1. Introduction
Polyphenols, are a diverse group of natural compounds found in plants. Their antioxidant properties make them valuable in combating the damage caused by free radicals, which are associated with aging and various diseases [
1,
2].
The subclasses of polyphenols, such as flavonoids, phenolic acids, and polyphenolic amides, each contribute different compounds with unique properties. These substances are present in a variety of foods, including fruits, vegetables, tea, coffee, red wine, and certain whole grains [
3,
4].
Consuming a diet rich in polyphenol-containing foods has been associated with potential health benefits, such as anti-inflammatory, anticancer, antiviral, and neuroprotective effects. However, as with any nutritional component, the specific health effects can depend on factors such as the type and amount of polyphenols consumed, as well as individual variations [
1,
2,
3,
4]. In a short communication, the focus is on predicting the toxicity properties of flavonoids using the pkCSM Database [
5].
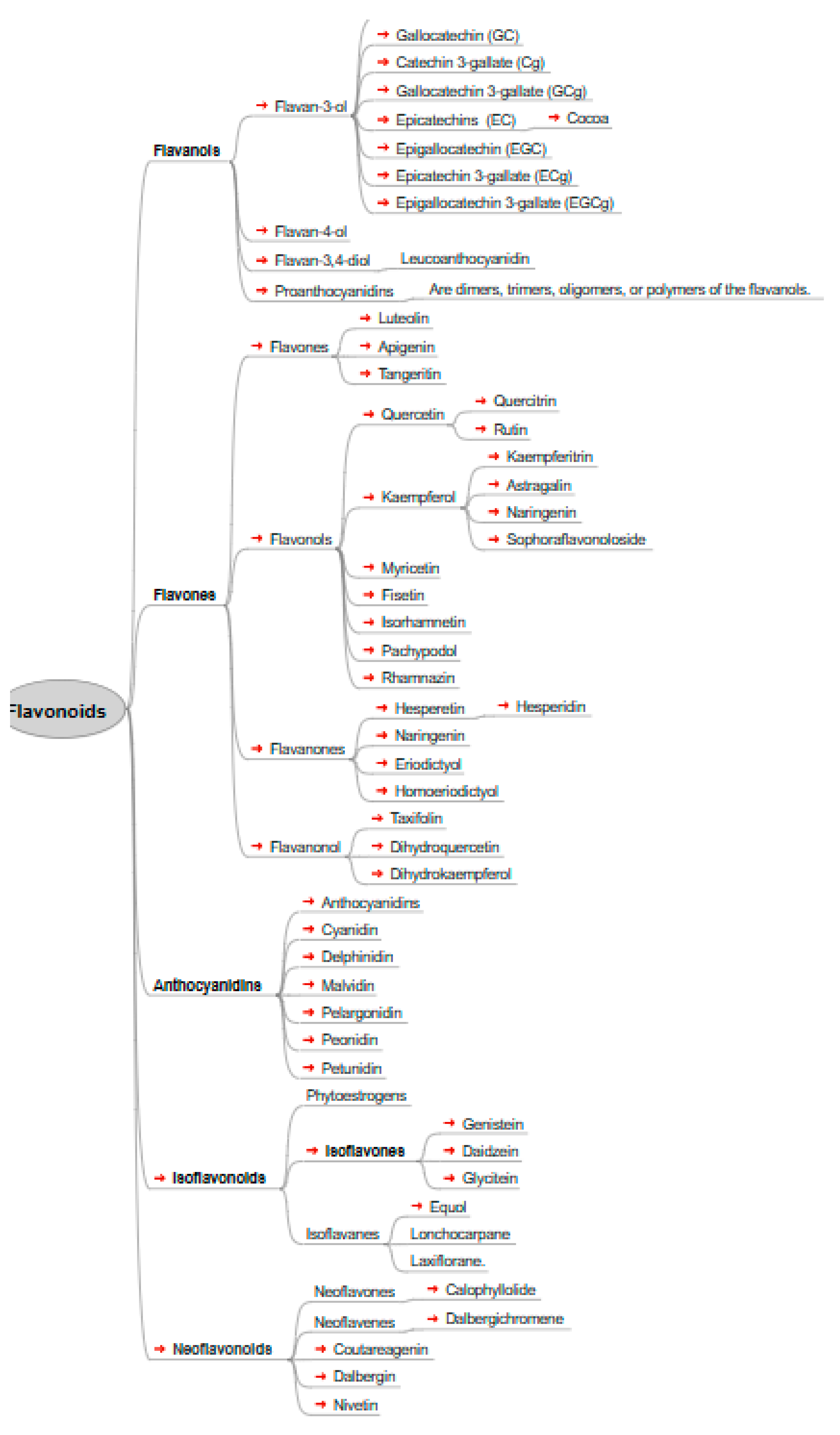
Flavonoids, also known as bioflavonoids, are polyphenolic secondary metabolites found in plants, commonly consumed in human diets due to their presence in fruits, vegetables, and other plant-based foods. The term "flavonoid" is derived from the Latin word "flavus," meaning yellow, reflecting their natural color [
6,
7,
8].
