Submitted:
15 December 2023
Posted:
18 December 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
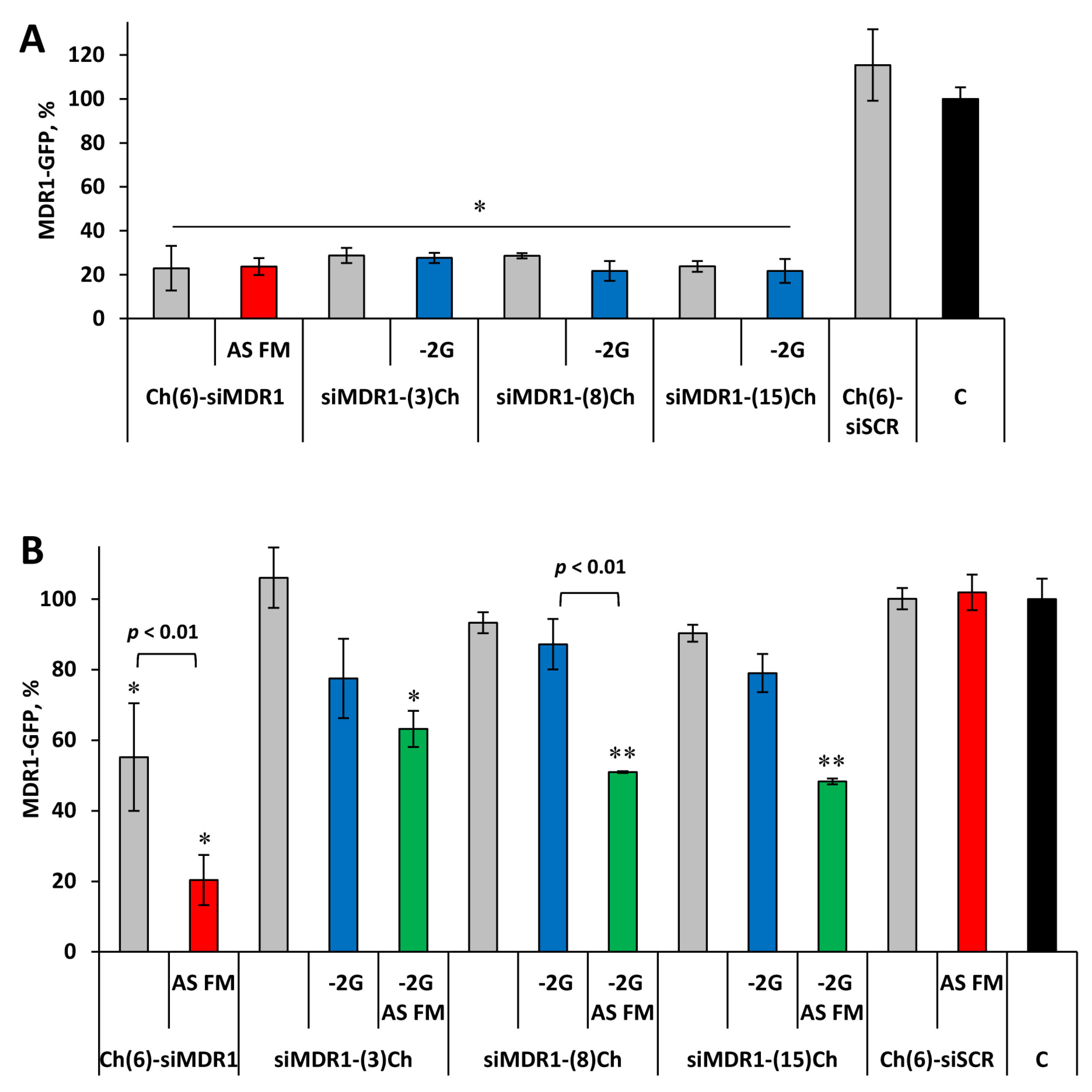
2.1. Silencing activity of cholesterol-modified siRNA under transfection
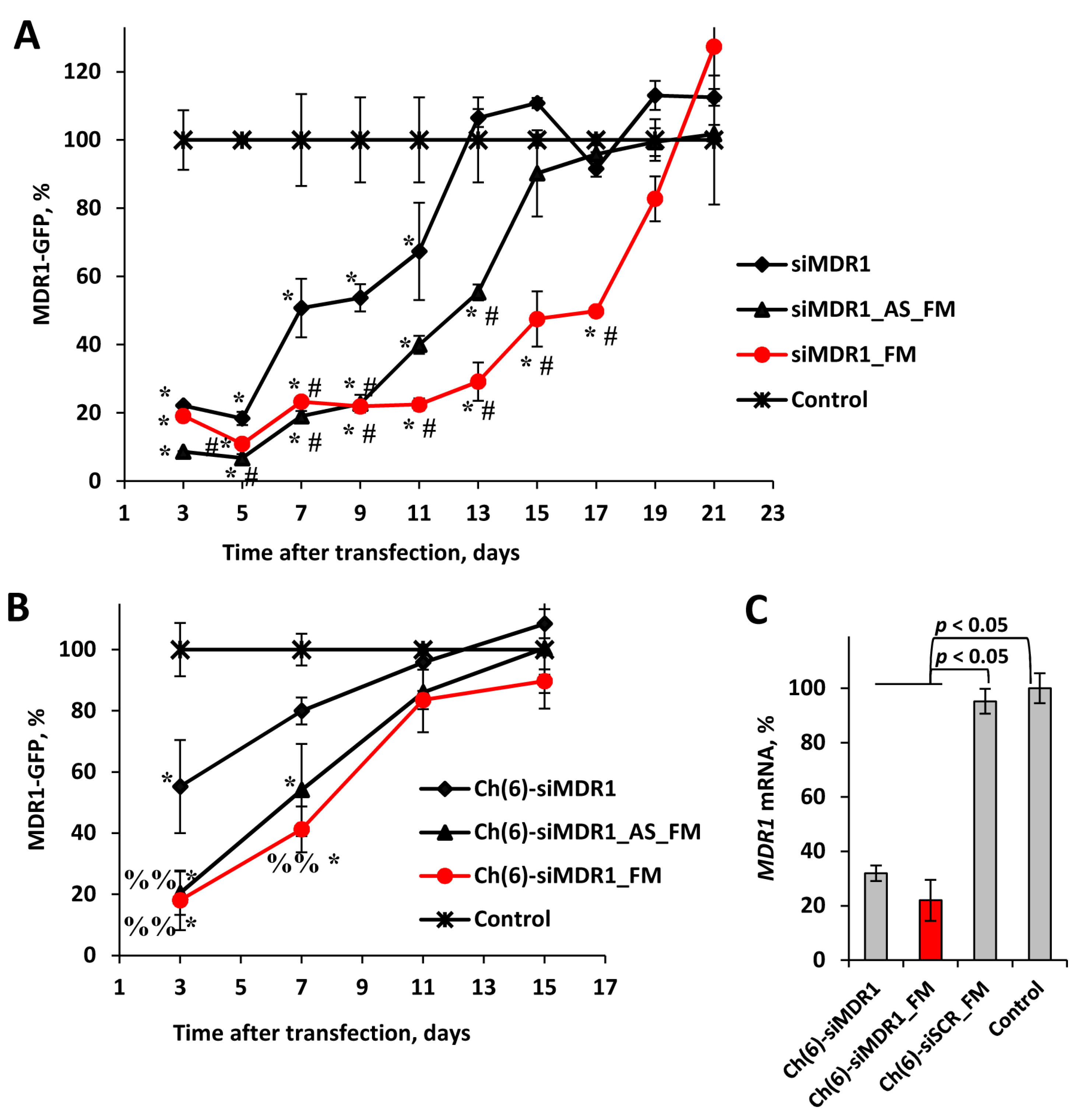
2.2. Silencing activity of cholesterol-modified siRNA in carrier-free mode
2.3. Silencing activity of selectively and fully modified siRNA
3. Discussion
4. Materials and Methods
4.1. Oligonucleotides
4.2. Cell cultures
4.3. Silencing activity assay using flow cytometry
4.4. Mice
4.5. Silencing activity assay in KB-8-5 xenograft tumors in SCID mice after intravenous administration
4.6. Statistical analyses
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fire, A.; Xu, S.; Montgomery, M.K.; Kostas, S.A.; Driver, S.E.; Mello, C.C. Potent and Specific Genetic Interference by Double-Stranded RNA in Caenorhabditis Elegans. Nature 1998, 391, 806–811. [CrossRef]
- Corydon, I.J.; Fabian-Jessing, B.K.; Jakobsen, T.S.; Jørgensen, A.C.; Jensen, E.G.; Askou, A.L.; Aagaard, L.; Corydon, T.J. 25 Years of Maturation: A Systematic Review of RNAi in the Clinic. Mol. Ther. - Nucleic Acids 2023, 33, 469–482. [CrossRef]
- Egli, M.; Manoharan, M. Chemistry, Structure and Function of Approved Oligonucleotide Therapeutics. Nucleic Acids Res. 2023, 51, 2529–2573. [CrossRef]
- Liu, C.; Shi, Q.; Huang, X.; Koo, S.; Kong, N.; Tao, W. MRNA-Based Cancer Therapeutics. Nat. Rev. Cancer 2023, 23, 526–543. [CrossRef]
- Chernikov, I. V.; Vlassov, V. V.; Chernolovskaya, E.L. Current Development of SiRNA Bioconjugates: From Research to the Clinic. Front. Pharmacol. 2019, 10, 444. [CrossRef]
- Salim, L.; Islam, G.; Desaulniers, J.P. Targeted Delivery and Enhanced Gene-Silencing Activity of Centrally Modified Folic Acid-SiRNA Conjugates. Nucleic Acids Res. 2020, 48, 75–85. [CrossRef]
