Submitted:
15 December 2023
Posted:
19 December 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
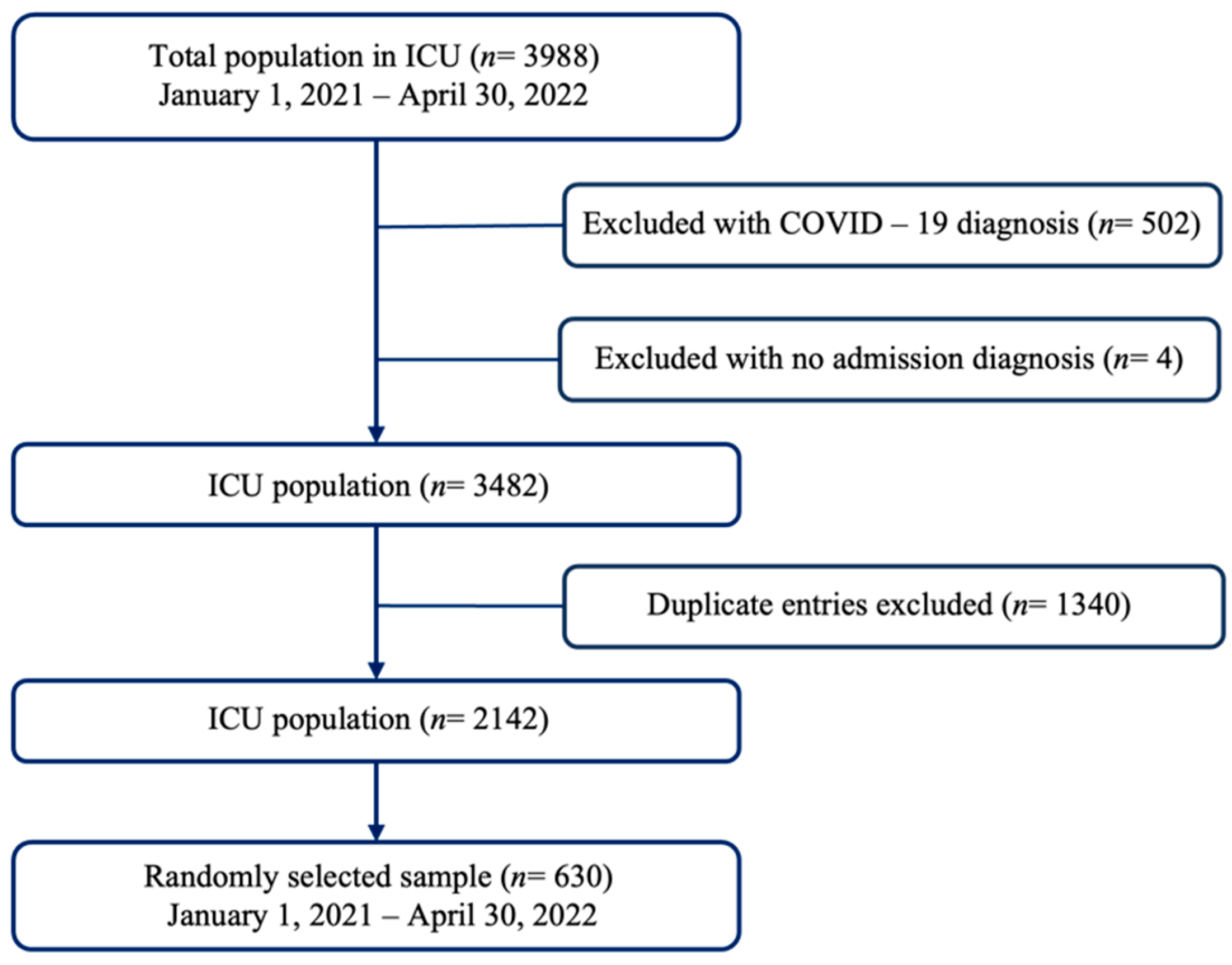
2.1. Design, Population, and Sample
2.2. Eligibility Criteria
2.3. Variables and Measurement
2.3.1. Explanatory Variables
2.3.2. Outcome Variable
2.4. Measurement Instruments
2.5. Ethical Considerations
2.6. Statistical Analysis
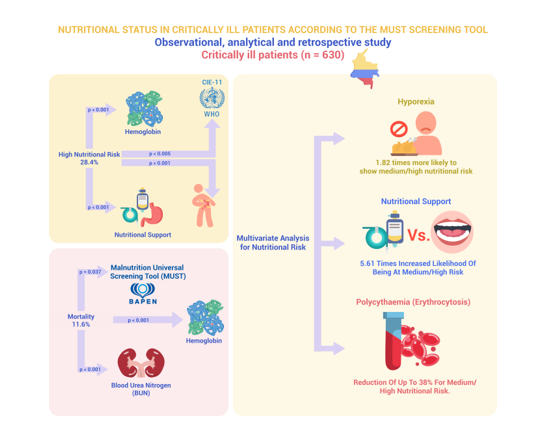
3. Results
3.1. Characterization of the Study Participants
3.2. Hematological and Biochemical Parameters Associated with Nutritional Risk
3.3. Morbidity, Gastrointestinal Symptoms, and Route of Nutritional Support Associated with Nutritional Risk
3.4. Factors Related to Mortality in the ICU
3.5. Multivariate Analysis for Nutritional Risk
4. Discussion
4.1. Diseases and Nutritional Risk by Sex
4.2. Hemoglobin and Electrolyte Values Associated with Nutritional Risk
4.3. Morbidity, Gastrointestinal Symptoms, and Nutritional Support Route Associated with Nutritional Risk
4.4. Nutritional Risk and Hematological and Biochemical Parameters Associated with Mortality in the ICU
4.5. MUST Nutritional Risk Associative Model
4.6. Study Limitations
5. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Robinson MK, Mogensen KM, Casey JD, McKane CK, Moromizato T, Rawn JD, Christopher KB. The relationship among obesity, nutritional status, and mortality in the critically ill. Critical Care Medicine. 1;43.1 (2015):87-100. [CrossRef]
- Ferrie S. What is nutritional assessment? A quick guide for critical care clinicians. Australian Critical Care. 33.3 (2020): 295-299. [CrossRef]
- Leij-Halfwerk S, Verwijs M.H, van Houdt S, Borkent J.W, Guaitoli P.R, Pelgrim T., ... & Manuel Consortium. Prevalence of protein-energy malnutrition risk in European older adults in community, residential and hospital settings, according to 22 malnutrition screening tools validated for use in adults≥ 65 years: a systematic review and meta-analysis. Maturitas, 126 (2019): 80-89. [CrossRef]