Figure 1.
displays the chemical structures of the main flavonoids studied in a particular investigation.
Figure 1.
displays the chemical structures of the main flavonoids studied in a particular investigation.
2. Material and Methods
The research conducted by Pires, Blundell, and Ascher involves the development and application of the pkCSM tool [
5], designed for predicting small-molecule pharmacokinetic and toxicity properties using graph-based signatures. In a specific application, the study investigates the toxicity properties of various flavonoids.
3. Results and Discussion
This communication offers a broad perspective on polyphenols, highlighting their diverse presence in plants and underscoring their antioxidant properties with associated health benefits. The main focus of the communication is the introduction of a short study centered on predicting the toxicity properties of flavonoids. This prediction is carried out using the pkCSM Database [
5], a tool designed for such computational analyses. The outcomes of this investigation are presented in
Table 1, providing a tabular representation of the results obtained. From these results collectively suggest that Hesperidin exhibits promising properties with a low indication of toxicity across various measures, making it a potentially favorable compound.
In the assessment of various flavonoids, Hesperidin emerges as a standout with exceptional potential and a notable lack of toxicity in both short and long-term evaluations. The specific toxicity parameters for Hesperidin are as follows:
Max. Tolerated Dose (Human) (log mg/kg/day): Hesperidin exhibits a low potential for toxicity, as indicated by the relatively low value of 0.525 log mg/kg/day for the maximum tolerated dose in humans.
Oral Rat Acute Toxicity (LD50) (mol/kg): The LD50 value of 2.506 mol/kg suggests a relatively low acute toxicity in oral exposure, with a higher dose needed to cause harm to 50% of test subjects.
Oral Rat Chronic Toxicity (LOAEL) (log mg/kg_bw/day): Hesperidin shows a favorable chronic toxicity profile with a LOAEL value of 3.167 log mg/kg_bw/day, signifying a higher dose before observing adverse effects.
Minnow Toxicity (log mM): In terms of minnow toxicity, Hesperidin demonstrates a low level of toxicity with a value of 7.131 log mM, suggesting a minimal impact on these aquatic organisms
In conclusion, the toxicity assessment of various flavonoids, with a specific focus on Hesperidin, reveals promising findings regarding its safety profile.
