Submitted:
15 December 2023
Posted:
18 December 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
2.1. Identification of antiphage systems in the genomes of 100 diverse P. aeruginosa clinical isolates
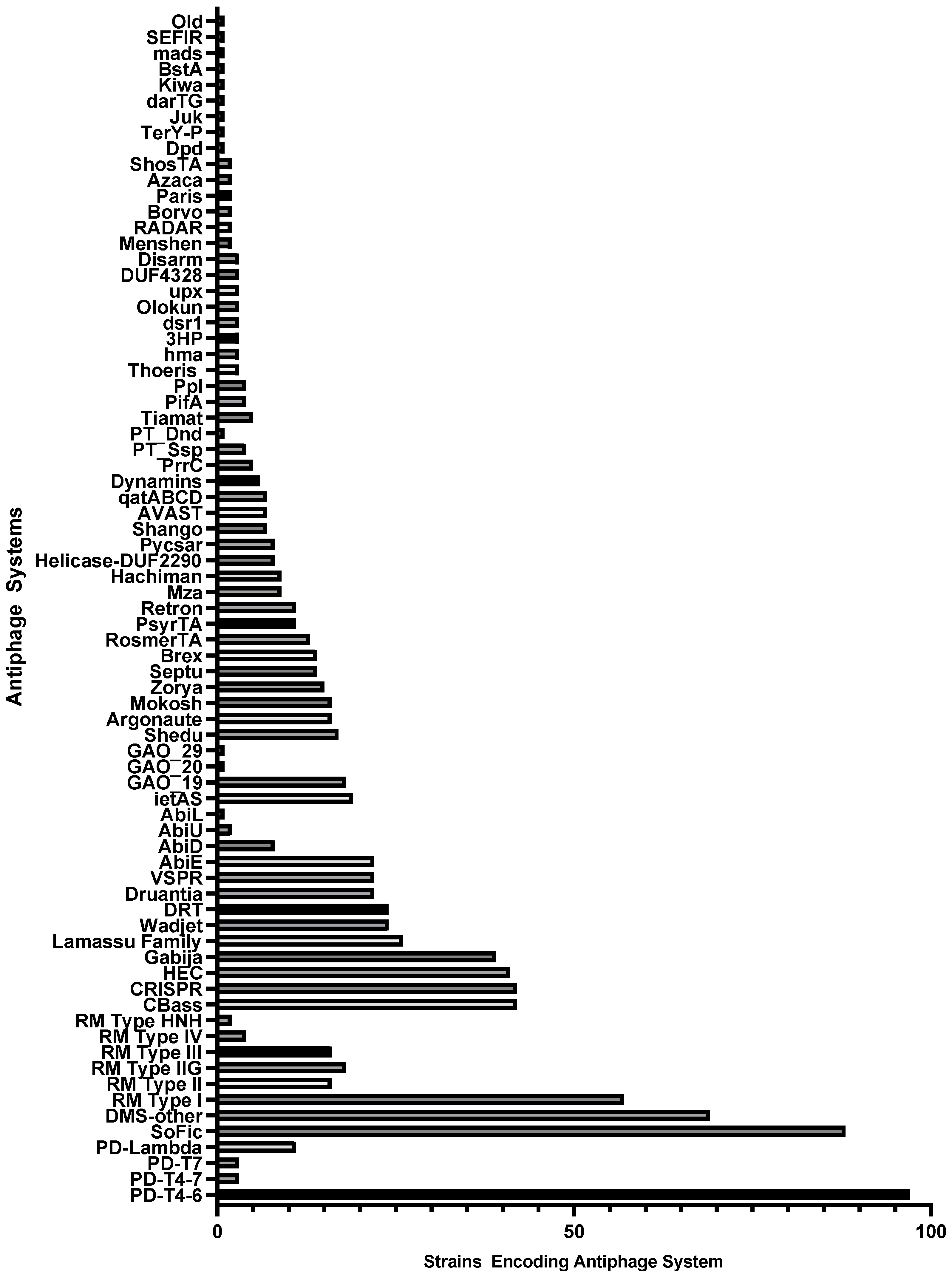
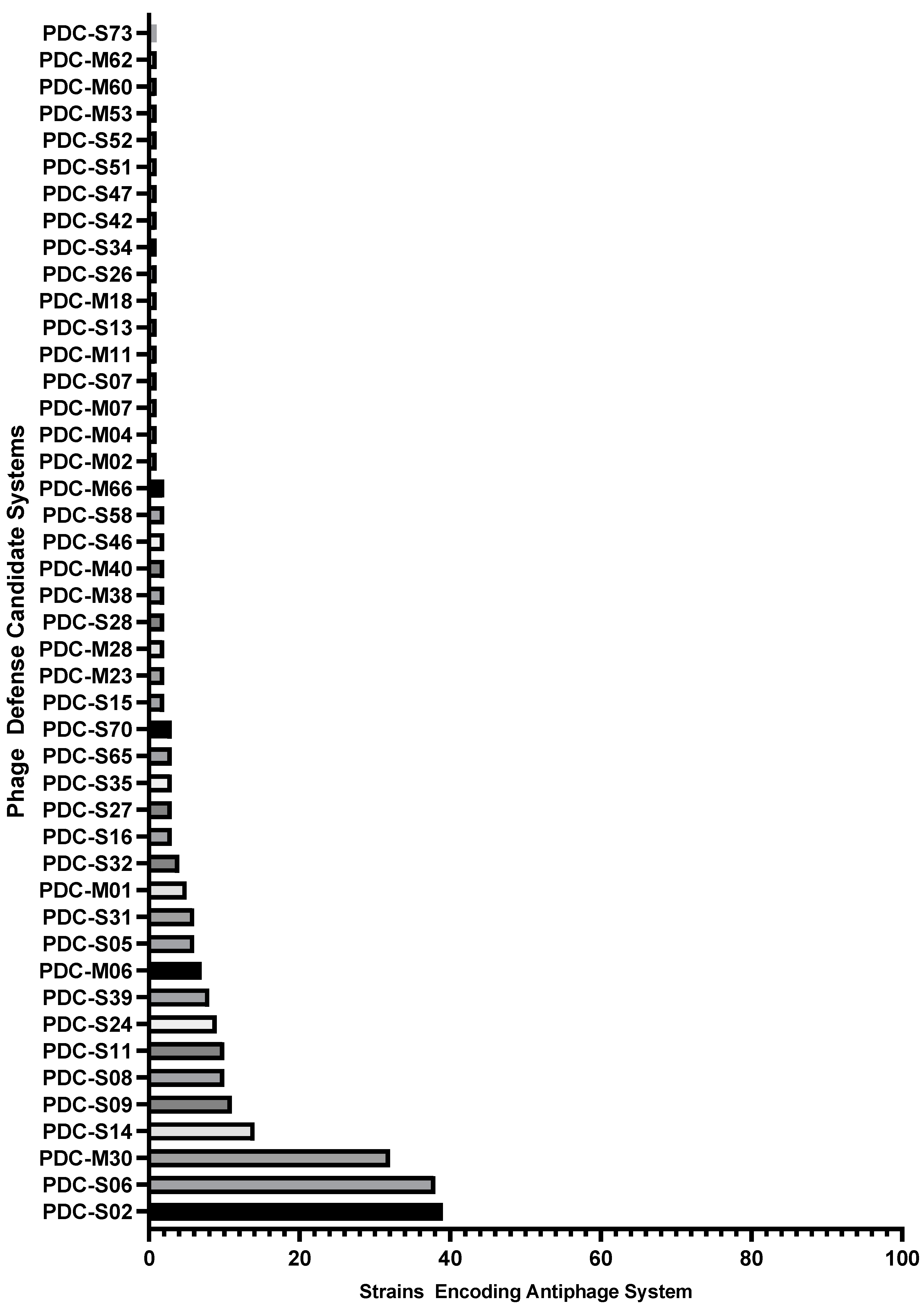
| Mechanism of Antiphage Action | System | Number of Strains Encoding | References |
|---|---|---|---|
| Nuclease Activity – Nucleic Acid Degradation | R-M systems | 67 | [18] |
| CRISPR-cas systems* | 42 | [42] | |
| Gabija | 39 | [17] | |
| Wadjet | 24 | [17,43] | |
| Druantia | 22 | [14,17] | |
| Shedu | 17 | [44] | |
| Mokosh | 16 | [7] | |
| Zorya* | 15 | [17] | |
| Septu | 14 | [14,17] | |
| mza | 9 | [19] | |
| qatABCD | 7 | [14,19] | |
| Ppl | 4 | [45] | |
| Olokun | 4 | [7] | |
| DISARM | 3 | [46] | |
| Upx | 3 | [19] | |
| Menshen | 2 | [7,47] | |
| Azaca | 2 | [7] | |
| Kiwa | 1 | [17,48] | |
| Old | 1 | [8] | |
| Abortive Infection or Cell Dormancy | PD Systems (T4-6, T4-7, T7, λ) | 100 | [10] |
| Abi systems (E, D, U, L) | 30 | [49,50,51,52] | |
| GAO_19 | 18 | [19] | |
| CBASS | 42 | [53] | |
| Lamassu | 26 | [7] | |
| ietAS | 19 | [19,26] | |
| RosmerTA | 13 | [7,54] | |
| PsyrTA | 11 | [7,55] | |
| Hachiman | 9 | [17,26,56] | |
| Helicase-DUF2290 | 8 | [8] | |
| Pycsar | 8 | [57] | |
| AVAST | 7 | [19,58] | |
| PrrC | 5 | [59,60] | |
| PifA | 4 | [61] | |
| Paris | 2 | [8] | |
| ShosTA | 2 | [7] | |
| darTG | 1 | [55,62] | |
| BstA | 1 | [63] | |
| Nucleic Acids Modification/Reverse Transcriptases/Expression Modification | DRT | 24 | [19] |
| Argonaute | 16 | [64,65,66] | |
| BREX | 14 | [67] | |
| Retrons | 11 | [68,69] | |
| PT_Ssp and PT_Dnd | 5 | [70] | |
| RADAR | 2 | [19] | |
| Dpd | 1 | [71] | |
| MADS | 1 | [72] | |
| Protein Modification | SoFic | 88 | [7] |
| Borvo | 2 | [7] | |
| TerY-P | 1 | [19] | |
| Other | Dynamins (Lysis Delayance) | 6 | [73] |
| Thoeris (NAD+ depletion) | 3 | [74] | |
| Dsr (NAD+ depletion) | 3 | [21] | |
| SEFIR (NAD+ depletion) | 1 | [7] | |
| Unknown | DMS other | 69 | [39] |
| HEC (Hma embedded candidates) | 41 | [39] | |
| Shango | 7 | [7] | |
| Tiamat | 5 | [7] | |
| Hma | 3 | [56] | |
| 3HP | 3 | [8] | |
| DUF4238 | 3 | [8] | |
| Juk | 1 | [75] |
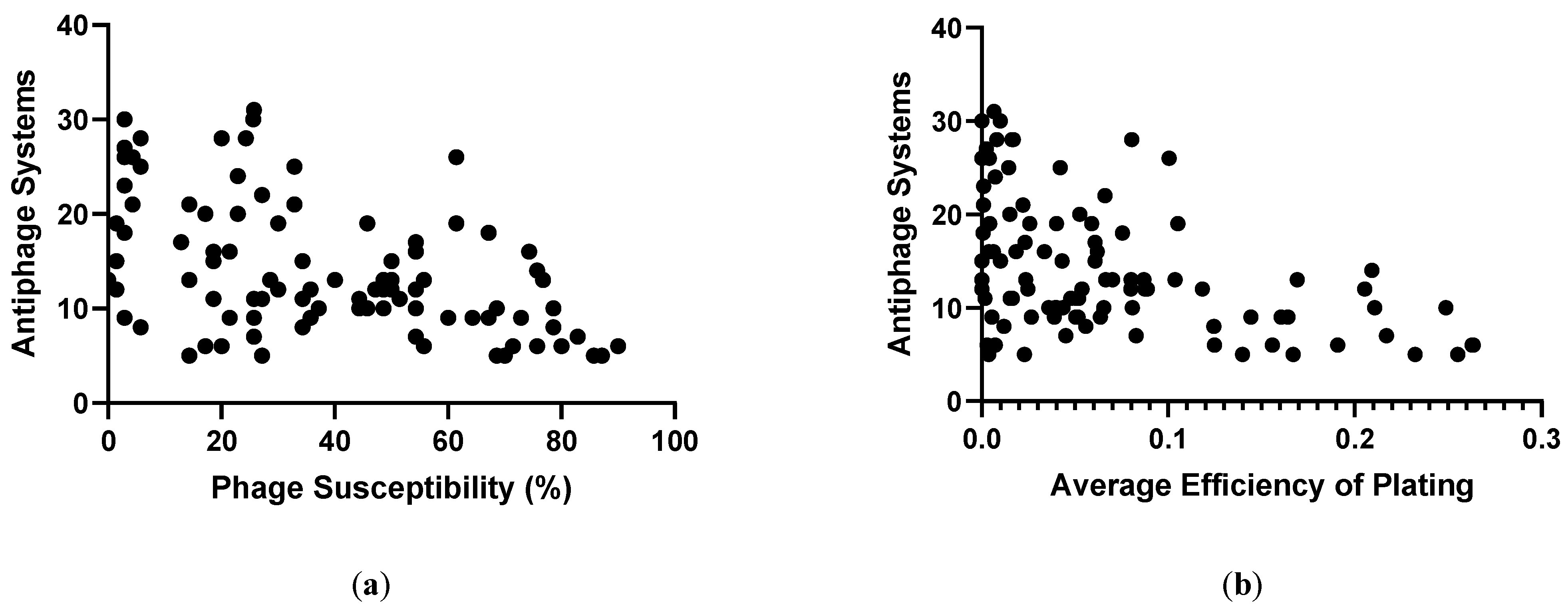
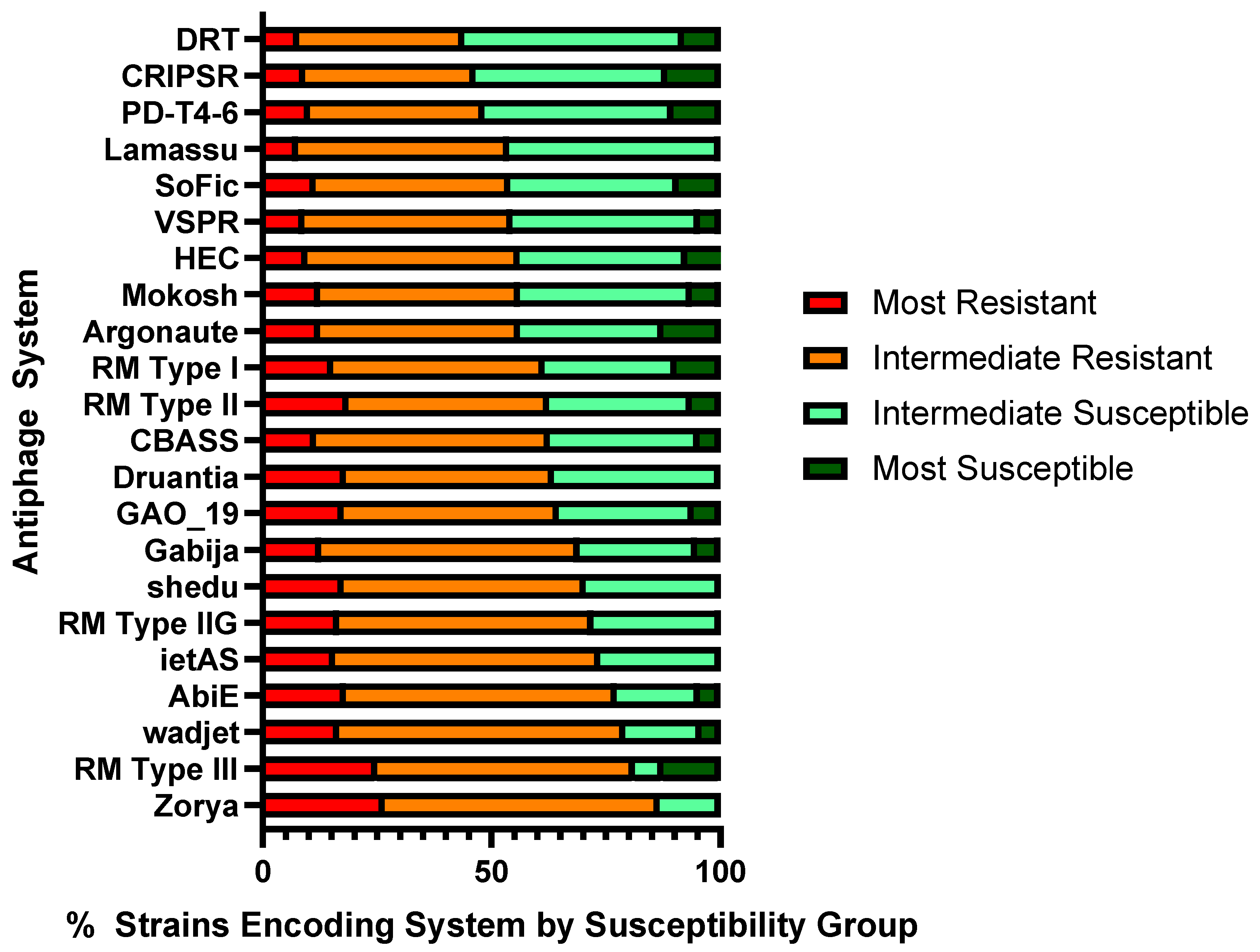
2.2. Phage susceptibility of the diverse P. aeruginosa strains
| Phage Susceptibility Group | Average Antiphage Systems/Strain |
|---|---|
| Most resistant 10% | 19.2 |
