Introduction:
Dermatophytes, a primarily pathogenic group of fungi, are accountable for the most prevalent fungal infections in humans, recognized as dermatophytosis or tinea. These fungi consist of closely related filamentous species with a specific inclination for infecting keratinized tissues, including the skin, hair, and nails. Among dermatophytes, Trichophyton rubrum holds the status of the most common causative species for these infections. Nevertheless, it's noteworthy that in specific regions of the world, there is a rising occurrence of infections attributed to other species, specifically T. interdigitale and T. mentagrophytes. (1). Terbinafine is the first-line treatment for Trichophyton infections, administered either topically or systemically depending on the severity of the case (2). Antifungal resistance is a broad term encompassing the inability of a fungal infection to respond positively to antifungal treatment (3). The emergence of resistant dermatophytosis, primarily evident in conditions like tinea corporis and cruris, has become a cause for global concern in recent years. In countries like India and other parts of Asia, resistance has surfaced as a clonal outbreak of terbinafine-resistant T. indotineae. (4,5). Consequently, this emergence has renewed research focus on dermatophytosis. Given the substantial increase in resistant fungal pathogens, attributed to the widespread use of antifungal agents for treating superficial mycoses, it is crucial to highlight the significance of antifungal resistance, paralleling the attention given to antibacterial and antiviral resistance. (6).
Table 1.
Antifungal drugs used in treatment of dermatophytosis and their target.
Table 1.
Antifungal drugs used in treatment of dermatophytosis and their target.
| Antifungal Class |
Drug Name |
Specific Target |
| Allylamines |
Naftifine |
Squalene epoxidase |
| Terbinafine |
| Azoles |
Imidazole: clotrimazole, miconazole, bifonazole, ketoconazole, luliconazole, lanoconazole
. |
Cytochrome P450, 14α- Lanosterol Demethylase. |
|
Triazole: itraconazole, fluconazole, efinaconazole, Albaconazole, posaconazole, ravuconazole, fosravuconazole, isavuconazole |
|
Tetrazole: Oteseconazole |
| Polyenes |
Amphotericin B |
Membrane barrier function. |
| Nystatin |
| Morpholines |
Amorolfine |
Sterol reductase and Isomerase. |
| Thiocarbamate |
Tolnaftate |
Squalene epoxidase |
| Others |
Griseofulvin |
Sliding of Microtubules. |
Antifungal resistance mechanisms:
Various mechanisms play a role in the emergence of antifungal resistance in fungal pathogens. Certain fungal species may exhibit an inherent or intrinsic resistance to antifungal drugs, meaning they are naturally unaffected by the antifungal agent even when treated with normally effective doses (7). As an example, initial resistance to allylamines like terbinafine in dermatophytes was initially documented in 2003 (8). Azoles, such as fluconazole, are naturally found to be ineffective against infections caused by molds like Aspergillus species. Alternatively, resistance that develops after exposure to antifungal drugs is known as secondary or acquired resistance. Azoles have a fungistatic effect (i.e., inhibit fungal growth), while allylamines are fungicidal, capable of killing the fungi (9, 10). Consequently, the development of acquired azole resistance in dermatophytes is more likely compared to allylamines. Furthermore, acquired resistance to fluconazole is frequently observed in yeasts, such as Candida albicans (11). Previous studies by our team indicates that treatment failures with terbinafine therapy may also be attributed to factors related to the host or the treatment itself, known as clinical resistance (12). These factors include an insufficient treatment regimen in terms of dosage and duration, as well as poor patient compliance (13).
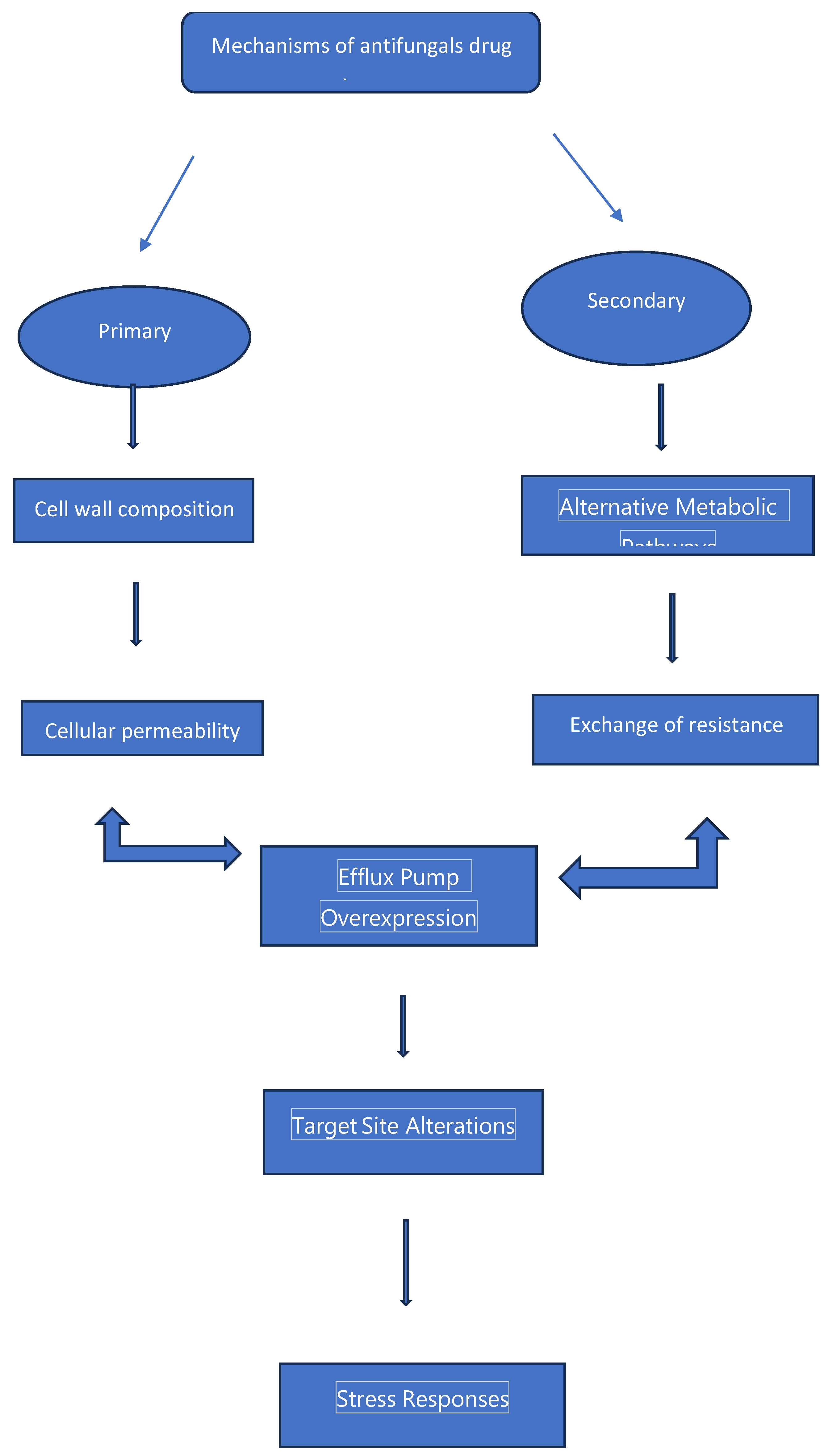
Figure 1.
Mechanisms of antifungal resistance.
Figure 1.
Mechanisms of antifungal resistance.
Assessment of antifungal resistance:
Microbial drug resistance occurs when a microorganism, such as a fungus, shows decreased sensitivity to an antifungal agent. The European Committee on Antimicrobial Susceptibility Testing (EUCAST) and the Clinical and Laboratory Standards Institute (CLSI) have developed a standardized in vitro approach to evaluate antifungal susceptibility in yeasts, molds, and dermatophytes. (14). This method involves measuring the minimum inhibitory concentration and comparing it to a clinical breakpoint (15). While there are established breakpoints for common pathogens that are specific to particular drugs and species, there are no such guidelines available for rare yeasts, molds, and dermatophytes (16). In the absence of specific breakpoints for uncommon fungi, epidemiological cutoff values(ECVs) are used to define the upper limit of susceptibility within the wild-type population (17,18). It's important to note that antifungal susceptibility testing can be challenging due to the unreliability of certain drugs (like caspofungin) and the behavior of certain species (such as Candida glabrata), making standardized testing less dependable in these cases (19). While the CLSI and EUCAST methods for determining minimum inhibitory concentrations may exhibit differences that result in distinct breakpoints, both approaches are grounded in the understanding of how antifungal drugs interact with fungi in animal models and patients. It is important to emphasize that there is no absolute, direct correlation between the in vitro minimum inhibitory concentration and a patient's clinical response. To address the challenge of response variability, drug resistance is categorized based on the likelihood of treatment success, uncertainty regarding its effectiveness, or the likelihood of treatment failure. Antifungal susceptibility testing serves as a valuable tool for identifying fungal strains that are prone to result in treatment failure and for monitoring changes in susceptibility or the emergence of new drug resistance mechanisms over time. It is worth noting that antifungal susceptibility testing is commonly used for assessing yeasts but is not officially recommended by the Infectious Diseases Society of America for evaluating Aspergillus species. (20).
Terbinafine resistance:
Terbinafine is commonly employed to effectively treat dermatophyte infections like onychomycosis. It's renowned for its high efficacy (21). Terbinafine resistance has mainly been linked to specific genetic mutations found in the squalene epoxidase (SQLE) gene (22). The primary target of terbinafine is the product of this gene. Interestingly, certain dermatophytes, such as T. rubrum and T. interdigitale, showcase a distinctive resistance mechanism by enhancing the expression of the salicylate 1-monooxygenase (salA) gene, thus increasing their resistance to terbinafine. Moreover, molds like Aspergillus fumigatus can acquire terbinafine resistance through the existence of extra-plasmid copies of the SQLE gene. (23). To uncover these innovative resistance mechanisms in fungal pathogens against terbinafine, the application of molecular biology techniques such as real-time polymerase chain reaction (RT-PCR), mutation analysis, and gene expression analysis can offer valuable insights. In India, a significant shift has been observed in the epidemiology of dermatophytosis. The incidence of T. rubrum infections has declined, whereas new strain types within the T. mentagrophytes/T. interdigitale complex, particularly T. mentagrophytes infections, have been increasing. (24). Furthermore, reports have emerged about a novel pathogen, Trichophyton indotineae, which is distinct from T. interdigitale (25). Despite these changes, a concerning trend of recalcitrant superficial dermatophytosis, primarily resistant to terbinafine (occurring in the range of 16-77%), has been increasingly documented in India (26). Various factors, such as the warm and humid climate, overcrowding, misdiagnosis, and improper usage of combined topical steroids and antimicrobial creams, could be contributing to the observed rise in terbinafine resistance. (27). The concern transcends the borders of India, as Trichophyton strains resistant to terbinafine have been documented in various nations, encompassing Japan, China, Denmark, Poland, Belgium, Germany, the USA, Canada, and the Middle East. (28). It is crucial to acknowledge that the underreporting of terbinafine resistance may occur because routine antifungal susceptibility testing (AFST) is not commonly conducted in clinical practice. (29). However, the high level of terbinafine resistance is a cause for concern, and addressing this growing problem should be a topic of discussion and action in global healthcare forums (30).
