1. Introduction
Attention to the role of epicardial adipose tissue (EAT) in the development of atrial fibrillation (AF) has increased in recent years. EAT plays several roles in the regulation of cardiac function and has a profound and complex interaction with the heart: it represents a storage of energy resources for the cardiac cells (in the older age), and it has thermogenesis properties (in the youngest)[
Fainberg 2018]; through endocrine and paracrine interactions it mediates inflammatory response and modulates the immune system[
Iacobellis 2004,
Iacobellis 2009]; finally, ganglionated plexi, key structures of the autonomic nervous system of the heart, are located in the EAT. It has peculiar characteristics that differentiate it from the other types of visceral adipose tissue; EAT shares properties with both white and brown adipose tissue, so that the term “beige” adipose tissue has been proposed to define it [
Sacks 2013]. Excess or dysregulation of EAT has shown to be involved in the pathogenesis of heart failure with preserved ejection fraction[
Van Woerden 2018,
Pugliese 2021] and coronary artery disease through an imbalance in the inflammatory state that promotes atherogenesis and the development of unstable plaques[
Hirata 2011,
Antonopoulos 2017]. The exact pathophysiology of the interplay between EAT and AF is not yet entirely clarified: dysfunctional or excessive EAT through the secretion of adipokines and cytokines favors an inflammatory milieu and the production of reactive oxygen species which in turn can promote fibrogenesis; on the other hand, it has been postulated that its proarrhythmic effects are mediated by modification in the expression and function of cardiac ion channels, resulting in arrhythmogenic electrical remodeling[
Friedman 2014,
Mahabadi 2014,
Wong 2016]. Indeed classic pro inflammatory cytokines – such as TNF alpha, IL 6 and IL 1 beta - as well as components of the inflammasome and of the immune response are overly expressed in the dysregulated EAT [
Mazurek 2003]. Catheter ablation is an effective and safe option for the treatment of AF, and it has earned strong recommendations in guidelines for rhythm control[
Hindricks 2021], also as first line option[
Saglietto 2021]. Nevertheless, the correct selection of the patients is fundamental in order to avoid referring to an invasive procedures patient who have high risk of recurrences. Aim of the study is to determine whether an increased volume of atrial EAT evaluated at routine pre-procedural imaging relates to the recurrence of AF after catheter ablation.
2. Materials and Methods
All patients undergoing AF cryoballoon transcatheter ablation from January 2019 to December 2021 with a preprocedural cardiac magnetic resonance (MRI) with sequences allowing for epicardial adipose tissue quantification were retrospectively included. Key exclusion criteria were age < 18 years and any contraindication to MRI. All patients signed a written informed consent. Arrhythmia recurrences were defined as ECG-documented recurrences at 12-lead ECG and 24-hour Holter recordings or as clinically recognized symptomatic episodes. All patients were routinely followed up with 24-hour ECG Holter recording at 3 and 12 months, outpatient visits and telephonic interview. The study was approved by the local ethical committee and conducted according to the declaration of Helsinki. Before the ablation procedure, all patients underwent a transesophageal echocardiography (TEE) to rule out left atrial appendage (LAA) thrombosis and cardiac MRI to assess LA anatomy. A 1.5-T scanner (Achieva, version 2.6, Philips Medical Systems, Eindhoven, The Netherlands) using a 32-channel body phased-array coil was used. Paramagnetic contrast medium (Prohance, gadoteridol, Bracco Imaging, Italy) was administered at a dose of 0.1 mmol/kg. The sequences acquired during the exam were: Angio-MRI (non ECG-gated, 4D time resolved with keyhole 4D-TRAK); LGE-MRI (free breathing navigator and ECG gated inversion recovery gradient-echo sequence). The ablation protocol adopted has been previously detailed elsewhere[
Ballatore 2023]. Briefly, through venous femoral accesses a diagnostic decapolar electrophysiological catheter was placed in the coronary sinus (CS), subsequently access to the LA was achieved through a patent foramen ovale or by single transseptal puncture and a 28-mm cryoballoon ablation catheter (Arctic Front Advance, Medtronic) and an inner lumen mapping catheter (Achieve, Medtronic) via a steerable 15Fr sheath (FlexCath Advance, Medtronic) were advanced in each pulmonary vein (PV) ostium. An activated clotting time > 350 s was maintained during the procedure. According to our standard approach, cryoenergy was delivered at each PV ostium for 180 seconds if PV isolation was achieved in less than 60 seconds, for 240 seconds for the other cases. Cryoenergy application was immediately stopped if the temperature reached -55°C or in case of loss of diaphragmatic stimulation during right PVs ablation.
Volume of left atrial epicardial adipose tissue was manually quantified on the acquired images by two trained radiologists (M.G and D.T.). Manual segmentations were performed on LGE-MRI images using open-source software 3D Slicer
(slicer.org). The primary outcome of the study was to correlate left atrial adipose tissue, evaluated at cardiac MRI, with recurrence after AF cryoballoon ablation. Both absolute LA epicardial adipose tissue and LA epicardial adipose tissue indexed on the LA were considered for the analyses.
