1. Introduction
The determination of the optimal breeding time in bitches frequently relies on assessment of serum progesterone (sP4) concentrations [
1,
2,
3]. This assessment serves multiple purposes, including the detection of reproductive abnormalities such as hypoluteoidism [
4,
5], the confirmation of luteolysis before parturition [
6,
7]. Throughout the estrus period in bitches, a characteristic rise in sP4 concentrations beyond the baseline is observed, frequently surpassing 1 ng/mL. Ovulation in the bitch take place roughly 36 to 50 hours following the LH peak [
8]. During this time, sP4 concentrations are approximately 2.02±0.18 ng/mL at the LH peak [
9], and they subsequently escalate to a range of 4.00 to 10.00 ng/mL on the ovulation day [
10]. Subsequent to ovulation in bitches, it is noteworthy that oocytes initially persist in an immature state recognized as primary oocytes. Fertilizable oocytes become evident 2 to 3 days after ovulation, as reported by Chastant-Maillard et al. [
11]. The complete maturation into the fertilization-ready stage occurred at 109 hours post-ovulation, in accordance with Reynaud et al. [
12]. Furthermore, existing research implies a maturation window of 96 to 108 hours for oocytes within the oviduct [
13]. Collectively, these findings underscore the paramount importance of precise ovulation detection to facilitate informed deliberations concerning mating or artificial insemination [
3].
Within the domain of veterinary practice, diverse techniques exist for quantifying sP4, encompassing radioimmunoassay (RIA) [
14,
15] liquid chromatography-tandem mass spectrometry (LC-MS) [
16,
17,
18], and chemiluminescence immunoassay (CLIA) [
15,
19,
20,
21]. Furthermore, recent progress encompasses the introduction of point-of-care sP4 measurement analyzers, exemplified by the rapid fluorescence immunochromatography assay (RFICA) and surface plasmon field-enhanced fluorescence spectroscopy (SPFS) [
22,
23,
24,
25].
However, it is crucial to acknowledge that outcomes of sP4 measurement frequently exhibit variations due to differences in laboratory methodologies and individual characteristics of bitches. Consequently, achieving precise determination of the optimal breeding time mandates the collection of numerous sequential blood samples throughout the proestrus and estrous phases. These samples function as a comparative benchmark against the gold standard or reference laboratory methodologies. With due regard to these factors, the present investigation endeavors to juxtapose sP4 outcomes obtained from a commercial POC analyzers specifically developed for expeditious sP4 assessment in bitches with those derived from chemiluminescent microparticle immunoassay (CMIA). The primary aim is to ascertain the precision and establish a standardized guideline for ascertaining the optimal breeding time.
2. Materials and Methods
Ethical approval
The guidelines concerning the appropriate care and utilization of animals received approval from the Animal Research Ethics Committee of the Faculty of Veterinary Medicine at Mahanakorn University of Technology, Thailand. To substantiate their validation, these guidelines have been identified with the specific approval code ACUC-MUT-2020/006. In accordance with the established ethical framework, the proprietors of the bitches demonstrated their concurrence to partake in the research by affixing their signatures to an official document.
Study period and location
Blood samples were procured for the purpose of analysis during the months of August 2022 and July 2023. This collection transpired at the Small Animal Teaching Hospital, Faculty of Veterinary Medicine, Mahanakorn University of Technology, Thailand, and similarly at Vet Home Polyclinic, Bangkok, Thailand.