Azole resistance:
Azoles represent the largest category of antifungal drugs. Presently, three generations of azoles are employed in clinical practice to treat dermatophytosis. The first generation of azoles includes an imidazole in their ring structure. Predominantly administered topically (except for ketoconazole), these compounds have restricted oral bioavailability and demonstrate high toxicity levels when given systemically. Conversely, the second and third generations of azoles are characterized by the triazole ring structure, distinguishing them from the imidazole composition found in the first generation. (31). The reported rate of azole resistance in dermatophytes is 19%. (32). The mechanisms of azole resistance in dermatophytes are not completely comprehended, but experimental studies have proposed potential mechanisms, including drug efflux. (33). The key aspect of high-level azole resistance in pathogenic fungi revolves around energy-dependent efflux facilitated by membrane-bound transporters, with a pivotal role played by the ATP Binding Cassette (ABC) transporter superfamily. These transporters have the capability to expel undesired substances from cells, and the resistance to triazoles in various fungi has been linked to the overexpression of specific ABC transporters, particularly those within the ABCG (PDR) family. (34,35). In dermatophytes, the existence of ABC transporters was confirmed in experimental studies, with evidence suggesting their involvement in pathogenicity, as seen in T. rubrum isolates with resistance to multiple antifungals (36). The 14α-lanosterol demethylase, which is essential for drug action, is governed by the Erg11 gene (37). In yeast, azole resistance is often linked to mechanisms like gene product overexpression and mutations in Erg11, but these specific mechanisms have not been observed in dermatophytes thus far (38). In response to environmental and drug-induced stress, dermatophytes have demonstrated the secretion of diverse proteins as part of their adaptive response. (39,40). Certain proteins, including heat shock proteins like hsp70, hsp90, and PacC, contribute significantly to the virulence of dermatophytes. (41). While the exact link between these proteins and drug resistance is not clearly understood, there is a hypothesis that stress adaptation enables the cell to withstand the presence of drugs and may contribute to the gradual development of more resilient resistance mechanisms over time. (42). Furthermore, exposure to antifungal and cytotoxic drugs at sub-inhibitory concentrations induces stress responses, leading to the increased expression of genes linked to cellular detoxification, drug efflux, and signaling pathways. This mechanism is likely to play a role in drug tolerance. Considering this, exploring the inhibition of heat shock proteins (Hsps) could be a potential avenue for future research in the development of antifungal drugs. (43). In light of the limited success in managing superficial fungal infections with existing therapies, newer triazole antifungal medications were developed with the goal of achieving higher efficacy and addressing resistant infections more effectively. There have been documented cases where tinea infections that were initially resistant to primary antifungal treatments, such as terbinafine and itraconazole, have shown positive responses to posaconazole and voriconazole, next-generation oral triazoles (44, 45,46,47). Nonetheless, it is crucial to emphasize that resistance to these more recent drugs may emerge. Posaconazole has demonstrated fungicidal activity against Candida and Aspergillus species in vitro and is sometimes utilized off-label for the treatment of superficial mycoses. However, environmental fungi, such as specific Aspergillus species rarely linked to onychomycosis, have been reported to exhibit resistance to posaconazole. This resistance is associated with mutations in the genes encoding 14 α-sterol demethylases (cyp51A and cyp51B), essential components in fungal sterol biosynthesis. (48). The development of resistance to these newer azole antifungals may be associated with cross-resistance from other triazoles, such as itraconazole or fluconazole, due to their comparable mechanisms of action. To address this concern and maintain the effectiveness of current antifungals, there is a requirement for the creation of novel drug targets and enhanced targeting strategies.
Griseofulvin:
Griseofulvin (GRI), the original systemic antifungal for treating dermatophytosis, rapidly accumulates in the stratum corneum (SC) but also experiences a rapid decline in concentration when treatment is stopped, as it lacks specific retention mechanisms within the SC. Additionally, perspiration can further wash GRI out of the SC. The prolonged treatment periods lasting weeks or even months, which are necessary for GRI in dermatophytosis therapy, can be attributed to its mechanism of action. This mechanism involves inhibiting microtubule aggregation and requires a significant amount of time to make dermatophytes nonviable, given their slow growth characteristics (49). Data from various countries have reported cases of Griseofulvin (GRI)-resistant dermatophyte isolates (50, 51,52,53). As an example, a recent study conducted in India indicated that GRI (generic name) demonstrated limited efficacy, with a mode of minimum inhibitory concentration (MIC) at 4 μg/mL. Approximately 98% of isolates in this study exhibited MICs equal to or greater than 2 μg/mL. Another study from the same country reported that GRI was the least effective drug in vitro, with a modal MIC of 32 μg/mL. In both studies, the predominant species was T. interdigitale. It is noteworthy that some researchers have observed that GRI's in vitro activity against T. mentagrophytes appears to be lower compared to its activity against T. rubrum. (54,55). While the exact mechanism of action for Griseofulvin (GRI) remains unspecified, there is evidence suggesting that when subjected to GRI, four Trichophyton species with disrupted MDR2 genes displayed increased levels of MDR4 transcripts, as observed. (56). Moreover, there has been documented evidence of elevated expression of Tru MDR1 in response to Griseofulvin (GRI) exposure. This indicates that efflux pumps may play a role in imparting resistance to GRI. (57).
