Submitted:
17 December 2023
Posted:
18 December 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Active Ingredients
2.2. Ex Vivo Experimentation
- -
- Untreated skin explants
- -
- Skin explant + vehicle 100%
- -
- Skin explant + vehicle (99.5%) + TPSE at 0.5%
2.3. In Vivo Trials
2.4. Statistical Analysis
3. Results
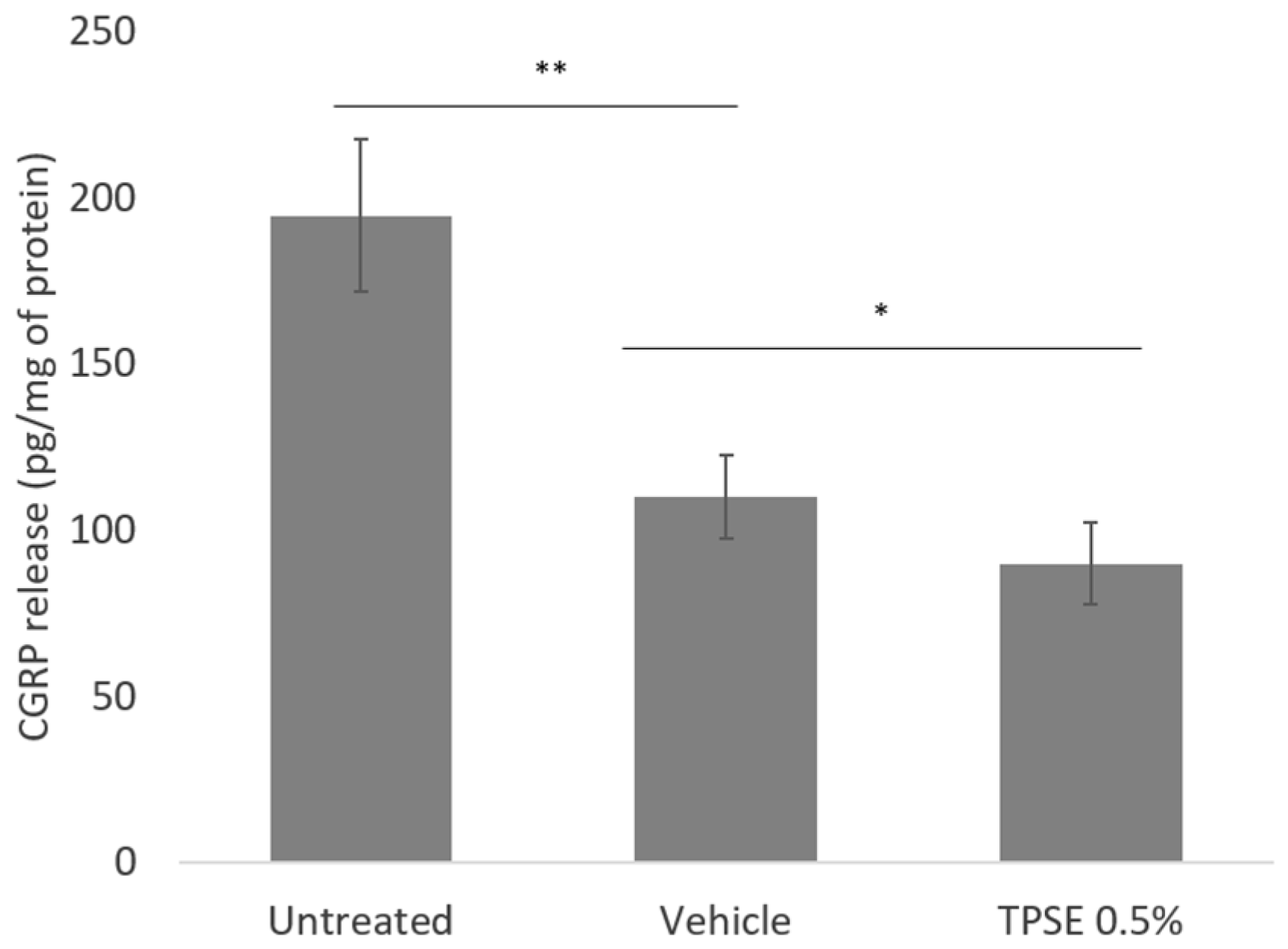
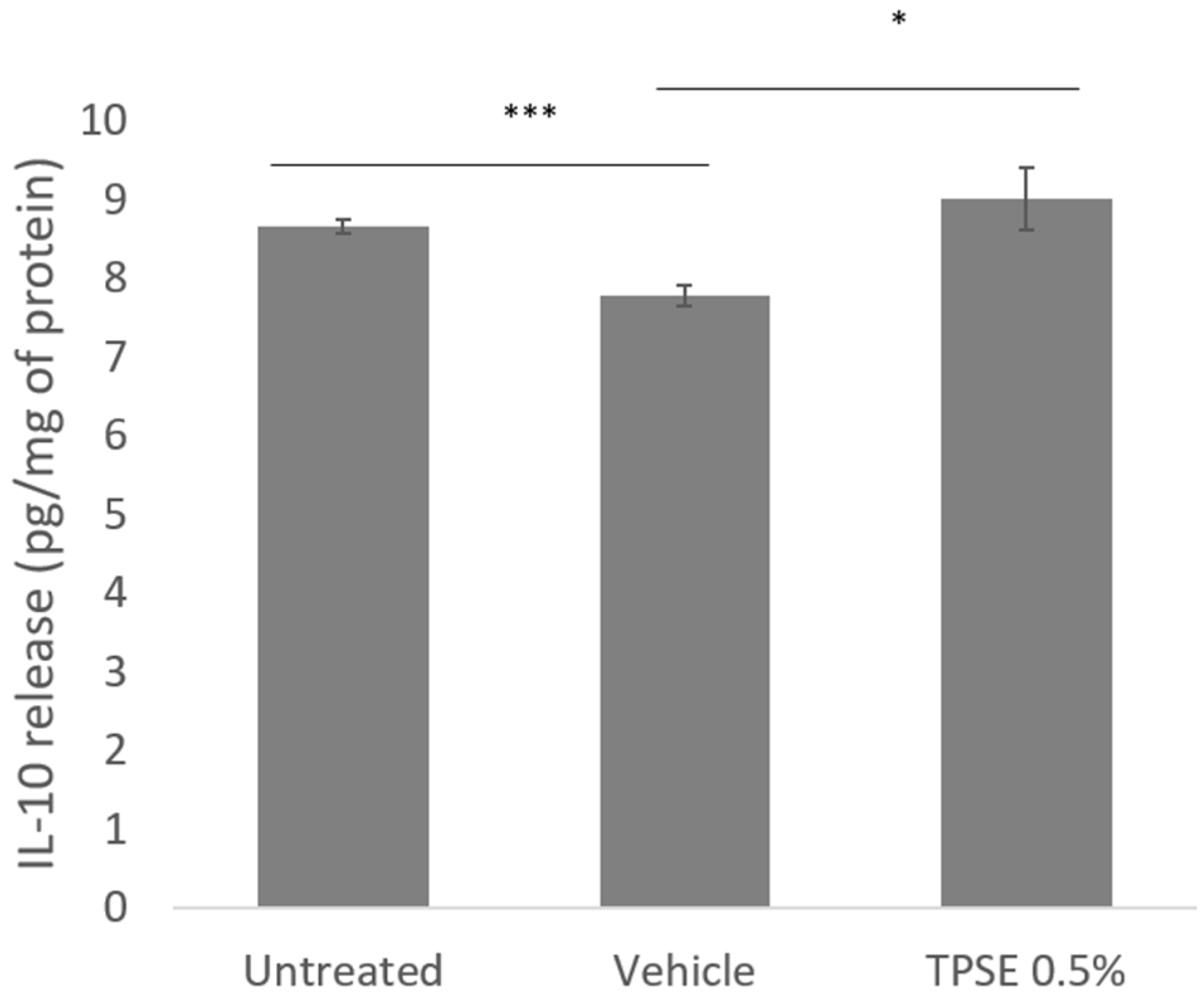
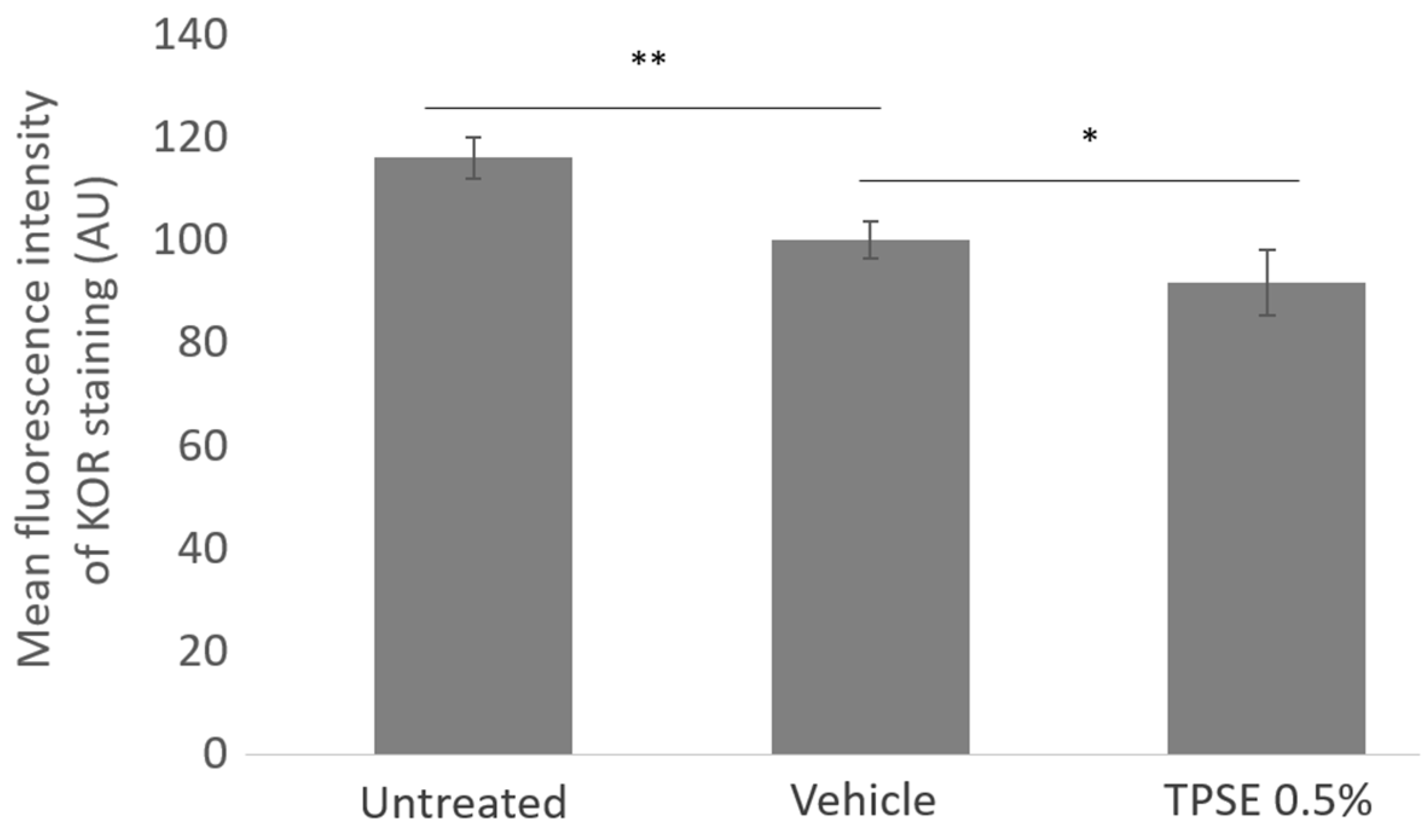
3.1. Ex Vivo Experimentation
3.2. Clinical Evaluation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Misery, L.; Ständer, S.; Szepietowski, J.C. Definition of Sensitive Skin: An Expert Position Paper from the Special Interest Group on Sensitive Skin of the International Forum for the Study of Itch. Acta Derm. Venereol. 2017, 97, 4–6. [Google Scholar] [CrossRef] [PubMed]
- Filaire, E.; Vialleix, C.; Cadoret, J.P.; Guénard, S.; Muller, C.; Dreux-Zhiga, A.; Berthon, J.Y. Characterization of reactive sensitive skin microbiota: effect of Halymenia durvillei (HD) extract treatment. Cosmetics. 2019, 6, 69. [Google Scholar] [CrossRef]
- Slominski, A.T.; Slominski, R.M.; Raman, C.; Chen, J.Y.; Athar, M.; Elmets, C. Neuroendocrine signaling in the skin with a special focus on the epidermal neuropeptides. A. J. Physiol. Cell Physiol. 2022, 1, C1757–C1776. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.S.; Pickens, S.; Burma, N.E.; Ibarra-Lecue, I.; Yang, H.; Xue, L.; Cook, C.; Hakimian, J.K.; Severino, A.L.; Lueptow, L.; et al. Kappa Opioid Receptors Drive a Tonic Aversive Component of Chronic Pain. J. Neurosci. 2019, 39, 4162–4178. [Google Scholar] [CrossRef] [PubMed]
- West, A.M.; Holleran, K.M.; Jones, S.R. Kappa Opioid Receptors Reduce Serotonin Uptake and Escitalopram Efficacy in the Mouse Substantia Nigra Pars Reticulata. Int. J. Mol. Sci. 2023, 24, 2080. [Google Scholar] [CrossRef]
