Submitted:
15 December 2023
Posted:
18 December 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
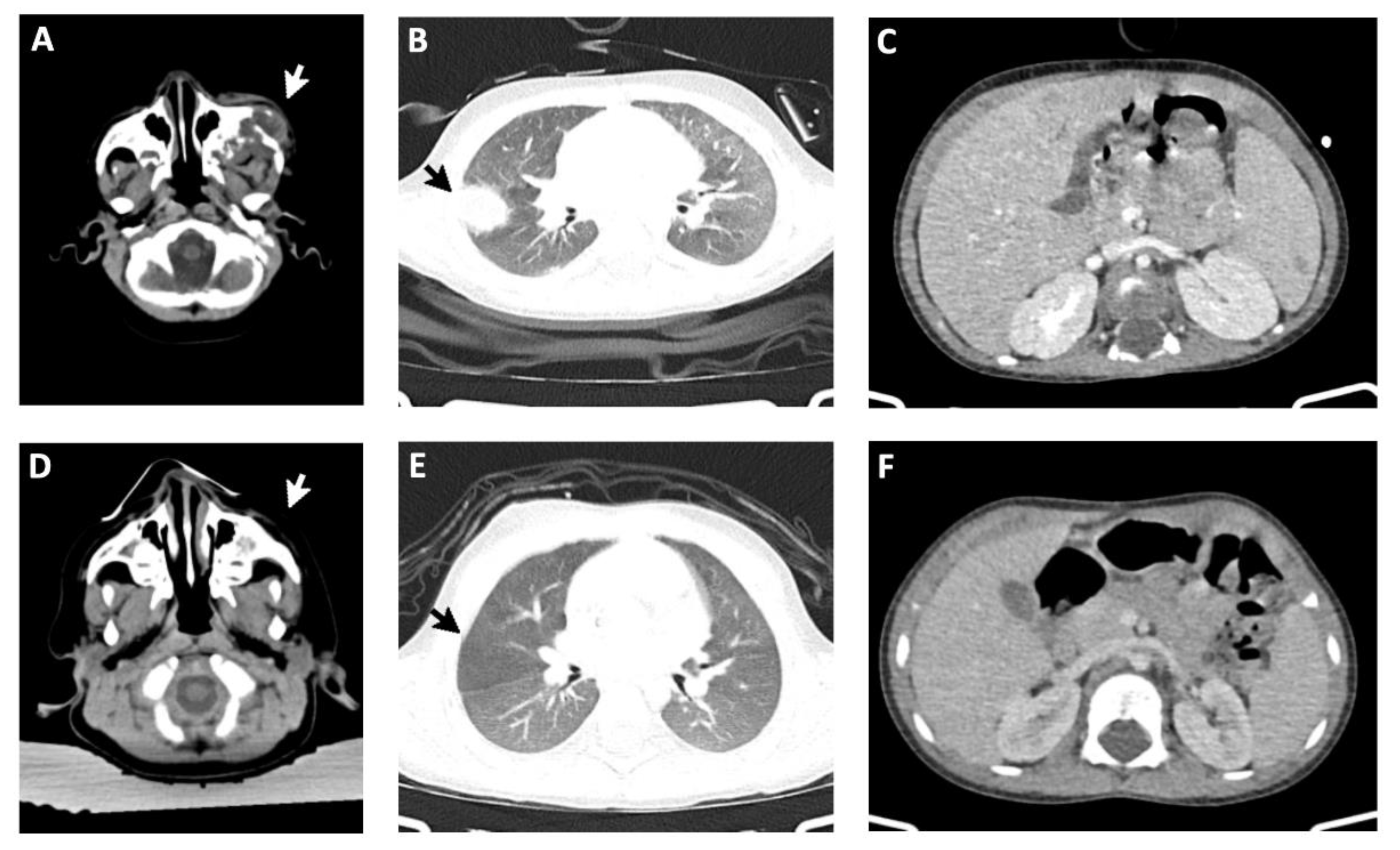
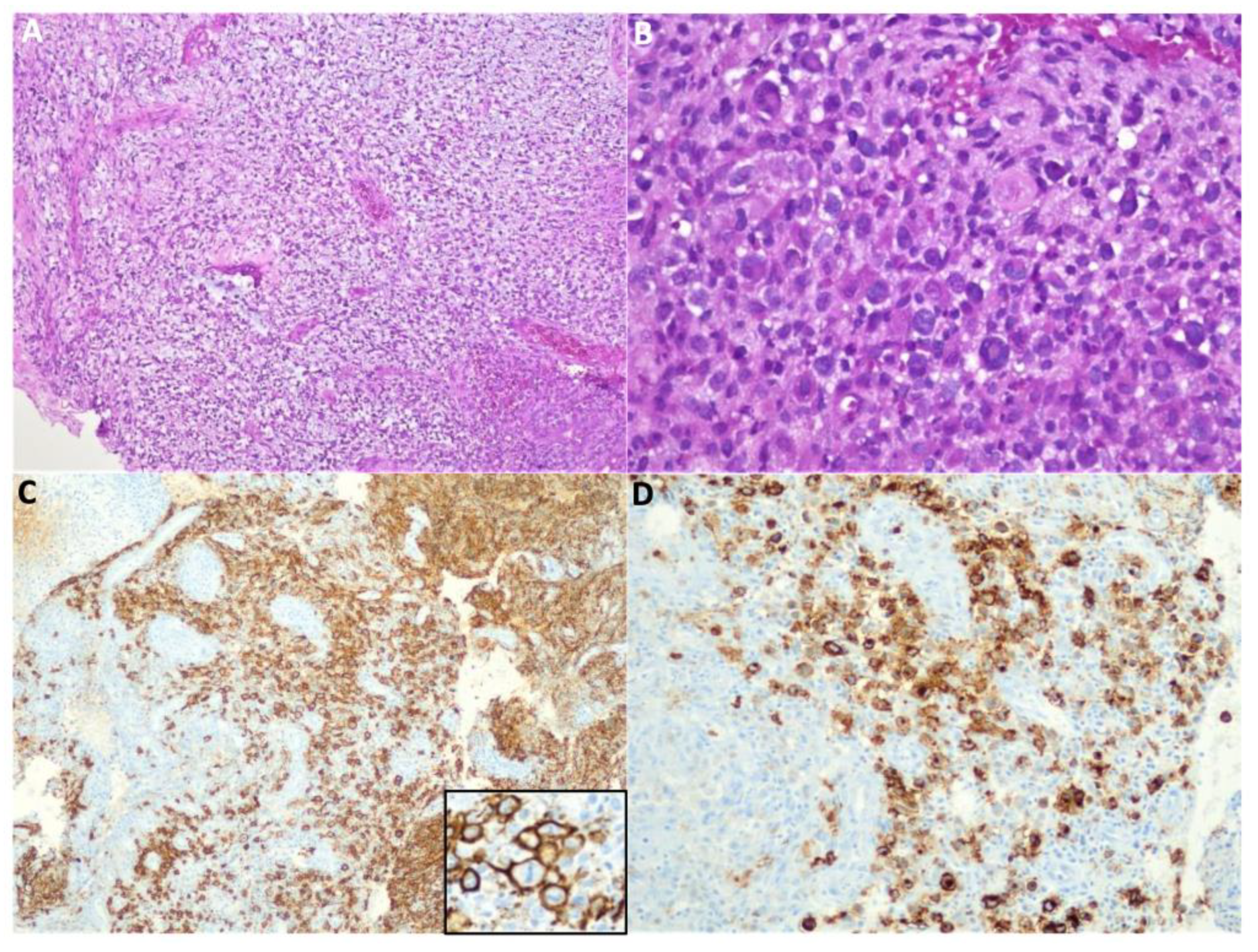
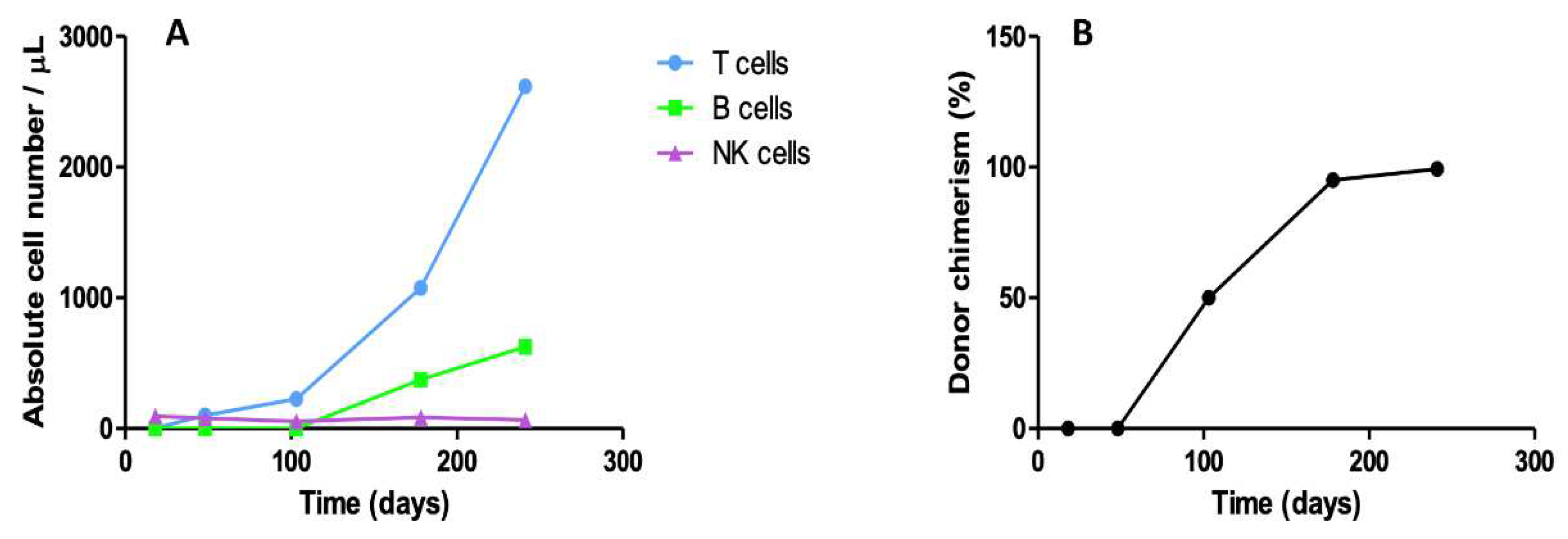
2. Detailed Case Description
3. Discussion
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- M. Albert, A. Lankester, A. Gennery, Primary Immunodeficiencies, in: The EBMT Handbook, Springer International Publishing, Cham, (2019): pp. 663–670. [CrossRef]
- M. van der Burg, A.R. Gennery, The expanding clinical and immunological spectrum of severe combined immunodeficiency. Educational paper, Eur J Pediatr. 170 (2011) 561–571. [CrossRef]
- W.J. Leonard, J.-X. Lin, J.J. O’Shea, The γc Family of Cytokines: Basic Biology to Therapeutic Ramifications, Immunity. 50 (2019) 832–850. [CrossRef]
- H.B. Gaspar, W. Qasim, E.G. Davies, K. Rao, P.J. Amrolia, P. Veys, How I treat severe combined immunodeficiency, Blood. 122 (2013) 3749–3758. [CrossRef]
- P. Mustillo, R.P.S. Bajwa, A.M. Termuhlen, K. Nicol, R. Scherzer, R. Jaffe, A.H. Filipovich, T.G. Gross, Tumor immune surveillance defect of X-linked severe combined immunodeficiency is not Epstein-Barr virus specific, Pediatr Blood Cancer. 51 (2008) 706–709. [CrossRef]
