Submitted:
15 December 2023
Posted:
18 December 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. The Genetics of PXE
2.1. Inheritance
2.2. The PXE Gene
3. Disease manifestations
3.1. Skin
3.2. Eyes
3.3. Vasculature
3.3.1. Coronary artery disease
3.3.2. Atherosclerosis
3.4. Heart
3.5. Dystrophic Cardiac Calcification
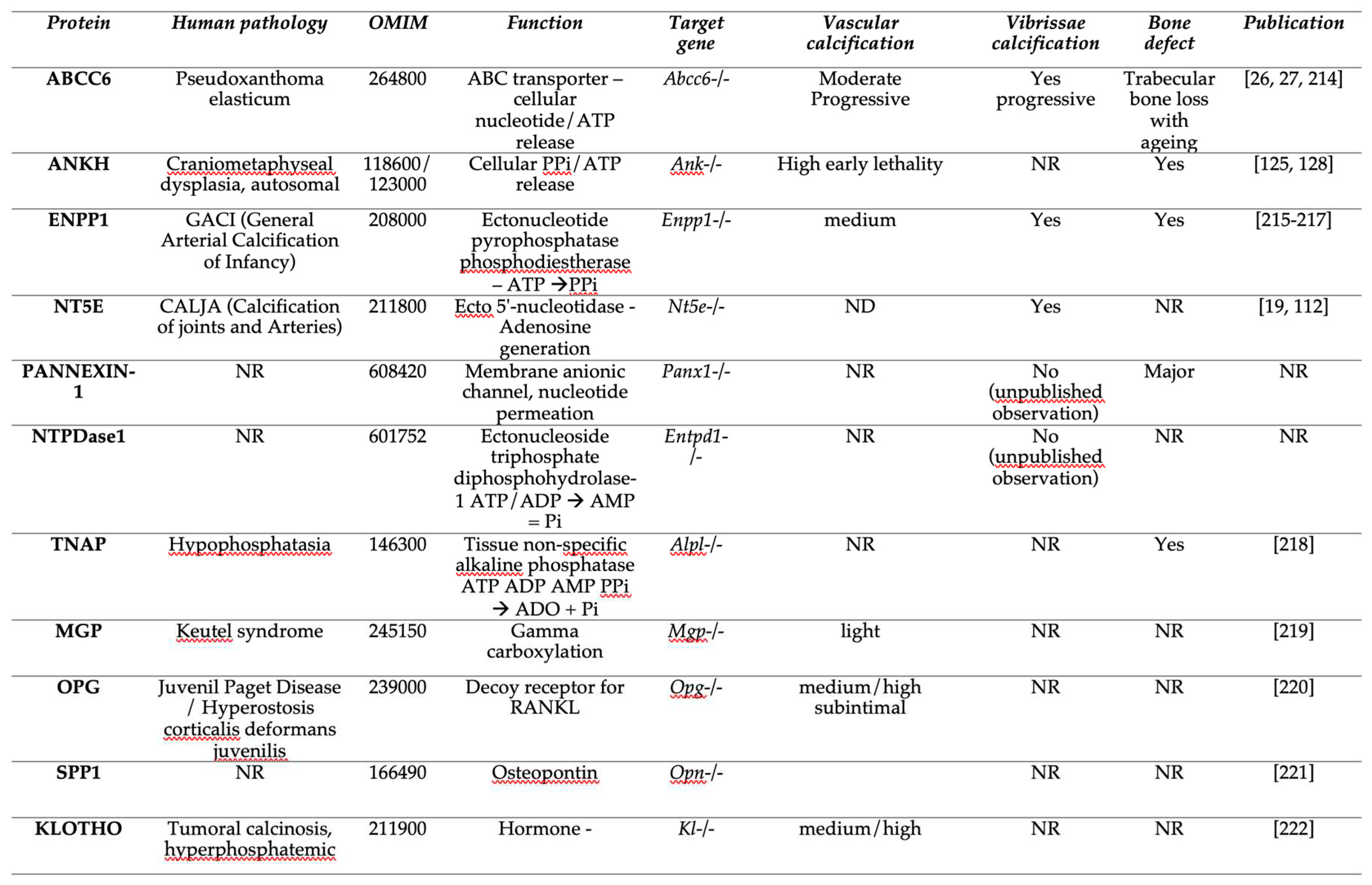
4. Animal models
5. The PXE pathophysiology is both metabolic and cellular
6. The search for the substrate(s)
6.1. A restricted substrate specificity
6.2. Vitamin K: logical but no joy
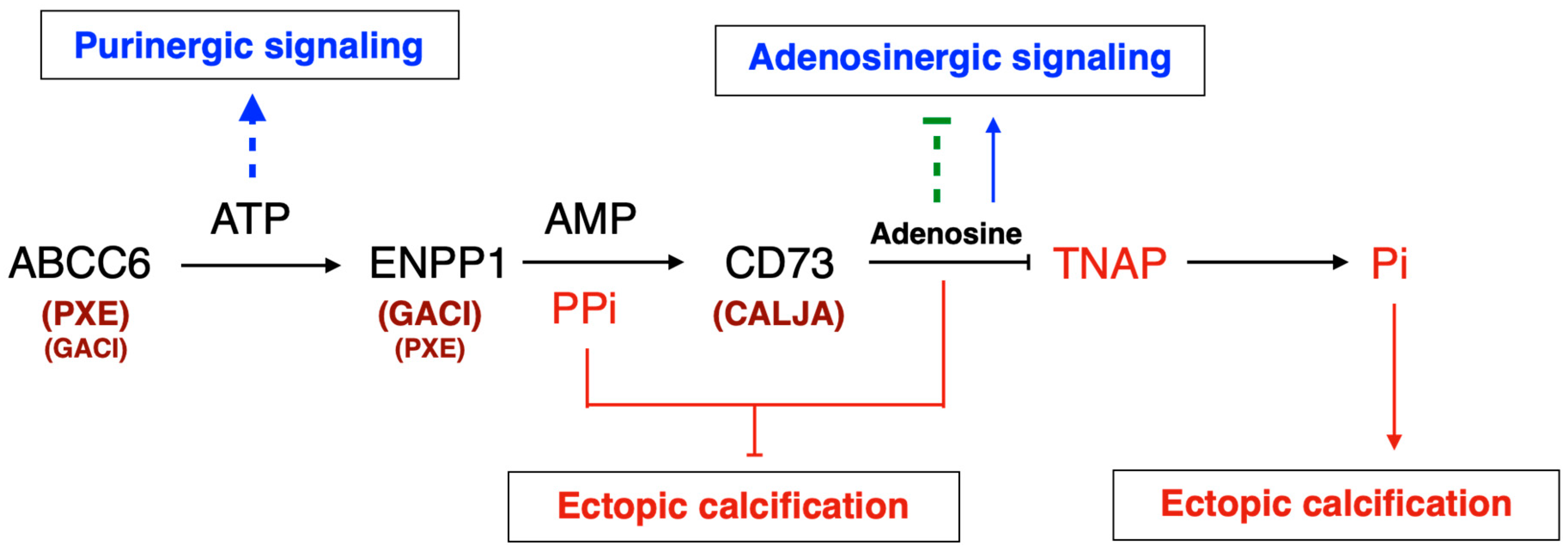
6.3. Adenosine
6.4. PPi
6.5. The question of ATP
6.6. Plasma PPi does not fully explain calcification susceptibility
- If a deficit in PPi production is essential to the etiology of both PXE and GACI and compensation appears to be a credible therapeutic possibility [12], plasma PPi does not correlate with calcification heterogeneity in humans [18] and mice [77]. Similarly, in a recent report investigating 78 patients and 69 heterozygous, Van Gils et al, found that neither phenotype manifestation/severity nor genotype correlated with plasma PPi [135].
- The liver expression of ABCC6 is necessary but not sufficient for calcification inhibition [19,80]. The question of how peripheral tissues contribute to calcification inhibition still remains unresolved; however, we discovered that bone marrow-derived ABCC6 is necessary to achieve full calcification inhibition without affecting plasma PPi levels (Brampton et al, Submitted).
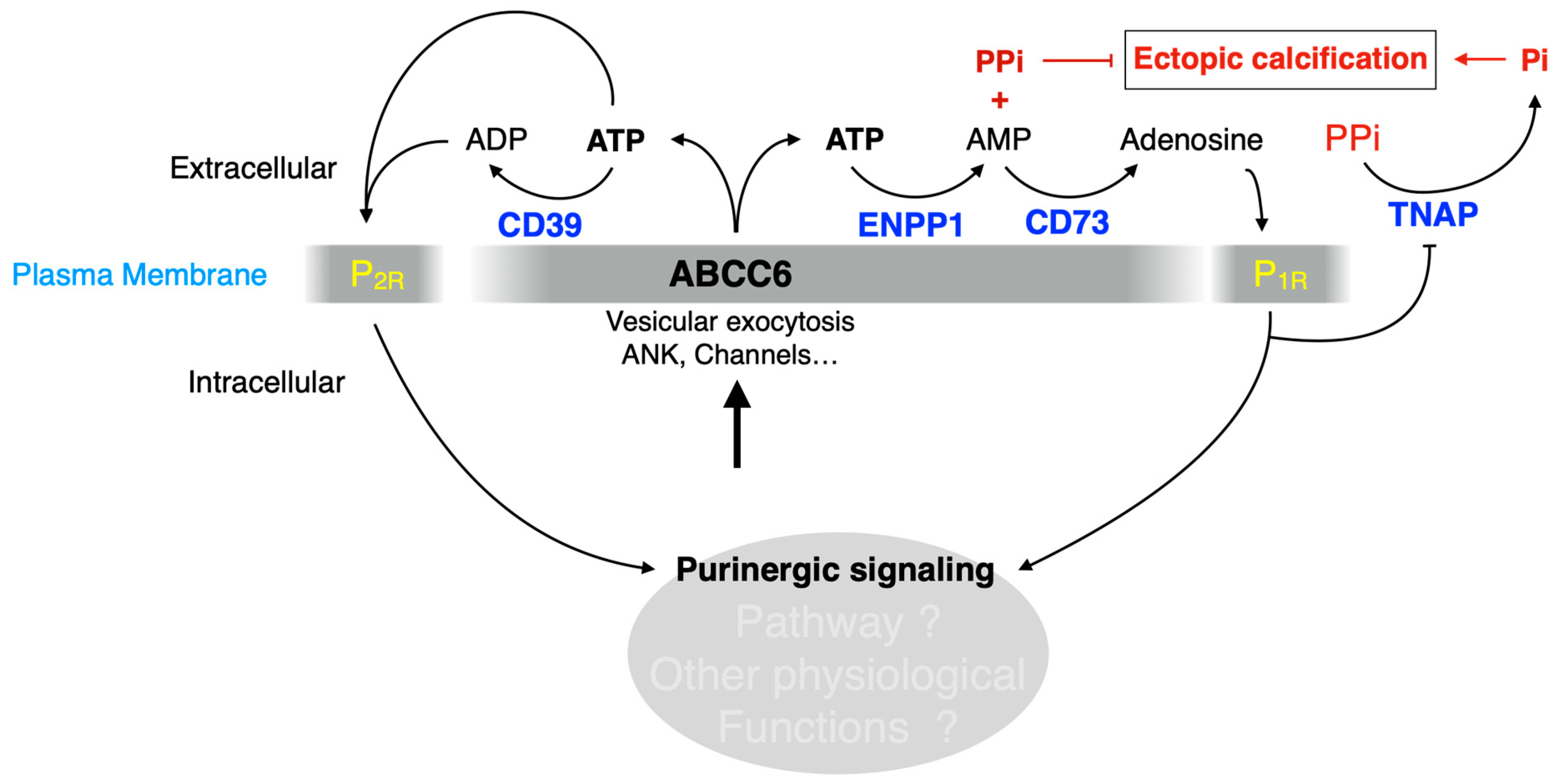
- Adding complexity to the relatively simplistic model shown on Figure 1, dermal fibroblasts of PXE patient also seem to display an impaired ability to generate PPi [136,137] and the crucial role of ANKH in the regulation of local PPi homeostasis [131] shows that in addition to ABCC6 keeping systemic PPi concentrations within the physiological range, extrahepatic PPi production (which can’t be assessed reliably as yet) is also a critical determinant of phenotypic outcome in PXE and GACI.