- Kauser, S.; Thody, A.J.; Schallreuter, K.U.; Gummer, C.L.; Tobin, D.J. Beta-endorphin as a regulator of human hair follicule melanocyte biology. J Invest Dermatol. 2004, 123, 184–195. [Google Scholar] [CrossRef] [PubMed]
- Sanders, K.M.; Akiyama, T. The vicious cycle of itch and anxiety. Neurosci. Biobehav. Rev. 2018, 87, 17–26. [Google Scholar] [CrossRef]
- Farage, M.A. Psychological aspects of sensitive skin: a vicious cycle. Cosmetics. 2022, 9, 78. [Google Scholar] [CrossRef]
- Hua, J.; Le Scanff, C.; Larue, J.; José, F.; Martin, J.C.; Devillers, L.; Filaire, E. Global stress response during a social response during a social stress test: impact of alexithymia and its subfactors. Psychoneuroendocrinology. 2014, 50, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Graubard, R.; Perez-Sanchez, A.; Katta, R. Stress and skin: an overview of mind body therapies as a treatment strategy in dermatology. Dermatol. Pract. Concept. 2021, 11, e2021091. [Google Scholar] [CrossRef]
- Filaire, E.; Duche, P.; Lac, G.; Robert, A. Saliva cortisol, physical exercise and training: influences of swimming and handball on cortisol concentrations in women. Eur. J. Appl. Physiol. Occup. Physiol. 1996, 74, 274–278. [Google Scholar] [CrossRef] [PubMed]
- Pruessner, J.C.; Wolf, O.T.; Hellhammer, D.H.; Buske-Kirschbaum, A.; von Auer, K.; Jobst, S.; Kaspers, F.; Kirschbaum, C. Free cortisol levels after awakening: a reliable biological marker for the assessment of adrenocortical activity. Life Sci. 1997, 61, 2539–2549. [Google Scholar] [CrossRef] [PubMed]
- Clow, A.; Hucklebridge, F.; Thorn, L. The cortisol awakening response in context. Int. Rev. Neurobiol. 2010, 93, 153–175. [Google Scholar] [CrossRef]
- Durguerian, A.; Filaire, E.; Drogou, C.; Sauvet, F.; Bougard, C.; Chennaoui, M. Hyperactivity of the Sympatho-Adrenomedullary System Without Any Modification of the Hypothalamic-Pituitary-Adrenal Axis After Food Restriction Among High-Level Weightlifters. Strength Cond. Res. 2018, 32, 1643–1655. [Google Scholar] [CrossRef]
