Submitted:
15 December 2023
Posted:
18 December 2023
You are already at the latest version
Abstract
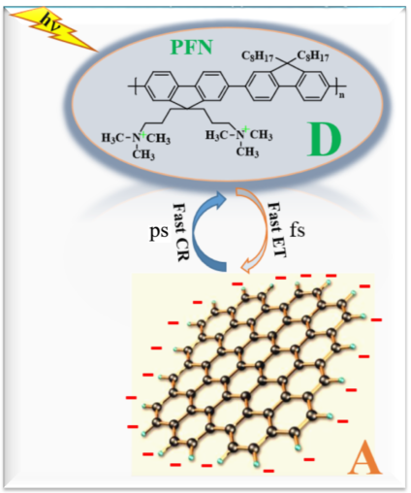
Keywords:
1. Introduction
2. Experimental Section
2.1. Materials
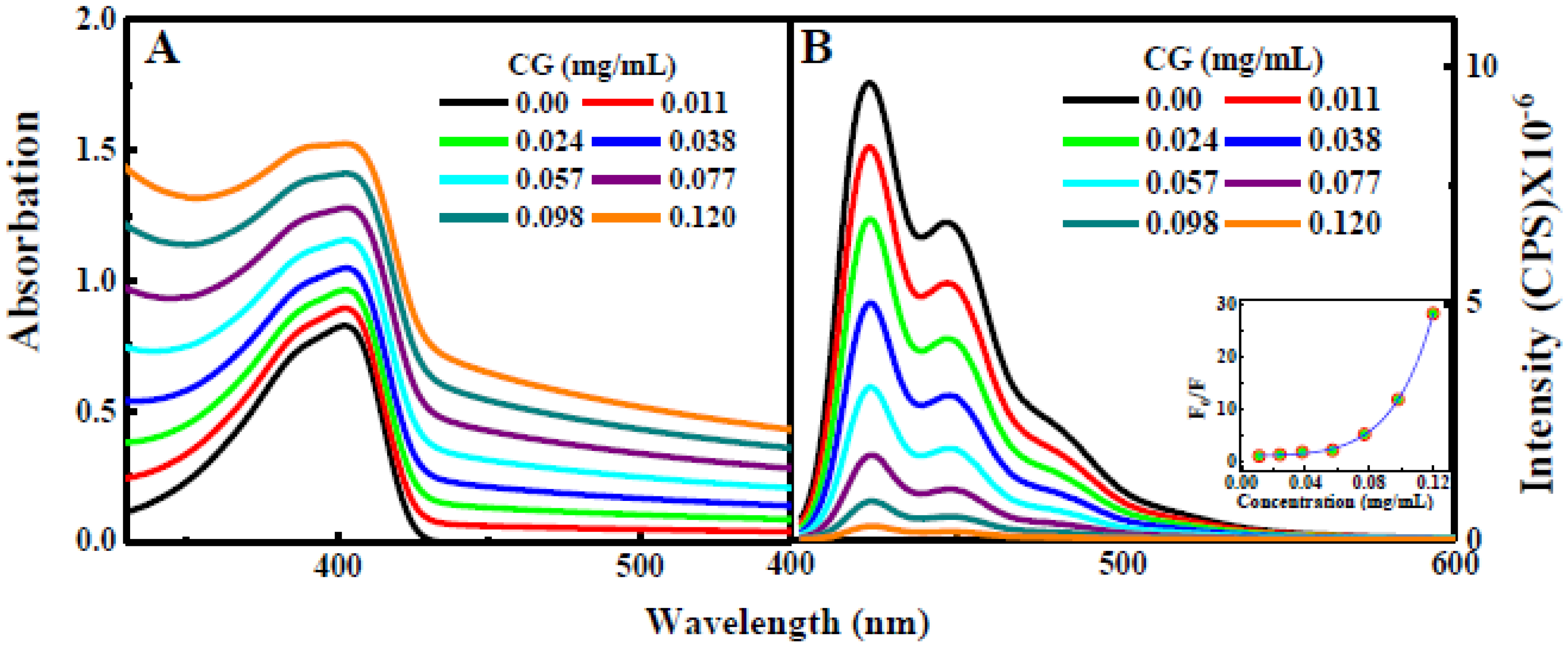
2.2. Steady-State Measurements
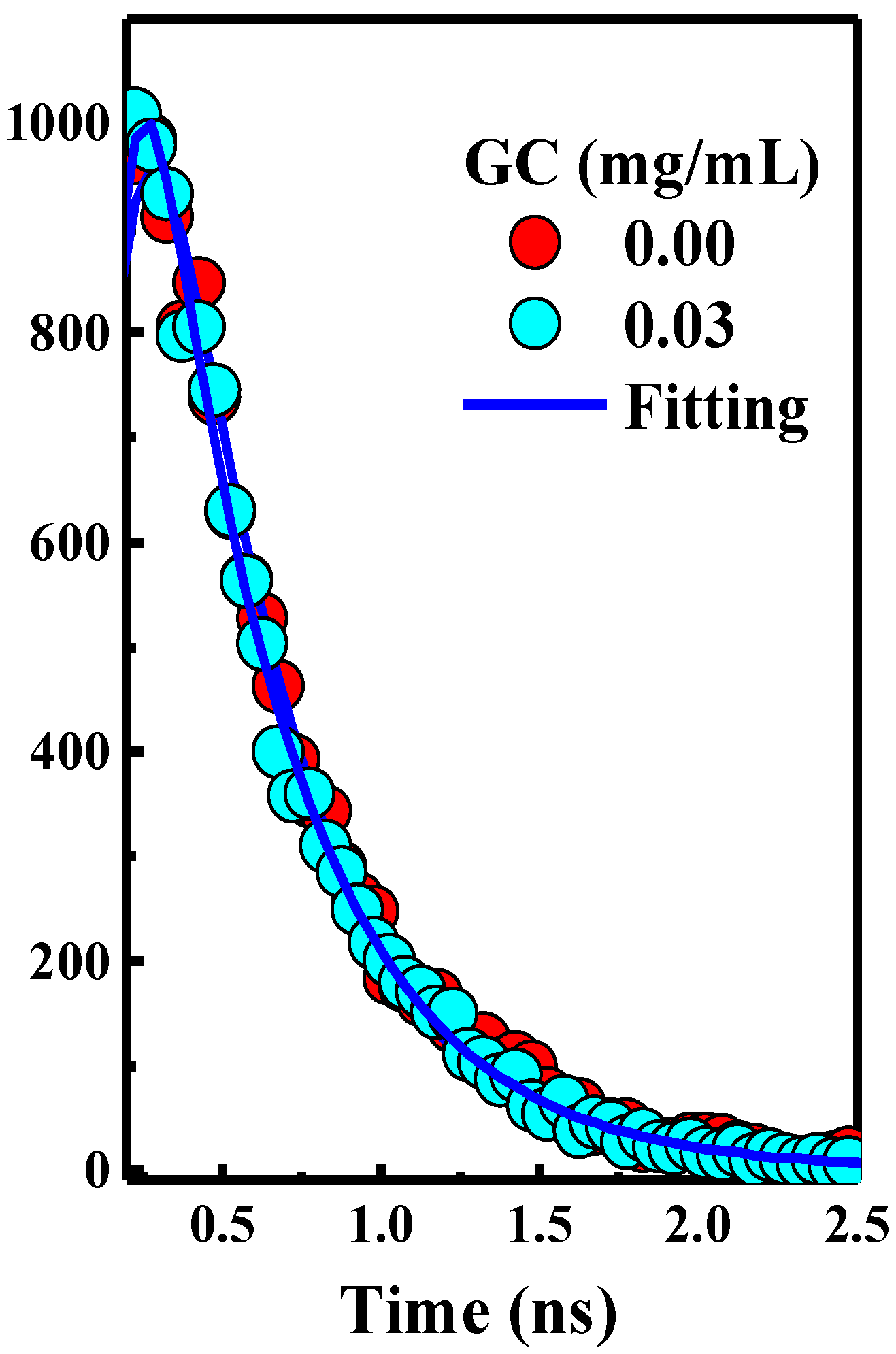
2.3. TCSPC Setup
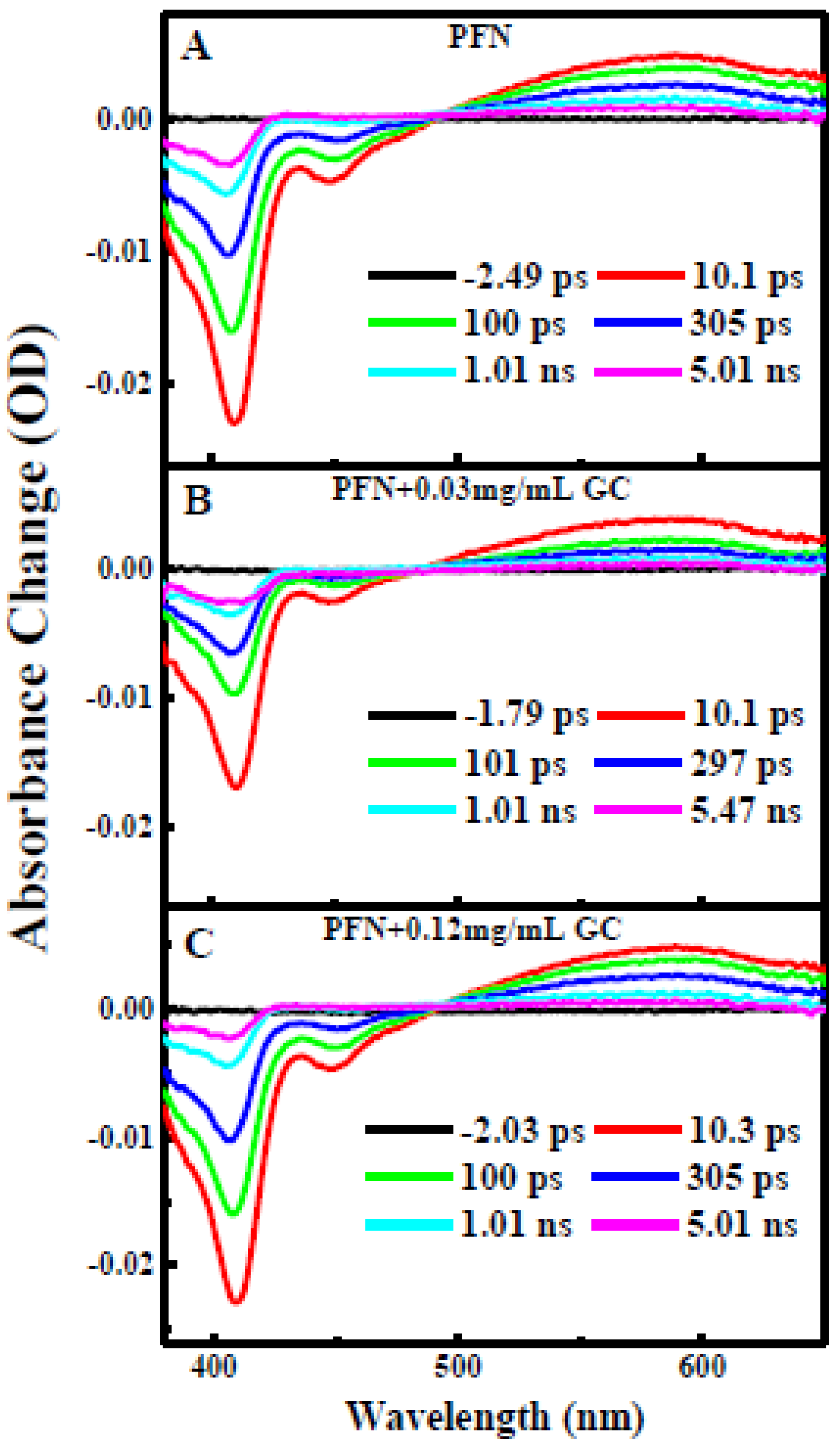
2.4. Femtosecond Broadband Transient Absorption (TA) Spectroscopy
 J pulse energy) generated in an optical parametric amplifier (Newport Spectra-Physics). The pump and probe pulses were overlapped within a 2-mm-thick cuvette cell containing PFN (0.4 OD) in the absence and presence of GC (0.03 and 0.12 mg/mL). In order to cover the transient spectra from a few hundred fs to
J pulse energy) generated in an optical parametric amplifier (Newport Spectra-Physics). The pump and probe pulses were overlapped within a 2-mm-thick cuvette cell containing PFN (0.4 OD) in the absence and presence of GC (0.03 and 0.12 mg/mL). In order to cover the transient spectra from a few hundred fs to  s time scales, a Helios and an EOS detection system were employed with time resolutions of 120 fs and 200 ps and detection limits of 5 ns and 1
s time scales, a Helios and an EOS detection system were employed with time resolutions of 120 fs and 200 ps and detection limits of 5 ns and 1  s, respectively [1].