- Nair, J.K.; Willoughby, J.L.S.; Chan, A.; Charisse, K.; Alam, M.R.; Wang, Q.; Hoekstra, M.; Kandasamy, P.; Kelin, A. V.; Milstein, S.; et al. Multivalent N -Acetylgalactosamine-Conjugated SiRNA Localizes in Hepatocytes and Elicits Robust RNAi-Mediated Gene Silencing. J. Am. Chem. Soc. 2014, 136, 16958–16961. [CrossRef]
- Chen, J.; Li, F.; Xu, Y.; Zhang, W.; Hu, Y.; Fu, Y.; Xu, W.; Ge, S.; Fan, X.; Lu, L. Cholesterol Modification of SDF-1-Specific SiRNA Enables Therapeutic Targeting of Angiogenesis through Akt Pathway Inhibition. Exp. Eye Res. 2019, 184, 64–71. [CrossRef]
- Zhou, J.; Lazar, D.; Li, H.; Xia, X.; Satheesan, S.; Charlins, P.; O’Mealy, D.; Akkina, R.; Saayman, S.; Weinberg, M.S.; et al. Receptor-Targeted Aptamer-SiRNA Conjugate-Directed Transcriptional Regulation of HIV-1. Theranostics 2018, 8, 1575–1590. [CrossRef]
- Cen, B.; Wei, Y.; Huang, W.; Teng, M.; He, S.; Li, J.; Wang, W.; He, G.; Bai, X.; Liu, X.; et al. An Efficient Bivalent Cyclic RGD-PIK3CB SiRNA Conjugate for Specific Targeted Therapy against Glioblastoma In Vitro and In Vivo. Mol. Ther. - Nucleic Acids 2018, 13, 220–232. [CrossRef]
- Malecova, B.; Burke, R.S.; Cochran, M.; Hood, M.D.; Johns, R.; Kovach, P.R.; Doppalapudi, V.R.; Erdogan, G.; Arias, J.D.; Darimont, B.; et al. Targeted Tissue Delivery of RNA Therapeutics Using Antibody-Oligonucleotide Conjugates (AOCs). Nucleic Acids Res. 2023, 51, 5901–5910. [CrossRef]
- Brown, K.M.; Nair, J.K.; Janas, M.M.; Anglero-Rodriguez, Y.I.; Dang, L.T.H.; Peng, H.; Theile, C.S.; Castellanos-Rizaldos, E.; Brown, C.; Foster, D.; et al. Expanding RNAi Therapeutics to Extrahepatic Tissues with Lipophilic Conjugates. Nat. Biotechnol. 2022, 40, 1500–1508. [CrossRef]