- Filaire, E.; Ferreira, J.P.; Oliveira, M.; Massart, A. Diurnal patterns of salivary alpha-amylase and cortisol secretion in female adolescent tennis players after 16 weeks of training. Psychoneuroendocrinology. 2013, 38, 1122–1132. [Google Scholar] [CrossRef] [PubMed]
- Pruessner, J.C.; Kirschbaum, C.; Meinlschmid, G.; Hellhammer, D.H. Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology. 2003, 28, 916–931. [Google Scholar] [CrossRef] [PubMed]
- Van Niekerk, J.K.; Huppert, F.A.; Herbert, J. Salivary cortisol and DHEA: association with measures of cognition and well-being in normal older men, and effects of three months of DHEA supplementation. Psychoneuroendocrinology. 2001, 26, 591–612. [Google Scholar] [CrossRef] [PubMed]
- Wilcox, R.R.; Granger, D.A.; Szanton, S.; Clark, F. Diurnal patterns and associations among salivary cortisol, DHEA and alpha-amylase in older adults. Physiol. Behav. 2014, 129, 11–16. [Google Scholar] [CrossRef]
- Hasegawa-Ohira, M.; Suguri, K.; Nomura, S. The Dehydroepiandrosterone Awakening Response as a Possible Index of Subjective Sleep Quality. Adv. Biomed. Engineering. 2016, 5, 132–136. [Google Scholar] [CrossRef]
- Lac, G. Saliva assays in clinical and research biology. Pathol. Biol. 2001, 49, 660–667. [Google Scholar] [CrossRef] [PubMed]
- Oskis, A.; Clow, A.; Thorn, L.; Loveday, C.; Hucklebridge, F. Differences between diurnal patterns of salivary cortisol and dehydroepiandrosterone in healthy female adolescents. Stress. 2012, 15, 110–113. [Google Scholar] [CrossRef] [PubMed]
- Viet, T.D.; Xuan, T.D.; Anh, L.H. 2. α-Amyrin and β-Amyrin Isolated from Celastrus hindsii Leaves and Their Antioxidant, Anti-Xanthine Oxidase, and Anti-Tyrosinase Potentials. Molecules. 2021, 26, 248. [Google Scholar] [CrossRef] [PubMed]