7. Altered ectonucleotidase activities associated with ABCC6 deficiency
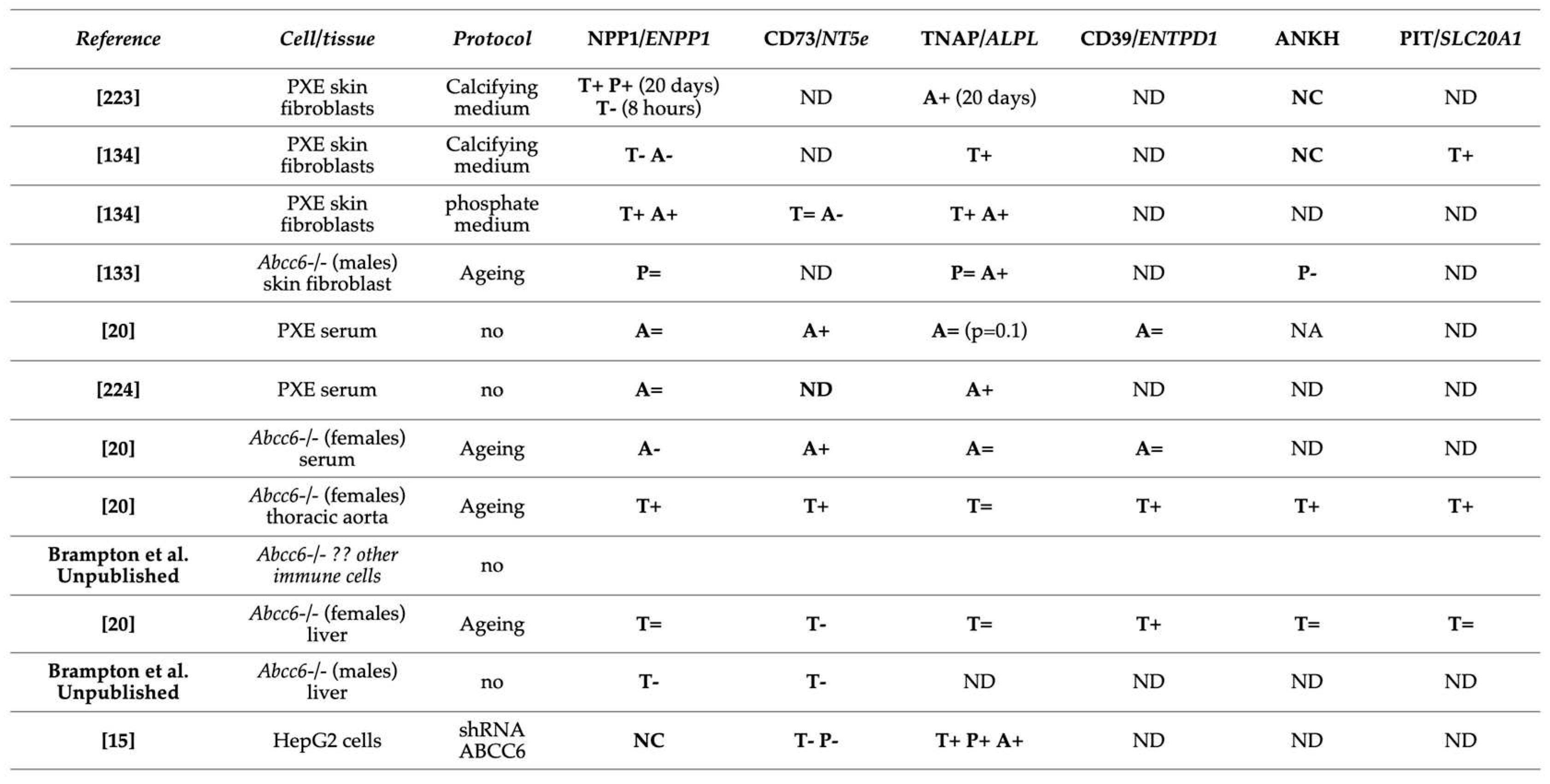
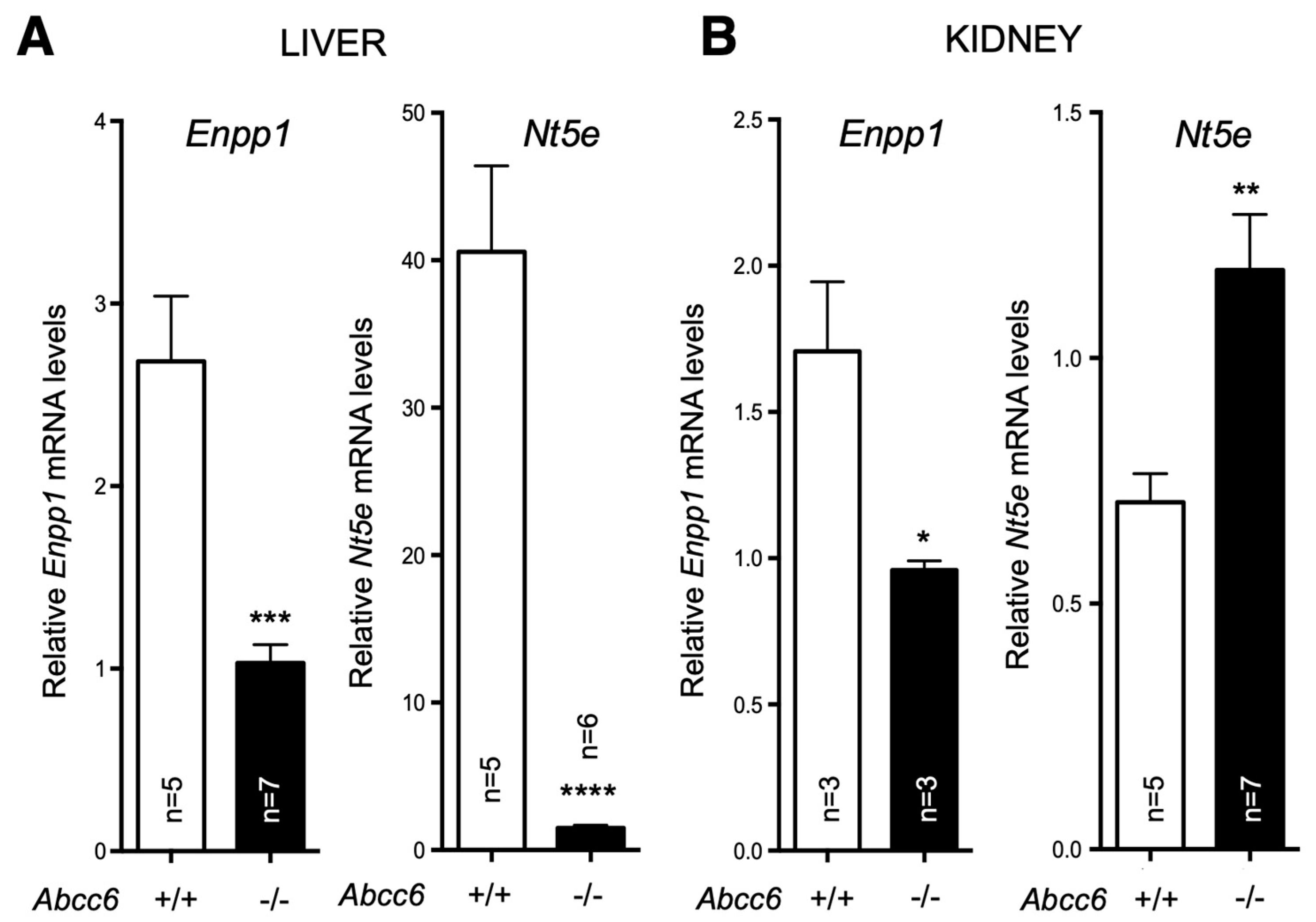
8. Impaired purinergic signaling – a connection to other PXE manifestations
8.1. Immune cells and inflammation
8.2. Vascular smooth muscle and endothelial cells – CD39
8.3. How does ABCC6 function relates to cardiac manifestations?
8.4. Compensatory mechanism?
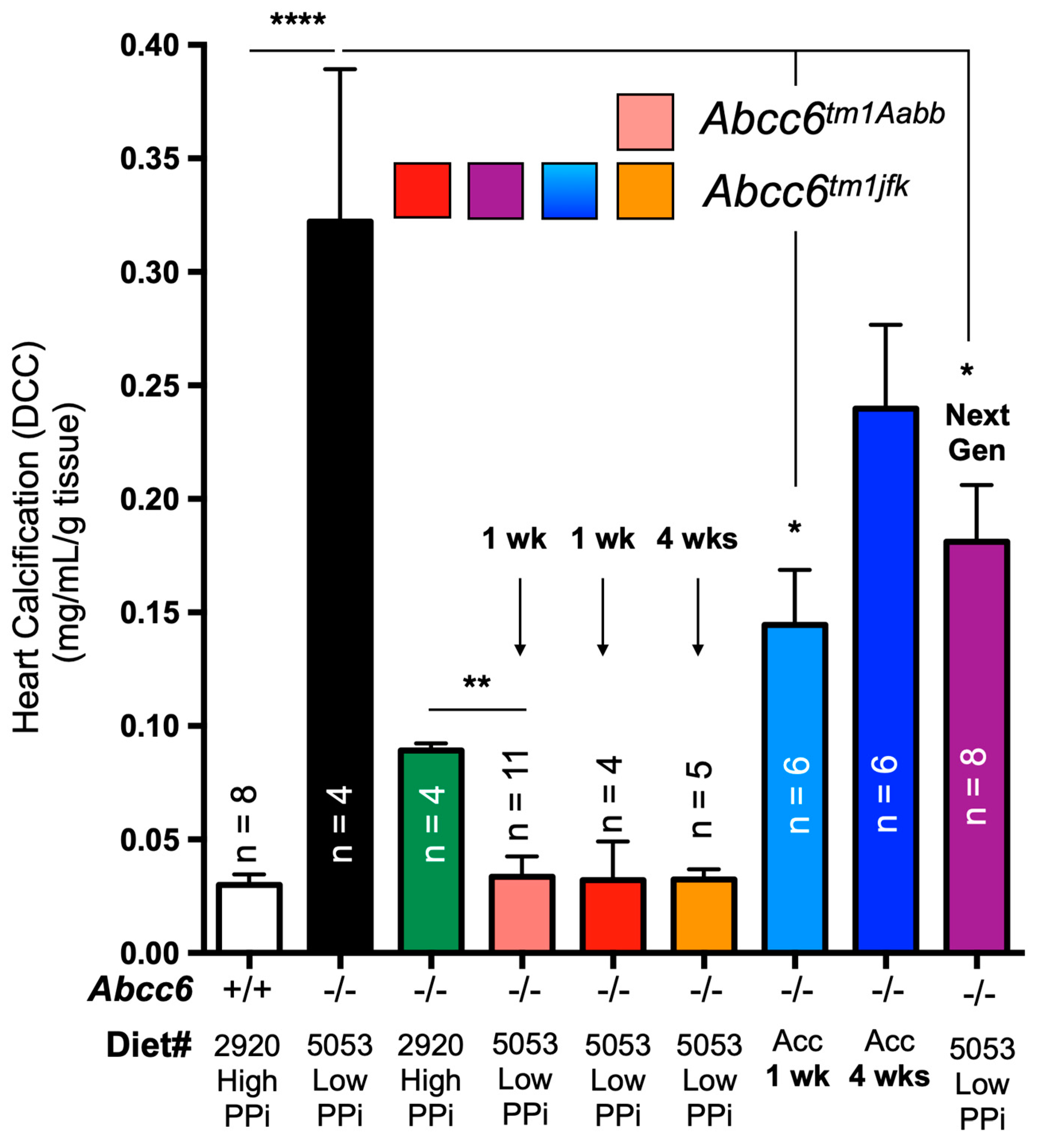
9. Pending questions and important future research directions
10. Conclusions
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Atzeni, F.; Sarzi-Puttini, P.; Bevilacqua, M. Calcium Deposition and Associated Chronic Diseases (Atherosclerosis, Diffuse Idiopathic Skeletal Hyperostosis, and Others). Rheum. Dis. Clin. North Am. 2006, 32, 413–426. [Google Scholar] [CrossRef] [PubMed]
- Bäck, M.; Aranyi, T.; Cancela, M.L.; Carracedo, M.; Conceição, N.; Leftheriotis, G.; Macrae, V.; Martin, L.; Nitschke, Y.; Pasch, A.; et al. Endogenous Calcification Inhibitors in the Prevention of Vascular Calcification: A Consensus Statement From the COST Action EuroSoftCalcNet. Front. Cardiovasc. Med. 2019, 5, 196. [Google Scholar] [CrossRef] [PubMed]
- Rutsch, F.; Nitschke, Y.; Terkeltaub, R. Genetics in arterial calcification: pieces of a puzzle and cogs in a wheel. Circ Res 2011, 109, 578–92. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, J.L.; Hutcheson, J.D.; Aikawa, E. Cardiovascular calcification: current controversies and novel concepts. Cardiovasc. Pathol. 2015, 24, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Ho, C.Y.; Shanahan, C.M. Medial Arterial Calcification: An Overlooked Player in Peripheral Arterial Disease. Arterioscler Thromb Vasc Biol 2016, 36, 1475–82. [Google Scholar] [CrossRef] [PubMed]
- Lanzer, P.; Boehm, M.; Sorribas, V.; Thiriet, M.; Janzen, J.; Zeller, T.; St Hilaire, C.; Shanahan, C. Medial vascular calcification revisited: review and perspectives. Eur. Heart J. 2014, 35, 1515–1525. [Google Scholar] [CrossRef] [PubMed]
- Villa-Bellosta, R. Vascular Calcification: Key Roles of Phosphate and Pyrophosphate. Int. J. Mol. Sci. 2021, 22, 13536. [Google Scholar] [CrossRef] [PubMed]
- Bergen, A.A.; Plomp, A.S.; Schuurman, E.J.; Terry, S.; Breuning, M.; Dauwerse, H.; Swart, J.; Kool, M.; Van Soest, S.; Baas, F.; et al. Mutations in ABCC6 cause pseudoxanthoma elasticum. Nat. Genet. 2000, 25, 228–231. [Google Scholar] [CrossRef] [PubMed]
- Le Saux, O.; Urban, Z.; Tschuch, C.; Csiszar, K.; Bacchelli, B.; Quaglino, D.; Pasquali-Ronchetti, I.; Pope, F.M.; Richards, A.; Terry, S.; et al. Mutations in a gene encoding an ABC transporter cause pseudoxanthoma elasticum. Nat. Genet. 2000, 25, 223–227. [Google Scholar] [CrossRef]
- Ringpfeil, F.; Lebwohl, M.G.; Christiano, A.M.; Uitto, J. Pseudoxanthoma elasticum: Mutations in the MRP6 gene encoding a transmembrane ATP-binding cassette (ABC) transporter. Proc. Natl. Acad. Sci. USA 2000, 97, 6001–6006. [Google Scholar] [CrossRef]
- Jansen, R.S.; Küçükosmanoğlu, A.; de Haas, M.; Sapthu, S.; Otero, J.A.; Hegman, I.E.M.; Bergen, A.A.B.; Gorgels, T.G.M.F.; Borst, P.; van de Wetering, K. ABCC6 prevents ectopic mineralization seen in pseudoxanthoma elasticum by inducing cellular nucleotide release. Proc. Natl. Acad. Sci. USA 2013, 110, 20206–20211. [Google Scholar] [CrossRef] [PubMed]
- Shimada, B.K.; Pomozi, V.; Zoll, J.; Kuo, S.; Martin, L.; Le Saux, O. ABCC6, Pyrophosphate and Ectopic Calcification: Therapeutic Solutions. Int. J. Mol. Sci. 2021, 22, 4555. [Google Scholar] [CrossRef] [PubMed]
- Jansen, R.S.; Duijst, S.; Mahakena, S.; Sommer, D.; Szeri, F.; Váradi, A.; Plomp, A.; Bergen, A.A.; Elferink, R.P.O.; Borst, P.; et al. ABCC6–Mediated ATP Secretion by the Liver Is the Main Source of the Mineralization Inhibitor Inorganic Pyrophosphate in the Systemic Circulation—Brief Report. Arter. Thromb. Vasc. Biol. 2014, 34, 1985–1989. [Google Scholar] [CrossRef]
- Markello, T.C.; Pak, L.K.; Hilaire, C.S.; Dorward, H.; Ziegler, S.G.; Chen, M.Y.; Chaganti, K.; Nussbaum, R.L.; Boehm, M.; Gahl, W.A. Vascular pathology of medial arterial calcifications in NT5E deficiency: Implications for the role of adenosine in pseudoxanthoma elasticum. Mol. Genet. Metab. 2011, 103, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Miglionico, R.; Armentano, M.F.; Carmosino, M.; Salvia, A.M.; Cuviello, F.; Bisaccia, F.; Ostuni, A. Dysregulation of gene expression in ABCC6 knockdown HepG2 cells. Cell. Mol. Biol. Lett. 2014, 19, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Le Saux, O.; Beck, K.; Sachsinger, C.; Silvestri, C.; Treiber, C.; Göring, H.H.; Johnson, E.W.; De Paepe, A.; Pope, F.M.; Pasquali-Ronchetti, I.; et al. A Spectrum of ABCC6 Mutations Is Responsible for Pseudoxanthoma Elasticum. Am. J. Hum. Genet. 2001, 69, 749–764. [Google Scholar] [CrossRef] [PubMed]
- Le Boulanger, G.; Labrèze, C.; Croué, A.; Schurgers, L.; Chassaing, N.; Wittkampf, T.; Rutsch, F.; Martin, L. An unusual severe vascular case of pseudoxanthoma elasticum presenting as generalized arterial calcification of infancy. Am. J. Med Genet. Part A 2009, 152A, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Nitschke, Y.; Baujat, G.; Botschen, U.; Wittkampf, T.; du Moulin, M.; Stella, J.; Le Merrer, M.; Guest, G.; Lambot, K.; Tazarourte-Pinturier, M.-F.; et al. Generalized Arterial Calcification of Infancy and Pseudoxanthoma Elasticum Can Be Caused by Mutations in Either ENPP1 or ABCC6. Am. J. Hum. Genet. 2012, 90, 25–39. [Google Scholar] [CrossRef] [PubMed]
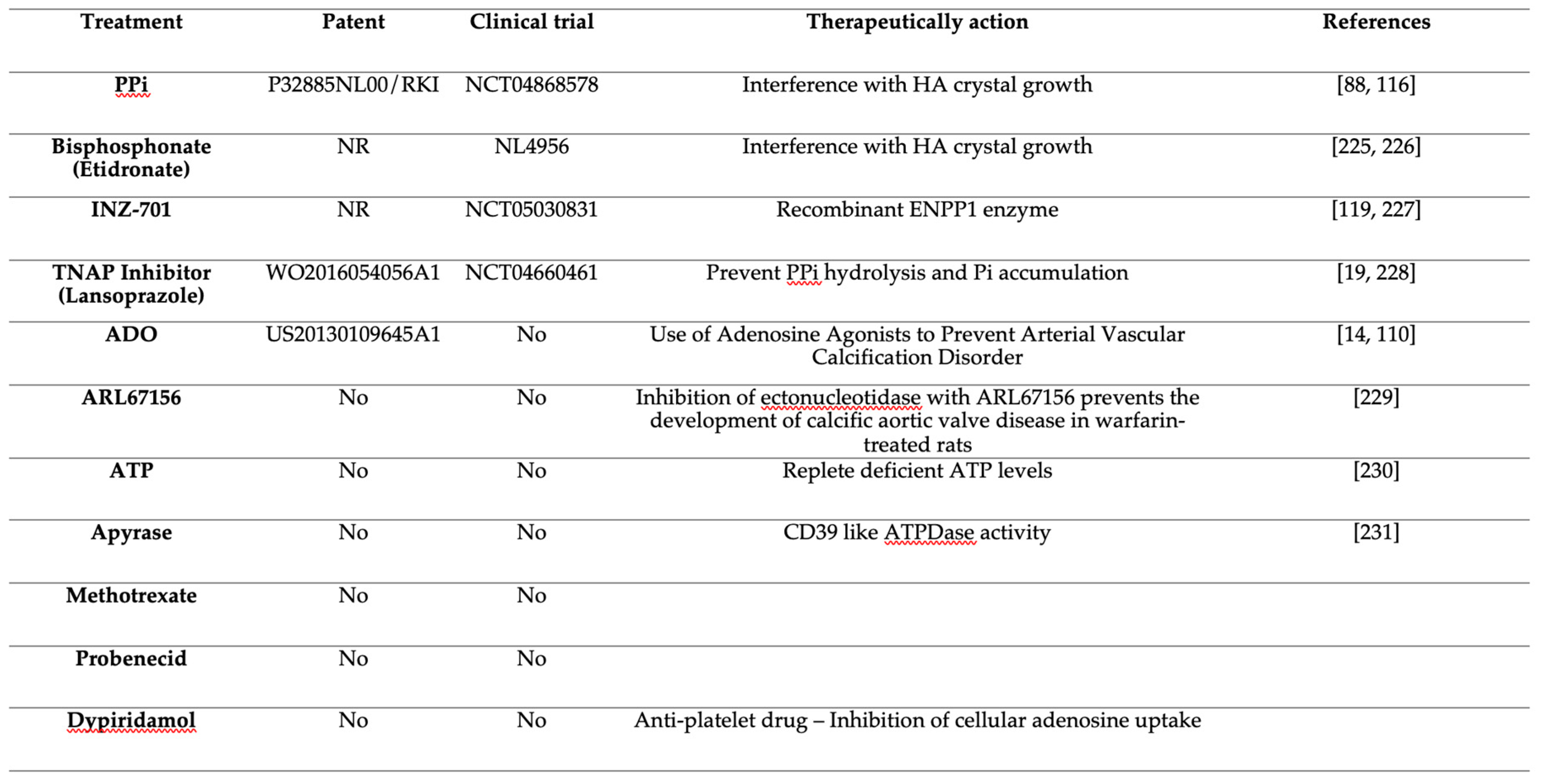
- Ziegler, S.G.; Ferreira, C.R.; MacFarlane, E.G.; Riddle, R.C.; Tomlinson, R.E.; Chew, E.Y.; Martin, L.; Ma, C.-T.; Sergienko, E.; Pinkerton, A.B.; et al. Ectopic calcification in pseudoxanthoma elasticum responds to inhibition of tissue-nonspecific alkaline phosphatase. Sci. Transl. Med. 2017, 9. [Google Scholar] [CrossRef]
- Kauffenstein, G.; Yegutkin, G.G.; Khiati, S.; Pomozi, V.; Le Saux, O.; Leftheriotis, G.; Lenaers, G.; Henrion, D.; Martin, L. Alteration of Extracellular Nucleotide Metabolism in Pseudoxanthoma Elasticum. J. Investig. Dermatol. 2018, 138, 1862–1870. [Google Scholar] [CrossRef]
- Darier, J. Pseudo-xanthome élastique. III ème congrès Intern de Dermat de Londres 1896, 289–295. [Google Scholar]
- Berlyne, G.M.; Bulmer, M.G.; Platt, R. The genetics of pseudoxanthoma elasticum1. Qjm: Int. J. Med. 1961, 30. [Google Scholar] [CrossRef]
- Pope, F.M. Two types of autosomal recessive pseudoxanthoma elasticum. Arch Dermatol 1974, 110, 209–212. [Google Scholar] [CrossRef] [PubMed]
- Neldner, K.H. Pseudoxanthoma elasticum. Int J Dermatol 1988, 27, 98–100. [Google Scholar] [CrossRef]
- Uitto, J.; Boyd, C.D.; Lebwohl, M.G.; Moshell, A.N.; Rosenbloom, J.; Terry, S. International Centennial Meeting on Pseudoxanthoma Elasticum: Progress in PXE Research. J. Investig. Dermatol. 1998, 110, 840–842. [Google Scholar] [CrossRef]
- Gorgels, T.G.; Hu, X.; Scheffer, G.L.; van der Wal, A.C.; Toonstra, J.; de Jong, P.T.; van Kuppevelt, T.H.; Levelt, C.N.; de Wolf, A.; Loves, W.J.; et al. Disruption of Abcc6 in the mouse: novel insight in the pathogenesis of pseudoxanthoma elasticum. Hum. Mol. Genet. 2005, 14, 1763–1773. [Google Scholar] [CrossRef] [PubMed]
- Klement, J.F.; Matsuzaki, Y.; Jiang, Q.-J.; Terlizzi, J.; Choi, H.Y.; Fujimoto, N.; Li, K.; Pulkkinen, L.; Birk, D.E.; Sundberg, J.P.; et al. Targeted Ablation of the Abcc6 Gene Results in Ectopic Mineralization of Connective Tissues. Mol. Cell. Biol. 2005, 25, 8299–8310. [Google Scholar] [CrossRef]
- Bergen, A.A. Pseudoxanthoma Elasticum: the End of the Autosomal Dominant Segregation Myth. J. Investig. Dermatol. 2006, 126, 704–705. [Google Scholar] [CrossRef] [PubMed]
- Struk, B.; Neldner, K.H.; Rao, V.S.; Jean, P.S.; Lindpaintner, K. Mapping of Both Autosomal Recessive and Dominant Variants of Pseudoxanthoma Elasticum to Chromosome 16p13.1. Hum. Mol. Genet. 1997, 6, 1823–1828. [Google Scholar] [CrossRef]