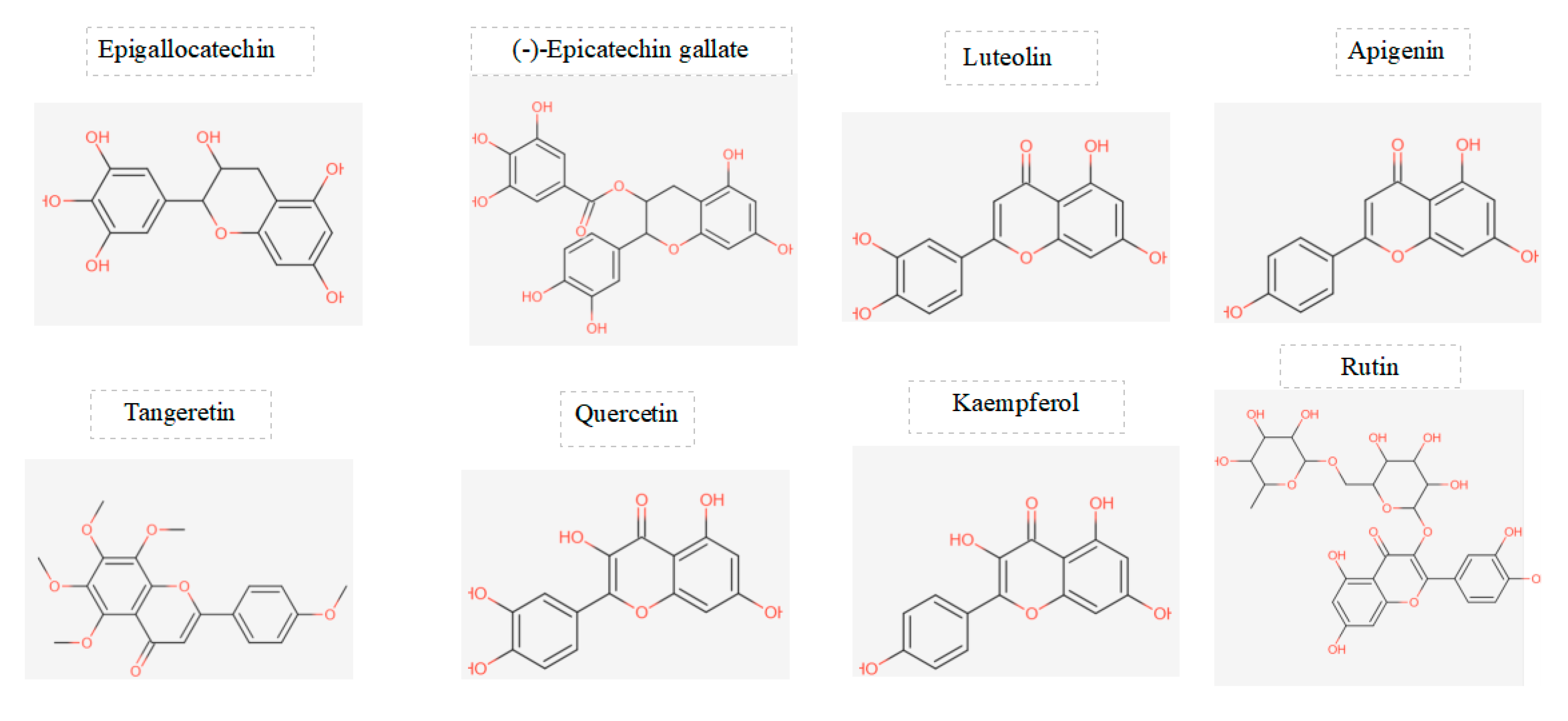
Figure 2.
displays the chemical structures of the main flavonoids studied in a particular investigation.
Figure 2.
displays the chemical structures of the main flavonoids studied in a particular investigation.
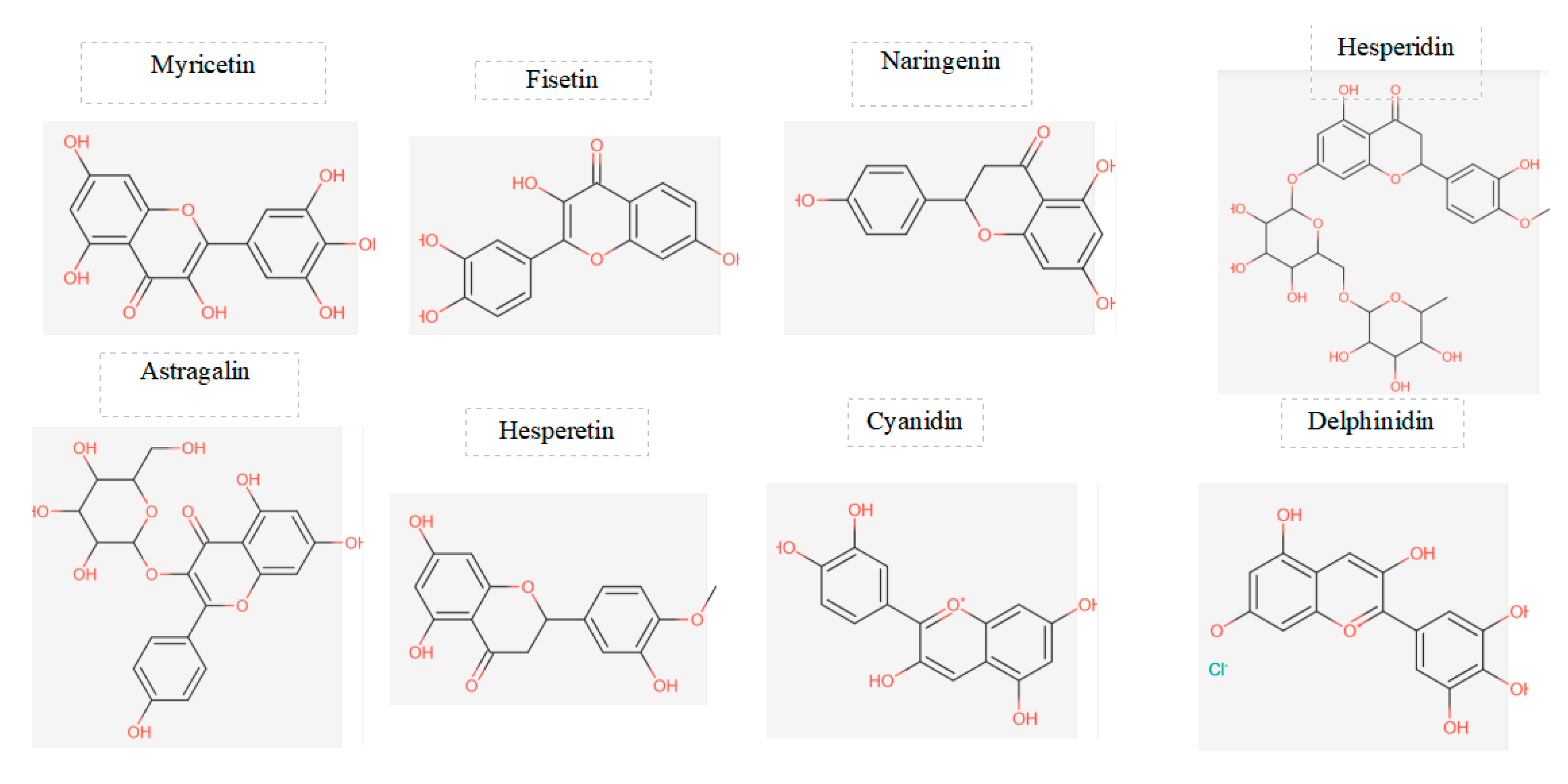
Figure 3.
displays the chemical structures of the main flavonoids studied in a particular investigation.