Development of Resistance to recently approved Triazoles antifungals:
In the realm of managing superficial fungal infections, a domain marked by a scarcity of truly effective therapies, the advent of newer triazole antifungals represents a significant stride forward. These innovative antifungal agents have been meticulously crafted to showcase heightened efficacy, addressing the limitations of existing treatments, and more effectively combating resistant infections. Amidst the medical landscape, instances abound where traditional approaches, such as primary antifungal therapies like terbinafine and itraconazole, have faced formidable challenges in the face of stubborn tinea infections. However, the emergence of Posaconazole and voriconazole, belonging to the newer triazole class, has ushered in a ray of hope. Reports abound detailing instances where these advanced antifungals have displayed remarkable effectiveness, providing a therapeutic lifeline for cases that previously resisted conventional treatments. This underscores the crucial role that ongoing advancements in antifungal research play in enhancing our ability to combat infections that, until recently, posed formidable challenges to effective management (6,45,.81,82). While the advent of novel antifungal drugs such as Posaconazole has undoubtedly brought about a transformative shift in the therapeutic landscape, the specter of resistance looms as a potential challenge. As with any new class of medications, there is a need for ongoing vigilance and research to monitor and comprehend the emergence of resistance to these promising treatments. Posaconazole, a cutting-edge oral triazole that has found application beyond its originally labeled use, particularly in addressing superficial mycoses, has shown considerable promise in vitro. Its fungicidal activity, notably against Candida and Aspergillus species, has been documented, marking a significant stride in the realm of antifungal research. However, it is crucial to acknowledge that the laboratory setting might not perfectly mirror the complexities of real-world clinical scenarios, where factors such as patient-specific variables and microbial adaptation may influence the efficacy of these drugs. As the medical community continues to explore and harness the potential of Posaconazole and similar agents, the dynamic interplay between drug efficacy and the development of resistance remains an area warranting meticulous investigation to ensure the sustained effectiveness of these valuable therapeutic tools (48). Despite the strides made in antifungal research, challenges persist in the form of environmental fungi, notably Aspergillus species, which have been identified as causative agents of onychomycosis. A notable setback arises in the context of Posaconazole, a potent antifungal belonging to the next-generation oral triazole class. Reports have surfaced, indicating instances of resistance to Posaconazole among environmental fungi, and this phenomenon has been linked to mutations in crucial genes encoding 14 alpha-sterol demethylases, specifically cyp51A and cyp51B. These demethylases play an indispensable role in the intricate process of fungal sterol biosynthesis. The emergence of mutations in these genetic components undermines the efficacy of Posaconazole, as alterations in these key genes interfere with the drug's ability to exert its fungicidal effect. This revelation not only underscores the adaptability of environmental fungi but also emphasizes the need for continuous scrutiny and adaptation of antifungal strategies to address evolving challenges in the realm of fungal infections. The pursuit of innovative solutions and a nuanced understanding of the genetic underpinnings of resistance remain imperative in the ongoing battle against the complexities of onychomycosis and related fungal afflictions (83,84). Moreover, within the spectrum of Candida species, including C. albicans, C. glabrata, C. tropicalis, and C. parapsilosis, there exists the potential for resistance to voriconazole, a triazole antifungal agent developed as a synthetic derivative of fluconazole. Voriconazole, although FDA-approved in 2002 for the treatment of cutaneous candidiasis, extends its utility through off-label applications to address various superficial fungal infections. However, the clinical landscape is not devoid of instances where certain Candida strains exhibit resistance to this therapeutic option. The intricate dynamics of resistance involve multifaceted factors, possibly including genetic variations within the Candida species that impede the drug's targeted action. As voriconazole's mechanism relies on inhibiting fungal ergosterol synthesis through interference with the cytochrome P450 enzyme system, alterations in these pathways could confer resistance. This underscores the imperative for ongoing research to dissect the molecular nuances of resistance mechanisms, allowing for the refinement of treatment strategies and the development of alternative approaches to effectively counteract Candida species that may elude the beneficial effects of voriconazole. In navigating the intricate landscape of antifungal therapy, a comprehensive understanding of resistance patterns is essential for optimizing treatment outcomes and ensuring the continued efficacy of available therapeutic options (85). The emergence of resistance to these newer azoles is a complex phenomenon, often attributed to cross-resistance stemming from the utilization of other triazoles like itraconazole or fluconazole. This is primarily because these compounds share a comparable mechanism of action, thereby creating a potential vulnerability in the face of evolving fungal resistance. In response to this challenge, the imperative arises for the exploration of innovative drug targets and the development of refined targeting strategies. This proactive approach is crucial to circumvent the pitfalls of cross-resistance and preserve the efficacy of existing antifungals. A notable candidate in this quest for enhanced antifungal strategies is ravuconazole, a novel triazole antifungal that has exhibited promising in vitro activity against a diverse array of fungal pathogens. Ravuconazole's spectrum of effectiveness extends to dermatophytes and various Candida species, showcasing its potential as a versatile therapeutic agent. Furthermore, its limited but noteworthy activity against non-dermatophyte molds (NDMs) such as S. brevicaulis and Fusarium species provides a glimpse into its broader applicability in addressing a spectrum of fungal infections. The exploration of such novel compounds not only diversifies the therapeutic arsenal but also holds the promise of overcoming existing challenges posed by drug resistance, thus paving the way for more effective management of fungal infections in diverse clinical settings (86). As of the current understanding, the resilience of clinical strains to ravuconazole or its prodrug fosravuconazole remains uncharted territory, with no documented instances of resistance reported to date. This absence of documented resistance is particularly noteworthy given the approval of fosravuconazole in Japan in 2018 for the treatment of tinea unguium, underscoring the efficacy and reliability of this antifungal agent in managing fungal infections. The rarity or nonexistence of resistance thus far serves as a positive indicator of the robustness of ravuconazole and its prodrug in clinical applications. However, vigilance and continued surveillance are paramount, considering the dynamic nature of microbial evolution and the potential for the development of resistance over time. The absence of documented resistance to ravuconazole and fosravuconazole opens avenues for their continued exploration and utilization in clinical settings, offering a valuable contribution to the ongoing efforts to combat fungal infections, especially those resistant to traditional therapies. Regular monitoring and research endeavors are essential to stay ahead of potential challenges and ensure the sustained effectiveness of ravuconazole in the evolving landscape of antifungal therapeutics (87).