Sample collection and progesterone measurements
A cohort of two-hundred and forty-seven bitches, encompassing a diverse spectrum of breeds including American bullies, English bulldog, French bulldogs, Shetland sheepdogs, Miniature american shepherds, Cavalier king charles spaniels, Chihuahuas, Pomeranians, Chow Chows, Akitas, and Pugs, was selected for the purpose of routine estrous observation followed by sequential natural mating or artificial insemination. The research endeavor took place concurrently at the Small Animal Teaching Hospital, Faculty of Veterinary Medicine, Mahanakorn University of Technology, Thailand, and Vet Home Polyclinic, Bangkok, Thailand. Blood samples were procured from all the bitches within a time span of 5 to 7 days subsequent to the commencement of vaginal swelling or discharge. The collected blood was allowed to undergo coagulation and was subsequently subjected to centrifugation at a force of 2,500 g for a duration of 15 min. Subsequently, the obtained sera were recovered, and two aliquots were prepared, one aliquot were meticulously prepared. One of these aliquots was promptly subjected to assessment for sP4 concentration through the utilization of CMIA. This process involved the application of an Architect i2000SR Immunoassay Analyzer along with the Architect Progesterone Reagent (Abbott Laboratories, Illinois, USA). The remaining aliquot was conserved at −20°C until its need arose for assessment via a commercial POC analyzer, namely Fuji Dri-Chem Immuno AU Cartridge v-PRG (Fujifilm corporation, Tokyo, Japan), however only 110 samples was evaluated by this POC. This process was conducted in adherence to the guidelines outlined by the manufacturer.
Statistical analysis
Mean, standard deviation (SD), 95% confidence interval (CI), minimum, and maximum value for sP4 concentration were meticulously documented. Quantification was meticulously carried out employing the commercial POC analyzer and CMIA across distinct phases of the bitch’s reproductive cycle, encompassing early-proestrus, LH peak, pre-ovulation, ovulation, and post-ovulation.
In the context of this investigation, the harmonization between values derived from commercial POC analyzer and those procured through the CMIA was meticulously established. This examination was executed by calculating Pearson’s correlation coefficient, Lin’s concordance correlation coefficient, and the bias correction factor. A correlation coefficient of ≤0.35 was interpreted as indicative of a low or weak correlation, while the range of 0.36-0.67 denoted a moderate correlation, 0.68- 0.89 indicated a high correlation, and values exceeding 0.90 signified a very high correlation [
26]. Furthermore, McBride [
27] outlined a classification of agreement strength based on the Lin’s concordance correlation coefficient: >0.99 as almost perfect; 0.95-0.99 as substantial; 0.90-0.96 as moderate; <0.90 as poor. Moreover, comprehensive Passing-Bablok regression and Bland-Altman analyses were executed for commercial POC and CMIA values. Furthermore, graphical representations were generated to vividly portray the acquired outcome. To determine the existence of a substantial distinction between the two means, a paired
t-test was utilized. All the analyses were meticulously performed utilizing free trial version of XLSTAT in Microsoft excel home and student edition (WA, USA). The designated significance level was set at
p< 0.05.
3. Results
The individual sP4 results of commercial POC analyzer comparison with CMIA was presented in
Table 1. The analysis revealed no significant difference (
p>0.05) was observed for the mean value of all samples.