- van Soest, S.; Swart, J.; Tijmes, N.; Sandkuijl, L.A.; Rommers, J.; Bergen, A.A. A Locus for Autosomal Recessive Pseudoxanthoma Elasticum, with Penetrance of Vascular Symptoms in Carriers, Maps to Chromosome 16p13.1. Genome Res. 1997, 7, 830–834. [Google Scholar] [CrossRef]
- Klein, I.; Sarkadi, B.; Varadi, A. An inventory of the human ABC proteins. Biochim Biophys Acta 1999, 1461, 237–62. [Google Scholar] [CrossRef] [PubMed]
- Stefková, J.; Poledne, R.; A Hubácek, J. ATP-binding cassette (ABC) transporters in human metabolism and diseases. Physiol Res 2004, 53, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Riordan, J.R.; Rommens, J.M.; Kerem, B.-S.; Alon, N.; Rozmahel, R.; Grzelczak, Z.; Zielenski, J.; Lok, S.; Plavsic, N.; Chou, J.-L.; et al. Identification of the Cystic Fibrosis Gene: Cloning and Characterization of Complementary DNA. Science 1989, 245, 1066–1073. [Google Scholar] [CrossRef] [PubMed]
- Ilias, A.; Urban, Z.; Seidl, T.L.; Le Saux, O.; Sinko, E.; Boyd, C.D.; Sarkadi, B.; Varadi, A. Loss of ATP-dependent transport activity in pseudoxanthoma elasticum-associated mutants of human ABCC6 (MRP6). J Biol Chem 2002, 277, 16860–7. [Google Scholar] [CrossRef] [PubMed]
- Le Saux, O.; Fülöp, K.; Yamaguchi, Y.; Iliás, A.; Szabó, Z.; Brampton, C.N.; Pomozi, V.; Huszár, K.; Arányi, T.; Váradi, A. Expression and In Vivo Rescue of Human ABCC6 Disease-Causing Mutants in Mouse Liver. PLOS ONE 2011, 6, e24738. [Google Scholar] [CrossRef]
- Pomozi, V.; Brampton, C.; Fülöp, K.; Chen, L.-H.; Apana, A.; Li, Q.; Uitto, J.; Le Saux, O.; Váradi, A. Analysis of Pseudoxanthoma Elasticum–Causing Missense Mutants of ABCC6 In Vivo ; Pharmacological Correction of the Mislocalized Proteins. J. Investig. Dermatol. 2014, 134, 946–953. [Google Scholar] [CrossRef] [PubMed]
- Pomozi, V.; Brampton, C.; Szeri, F.; Dedinszki, D.; Kozak, E.; van de Wetering, K.; Hopkins, H.; Martin, L.; Varadi, A.; Le Saux, O. Functional Rescue of ABCC6 Deficiency by 4-Phenylbutyrate Therapy Reduces Dystrophic Calcification in Abcc6(-/-) Mice. J Invest Dermatol 2017, 137, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Chassaing, N.; Martin, L.; Calvas, P.; Le Bert, M.; Hovnanian, A. Pseudoxanthoma elasticum: a clinical, pathophysiological and genetic update including 11 novel ABCC6 mutations. J. Med Genet. 2005, 42, 881–892. [Google Scholar] [CrossRef] [PubMed]
- Plomp, A.S.; Toonstra, J.; Bergen, A.A.; van Dijk, M.R.; de Jong, P.T. Proposal for updating the pseudoxanthoma elasticum classification system and a review of the clinical findings. Am. J. Med Genet. Part A 2010, 152, 1049–1058. [Google Scholar] [CrossRef]
- Weenink, A.; Dijkman, G.; Demeijer, P. Pseudoxanthoma elasticum and its complications: two case reports. Neth. J. Med. 1996, 49, 24–29. [Google Scholar] [CrossRef]
- Doyne, R. Chorioidal and retinal changes the result of blows on the eye. Trans Ophthalmol Soc UK 1889, 9, 128. [Google Scholar]
- Knapp, H. On the formation of dark angioid streaksas an unusual metamorphosis of retinal hemorrhage. Arch Ophthalmol 1892, 21, 289–292. [Google Scholar]
- Groenblad, E. Angioid streaks: pseudoxanthoma elasticum: vorloeufige mitteilung. Acta Ophthalmol 1929, 7, 329. [Google Scholar] [CrossRef]
- Gass, J.D. “Comet” lesion: an ocular sign of pseudoxanthoma elasticum. Retina 2003, 23, 729–30. [Google Scholar] [CrossRef] [PubMed]
- Finger, R.P.; Issa, P.C.; Schmitz-Valckenberg, S.; Holz, F.G.; Scholl, H.N. Long-term effectiveness of intravitreal bevacizumab for choroidal neovascularization secondary to angioid streaks in pseudoxanthoma elasticum. Retina 2011, 31, 1268–1278. [Google Scholar] [CrossRef]
- Hess, K.; Raming, K.; Issa, P.C.; Herrmann, P.; Holz, F.G.; Pfau, M. Inner retinal degeneration associated with optic nerve head drusen in pseudoxanthoma elasticum. Br. J. Ophthalmol. 2021, 107, 570–575. [Google Scholar] [CrossRef] [PubMed]
- Brampton, C.; Pomozi, V.; Chen, L.-H.; Apana, A.; McCurdy, S.; Zoll, J.; Boisvert, W.A.; Lambert, G.; Henrion, D.; Blanchard, S.; et al. ABCC6 deficiency promotes dyslipidemia and atherosclerosis. Sci. Rep. 2021, 11, 1–16. [Google Scholar] [CrossRef]
- Lefthériotis, G.; Omarjee, L.; Le Saux, O.; Henrion, D.; Abraham, P.; Prunier, F.; Willoteaux, S.; Martin, L. The vascular phenotype in Pseudoxanthoma elasticum and related disorders: contribution of a genetic disease to the understanding of vascular calcification. Front. Genet. 2013, 4, 4. [Google Scholar] [CrossRef]
- Vasseur, M.; Carsin-Nicol, B.; Ebran, J.; Willoteaux, S.; Martin, L.; Lefthériotis, G. Carotid Rete Mirabile and Pseudoxanthoma Elasticum: An Accidental Association? Eur. J. Vasc. Endovasc. Surg. 2011, 42, 292–294. [Google Scholar] [CrossRef]
- Omarjee, L.; Fortrat, J.-O.; Larralde, A.; Le Pabic, E.; Kauffenstein, G.; Laot, M.; Navasiolava, N.; Mention, P.-J.; Linares, J.L.C.; Valdivielso, P.; et al. Internal Carotid Artery Hypoplasia: A New Clinical Feature in Pseudoxanthoma Elasticum. J. Stroke 2019, 21, 108–111. [Google Scholar] [CrossRef]
- Kranenburg, G.; De Jong, P.A.; Mali, W.P.; Attrach, M.; Visseren, F.L.J.; Spiering, W. Prevalence and severity of arterial calcifications in pseudoxanthoma elasticum (PXE) compared to hospital controls. Novel insights into the vascular phenotype of PXE. Atherosclerosis 2017, 256, 7–14. [Google Scholar] [CrossRef]
- Kevorkian, J.P.; Masquet, C.; Kural-Menasche, S.; Le Dref, O.; Beaufils, P. New report of severe coronary artery disease in an eighteen-year-old girl with pseudoxanthoma elasticum. Case report and review of the literature. Angiology 1997, 48, 735–41. [Google Scholar] [CrossRef] [PubMed]
- Kocaman, S.; Tavil, Y.; Yalcin, M. Severe coronary artery disease in a 21-year-old girl with pseudoxanthoma elasticum anomalous origin of the right coronary artery. Acta Cardiol. 2007, 62, 417–419. [Google Scholar] [CrossRef] [PubMed]
- Miwa, K.; Higashikata, T.; Mabuchi, H. Intravascular ultrasound findings of coronary wall morphology in a patient with pseudoxanthoma elasticum. Heart 2004, 90, e61–e61. [Google Scholar] [CrossRef] [PubMed]
- Lefthériotis, G.; Abraham, P.; Le Corre, Y.; Le Saux, O.; Henrion, D.; Ducluzeau, P.H.; Prunier, F.; Martin, L. Relationship between ankle brachial index and arterial remodeling in pseudoxanthoma elasticum. J. Vasc. Surg. 2011, 54, 1390–1394. [Google Scholar] [CrossRef]
- Leftheriotis, G.; Kauffenstein, G.; Hamel, J.F.; Abraham, P.; Le Saux, O.; Willoteaux, S.; Henrion, D.; Martin, L. The Contribution of Arterial Calcification to Peripheral Arterial Disease in Pseudoxanthoma Elasticum. PLOS ONE 2014, 9, e96003. [Google Scholar] [CrossRef] [PubMed]
- Prunier, F.; Terrien, G.; Le Corre, Y.; Apana, A.L.Y.; Bière, L.; Kauffenstein, G.; Furber, A.; Bergen, A.A.B.; Gorgels, T.G.M.F.; Le Saux, O.; et al. Pseudoxanthoma Elasticum: Cardiac Findings in Patients and Abcc6-Deficient Mouse Model. PLOS ONE 2013, 8, e68700. [Google Scholar] [CrossRef] [PubMed]
- Köblös, G.; Andrikovics, H.; Prohászka, Z.; Tordai, A.; Váradi, A.; Arányi, T. The R1141X Loss-of-Function Mutation of the ABCC6 Gene Is a Strong Genetic Risk Factor for Coronary Artery Disease. Genet. Test. Mol. Biomarkers 2010, 14, 75–78. [Google Scholar] [CrossRef]
- Trip, M.D.; Smulders, Y.M.; Wegman, J.J.; Hu, X.; Boer, J.M.; Brink, J.B.T.; Zwinderman, A.H.; Kastelein, J.J.; Feskens, E.J.; Bergen, A.A.; et al. Frequent mutation in the ABCC6 gene (R1141X) is associated with a strong increase in the prevalence of coronary artery disease. Circ. 2002, 106, 773–775. [Google Scholar] [CrossRef]
- Hornstrup, L.S.; Tybjærg-Hansen, A.; Haase, C.L.; Nordestgaard, B.G.; Sillesen, H.; Grande, P.; Frikke-Schmidt, R.; Christoffersen, M.; Schnohr, P.; Jensen, G.B.; et al. Heterozygosity for R1141X in ABCC6 and Risk of Ischemic Vascular Disease. Circ. Cardiovasc. Genet. 2011, 4, 534–541. [Google Scholar] [CrossRef]