- Agarwal E, Ferguson M, Banks M, Batterham M, Bauer J, Capra S. & Isenring E. Malnutrition and poor food intake are associated with prolonged hospital stay, frequent readmissions, and greater in-hospital mortality: results from the Nutrition Care Day Survey 2010. Clinical nutrition, 32. 5 (2013): 737-745. [CrossRef]
- Barker L.A, Gout B.S., & Crowe T.C. Hospital malnutrition: prevalence, identification and impact on patients and the healthcare system. International journal of environmental research and public health, 8.2 (2011): 514-527. [CrossRef]
- Lam N.V, Sulo S, Nguyen H.A, Nguyen T.N, Brunton C, Duy N.N & Nguyen H.B. High prevalence and burden of adult malnutrition at a tertiary hospital: An opportunity to use nutrition-focused care to improve outcomes. Clinical Nutrition Open Science, 40 (2021): 79-88. [CrossRef]
- Allard J.P, Keller H, Jeejeebhoy K.N, Laporte M, Duerksenm D.R, Gramlich L, & Lou, W. Malnutrition at hospital admission—contributors and effect on length of stay: a prospective cohort study from the Canadian Malnutrition Task Force. Journal of Parenteral and Enteral Nutrition, 40.4 (2016): 487-497. DOI: . [CrossRef]
- Correia M.I.T, Perman M.I & Waitzberg D.L. Hospital malnutrition in Latin America: A systematic review. Clinical nutrition, 36.4 (2017): 958-967. [CrossRef]
- Correia, M.I.T.D. Nutrition screening vs nutrition assessment: what’s the difference?. Nutrition in Clinical Practice. 33.1 (2018): 62-72. [CrossRef]
- Guzman, M.A., & Calamba, P.G.S. Level Of Knowledge, Attitudes And Practices On Adult Patient’s Hospital Malnutrition Among Resident Physicians In A Tertiary Government Hospital. Clinical Nutrition ESPEN, 54 (2023) 525. Available online: https://clinicalnutritionespen.com/article/S2405-4577(22)00698-2/pdf (accessed on 8 september 2023).
