to the Van der Walls bond increases the stability of the high-energy compounds. We also found that the position of salts in respect of 3-amino-5-[(2, 4, 6-trinitrophenyl) amino]-1H-1, 2, 4-triazole is salt size dependent. The relatively small salt molecules could be placed among the loophole formed by Phenyl and Triazole rings, while the rest could be displaced above this ring where the formation of Van der Walls bonds is possible. The H transfer from salt molecules to 3-amino-5-[(2, 4, 6-trinitrophenyl) amino]-1H-1, 2, 4-triazole process could take place. Referring to the results of our studies, we predicted that the H transfer could not influence the thermal and chemical stability of 3-amino-5-[(2, 4, 6-trinitrophenyl) amino]-1H-1, 2, 4-triazole with salts.
Calculated values of hardness indexes indicate the high stability of 3-amino-5-[(2, 4, 6-trinitrophenyl) amino]-1H-1, 2, 4-triazole with salts. The exception is compounds with Picrate in which the Van der Waals and strong ionic bonds are not formed.
Referring to the results of the analysis of the HOMO-LUMO gap, and chemical hardness, we state that only properly chosen salts could significantly increase the chemical stability of the aminated compound with a triazole ring. The properly chosen salts could reduce sensitivity to shock stimuli of the above compounds. This sensitivity of 3-amino-5-[(2, 4, 6-trinitrophenyl) amino]-1H-1, 2, 4-triazole will decrease NH3 which indicates oxygen balance study presented in this paper.
The larger detonation pressure and velocity, in some cases remarkable, than that of 3-amino-5-[(2, 4, 6-trinitrophenyl) amino]-1H-1, 2, 4-triazole allow us to predict the improving energetic properties of the aminated compound due to agglomeration.
Overall, our study showed that perchlorate anion in high-energy materials could be exchanged with aminated compounds.
Author Contributions
Conceptualization, J.S. and J.T.; methodology, J.T.; validation, J.S., and J.T.; formal analysis, J.S., and J.T..; investigation, J.S.; resources, J.T..; data curation, J.S., and J.T; writing—original draft preparation, J.S., and J.T.; writing—review and editing, J.S., and J.T.. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The data supporting reported results can be received under request for authors.
Acknowledgments
The numerical calculations with GAUSSIAN09 package were performed on the resources of the Information Technology Research Center of Vilnius University.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Akhavan, J. The Chemistry of Explosives, 4th ed.; RSC, London, UK, 2022, 359 pp. ISBN: 978-1-83916-446-0.
- Agrawal, J.P.; Hodgson, R. Organic Chemistry of Explosives. Wiley Interscience, N.Y., 2007, 417pp. ISBN: 978-0-470-02967-1.
- Green Energetic Materials. (Brinck, T., Ed.) Wiley, Chichester, UK, 2014, 290 pp. ISBN: 978-1-119-94129-3.
- Klapötke, T.M. Chemistry of High-Energy Materials. Walter de Gruyter, Berlin, 2012. pp. 141-142. ISBN 978-3110273588.
- Agrawal, P.; Dodke, V.S. Validation of approaches (Salt Formation & Introduction of Amino Group/s) for Imparting/Improving Thermal Stability of Explosives, Part I. Propellants Explos. Pyrotech., 2022, 47, e202100265. [CrossRef]
- Agrawal, P.; Dodke, V.S. Validation of approaches (introduction of conjugation & condensation with triazole ring/s) for imparting/improving thermal stability of explosives, Part II, FirePhysChem, 2023, 3, 1-10. [CrossRef]
- Tang, Y.; He, C.; Imler, G.H.; Parrish, D.A.; Shreeve, J.N.M. Aminonitro groups surrounding a fused pyrazolotriazine ring: a superior thermally stable and insensitive energetic material. ACS Appl. Energy Mater. 2019, 2, 2263-2267. [CrossRef]