- Kuzaj, P.; Kuhn, J.; Dabisch-Ruthe, M.; Faust, I.; Götting, C.; Knabbe, C.; Hendig, D. ABCC6- a new player in cellular cholesterol and lipoprotein metabolism? Lipids Heal. Dis. 2014, 13, 118–118. [Google Scholar] [CrossRef] [PubMed]
- Peloso, G.M.; Demissie, S.; Collins, D.; Mirel, D.B.; Gabriel, S.B.; Cupples, L.A.; Robins, S.J.; Schaefer, E.J.; Brousseau, M.E. Common genetic variation in multiple metabolic pathways influences susceptibility to low HDL-cholesterol and coronary heart disease. J. Lipid Res. 2010, 51, 3524–3532. [Google Scholar] [CrossRef] [PubMed]
- Pisciotta, L.; Tarugi, P.; Borrini, C.; Bellocchio, A.; Fresa, R.; Guerra, D.; Quaglino, D.; Ronchetti, I.; Calandra, S.; Bertolini, S. Pseudoxanthoma elasticum and familial hypercholesterolemia: A deleterious combination of cardiovascular risk factors. Atherosclerosis 2010, 210, 173–176. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Near, S.; Young, K.; Connelly, P.W.; Hegele, R.A. ABCC6 gene polymorphism associated with variation in plasma lipoproteins. J. Hum. Genet. 2001, 46, 699–705. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.-D.; Terbah, M.; Daudon, P.; Martin, L. Left Ventricular Systolic and Diastolic Function by Echocardiogram in Pseudoxanthoma Elasticum. Am. J. Cardiol. 2006, 97, 1535–1537. [Google Scholar] [CrossRef] [PubMed]
- Miki, K.; Yuri, T.; Takeda, N.; Takehana, K.; Iwasaka, T.; Tsubura, A. An autopsy case of pseudoxanthoma elasticum: histochemical characteristics. Med Mol. Morphol. 2007, 40, 172–177. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Lopez, F.; Llorian, A.; Ferrer-Roca, O.; Betriu, A.; Sanz, G. Restrictive Cardiomyopathy in Pseudoxanthoma Elasticum. Chest 1980, 78, 113–115. [Google Scholar] [CrossRef]
- Lebwohl, M.G.; Distefano, D.; Prioleau, P.G.; Uram, M.; Yannuzzi, L.A.; Fleischmajer, R. Pseudoxanthoma Elasticum and Mitral-Valve Prolapse. New Engl. J. Med. 1982, 307, 228–231. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, K.; Uno, K.; Fujii, T.; Mukai, M.; Handa, S. Mitral Stenosis in Pseudoxanthoma Elasticum. Chest 1992, 101, 1706–1707. [Google Scholar] [CrossRef]
- Vanakker, O.M.; Leroy, B.P.; Coucke, P.; Bercovitch, L.G.; Uitto, J.; Viljoen, D.; Terry, S.F.; Van Acker, P.; Matthys, D.; Loeys, B.; et al. Novel clinico-molecular insights in pseudoxanthoma elasticum provide an efficient molecular screening method and a comprehensive diagnostic flowchart. Hum. Mutat. 2007, 29, 205–205. [Google Scholar] [CrossRef]
- Basso, C.; Boschello, M.; Perrone, C.; Mecenero, A.; Cera, A.; Bicego, D.; Thiene, G.; De Dominicis, E. An echocardiographic survey of primary school children for bicuspid aortic valve. Am. J. Cardiol. 2004, 93, 661–663. [Google Scholar] [CrossRef]
- Mungrue, I.N.; Zhao, P.; Yao, Y.; Meng, H.; Rau, C.; Havel, J.V.; Gorgels, T.G.; Bergen, A.A.; MacLellan, W.R.; Drake, T.A.; et al. Abcc6 Deficiency Causes Increased Infarct Size and Apoptosis in a Mouse Cardiac Ischemia-Reperfusion Model. Arter. Thromb. Vasc. Biol. 2011, 31, 2806–2812. [Google Scholar] [CrossRef]
- Eaton, G.J.; Custer, R.P.; Johnson, F.N.; Stabenow, K.T. Dystrophic cardiac calcinosis in mice: genetic, hormonal, and dietary influences. Am J Pathol 1978, 90, 173–186. [Google Scholar]
- Ivandic, B.T.; Qiao, J.H.; Machleder, D.; Liao, F.; A Drake, T.; Lusis, A.J. A locus on chromosome 7 determines myocardial cell necrosis and calcification (dystrophic cardiac calcinosis) in mice. Proc. Natl. Acad. Sci. USA 1996, 93, 5483–5488. [Google Scholar] [CrossRef] [PubMed]
- Aherrahrou, Z.; Doehring, L.C.; Ehlers, E.-M.; Liptau, H.; Depping, R.; Linsel-Nitschke, P.; Kaczmarek, P.M.; Erdmann, J.; Schunkert, H. An Alternative Splice Variant in Abcc6, the Gene Causing Dystrophic Calcification, Leads to Protein Deficiency in C3H/He Mice. J. Biol. Chem. 2008, 283, 7608–7615. [Google Scholar] [CrossRef] [PubMed]
- Meng, H.; Vera, I.; Che, N.; Wang, X.; Wang, S.S.; Ingram-Drake, L.; Schadt, E.E.; Drake, T.A.; Lusis, A.J. Identification of Abcc6 as the major causal gene for dystrophic cardiac calcification in mice through integrative genomics. Proc. Natl. Acad. Sci. USA 2007, 104, 4530–4535. [Google Scholar] [CrossRef]
- Le Corre, Y.; Le Saux, O.; Froeliger, F.; Libouban, H.; Kauffenstein, G.; Willoteaux, S.; Leftheriotis, G.; Martin, L. Quantification of the Calcification Phenotype of Abcc6-Deficient Mice with Microcomputed Tomography. Am. J. Pathol. 2012, 180, 2208–2213. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Guo, H.; Chou, D.W.; Berndt, A.; Sundberg, J.P.; Uitto, J. Mouse Models for Pseudoxanthoma Elasticum: Genetic and Dietary Modulation of the Ectopic Mineralization Phenotypes. PLOS ONE 2014, 9, e89268. [Google Scholar] [CrossRef] [PubMed]
- Morikane, S.; Ishida, K.; Taniguchi, T.; Ashizawa, N.; Matsubayashi, M.; Kurita, N.; Kobashi, S.; Iwanaga, T. Identification of a DBA/2 Mouse Sub-strain as a Model for Pseudoxanthoma Elasticum-Like Tissue Calcification. Biol. Pharm. Bull. 2023, 46, 1737–1744. [Google Scholar] [CrossRef]
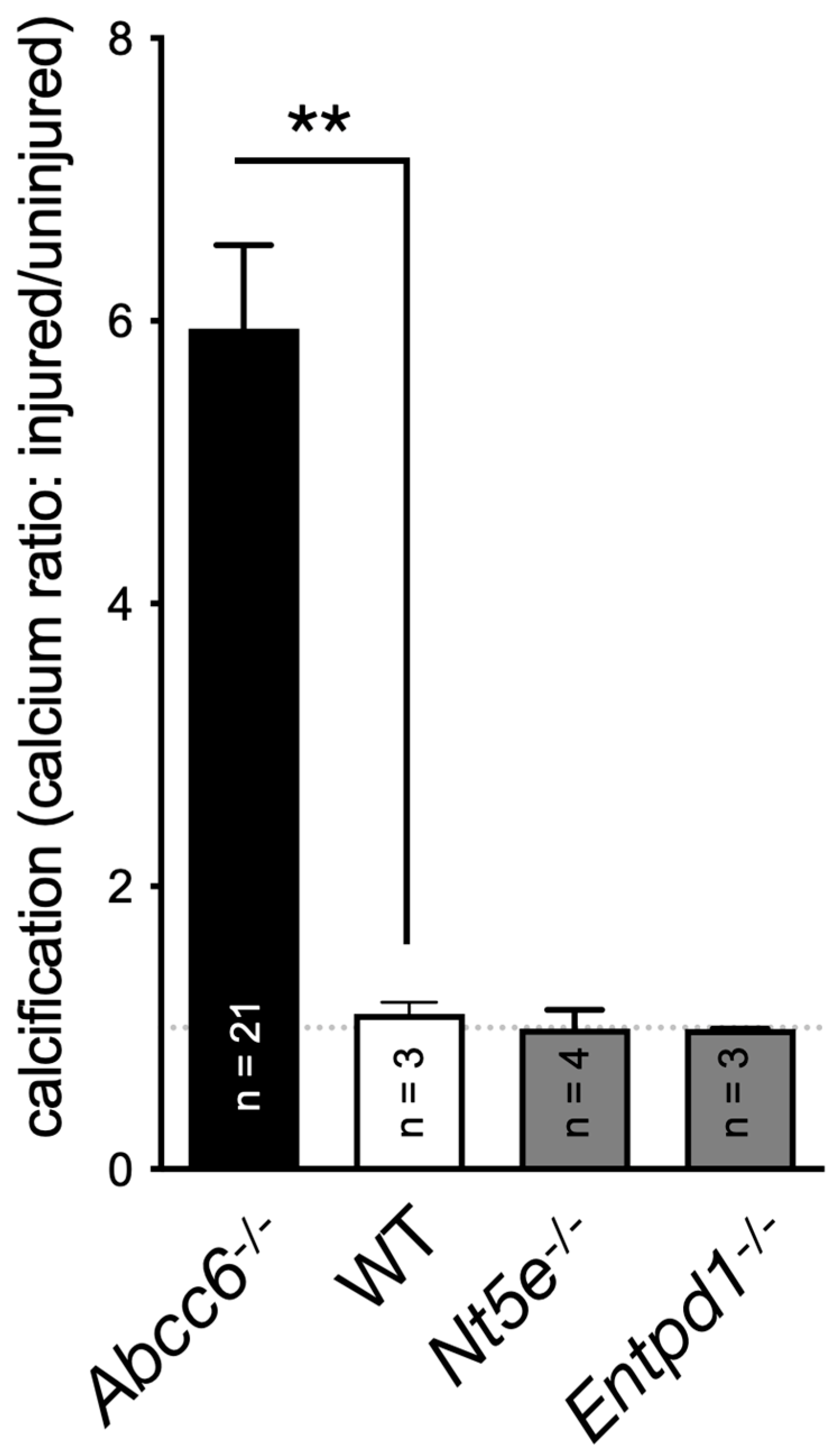
- Brampton, C.; Aherrahrou, Z.; Chen, L.-H.; Martin, L.; Bergen, A.A.; Gorgels, T.G.; Erdfdi, J.; Schunkert, H.; Szabó, Z.; Váradi, A.; et al. The Level of Hepatic ABCC6 Expression Determines the Severity of Calcification after Cardiac Injury. Am. J. Pathol. 2014, 184, 159–170. [Google Scholar] [CrossRef]
- Dedinszki, D.; Szeri, F.; Kozák, E.; Pomozi, V.; Tőkési, N.; Mezei, T.R.; Merczel, K.; Letavernier, E.; Tang, E.; Le Saux, O.; et al. Oral administration of pyrophosphate inhibits connective tissue calcification. EMBO Mol. Med. 2017, 9, 1463–1470. [Google Scholar] [CrossRef]
- Pomozi, V.; Brampton, C.; van de Wetering, K.; Zoll, J.; Calio, B.; Pham, K.; Owens, J.B.; Marh, J.; Moisyadi, S.; Váradi, A.; et al. Pyrophosphate Supplementation Prevents Chronic and Acute Calcification in ABCC6-Deficient Mice. Am. J. Pathol. 2017, 187, 1258–1272. [Google Scholar] [CrossRef]
- Omarjee, L.; Roy, C.; Leboeuf, C.; Favre, J.; Henrion, D.; Mahe, G.; Leftheriotis, G.; Martin, L.; Janin, A.; Kauffenstein, G. Evidence of Cardiovascular Calcification and Fibrosis in Pseudoxanthoma Elasticum Mouse Models Subjected to DOCA-Salt Hypertension. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef]
- Brampton, C.; Yamaguchi, Y.; Vanakker, O.; Van Laer, L.; Chen, L.-H.; Thakore, M.; De Paepe, A.; Pomozi, V.; Szabó, P.T.; Martin, L.; et al. Vitamin K does not prevent soft tissue mineralization in a mouse model of pseudoxanthoma elasticum. Cell Cycle 2011, 10, 1810–1820. [Google Scholar] [CrossRef]
- Ibold, B.; Tiemann, J.; Faust, I.; Ceglarek, U.; Dittrich, J.; Gorgels, T.G.M.F.; Bergen, A.A.B.; Vanakker, O.; Van Gils, M.; Knabbe, C.; et al. Genetic deletion of Abcc6 disturbs cholesterol homeostasis in mice. Sci. Rep. 2021, 11, 1–13. [Google Scholar] [CrossRef]
- Garcia-Fernandez, M.I.; Gheduzzi, D.; Boraldi, F.; Paolinelli, C.D.; Sanchez, P.; Valdivielso, P.; Morilla, M.J.; Quaglino, D.; Guerra, D.; Casolari, S.; et al. Parameters of oxidative stress are present in the circulation of PXE patients. Biochim. et Biophys. Acta (BBA) - Mol. Basis Dis. 2008, 1782, 474–481. [Google Scholar] [CrossRef]
- Li, Q.; Jiang, Q.; Uitto, J. Pseudoxanthoma elasticum: oxidative stress and antioxidant diet in a mouse model (Abcc6-/-). J Invest Dermatol 2008, 128, 1160–4. [Google Scholar] [CrossRef]
- Pasquali-Ronchetti, I.; Garcia-Fernandez, M.I.; Boraldi, F.; Quaglino, D.; Gheduzzi, D.; Paolinelli, C.D.V.; Tiozzo, R.; Bergamini, S.; Ceccarelli, D.; Muscatello, U. Oxidative stress in fibroblasts from patients with pseudoxanthoma elasticum: possible role in the pathogenesis of clinical manifestations. J. Pathol. 2005, 208, 54–61. [Google Scholar] [CrossRef]
- Kauffenstein, G.; Pizard, A.; Le Corre, Y.; Vessières, E.; Grimaud, L.; Toutain, B.; Labat, C.; Mauras, Y.; Gorgels, T.; Bergen, A.; et al. Disseminated Arterial Calcification and Enhanced Myogenic Response Are Associated With Abcc6 Deficiency in a Mouse Model of Pseudoxanthoma Elasticum. Arter. Thromb. Vasc. Biol. 2014, 34, 1045–1056. [Google Scholar] [CrossRef]