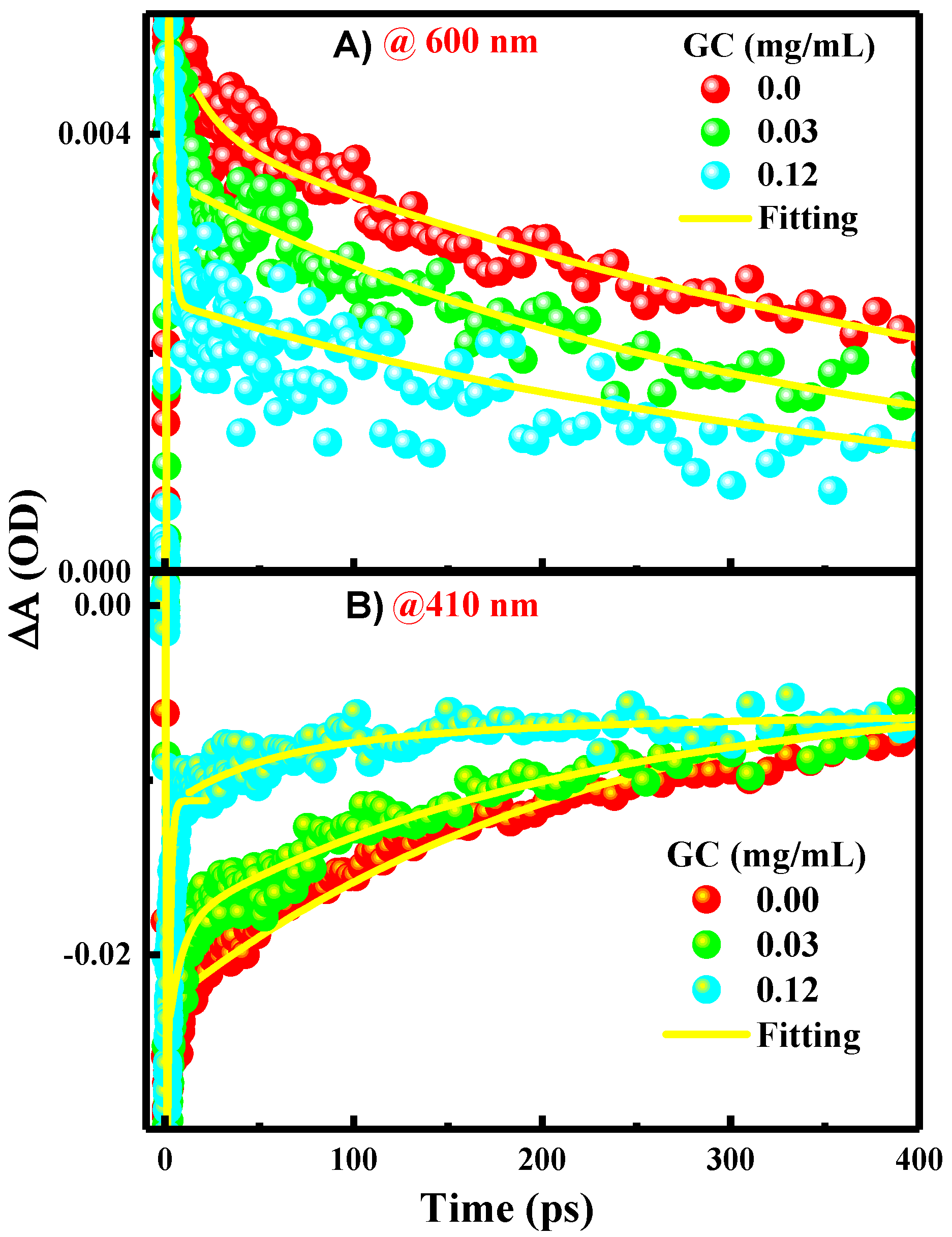
s, respectively [1].3. Results and Discussion
4. Conclusion
Acknowledgments
Conflicts of Interest
References
- Alsam, A. A; Aly, S. M.; Usman, A.; Parida, R. M.; Del Gobbo, S.; Alarousu, E.; Mohammed, O.F. Bimolecular Excited-State Electron Transfer with Surprisingly Long-Lived Radical Ions, J. Phys. Chem. C 2015, 119, 21896–21903. [Google Scholar] [CrossRef]
- Liu, C.; Shao, L.; Chen, S.; Hu, Z.; Cai, H.; Huang, F. Recent Progress in π-Conjugated Polymers for Organic Photovoltaics: Solar Cells and Photodetectors., Prog. Polym. Sci., 2023, 143, 101711–101715.
- Facile Synthesis of Key Building Blocks of D18 Series Conjugated Polymers for High-Performance Polymer Solar Cells Zhong, X.; T. W. Chen; Yan, L.; You* W. ACS Appl. Polym. Mater., 2023, 5, 3–1937.
- Skotheim, T. A.; Reynolds, J. R. Skotheim, T. A.; Reynolds, J. R. Handbook of Conducting Polymers, CRC, Boca Raton, FL, 2007, 2, 3rd ed.
- McQuade, D. T.; Pullen, A. E.; Swager, T. M. Conjugated Polymer-Based Chemical Sensors. Chem. Rev. 2000, 100, 2537–2574. [Google Scholar] [CrossRef] [PubMed]