Statistical Analysis
Categorical variables are reported as percentage. Continuous variables are reported as mean ± standard deviation (SD) or median and interquartile range (IQR). Student’s t-test and Fisher exact test were used to compare continuous and categorical variables between groups, respectively. A receiver operating characteristic (ROC) curve was used to analyze the relationship between AF recurrences and the absolute LA EAT volume and the LA EAT volume indexed on the LA. The strength of the predictive ability of the variables in exam was evaluated with the area under the curve (AUC): a value greater than 0.7 was considered indicative of an acceptable association. Youden index was used to identify the best cut-offs at ROC analyses. Survival analyses were performed with Kaplan-Meier curves and log-rank test comparing two groups stratified on the cut-off identified at the ROC analysis. Univariate and multivariate Cox regression analyses were used to identify predictors of AF after ablation. A two-tailed p value<0.05 was considered statistically significant. All analyses were performed using R software version 4.2.2 (R Foundation for Statistical Computing, Vienna, Austria).
3. Results
Segmentation and quantification of LA EAT at cardiac MRI could not be achieved in one case of the 50 enrolled patients. Baseline characteristics of the study population are reported in
Table 1. The mean age was 59.6 years, 38% of patients were female, and the majority suffered of paroxysmal AF (86%); the mean number of previous AADs (effective, not-effective or not-tolerated) or rate control agents was 1.7 per patient. At baseline echocardiography mean ejection fraction was 61%. At MRI mean indexed LA volume was 43.3 ml/mq. After a median