Submitted:
19 December 2023
Posted:
19 December 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Cell Culture and Insects
2.2. Immunofluorescence
2.3. Quantitative RT-PCR (qRT-PCR)
2.4. Synthesis of BmAurora B-specific siRNA fragments
2.5. Cell transfection
2.6. Statistics
3. Results
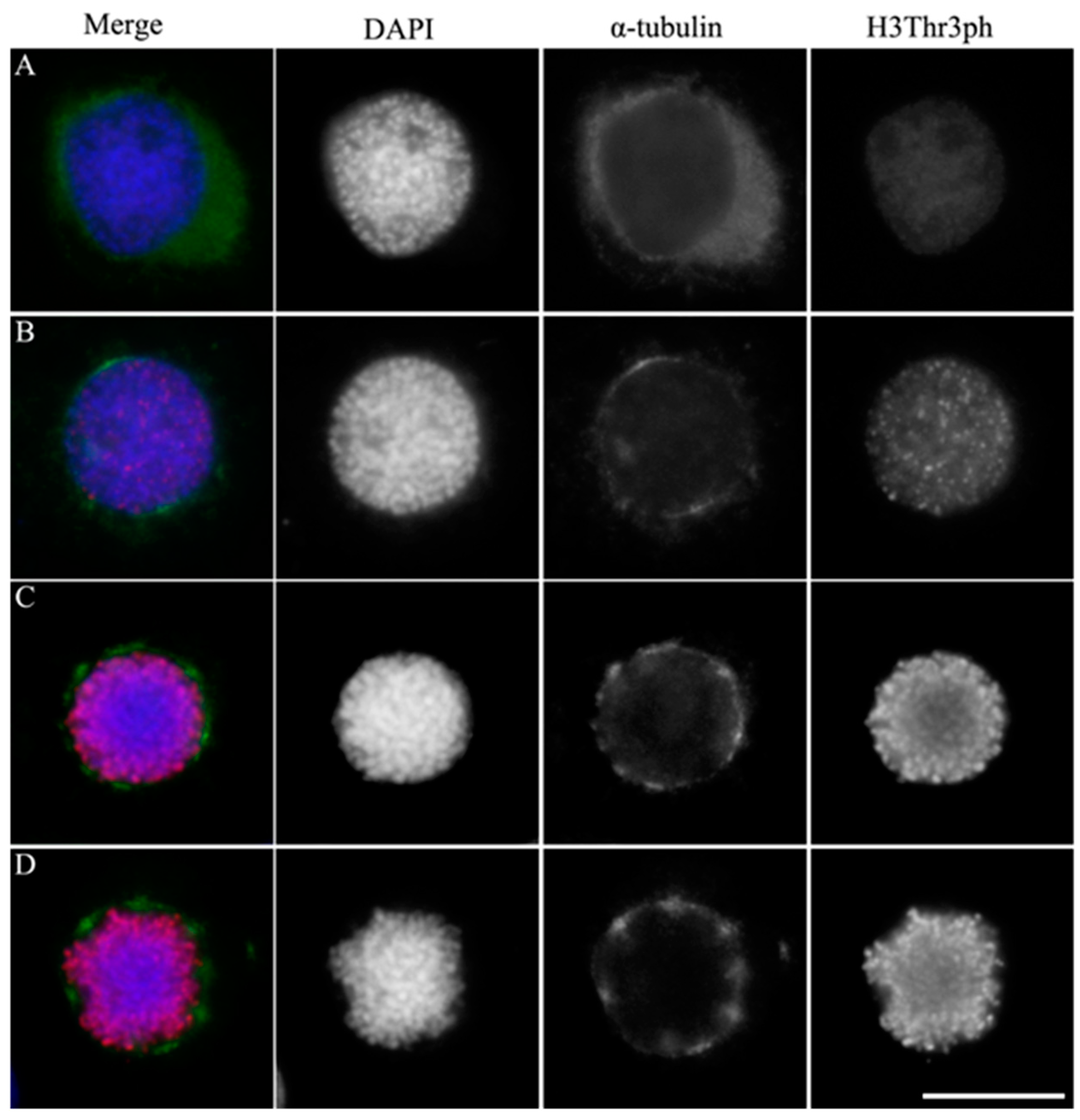
3.1. Spindle microtubule assembly in BmN4 cells
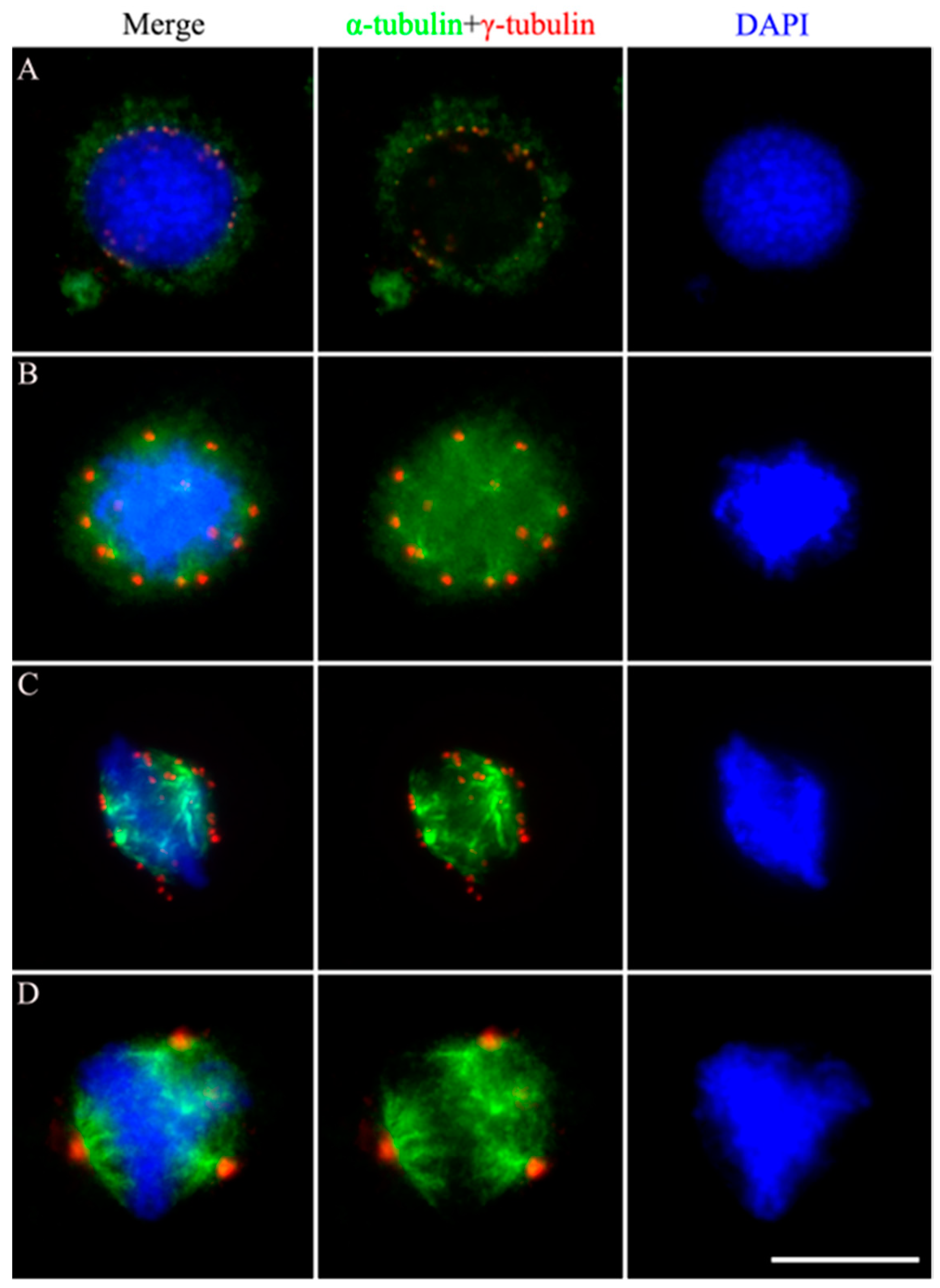
3.2. Co-localisation of α-tubulin and γ-tubulin displays square spindles in metaphase BmN4 cells.
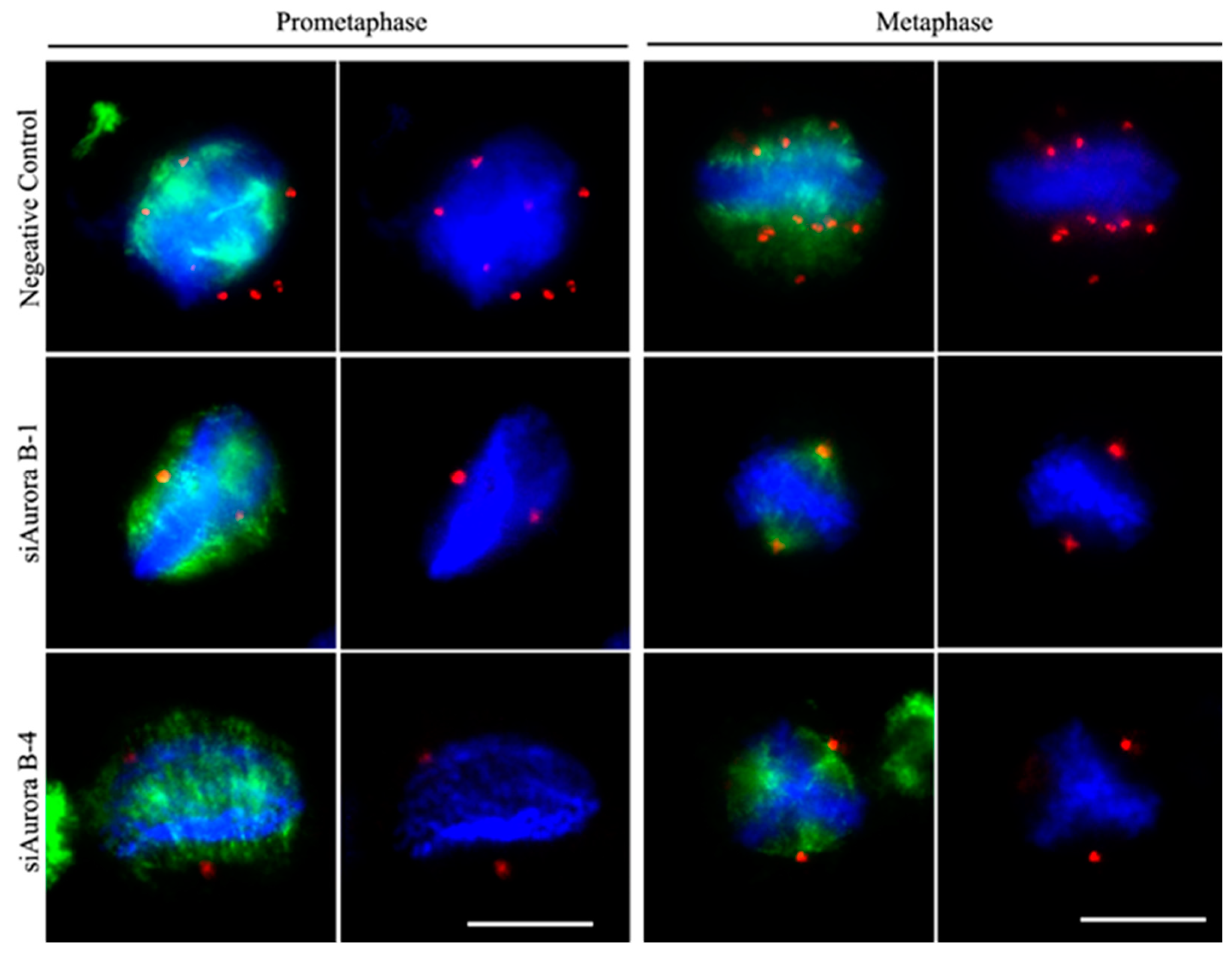
3.4. BmAurora B-depleted cells do not form square spindles in metaphase
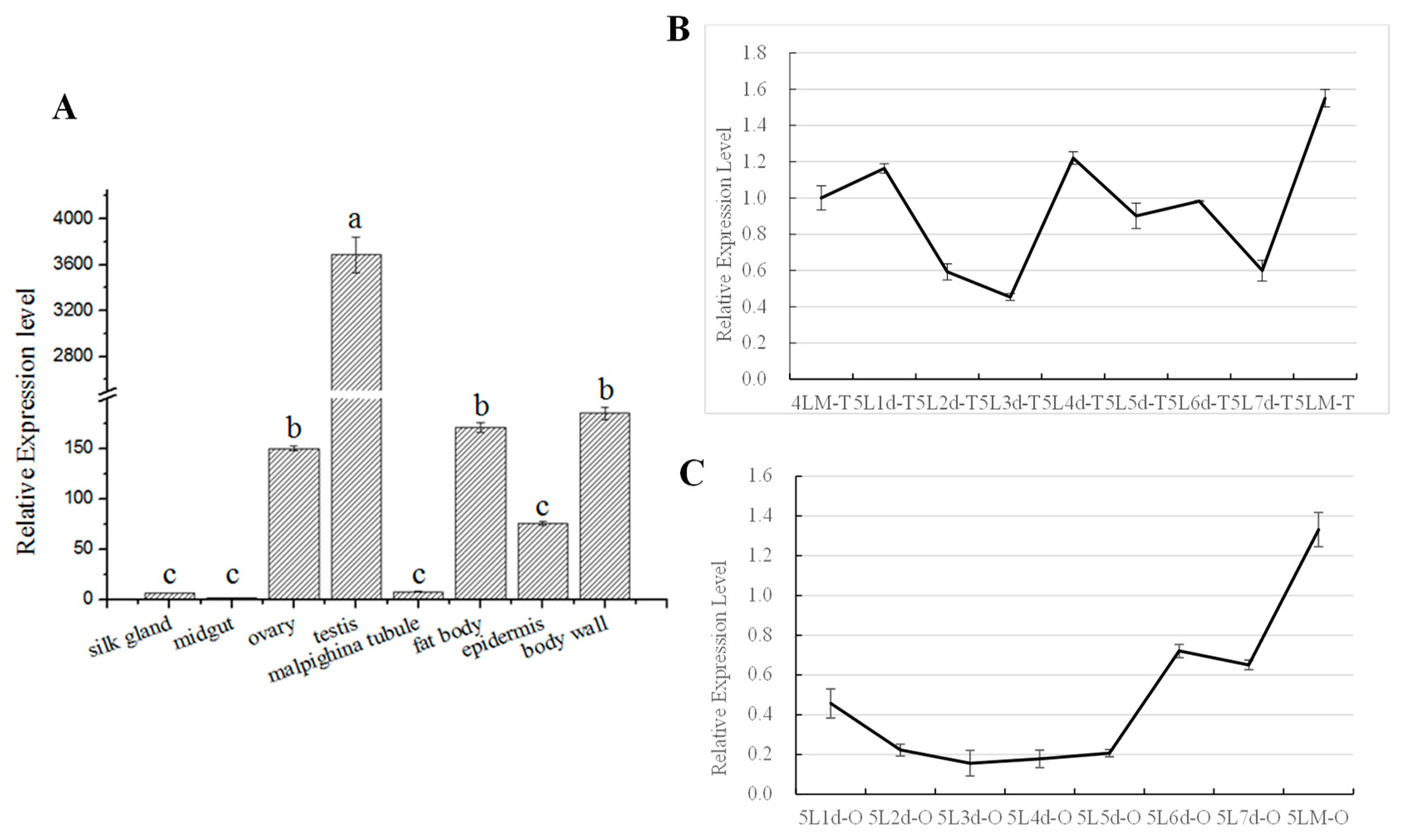
3.5. Spatiotemporal analysis of BmAurora B expression in silkworm larval tissues
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Helmke, K.J.; Heald, R.; Wilbur, J.D. Interplay between spindle architecture and function. Intern. Rev. Cell Mol. Biol. 2013, 306, 83–125. [Google Scholar] [CrossRef]
- Bornens, M. Centrosome organization and functions. Curr. Opin. Struc. Biol. 2021, 66, 199–206. [Google Scholar] [CrossRef]
- Rosselló, C.A.; Lindström, L.; Eklund, G.; Corvaisier, M.; Kristensson, M.A. γ-Tubulin-γ-tubulin interactions as the basis for the formation of a meshwork. Int. J. Mol. Sci. 2018, 19. [Google Scholar] [CrossRef]