- Tang, Y.; An, Z.; Chinnam, A.K.; Staples, R.J.; Shreeve, J.N.M. Very thermostable energetic materials based on a fused-triazole: 3, 6-diamino-1 H-[1,2,4] triazolo [4, 3- b][1, 2, 4] triazole. New J. Chem., 2021, 45, 85-91. [CrossRef]
- Tamuliene, J.; Sarlauskas, J.; Bekesiene, S. Modeling and investigation of new explosive materials based on N-(3, 5-dimethyl-2, 4, 6-trinitrophenyl)-1 H-1, 2, 4-triazol-3-amine. J. Mol. Model. 2017, 23, 1-6. [CrossRef]
- Coburn, M.D.; Jackson, T.E. Picrylamino-substituted heterocycles. III. 1, 2, 4-triazoles. J. Heterocycl. Chem., 1968, 5, 199-203. [CrossRef]
- Yigiter, A.O.; Atakol, M.K.; Levent Aksu, M.; Atakol, O. Thermal characterization and theoretical and experimental comparison of picryl chloride derivatives of heterocyclic energetic compounds. J. Therm. Anal. Calorim., 2017, 127, 2199-2213. [CrossRef]
- Tamulienė, J., Šarlauskas, J., Bekešienė, S. Influence of Nitro Group Substitutes to the Stability and Energetic Properties of N-(2, 4, 6-trinitrophenyl)-1H-1, 2, 4-triazol-3-amine. Am. J. Analyt. Chem., 2017, 8, 125-141. [CrossRef]
- Chioato, Z.L.; Klapötke, T.M.; Mieskes, F.; Stierstorfer, J.; Weyrauther, M. (Picrylamino)-1, 2, 4-triazole Derivatives–Thermally Stable Explosives. Eur. J. Inorg. Chem., 2016, 7, 956-962. [CrossRef]
- Mitchell, A.R.; Coburn, M.D.; Schmidt, R.D.; Pagoria, P.F.; Lee, Gregory S. Advances in the chemical conversion of surplus energetic materials to higher value products. Thermochim. Acta, 2002, 384, 205–217. [CrossRef]
- Finch, A.; Gardner, P.J.; Smith, A.E. Thermochemistry of picrates. I. The standard enthalpy of formation of ammonium picrate. Thermochim. Acta, 1981, 49, 281-285. [CrossRef]
- Liu, L.; Li, F.; Tan, L.; Ming, L.; Yi, Y. Effects of Nanometer Ni, Cu, Al and NiCu Powders on the Thermal Decomposition of Ammonium Perchlorate. Propellants, Explos. Pyrotech. 2004, 29, 34–38. [CrossRef]
- Boggs, T.L. Deflagration Rate, Surface Structure and Subsurface Profile of Self-Deflagrating Single Crystals of Ammonium Perchlorate. AIAA J., 1970, 8, 867–873. [CrossRef]
- Dendage, P.S.; Sarwade, D.B.; Asthana, S.N.; Singh, H. Hydrazinium nitroformate (HNF) and HNF based propellants: A review. J. Energ. Mater., 2001, 19, 41-78. [CrossRef]
- Jadhav, H.S.; Talawar, M.B.; Dhavale, D.D.; Asthana, S.N.; Krishnamurthy, V.N. Synthesis, characterization and thermal behavior of hydrazinium nitroformate (HNF) and its new N-alkyl substituted derivatives. Indian J. Chem. Technol., 2005, 12, 187-199.
- Tummers, M.J.; van der Heijden, A.E.D.M.; van Veen, E.H. Selection of burning rate modifiers for hydrazinium nitroformate. Combust. Flame, 2012, 159, 882-886. [CrossRef]
- Rossi, M.J.; Bottaro, J.C.; McMillen, D.F.The Thermal Decomposition of the New Energetic Material Ammoniumdinitramide (NH4N(NO2)2) in Relation to Nitramide (NH2NO2) and NH4NO3. Int. J. Chem. Kinet. 1993, 25, 549-570. [CrossRef]
- Luk’yanov, O.A.; Shlykova, N.I.; Tartakovsky, V.A. Dinitramide and its salts. Russ. Chem. Bull. 1994, 43, 1680–1683. [CrossRef]
- Langlet, A.; Wingborg, N.; Ostmark, H. ADN: A new high performance oxidizer for solid propellants. Int. J. Energ. Mater. Chem. Propuls., 1997, 4, 616-626. [CrossRef]
- Muravyev, N.V.; Koga, N.; Meerov D.B.; Pivkina A.N. Kinetic analysis of overlapping multistep thermal decomposition comprising exothermic and endothermic processes: thermolysis of ammonium dinitramide. Phys. Chem. Chem. Phys., 2017,19, 3254-3264. [CrossRef]