- Kirkland LL, Shaughnessy E. Recognition and prevention of nosocomial malnutrition: a review and a call to action. Am J Med; 130.12 (2017):1345e50. [CrossRef]
- Marshall AP, Takefala T, Williams LT, Spencer A, Grealish L, Roberts S. Health practitioner practices and their influence on nutritional intake of hospitalised patients. Int J Nurs Sci. 6.2 (2019):162e8. [CrossRef]
- Weijs PJ, Looijaard WG, Dekker IM, Stapel SN, Girbes AR, Oudemans-van Straaten HM, et al. Low skeletal muscle area is a risk factor for mortality in mechanically ventilated critically ill patients. Crit Care. 18 (2014):R12. [CrossRef]
- Mogensen KM, Robinson MK, Casey JD, Gunasekera NS, Moromizato T, Rawn JD, Christopher KB. Nutritional Status and Mortality in the Critically Ill. Crit Care Med. 43.12 (2015):2605-15. [CrossRef]
- Jensen GL, Cederholm T, Correia ITD, Gonzalez MC, Fukushima R, Higashiguchi T. GLIM criteria for the diagnosis of malnutrition: a consensus report from the global clinical nutrition community. JPEN (J Parenter Enteral Nutr) 43.1 (2019): 32e40. [CrossRef]
- Inciong, J.F.B., Chaudhary, A., Hsu, H. S., Joshi, R., Seo, J. M., Trung, L. V., ... & Usman, N. Hospital malnutrition in northeast and southeast Asia: A systematic literature review. Clinical nutrition ESPEN, 39 (2020): 30-45. [CrossRef]
- Inciong JFB, Chaudhary A, Hsu HS, Joshi R, Seo JM, Trung LV, Ungpinitpong W, Usman N, Pradelli L, Omaralsaleh AJ. Economic burden of hospital malnutrition: A cost-of-illness model. Clin Nutr ESPEN. 48 (2022): 342-350. [CrossRef]
- Cederholm T, Bosaeus I, Barazzoni R, Bauer J, Van Gossum A, Klek S, Muscaritoli M, Nyulasi I, Ockenga J, Schneider SM, de van der Schueren MA, Singer P. Diagnostic criteria for malnutrition - An ESPEN Consensus Statement. Clin Nutr. 34.3 (2015):335-40. [CrossRef]
- Ceniccola G.D,.C. Relevance of AND-ASPEN criteria of malnutrition to predict hospital mortality in Holanda T.P, Pequeno R.S.F, Mendonça V.S, Oliveira A.B.M, Carvalho L.S.F., & Araujo W.M critically ill patients: A prospective study. Journal of critical care, 44 (2018): 398-403. [CrossRef]
- Fontes D, Generoso Sde V, Toulson Davisson Correia MI. Subjective global assessment: a reliable nutritional assessment tool to predict outcomes in critically ill patients. Clin Nutr. 33.2 (2014):291-5. [CrossRef]
- Atalay BG, Yagmur C, Nursal TZ, Atalay H, Noyan T. Use of subjective global assessment and clinical outcomes in critically ill geriatric patients receiving nutrition support. JPEN J Parenter Enteral Nutr. 32.4 (2008):454-9. [CrossRef]
- Lew CCH, Yandell R, Fraser RJL, Chua AP, Chong MFF, Miller M. Association Between Malnutrition and Clinical Outcomes in the Intensive Care Unit: A Systematic Review [Formula: see text]. JPEN J Parenter Enteral Nutr. 41.5 (2017):744-758. [CrossRef]
- Díaz G, T D Correia MI, Gonzalez MC, Reyes M. The global leadership initiative on malnutrition criteria for the diagnosis of malnutrition in patients admitted to the intensive care unit: A systematic review and meta-analysis. Clin Nutr. 42.2 (2023):182-189. [CrossRef]
- Theilla, M., Rattanachaiwong, S., Kagan, I., Rigler, M., Bendavid, I., & Singer, P. (2021). Validation of GLIM malnutrition criteria for diagnosis of malnutrition in ICU patients: An observational study. Clinical Nutrition. 40.5 (2021): 3578-3584. [CrossRef]
- Stratton RJ, Hackston A, Longmore D, Dixon R, Price S, Stroud M, et al. Malnutrition in hospital outpatients and inpatients: prevalence, concurrent validity and ease of use of the ‘malnutrition universal screening tool’ (‘MUST’) for adults. British Journal of Nutrition. Cambridge University Press; 92.5 (2004): 799–808. [CrossRef]