- Xing, X.J.; Liu, X.G.; Yue He, Y.L.; Zhang, C.L.; Tang, H.W.; Pang, D.W. Amplified Fluorescent Sensing of DNA Using Graphene Oxide and a Conjugated Cationic Polymer. Biomacromolecules 2013, 14, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Taranekar, P.; Reynolds, J.R.; Schanze, K.S. Conjugated Polyelectrolytes: Synthesis, Photophysics, and Applications. Angew. Chem. Int. Ed. 2009, 48, 4300. [Google Scholar] [CrossRef] [PubMed]
- Schanze, K.S.; Shelton, A.H. Functional Polyelectrolytes. Langmuir 2009, 25, 13698–13702. [Google Scholar] [CrossRef] [PubMed]
- Kang, R.; Oh, S.H.; Kim, D.Y. Influence of the Ionic Functionalities of Polyfluorene Derivatives as a Cathode Interfacial Layer on Inverted Polymer Solar Cells. ACS Appl. Mater. Interfaces 2014, 6, 6227–6236. [Google Scholar] [CrossRef]
- Ho, H.A.; Leclerc, M. Optical Sensors Based on Hybrid Aptamer/Conjugated Polymer Complexes. J. Am. Chem. Soc. 2004, 126, 1384–1387. [Google Scholar] [CrossRef]
- Feng, F.; Wang, H.; Han, L.; Wang, S. Fluorescent Conjugated Polyelectrolyte as an Indicator for Convenient Detection of DNA Methylation. J. Am. Chem. Soc. 2008, 130, 11338–11343. [Google Scholar] [CrossRef]
- Yi, C.; Song, B.; Tian, W.; Cui, X.; Qi, Q.; Jiang, W.; Qi, Z.; Sun, Y. Fluorescent Sensor of Fluorene Derivatives Having Phosphonic Acid as a Fluorogenic Ionophore: Synthesis and Static Quenched Properties for Fe(III). Tetrahedron Lett. 2014, 55, 5119–5123. [Google Scholar] [CrossRef]