follow-up of 16.0 months (IQR: 12.0-21.8), AF relapsed in 17 patients (34%). No statistically significant differences in baseline characteristics were found between patients with and without recurrences, with the exception of greater use of beta-blockers at baseline (79% vs. 47%, p-value=0.019) in patients free from arrhythmic recurrences.
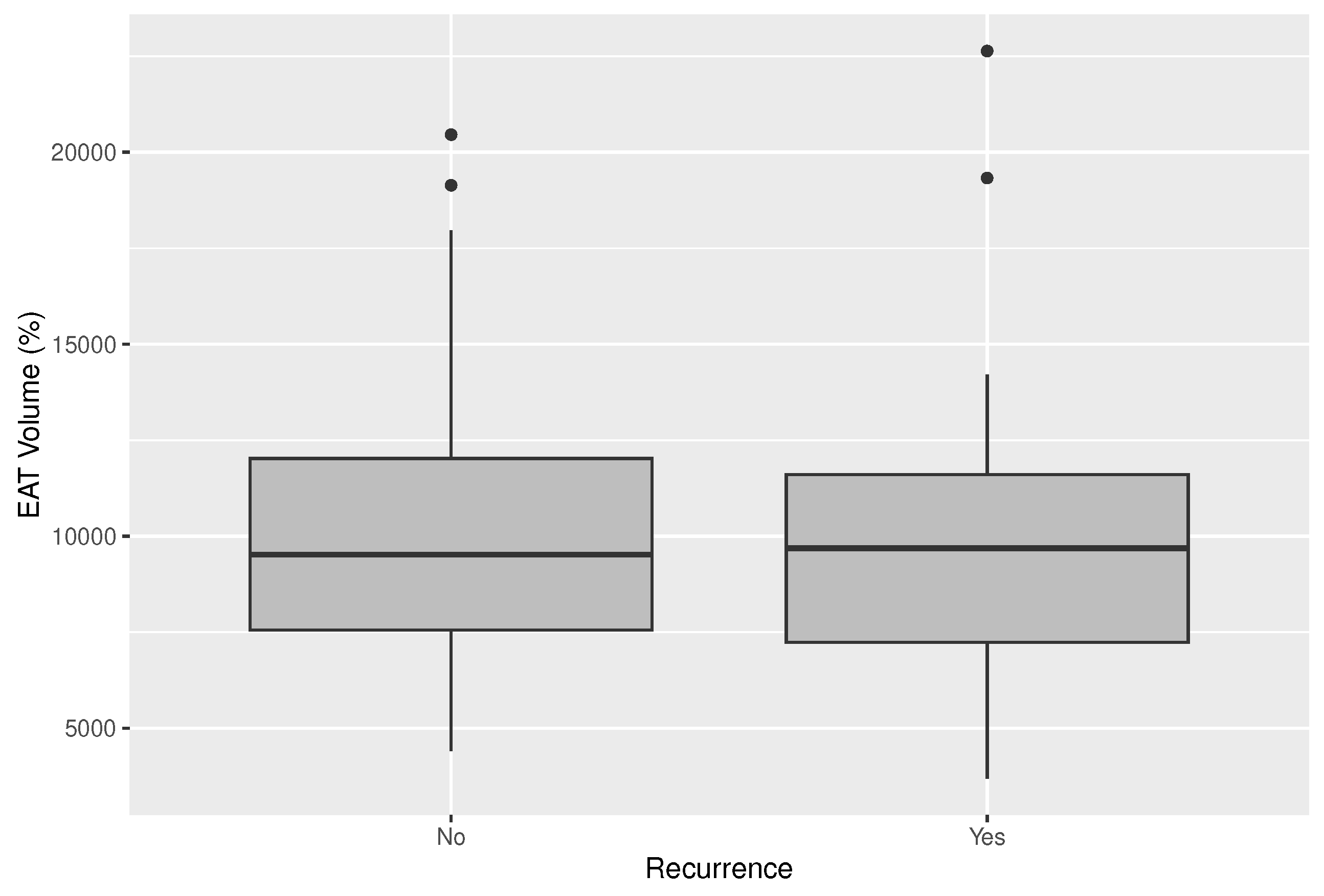
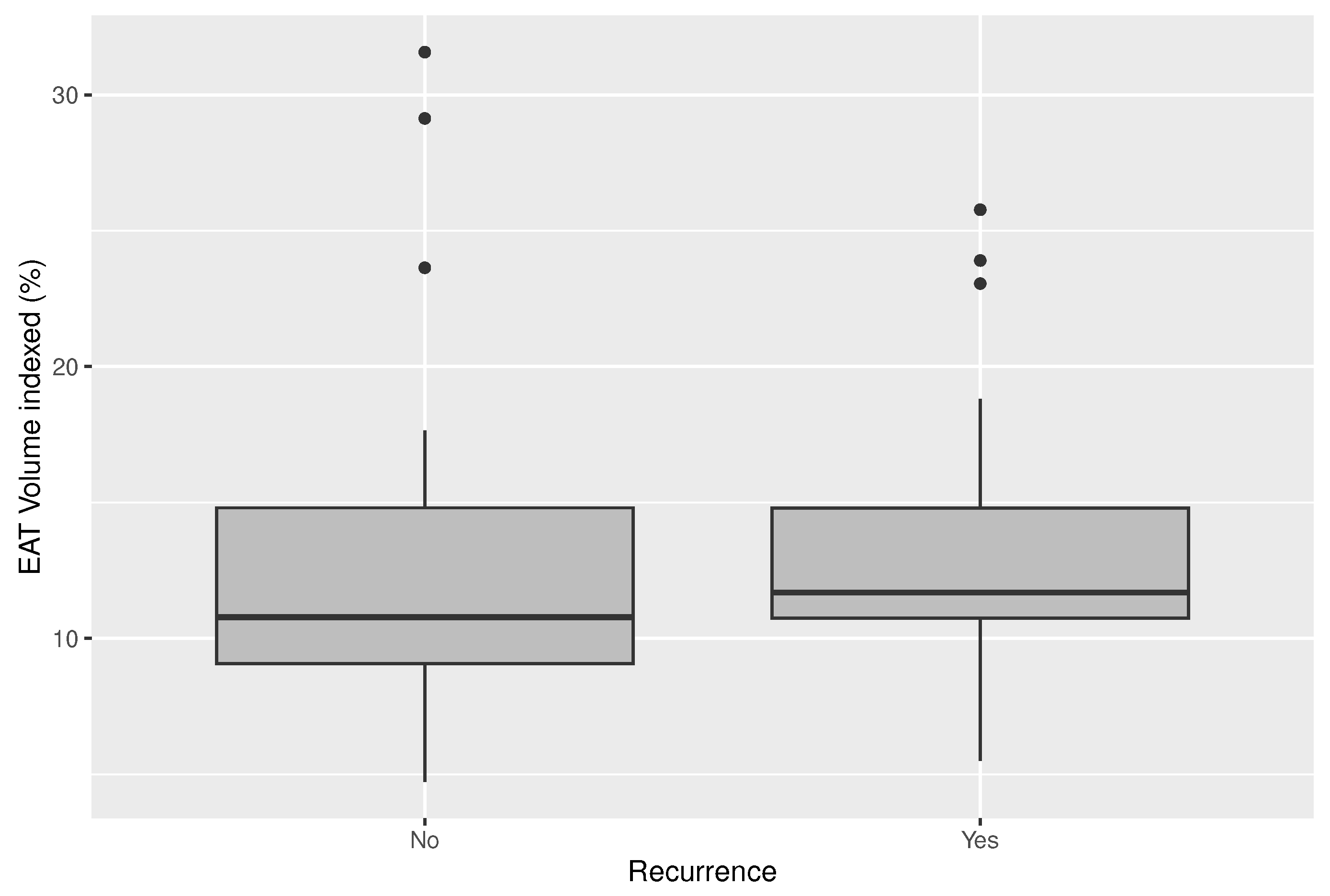
Mean EAT volume was 10.3 ml and the EAT volume indexed on the LA (EATi) was 13.2%. Absolute volume of EAT did not diverge in patients with and without AF recurrences (10.35 ml vs. 10.29 ml; p-value=0.963;
Figure 1), whereas, the indexed volume, was lower, albeit non statistically significant, in patients not suffering relapses during follow-up (12.77% vs. 14.06%; p-value=0.467) (
Figure 2)
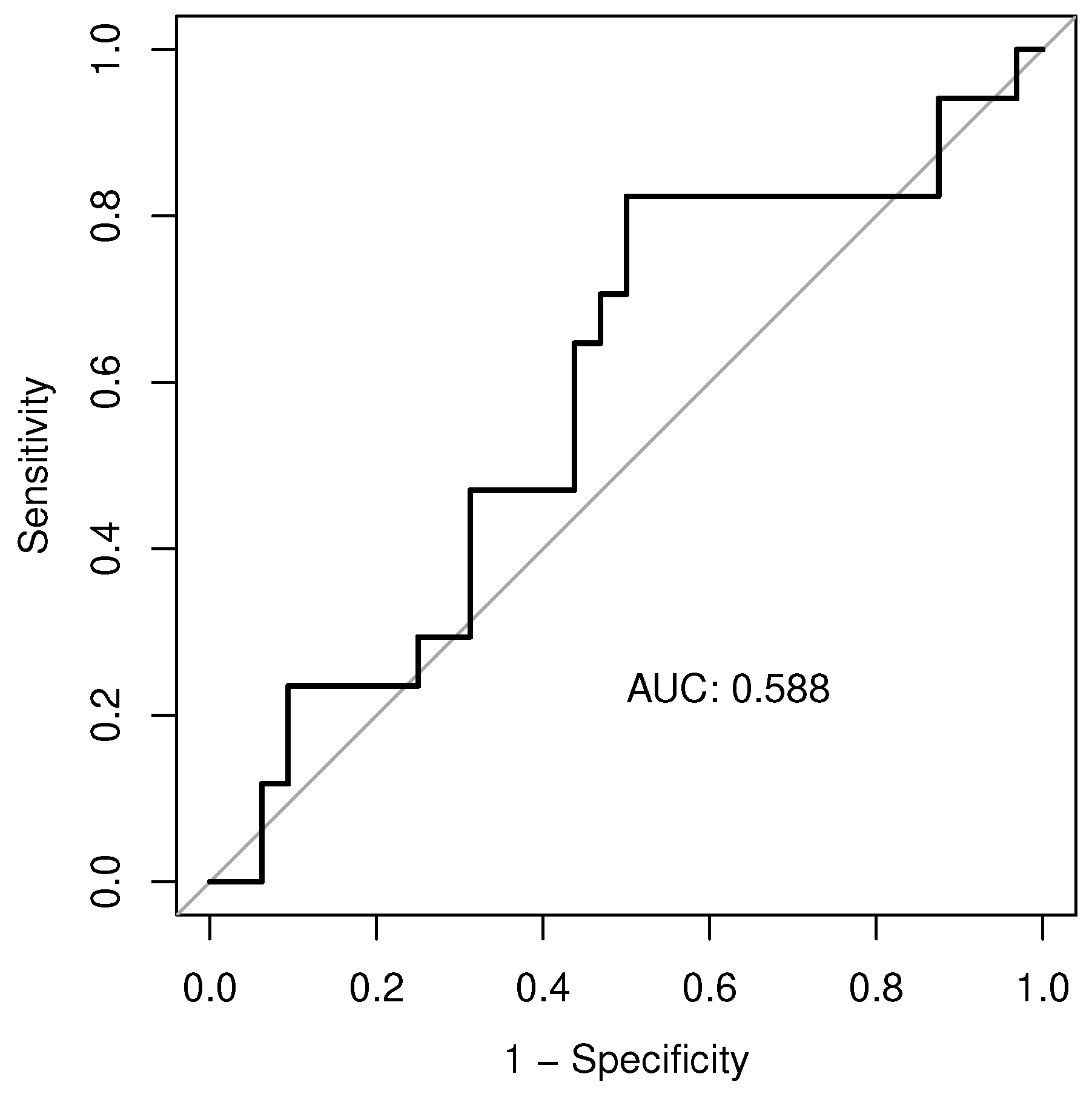
ROC curve testing the ability of EATi to predict AF recurrence after cryoballoon ablation (
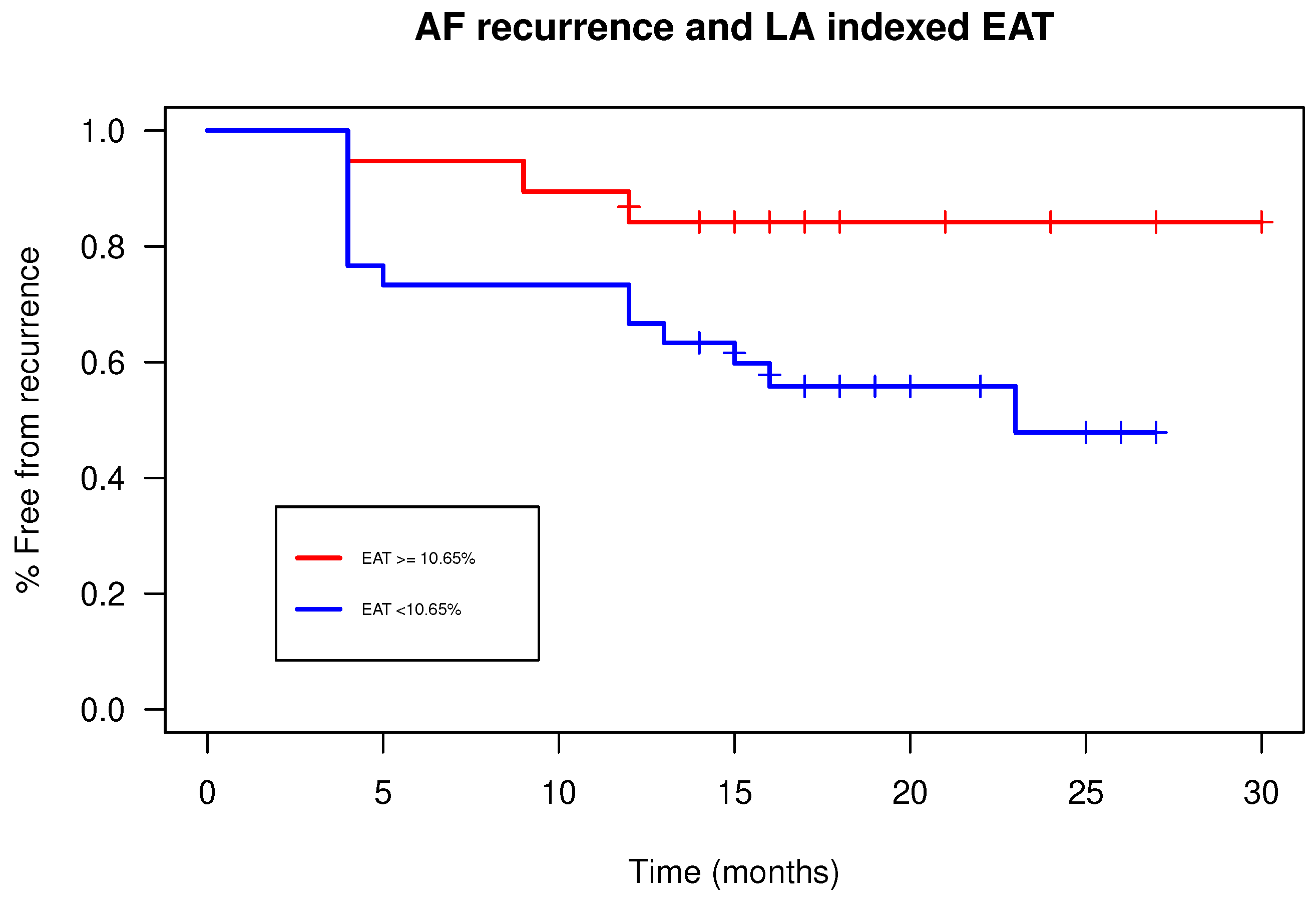
Figure 3) showed a sub-optimal performance (AUC: 0.588). The finest identified cut-off, able to predict patients with AF recurrence, was 10.65% with a sensitivity of 0.5, a specificity of 0.82, a positive predictive value of 0.59 and a negative predictive value of 0.76. Patients with an EATi value lower than the identified cut-off (10.65%) showed greater survival free from arrhythmias than patients with a value above the cut-off (84% vs. 48%; p-value=0.04;
Figure 4).
Figures S1 and S2 (see Supplementary Material) illustrate the ROC curve and subsequent Kaplan-Meier analysis stratified by absolute LA EAT volume.