| Intermediate resistant | 16.8 |
| Intermediate susceptible | 11.9 |
| Most susceptible 10% | 7.6 |
3. Discussion
4. Materials and Methods
4.1. Bacterial strains used in this study
4.2. Phages used in this work
4.3. Handling of bacterial cultures and phages
4.4. Analysis of bacterial genomes for identification of antiphage systems
4.5. Phage susceptibility testing
4.6. Determination of phage susceptibility groups
4.7. Correlation of antiphage systems with phage susceptibility
4.8. Correlation of antiphage systems with average efficiency of plating
4.9. Assessment of prevalence of antiphage systems in phage susceptibility groups
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nasir, A.; Romero-Severson, E.; Claverie, J.M. Investigating the concept and origin of viruses. Trends Microbiol 2020, 28, 959–967. [Google Scholar] [CrossRef]
- Kortright, K.E.; Done, R.E.; Chan, B.K.; Souza, V.; Turner, P.E. Selection for phage resistance reduces virulence of Shigella flexneri. Appl Environ Microbiol 2022, 88, e0151421. [Google Scholar] [CrossRef]
- Tang, M.; Huang, Z.; Zhang, X.; Kong, J.; Zhou, B.; Han, Y.; Zhang, Y.; Chen, L.; Zhou, T. Phage resistance formation and fitness costs of hypervirulent Klebsiella pneumoniae mediated by K2 capsule-specific phage and the corresponding mechanisms. Front Microbiol 2023, 14, 1156292. [Google Scholar] [CrossRef] [PubMed]
- Hasan, M.; Ahn, J. Evolutionary dynamics between phages and bacteria as a possible approach for designing effective phage therapies against antibiotic-resistant bacteria. Antibiotics (Basel) 2022, 11. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.C.; Laderman, E.; Huiting, E.; Zhang, C.; Davidson, A.; Bondy-Denomy, J. Core defense hotspots within Pseudomonas aeruginosa are a consistent and rich source of anti-phage defense systems. Nucleic Acids Res 2023, 51, 4995–5005. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Fan, H.; Tong, Y. Unveil the Secret of the bacteria and phage arms race. Int J Mol Sci 2023, 24. [Google Scholar] [CrossRef] [PubMed]
- Millman, A.; Melamed, S.; Leavitt, A.; Doron, S.; Bernheim, A.; Hor, J.; Garb, J.; Bechon, N.; Brandis, A.; Lopatina, A.; et al. An expanded arsenal of immune systems that protect bacteria from phages. Cell Host Microbe 2022, 30, 1556–1569. [Google Scholar] [CrossRef] [PubMed]
- Rousset, F.; Depardieu, F.; Miele, S.; Dowding, J.; Laval, A.L.; Lieberman, E.; Garry, D.; Rocha, E.P.C.; Bernheim, A.; Bikard, D. Phages and their satellites encode hotspots of antiviral systems. Cell Host Microbe 2022, 30, 740–753. [Google Scholar] [CrossRef]