- Moore, E.M.; Wagner, C.; Komarnytsky, S. The Enigma of Bioactivity and Toxicity of Botanical Oils for Skin Care. Front. Pharmacol. Sec. Ethnopharmacol. 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Tomar, M.; Bhuyan, D.J.; Punia, S.; Grasso, S. Tomato (Solanum lycopersicum L.) seed: A review on bioactives and biomedical activities. Biomed. Pharmacother. 2021, 142, 112018. [Google Scholar] [CrossRef] [PubMed]
- Bauer, S.; Schulte, E.; Their, H. Composition of the surface wax from tomatoes: II. Quantification of the components at the ripe red stage and during ripening. Eur. Food Res. Technol. 2004; 219, 487–491. [Google Scholar] [CrossRef]
- Gleize, B.; Steib, M.; André, E.; Reboul, E. Simple and fast HPLC method for simultaneous determination of retinol, tocopherols, coenzyme Q10 and carotenoids in complex samples. Food Chemistry. 2012, 134, 2560–2564. [Google Scholar] [CrossRef] [PubMed]
- Frosch, P.J.; Duhring, A.M.K. A method for appraising the stinging capacity of topically applied substances. J. Soc. Cosmet. Chem. 1977, 28, 197–209. [Google Scholar]
- Cole, A.S.; Eastao, J.E. Biochemistry and Oral Biology, 2nd ed.; Wright, London, 1988; p.476.
- Mayer, J.D.; Gaschke, Y.N. The experience and meta-experience of mood. Int. J. Pers. Soc. Psychol. 1988, 55, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Niedenthal, P.; Dalle, N. Emotional response categorization during naturally induced emotions. Eur. J. Soc. Psychol. 2001, 31, 737–742. [Google Scholar] [CrossRef]
- Fiet, J.; Passat, P.; Guechot, J.; Gourmel, B.; Villette J., M.; Cathelineau, G. Interet du dosage du cortisol dans la salive. Nouvelle Presse Medicale. 1981, 10, 2664. [Google Scholar]
- Seeman, T.E.; Robbins, R.J. Aging and hypothalamic-pituitary-adrenal response to challenge in humans. Endocr. Rev. 1994, 15, 233–260. [Google Scholar]
- Manav, V.; Karaali, M.G.; Erdem, O.; Aksu, A.E.K. Association between biophysical properties and anxiety in patients with sensitive skin. Skin Res. Technol. 2022, 28, 556–563. [Google Scholar] [CrossRef] [PubMed]