Table 2 shows univariate and multivariate Cox regression analyses for AF recurrences. At multivariate analysis EATi above the cut-off of 10.65% was independently associated to an increased risk of AF recurrences (HR 4.5; 95%CI: 1.2-17.0; adjusted for BSA, diabetes, age and beta-blockers use). Diabetes also emerged as an independent risk factor of cryoballoon catheter ablation failure (HR 5.5; 95%CI:1.2-24.4), whereas beta-blockers showed a protective effect (HR 0.31; 95%CI: 0.10-0.96).
4. Discussion
The main findings of the present study are:
evaluation of left atrial adipose tissue by means of routine preprocedural cardiac MRI imaging is feasible;
levels of left atrial adipose tissue indexed on LA volume are associated with a higher risk of arrhythmic recurrence after AF cryoballoon catheter ablation.
Identification of the optimal candidates for catheter ablation of AF is of paramount importance. Assessment of the risk of AF recurrence after the procedure is a fundamental step in the management of patients with AF. More importantly, in case of modifiable risk factors, such as obesity, patients can be encouraged to improve lifestyle habits, delaying ablation when and whether these risk factors have been controlled. Recently, web based risk scores have been made freely available to physicians and patients to predict the probability of AF recurrence after catheter ablation (
AFA-RECUR)[
Saglietto 2023]. Interestingly, BMI has a deep impact on the risk of arrhythmic recurrence and reduction in body weight is associated with a similar decrease in arrhythmic risk. Bearing in mind the recent extension of gliflozin use for the treatment of heart failure, greater attention to the metabolic aspects should be posed also for AF patients: indeed, SGLT2 inhibitors have shown to reduce the risk of new onset AF along with a reduction in body weight[
Chahine 2022,