- Georjon, H.; Bernheim, A. The highly diverse antiphage defence systems of bacteria. Nat Rev Microbiol 2023, 21, 686–700. [Google Scholar] [CrossRef]
- Vassallo, C.N.; Doering, C.R.; Littlehale, M.L.; Teodoro, G.I.C.; Laub, M.T. A functional selection reveals previously undetected anti-phage defence systems in the E. coli pangenome. Nat Microbiol 2022, 7, 1568–1579. [Google Scholar] [CrossRef]
- Macdonald, E.; Wright, R.; Connolly, J.P.R.; Strahl, H.; Brockhurst, M.; van Houte, S.; Blower, T.R.; Palmer, T.; Mariano, G. The novel anti-phage system Shield co-opts an RmuC domain to mediate phage defense across Pseudomonas species. PLoS Genet 2023, 19, e1010784. [Google Scholar] [CrossRef] [PubMed]
- Akritidou, K.; Thurtle-Schmidt, B.H. OLD family nuclease function across diverse anti-phage defense systems. Front Microbiol 2023, 14, 1268820. [Google Scholar] [CrossRef] [PubMed]
- Fernández-García, L.; Song, S.; Kirigo, J.; Battisti, M.E.; Huelgas-Méndez, D.; García-Contreras, R.; Petersen, M.E.; Tomás, M.; Wood, T.K. Toxin/antitoxin systems induce persistence and work in concert with restriction/modification systems to inhibit phage. bioRxiv 2023, 2023.2002.2025.529695. [Google Scholar] [CrossRef]
- Wang, S.; Sun, E.; Liu, Y.; Yin, B.; Zhang, X.; Li, M.; Huang, Q.; Tan, C.; Qian, P.; Rao, V.B.; et al. Landscape of new nuclease-containing antiphage systems in Escherichia coli and the counterdefense roles of bacteriophage T4 genome modifications. J Virol 2023, 97, e0059923. [Google Scholar] [CrossRef]
- Wein, T.; Johnson, A.G.; Millman, A.; Lange, K.; Yirmiya, E.; Hadary, R.; Garb, J.; Steinruecke, F.; Hill, A.B.; Kranzusch, P.J.; et al. CARD-like domains mediate anti-phage defense in bacterial gasdermin systems. bioRxiv 2023, 2023.2005.2028.542683. [Google Scholar] [CrossRef]
- Bravo, J.P.K.; Aparicio-Maldonado, C.; Nobrega, F.L.; Brouns, S.J.J.; Taylor, D.W. Structural basis for broad anti-phage immunity by DISARM. Nat Commun 2022, 13, 2987. [Google Scholar] [CrossRef]
- Doron, S.; Melamed, S.; Ofir, G.; Leavitt, A.; Lopatina, A.; Keren, M.; Amitai, G.; Sorek, R. Systematic discovery of antiphage defense systems in the microbial pangenome. Science 2018, 359. [Google Scholar] [CrossRef]
- Dupuis, M.E.; Villion, M.; Magadan, A.H.; Moineau, S. CRISPR-Cas and restriction-modification systems are compatible and increase phage resistance. Nat Commun 2013, 4, 2087. [Google Scholar] [CrossRef]