- Duarte, A.; Pu, K.-Y.; Liu, B.; Bazan, G.C. Recent Advances in Conjugated Polyelectrolytes for Emerging Optoelectronic Applications. Chem. Mater. 2011, 23, 501–515. [Google Scholar] [CrossRef]
- Alsam, A.A.; Adhikari, A.; Parida, M.R.; Aly, S.M.; Bakr, O.M.; and Mohammed, O.F. Bane of Hydrogen-Bond Formation on the Photoinduced Charge-Transfer Process in Donor-Acceptor Systems, J. Phys. Chem. C 2017, 121, 7837–7843. [Google Scholar] [CrossRef]
- Lee, K.; Kim, H. J.; Kim, J. Design Principle of Conjugated Polyelectrolytes to Make Them Water-Soluble and Highly Emissive. Adv. Funct. Mater. 2012, 22, 1076–1086. [Google Scholar] [CrossRef]
- Closs, G. L.; Miller, J. R. Intramolecular Long-Distance Electron-Transfer in Organic Molecules. Science 1988, 240, 440–447. [Google Scholar] [CrossRef]
- Kim, I.; Kyhm, K.; Kang, M.; Woo, H. Y. Ultrafast Combined Dynamics of Förster Resonance Energy Transfer and Transient Quenching in Cationic Polyfluorene/Fluorescein-Labelled Single-Stranded DNA Complex. J. Lumin. 2014, 149, 185–189. [Google Scholar] [CrossRef]
- Clarke, T. M.; Durrant, J. R. Charge Photogeneration in Organic Solar Cells. Chem. Rev. 2010, 110, 6736–6767. [Google Scholar] [CrossRef]