- Rasmussen, M.R.; Nielsen, K.L.; Laursen, M.R.; Nielsen, C.B.; Svendsen, P.; Dimke, H.; Christensen, E.I.; Johannsen, M.; Moestrup, S.K. Untargeted Metabolomics Analysis of ABCC6-Deficient Mice Discloses an Altered Metabolic Liver Profile. J. Proteome Res. 2016, 15, 4591–4600. [Google Scholar] [CrossRef]
- Lofaro, F.D.; Boraldi, F.; Garcia-Fernandez, M.; Estrella, L.; Valdivielso, P.; Quaglino, D. Relationship Between Mitochondrial Structure and Bioenergetics in Pseudoxanthoma elasticum Dermal Fibroblasts. Front. Cell Dev. Biol. 2020, 8. [Google Scholar] [CrossRef]
- Martin, L.J.; Lau, E.; Singh, H.; Vergnes, L.; Tarling, E.J.; Mehrabian, M.; Mungrue, I.; Xiao, S.; Shih, D.; Castellani, L.; et al. ABCC6 Localizes to the Mitochondria-Associated Membrane. Circ. Res. 2012, 111, 516–520. [Google Scholar] [CrossRef]
- Gentile, D.; Natale, M.; Lazzerini, P.E.; Capecchi, P.L.; Laghi-Pasini, F. The role of P2X7 receptors in tissue fibrosis: a brief review. Purinergic Signal. 2015, 11, 435–440. [Google Scholar] [CrossRef] [PubMed]
- Kauffenstein, G.; Tamareille, S.; Prunier, F.; Roy, C.; Ayer, A.; Toutain, B.; Billaud, M.; Isakson, B.E.; Grimaud, L.; Loufrani, L.; et al. Central Role of P2Y 6 UDP Receptor in Arteriolar Myogenic Tone. Arter. Thromb. Vasc. Biol. 2016, 36, 1598–1606. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, G.; Cronstein, B. Signaling pathways involving adenosine A2A and A2B receptors in wound healing and fibrosis. Purinergic Signal. 2016, 12, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.-M.; Zhang, N.; Li, J.-S.; Yang, Z.-H.; Huang, X.-L.; Yang, X.-F. Purinergic receptors mediate endothelial dysfunction and participate in atherosclerosis. Purinergic Signal. 2022, 19, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Beck, K.; Hayashi, K.; Dang, K.; Hayashi, M.; Boyd, C.D. Analysis of ABCC6 (MRP6) in normal human tissues. Histochem. 2005, 123, 517–528. [Google Scholar] [CrossRef] [PubMed]
- Beck, K.; Hayashi, K.; Nishiguchi, B.; Le Saux, O.; Hayashi, M.; Boyd, C.D. The Distribution of Abcc6 in Normal Mouse Tissues Suggests Multiple Functions for this ABC Transporter. J. Histochem. Cytochem. 2003, 51, 887–902. [Google Scholar] [CrossRef] [PubMed]
- Le Saux, O.; Bunda, S.; VanWart, C.M.; Douet, V.; Got, L.; Martin, L.; Hinek, A. Serum Factors from Pseudoxanthoma Elasticum Patients Alter Elastic Fiber Formation In Vitro. J. Investig. Dermatol. 2006, 126, 1497–1505. [Google Scholar] [CrossRef]
- Jiang, Q.; Li, Q.; Uitto, J. Aberrant Mineralization of Connective Tissues in a Mouse Model of Pseudoxanthoma Elasticum: Systemic and Local Regulatory Factors. J. Investig. Dermatol. 2007, 127, 1392–1402. [Google Scholar] [CrossRef]
- Jiang, Q.; Endo, M.; Dibra, F.; Wang, K.; Uitto, J. Pseudoxanthoma Elasticum Is a Metabolic Disease. J. Investig. Dermatol. 2009, 129, 348–354. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Oldenburg, R.; Otsuru, S.; Grand-Pierre, A.E.; Horwitz, E.M.; Uitto, J. Parabiotic heterogenetic pairing of Abcc6-/-/Rag1-/- mice and their wild-type counterparts halts ectopic mineralization in a murine model of pseudoxanthoma elasticum. Am J Pathol 2010, 176, 1855–62. [Google Scholar] [CrossRef]
- Ronchetti, I.; Boraldi, F.; Annovi, G.; Cianciulli, P.; Quaglino, D. Fibroblast involvement in soft connective tissue calcification. Front. Genet. 2013, 4, 22. [Google Scholar] [CrossRef] [PubMed]
- Passi, A.; Albertini, R.; Contri, M.B.; de Luca, G.; de Paepe, A.; Pallavicini, G.; Ronchetti, I.P.; Tiozzo, R. Proteoglycan alterations in skin fibroblast cultures from patients affected with pseudoxanthoma elasticum. Cell Biochem Funct 1996, 14, 111–20. [Google Scholar] [CrossRef]
- Quaglino, D.; Sartor, L.; Garbisa, S.; Boraldi, F.; Croce, A.; Passi, A.; De Luca, G.; Tiozzo, R.; Pasquali-Ronchetti, I. Dermal fibroblasts from pseudoxanthoma elasticum patients have raised MMP-2 degradative potential. Biochim. et Biophys. Acta (BBA) - Mol. Basis Dis. 2005, 1741, 42–47. [Google Scholar] [CrossRef]
- Tiemann, J.; Wagner, T.; Lindenkamp, C.; Plümers, R.; Faust, I.; Knabbe, C.; Hendig, D. Linking ABCC6 Deficiency in Primary Human Dermal Fibroblasts of PXE Patients to p21-Mediated Premature Cellular Senescence and the Development of a Proinflammatory Secretory Phenotype. Int. J. Mol. Sci. 2020, 21, 9665. [Google Scholar] [CrossRef]
- Belinsky, M.G.; Chen, Z.-S.; Shchaveleva, I.; Zeng, H.; Kruh, G.D. Characterization of the drug resistance and transport properties of multidrug resistance protein 6 (MRP6, ABCC6). Cancer Res. 2002, 62, 6172–6177. [Google Scholar]
- Vanakker, O.M.; Martin, L.; Gheduzzi, D.; Leroy, B.P.; Loeys, B.L.; Guerci, V.I.; Matthys, D.; Terry, S.F.; Coucke, P.J.; Pasquali-Ronchetti, I.; et al. Pseudoxanthoma Elasticum-Like Phenotype with Cutis Laxa and Multiple Coagulation Factor Deficiency Represents a Separate Genetic Entity. J. Investig. Dermatol. 2007, 127, 581–587. [Google Scholar] [CrossRef] [PubMed]
- Vanakker, O.M.; Martin, L.; Schurgers, L.J.; Quaglino, D.; Costrop, L.; Vermeer, C.; Pasquali-Ronchetti, I.; Coucke, P.J.; De Paepe, A. Low serum vitamin K in PXE results in defective carboxylation of mineralization inhibitors similar to the GGCX mutations in the PXE-like syndrome. Mod. Pathol. 2010, 90, 895–905. [Google Scholar] [CrossRef]
- Borst, P.; van de Wetering, K.; Schlingemann, R. Does the absence of ABCC6 (multidrug resistance protein 6) in patients with Pseudoxanthoma elasticum prevent the liver from providing sufficient vitamin K to the periphery? Cell Cycle 2008, 7, 1575–9. [Google Scholar] [CrossRef]
- Gorgels, T.G.M.F.; Waarsing, J.H.; Herfs, M.; Versteeg, D.; Schoensiegel, F.; Sato, T.; Schlingemann, R.O.; Ivandic, B.; Vermeer, C.; Schurgers, L.J.; et al. Vitamin K supplementation increases vitamin K tissue levels but fails to counteract ectopic calcification in a mouse model for pseudoxanthoma elasticum. J. Mol. Med. 2011, 89, 1125–1135. [Google Scholar] [CrossRef]
- Jiang, Q.; Li, Q.; Grand-Pierre, A.E.; Schurgers, L.J.; Uitto, J. Administration of vitamin K does not counteract the ectopic mineralization of connective tissues in Abcc6 (-/-) mice, a model for pseudoxanthoma elasticum. Cell Cycle 2011, 10, 701–7. [Google Scholar] [CrossRef]
- Fülöp, K.; Jiang, Q.; Wetering, K.V.; Pomozi, V.; Szabó, P.T.; Arányi, T.; Sarkadi, B.; Borst, P.; Uitto, J.; Váradi, A. ABCC6 does not transport vitamin K3-glutathione conjugate from the liver: Relevance to pathomechanisms of pseudoxanthoma elasticum. Biochem. Biophys. Res. Commun. 2011, 415, 468–471. [Google Scholar] [CrossRef] [PubMed]
- Hilaire, C.S.; Ziegler, S.G.; Markello, T.C.; Brusco, A.; Groden, C.; Gill, F.; Carlson-Donohoe, H.; Lederman, R.J.; Chen, M.Y.; Yang, D.; Siegenthaler, M.P.; Arduino, C.; Mancini, C.; Freudenthal, B.; Stanescu, H.C.; Zdebik, A.A.; Chaganti, R.K.; Nussbaum, R.L.; Kleta, R.; Gahl, W.A.; Boehm, M. NT5E mutations and arterial calcifications. N Engl J Med 2011, 364, 432–42. [Google Scholar] [CrossRef]
- Zimmermann, H. 5′-Nucleotidase: molecular structure and functional aspects. Biochem. J. 1992, 285, 345–365. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Price, T.P.; Sundberg, J.P.; Uitto, J. Juxta-articular joint-capsule mineralization in CD73 deficient mice: similarities to patients with NT5E mutations. Cell Cycle 2014, 13, 2609–15. [Google Scholar] [CrossRef]
- Joolharzadeh, P.; St. Hilaire, C. CD73 (Cluster of Differentiation 73) and the Differences Between Mice and Humans. Arter. Thromb. Vasc. Biol. 2019, 39, 339–348. [Google Scholar] [CrossRef]
- Szabó, Z.; Váradi, A.; Li, Q.; Uitto, J. ABCC6 does not transport adenosine — Relevance to pathomechanism of pseudoxanthoma elasticum. Mol. Genet. Metab. 2011, 104, 421. [Google Scholar] [CrossRef] [PubMed]
- Nitschke, Y.; Rutsch, F. Generalized arterial calcification of infancy and pseudoxanthoma elasticum: two sides of the same coin. Front. Genet. 2012, 3, 302. [Google Scholar] [CrossRef]