- Akhmanova, A.; Kapitein, L.C. Mechanisms of microtubule organization in differentiated animal cells. Nat. Rev. Mol. Cell Biol. 2022, 23, 541–558. [Google Scholar] [CrossRef]
- Zhang, H.; Dawe, R.K. Mechanisms of plant spindle formation. Chromosome Res. 2011, 19, 335–344. [Google Scholar] [CrossRef]
- Mogessie, B.; Scheffler, K.; Schuh, M. Assembly and positioning of the oocyte meiotic spindle. Annu Rev. Cell Dev. Biol. 2018, 34, 381–403. [Google Scholar] [CrossRef]
- Meunier, S.; Vernos, I. Microtubule assembly during mitosis - from distinct origins to distinct functions? J. Cell Sci. 2012, 125, 2805–2814. [Google Scholar] [CrossRef]
- Meunier, S.; Vernos, I. Acentrosomal microtubule assembly in mitosis: the where, when, and how. Trends Cell Biol. 2016, 26, 80–87. [Google Scholar] [CrossRef]
- Clarke, P.R.; Zhang, C. Spatial and temporal coordination of mitosis by Ran GTPase. Nature Rev. Mol. Cell Biol. 2008, 9, 464–477. [Google Scholar] [CrossRef]
- Carmena, M.; Wheelock, M.; Funabiki, H.; Earnshaw, W.C. The chromosomal passenger complex (CPC): from easy rider to the godfather of mitosis. Nature Rev. Mol. Cell Biol. 2012, 13, 789–803. [Google Scholar] [CrossRef]
- Carazo-Salas, R.E.; Guarguaglini, G.; Gruss, O.J.; Segref, A.; Karsenti, E.; Mattaj, I.W. Generation of GTP-bound Ran by RCC1 is required for chromatin-induced mitotic spindle formation. Nature 1999, 400, 178–181. [Google Scholar] [CrossRef]