- Wu, C.; Xu, Q. H. Enhanced One- and Two-Photon Excitation Emission of a Porphyrin Photosensitizer by FRET from a Conjugated Polyelectrolyte. Macromol. Rapid Comm. 2009, 30, 504–508. [Google Scholar] [CrossRef]
- Zhao M-Y, Tang Y-F, Han G-Z. Recent Advances in the Synthesis of Aromatic Azo Compounds. Molecules 2023, 28, 6741. [CrossRef]
- Nurul A. R, Zuhair J, Synthesis, chemical identification and biological application of Azo-based molecules containing different terminal group: A review. journal of molecular structure 2023, 1284, 135329–13533. [CrossRef]
- Rosspeintner, A.; Angulo, G.; Vauthey, E. Bimolecular Photoinduced Electron Transfer Beyond the Diffusion Limit: The Rehm−Weller Experiment Revisited with Femtosecond Time Resolution. J. Am. Chem. Soc. 2014, 136, 2026–2032. [Google Scholar] [CrossRef]
- Stork, M.; Gaylord, B. S.; Alan J., Heeger; Bazan, G. C. Energy Transfer in Mixtures of Water-Soluble Oligomers: Effect of Charge, Aggregation, and Surfactant Complexation. Adv. Mater. 2002, 14, 361–366. [Google Scholar] [CrossRef]
- Srinivasan, M. V.; Ito, M.; Kumar, P.; Abhirami, K.; Tsuda, N.; Yamada, J.; Shin, P.-K.; Ochiai, S. Performance Evaluation of an Organic Thin-Film Solar Cell of PTB7:PC71BM with an Alcohol-Soluble Polyelectrolyte Interlayer Prepared Using the Spray-Coating Method. Ind. Eng. Chem. Res. 2015, 54, 181–187. [Google Scholar] [CrossRef]