- Cartwright, M.M.; Kamen, T.; Desai, S.R. The Psychosocial Burden of Skin Disease and Dermatology Care Insights Among Skin of Color Consumers. Dermatol. 2023, 1, 1027–1033. [Google Scholar] [CrossRef]
- Cabannes, M.; Risselada, C.; Chaisemartin, L.; Pasquet, J.; Couval, E.; Berthon, J.Y.; Filaire, E. Increase in subjective well-being and psychological health after application of C8-silk lipoamino acid functionalized pigments included in a foundation. Int. J. Cosm. Sci. 2019, 41, 489–495. [Google Scholar] [CrossRef]
- Abdelhadi, S.; Nordlind, K.; Johansson, B.; Theodorsson, E.; Holst, M.; Lönndahl, L. Expression of calcitonin gene-related peptide in atopic dermatitis and correlation with distress. Immunopharmacol. Immunotoxicol. 2023, 7, 1–6. [Google Scholar] [CrossRef]
- Bigliardi, P.L.; Dancik, Y.; Neumann, C.; Bigliardi-Qi, M. Opioids and Skin Homeostasis, Regeneration and Ageing-What’s the Evidence? Exp. Dermatol. 2016, 25, 586–591. [Google Scholar] [CrossRef]
- Sattayakhom, A.; Wichit, S.; Koomhin, P. The Effects of Essential Oils on the Nervous System: A Scoping Review. Molecules. 2023, 28, 3771. [Google Scholar] [CrossRef]
- Min-Kyung, K. Brain waves and emotional responses, according to color stimulation of eye shadows using contrast and similar color arrangement. As. J. Beauty Cosmetol. 2018, 16, 509–521. [Google Scholar]
- Gervason, S.; Napoli, M.; Dreux-Zhiga, A.; Lazzarelli, C.; Garcier, A.; Briand, A.; Thepot, A.; Berthon, J.Y.; Filaire, E. Attenuation of negative effects of senescence in human skin using an extract from Sphingomonas hydrophobicum: development of new skin care solution. Int. J. Cosmetic Sci. 2019, 41, 391–397. [Google Scholar] [CrossRef]
- Levy, L.; Emer, J. Emotional benefit of cosmetic camouflage in the treatment of facial skin conditions: personal experience and review. Clin. Cosmet. Investig. Dermatology. 2012, 5, 173–182. [Google Scholar]