- Kaláb, P.; Pralle, A.; Isacoff, E.Y.; Heald, R.; Weis, K. Analysis of a RanGTP-regulated gradient in mitotic somatic cells. Nature 2006, 440, 697–701. [Google Scholar] [CrossRef]
- Nachury, M.V.; Maresca, T.J.; Salmon, W.C.; Waterman-Storer, C.M.; Heald, R.; Weis, K. Importin beta is a mitotic target of the small GTPase Ran in spindle assembly. Cell 2001, 104, 95–106. [Google Scholar] [CrossRef]
- Sampath, S.C.; Ohi, R.; Leismann, O.; Salic, A.; Pozniakovski, A.; Funabiki, H. The chromosomal passenger complex is required for chromatin-induced microtubule stabilization and spindle assembly. Cell 2004, 118, 187–202. [Google Scholar] [CrossRef]
- Tseng, B.S.; Tan, L.; Kapoor, T.M.; Funabiki, H. Dual detection of chromosomes and microtubules by the chromosomal passenger complex drives spindle assembly. Dev. Cell 2010, 18, 903–912. [Google Scholar] [CrossRef]
- Pu, X.; Hong, X.; Chen, M.; Lu, C. Studies on mitosis and chromosomes of BmN cells. Science of Sericulture 2003, 29, 136–141, (In Chinese with English abstract). [Google Scholar]
- Drinnenberg, I.A.; deYoung, D.; Henikoff, S.; Malik, H.S. Recurrent loss of CenH3 is associated with independent transitions to holocentricity in insects. eLife 2014, 3, e03676. [Google Scholar] [CrossRef]