- Benizri, S.; Gissot, A.; Martin, A.; Vialet, B.; Grinstaff, M.W.; Barthélémy, P. Bioconjugated Oligonucleotides: Recent Developments and Therapeutic Applications. Bioconjug. Chem. 2019, 30, 366–383. [CrossRef]
- Chernikov, I. V.; Gladkikh, D. V.; Meschaninova, M.I.; Ven’yaminova, A.G.; Zenkova, M.A.; Vlassov, V. V.; Chernolovskaya, E.L. Cholesterol-Containing Nuclease-Resistant SiRNA Accumulates in Tumors in a Carrier-Free Mode and Silences MDR1 Gene. Mol. Ther. - Nucleic Acids 2017, 6, 209–220. [CrossRef]
- Engelbeen, S.; Pasteuning-Vuhman, S.; Boertje-Van Der Meulen, J.; Parmar, R.; Charisse, K.; Sepp-Lorenzino, L.; Manoharan, M.; Aartsma-Rus, A.; Van Putten, M. Efficient Downregulation of Alk4 in Skeletal Muscle After Systemic Treatment with Conjugated SiRNAs in a Mouse Model for Duchenne Muscular Dystrophy. Nucleic Acid Ther. 2023, 33, 26–34. [CrossRef]
- Davis, S.M.; Hariharan, V.N.; Lo, A.; Turanov, A.A.; Echeverria, D.; Sousa, J.; McHugh, N.; Biscans, A.; Alterman, J.F.; Karumanchi, S.A.; et al. Chemical Optimization of SiRNA for Safe and Efficient Silencing of Placental SFLT1. Mol. Ther. - Nucleic Acids 2022, 29, 135–149. [CrossRef]
- Biscans, A.; Coles, A.; Haraszti, R.; Echeverria, Di.; Hassler, M.; Osborn, M.; Khvorova, A. Diverse Lipid Conjugates for Functional Extra-Hepatic SiRNA Delivery in Vivo. Nucleic Acids Res. 2019, 47, 1082–1096. [CrossRef]
- Egli, M.; Schlegel, M.K.; Manoharan, M. Acyclic (S)-Glycol Nucleic Acid (S-GNA) Modification of SiRNAs Improves the Safety of RNAi Therapeutics While Maintaining Potency. Rna 2023, 29, 402–414. [CrossRef]
- Matsuda, S.; Bala, S.; Liao, J.Y.; Datta, D.; Mikami, A.; Woods, L.; Harp, J.M.; Gilbert, J.A.; Bisbe, A.; Manoharan, R.M.; et al. Shorter Is Better: The α-(l)-Threofuranosyl Nucleic Acid Modification Improves Stability, Potency, Safety, and Ago2 Binding and Mitigates Off-Target Effects of Small Interfering RNAs. J. Am. Chem. Soc. 2023, 145, 19691–19706. [CrossRef]