- Aly, S. M.; Parida, M. R.; Alarousu, E.; Mohammed, O. F. Ultrafast Electron Injection at the Cationic Porphyrin-Graphene Interface Assisted by Molecular Flattening. Chem. Commun. 2014, 50, 10452–10455. [Google Scholar] [CrossRef]
- Mohammed, O. F.; Banerji, N.; Lang, B.; Nibbering, E. T. J.; Vauthey, E. Photoinduced Bimolecular Electron Transfer Investigated by Femtosecond Time-Resolved Infrared Spectroscopy. , J. Phys. Chem. A 2006, 110, 13676–13680. [Google Scholar] [CrossRef]
- Alsam A. A.; Aly, S. M.; Parida R. M.; Alarousu E.; Cao, Z.; Cavallo, L.; Mohammed, F. O., Real-time observation of intersystem crossing induced by charge recombination during bimolecular electron transfer reactions. Dyes and Pigments 2017, 136, 881–886. [CrossRef]
- Pinto, S. M.; Burrows, H. D.; Pereira, M. M.; Fonseca, S. M.; Dias, F. B.; Mallavia, R.; Tapia, M. J. Singlet-Singlet Energy Transfer in Self-Assembled Systems of the Cationic Poly{9,9-bis [6-N,N,N-trimethylammonium)hexyl]fluorene-co-1,4-phenylene} with Oppositely Charged Porphyrins. J. Phys. Chem. B 2009, 113, 16093–16100. [Google Scholar] [CrossRef]
- Jiang, J.; Alsam, A. A.; Wang, S.; Aly, S. M.; Pan, Z.; Mohammed, F. O.; and Schanze, S. K. Effect of Conjugation Length on Photoinduced Charge Transfer in π-Conjugated Oligomer-Acceptor Dyads, J. Phys. Chem. A 2017, 121, 4891–4901. [Google Scholar] [CrossRef]