- Papapietro K, Méndez C, Matos AA, et al. Current clinical nutrition practices in critically ill patients in Latin America: a multinational observational study. Crit Care. 21 (2017): 227. [CrossRef]
- Abate, Semagn Mekonnen, et al. Prevalence and outcomes of Malnutrition among hospitalized COVID-19 patients: A systematic review and meta-analysis. Clinical Nutrition ESPEN 43 (2021): 174-183. [CrossRef]
- Castro-Vega I, Veses-Martín S, Cantero-Llorca J, Salom-Vendrell C, Bañuls C, Hernández-Mijares A. Validación del cribado nutricional Malnutrition Screening Tool comparado con la valoración nutricional completa y otros cribados en distintos ámbitos sociosanitarios. Nutr. Hosp. 2018 [Internet]; 35(2):351-358. Available online: https://scielo.isciii.es/scielo.php?script=sci_arttext&pid=S0212-16112018000200351 (accessed on 26 jun 2023).
- British Association of Parenteral and Enteral Nutrition – BAPEN. Malnutrition Universal Screening Tool (Instrumento universal para el cribado de la malnutrición) [Internet]. 2003 Available online: https://www.bapen.org.uk/images/pdfs/must/spanish/must-toolkit.pdf (accessed on 25 august 2023).
- Savin, Z., Kupershmidt, A., Phollan, D., Lazarovich, A., Rosenzweig, B., Shashar, R., ... & Mano, R. The role of malnutrition universal screening tool in predicting outcomes after radical cystectomy. Surgical Oncology. 49 (2023): 101962. [CrossRef]
- Park J, Jeon K, Chung C.R, Yang J.H, Cho Y.H, Cho J., Et al. nationwide analysis of intensive care unit admissions, 2009–2014–The Korean ICU National Data (KIND) study. Journal of Critical Care, 44 (2018): 24-30. [CrossRef]
- Thompson K, Finfer S, Woodward M, Leong R, Liu B. Sex differences in sepsis hospitalisations and outcomes in older women and men: a prospective cohort study. Journal of Infection 84.6 (2022): 770-776. [CrossRef]
- Jaitovich A, Khan M, Itty R, Chieng H, Dumas C, Nadendla P., Et al. ICU admission muscle and fat mass, survival, and disability at discharge: a prospective cohort study. Chest, 155.2 (2019): 322-330. [CrossRef]
- Kumar SI, Doo K, Sottilo-Brammeier J, Lane C, Liebler JM. Super Obesity in the Medical Intensive Care Unit. J Intensive Care Med. 35.5 (2020):478-484. [CrossRef]
- Anesi G, Savarimuthu S, Invernizzi J, Hyman R, Ramkillawan A, Eddey C., Et al. ICU Mortality Across Prepandemic and Pandemic Cohorts in a Resource-Limited Setting: A Critical Care Resiliency Analysis From South Africa. CHEST Critical Care, 1.1 (2023):100005. [CrossRef]
- Parent B, Seaton M, and O’Keefe G.E. "Biochemical markers of nutrition support in critically ill trauma victims." Journal of Parenteral and Enteral Nutrition 42.2 (2018): 335-342. [CrossRef]
- Drews R. Critical issues in hematology: anemia, thrombocytopenia, coagulopathy, and blood product transfusions in critically ill patients. Clinics in chest medicine 24.4 (2003): 607-622. [CrossRef]
- Vincent J, Baron J.F, Reinhart K, Gattinoni L, Thijs L, Webb A., Et al. Anemia and blood transfusion in critically ill patients. Jama, 288. 12 (2002): 1499-1507. [CrossRef]
- Go AS, Yang J, Ackerson LM, Lepper K, Robbins S, Massie BM, Shlipak MG. Hemoglobin level, chronic kidney disease, and the risks of death and hospitalization in adults with chronic heart failure: the Anemia in Chronic Heart Failure: Outcomes and Resource Utilization (ANCHOR) Study. Circulation. 13;113. 23 (2006): 2713-23. [CrossRef]
- Salisbury AC, Alexander KP, Reid KJ, Masoudi FA, Rathore SS, Wang TY, Bach RG, Marso SP, Spertus JA, Kosiborod M. Incidence, correlates, and outcomes of acute, hospital-acquired anemia in patients with acute myocardial infarction. Circ Cardiovasc Qual Outcomes. 3(4) (2010):337-46. [CrossRef]
- Mehdi U, Toto RD. Anemia, diabetes, and chronic kidney disease. Diabetes Care. 2009 Jul; 32.7 (2009):1320-6. DOI: https://doi.org/10.2337%2Fdc08-0779.