Pandey 2021]. These effects are, at least partially, mediated by a reduction of epicardial adipose tissue. Another new oral antidiabetic drug, semaglutide, recently showed weight lowering properties, but its effect on prevention of atrial arrhythmias is still unclear[
Lincoff 2023]. On these bases, evaluation of left atrial adipose tissue may play an important role in the management of patients with AF, both in adding a parameter to assess in search of the likelihood of AF recurrence than in identifying the correct timing of the procedure once other risk factors are controlled, in order to avoid futile procedures.
Previous literature on the topic presents great heterogeneity on both the methods of EAT quantification and the parameters analyzed. The preferred imaging modality adopted in previous studies was cardiac CT, whereas cardiac MRI has been seldom used; thickness of EAT as well as total, and LA volume of EAT have been proposed. A previous meta-analysis by Shamloo[
Seperi Shamloo 2019] summarized current evidence by grouping studies on the basis of the variable analyzed: the Authors found a significant differences in LA EAT volume, analyzed at CT, in patients with and without AF recurrences after catheter ablation, however the difference was in crude values, not corrected for other risk factors that may have potentially driven the association. A more recent systematic review[
Chen 2022] meta analyzed relative risk and hazard ratio of several EAT parameters and AF recurrence after catheter ablation suggesting that relative EAT thickness but not EAT volume measures related with an increased risk of recurrence. More specifically this relation was more evident in younger patients (less than 60 years old), Asian AF population and in case of longer follow-up.