- Mon, H.; Lee, J.M.; Mita, K.; Goldsmith, M.R.; Kusakabe, T. Chromatin-induced spindle assembly plays an important role in metaphase congression of silkworm holocentric chromosomes. Insect Biochem. Mol. Biol. 2014, 45, 40–50. [Google Scholar] [CrossRef]
- Vanpoperinghe, L.; Carlier-Grynkorn, F.; Cornilleau, G.; Kusakabe, T.; Drinnenberg, I.A.; Tran, P.T. Live-cell imaging reveals square shape spindles and long mitosis duration in the silkworm holocentric cells. MicroPub. Biol. 1791. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods (San Diego, Calif.) 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Giet, R.; Glover, D.M. Drosophila aurora B kinase is required for histone H3 phosphorylation and condensin recruitment during chromosome condensation and to organize the central spindle during cytokinesis. J. Cell Biol. 2001, 152, 669–682. [Google Scholar] [CrossRef]
- Bai, X.; Melesse, M.; Sorensen Turpin, C.G.; Sloan, D.E.; Chen, C.Y.; Wang, W.C.; Lee, P.Y.; Simmons, J.R.; Nebenfuehr, B.; Mitchell, D.; Klebanow, L.R.; Mattson, N.; Betzig, E.; Chen, B.C.; Cheerambathur, D.; Bembenek, J.N. Aurora B functions at the apical surface after specialized cytokinesis during morphogenesis in C. elegans. Dev. (Cambridge, England) 2020, 147. [Google Scholar] [CrossRef]