- Yang, H.; Chen, F.; Hu, Y.; Lu, Q.; Xiao, L..; Wang, Y.; Zhao, F.; Jiang, W.; Hao, G. A review on surface coating strategies for anti-hygroscopic of high energy oxidizer ammonium dinitramide. Def. Technol., 2023 (In Press, available online: 26 August 2023). [CrossRef]
- Chaplygin, D.A.; Larin, A.A.; Muravyev, N.V.; Meerov, D.B.; Kosareva, E.K.; Kiselev, V.G.; Pivkina, A.N.; Ananyev, I.V.; Fershtat, L. L. Nitrogen-rich metal-free salts: a new look at the 5-(trinitromethyl) tetrazolate anion as an energetic moiety. Dalton Transactions, 2021, 50, 13778-13785. [CrossRef]
- Ma, Q.; Fan, G.; Liao, L.; Lu, H.; Chen, Y.; Huang, J. Thermally Stable Energetic Salts Composed of Heterocyclic Anions and Cations Based on 3, 6, 7-Triamino-7 H-s-triazolo [5, 1-c]-s-triazole: Synthesis and Intermolecular Interaction Study. ChemPlusChem, 2017, 82, 474-482. [CrossRef]
- Li, Y.; Huang, H.; Lin, X.; Pan, R.; Yang, J. Oxygen-rich anion based energetic salts with high detonation performances. RSC Adv., 2016, 6, 54310-54317. [CrossRef]
- Fischer, N.; Fischer, D.; Klapötke, T.M.; Piercey, D.G.; Stierstorfer, J. Pushing the limits of energetic materials – the synthesis and characterization of dihydroxylammonium 5,5′-bistetrazole-1,1′-diolate. J. Mater. Chem., 2012, 22, 20418–20422. [CrossRef]
- Fischer, N.; Klapötke, T.M.; Reymann, M.; Stierstorfer, J.: Nitrogen-Rich Salts of 1H,1′H-5,5′-Bitetrazole-1,1′-diol: Energetic Materials with High Thermal Stability. Eur. J. Inorg. Chem. 2013, 12, 2167–2180. [CrossRef]
- Huang, H., Shi, Y., & Yang, J. Thermal characterization of the promising energetic material TKX-50. J. Therm. Anal. Calorim., 2015, 21, 705-709. [CrossRef]
- Klapötke, T.M.; Cudziło, S.; Trzciński, W.A.; Paszula, J.; Bauer, L.; Riedelsheimer, C.; Lechner, J.T. Performance of TKX-50 in thermobaric explosives. Propellants Explos. Pyrotech., 2023, 48, e202300010. [CrossRef]
- Golenko, Y.D.; Topchiy, M.A.; Asachenko, A.F.; Nechaev, M.S.; Pleshakov, D.V. Optimization Studies on Synthesis of TKX-50. Chin. J. Chem., 2017, 35, 98-102. [CrossRef]
- Šarlauskas, J.; Tamulienė, J. Preparation and Characterization of Cationic Energetic Salts of 5-Amino-3-[(2, 4, 6-trinitrophenyl) amino]-1H-1, 2, 4-triazole (APATO). Cent. Eur. J. Energ., 2022, 19, 311-325. [CrossRef]
- Niziński, P.; Błażewicz, A.; Kończyk, J.; Michalski, R. Perchlorate–properties, toxicity and human health effects: an updated review. Rev. Environ. Health, 2021, 36, 199-222. [CrossRef]
- Pleus, R.C.; Corey, L.M.. Environmental exposure to perchlorate: a review of toxicology and human health. Toxicol. Appl. Pharmacol., 2018, 358, 102-109. [CrossRef]
- Oxley, J.C., Smith, J.L., Porter, M.M., Yekel, M.J., Canaria, J.A. Potential Biocides: Iodine-Producing Pyrotechnics. Propellants Explos. Pyrotech. 2017, 42, 960-973. [CrossRef]
- Oxley, J.C. The Chemistry of Explosives. In Zukas, J.A., Walters, W.P., Eds.; Explosive Effects and Applications. High-Pressure Shock Compression of Condensed Matter., Springer, New York, NY, 1998; pp. 137-172. ISBN978-0-387-95558-2. [CrossRef]
- He, P., Zhang, J. G., Yin, X., Wu, J. T., Wu, L., Zhou, Z. N., & Zhang, T. L. Energetic Salts Based on Tetrazole N-oxide. Chemistry–A European Journal, 2016, 22, 7670-7685. [CrossRef]
- Wurzenberger, M.H.; Szimhardt, N.; Stierstorfer, J. Nitrogen-rich copper (II) bromate complexes: an exotic class of primary explosives. Inorg. Chem., 2018, 57, 7940-7949. [CrossRef]
- Tamuliene, J.; Sarlauskas, J. Impact of Incremental Methylene Groups on the Energetic Properties of Aromatic Nitramines. Energies, 2023, 16, 3117. [CrossRef]
- Becke, A.D. Density functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648. [CrossRef]