- Liu, W.; Iwamoto, N.; Marappan, S.; Luu, K.; Tripathi, S.; Purcell-Estabrook, E.; Shelke, J.D.; Shah, H.; Lamattina, A.; Pan, Q.; et al. Impact of Stereopure Chimeric Backbone Chemistries on the Potency and Durability of Gene Silencing by RNA Interference. Nucleic Acids Res. 2023, 51, 4126–4147. [CrossRef]
- Volkov, A.A.; Kruglova, N.S.; Meschaninova, M.I.; Venyaminova, A.G.; Zenkova, M.A.; Vlassov, V. V.; Chernolovskaya, E.L. Selective Protection of Nuclease-Sensitive Sites in SiRNA Prolongs Silencing Effect. Oligonucleotides 2009, 19, 191–202. [CrossRef]
- Foster, D.J.; Brown, C.R.; Shaikh, S.; Trapp, C.; Schlegel, M.K.; Qian, K.; Sehgal, A.; Rajeev, K.G.; Jadhav, V.; Manoharan, M.; et al. Advanced SiRNA Designs Further Improve In Vivo Performance of GalNAc-SiRNA Conjugates. Mol. Ther. 2018, 26, 708–717. [CrossRef]
- Petrova, N.S.; Chernikov, I. V.; Meschaninova, M.I.; Dovydenko, I.S.; Venyaminova, A.G.; Zenkova, M.A.; Vlassov, V. V.; Chernolovskaya, E.L. Carrier-Free Cellular Uptake and the Gene-Silencing Activity of the Lipophilic SiRNAs Is Strongly Affected by the Length of the Linker between SiRNA and Lipophilic Group. Nucleic Acids Res. 2012, 40, 2330–2344. [CrossRef]
- Chernikov, I. V.; Gladkikh, D. V.; Karelina, U.A.; Meschaninova, M.I.; Ven’yaminova, A.G.; Vlassov, V. V.; Chernolovskaya, E.L. Trimeric Small Interfering RNAs and Their Cholesterol-Containing Conjugates Exhibit Improved Accumulation in Tumors, but Dramatically Reduced Silencing Activity. Molecules 2020, 25, 1877. [CrossRef]
- Chernikov, I. V.; Gladkikh, D. V.; Meschaninova, M.I.; Karelina, U.A.; Ven’Yaminova, A.G.; Zenkova, M.A.; Vlassov, V. V.; Chernolovskaya, E.L. Fluorophore Labeling Affects the Cellular Accumulation and Gene Silencing Activity of Cholesterol-Modified SiRNAs in Vitro. Nucleic Acid Ther. 2019, 29, 33–43. [CrossRef]