Resistance to topical antifungal agents:
Topical antifungals play a pivotal role in the therapeutic landscape for dermatophytosis, offering a localized and effective approach to treatment. Noteworthy among these are various formulations such as amorolfine (widely utilized in Europe), tavaborole (a prominent choice in the USA), ciclopirox (deployed in both the USA and Canada), efinaconazole (utilized in the USA, Canada, and Japan), and luliconazole (a staple in Japan). The widespread use of these topicals stems from their inherent advantages, including a reduced risk of systemic adverse events and limited potential for drug interactions. In the realm of dermatophyte susceptibility to topical antifungals, the emergence of natural mutants exhibiting low susceptibility is a rare occurrence. The available literature underscores the scarcity of reports documenting resistance to these topical drugs, further highlighting their effectiveness in managing dermatophytosis. This rarity suggests that the topical antifungals mentioned have maintained their efficacy against natural dermatophyte strains. However, it is essential to acknowledge the dynamic nature of fungal evolution, and one notable exception is the documented in vitro resistance to tavaborole. This resistance has been observed in T. rubrum, encompassing both reference strains and clinical isolates. This finding emphasizes the need for ongoing surveillance and research to monitor potential shifts in susceptibility patterns and to guide clinicians in adapting treatment strategies to address emerging challenges in the effective management of dermatophytosis (88).
Antifungals cross resistance:
Mutants resistant to terbinafine (TRB) display cross-resistance to other squalene epoxidase (SQLE) inhibitors, aligning with the mechanism associated with SQLE gene mutations. It is noteworthy that amorolfine (AMF) has a restricted inhibitory effect on squalene epoxidase, alongside its influence on C-14 reductase and C8 isomerase. (58,59). Research conducted by Ghelardi et al. (2014) revealed that successive sub-culturing with subinhibitory drug concentrations resulted in notable elevations in minimum inhibitory concentrations (MICs) for terbinafine (TRB), itraconazole (ITR), and amorolfine (AMF), with resistance emerging at high frequencies. (60). Nevertheless, there were no identified ciclopirox (CPX) resistant mutants. Different resistant mutants exhibited varying degrees of cross-resistance, indicating mechanisms beyond target gene modification, possibly involving efflux transporters—though the reversal of resistance after nonselective media passages challenges this concept. In a separate study, strains cultured with fluconazole (FLU) demonstrated elevated minimum inhibitory concentrations (MICs) for both FLU and itraconazole (ITR), while those cultured with ITR exhibited higher MICs for both ITR and FLU, suggesting the presence of cross-resistance. (61)
Emerging Fungal Pathogens with Multiple Drug Resistance:
Although infrequent, the detection of outbreaks involving multidrug-resistant Trichophyton isolates has been demonstrated through comprehensive whole-genome sequencing and antifungal susceptibility testing (AFST) (62,63,64). Isolates of Trichophyton from India, belonging to the Trichophyton mentagrophytes/interdigitale complex, have demonstrated resistance to multiple antifungal agents. This resistance includes terbinafine, griseofulvin, and azoles such as itraconazole, fluconazole, and voriconazole. (65,66). Remarkably, instances of T. mentagrophytes infections resistant to terbinafine have been successfully treated with topical ciclopirox olamine and miconazole. Recent updates from the Centers for Disease Control and Prevention (CDC) have highlighted the rise of multidrug-resistant Candida and Aspergillus species. (67). This includes instances of acquired resistance to azoles and echinocandins, both individually and in combination, as observed in Candida glabrata (68). Furthermore, Candida auris, an emerging pathogen known for its tendency to induce severe invasive bloodstream infections and high mortality rates in over 20 countries, has exhibited resistance to multiple categories of antifungal agents. (69). In vitro studies have shown the potential efficacy of triazoles like ravuconazole for managing drug-resistant candidiasis, offering an alternative treatment avenue (70).
How to overcome antifungal resistance:
Multiple tactics have been employed to address antifungal resistance, encompassing higher antifungal dosages, enhanced drug delivery systems to optimize existing antifungal efficacy, the utilization of combined antifungal treatments, surgical intervention for isolated infections, immunomodulation, and the exploration of experimental antifungal agents (71). Dose intensity, although not formally studied through controlled clinical trials, is supported by clinical evidence that suggests high-dose fluconazole as a safe and effective treatment for susceptible dose-dependent Candida species. Recent pharmacodynamic investigations further reinforce the logic behind increasing fluconazole dosages for managing such Candida strains. Notably, findings from animal infection models, conducted by Andes and colleagues as well as Louie and colleagues, indicate that the mycological effectiveness of fluconazole is associated with achieving an area-under-the-curve (AUC) to minimum inhibitory concentration (MIC) ratio within the range of 25 to 50 (72,73).