- Pagès, S.; Lang, B.; Vauthey, E. Ultrafast Excited State Dynamics of the Perylene Radical Cation Generated upon Bimolecular Photoinduced Electron Transfer Reaction. J. Phys. Chem. A 2006, 110, 7547–7553. [Google Scholar] [CrossRef]
- Keizer, J. Nonlinear Fluorescence Quenching and the Origin of Positive Curvature in Stern-Volmer Plots. Journal of American Chemical Society 1983, 105, 1494–1498. [Google Scholar] [CrossRef]
- Lakowicz, J. R. Principles of Fluorescence Spectroscopy. Springer Science+Business Media, LLC, Singapore, third edn., 2006.
- Tvrdy, K.; Frantsuzov, P. A.; Kamat, P. V. Photoinduced Electron Transfer from Semiconductor Quantum Dots to Metal Oxide Nanoparticles. Proc. Natl. Acad. Sci. USA 2010, 108, 29–34. [Google Scholar] [CrossRef]
- Wang, E.; Ma, Z.; Zhang, Z.; Henriksson, P.; Inganas, O.; Zhang, F.; Andersson, M. R. An Isoindigo-Based Low Band Gap Polymer for Efficient Polymer Solar Cells with High Photo-voltage. Chem. Commun. 2011, 47, 4908–4910. [Google Scholar] [CrossRef]
- Rehm, D.; Weller, A. Kinetics of Fluorescence Quenching by Electron and H-Atom Transfer. Isr. J. Chem. 1970, 8, 259–271. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