- C. Maas, R. Lüftinger, W. Krois, S. Matthes-Martin, G. Bayer, K. Boztug, M. Metzelder, EBV-positive B-cell lymphoma manifestation of the liver in an infant with RAG1 severe combined immunodeficiency disease, Pediatr Blood Cancer. 65 (2018) e27258. [CrossRef]
- M. Migliavacca, A. Assanelli, M. Ponzoni, R. Pajno, F. Barzaghi, F. Giglio, F. Ferrua, M. Frittoli, I. Brigida, F. Dionisio, R. Nicoletti, M. Casiraghi, M.G. Roncarolo, C. Doglioni, J. Peccatori, F. Ciceri, M.P. Cicalese, A. Aiuti, First Occurrence of Plasmablastic Lymphoma in Adenosine Deaminase-Deficient Severe Combined Immunodeficiency Disease Patient and Review of the Literature, Front Immunol. 9 (2018). [CrossRef]
- S. Sharma, R.K. Pilania, G. Anjani, M. Sudhakar, K. Arora, R. Tyagi, M. Dhaliwal, P. Vignesh, A. Rawat, S. Singh, Lymphoproliferation in Inborn Errors of Immunity: The Eye Does Not See What the Mind Does Not Know, Front Immunol. 13 (2022). [CrossRef]