Due to the challenging and persistent nature of numerous fungal infections, the idea of combining multiple antifungal agents has gained traction as a strategy to improve treatment effectiveness, mitigate the development of resistance, and potentially lessen adverse effects. It's important to note that, except for cryptococcal meningitis, there is currently no clinical evidence supporting the superiority of combination antifungal therapy over single-drug treatments for refractory fungal infections (74). The scarce data currently available indicate that the combination of multiple antifungal agents might hold the potential to hinder the onset of secondary resistance or decrease the probability of selecting fungal strains with inherent resistance. Among the few studies published, a majority suggest that combinations incorporating amphotericin B and flucytosine have proven effective in diminishing the emergence of flucytosine resistance. (75).
The growing complexities associated with antifungal resistance and the emergence of progressively resistant fungal strains have intensified the urgent requirement for the innovation of novel antifungal treatments. Presently, several new antifungal compounds are undergoing assessments in phase II to III clinical trials. (76,77, 78). Among these, several innovative triazole antifungals, such as voriconazole, posaconazole, and potentially ravuconazole, demonstrate promising efficacy in both in vitro and in vivo settings against specific yeast and mold species resistant to fluconazole and itraconazole. The convenience of these agents being available in oral formulations allows for extended outpatient therapy. However, given that all these antifungals share the same target site (14α-demethylase) and considering the frequent involvement of broad-substrate efflux pumps in azole resistance, there are concerns about the potential for cross-resistance between older and newer triazoles. (79).
Emerging technologies such as lasers, photodynamic therapy, and iontophoresis require comprehensive, well-established research to validate their efficacy in treating and managing tinea infections. With the risk of overusing antifungal medications contributing to the development of antifungal resistance, there is an increasing demand for the implementation of antifungal stewardship programs (AFS). (80).
Conclusion:
Considerable progress has been made in advancing our understanding of the biology and diagnosis of antifungal drug resistance. However, the complex medical situations faced by patients dealing with resistant mycoses make it challenging to establish straightforward methods for identifying, preventing, and addressing antifungal drug resistance at this time. Ongoing research in both preclinical and clinical realms offers the potential to shed light on the significance of routine in-vitro susceptibility testing in cases of refractory mycoses and to explore innovative clinical approaches aimed at combating antifungal drug resistance in pathogenic fungi.
References
- Singh, A., Masih, A., Khurana, A., et al., 2018. High terbinafine resistance in Trichophyton interdigitale isolates in Delhi, India harbouring mutations in the squalene epoxidase gene. Mycoses 61, 477–484.
- Saunte, D.M.; Arendrup, M.C. Månedsbladet Rationel Farmakoterapi 2015, nr. 3. Svampeinfektioner i Hud, Hår og Negle.
- White TC, Marr KA, Bowden RA. 1998. Clinical, cellular, and molecular factors that contribute to antifungal drug resistance. Clin Microbiol Rev 11: 382–402. [CrossRef]
- Jabet A, Brun S, Normand AC, Imbert S, Akhoundi M, Dannaoui E, Audiffred L, Chasset F, Izri A, Laroche L, Piarroux R, Bachmeyer C, Hennequin C, Sabater AM. Extensive Dermatophytosis Caused by Terbinafine-Resistant Trichophyton indotineae, France. Emerg Infect Dis. 2022 Jan;28(1):229-233. [CrossRef]
- Bishnoi, A., Vinay, K., Dogra, S., 2018. Emergence of recalcitrant dermatophytosis in India. Lancet Infect. Dis. 18, 250–251. [CrossRef]
- Gupta AK, Renaud HJ, Quinlan EM, et al. The growing problem of antifungal resistance in onychomycosis and other superficial mycoses. Am J Clin Dermatol. 2021;22(2): 149–157. [CrossRef]
- Antifungal resistance j fungal diseases j CDC [Internet]. 2020. [cited 2021 Apr 19].
- Mukherjee PK, Leidich SD, Isham N, et al. Clinical Trichophyton rubrum strain exhibiting primary resistance to terbinafine. Antimicrob Agents Chemother. 2003;47(1): 82–86. [CrossRef]
- Ghannoum M. Azole resistance in dermatophytes: prevalence and mechanism of action. J Am Podiatr Med Assoc. 2016;106(1):79–86. [CrossRef]
- Jiang Y, Luo W, Verweij PE, et al. regional differences in antifungal susceptibility of the prevalent dermatophyte Trichophyton rubrum. Mycopathologia. 2021;186(1):53–70. [CrossRef]
- Marichal P, Koymans L, Willemsens S, et al. Contribution of mutations in the cytochrome P450 14alpha-demethylase (Erg11p, Cyp51p) to azole resistance in Candida albicans.
- Ghannoum M, Isham N. Fungal nail infections (onychomycosis): a never-ending story? PLoS Pathog. 2014;10(6): e1004105.
- Yadav A., Jain K., Wang Y., Pawar K., Kaur H., Sharma H.K., Tripathy V., Singh A., Xu J., Chowdhary A. Candida auris on Apples: Diversity and Clinical Significance. mBio. 2022;13:e0051822.