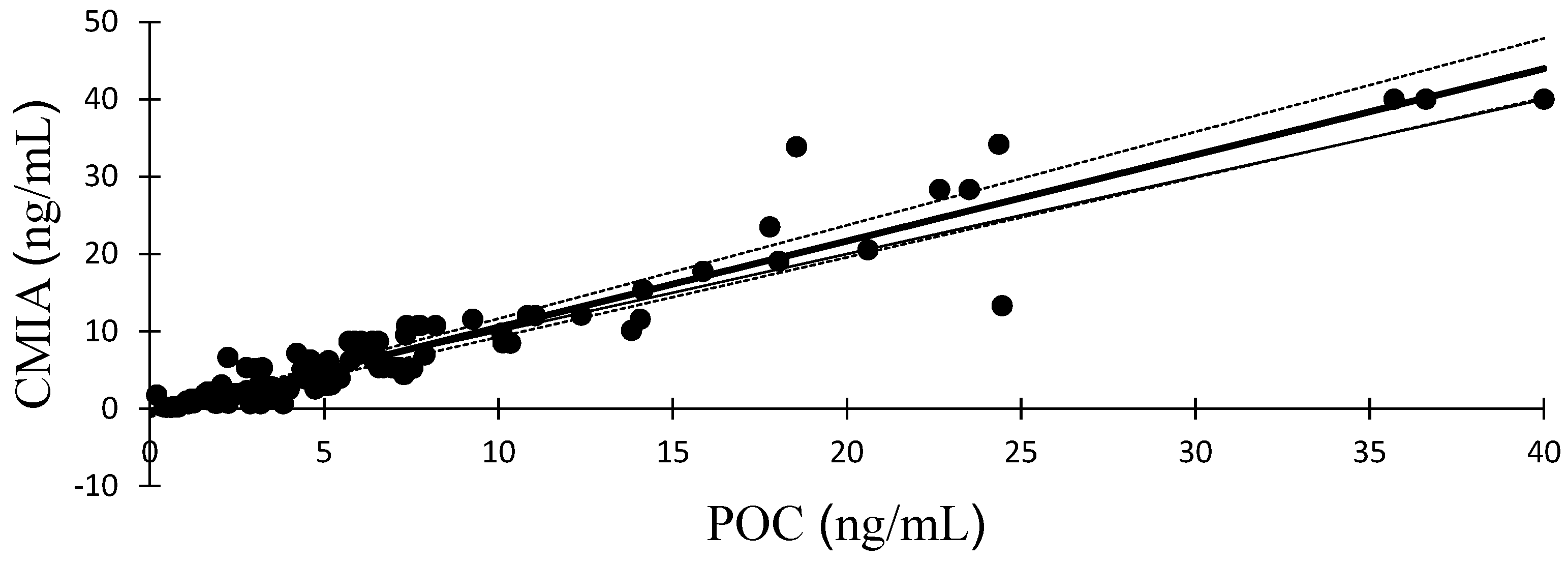
Figure 1 presents a visual representation of the Passing-Bablok regression plot, illustrating a comparison between serum progesterone measurements acquired from POC analyzer and those derived from CMIA. The associated regression equations and correlation coefficient (r) are provided as follows: y = 1.114x–0.597. The analysis of Bland-Altman plots is shown in
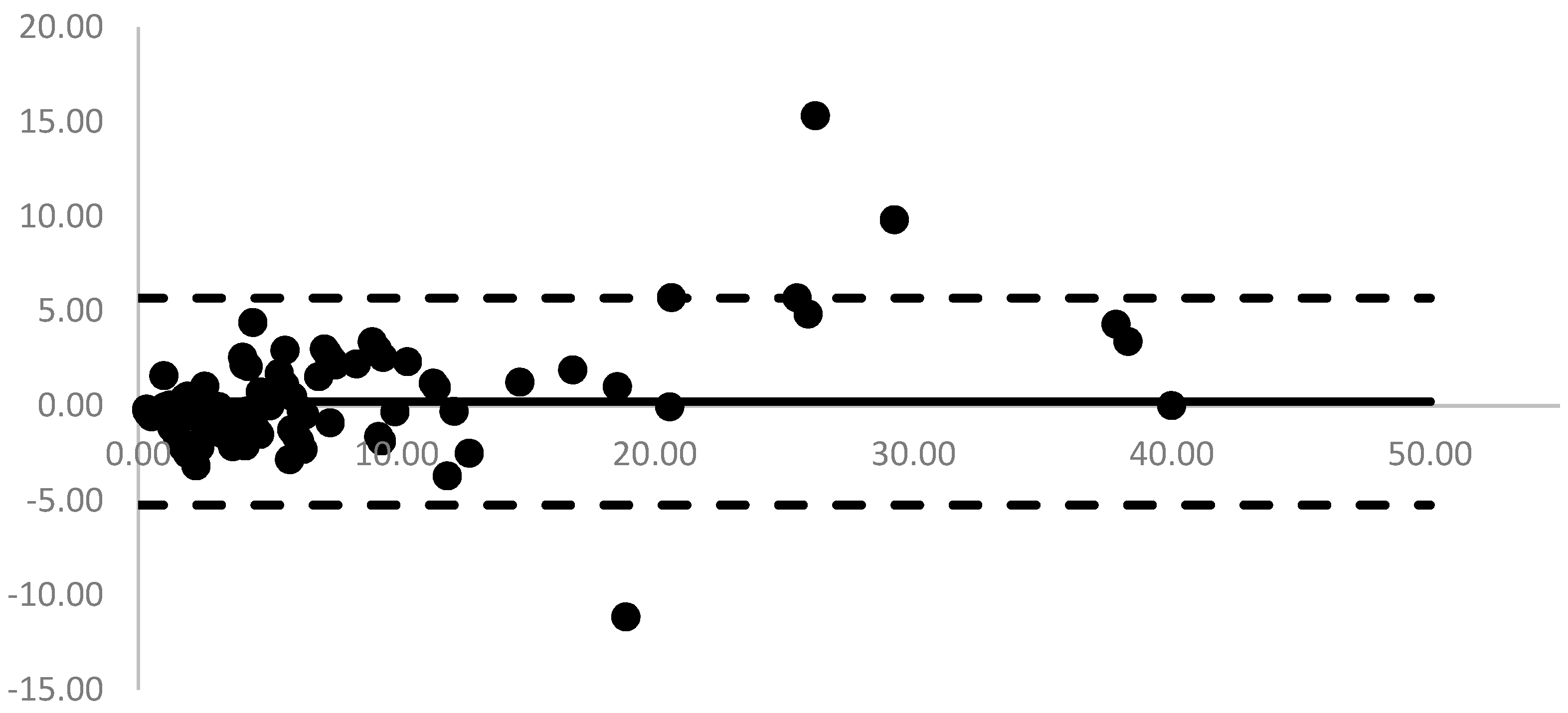
Figure 2. Mean (range) biases between POC analyzer and CMIA were 0.22 ng/mL (-5.24 to 5.69 ng/mL).
The means, SD, 95% CI and range of sP4 concentrations, as determined by CMIA and POC analyzer during the early-proestrus, LH peak, pre-ovulation, ovulation and post-ovulation periods of the bitch, were presented in Table 3.
4. Discussion
Several researchers opt for employing reference standards such as RIA [
15,
28] or LC-MS [
29] for sP4 measurement in bitches. However, the inconvenience associated with RIA stems from the requirement for specialized laboratory equipment, notably radioactive substances, which have the potential to jeopardize both laboratory personnel and the environment. At present, CLIA is displacing RIA in a multitude of diagnostic laboratories, gaining traction among veterinary practitioners as a preferred method for quantifying progesterone [
30,
31]. The CMIA represents a sophisticated and advanced iteration of CLIA, with practical implementation in veterinary reference laboratories throughout Thailand [
22].
The expeditious determination of sP4 concentrations holds pivotal significance in facilitating precise diagnoses and well-informed clinical decisions, especially in contexts such as mating or the meticulous management of cesarean sections. In response to the multifaceted demands of diagnostic accuracy and sound decision-making, veterinary practitioners consider the adoption of commercial POC immunologic analyzer indispensable. This device, commonly known as an in-house progesterone measurement analyzer, caters to these requisites. Present study was conducted an evaluation of the commercial POC analyzer, an automated fluorescence immunoassay analyzer. This innovative technology provides a method for in-hospital sP4 concentration measurements through the utilization of surface plasmon field-enhanced fluorescence spectroscopy. The advanced analyzer distinguished itself by obviating the requirement for washing of surplus fluorescent beads, resulting in a streamlined process. This attribute not only enhances efficiency but also yields a compact immunodiagnostic analyzer capable of providing quantitative results in approximately 10 min. The rapid processing time and likely portable of this analyzer render it a valuable instrument for veterinarians, facilitating the utilization of sP4 concentrations in prompt and effective patient management.