- Tang, Q.; Sousa, J.; Echeverria, D.; Fan, X.; Hsueh, Y.C.; Afshari, K.; MeHugh, N.; Cooper, D.A.; Vangjeli, L.; Monopoli, K.; et al. RNAi-Based Modulation of IFN-γ Signaling in Skin. Mol. Ther. 2022, 30, 2709–2721. [CrossRef]
- Osborn, M.F.; Coles, A.H.; Biscans, A.; Haraszti, R.A.; Roux, L.; Davis, S.; Ly, S.; Echeverria, D.; Hassler, M.R.; Godinho, B.M.D.C.; et al. Hydrophobicity Drives the Systemic Distribution of Lipid-Conjugated SiRNAs via Lipid Transport Pathways. Nucleic Acids Res. 2019, 47, 1070–1081. [CrossRef]
- Biscans, A.; Coles, A.; Haraszti, R.; Echeverria, Di.; Hassler, M.; Osborn, M.; Khvorova, A. Diverse Lipid Conjugates for Functional Extra-Hepatic SiRNA Delivery in Vivo. Nucleic Acids Res. 2019, 47, 1082–1096. [CrossRef]
- Biscans, A.; Caiazzi, J.; Davis, S.; McHugh, N.; Sousa, J.; Khvorova, A. The Chemical Structure and Phosphorothioate Content of Hydrophobically Modified SiRNAs Impact Extrahepatic Distribution and Efficacy. Nucleic Acids Res. 2020, 48, 7665–7680. [CrossRef]
- Godinho, B.M.D.C.; Gilbert, J.W.; Haraszti, R.A.; Coles, A.H.; Biscans, A.; Roux, L.; Nikan, M.; Echeverria, D.; Hassler, M.; Khvorova, A. Pharmacokinetic Profiling of Conjugated Therapeutic Oligonucleotides: A High-Throughput Method Based Upon Serial Blood Microsampling Coupled to Peptide Nucleic Acid Hybridization Assay. Nucleic Acid Ther. 2017, 27, 323–334. [CrossRef]
- Wolfrum, C.; Shi, S.; Jayaprakash, K.N.; Jayaraman, M.; Wang, G.; Pandey, R.K.; Rajeev, K.G.; Nakayama, T.; Charrise, K.; Ndungo, E.M.; et al. Mechanisms and Optimization of in Vivo Delivery of Lipophilic SiRNAs. Nat. Biotechnol. 2007, 25, 1149–1157. [CrossRef]