- Ferrandiz, N.; Barroso, C.; Telecan, O.; Shao, N.; Kim, H.M.; Testori, S.; Faull, P.; Cutillas, P.; Snijders, A.P.; Colaiácovo, M.P.; Martinez-Perez, E. Spatiotemporal regulation of Aurora B recruitment ensures release of cohesion during C. elegans oocyte meiosis. Nat. Commun. 2018, 9, 834. [Google Scholar] [CrossRef]
- Divekar, N.S.; Davis-Roca, A.C.; Zhang, L.; Dernburg, A.F.; Wignall, S.M. A degron-based strategy reveals new insights into Aurora B function in C. elegans. PLoS Genet. 2021, 17, e1009567. [Google Scholar] [CrossRef]
- Oakley, B.R.; Paolillo, V.; Zheng, Y. γ-Tubulin complexes in microtubule nucleation and beyond. Mol. Biol. Cell 2015, 26, 2957–2962. [Google Scholar] [CrossRef]
- Bahtz, R.; Seidler, J.; Arnold, M.; Haselmann-Weiss, U.; Antony, C.; Lehmann, W.D.; Hoffmann, I. GCP6 is a substrate of Plk4 and required for centriole duplication. J. Cell Sci. 2012, 125 Pt 2, 486–496. [Google Scholar] [CrossRef]
- Farache, D.; Emorine, L.; Haren, L.; Merdes, A. Assembly and regulation of γ-tubulin complexes. Open Biol. 2018, 8. [Google Scholar] [CrossRef]
- Bishop, J.D.; Han, Z.; Schumacher, J.M. The Caenorhabditis elegans Aurora B kinase AIR-2 phosphorylates and is required for the localization of a BimC kinesin to meiotic and mitotic spindles. Mol. Biol. Cell. 2005, 16, 742–756. [Google Scholar] [CrossRef]