- Arendrup MC, Cuenca-Estrella M, Lass-Florl C, Hope WW. Breakpoints for antifungal agents: an update from EUCAST focussing on echinocandins against Candida spp and triazoles against Aspergillus spp. Drug Resist Updat 2013; 16: 81–95. [CrossRef]
- Pfaller MA, Andes D, Arendrup MC, et al. Clinical breakpoints for voriconazole and Candida spp revisited: review of microbiologic, molecular, pharmacodynamic, and clinical data as they pertain to the development of species-specific interpretive criteria. Diagn Microbiol Infect Dis 2011; 70: 330–43. [CrossRef]
- Pfaller MA, Diekema DJ, Andes D, et al. Clinical breakpoints for the echinocandins and candida revisited: integration of molecular, clinical, and microbiological data to arrive at species-specific interpretive criteria. Drug Resist Updat 2011; 14: 164–76. [CrossRef]
- Espinel-Ingroff A, Pfaller MA, Bustamante B, et al. Multilaboratory study of epidemiological cutoff values for detection of resistance in eight Candida species to fluconazole, posaconazole, and voriconazole. Antimicrob Agents Chemother 2014; 58: 2006–12. [CrossRef]
- Turnidge J, Kahlmeter G, Kronvall G. Statistical characterisation of bacterial wild-type MIC value distributions and the determination of epidemiological cut-off values. Clin Microbiol Infect 2006; 12: 418–25. [CrossRef]
- Espinel-Ingroff A, Arendrup MC, Pfaller MA, et al. Interlaboratory variability of caspofungin MICs for Candida spp using CLSI and EUCAST methods: should the clinical laboratory be testing this agent? Antimicrob Agents Chemother 2013; 57: 5836–42.
- Perlin DS, Rautemaa-Richardson R, Alastruey-Izquierdo A. The global problem of antifungal resistance: prevalence, mechanisms, and management. Lancet Infect Dis. 2017 Dec;17(12): e383-e392. Epub 2017 Jul 31. PMID: 28774698. [CrossRef]
- Crawford F, Young P, Godfrey C, et al. Oral treatments for toenail onychomycosis: a systematic review. Arch Dermatol. 2002; 138:811–816.
- Singh A, Masih A, Khurana A, et al. High terbinafine resistance in Trichophyton interdigitale isolates in Delhi, India harbouring mutations in the squalene epoxidase gene. Mycoses. 2018;61(7):477–484.
- Santos HL, Lang EAS, Segato F, et al. Terbinafine resistance conferred by multiple copies of the salicylate 1- monooxygenase gene in Trichophyton rubrum. Med Mycol. 2018;56(3):378–381. [CrossRef]
- Singh A, Masih A, Monroy-Nieto J, et al. A unique multidrug-resistant clonal Trichophyton population distinct from Trichophyton mentagrophytes/Trichophyton interdigitale complex causing an ongoing alarming dermatophytosis outbreak in India: genomic insights and resistance profile. Fungal Genet Biol. 2019;133(103266): 103266. [CrossRef]
- Kano R, Kimura U, Kakurai M, et al. Trichophyton indotineae sp. nov.: a new highly terbinafine-resistant anthropophilic dermatophyte species. Mycopathologia. 2020;185(6): 947–958. [CrossRef]
- Ebert A, Monod M, Salamin K, et al. Alarming India-wide phenomenon of antifungal resistance in dermatophytes: a multicentre study. Mycoses. 2020;63(7):717–728. [CrossRef]
- Salehi Z, Shams-Ghahfarokhi M, Razzaghi-Abyaneh M. Antifungal drug susceptibility profile of clinically important dermatophytes and determination of point mutations in terbinafine-resistant isolates. Eur J Clin Microbiol Infect Dis. 2018;37(10):1841–1846. [CrossRef]
- Nenoff P, Verma SB, Ebert A, Süß A, Fischer E, Auerswald E, et al. Spread of terbinafine-resistant Trichophyton mentagrophytes type VIII (India) in Germany—“the tip of the iceberg? J Fungi (Basel). 2020; 6:207.
- Saunte D, Pereiro-Ferreiros M, Rodrıguez-Cerdeira C, et al. Emerging antifungal treatment failure of dermatophytosis in Europe: take care or it may become endemic. J Eur Acad Dermatol Venereol. 2021;35(7):1582–1586. [CrossRef]
- Gaurav V, Bhattacharya SN, Sharma N, et al. Terbinafine resistance in dermatophytes: time to revisit alternate antifungal therapy. J Mycol Med. 2021;31(1):101087. [CrossRef]
- Mast, N., Zheng, W., Stout, C.D., et al., 2013. Antifungal azoles: structural insights into undesired tight binding to cholesterol metabolizing CYP46A1. Mol. Pharmacol. 84, 86–94. [CrossRef]
- Pai V, Ganavalli A, Kikkeri NN. Antifungal resistance in dermatology. Indian J Dermatol. 2018;63(5):361–368. [CrossRef]
- Coleman, J.J1., Mylonakis, E., 2009. Efflux in fungi : la pièce de résistance. PLoSPathog. 5, e1000486.
- Martinez-Rossi, N.M., Bitencourt, T.A., Peres, N.T.A., Lang, E.A.S., Gomes, E.V., Quaresemin, N.R., Martins, M.P., Lopes, L., Rossi, A., 2018. Dermatophyte resistance to antifungal drugs: mechanisms and prospectus. Front. Microbiol. 9, 1108. [CrossRef]
- Wilkens, S., 2015. Structure and mechanism of ABC transporters. F1000Prime Rep. 7, 14. [CrossRef]
- Fachin, A.L., Ferreira-Nozawa, M.S., Maccheroni Jr, W., et al., 2006. Role of the ABC transporter TruMDR2 in terbinafine, 4- nitroquinoline N-oxide and ethidium bromide susceptibility in Trichophyton rubrum. J. Med. Microbiol. 55 (Pt 8), 1093–1099.