- Similowski T, Agustí A, MacNee W, Schönhofer B. The potential impact of anaemia of chronic disease in COPD. Eur Respir J ;27 (2006):390–396. DOI: . [CrossRef]
- Khamiees M, Raju P, DeGirolamo A, AmoatengAdjepong Y, Manthous CA. Predictors of extubation outcome in patients who have successfully completed a spontaneous breathing trial. Chest. 120 (2001):1262–1270. [CrossRef]
- Rasmussen L, Christensen S, Lenler-Petersen P, Johnsen SP. Anemia and 90-day mortality in COPD patients requiring invasive mechanical ventilation. Clin Epidemiol. 17;3 (2010):1-5. [CrossRef]
- Hayden SJ, Albert TJ, Watkins TR, Swenson ER. Anemia in critical illness: insights into etiology, consequences, and management. Am J Respir Crit Care Med. 15;185(10) (2012):1049-57. [CrossRef]
- Zaher S. "Observational study to assess the relationship between enteral nutrition delivery and nutritional biomarkers among mechanically ventilated critically ill patients." Saudi Journal of Biological Sciences 29.12 (2022): 103466. DOI: . [CrossRef]
- Son D.H, Kim K.S, Lee H.S, Lee JW., & Shin C.S. Derivation and validation of a new nutritional index for predicting 90 days mortality after ICU admission in a Korean population. Journal of the Formosan Medical Association, 119.8 (2020): 1283-1291. [CrossRef]
- Wu L, Yan Q, Mai H, Song J, Ye L, Che X, & Wang L. Does the Geriatric Nutritional Risk Index Play a Predictive Role in Postoperative Atrial Fibrillation and Outcomes in Cardiac Surgery?. Journal of Cardiothoracic and Vascular Anesthesia, 37.1 (2023): 58-64. [CrossRef]
- Stratton R, Beggs E, Holmes E, Burden S, & Cawood A. National survey of malnutrition and nutritional care in adults. (2021): [Internet]. Available online: https://www.bapen.org.uk/pdfs/reports/mag/national-survey-of-malnutrition-and-nutritional-care-2020.pdf (accesed on 10 july 2023).