- Gao, L.; Altae-Tran, H.; Bohning, F.; Makarova, K.S.; Segel, M.; Schmid-Burgk, J.L.; Koob, J.; Wolf, Y.I.; Koonin, E.V.; Zhang, F. Diverse enzymatic activities mediate antiviral immunity in prokaryotes. Science 2020, 369, 1077–1084. [Google Scholar] [CrossRef]
- Tal, N.; Millman, A.; Stokar-Avihail, A.; Fedorenko, T.; Leavitt, A.; Melamed, S.; Yirmiya, E.; Avraham, C.; Brandis, A.; Mehlman, T.; et al. Bacteria deplete deoxynucleotides to defend against bacteriophage infection. Nat Microbiol 2022, 7, 1200–1209. [Google Scholar] [CrossRef]
- Garb, J.; Lopatina, A.; Bernheim, A.; Zaremba, M.; Siksnys, V.; Melamed, S.; Leavitt, A.; Millman, A.; Amitai, G.; Sorek, R. Multiple phage resistance systems inhibit infection via SIR2-dependent NAD(+) depletion. Nat Microbiol 2022, 7, 1849–1856. [Google Scholar] [CrossRef] [PubMed]
- Kronheim, S.; Daniel-Ivad, M.; Duan, Z.; Hwang, S.; Wong, A.I.; Mantel, I.; Nodwell, J.R.; Maxwell, K.L. A chemical defence against phage infection. Nature 2018, 564, 283–286. [Google Scholar] [CrossRef]
- Bernheim, A.; Millman, A.; Ofir, G.; Meitav, G.; Avraham, C.; Shomar, H.; Rosenberg, M.M.; Tal, N.; Melamed, S.; Amitai, G.; et al. Prokaryotic viperins produce diverse antiviral molecules. Nature 2021, 589, 120–124. [Google Scholar] [CrossRef]
- Hardy, A.; Kever, L.; Frunzke, J. Antiphage small molecules produced by bacteria - beyond protein-mediated defenses. Trends Microbiol 2023, 31, 92–106. [Google Scholar] [CrossRef] [PubMed]
- Habusha, M.; Tzipilevich, E.; Fiyaksel, O.; Ben-Yehuda, S. A mutant bacteriophage evolved to infect resistant bacteria gained a broader host range. Mol Microbiol 2019, 111, 1463–1475. [Google Scholar] [CrossRef]
- Stokar-Avihail, A.; Fedorenko, T.; Hor, J.; Garb, J.; Leavitt, A.; Millman, A.; Shulman, G.; Wojtania, N.; Melamed, S.; Amitai, G.; et al. Discovery of phage determinants that confer sensitivity to bacterial immune systems. Cell 2023, 186, 1863–1876. [Google Scholar] [CrossRef]
- Watson, B.N.J.; Easingwood, R.A.; Tong, B.; Wolf, M.; Salmond, G.P.C.; Staals, R.H.J.; Bostina, M.; Fineran, P.C. Different genetic and morphological outcomes for phages targeted by single or multiple CRISPR-Cas spacers. Philos Trans R Soc Lond B Biol Sci 2019, 374, 20180090. [Google Scholar] [CrossRef]
- Gao, Z.; Feng, Y. Bacteriophage strategies for overcoming host antiviral immunity. Front Microbiol 2023, 14, 1211793. [Google Scholar] [CrossRef]