Figure 3.
displays the chemical structures of the main flavonoids studied in a particular investigation.
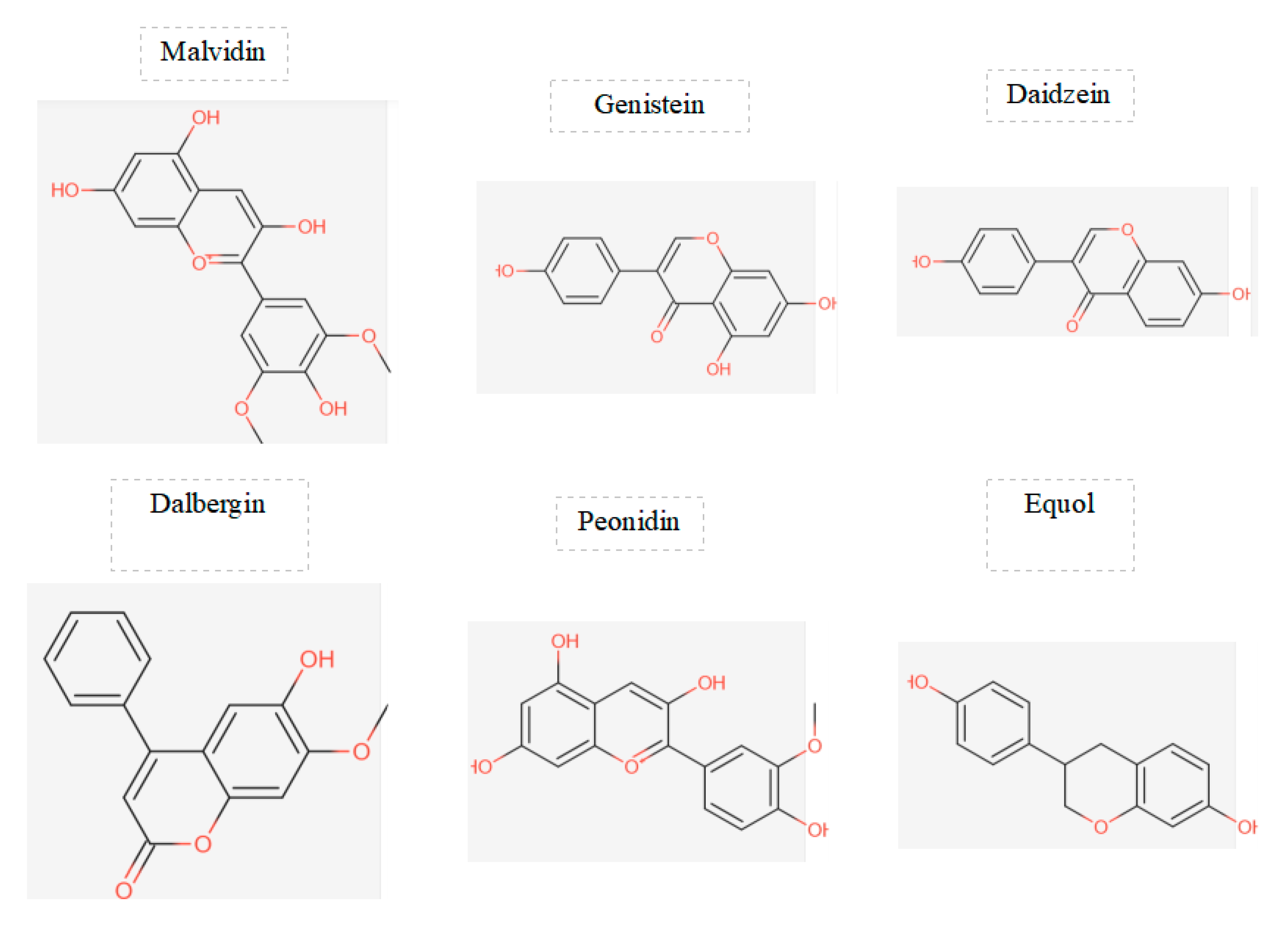
Figure 4.
displays the chemical structures of the main flavonoids studied in a particular investigation.
Figure 4.
displays the chemical structures of the main flavonoids studied in a particular investigation.
Table 1.
the comparison of predicted toxicity properties of flavonoids using the pkCSM Database.
Table 1.
the comparison of predicted toxicity properties of flavonoids using the pkCSM Database.
| Compounds |
AMES toxicity |
Max.
tolerated
dose (human) (log mg/kg/day) |
Oral Rat Acute Toxicity (LD50) (mol/kg) |
Oral Rat Chronic Toxicity (LOAEL) (log mg/kg_bw/day) |
Hepatotoxicity |
Skin
Sensitisation |
T.