- Pomozi, V.; Julian, C.B.; Zoll, J.; Pham, K.; Kuo, S.; Tőkési, N.; Martin, L.; Váradi, A.; Le Saux, O. Dietary Pyrophosphate Modulates Calcification in a Mouse Model of Pseudoxanthoma Elasticum: Implication for Treatment of Patients. J. Investig. Dermatol. 2018, 139, 1082–1088. [Google Scholar] [CrossRef]
- Cheng, Z.; O'Brien, K.; Howe, J.; Sullivan, C.; Schrier, D.; Lynch, A.; Jungles, S.; Sabbagh, Y.; Thompson, D. INZ-701 Prevents Ectopic Tissue Calcification and Restores Bone Architecture and Growth in ENPP1-Deficient Mice. J. Bone Miner. Res. 2020, 36, 1594–1604. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, I.J.; Cheng, Z.; Ralph, D.; O'Brien, K.; Flaman, L.; Howe, J.; Thompson, D.; Uitto, J.; Li, Q.; Sabbagh, Y. INZ-701, a recombinant ENPP1 enzyme, prevents ectopic calcification in an Abcc6(-/-) mouse model of pseudoxanthoma elasticum. Exp Dermatol 2022. [Google Scholar] [CrossRef] [PubMed]
- Roman, R.M.; Wang, Y.; Lidofsky, S.D.; Feranchak, A.P.; Lomri, N.; Scharschmidt, B.F.; Fitz, J.G. Hepatocellular ATP-binding Cassette Protein Expression Enhances ATP Release and Autocrine Regulation of Cell Volume. J. Biol. Chem. 1997, 272, 21970–21976. [Google Scholar] [CrossRef]
- Reisin, I.; Prat, A.; Abraham, E.; Amara, J.; Gregory, R.; Ausiello, D.; Cantiello, H. The cystic fibrosis transmembrane conductance regulator is a dual ATP and chloride channel. J. Biol. Chem. 1994, 269, 20584–20591. [Google Scholar] [CrossRef] [PubMed]
- Sabirov, R.Z.; Okada, Y. ATP release via anion channels. Purinergic Signal 2005, 1, 311–28. [Google Scholar] [CrossRef] [PubMed]
- Tu, J.; Le, G.; Ballard, H.J. Involvement of the cystic fibrosis transmembrane conductance regulator in the acidosis-induced efflux of ATP from rat skeletal muscle. J. Physiol. 2010, 588, 4563–4578. [Google Scholar] [CrossRef]
- Praetorius, H.A.; Leipziger, J. ATP release from non-excitable cells. Purinergic Signal. 2009, 5, 433–446. [Google Scholar] [CrossRef] [PubMed]
- Ho, A.M.; Johnson, M.D.; Kingsley, D.M. Role of the Mouse ank Gene in Control of Tissue Calcification and Arthritis. Science 2000, 289, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Nürnberg, P.; Thiele, H.; Chandler, D.; Höhne, W.; Cunningham, M.L.; Ritter, H.; Leschik, G.; Uhlmann, K.; Mischung, C.; Harrop, K.; et al. Heterozygous mutations in ANKH, the human ortholog of the mouse progressive ankylosis gene, result in craniometaphyseal dysplasia. Nat. Genet. 2001, 28, 37–41. [Google Scholar] [CrossRef]
- Reichenberger, E.; Tiziani, V.; Watanabe, S.; Park, L.; Ueki, Y.; Santanna, C.; Baur, S.T.; Shiang, R.; Grange, D.K.; Beighton, P.; et al. Autosomal Dominant Craniometaphyseal Dysplasia Is Caused by Mutations in the Transmembrane Protein ANK. Am. J. Hum. Genet. 2001, 68, 1321–1326. [Google Scholar] [CrossRef]
- Szeri, F.; Lundkvist, S.; Donnelly, S.; Engelke, U.F.H.; Rhee, K.; Williams, C.J.; Sundberg, J.P.; Wevers, R.A.; Tomlinson, R.E.; Jansen, R.S.; et al. The membrane protein ANKH is crucial for bone mechanical performance by mediating cellular export of citrate and ATP. PLOS Genet. 2020, 16, e1008884. [Google Scholar] [CrossRef] [PubMed]
- Szeri, F.; Niaziorimi, F.; Donnelly, S.; Fariha, N.; Tertyshnaia, M.; Patel, D.; Lundkvist, S.; van de Wetering, K. The Mineralization Regulator ANKH Mediates Cellular Efflux of ATP, Not Pyrophosphate. J. Bone Miner. Res. 2022, 37, 1024–1031. [Google Scholar] [CrossRef] [PubMed]
- Le Saux, O.; Martin, L.; Aherrahrou, Z.; Leftheriotis, G.; Váradi, A.; Brampton, C.N. The molecular and physiological roles of ABCC6: more than meets the eye. Front. Genet. 2012, 3, 289. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Kingman, J.; van de Wetering, K.; Tannouri, S.; Sundberg, J.P.; Uitto, J. Abcc6 Knockout Rat Model Highlights the Role of Liver in PPi Homeostasis in Pseudoxanthoma Elasticum. J. Investig. Dermatol. 2017, 137, 1025–1032. [Google Scholar] [CrossRef]
- Van Gils, M.; Depauw, J.; Coucke, P.J.; Aerts, S.; Verschuere, S.; Nollet, L.; Vanakker, O.M. Inorganic Pyrophosphate Plasma Levels Are Decreased in Pseudoxanthoma Elasticum Patients and Heterozygous Carriers but Do Not Correlate with the Genotype or Phenotype. J. Clin. Med. 2023, 12, 1893. [Google Scholar] [CrossRef] [PubMed]
- Boraldi, F.; Bartolomeo, A.; Li, Q.; Uitto, J.; Quaglino, D. Changes in dermal fibroblasts from Abcc6(-/-) mice are present before and after the onset of ectopic tissue mineralization. J Investig. Dermatol 2014, 134, 1855–1861. [Google Scholar]
- Dabisch-Ruthe, M.; Kuzaj, P.; Götting, C.; Knabbe, C.; Hendig, D. Pyrophosphates as a major inhibitor of matrix calcification in Pseudoxanthoma elasticum. J. Dermatol. Sci. 2014, 75, 109–120. [Google Scholar] [CrossRef]
- Boraldi, F.; Costa, S.; Rabacchi, C.; Ciani, M.; Vanakker, O.; Quaglino, D. Can APOE and MTHFR polymorphisms have an influence on the severity of cardiovascular manifestations in Italian Pseudoxanthoma elasticum affected patients? Mol. Genet. Metab. Rep. 2014, 1, 477–482. [Google Scholar] [CrossRef] [PubMed]
- De Vilder, E.Y.G.; Hosen, M.J.; Martin, L.; De Zaeytijd, J.; Leroy, B.P.; Ebran, J.M.; Coucke, P.J.; De Paepe, A.; Vanakker, O.M. VEGFA variants as prognostic markers for the retinopathy in pseudoxanthoma elasticum. Clin Genet 2020, 98, 74–79. [Google Scholar] [CrossRef]
- Hendig, D.; Knabbe, C.; Götting, C. New insights into the pathogenesis of pseudoxanthoma elasticum and related soft tissue calcification disorders by identifying genetic interactions and modifiers. Front. Genet. 2013, 4, 114. [Google Scholar] [CrossRef]
- Hosen, M.J.; Van Nieuwerburgh, F.; Steyaert, W.; Deforce, D.; Martin, L.; Leftheriotis, G.; De Paepe, A.; Coucke, P.J.; Vanakker, O.M. Efficiency of Exome Sequencing for the Molecular Diagnosis of Pseudoxanthoma Elasticum. J. Investig. Dermatol. 2015, 135, 992–998. [Google Scholar] [CrossRef]
- Luo, H.; Faghankhani, M.; Cao, Y.; Uitto, J.; Li, Q. Molecular Genetics and Modifier Genes in Pseudoxanthoma Elasticum, a Heritable Multisystem Ectopic Mineralization Disorder. J. Investig. Dermatol. 2020, 141, 1148–1156. [Google Scholar] [CrossRef]
- Vanakker, O.M.; Hosen, M.J.; De Paepe, A. The ABCC6 transporter: what lessons can be learnt from other ATP-binding cassette transporters? Front. Genet. 2013, 4, 203. [Google Scholar] [CrossRef] [PubMed]
- Moorhead, W.J.; Chu, C.C.; Cuevas, R.A.; Callahan, J.T.; Wong, R.; Regan, C.; Boufford, C.K.; Sur, S.; Liu, M.; Gomez, D.; MacTaggart, J.N.; Kamenskiy, A.; Boehm, M.; Hilaire, C.S. Dysregulation of FOXO1 (Forkhead Box O1 Protein) Drives Calcification in Arterial Calcification due to Deficiency of CD73 and Is Present in Peripheral Artery Disease. Arterioscler Thromb Vasc Biol 2020, 40, 1680–1694. [Google Scholar] [CrossRef] [PubMed]
- Snider, N.T.; Griggs, N.W.; Singla, A.; Moons, D.S.; Weerasinghe, S.V.; Lok, A.S.; Ruan, C.; Burant, C.F.; Conjeevaram, H.S.; Omary, M.B. CD73 (ecto-5′-nucleotidase) hepatocyte levels differ across mouse strains and contribute to mallory-denk body formation. J. Hepatol. 2013, 58, 1790–1800. [Google Scholar] [CrossRef] [PubMed]
- Pomozi, V.; Brampton, C.; van de Wetering, K.; Zoll, J.; Calio, B.; Pham, K.; Owens, J.B.; Marh, J.; Moisyadi, S.; Váradi, A.; et al. Pyrophosphate Supplementation Prevents Chronic and Acute Calcification in ABCC6-Deficient Mice. Am. J. Pathol. 2017, 187, 1258–1272. [Google Scholar] [CrossRef] [PubMed]
- Martinelli, F.; Cuviello, F.; Pace, M.C.; Armentano, M.F.; Miglionico, R.; Ostuni, A.; Bisaccia, F. Extracellular ATP Regulates CD73 and ABCC6 Expression in HepG2 Cells. Front. Mol. Biosci. 2018, 5, 75. [Google Scholar] [CrossRef]
- Hart, M.L.; Much, C.; Gorzolla, I.C.; Schittenhelm, J.; Kloor, D.; Stahl, G.L.; Eltzschig, H.K. Extracellular Adenosine Production by Ecto-5′-Nucleotidase Protects During Murine Hepatic Ischemic Preconditioning. Gastroenterology 2008, 135, 1739–1750. [Google Scholar] [CrossRef]