- Yan, Y.; Zheng, J.; Zhang, X.; Yin, Y. dbAPIS: a database of anti-prokaryotic immune system genes. Nucleic Acids Res 2023. [Google Scholar] [CrossRef]
- Dublanchet, A.; Bourne, S. The epic of phage therapy. Can J Infect Dis Med Microbiol 2007, 18, 15–18. [Google Scholar] [CrossRef] [PubMed]
- Nikolich, M.P.; Filippov, A.A. Bacteriophage therapy: developments and directions. Antibiotics (Basel) 2020, 9. [Google Scholar] [CrossRef]
- Polaska, M.; Sokolowska, B. Bacteriophages-a new hope or a huge problem in the food industry. AIMS Microbiol 2019, 5, 324–346. [Google Scholar] [CrossRef]
- McCallin, S.; Sacher, J.C.; Zheng, J.; Chan, B.K. Current state of compassionate phage therapy. Viruses 2019, 11. [Google Scholar] [CrossRef] [PubMed]
- Lewis, J.M.; Sagona, A.P. Armed phages are heading for clinical trials. Nat Microbiol 2023, 8, 1191–1192. [Google Scholar] [CrossRef] [PubMed]
- Hitchcock, N.M.; Devequi Gomes Nunes, D.; Shiach, J.; Valeria Saraiva Hodel, K.; Dantas Viana Barbosa, J.; Alencar Pereira Rodrigues, L.; Coler, B.S.; Botelho Pereira Soares, M.; Badaro, R. Current clinical landscape and global potential of bacteriophage therapy. Viruses 2023, 15. [Google Scholar] [CrossRef]
- Abedon, S.T.; Danis-Wlodarczyk, K.M.; Wozniak, D.J. Phage cocktail development for bacteriophage therapy: toward improving spectrum of activity breadth and depth. Pharmaceuticals (Basel) 2021, 14, 1019. [Google Scholar] [CrossRef]
- Li, C.; Shi, T.; Sun, Y.; Zhang, Y. A novel method to create efficient phage cocktails via use of phage-resistant bacteria. Appl Environ Microbiol 2022, 88, e0232321. [Google Scholar] [CrossRef]
- Hochhauser, D.; Millman, A.; Sorek, R. The defense island repertoire of the Escherichia coli pan-genome. PLoS Genet 2023, 19, e1010694. [Google Scholar] [CrossRef]
- Payne, L.J.; Meaden, S.; Mestre, M.R.; Palmer, C.; Toro, N.; Fineran, P.C.; Jackson, S.A. PADLOC: a web server for the identification of antiviral defence systems in microbial genomes. Nucleic Acids Res 2022, 50, W541–W550. [Google Scholar] [CrossRef]
- Lebreton, F.; Snesrud, E.; Hall, L.; Mills, E.; Galac, M.; Stam, J.; Ong, A.; Maybank, R.; Kwak, Y.I.; Johnson, S.; et al. A panel of diverse Pseudomonas aeruginosa clinical isolates for research and development. JAC Antimicrob Resist 2021, 3, dlab179. [Google Scholar] [CrossRef]
- Payne, L. PADLOC-DB v2.0.0. 2023, Available online: https://github.com/padlocbio/padloc-db/releases.