- Dunning Jr. T.H, Gaussian basis sets for use in correlated molecular calculations. I. The atoms boron through neon and hydrogen. J. Chem. Phys. 1989, 90, 1007. [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J. R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; Li, X.; Caricato, M.; Marenich, A.; Bloino, J.; Janesko, B.G.; Gomperts, R.; Mennucci, B.; Hratchian, H.P.; Ortiz, J.V.; Izmaylov, A.F.; Sonnenberg, J.L.; Williams-Young, D.; Ding, F.; Lipparini, F.; Egidi, F.; Goings, J.; Peng, B.; Petrone, A.; Henderson, T.; Ranasinghe, D.; Zakrzewski, V.G.; Gao, J.; Rega, N.; Zheng, G.; Liang, W.; Hada, M.; Ehar, M.; Toyota, K.; Fukuda, R.; Hasegawa, J.; Ishida, M.; Nakajima, T.; Honda, Y.; Kitao, O.; Nakai, H.; Vreven, T.; Throssell, K.; Montgomery, J.A.Jr., Peralta, J.E.; Ogliaro, F.; Bearpark, M.; Heyd, J.J.; Brothers, E.; Kudin, K.N.; Staroverov, V.N.; Keith, T.; Kobayashi, R.; Normand, J.; Raghavachari, K.; Rendell, A.; Burant, J.C.; Iyengar, S.S.; Tomasi, J.; Cossi, M.; Millam, J.M.;, Klene, M.; Adamo, C.; Cammi, R.; Ochterski, J.W.; Martin, R.L.; Morokuma, K.; Farkas, O.; Foresman, J.B., Fox, D.J. Gaussian, Inc., Wallingford CT. 2016., 139pp. URL: https://www.cwu.edu/chemistry/sites/cts.cwu.edu.chemistry/files/documents/Gaussian_09_ReferenceManual.pdf (accessed: 26.03.2023).
- Cardia, R.; Malloci, G.; Mattoni, A.; Cappellini, G. Effects of TIPS-Functionalization and Perhalogenation on the Electronic, Optical, and Transport Properties of Angular and Compact Dibenzochrysene. J. Phys. Chem. A 2014, 118, 5170–5177. [CrossRef]
- Cardia, R.; Malloci, G.; Rignanese, G.M.; Blasé, X.; Molteni, E.; Cappellini, G. Electronic and optical properties of hexathiapentacene in the gas and crystal phases. Phys. Rev. B 2016, 93, 235132. [CrossRef]
- Dardenne, N.; Cardia, R.; Li, J.; Malloci, G.; Cappellini, G.; Blasé, X.; Charlier, J.C.; Rignanese, G. Tuning Optical Properties of Dibenzochrysenes by Functionalization: A Many-Body Perturbation Theory Study. Phys. Chem. C 2017, 121, 24480–24488. [CrossRef]
- Antidormi, A.; Aprile, G.; Cappellini, G.; Cara, E.; Cardia, R.; Colombo, L.; Farris, R.; d’Ischia, M.; Mehrabanian, M.; Melis, C.; et al. Physical and Chemical Control of Interface Stability in Porous Si–Eumelanin Hybrids. J. Phys. Chem. C 2018, 122, 28405–28415. [CrossRef]
- Mocci, P.; Cardia, R.; Cappellini, G. Inclusions of Si-atoms in Graphene nanostructures: A computational study on the ground-state electronic properties of Coronene and Ovalene. J. Phys. Conf. Ser. 2018, 956, 012020. [CrossRef]
- Mocci, P.; Cardia, R.; Cappellini, G. Si-atoms substitutions effects on the electronic and optical properties of coronene and ovalene. New J. Phys. 2018, 20, 113008. [CrossRef]
- Kumar, A.; Cardia, R.; Cappellini, G. Electronic and optical properties of chromophores from bacterial cellulose. Cellulose 2018, 25, 2191–2203. [CrossRef]
- Szafran, M.; Koput, J. Ab initio and DFT calculations of structure and vibrational spectra of pyridine and its isotopomers. J. Mol. Struct. 2001, 565, 439–448. [CrossRef]
- Begue, D.; Carbonniere, P.; Pouchan, C. Calculations of Vibrational Energy Levels by Using a Hybrid ab Initio and DFT Quartic Force Field: Application to Acetonitrile. J. Phys. Chem. A 2005, 109, 4611–4616. [CrossRef]
- Parthasarathi, R.; Padmanabhan, J.; Subramanian, V.; Maiti, B.; Chattaraj, P.K. Toxicity analysis of 3,3′,4,4′,5-pentachloro biphenyl through chemical reactivity and selectivity profiles. Curr. Sci. 2004, 86, 535–542. Available online: https://www.jstor.org/stable/24107906 (accessed on 30 January 2023).