- Chua, I. Quinti, B. Grimbacher, Lymphoma in common variable immunodeficiency: interplay between immune dysregulation, infection and genetics, Curr Opin Hematol. 15 (2008) 368–374. [CrossRef]
- C. Cunningham-Rundles, P. Lieberman, G. Hellman, R.S.K. Chaganti, Non-hodgkin lymphoma in common variable immunodeficiency, Am J Hematol. 37 (1991) 69–74. [CrossRef]
- C. Wehr, L. Houet, S. Unger, G. Kindle, S. Goldacker, B. Grimbacher, A. Caballero Garcia de Oteyza, R. Marks, D. Pfeifer, A. Nieters, M. Proietti, K. Warnatz, A. Schmitt-Graeff, Altered Spectrum of Lymphoid Neoplasms in a Single-Center Cohort of Common Variable Immunodeficiency with Immune Dysregulation, J Clin Immunol. 41 (2021) 1250–1265. [CrossRef]
- C. Schuetz, et al. An Immunodeficiency Disease with RAG Mutations and Granulomas, New England Journal of Medicine. 358 (2008) 2030–2038. [CrossRef]
- R. Albar, M. Mahdi, F. Alkeraithe, K.N. Almufarriji, Epstein-Barr virus associated with high-grade B-cell lymphoma in nude severe combined immunodeficiency, BMJ Case Rep. 12 (2019) e227715. [CrossRef]
- Cohen JM, Sebire NJ, Harvey J, et al. Successful treatment of lymphoproliferative disease complicating primary immunodefi- ciency/immunodysregulatory disorders with reduced-intensity allo- geneic stem-cell transplantation. Blood 2007;110:2209–2214. [CrossRef]
- Brandau O, Schuster V, Weiss M, et al. Epstein-Barrvirus-negative boys with non-Hodgkin lymphoma are mutated in the SH2D1A gene, as are patients with X-linked lymphoproliferative disease (XLP). Hum Mol Genet 1999;8:2407–2413. [CrossRef]
- Strahm B, Rittweiler K, Duffner U, et al. Recurrent B-cell non- Hodgkin’s lymphoma in two brothers with X-linked lymphoproli- ferative disease without evidence for Epstein-Barr virus infection. Br J Haematol 2000;108:377–382. [CrossRef]
- G. Klein, E. Klein, Surveillance against tumors—is it mainly immunological, Immunol Lett. 100 (2005) 29–33. [CrossRef]
- Slatter, M. A., Angus, B., Windebank, K., Taylor, A., Meaney, C., Lester, T., Norbury, G., Hambleton, S., Abinun, M., Flood, T. J., Cant, A. J., & Gennery, A. R. (2011). Polymorphous lymphoproliferative disorder with Hodgkin-like features in common γ-chain-deficient severe combined immunodeficiency. The Journal of allergy and clinical immunology, 127(2), 533–535. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