- Burnstock, G.; Knight, G.E.; Greig AV, H. Purinergic Signaling in Healthy and Diseased Skin. J. Investig. Dermatol. 2012, 132, 526–546. [Google Scholar] [CrossRef]
- Burnstock, G.; Pelleg, A. Cardiac purinergic signalling in health and disease. Purinergic Signal. 2014, 11, 1–46. [Google Scholar] [CrossRef]
- Burnstock, G.; Vaughn, B.; Robson, S.C. Purinergic signalling in the liver in health and disease. Purinergic Signal. 2013, 10, 51–70. [Google Scholar] [CrossRef]
- Jain, S.; Jacobson, K.A. Purinergic signaling in diabetes and metabolism. Biochem. Pharmacol. 2020, 187, 114393–114393. [Google Scholar] [CrossRef]
- Borea, P.A.; Gessi, S.; Merighi, S.; Varani, K. Adenosine as a Multi-Signalling Guardian Angel in Human Diseases: When, Where and How Does it Exert its Protective Effects? Trends Pharmacol. Sci. 2016, 37, 419–434. [Google Scholar] [CrossRef] [PubMed]
- Linden, J. Adenosine in Tissue Protection and Tissue Regeneration: TABLE 1. Mol. Pharmacol. 2005, 67, 1385–1387. [Google Scholar] [CrossRef] [PubMed]
- Burnstock, G. Purinergic Signaling in the Cardiovascular System. Circ. Res. 2017, 120, 207–228. [Google Scholar] [CrossRef]
- Burnstock, G.; Knight, G.E. Cellular distribution and functions of P2 receptor subtypes in different systems. Int Rev Cytol 2004, 240, 31–304. [Google Scholar]
- Idzko, M.; Ferrari, D.; Riegel, A.-K.; Eltzschig, H.K. Extracellular nucleotide and nucleoside signaling in vascular and blood disease. Blood 2014, 124, 1029–1037. [Google Scholar] [CrossRef] [PubMed]
- Eltzschig, H.K.; Sitkovsky, M.V.; Robson, S.C. Purinergic Signaling during Inflammation. New Engl. J. Med. 2012, 367, 2322–2333. [Google Scholar] [CrossRef]
- Agrawal, A.; Jørgensen, N.R. Extracellular purines and bone homeostasis. Biochem. Pharmacol. 2021, 187, 114425. [Google Scholar] [CrossRef]
- Eltzschig, H.K.; Sitkovsky, M.V.; Robson, S.C. Purinergic Signaling during Inflammation. New Engl. J. Med. 2013, 368, 1260–1260. [Google Scholar] [CrossRef]
- Hechler, B.; Gachet, C. Purinergic Receptors in Thrombosis and Inflammation. Arter. Thromb. Vasc. Biol. 2015, 35, 2307–2315. [Google Scholar] [CrossRef] [PubMed]
- Linden, J.; Koch-Nolte, F.; Dahl, G. Purine Release, Metabolism, and Signaling in the Inflammatory Response. Annu Rev Immunol 2019, 37, 325–347. [Google Scholar] [CrossRef]
- Zuccarini, M.; Giuliani, P.; Caciagli, F.; Ciccarelli, R.; Di Iorio, P. In Search of a Role for Extracellular Purine Enzymes in Bone Function. Biomolecules 2021, 11, 679. [Google Scholar] [CrossRef] [PubMed]
- Idzko, M.; Ferrari, D.; Eltzschig, H.K. Nucleotide signalling during inflammation. Nature 2014, 509, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Henaut, L.; Sanchez-Nino, M.D.; Castillo, G.A.-E.; Sanz, A.B.; Ortiz, A. Targeting local vascular and systemic consequences of inflammation on vascular and cardiac valve calcification. Expert Opin. Ther. Targets 2015, 20, 89–105. [Google Scholar] [CrossRef]
- Lee, S.J.; Lee, I.-K.; Jeon, J.-H. Vascular Calcification—New Insights into Its Mechanism. Int. J. Mol. Sci. 2020, 21, 2685. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Sun, Z.; Zhang, L.; Yan, J.; Shao, C.; Jing, L.; Li, L.; Wang, Z. Role of Macrophages in the Progression and Regression of Vascular Calcification. Front. Pharmacol. 2020, 11, 661. [Google Scholar] [CrossRef] [PubMed]
- Villa-Bellosta, R.; Hamczyk, M.R.; Andrés, V. Alternatively activated macrophages exhibit an anticalcifying activity dependent on extracellular ATP/pyrophosphate metabolism. Am. J. Physiol. Physiol. 2016, 310, C788–C799. [Google Scholar] [CrossRef]
- Mention, P.-J.; Lacoeuille, F.; Leftheriotis, G.; Martin, L.; Omarjee, L. 18F-Flurodeoxyglucose and 18F-Sodium Fluoride Positron Emission Tomography/Computed Tomography Imaging of Arterial and Cutaneous Alterations in Pseudoxanthoma Elasticum. Circ. Cardiovasc. Imaging 2018, 11, e007060. [Google Scholar] [CrossRef]
- Omarjee, L.; Mention, P.J.; Janin, A.; Kauffenstein, G.; Pabic, E.L.; Meilhac, O.; Blanchard, S.; Navasiolava, N.; Leftheriotis, G.; Couturier, O.; Jeannin, P.; Lacoeuille, F.; Martin, L. Assessment of Inflammation and Calcification in Pseudoxanthoma Elasticum Arteries and Skin with 18F-FluroDeoxyGlucose and 18F-Sodium Fluoride Positron Emission Tomography/Computed Tomography Imaging: The GOCAPXE Trial. J Clin Med 2020, 9. [Google Scholar] [CrossRef]
- Peng, S.; Sun, X.; Wang, X.; Wang, H.; Shan, Z.; Teng, W.; Li, C. Myeloid related proteins are up-regulated in autoimmune thyroid diseases and activate toll-like receptor 4 and pro-inflammatory cytokines in vitro. Int. Immunopharmacol. 2018, 59, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Bowen, A.R.; Götting, C.; LeBoit, P.E.; McCalmont, T.H. Pseudoxanthoma elasticum-like fibers in the inflamed skin of patients without pseudoxanthoma elasticum. J. Cutan. Pathol. 2007, 34, 777–781. [Google Scholar] [CrossRef]
- Rabin, J.; Zhao, Y.; Mostafa, E.; Al-Suqi, M.; Fleischmann, E.; Conaway, M.R.; Mann, B.J.; Chhabra, P.; Brayman, K.L.; Krupnick, A.; et al. Regadenoson for the treatment of COVID-19: A five case clinical series and mouse studies. PLOS ONE 2023, 18, e0288920. [Google Scholar] [CrossRef] [PubMed]
- Casemayou, A.; Belliere, J.; Letavernier, E.; Colliou, E.; El Hachem, H.; Zarowski, J.; Bazin, D.; Kounde, C.; Piedrafita, A.; Feuillet, G.; et al. Abcc6 deficiency prevents rhabdomyolysis-induced acute kidney injury. Sci. Rep. 2023, 13, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Kauffenstein, G.; Drouin, A.; Thorin-Trescases, N.; Bachelard, H.; Robaye, B.; D'Orléans-Juste, P.; Marceau, F.; Thorin. ; Sévigny, J. NTPDase1 (CD39) controls nucleotide-dependent vasoconstriction in mouse. Cardiovasc. Res. 2009, 85, 204–213. [Google Scholar] [CrossRef] [PubMed]
- Nitschke, Y.; Rutsch, F. Genetics in Arterial Calcification: Lessons Learned From Rare Diseases. Trends Cardiovasc. Med. 2012, 22, 145–149. [Google Scholar] [CrossRef] [PubMed]
- Tyson, K.L.; Reynolds, J.L.; McNair, R.; Zhang, Q.; Weissberg, P.L.; Shanahan, C.M. Osteo/Chondrocytic Transcription Factors and Their Target Genes Exhibit Distinct Patterns of Expression in Human Arterial Calcification. Arter. Thromb. Vasc. Biol. 2003, 23, 489–494. [Google Scholar] [CrossRef] [PubMed]
- Towler, D.A. Inorganic pyrophosphate: a paracrine regulator of vascular calcification and smooth muscle phenotype. Arterioscler Thromb Vasc Biol 2005, 25, 651–4. [Google Scholar] [CrossRef] [PubMed]
- Prosdocimo, D.A.; Douglas, D.C.; Romani, A.M.; O'Neill, W.C.; Dubyak, G.R.; Villa-Bellosta, R.; Hamczyk, M.R.; Andrés, V.; Trueblood, K.E.; Mohr, S.; et al. Autocrine ATP release coupled to extracellular pyrophosphate accumulation in vascular smooth muscle cells. Am. J. Physiol. Physiol. 2009, 296, C828–C839. [Google Scholar] [CrossRef]
- Beck, K.; Dang, K.; Boyd, C.D. The tissue distribution of murine Abcc6 (Mrp6) during embryogenesis indicates that the presence of Abcc6 in elastic tissues is not required for elastic fiber assembly. Histochem. J. 2005, 36, 167–170. [Google Scholar] [CrossRef]
- Villa-Bellosta, R.; O’neill, W.C. Pyrophosphate deficiency in vascular calcification. Kidney Int. 2018, 93, 1293–1297. [Google Scholar] [CrossRef] [PubMed]
- Buchet, R.; Tribes, C.; Rouaix, V.; Doumeche, B.; Fiore, M.; Wu, Y.; Magne, D.; Mebarek, S. Hydrolysis of Extracellular ATP by Vascular Smooth Muscle Cells Transdifferentiated into Chondrocytes Generates P(i) but Not PP(i). Int J Mol Sci 2021, 22. [Google Scholar] [CrossRef] [PubMed]
- Pingel, S.; Pausewang, K.S.; Passon, S.G.; Blatzheim, A.K.; Gliem, M.; Issa, P.C.; Hendig, D.; Horlbeck, F.; Tuleta, I.; Nickenig, G.; et al. Increased vascular occlusion in patients with pseudoxanthoma elasticum. Vasa 2017, 46, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Rau, C.D.; Wang, J.; Avetisyan, R.; Romay, M.C.; Martin, L.; Ren, S.; Wang, Y.; Lusis, A.J. Mapping genetic contributions to cardiac pathology induced by Beta-adrenergic stimulation in mice. Circ. Cardiovasc. Genet. 2015, 8, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Rumney, R.M.H.; Róg, J.; Chira, N.; Kao, A.P.; Al-Khalidi, R.; Górecki, D.C. P2X7 Purinoceptor Affects Ectopic Calcification of Dystrophic Muscles. Front. Pharmacol. 2022, 13, 935804. [Google Scholar] [CrossRef]