In present study, the correlation analysis between commercial POC analyzer and CMIA revealed Pearsons’ correlation coefficients that exceeded the significant threshold 0.90 (
Table 2). This outcome unequivocally signifies robust and meaningful associations [
26]. This finding provides compelling evidence of the inherent reliability in the measurements of sP4 concentrations acquired from this POC analyzer. However, the assessment of the strength of agreement, conducted through the Lin’s concordance correlation coefficient, exhibited variations across the analyzers as substantial level [
27].
Table 3 was presented the mean, SD, 95% CI and range for serum progesterone concentration with quantification compared between the commercial POC analyzer and CMIA for estimates during the early proestrus, LH peak, pre-ovulation, ovulation and post-ovulation periods of the bitch. This table displays discernible sets of values, denoted by lowercase letters, meticulously organized in the respective rows for POC analyzer. Each of these values, associated with a distinct superscript, demonstrates a statistically significant difference (
p<0.05) when compared to the reference CMIA values. Conversely, the uppercase lettered values, presented similarly with their own set of superscripts, demonstrate no statistically significant difference
(p>0.05). The absence of a significant difference indicates that these results are comparable to CMIA results. Importantly, the mean for all periods in POC analyzer revealed no difference from CMIA. However, the ovulation period of meticulously estrous cycle for POC analyzer showed no significant difference.
The mean, 95%CI and minimum-maximum of sP4 concentrations obtained at the estimated time of ovulation in the present study are of particular significance due to their potential implications in reproductive management. Moreover, considerable variations in sP4 concentrations were observed depending on the factor that could potentially lead to confusion among veterinary professionals. For instance, when a single plasma sample was divided into 7 aliquots and subjected to sP4 concentration assays using various techniques across 7 independent laboratories, resulting mean sP4 concentrations were as follows: 4.6, 3.6, 6.8, 7.2, 3.9, 9.2, and 5.2 ng/mL [
32]. Recent investigations by Bonte et al. [
33], Tahir et al. [
34], Schmicke et al. [
31] underscore the substantial variations in sP4 concentrations during the estimated time of ovulation, contingent upon the assay employed. However, the sP4 concentrations determined using CMIA at the estimated time of ovulation in present study (mean±SD = 7.05±1.41 ng/mL; 95% CI = 6.69 to 7.40 ng/mL; range = 5.07 to 9.78 ng/mL) displayed no statistically significant difference compared to this POC analyzer (mean±SD = 6.14±2.03 ng/mL; 95% CI = 5.37 to 6.92 ng/mL; range = 2.24 to 10.35 ng/mL). Consequently, our findings hold the potential to guide optimal breeding time management, with implications based on the 95% CI and range (minimum-maximum) of sP4 results (
Table 4). Furthermore, this research suggests avenues for further studies, particularly in the realm of investigating specific refinements to sP4 assessment. These refinements aim to bolster veterinary practice and enhance reproductive management by providing more precise and reliable tools. In alignment with this objective, the guidelines presented herein were meticulously crafted. They were developed by aligning with established CMIA guidelines and adapted them using the range and 95% CI derived from each set of results (
Table 4).
Furthermore, this investigation aims to comprehensively assess 63 bitches to determine ovulation day and predict the optimal breeding date. This involved utilizing both CMIA and POC analysis methods during proestrus and estrus periods before breeding, aiming to refine the prediction accuracy. At or near the ovulation phase, these animals received artificial insemination two to four days later, aligning with the determined optimal breeding window. The results revealed a promising outcome, with 55 out of 63 bitches successfully conceiving, resulting in an 87.30% pregnancy rate. Additionally, 52 out of 63 bitches achieved parturition, culminating in an 82.54% parturition rate, indicating the accuracy of the predicted breeding window. These findings underscore the effectiveness of the CMIA and POC analyzers and their pivotal role in enhancing breeding and parturition outcomes. The shorter turnaround time offered by the POC analyzer expedites decision-making in breeding management, benefiting both veterinary practitioners and breeders by facilitating more precise and timely breeding decisions aligned with optimal fertility windows.