- Makarova, K.S.; Wolf, Y.I.; Iranzo, J.; Shmakov, S.A.; Alkhnbashi, O.S.; Brouns, S.J.J.; Charpentier, E.; Cheng, D.; Haft, D.H.; Horvath, P.; et al. Evolutionary classification of CRISPR-Cas systems: a burst of class 2 and derived variants. Nat Rev Microbiol 2020, 18, 67–83. [Google Scholar] [CrossRef]
- Deep, A.; Gu, Y.; Gao, Y.Q.; Ego, K.M.; Herzik, M.A., Jr.; Zhou, H.; Corbett, K.D. The SMC-family Wadjet complex protects bacteria from plasmid transformation by recognition and cleavage of closed-circular DNA. Mol Cell 2022, 82, 4145–4159. [Google Scholar] [CrossRef] [PubMed]
- Loeff, L.; Walter, A.; Rosalen, G.T.; Jinek, M. DNA end sensing and cleavage by the Shedu anti-phage defense system. bioRxiv 2023, 2023.2008.2010.552762. [Google Scholar] [CrossRef]
- Wang, S.; Sun, E.; Liu, Y.; Yin, B.; Zhang, X.; Li, M.; Huang, Q.; Tan, C.; Qian, P.; Rao, V.B.; et al. The complex roles of genomic DNA modifications of bacteriophage T4 in resistance to nuclease-based defense systems of E. coli. bioRxiv 2022, 2022.2006.2016.496414. [Google Scholar] [CrossRef]
- Ofir, G.; Melamed, S.; Sberro, H.; Mukamel, Z.; Silverman, S.; Yaakov, G.; Doron, S.; Sorek, R. DISARM is a widespread bacterial defence system with broad anti-phage activities. Nat Microbiol 2018, 3, 90–98. [Google Scholar] [CrossRef]
- Mariano, G.; Blower, T.R. Conserved domains can be found across distinct phage defence systems. Mol Microbiol 2023, 120, 45–53. [Google Scholar] [CrossRef]
- Todeschini, T.C.; Wu, Y.; Naji, A.; Mondi, R.; Nobrega, F.L. Kiwa rescues RecBCD for anti-phage activity. bioRxiv 2023, 2023.2002.2026.530102. [Google Scholar] [CrossRef]
- Dy, R.L.; Przybilski, R.; Semeijn, K.; Salmond, G.P.; Fineran, P.C. A widespread bacteriophage abortive infection system functions through a Type IV toxin-antitoxin mechanism. Nucleic Acids Res 2014, 42, 4590–4605. [Google Scholar] [CrossRef]
- Garvey, P.; Fitzgerald, G.F.; Hill, C. Cloning and DNA sequence analysis of two abortive infection phage resistance determinants from the lactococcal plasmid pNP40. Appl Environ Microbiol 1995, 61, 4321–4328. [Google Scholar] [CrossRef]
- Dai, G.; Su, P.; Allison, G.E.; Geller, B.L.; Zhu, P.; Kim, W.S.; Dunn, N.W. Molecular characterization of a new abortive infection system (AbiU) from Lactococcus lactis LL51-1. Appl Environ Microbiol 2001, 67, 5225–5232. [Google Scholar] [CrossRef]
- Deng, Y.M.; Liu, C.Q.; Dunn, N.W. Genetic organization and functional analysis of a novel phage abortive infection system, AbiL, from Lactococcus lactis. J Biotechnol 1999, 67, 135–149. [Google Scholar] [CrossRef]
- Millman, A.; Melamed, S.; Amitai, G.; Sorek, R. Diversity and classification of cyclic-oligonucleotide-based anti-phage signalling systems. Nat Microbiol 2020, 5, 1608–1615. [Google Scholar] [CrossRef] [PubMed]
- Kelly, A.; Arrowsmith, T.J.; Went, S.C.; Blower, T.R. Toxin-antitoxin systems as mediators of phage defence and the implications for abortive infection. Curr Opin Microbiol 2023, 73, 102293. [Google Scholar] [CrossRef] [PubMed]
- Sberro, H.; Leavitt, A.; Kiro, R.; Koh, E.; Peleg, Y.; Qimron, U.; Sorek, R. Discovery of functional toxin/antitoxin systems in bacteria by shotgun cloning. Mol Cell 2013, 50, 136–148. [Google Scholar] [CrossRef]
- Payne, L.J.; Todeschini, T.C.; Wu, Y.; Perry, B.J.; Ronson, C.W.; Fineran, P.C.; Nobrega, F.L.; Jackson, S.A. Identification and classification of antiviral defence systems in bacteria and archaea with PADLOC reveals new system types. Nucleic Acids Res 2021, 49, 10868–10878. [Google Scholar] [CrossRef]
- Tal, N.; Morehouse, B.R.; Millman, A.; Stokar-Avihail, A.; Avraham, C.; Fedorenko, T.; Yirmiya, E.; Herbst, E.; Brandis, A.; Mehlman, T.; et al. Cyclic CMP and cyclic UMP mediate bacterial immunity against phages. Cell 2021, 184, 5728–5739. [Google Scholar] [CrossRef]
- Boyle, T.A.; Hatoum-Aslan, A. Recurring and emerging themes in prokaryotic innate immunity. Curr Opin Microbiol 2023, 73, 102324. [Google Scholar] [CrossRef]