- Tamuliene, J.; Sarlauskas, J. Computational Studies of Energetic Property Peculiarities in Trinitrophenyl-Substituted Nitramines. Energies, 2023, 16, 5180. [CrossRef]
- Urizar, M.J.; James, E., Jr.; Smith, L.C. Detonation velocity of pressed TNT. Phys. Fluids 1961, 4, 262–274. [CrossRef]
- Türker, L. Velocity of detonation—A mathematical model. Acta Chim. Slov. 2010, 57, 288–296.
- Kamlet, M.J.; Jacobs, S.J. Chemistry of Detonations. I. Simple Method for Calculating Detonation Properties of CHNO Explosives. J. Chem. Phys. 1968, 48, 23–55. [CrossRef]
- Zohari, N.; Montazeri, M.; Hosseini, S.G. Estimation of the Detonation Pressure of Co-crystal Explosives through a Novel, Simple and Reliable Model. Cent. Eur. J. Energ. Mater. 2020, 17, 492-505. [CrossRef]
- Wen, L.; Wang, B.; Yu, T.; Lai, W.; Shi, J.; Liu, M.; Liu, Y. Accelerating the search of CHONF-containing highly energetic materials by combinatorial library design and high-throughput screening. Fuel. 2022, 310, 122241. [CrossRef]
- Li, Y., Wang, X.; Xue, M. Touching the density limits of energetic materials by molecular design. New J. Chem. 2023, 47, 19191-19201. [CrossRef]
- Klapötke, T.M.; Ang, H.G. Estimation of the Crystalline Density of Nitramine (N-NO2 based) High Energy Density Materials (HEDM). Propellants Explos. Pyrotech, 2001, 26, 221-224. [CrossRef]
- Feng, B.; Fan, L.W.; Zeng, Y. Atomistic insights into the synergistic effects of tensile strain and hydroxyl group on increasing the thermal conductivity of monohydric alcohols as latent heat storage materials. Mater. Today Commun. 2020, 25, 101335. [CrossRef]
- McNeil, S.A.; Kelley, S.P., Beg, C.; Cook, H.; Rogers, R.D.; Nikles, D.E. Cocrystals of 10-methylphenthiazine and 1,3-dinitrobenzene: implications for the optical sensing of tnt-based explosives. ACS Appl. Mater. Interfaces, 2013, 5, 7647-7653. [CrossRef]
- Baxter, A.F.; Martin, I., Christe, K.O.; Haiges, R. Formamidinium Nitroformate: An Insensitive RDX Alternative. J. Am. Chem. Soc. 2018, 140, 15089−15098. [CrossRef]
- Şen, N.; Nazir, H.; Atҫeken, N.; Hope, K.S.; Acar, N.; Atakol, O. Synthesis, characterisation and energetic performance of insensitive energetic salts formed between picric acid and 2, 3-diaminotoluene, 2, 4-diaminotoluene. J. Mol. Struct. 2020, 1205, 127580. [CrossRef]
- Panja, S.K.; Dwivedi, N.; Noothalapati, H.; Shigeto, S.; Sikder, A.K.; Saha, A.; Sunkari, S.S.; Saha, S. (2015). Significance of weak interactions in imidazolium picrate ionic liquids: spectroscopic and theoretical studies for molecular level understanding. Phys. Chem. Chem. Phys. 2015, 17, 18167-18177. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).