5. Conclusions
The utilization of commercial POC immunologic analyzer for progesterone measurement emerges as a valuable clinical tool in the precise determination of the optimal timing for natural mating or artificial insemination in bitches. Moreover, the broader adoption of this advanced technology within the veterinary community and among breeders has the potential to significantly enhance the quality of breeding decisions, thereby contributing to the overall improvement of the dog breeding process.
Author Contributions
Conceptualization: T.S., S.W., S.A., W.P. and S.R.; Methodology: T.S., S.W. and S.R.; Original draft preparation: T.S. and S.R.; Writing and editing: T.S., S.A. and S.R.; Funding acquisition: S.W. and S.R.; Formal; analysis: T.S., S.W. and S.R. All authors have read and agreed to the published version of the manuscript.
Acknowledgements
The present study received partial financial backing Mahanakorn University of Technology (Thailand) and JF Advance Med Co., Ltd. (Thailand) through the allocation of funds provided by the Veterinary Research Grant under Contract No. Immuno-002-2022. Furthermore, the authors extend their sincere appreciation to Bangkok R.I.A. Co., Ltd. (Thailand) for their valuable support in the field of CMIA.
Conflicts of Interest
The authors declare that they have no competing interests.
References
- Gropetti, D.; Aralla, M.; Bronzo, V.; Bosi, G.; Pecile, A.; Arrighi, S. Periovulatory time in the bitch: What’s new to know? Comparison between ovarian histology and clinical features. Anim. Reprod. Sci. 2015, 152, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Rota, A.; Vannozzi, I.; Marianelli, S.; Gavazza, A.; Lubas, G. Laboratory and clinical evaluation of a FEIA method for canine serum progesterone assay. Reprod. Domest. Anim. 2016, 51, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Conley, A.J.; Gonzales, K.; Erb, H.N.; Christensen, B.W. Progesterone analysis in canine breeding management. Vet Clin Am Small Anim Pract. 2023, 53, 931–949. [Google Scholar] [CrossRef] [PubMed]
- Tibold, A.; Thuroczy, J. Progesterone, oestra- diol, FSH and LH concentrations in serum of progesterone-treated pregnant bitches with suspected luteal insufficiency. Reprod. Domest. Anim. 2009, 44, 129–132. [Google Scholar] [CrossRef] [PubMed]
- Johnson, C.A. High-risk pregnancy and hypoluteoidism in the bitch. Theriog. 2008, 70, 1424–1430. [Google Scholar] [CrossRef] [PubMed]
- De Cramer, K.G.M.; Nöthling, J.O. The precision of predicting the time of onset of parturition in the bitch using the level of progesterone in plasma during the preparturient period. Theriog. 2018, 107, 21–218. [Google Scholar] [CrossRef] [PubMed]
- Roos, J.; Maenhoudt, C.; Zilberstein, L.; Mir, F.; Borges, P.; Further, E.; Niewiadomska, Z.; Nudelmann, N.; Fontbonne, A. Neonatal puppy survival after planned caesarean section in the bitch using aglepristone as a primer: a retrospective study on 74 cases. Reprod Domest Anim 2018, 53 (Suppl. S3), 85–95. [Google Scholar] [CrossRef] [PubMed]
- Concannon, P.W. Canine physiology of reproduction. In Small Animal Reproduction and Infertility; Burk TJ, Ed.; Lea & Febiger: Philadelphia USA, 1986; pp. 23–77. [Google Scholar]