- Jabbar, M.A.; Snyder, L. Genetic and physiological studies of an Escherichia coli locus that restricts polynucleotide kinase- and RNA ligase-deficient mutants of bacteriophage T4. J Virol 1984, 51, 522–529. [Google Scholar] [CrossRef]
- Kaufmann, G. Anticodon nucleases. Trends Biochem Sci 2000, 25, 70–74. [Google Scholar] [CrossRef]
- Cram, D.; Ray, A.; Skurray, R. Molecular analysis of F plasmid pif region specifying abortive infection of T7 phage. Mol Gen Genet 1984, 197, 137–142. [Google Scholar] [CrossRef]
- LeRoux, M.; Srikant, S.; Teodoro, G.I.C.; Zhang, T.; Littlehale, M.L.; Doron, S.; Badiee, M.; Leung, A.K.L.; Sorek, R.; Laub, M.T. The DarTG toxin-antitoxin system provides phage defence by ADP-ribosylating viral DNA. Nat Microbiol 2022, 7, 1028–1040. [Google Scholar] [CrossRef]
- Owen, S.V.; Wenner, N.; Dulberger, C.L.; Rodwell, E.V.; Bowers-Barnard, A.; Quinones-Olvera, N.; Rigden, D.J.; Rubin, E.J.; Garner, E.C.; Baym, M.; et al. Prophages encode phage-defense systems with cognate self-immunity. Cell Host Microbe 2021, 29, 1620–1633. [Google Scholar] [CrossRef]
- Burroughs, A.M.; Ando, Y.; Aravind, L. New perspectives on the diversification of the RNA interference system: insights from comparative genomics and small RNA sequencing. Wiley Interdiscip Rev RNA 2014, 5, 141–181. [Google Scholar] [CrossRef]
- Chakravarti, A.; Patel, D.J. Atypical bacterial Argonautes regulate antiphage defense. Cell Res 2023, 33, 655–656. [Google Scholar] [CrossRef]
- Makarova, K.S.; Wolf, Y.I.; van der Oost, J.; Koonin, E.V. Prokaryotic homologs of Argonaute proteins are predicted to function as key components of a novel system of defense against mobile genetic elements. Biol Direct 2009, 4, 29. [Google Scholar] [CrossRef] [PubMed]
- Goldfarb, T.; Sberro, H.; Weinstock, E.; Cohen, O.; Doron, S.; Charpak-Amikam, Y.; Afik, S.; Ofir, G.; Sorek, R. BREX is a novel phage resistance system widespread in microbial genomes. EMBO J 2015, 34, 169–183. [Google Scholar] [CrossRef]
- Millman, A.; Bernheim, A.; Stokar-Avihail, A.; Fedorenko, T.; Voichek, M.; Leavitt, A.; Oppenheimer-Shaanan, Y.; Sorek, R. Bacterial retrons function in anti-phage defense. Cell 2020, 183, 1551–1561. [Google Scholar] [CrossRef] [PubMed]
- Mestre, M.R.; Gonzalez-Delgado, A.; Gutierrez-Rus, L.I.; Martinez-Abarca, F.; Toro, N. Systematic prediction of genes functionally associated with bacterial retrons and classification of the encoded tripartite systems. Nucleic Acids Res 2020, 48, 12632–12647. [Google Scholar] [CrossRef] [PubMed]
- Xiong, X.; Wu, G.; Wei, Y.; Liu, L.; Zhang, Y.; Su, R.; Jiang, X.; Li, M.; Gao, H.; Tian, X.; et al. SspABCD-SspE is a phosphorothioation-sensing bacterial defence system with broad anti-phage activities. Nat Microbiol 2020, 5, 917–928. [Google Scholar] [CrossRef] [PubMed]
- Thiaville, J.J.; Kellner, S.M.; Yuan, Y.; Hutinet, G.; Thiaville, P.C.; Jumpathong, W.; Mohapatra, S.; Brochier-Armanet, C.; Letarov, A.V.; Hillebrand, R.; et al. Novel genomic island modifies DNA with 7-deazaguanine derivatives. Proc Natl Acad Sci U S A 2016, 113, E1452–1459. [Google Scholar] [CrossRef] [PubMed]
- Maestri, A.; Pursey, E.; Chong, C.; Pons, B.J.; Gandon, S.; Custodio, R.; Chisnall, M.; Grasso, A.; Paterson, S.; Baker, K.; et al. Bacterial defences interact synergistically by disrupting phage cooperation. bioRxiv 2023, 2023.2003.2030.534895. [Google Scholar] [CrossRef]
- Guo, L.; Sattler, L.; Shafqat, S.; Graumann, P.L.; Bramkamp, M. A Bacterial dynamin-like protein confers a novel phage resistance strategy on the population level in Bacillus subtilis. mBio 2021, 13, e0375321. [Google Scholar] [CrossRef] [PubMed]
- Ka, D.; Oh, H.; Park, E.; Kim, J.H.; Bae, E. Structural and functional evidence of bacterial antiphage protection by Thoeris defense system via NAD(+) degradation. Nat Commun 2020, 11, 2816. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Guan, J.; Hareendranath, S.; Crawford, E.; Agard, D.A.; Makarova, K.S.; Koonin, E.V.; Bondy-Denomy, J. A family of novel immune systems targets early infection of nucleus-forming jumbo phages. bioRxiv 2022, 2022.2009.2017.508391. [Google Scholar] [CrossRef]