Pyriformis
toxicity
(log ug/L) |
Minnow
toxicity
(log mM) |
| Epigallocatechin |
No |
0.506 |
2.492 |
2.927 |
no |
no |
0.286 |
4.235 |
| Luteolin |
No |
0.499 |
2.455 |
2.409 |
no |
no |
0.326 |
3.169 |
| (-)-Epicatechin gallate |
No |
0.449 |
2.558 |
2.777 |
no |
no |
0.285 |
6.146 |
| Apigenin |
No |
0.328 |
2.45 |
2.298 |
no |
no |
0.38 |
2.432 |
| Tangeritin |
No |
0.385 |
2.368 |
0.944 |
no |
no |
0.355 |
0.144 |
| Quercetin |
No |
0.499 |
2.471 |
2.612 |
no |
no |
0.288 |
3.721 |
| Kaempferol |
No |
0.531 |
2.449 |
2.505 |
no |
no |
0.312 |
2.885 |
| Rutin |
No |
0.452 |
2.491 |
3.673 |
no |
no |
0.285 |
7.677 |
| Myricetin |
No |
0.51 |
2.497 |
2.718 |
no |
no |
0.286 |
5.023 |
| Fisetin |
No |
0.579 |
2.465 |
1.921 |
No |
No |
0.376 |
2.273 |
| Astragalin |
No |
0.582 |
2.546 |
4.53 |
No |
No |
0.285 |
6.735 |
| Naringenin |
No |
-0.176 |
1.791 |
1.944 |
No |
No |
0.369 |
2.136 |
| Hesperidin |
No |
0.525 |
2.506 |
3.167 |
No |
No |
0.285 |
7.131 |
| Hesperetin |
No |
0.25 |
2.042 |
2.605 |
No |
No |
0.39 |
2.305 |
| Cyanidin |
No |
0.497 |
2.464 |
2.542 |
No |
No |
0.29 |
2.548 |
| Delphinidin |
No |
0.503 |
2.548 |
3.09 |
No |
No |
0.286 |
3.85 |
| Malvidin |
No |
0.554 |
2.346 |
2.412 |
No |
No |
0.327 |
1.224 |
| Genistein |
No |
0.478 |
2.268 |
2.189 |
No |
No |
0.377 |
No |
| Daidzein |
No |
0.187 |
2.164 |
1.187 |
No |
No |
0.693 |
1.035 |
| Dalbergin |
No |
0.097 |
2.012 |
0.889 |
No |
No |
0.467 |
0.581 |
| Peonidin |
No |
0.568 |
2.408 |
2.434 |
No |
No |
0.319 |
1.409 |
| Equol |
No |
-0.497 |
2.384 |
1.924 |
No |
No |
0.849 |
1.76 |
4. Conclusions
Among the compounds studied, hesperidin stands out, showing excellent potential properties and demonstrating low toxicity in both short- and long-term evaluations. Parameters, including Max. Tolerated Dose (Human), Acute Oral Rat Toxicity (LD50), Chronic Oral Rat Toxicity (LOAEL), and Minnow Toxicity collectively contribute to a favorable characterization of hesperidin.The low Max. tolerated dose (human) suggests minimal potential for harm to humans, while the acute oral rat toxicity (LD50) indicates a relatively low level of acute toxicity. The chronic oral toxicity value for rats (LOAEL) suggests a higher dose threshold for observing adverse effects over a prolonged period. Furthermore, the low toxicity of minnows highlights the minimal impact of hesperidin on aquatic organisms.
These findings collectively position hesperidin as a promising candidate among flavonoids, demonstrating not only its potential health benefits but also its safety with minimal adverse effects. Further research and exploration of the therapeutic potential of hesperidin, taking into account its favorable toxicity profile, could contribute to its use in various applications, including pharmaceutical and nutritional contexts.
Author Contributions
Protocol designed by IVF . All authors read and approved the final manuscript.
Conflicts of Interest
Authors declare that they do not have any conflict of interest
References
- Abbas, M., Saeed, F., Anjum, F. M., Afzaal, M., Tufail, T., Bashir, M. S., ... & Suleria, H. A. R. (2017). Natural polyphenols: An overview. International Journal of Food Properties, 20(8), 1689-1699. [CrossRef]
- Scalbert, A., Johnson, I. T., & Saltmarsh, M. (2005). Polyphenols: antioxidants and beyond. The American journal of clinical nutrition, 81(1), 215S-217S. [CrossRef]
- Williamson, G. (2017). The role of polyphenols in modern nutrition. Nutrition bulletin, 42(3), 226-235. [CrossRef]
- Rasouli, H., Farzaei, M. H., & Khodarahmi, R. (2017). Polyphenols and their benefits: A review. International Journal of Food Properties, 20(sup2), 1700-1741. [CrossRef]
- Pires, D. E., Blundell, T. L., & Ascher, D. B. (2015). pkCSM: predicting small-molecule pharmacokinetic and toxicity properties using graph-based signatures. Journal of medicinal chemistry, 58(9), 4066-4072. [CrossRef]
- Panche, A. N., Diwan, A. D., & Chandra, S. R. (2016). Flavonoids: an overview. Journal of nutritional science, 5, e47. [CrossRef]
- DHarborne, J. B., & Mabry, T. J. (2013). The flavonoids: advances in research.
- Havsteen, B. H. (2002). The biochemistry and medical significance of the flavonoids. Pharmacology & therapeutics, 96(2-3), 67-202. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).