- Ly, S.; Navaroli, D.M.; Didiot, M.-C.; Cardia, J.; Pandarinathan, L.; Alterman, J.F.; Fogarty, K.; Standley, C.; Lifshitz, L.M.; Bellve, K.D.; et al. Visualization of Self-Delivering Hydrophobically Modified SiRNA Cellular Internalization. Nucleic Acids Res. 2017, 45, 15–25. [CrossRef]
- Chernikov, I. V.; Karelina, U.A.; Meschaninova, M.I.; Ven’yaminova, A.G.; Zenkova, M.A.; Vlassov, V. V.; Chernolovskaya, E.L. Investigation of the Internalization of Fluorescently Labeled Lipophilic SiRNA into Cultured Tumor Cells. Russ. J. Bioorganic Chem. 2019, 45, 766–773. [CrossRef]
- Alterman, J.F.; Hall, L.M.; Coles, A.H.; Hassler, M.R.; Didiot, M.-C.; Chase, K.; Abraham, J.; Sottosanti, E.; Johnson, E.; Sapp, E.; et al. Hydrophobically Modified SiRNAs Silence Huntingtin MRNA in Primary Neurons and Mouse Brain. Mol. Ther. - Nucleic Acids 2015, 4, e266. [CrossRef]
- Wada, S.; Yasuhara, H.; Wada, F.; Sawamura, M.; Waki, R. Evaluation of the Effects of Chemically Different Linkers on Hepatic Accumulations , Cell Tropism and Gene Silencing Ability of Cholesterol-Conjugated Antisense Oligonucleotides. J. Control. Release 2016, 226, 57–65. [CrossRef]
- Hassler, M.R.; Turanov, A.A.; Alterman, J.F.; Haraszti, R.A.; Coles, A.H.; Osborn, M.F.; Echeverria, D.; Nikan, M.; Salomon, W.E.; Roux, L.; et al. Comparison of Partially and Fully Chemically-Modified SiRNA in Conjugate-Mediated Delivery in Vivo. Nucleic Acids Res. 2018, 46, 2185–2196. [CrossRef]
- Nair, J.K.; Attarwala, H.; Sehgal, A.; Wang, Q.; Aluri, K.; Zhang, X.; Gao, M.; Liu, J.; Indrakanti, R.; Schofield, S.; et al. Impact of Enhanced Metabolic Stability on Pharmacokinetics and Pharmacodynamics of GalNAc-SiRNA Conjugates. Nucleic Acids Res. 2017, 45, 10969–10977. [CrossRef]
- Laurent, Q.; Martinent, R.; Moreau, D.; Winssinger, N.; Sakai, N.; Matile, S. Oligonucleotide Phosphorothioates Enter Cells by Thiol-Mediated Uptake. Angew. Chemie - Int. Ed. 2021, 60, 19102–19106. [CrossRef]