- Pelegrin, P.; Surprenant, A. Dynamics of macrophage polarization reveal new mechanism to inhibit IL-1beta release through pyrophosphates. EMBO J 2009, 28, 2114–27. [Google Scholar] [CrossRef]
- Sassi, Y.; Ahles, A.; Truong, D.-J.J.; Baqi, Y.; Lee, S.-Y.; Husse, B.; Hulot, J.-S.; Foinquinos, A.; Thum, T.; Müller, C.E.; et al. Cardiac myocyte–secreted cAMP exerts paracrine action via adenosine receptor activation. J. Clin. Investig. 2014, 124, 5385–5397. [Google Scholar] [CrossRef] [PubMed]
- Bonner, F.; Borg, N.; Jacoby, C.; Temme, S.; Ding, Z.; Flogel, U.; Schrader, J. Ecto-5'-nucleotidase on immune cells protects from adverse cardiac remodeling. Circ Res 2013, 113, 301–12. [Google Scholar] [CrossRef]
- Borg, N.; Alter, C.; Görldt, N.; Jacoby, C.; Ding, Z.; Steckel, B.; Quast, C.; Bönner, F.; Friebe, D.; Temme, S.; et al. CD73 on T Cells Orchestrates Cardiac Wound Healing After Myocardial Infarction by Purinergic Metabolic Reprogramming. Circ. 2017, 136, 297–313. [Google Scholar] [CrossRef]
- Rostand, S.G.; Sanders, C.; Kirk, K.A.; Rutsky, E.A.; Fraser, R.G. Myocardial calcification and cardiac dysfunction in chronic renal failure. Am J Med 1988, 85, 651–7. [Google Scholar] [CrossRef]
- Shackley, B.S.; Nguyen, T.P.; Shivkumar, K.; Finn, P.J.; Fishbein, M.C. Idiopathic massive myocardial calcification: a case report and review of the literature. Cardiovasc. Pathol. 2011, 20, e79–e83. [Google Scholar] [CrossRef] [PubMed]
- A Bridge, J.; McManus, B.M.; Remmenga, J.; Cuppage, F.P. Complete heart block in the 18p--syndrome. Congenital calcification of the atrioventricular node. Arch Pathol Lab Med 1989, 113, 539–541. [Google Scholar] [PubMed]
- Henderson, R.R.; Santiago, L.M.; Spring, D.A.; Harrington, A.R. Metastatic Myocardial Calcification in Chronic Renal Failure Presenting as Atrioventricular Block. New Engl. J. Med. 1971, 284, 1252–1253. [Google Scholar] [CrossRef] [PubMed]
- Pillai, I.C.; Li, S.; Romay, M.; Lam, L.; Lu, Y.; Huang, J.; Dillard, N.; Zemanova, M.; Rubbi, L.; Wang, Y.; et al. Cardiac Fibroblasts Adopt Osteogenic Fates and Can Be Targeted to Attenuate Pathological Heart Calcification. Cell Stem Cell 2016, 20, 218–232. [Google Scholar] [CrossRef] [PubMed]
- Bertulezzi, G.; Paris, R.; Moroni, M.; Porta, C.; Nastasi, G.; Amadeo, A. Atrial septal aneurysm in a patient with pseudoxanthoma elasticum. Acta Cardiol 1998, 53, 223–225. [Google Scholar] [PubMed]
- Fang, M.L.; Astarita, R.W.; Steinman, H.K. Cardiac Calcifications and Yellow Papules in a Young Man. Arch. Dermatol. 1988, 124, 1563–1564. [Google Scholar] [CrossRef]
- Farmakis, D.; Vesleme, V.; Papadogianni, A.; Tsaftaridis, P.; Kapralos, P.; Aessopos, A. Aneurysmatic dilatation of ascending aorta in a patient with beta-thalassemia and a pseudoxanthoma elasticum-like syndrome. Ann Hematol 2004, 83, 596–9. [Google Scholar] [CrossRef] [PubMed]
- Giovannoni, I.; Callea, F.; Travaglini, L.; Amodeo, A.; Cogo, P.; Secinaro, A.; Bizzarri, C.; Cutrera, R.; El Hachem, M.; Francalanci, P. Heart transplant and 2-year follow up in a child with generalized arterial calcification of infancy. Eur. J. Pediatr. 2014, 173, 1735–1740. [Google Scholar] [CrossRef]
- Nolte, K. Sudden cardiac death owing to pseudoxanthoma elasticum: A case report. Hum. Pathol. 2000, 31, 1002–1004. [Google Scholar] [CrossRef]
- Przybojewski, J.Z.; Maritz, F.; A Tiedt, F.; Van Der Walt, J.J. Pseudoxanthoma elasticum with cardiac involvement. A case report and review of the literature. S Afr Med J. 1981, 59, 268–275. [Google Scholar]
- El-Brolosy, M.A.; Stainier, D.Y.R. Genetic compensation: A phenomenon in search of mechanisms. PLoS Genet. 2017, 13, e1006780. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Uitto, J. The mineralization phenotype in Abcc6 ( -/- ) mice is affected by Ggcx gene deficiency and genetic background--a model for pseudoxanthoma elasticum. J Mol Med 2010, 88, 173–81. [Google Scholar] [CrossRef] [PubMed]
- Fleisch, H.; Russell, R.G.G.; Straumann, F. Effect of Pyrophosphate on Hydroxyapatite and Its Implications in Calcium Homeostasis. Nature 1966, 212, 901–903. [Google Scholar] [CrossRef] [PubMed]
- Heinonen, J.K. Biological Role of Inorganic Pyrophosphate; Springer Science and Business Media LLC: Dordrecht, GX, Netherlands, 2001; ISBN 9781461355519. [Google Scholar]
- Hsu, V.M.; Kozák, E.; Li, Q.; Bocskai, M.; Schlesinger, N.; Rosenthal, A.; McClure, S.T.; Kovács, L.; Bálint, L.; Szamosi, S.; et al. Inorganic pyrophosphate is reduced in patients with systemic sclerosis. Rheumatology 2021, 61, 1158–1165. [Google Scholar] [CrossRef] [PubMed]
- O'Neill, W.C.; Lomashvili, K.A.; Malluche, H.H.; Faugere, M.-C.; Riser, B.L. Treatment with pyrophosphate inhibits uremic vascular calcification. Kidney Int. 2011, 79, 512–517. [Google Scholar] [CrossRef] [PubMed]
- Orriss, I.R.; Arnett, T.R.; Russell, R.G.G. Pyrophosphate: a key inhibitor of mineralisation. Curr. Opin. Pharmacol. 2016, 28, 57–68. [Google Scholar] [CrossRef]
- Väärämäki, S.; Pelttari, S.; Uusitalo, H.; Tökési, N.; Váradi, A.; Nevalainen, P.I. Pyrophosphate Treatment in Pseudoxanthoma Elasticum (PXE)-Preventing ReOcclusion After Surgery for Critical Limb Ischaemia. Surgical Case Reports 2019, 2, 1–3. [Google Scholar] [CrossRef]
- Ralph, D.; Nitschke, Y.; Levine, M.A.; Caffet, M.; Wurst, T.; Saeidian, A.H.; Youssefian, L.; Vahidnezhad, H.; Terry, S.F.; Rutsch, F.; et al. ENPP1 variants in patients with GACI and PXE expand the clinical and genetic heterogeneity of heritable disorders of ectopic calcification. PLOS Genet. 2022, 18, e1010192. [Google Scholar] [CrossRef]
- Laurain, A.; Rubera, I.; Razzouk-Cadet, M.; Bonnafous, S.; Albuquerque, M.; Paradis, V.; Patouraux, S.; Duranton, C.; Lesaux, O.; Lefthériotis, G.; et al. Arterial Calcifications in Patients with Liver Cirrhosis Are Linked to Hepatic Deficiency of Pyrophosphate Production Restored by Liver Transplantation. Biomedicines 2022, 10, 1496. [Google Scholar] [CrossRef]
- Letavernier, E.; Bouderlique, E.; Zaworski, J.; Martin, L.; Daudon, M. Pseudoxanthoma Elasticum, Kidney Stones and Pyrophosphate: From a Rare Disease to Urolithiasis and Vascular Calcifications. Int. J. Mol. Sci. 2019, 20, 6353. [Google Scholar] [CrossRef]
- Letavernier, E.; Kauffenstein, G.; Huguet, L.; Navasiolava, N.; Bouderlique, E.; Tang, E.; Delaitre, L.; Bazin, D.; de Frutos, M.; Gay, C.; et al. ABCC6 Deficiency Promotes Development of Randall Plaque. J. Am. Soc. Nephrol. 2018, 29, 2337–2347. [Google Scholar] [CrossRef] [PubMed]
- Addison, W.N.; Azari, F.; Sørensen, E.S.; Kaartinen, M.T.; McKee, M.D. Pyrophosphate Inhibits Mineralization of Osteoblast Cultures by Binding to Mineral, Up-regulating Osteopontin, and Inhibiting Alkaline Phosphatase Activity. J. Biol. Chem. 2007, 282, 15872–15883. [Google Scholar] [CrossRef] [PubMed]
- Plomp, A.S.; Bergen, A.A.; Florijn, R.J.; Terry, S.F.; Toonstra, J.; van Dijk, M.R.; de Jong, P.T. Pseudoxanthoma elasticum: Wide phenotypic variation in homozygotes and no signs in heterozygotes for the c.3775delT mutation in ABCC6. Anesthesia Analg. 2009, 11, 852–858. [Google Scholar] [CrossRef] [PubMed]
- Uitto, J.; Jiang, Q.; Váradi, A.; Bercovitch, L.G.; Terry, S.F. Pseudoxanthoma elasticum: diagnostic features, classification and treatment options. Expert Opin. Orphan Drugs 2014, 2, 567–577. [Google Scholar] [CrossRef]
- Lau, W.L.; Liu, S.; Vaziri, N.D. Chronic Kidney Disease Results in Deficiency of ABCC6, the Novel Inhibitor of Vascular Calcification. Am. J. Nephrol. 2014, 40, 51–55. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