- Rizzi, V.; Gubitosa, J.; Fini, P.; Cosma, P. Neurocosmetics in Skincare-the Fascinating World of Skin–Brain Connection: A Review to Explore Ingredients, Commercial Products for Skin Aging, and Cosmetic Regulation. Cosmetics. 2021, 8, 66. [Google Scholar] [CrossRef]
- Joseph, N.T.; Jiang, Y.; Zilioli, S. Momentary emotions and salivary cortisol: a systematic review and meta-analysis of ecological momentary assessment studies. Neurosc. Behav. Rev. 2021, 125, 365–379. [Google Scholar] [CrossRef] [PubMed]
- Fukui, H.; Toyoshima, K.; Komaki, R. Psychological and neuroendocrinological effects of odour of saffron (Crocus sativus). Phytomed. 2011, 18, 726–730. [Google Scholar] [CrossRef]
- Watanabe, E.; Kuchta, K.; Kimura, M.; Rauwald, H.W.; Kamei, T.; Imanishi, J. Effects of bergamot (Citrus bergamia (Risso) Wright & Arn.) essential oil aromatherapy on mood states, parasympathetic nervous system activity, and salivary cortisol levels in 41 healthy females. Forsch. Komplement. Med. 2015, 22, 43–49. [Google Scholar]
| Group A (Placebo) | Group B (TPSE) | |
|---|---|---|
|
Erythrosis Intensity D0 D28 |
2.10 ± 0.97 1.95 ± 1.01 |
1.62 ± 0.77 1.15 ± 0.58*** |
|
Erythrosis extent D0 D28 |
1.65 ± 0.71 1.60 ± 0.70 |
1.60 ± 0.82 1.38 ± 0.77** |
| Clinical scoring for skin homogeneity analysis | D0 | D28 |
|---|---|---|
|
Group A (Placebo) Group B (TPSE) |
1.45 ± 1.07 1.36 ± 0.73 |
1.30 ± 0.95 1.12 ± 0.70*** |
| Group A (Placebo) | Group B (TPSE) | |
|---|---|---|
| D0 | ||
|
CAR AUC Cort DAR AUC DHEA |
5.95 ± 0.18 38.95 ± 0.85 0.18 ± 0.03 7.82 ± 0.10 |
6.34 ± 0.15 39.04 ± 0.61 0.18 ± 0.01 7.86 ± 0.07 |
| D28 | ||
|
CAR AUC Cort DAR AUC DHEA |
6.56 ± 0.16 39.26 ± 0.79 0.27 ± 0.06 7.58 ± 0.15 |
5.43 ± 0.20*** 35.36 ± 0.62** 0.36 ± 0.04** 8.08 ± 0.09** |
| Group A (Placebo) | Group B (TPSE) | |
|---|---|---|
| D0 D28 |
47.3 ± 2.5 41.4 ± 2.6 |
44.0 ± 1.349.0 ± 1.4** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