- Kutzler, M.A.; Mohammed, H.O.; Lamb, S.V.; Meyers-Wallen, V.N. Accuracy of canine parturition date prediction from the initial rise in preovulatory progesterone concentration. Theriog. 2003, 60, 1187–1196. [Google Scholar] [CrossRef] [PubMed]
- Edens, M.S.D.; Heath, A.M. Chapter 2 – Breeding management in the bitch and queen. In Small Animal Theriogenology; Root Kustritz, M.V., Messonnier, S.P., Eds.; Butterworth-Heinemann: Saint Louis, USA, 2003; p. 33. [Google Scholar]
- Chastant-Maillard, S.; Viaris de Lesegno, C.; Chebrout, M.; Thoumire, S.; Meylheuc, T.; Fontbonne, A.; Chodkiewicz, M.; Saint-Dizier, M.; Reynaud, K. The canine oocyte: uncommon features of in vivo and in vitro maturation. Reprod. Fertil. Dev. 2011, 23, 391–402. [Google Scholar] [CrossRef]
- Reynaud, K.; Saint-Dizier, M.; Tahir, M.Z.; Havard, T.; Harichaux, G.; Labas, V.; Thoumire, S.; Fontbonne, A.; Grimard, B.; Chastant-Maillard, S. Progesterone plays a critical role in canine oocyte maturation and fertilization. Biol. Reprod. 2015, 93, 1–9. [Google Scholar] [CrossRef]
- Tsutsui, T.; Takahashi, F.; Hori, T.; Kawakami, E.; Concannon, P.W. Prolonged duration of fertility of dog ova. Reprod. Domest. Anim. 2009, 44, 230–233. [Google Scholar] [CrossRef]
- Skenandore, C.S.; Pineda, A.; Bahr, J.M.; Newell- Fugate, A.E.; Cardoso, F.C. Evaluation of a commercially available radioimmunoassay and enzyme immunoassay for the analysis of progesterone and estradiol and the comparison of two extraction efficiency methods. Domest. Anim. Endocrinol. 2017, 60, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Tal, S.; Mazaki-Tovi, M.; Druker, S.; Novak, S.; Raz, T.; Aroch, I. Evaluation of two chemiluminescent assays compared with radioimmunoassay for serum pro- gesterone measurement in bitches. Theriog. 2020, 147, 116–123. [Google Scholar] [CrossRef]
- Trabert, B.; Falk, R.T.; Stanczyk, F.Z.; McGlynn, K.A.; Brinton, L.A.; Xu, X. Reproducibility of an assay to measure serum progesterone metabolites that may be related to breast cancer risk using liquid chromatography-tandem mass spectrometry. Horm. Mol. Biol. Clin. Investig. 2015, 23, 79–84. [Google Scholar] [CrossRef]
- Patton, P.E.; Lim, J.Y.; Hickok, L.R.; Kettel, L.M.; Larson, J.M.; Pau, K.Y. Precision of progesterone measurements with the use of automated immunoassay analyzers and the impact on clinical decisions for in vitro fertilization. Fertil. Steril. 2014, 101, 1629–1636. [Google Scholar] [CrossRef]
- Sasaki, M.; Ochiai, H.; Takahashi, K.; Suzuki, R.; Minato, K.; Fujikata, A. Development and validation of LC-MS/MS assay for the quantification of progesterone in rat plasma and its application to pharmacokinetic studies. Drug Res. (Stuttg.) 2015, 65, 484–489. [Google Scholar] [CrossRef] [PubMed]
- Nöthling, J.O.; de Cramer, K.G.M. Comparing the values of progesterone in the blood of bitches as measured with a chemiluminescence immunoassay and a radioimmu- noassay. Reprod. Domest. Anim. 2018, 53, 1136–1141. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Zhao, M.; No, J.; Nam, Y.; Im, G.S.; Hur, T.Y. Dog cloning with in vivo matured oocytes obtained using electric chemiluminescence immunoassay-predicted ovulation method. PloS One 2017, 12, e0173735. [Google Scholar] [CrossRef]
- Gloria, A.; Contri, A.; Carluccio, A.; Robbe, D. Blood periovulatory progesterone quantification using different techniques in the dog. Anim Reprod Sci. 2018, 192, 179–184. [Google Scholar] [CrossRef]