- Morton M, Patterson J, Sciuva J, Perni, J, Backes F, Nagel C y Chambers LM. Malnutrition, sarcopenia, and cancer cachexia in gynecologic cancer. Gynecologic Oncology 175 (2023): 142-155. [CrossRef]
- Allen B. y Saunders J. Malnutrition and undernutrition: causes, consequences, assessment and management, Medicine. 51 (2023): 461 - 468. [CrossRef]
- Razon A.H, Haque M.I, Ahmed M.F., & Ahmad T. Assessment of dietary habits, nutritional status and common health complications of older people living in rural areas of Bangladesh. Heliyon, 8(2) (2022). [CrossRef]
- Rosato E, Gigante A, Pellicano C, Colalillo A, Alunni-Fegatelli D., & Muscaritoli M. Phase angle, nutritional status, and mortality in systemic sclerosis: An exploratory pilot study. Nutrition 107 (2023): 111946. [CrossRef]
- Lima J, Dias A.J.B, Burgel C.F, Bernardes S, Gonzalez M.C., & Silva F.M. Complementarity of nutritional screening tools to GLIM criteria on malnutrition diagnosis in hospitalised patients: A secondary analysis of a longitudinal study. Clinical Nutrition, 41.10 (2022): 2325-2332. [CrossRef]
- Pearcy J, Agarwal E, Isenring E, Somani A, Wright C., & Shankar B. Ward-based nutrition care practices and a snapshot of patient care: Results from nutritionDay in the ICU. Clinical nutrition ESPEN 41 (2021): 340-345. [CrossRef]
- McClave S.A, Taylor B.E, Martindale R.G, Warren M.M, Johnson D.R, Braunschweig C & Compher C. Guidelines for the provision and assessment of nutrition support therapy in the adult critically ill patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (ASPEN). JPEN. Journal of parenteral and enteral nutrition, 40(2) (2016): 159-211. [CrossRef]
- Burch N. Artificial nutrition support. Medicine, 51(7) (2023): 474-479. [CrossRef]
| Hematological parameter | ||
| Hemoglobin | Normal range | 12.3–15.3 g/dL |
| Anemia | <12.3 g/dL | |
| Polycythemia | >15.3 g/dL | |
| Leukocyte levels | Normal range | 4.5–11 × 103/uL |
| Leukocytosis | >11 × 103/uL | |
| Leukopenia | <4.5 × 103/uL | |
| Lymphocyte levels | Normal range | 1.0– 4.8 × 103/uL |
| Lymphocytosis | >4.8 × 103/uL | |
| Lymphopenia | <1.0 × 103/uL | |
| Biochemical parameters | ||
| Urea nitrogen levels | Normal range | 7–20 mg/dL |
| Increased | >20 mg/dL | |
| Decreased | <7 mg/dL | |
| Creatinine levels | Normal range | 0.7–1.4 mg/dl |
| Increased | >1.4 mg/dl | |
| Decreased | <0.7 mg/dl | |
| Chlorine levels | Normal range | 96 – 106 mEq/L |
| Hyperchloremia | >106 mEq/L | |
| Hypochloremia | <96 mEq/L | |
| Potassium levels | Normal kalemia | 3.5 – 5.0 mEq/L |
| Hyperkalemia | >5 mEq/L | |
| Hypokalemia | <3.5 mEq/L | |
| Sodium levels | Normal range | 135 – 145 mEq/L |
| Hypernatremia | >145 mEq/L | |
| Hyponatremia | <135 mEq/L | |
| Variables | Total x̄ (±SD) n (%) | Female x̄ (±SD) 289 (45.87%) | Male x̄ (±SD) 341 (54.13%) | p |
|---|---|---|---|---|
| Age | 64.75 (± 16.21) | 63.32 (± 18.08) | 65.97 (± 14.35) | 0.041 |
| Cause of admission ICD-11 | ||||
| Circulatory and respiratory diseases | 315 (50) | 123 (39) | 192 (61) | 0.011 |
| Neoplasms | 107 (17) | 50 (46.7) | 57 (53.3) | |