- Meeske, A.J.; Nakandakari-Higa, S.; Marraffini, L.A. Cas13-induced cellular dormancy prevents the rise of CRISPR-resistant bacteriophage. Nature 2019, 570, 241–245. [Google Scholar] [CrossRef] [PubMed]
- Bertozzi Silva, J.; Storms, Z.; Sauvageau, D. Host receptors for bacteriophage adsorption. FEMS Microbiol Lett 2016, 363, fnw002. [Google Scholar] [CrossRef] [PubMed]
- Nasrin, S.; Hegerle, N.; Sen, S.; Nkeze, J.; Sen, S.; Permala-Booth, J.; Choi, M.; Sinclair, J.; Tapia, M.D.; Johnson, J.K.; et al. Distribution of serotypes and antibiotic resistance of invasive Pseudomonas aeruginosa in a multi-country collection. BMC Microbiol 2022, 22, 13. [Google Scholar] [CrossRef]
- Hynes, A.P.; Rousseau, G.M.; Agudelo, D.; Goulet, A.; Amigues, B.; Loehr, J.; Romero, D.A.; Fremaux, C.; Horvath, P.; Doyon, Y.; et al. Widespread anti-CRISPR proteins in virulent bacteriophages inhibit a range of Cas9 proteins. Nat Commun 2018, 9, 2919. [Google Scholar] [CrossRef]
- Chen, Y.; Batra, H.; Dong, J.; Chen, C.; Rao, V.B.; Tao, P. Genetic engineering of bacteriophages against infectious diseases. Front Microbiol 2019, 10, 954. [Google Scholar] [CrossRef]
- Hobbs, S.J.; Wein, T.; Lu, A.; Morehouse, B.R.; Schnabel, J.; Leavitt, A.; Yirmiya, E.; Sorek, R.; Kranzusch, P.J. Phage anti-CBASS and anti-Pycsar nucleases subvert bacterial immunity. Nature 2022, 605, 522–526. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Garushyants, S.K.; Hurk, A.v.d.; Aparicio-Maldonado, C.; Kushwaha, S.K.; King, C.M.; Ou, Y.; Todeschini, T.C.; Clokie, M.R.J.; Millard, A.M.; et al. Synergistic anti-phage activity of bacterial defence systems. bioRxiv 2023, 2022.2008.2021.504612. [Google Scholar] [CrossRef]
- Tesson, F.; Bernheim, A. Synergy and regulation of antiphage systems: toward the existence of a bacterial immune system? Curr Opin Microbiol 2023, 71, 102238. [Google Scholar] [CrossRef] [PubMed]
- Borges, A.L.; Castro, B.; Govindarajan, S.; Solvik, T.; Escalante, V.; Bondy-Denomy, J. Bacterial alginate regulators and phage homologs repress CRISPR-Cas immunity. Nat Microbiol 2020, 5, 679–687. [Google Scholar] [CrossRef] [PubMed]
- Ngiam, L.; Weynberg, K.D.; Guo, J. The presence of plasmids in bacterial hosts alters phage isolation and infectivity. ISME Commun 2022, 2, 75. [Google Scholar] [CrossRef]
- Kutter, E. Phage host range and efficiency of plating. Methods Mol Biol 2009, 501, 141–149. [Google Scholar] [CrossRef]
- Gaborieau, B.; Vaysset, H.; Tesson, F.; Charachon, I.; Dib, N.; Bernier, J.; Dequidt, T.; Georjon, H.; Clermont, O.; Hersen, P.; et al. Predicting phage-bacteria interactions at the strain level from genomes. bioRxiv 2023, 2023.2011.2022.567924. [Google Scholar] [CrossRef]
- Lam, J.S.; Taylor, V.L.; Islam, S.T.; Hao, Y.; Kocincova, D. Genetic and functional diversity of Pseudomonas aeruginosa lipopolysaccharide. Front Microbiol 2011, 2, 118. [Google Scholar] [CrossRef]
- DebRoy, C.; Fratamico, P.M.; Yan, X.; Baranzoni, G.; Liu, Y.; Needleman, D.S.; Tebbs, R.; O’Connell, C.D.; Allred, A.; Swimley, M.; et al. Comparison of O-antigen gene clusters of all O-serogroups of Escherichia coli and proposal for adopting a new nomenclature for O-typing. PLoS One 2016, 11, e0147434. [Google Scholar] [CrossRef]
- Mencke, J.L.; He, Y.; Filippov, A.A.; Nikolich, M.P.; Belew, A.T.; Fouts, D.E.; McGann, P.T.; Swierczewski, B.E.; Getnet, D.; Ellison, D.W.; et al. Identification and characterization of vB_PreP_EPr2, a lytic bacteriophage of pan-drug resistant Providencia rettgeri. Viruses 2022, 14, 708. [Google Scholar] [CrossRef]
- Sergueev, K.V.; Filippov, A.A.; Farlow, J.; Su, W.; Kvachadze, L.; Balarjishvili, N.; Kutateladze, M.; Nikolich, M.P. Correlation of host range expansion of therapeutic bacteriophage Sb-1 with allele state at a hypervariable repeat locus. Appl Environ Microbiol 2019, 85, e01209–19. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