- Kunanusont, N.; Punyadarsaniya, D.; Ruenphet, S. Accuracy and precision guidelines for optimal breeding time in bitches using in-house progesterone measurement compared with chemiluminescent microparticle immunoassay. Vet. World 2021, 14, 585–588. [Google Scholar] [CrossRef]
- Fontbonne, A.; Maenhoudt, C.; Thoumire, S.; Roos, J.; Niewiadomska, Z.; Robiteau, G.; Rousseliere, E.; Buronfosse, T. Evaluation of surface plasmon field-enhanced fluorescence spectroscopy for rapid measurement of progesterone concentration in bitches. Am. J. Vet. Res. 2021, 82, 417–424. [Google Scholar] [CrossRef] [PubMed]
- Milani, C.; Boscato, E.; Gabai, G.; Badon, T.; Schrank, M.; Sontas, H.; Romagnoli, S.; Mollo, A. Analytical and clinical performance of a fluorescence enzyme immunoassay for progesterone and determination of ovulation day in bitches. J. Vet. Diag. Invest. 2022, 34, 977–982. [Google Scholar] [CrossRef] [PubMed]
- Hussein, H.A.; Sculer, G.; Wehrend, A. Comparison of three progesterone quantification methods using blood samples drawn from bitches during the periovulatory phase. Vet. World. 2022, 15, 119–123. [Google Scholar] [CrossRef]
- Taylor, R. Interpretation of the correlation coeffi- cient: A basic review. J. Diagn. Med. Sonogr. 1990, 6, 35–39. [Google Scholar] [CrossRef]
- McBride, G.B. A Proposal for Strength-of-Agreement Criteria for Lin’S Concordance Correlation Coefficient. NIWA Client Report: HAM2005-062; National Institute of Water & Atmospheric Research: Hamilton, New Zealand. May 2005.
- Brugger, N.; Otzdorff, C.; Walter, B.; Hoffmann, B.; Braun, J. Quantitative determination of progesterone (P4) in canine blood serum using an enzyme-linked fluorescence assay. Reprod. Domest. Anim. 2011, 46, 870–873. [Google Scholar] [CrossRef] [PubMed]
- Idexx Laboratories Inc. Catalyst progesterone for in- house measurement of progesterone in plasma from bitches. Available online: www.idexx.com/files/catalyst-progesterone-white-paper-en.pdf (accessed on 14 December 2020).
- Rota, A.; Charles, C.; Cucuzza, A.S.; Pregel, P. Diagnostic efficiency of a single progesterone determi- nation to assess full-term pregnancy in the bitch. Reprod. Domest. Anim. 2015, 50, 1028–1031. [Google Scholar] [CrossRef] [PubMed]
- Schmicke, M.; Urhausen, C.; Wolf, K.; Schmidt, S.; Günzel-Apel, A.R. Evaluation of the blood progesterone concentration in the bitch measured by chemiluminescence immunoassay at the day of ovulation. Tierarztl. Prax. Ausg. K Kleintiere Heimtiere. 2016, 44, 317–322. [Google Scholar] [PubMed]
- Fraser, N.; Wilboen, R.R.; Schultz, W.; Randall, J.; Pew, C.; Greer, M. Comparative progesterone assay. Clin. Theriog. 2015, 7, 207–209. [Google Scholar]
- Bonte, T.; Rosset, E.; Combe, P. Performance evaluation of BioMerieux Vidas automated immunoassays for the determination of plasma progesterone in dogs, in Proceedings. 20th Eur Vet Soc Small Anim Reprod Cong. 2017, 43.
- Tahir, M.Z.; Thoumire, S.; Raffaelli, M.; Grimard, B.; Reynaud, K.; Chastant-Maillard, S. Effect of blood handling conditions on progesterone assay results obtained by chemiluminescence in the bitch. Domest Anim Endocrinol. 2013, 45, 141–144. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).