| Nervous and musculoskeletal diseases and trauma | 83 (13.1) | 45 (54.2) | 38 (45.8) | |
| Endocrine and digestive diseases | 47 (7.5) | 28 (59.6) | 19 (40.4) | |
| Infectious or parasitic diseases | 33 (5.2) | 17 (51.5) | 16 (48.5) | |
| Other diseases | 45 (7.1) | 26 (57.8) | 19 (42.2) | |
| Days of hospitalization | 6.06 (8.49) | 6.25 (10.24) | 5.90 (6.69) | 0.612 |
| Deaths | 73 (11.6) | 35 (48) | 38 (52.) | 0.706 |
| Weight | 66.35 (± 13.78) | 61.95 (± 12.55) | 70.08 (± 13.68) | <0.001 |
| BMI | 24.76 (± 4.75) | 24.83 (± 4.98) | 24.70 (± 4.57) | 0.746 |
| Nutritional risk | ||||
| Low risk | 383 (60.8) | 161 (42) | 222 (58) | 0.054 |
| Medium risk | 68 (10.8) | 36 (52.9) | 32 (47.1) | |
| High risk | 179 (28.4) | 92 (51.4) | 87 (48.6) | |
| Laboratory tests | x̄ (±SD) | MUST scale | |||
|---|---|---|---|---|---|
| Low risk | Medium risk | High risk | p | ||
| Hemoglobin (g/dL) | 11.46 (2.60) | 11.79 (2.67) | 11.21 (2.55) | 10.84 (2.32) | <0.001 |
| Hematocrit (%) | 35.37 (7.68) | 36.35 (7.80) | 34.76 (7.91) | 33.49 (6.98) | <0.001 |
| Leukocytes (x103/uL) | 10.68 (17.22) | 10.21 (11.05) | 9.14 (5.82) | 12.27 (27.73) | 0.308 |
| Lymphocytes (x103/uL) | 2.01 (9.77) | 2.08 (10.86) | 1.43 (0.70) | 2.04 (8.89) | 0.876 |
| BUN (mg/dL) | 25.63 (18.41) | 25.40 (18.49) | 23.72 (16.68) | 26.83 (18.88) | 0.460 |
| Creatinine (mg/dL) | 1.61 (2.08) | 1.65 (2.08) | 1.67 (2.25) | 1.50 (1.99) | 0.716 |
| Chlorine (mEq/L) | 104.09 (7.89) | 104.45 (5.99) | 104.89 (4.27) | 103.02 (11.51) | 0.091 |
| Potassium (mEq/L) | 4.15 (0.64) | 4.15 (0.59) | 4.14 (0.61) | 4.14 (0.75) | 0.971 |
| Sodium (mEq/L) | 139.13 (5.03) | 139.28 (5.42) | 139.61 (3.15) | 138.62 (4.68) | 0.251 |
| MUST scale nutritional risk | High risk n (%) |
Medium risk n (%) |
Low risk n (%) |
Total | p |
|---|---|---|---|---|---|
| 179 (28.41) | 68 (10.8) | 383 (60.8) | 630 | ||
| Cause of admission ICD -11 | |||||
| Circulatory and respiratory diseases | 68 (21.6) | 36 (11.4) | 211 (67) | 315 (50) | 0.005 |
| Neoplasms | 41 (38.3) | 9 (8.4) | 57 (53.3) | 107 (17) | |
| Nervous and musculoskeletal diseases and trauma | 20 (24.1) | 9 (10.8) | 54 (65.1) | 83 (13.2) | |
| Endocrine and digestive diseases | 18 (38.3) | 7 (15) | 22 (46.8) | 47 (7.5) | |
| Infectious or parasitic diseases | 14 (42.4) | 1 (3) | 18 (54.6) | 33 (5.2) | |
| Other diseases | 18 (40) | 6 (13.3) | 21 (46.7) | 45 (7.1) | |
| Gastrointestinal symptoms | |||||
| Presence of hyporexia | 65 (47.8) | 8 (5.9) | 63 (46.3) | 136 (21.6) | <0.001 |
| Absence of hyporexia | 114 (23.1) | 60 (12.2) | 320 (64.8) | 494 (78.4) | |
| Presence of abdominal distension | 73 (36.1) | 21 (10.4) | 108 (53.5) | 202 (32.1) | 0.012 |
| Absence of abdominal distension | 106 (24.8) | 47 (11) | 275 (64.3) | 428 (67.9) | |
| Presence of diarrhea | 59 (43.7) | 10 (7.4) | 66 (48.9) | 135 (21.4) | <0.001 |
| Absence of diarrhea | 120 (24.2) | 58 (11.7) | 317 (64) | 495 (78.6) | |
| Presence of abdominal pain | 89 (37.6) | 20 (8.4) | 128 (54) | 237 (37.6) | <0.001 |
| Absence of abdominal pain | 90 (22.9) | 48 (12.2) | 255 (64.9) | 393 (62.4) | |
| Presence of swallowing difficulties | 70 (45.7) | 5 (3.3) | 78 (51) | 153 (24.3) | <0.001 |