- Shmushkovich, T.; Monopoli, K.R.; Homsy, D.; Leyfer, D.; Betancur-Boissel, M.; Khvorova, A.; Wolfson, A.D. Functional Features Defining the Efficacy of Cholesterol-Conjugated, Self-Deliverable, Chemically Modified SiRNAs. Nucleic Acids Res. 2018, 46, 10905–10916. [CrossRef]
- Zenkov, A.N.; Scvortsova, N. V.; Chernolovskaya, E.L.; Pospelova, T.I.; Vlassov, V. V. Expression of the MDR1 and MRP Genes in Patients with Lymphoma with Primary Bone Marrow Involvement. Nucleosides, Nucleotides and Nucleic Acids 2004, 23, 843–847. [CrossRef]
- Meschaninova, M.I.; Novopashina, D.S.; Semikolenova, O.A.; Silnikov, V.N.; Venyaminova, A.G. Novel Convenient Approach to the Solid-Phase Synthesis of Oligonucleotide Conjugates. Molecules 2019, 24, 1–14. [CrossRef]
- Evdokimov, A.; Petruseva, I.; Tsidulko, A.; Koroleva, L.; Serpokrylova, I.; Silnikov, V.; Lavrik, O. New Synthetic Substrates of Mammalian Nucleotide Excision Repair System. Nucleic Acids Res. 2013, 41. [CrossRef]
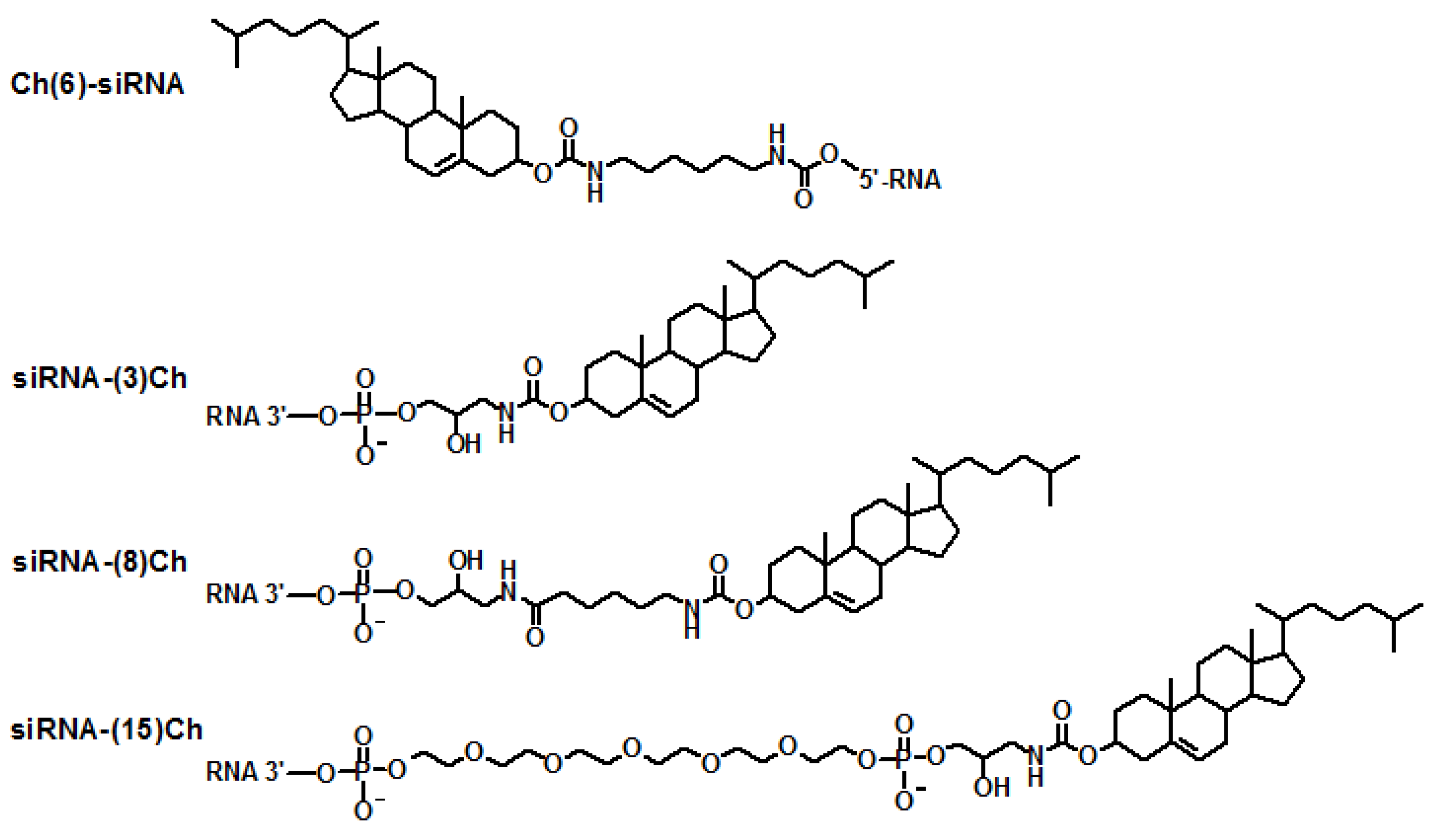
| Designation1 | Sequence 5' - 3' 2 |
|---|---|
| MDR1 S | GGCUUmGACmAAGUUmGUmAUmAUmGG |
| Ch(6)-MDR1 S | Ch(6)-GGCUUmGACmAAGUUmGUmAUmAUmGG |
| MDR1-(n)Ch S, n=3, 8, 15 | GGCUUmGACmAAGUUmGUmAUmAUmGG-(n)Ch |
| MDR1_-2G-(n)Ch S, n=3, 8, 15 | GGCUUmGACmAAGUUmGUmAUmAUm-(n)Ch |
| MDR1_FM S | GmGmCmUmUmGmAfCmAfAfGfUmUmGmUmAmUmAmUmGmGm |
| MDR1 AS | AUmAUmACmAACUUmGUCmAAGCCmAA |
| MDR1_FM AS | AmUfAmUmAmCfAmAmCmUmUmGmUmCfAmAfGmCmCmAmAm |
| SCRm S | CmAAGUCUCGUmAUmGUmAGUmGGUU |
| SCRm AS | CCmACUmACmAUmACGAGACUUmGUU |
| Designation | IC50, nM1 |
|---|---|
| siMDR1 | 4.4±1.9# |
| siMDR1_AS_FM | 1.2±0.3##, ** |
| siMDR1_FM | 0.5±0.3##, ** |
| Ch(6)-siMDR1 | 15.3±5.7* |
| Ch(6)-siMDR1_AS_FM | 3.3±2.2# |
| Ch(6)-siMDR1_FM | 0.3±0.1##, ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