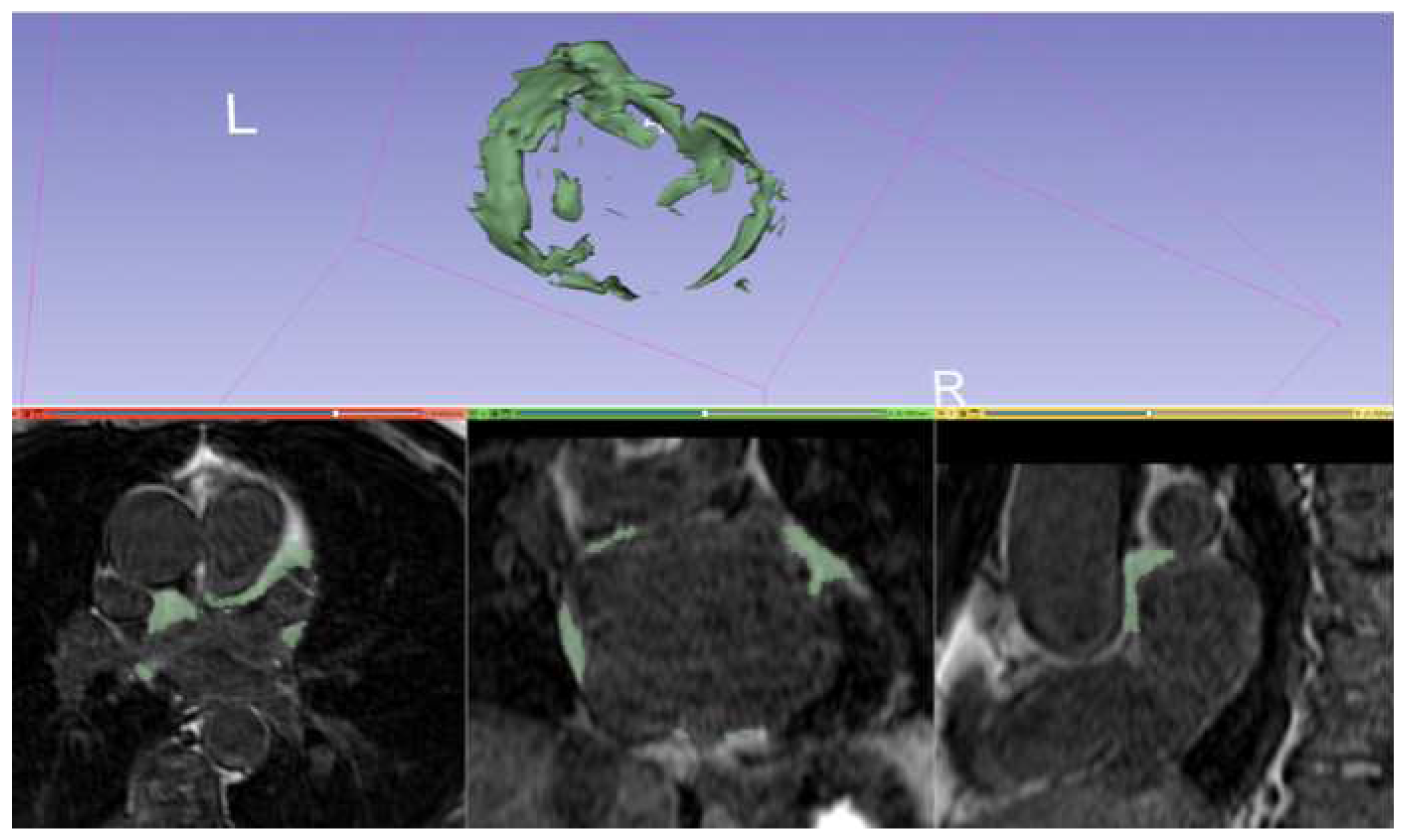
Only few works have focused on the evaluation of LA EAT with cardiac MRI[
Chahine 2022,
Nakamori 2018]. The present study focused exclusively on the analysis of epicardial adipose tissue at the left atrium level (
Figure 5): due to the reduced left atrial thickness the distinction between epicardial and pericardial adipose tissue is challenging and could have entailed a less accurate quantification; on the other hand, left atrial epicardial adipose tissue embraces ganglionated plexi, which play an important role in AF arrhythmogenesis. Indeed, we found that an amount of LA EAT equal or superior to 10.65% of the total volume of the LA was independently associated with the risk of AF recurrence after cryoballoon ablation. These findings are consistent with those recently published by Chahine et al. with a slightly different cut-off[
Chahine 2022]. In fact, quantification of atrial adipose tissue by means of cardiac MRI is surely feasible if the preprocedural imaging is focused on LA, without the need of acquisition of the whole cardiac volume, significantly shortening examination time. From a practical point of view, this is very important as it allows to implement routine evaluation of this parameter without changing current protocol or adding further time-consuming acquisitions.
4.1. Limitations
This work presents the following limitations. The population in exam is small and includes mostly patients with paroxysmal AF, preventing generalization of the results to the entire AF population. The low numerosity may result in statistical under-power preventing to detect significant differences. In our cohort all patients underwent cryoballoon ablation: therefore, it is unclear whether the association will remain valid in case of another ablation energy.