| Absence of swallowing difficulties | 109 (22.9) | 63 (13.2) | 305 (63.9) | 477 (75.7) | |
| Presence of emesis | 86 (39.5) | 16 (7.3) | 116 (53.2) | 218 (34.6) | <0.001 |
| Absence of emesis | 93 (22.6) | 52 (12.6) | 267 (64.8) | 412 (65.4) | |
| Nutritional support route | |||||
| Oral route | 125 (23.5) | 64 (12.1) | 342 (64.4) | 531 (84.3) | <0.001 |
| Enteral route | 37 (48.1) | 4 (5.2) | 36 (46.8) | 77 (12.2) | |
| Parenteral route | 17 (77.3) | 0 | 5 (22.7) | 22 (3.5) | |
| Variables | Alive x̄ (±SD) n (%) |
Dead x̄ (±SD) n (%) |
p |
|---|---|---|---|
| Nutritional risk according to MUST | |||
| High risk | 149 (26.8) | 30 (41.1) | 0.037 |
| Medium risk | 62 (11.1) | 6 (8.2) | |
| Low risk | 346 (62.1) | 37 (50.7) | |
| Hematological parameters | |||
| Hemoglobin (g/dL) | 11.60 (2.63) | 10.39 (20.06) | < 0.001 |
| Leukocytes (x103/uL) | 10.52 (17.92) | 11.85 (10.52) | 0.533 |
| Lymphocytes (x103/uL) | 2.12 (10.39) | 1.10 (0.56) | 0.396 |
| Biochemical parameters | |||
| BUN (mg/dL) | 24.32 (16.89) | 35.45 (25.33) | < 0.001 |
| Creatinine (mg/dL) | 1.55 (2.06) | 2.02 (2.08) | 0.068 |
| Chlorine (mEq/L) | 104.15 (6.78) | 103.61 (13.21) | 0.728 |
| Potassium (mEq/L) | 4.13 (0.62) | 4.23 (0.77) | 0.318 |
| Sodium (mEq/L) | 139.17 (4.73) | 138.78 (6.86) | 0.631 |
| Raw estimate (unadjusted) | Adjusted estimate | |||||||
|---|---|---|---|---|---|---|---|---|
| Variables | Low Nutritional Risk | Medium / High Nutritional Risk | OR | 95% CI | p | OR | 95% CI | p |
| Hyporexia | ||||||||
| No | 320 (64.78) | 174 (35.22) | 1 (Ref.) | - | - | 1 (Ref.) | - | - |
| Yes | 63 (46.32) | 73 (53.68) | 0.47 | 0.32 - 0.69 | 0.000 | 1.82 | 1.22 - 2.71 | 0.003 |
| Interpretation of hemoglobin levels | ||||||||
| Normal range | 122 (65.95) | 63 (34.05) | 1 (Ref.) | - | - | 1 (Ref.) | - | - |
| Polycythemia | 45 (83.33) | 9 (16.67) | 0.39 | 0.18 - 0.84 | 0.017 | 0.38 | 0.17 - 0.84 | 0.017 |
| Anemia | 216 (55.24) | 175 (44.76) | 1.57 | 1.09 - 2.26 | 0.015 | 1.31 | 0.89 - 1.91 | 0.168 |
| Nutritional support route | ||||||||
| Oral support | 342 (64.41) | 189 (35.59) | 1 (Ref.) | - | - | 1 (Ref.) | - | - |
| Enteral support | 36 (46.75) | 41 (53.25) | 2.06 | 1.27 - 3.34 | 0.003 | 1.85 | 1.13 - 3.04 | 0.015 |
| Parenteral support | 5 (22.73) | 17 (77.27) | 6.15 | 2.23 - 16.94 | 0.000 | 5.61 | 2.00 - 15.74 | 0.001 |
| Evaluation of the associative model for nutritional risk | ||||||||
| Reference test | ||||||||
| Diagnostic test | Sick | Healthy | Total | |||||
| Positive | 98 | 69 | 167 | |||||
| Negative | 149 | 314 | 463 | |||||
| Total | 247 | 383 | 630 | |||||
| Value | CI (95%) | |||||||
| Sensitivity (%) | 39.68 | 33.37 | 45.98 | |||||
| Specificity (%) | 81.98 | 78.00 | 85.96 | |||||
| Validity index (%) | 65.40 | 61.60 | 69.19 | |||||
| Predictive value + | 58.68 | 50.92 | 66.45 | |||||
| Predictive value − | 67.82 | 63.46 | 72.18 | |||||
| Prevalence (%) | 39.21 | 35.31 | 43.10 | |||||
| Youden index | 0.22 | 0.14 | 0.29 | |||||
| Likelihood ratio + | 2.20 | 1.69 | 2.87 | |||||
| Likelihood ratio − | 0.74 | 0.66 | 0.82 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