- Kassamaly, I.T.; Cornilleau, G.; Drinnenberg, I.A.; Tran, P.T. Kinesin-5 and kinesin-14 are partially antagonistic in spindle assembly in the holocentric silkworm B. mori cells. MicroPubl. Biol. 0006. [Google Scholar] [CrossRef]
- Honda, R.; Körner, R.; Nigg, E.A. Exploring the functional interactions between Aurora B, INCENP, and survivin in mitosis. Mol. Biol. Cell 2003, 14, 3325e3341. [Google Scholar] [CrossRef]
- Shao, H.; Huang, Y.; Zhang, L.; Yuan, K.; Chu, Y.; Dou, Z.; Jin, C.; Garcia-Barrio, M.; Liu, X.; Yao, X. Spatiotemporal dynamics of Aurora B-PLK1-MCAK signaling axis orchestrates kinetochore bi-orientation and faithful chromosome segregation. Sci. Rep. 2015, 5, 12204. [Google Scholar] [CrossRef]
- Gadea, B.B.; Ruderman, J.V. Aurora B is required for mitotic chromatin-induced phosphorylation of Op18/Stathmin. Proc. Natl. Acad. Sci. USA. 2006, 103, 4493–4498. [Google Scholar] [CrossRef]
- Muñoz-Barrera, M.; Monje-Casas, F. Increased Aurora B activity causes continuous disruption of kinetochore–microtubule attachments and spindle instability. Proc. Natl. Acad. Sci. USA. 2014, 111, E3996–E4005. [Google Scholar] [CrossRef]
- Gang, X.; Qian, W.; Zhang, T.; Yang, X.; Xia, Q.; Cheng, D. Aurora B kinase is required for cell cycle progression in silkworm. Gene 2017, 599, 60–67. [Google Scholar] [CrossRef]