5. Conclusions
LA EAT volume indexed on the LA volume independently related to arrhythmia recurrence after AF cryoballoon catheter ablation. Prospective studies with larger cohorts are warranted to assess the role of the evaluation of LA EAT volume for referral of patients to catheter ablation.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on
Preprints.org, Figure S1: ROC curve analysis for the absolute LA EAT volume. Finest cut-off identified with the Youden index wass 19.2 ml with a sensitivity of 0.12, a specificity of 0.97, positive predictive value of 0.67 and negative predictive value of 0.68.; Figure S2: Kaplan-Meier arrhythmia-free survival curves stratified by the absolute LA EAT volume above o below 19.2 ml, showing no statistically significant difference (62% vs 33%; p-value=0.2).
Author Contributions
Conceptualization A.B., M.A., M.G., R.F.; methodology, H.X.; software, D.T., F.G., and M.G.; formal analysis, A.B., H.X., D.T. and M.G..; investigation, A.B., H.X. and S.M.; data curation, H.X., S.M.; writing—original draft preparation, A.b. and M.A.; writing—review and editing F.G., A.S., L.C., E.R. and G.M.D.F; visualization, X.X.; supervision, M.A., R.F., G.M.D.F; project administration, M.A.; funding acquisition, M.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by a grant from the Italian Ministry of Health: “Giovani Ricercatori—Ricerca Finalizzata”, project number GR-2016-02362088; the funder had no role in analyzing the data of this study.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Ethics Committee of A.O.U Città della Salute e della Scienza, Turin (“Crioablazione della Fibrillazione Atriale: valutazione degli effetti in vivo e indici predittivi di fibrosi" Prot. 0042583).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.
Conflicts of Interest
MA is consultant for Biosense Webster and Boston Scientific, clinical proctor for Medtronic and has received educational grants from Abbott.
References
- Fainberg 2018. Fainberg, H.P.; Birtwistle, M.; Alagal, R.; Alhaddad, A.; Pope, M.; Davies, G.; Woods, R.; Castellanos, M.; May, S.T.; Ortori, C.A.; et al. Transcriptional Analysis of Adipose Tissue during Development Reveals Depot-Specific Responsiveness to Maternal Dietary Supplementation. Sci. Rep. 2018, 8. [CrossRef]
- Iacobellis 2004. Iacobellis, G.; Pistilli, D.; Gucciardo, M.; Leonetti, F.; Miraldi, F.; Brancaccio, G.; Gallo, P.; Tiziana Di Gioia, C.R. Adiponectin Expression in Human Epicardial Adipose Tissue in Vivo Is Lower in Patients with Coronary Artery Disease. Cytokine 2005, 29, 251–255. [CrossRef]
- Iacobellis 2009. Iacobellis, G.; Di Gioia, C.R.; Di Vito, M.; Petramala, L.; Cotesta, D.; De Santis, V.; Vitale, D.; Tritapepe, L.; Letizia, C. Epicardial Adipose Tissue and Intracoronary Adrenomedullin Levels in Coronary Artery Disease. Horm. Metab. Res. 2009, 41, 855–860. [CrossRef]
- Sacks 2013. Sacks, H.S.; Fain, J.N.; Bahouth, S.W.; Ojha, S.; Frontini, A.; Budge, H.; Cinti, S.; Symonds, M.E. Adult Epicardial Fat Exhibits Beige Features. J. Clin. Endocrinol. Metab. 2013, 98, E1448–E1455. [CrossRef]
- Van Woerden 2018. Van Woerden, G.; Gorter, T.M.; Westenbrink, B.D.; Willems, T.P.; van Veldhuisen, D.J.; Rienstra, M. Epicardial Fat in Heart Failure Patients with Mid-Range and Preserved Ejection Fraction. Eur. J. Heart Fail. 2018, 20, 1559–1566. [CrossRef]
- Pugliese 2021. Pugliese, N.R.; Paneni, F.; Mazzola, M.; De Biase, N.; Del Punta, L.; Gargani, L.; Mengozzi, A.; Virdis, A.; Nesti, L.; Taddei, S.; et al. Impact of Epicardial Adipose Tissue on Cardiovascular Haemodynamics, Metabolic Profile, and Prognosis in Heart Failure. Eur. J. Heart Fail. 2021, 23, 1858–1871. [CrossRef]
- Hirata 2011. Hirata, Y.; Tabata, M.; Kurobe, H.; Motoki, T.; Akaike, M.; Nishio, C.; Higashida, M.; Mikasa, H.; Nakaya, Y.; Takanashi, S.; et al. Coronary Atherosclerosis Is Associated with Macrophage Polarization in Epicardial Adipose Tissue. J. Am. Coll. Cardiol. 2011, 58, 248–255. [CrossRef]
- Antonopoulos 2017. Antonopoulos, A.S.; Sanna, F.; Sabharwal, N.; Thomas, S.; Oikonomou, E.K.; Herdman, L.; Margaritis, M.; Shirodaria, C.; Kampoli, A.M.; Akoumianakis, I.; et al. Detecting Human Coronary Inflammation by Imaging Perivascular Fat. Sci. Transl. Med. 2017, 9. [CrossRef]
- Friedman 2014. Friedman, D.J.; Wang, N.; Meigs, J.B.; Hoffmann, U.; Massaro, J.M.; Fox, C.S.; Magnani, J.W. Pericardial Fat Is Associated with Atrial Conduction: The Framingham Heart Study. J. Am. Heart Assoc. 2014, 3. [CrossRef]
- Mahabadi 2014. Mahabadi, A.A.; Lehmann, N.; Kälsch, H.; Bauer, M.; Dykun, I.; Kara, K.; Moebus, S.; Jöckel, K.H.; Erbel, R.; Möhlenkamp, S. Association of Epicardial Adipose Tissue and Left Atrial Size on Non-Contrast CT with Atrial Fibrillation: The Heinz Nixdorf Recall Study. Eur. Hear. journal. Cardiovasc. Imaging 2014, 15, 863–869. [CrossRef]
- Wong 2016. 10. Wong, C.X.; Sun, M.T.; Odutayo, A.; Emdin, C.A.; Mahajan, R.; Lau, D.H.; Pathak, R.K.; Wong, D.T.; Selvanayagam, J.B.; Sanders, P.; et al. Associations of Epicardial, Abdominal, and Overall Adiposity With Atrial Fibrillation. Circ. Arrhythm. Electrophysiol. 2016, 9. [CrossRef]
- Mazurek 2003. Mazurek, T.; Zhang, L.F.; Zalewski, A.; Mannion, J.D.; Diehl, J.T.; Arafat, H.; Sarov-Blat, L.; O’Brien, S.; Keiper, E.A.; Johnson, A.G.; et al. Human Epicardial Adipose Tissue Is a Source of Inflammatory Mediators. Circulation 2003, 108, 2460–2466. [CrossRef]
- Hindricks 2021. Hindricks, G.; Potpara, T.; Dagres, N.; Arbelo, E.; Bax, J.J.; Blomström-Lundqvist, C.; Boriani, G.; Castella, M.; Dan, G.-A.; Dilaveris, P.E.; et al. 2020 ESC Guidelines for the Diagnosis and Management of Atrial Fibrillation Developed in Collaboration with the European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2021, 42, 373–498. [CrossRef]
- Saglietto 2021. Saglietto, A.; Gaita, F.; De Ponti, R.; De Ferrari, G.M.; Anselmino, M. Catheter Ablation vs. Anti-Arrhythmic Drugs as First-Line Treatment in Symptomatic Paroxysmal Atrial Fibrillation: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. Front. Cardiovasc. Med. 2021, 8. [CrossRef]
- Ballatore 2023. Ballatore, A.; Negrello, E.; Gatti, M.; Matta, M.; Desalvo, P.; Marcialis, L.; Marconi, S.; Tore, D.; Magnano, M.; Bissolino, A.; et al. Evaluation of Pulmonary Vein Fibrosis Following Cryoballoon Ablation of Atrial Fibrillation: A Semi-Automatic MRI Analysis. J. Cardiovasc. Dev. Dis. 2023, 10, 396. [CrossRef]
- Saglietto 2023. Saglietto, A.; Gaita, F.; Blomstrom-Lundqvist, C.; Arbelo, E.; Dagres, N.; Brugada, J.; Maggioni, A. Pietro; Tavazzi, L.; Kautzner, J.; De Ferrari, G.M.; et al. AFA-Recur: An ESC EORP AFA-LT Registry Machine-Learning Web Calculator Predicting Atrial Fibrillation Recurrence after Ablation. Europace 2023, 25, 92–100. [CrossRef]
- Chahine 2022. Chahine, Y.; Macheret, F.; Ordovas, K.; Kim, J.; Boyle, P.M.; Akoum, N. MRI-Quantified Left Atrial Epicardial Adipose Tissue Predicts Atrial Fibrillation Recurrence Following Catheter Ablation. Front. Cardiovasc. Med. 2022, 9, 1045742. [CrossRef]
- Pandey 2021. Pandey, A.K.; Okaj, I.; Kaur, H.; Belley-Cote, E.P.; Wang, J.; Oraii, A.; Benz, A.P.; Johnson, L.S.B.; Young, J.; Wong, J.A.; et al. Sodium-Glucose Co-Transporter Inhibitors and Atrial Fibrillation: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Am. Heart Assoc. 2021, 10, 22222. [CrossRef]
- Lincoff 2023. Lincoff, A.M.; Brown-Frandsen, K.; Colhoun, H.M.; Deanfield, J.; Emerson, S.S.; Esbjerg, S.; Hardt-Lindberg, S.; Hovingh, G.K.; Kahn, S.E.; Kushner, R.F.; et al. Semaglutide and Cardiovascular Outcomes in Obesity without Diabetes. N. Engl. J. Med. 2023. [CrossRef]
- Seperi Shamloo 2019. Sepehri Shamloo, A.; Dagres, N.; Dinov, B.; Sommer, P.; Husser-Bollmann, D.; Bollmann, A.; Hindricks, G.; Arya, A. Is Epicardial Fat Tissue Associated with Atrial Fibrillation Recurrence after Ablation? A Systematic Review and Meta-Analysis. IJC Hear. Vasc. 2019, 22, 132–138. [CrossRef]
- Chen 2022. Chen, J.; Mei, Z.; Yang, Y.; Dai, C.; Wang, Y.; Zeng, R.; Liu, Q. Epicardial Adipose Tissue Is Associated with Higher Recurrence Risk after Catheter Ablation in Atrial Fibrillation Patients: A Systematic Review and Meta-Analysis. BMC Cardiovasc. Disord. 2022, 22, 1–10. [CrossRef]
- Nakamori 2018. Nakamori, S.; Nezafat, M.; Ngo, L.H.; Manning, W.J.; Nezafat, R. Left Atrial Epicardial Fat Volume Is Associated With Atrial Fibrillation: A Prospective Cardiovascular Magnetic Resonance 3D Dixon Study. J. Am. Heart Assoc. 2018, 7. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).