- Willems, E.; Dedobbeleer, M.; Digregorio, M.; Lombard, A.; Lumapat, P.N.; Rogister, B. The functional diversity of Aurora kinases: a comprehensive review. Cell Div. 2018, 13, 7. [Google Scholar] [CrossRef]
- Dephoure, N.; Zhou, C.; Villén, J.; Beausoleil, S.A.; Bakalarski, C.E.; Elledge, S.J.; Gygi, S.P. A quantitative atlas of mitotic phosphorylation. Proc. Natl. Acad. Sci. USA. 2008, 105, 10762–10767. [Google Scholar] [CrossRef]
- Collette, K.S.; Petty, E.L.; Golenberg, N.; Bembenek, J.N.; Csankovszki, G. Different roles for Aurora B in condensin targeting during mitosis and meiosis. J. Cell Sci. 2011, 124 Pt 21, 3684–3694. [Google Scholar] [CrossRef]

| Primer | Primer sequence (5’-3’) | Purpose |
|---|---|---|
| BmAurora B-RT-F | GGCCAAGGCAAATTCGGACATGTT | qRT-PCR |
| BmAurora B-RT-R | CGTCCTTGGGGTGAATTTGTGAGATGTT | |
| BmA3-RT-F | ATGTGCGACGAAGAAGTTGC | |
| BmA3-RT-R | GTCTCCTACGTACGAGTCCT | |
| siBmAurora B-1-s | CCAGAAAGUAAAGCAGCAATT | siRNA synthesis |
| siBmAurora B-1-as | UUGCUGCUUUACUUUCUGGTT | |
| siBmAurora B-2-s | GGGAAAGCCUCCAUUUGAATT | |
| siBmAurora B-2-as | UUCAAAUGGAGGCUUUCCCTT | |
| siBmAurora B-3-s | CCUGAUGGAGCCAAGGAUUTT | |
| siBmAurora B-3-as | AAUCCUUGGCUCCAUCAGGTT | |
| siBmAurora B-4-s | GCCAAGGAUUUGAUCUCAATT | |
| siBmAurora B-4-as | UUGAGAUCAAAUCCUUGGCTT | |
| NC-FAM-s | UUCUCCGAACGUGUCACGUTT | |
| NC-FAM-as | ACGUGACACGUUCGGAGAATT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