- Feng, W., Yang, J., Xi, Z., et al., 2017. Mutations and/or overexpression of ERG4 and ERG11 genes in clinical azoles-resistant isolates of Candida albicans. Microb. Drug Resist. 23, 563–570.
- Xiang, M.J., Liu, J.Y., Ni, P.H., et al., 2013. Erg11 mutations associated with azole resistance in clinical isolates of Candida albicans. FEMS Yeast Res. 13 (4), 386–393. [CrossRef]
- Peres, N.T., Sanches, P.R., Falcão, J.P., et al., 2010. Transcriptional profiling reveals the expression of novel genes in response to various stimuli in the human dermatophyte Trichophyton rubrum. BMC Microbiol. 10, 39. [CrossRef]
- Paião, F.G., Segato, F., Cursino-Santos, J.R., et al., 2007. Analysis of Trichophyton rubrum gene expression in response to cytotoxic drugs. FEMS Microbiol. Lett. 271, 180–186 Epub 2007 Apr 10.
- Martinez-Rossi, N.M., Peres, N.T., Rossi, A., 2017. Pathogenesis of dermatophytosis: sensing the host tissue. Mycopathologia 182, 215–227. [CrossRef]
- Perlin, D.S., Shor, E., Zhao, Y., 2015. Update on antifungal drug resistance. Curr. Clin. Microbiol. Rep. 2, 84–85. [CrossRef]
- Martinez-Rossi, Nilce M., Tiago, R., et al., 2016. Heat shock proteins in dermatophytes: current advances and perspectives. Curr. Genom. 17, 99–111.
- Hsieh A, Quenan S, Riat A, et al. A new mutation in the SQLE gene of Trichophyton mentagrophytes associated to terbinafine resistance in a couple with disseminated tinea corporis. J Mycol Med. 2019;29(4):352–355. [CrossRef]
- Gaurav V, Bhattacharya SN, Sharma N, et al. Terbinafine resistance in dermatophytes: time to revisit alternate antifungal therapy. J Mycol Med. 2021;31(1):101087. [CrossRef]
- Nofal A, Fawzy MM, El-Hawary EE. Successful treatment of resistant onychomycosis with voriconazole in a liver transplant patient. Dermatol Ther. 2020;33: e14014. [CrossRef]
- Gu D, Hatch M, Ghannoum M, et al. Treatment-resistant dermatophytosis: a representative case highlighting an emerging public health threat. JAAD Case Rep. 2020;6(11): 1153–1155. [CrossRef]
- Farowski F, Vehreschild JJ, Cornely OA. Posaconazole: a next generation triazole antifungal. Future Microbiol. 2007; 2(3):231–243. [CrossRef]
- Epstein, W.L., Shah, V.P., Riegelman, S., 1972. Griseofulvin levels in stratum corneum. Study after oral administration in man. Arch. Dermatol. 106, 344–348. [CrossRef]
- Yenişehirli, G1., Tunçoğlu, E., Yenişehirli, A., Bulut, Y., 2013. In vitro activities of antifungal drugs against dermatophytes isolated in Tokat, Turkey. Int. J. Dermatol. 52, 1557–1560. [CrossRef]
- Chadeganipour, M1., Nilipour, S., Havaei, A., 2004. In vitro evaluation of griseofulvin against clinical isolates of dermatophytes from Isfahan. Mycoses 47, 503–507. [CrossRef]
- Ghannoum, M.A., Chaturvedi, V., Espinel-Ingroff, A., et al., 2004. Intra- and interlaboratory study of a method for testing the antifungal susceptibilities of dermatophytes. J. Clin. Microbiol. 7, 2977–2979. [CrossRef]
- Mistik, S., Ferahbas, A., Koc, A.N., Ayangil, D., Ozturk, A., 2006. What defines the quality of patient care in tinea pedis? J. Eur. Acad. Dermatol. Venereol. 20 (2), 158–165.
- Rudramurthy, S.M., Shankarnarayan, S.A., Dogra, S., et al., 2018. Mutation in the squalene epoxidase gene of Trichophyton interdigitale and Trichophyton rubrum associated with Allylamine resistance. Antimicrob. Agents Chemother. 62, e02522–e2617. [CrossRef]
- Korting, H.C., Rosenkranz, S., 1990. In vitro susceptibility of dermatophytes from Munich to griseofulvin, miconazole and ketoconazole. Mycoses 33, 136–139. [CrossRef]
- Martins, M.P., Franceschini, A.C.C., Jacob, T.R., et al., 2016. Compensatory expression of multidrug-resistance genes encoding ABC transporters in dermatophytes. 65, 605–610. [CrossRef]
- Cervelatti, E.P., Fachin, A.L., Ferreira-Nozawa, M.S., et al., 2006. Molecular cloning and characterization of a novel ABC transporter gene in the human pathogen Trichophyton rubrum. Med. Mycol. 44, 141–147. [CrossRef]
- Osborne, C.S., Hofbauer, B., Favre, B., et al., 2003. In vitro analysis of the ability of Trichophyton rubrum to become resistant to terbinafine. Antimicrob. Agents Chemother. 47, 3634–3636. [CrossRef]
- Favre, B., Ryder, N.S., 1996. Characterization of squalene epoxidase activity from the dermatophyte Trichophyton rubrum and its inhibition by terbinafine and other antimycotic agents. Antimicrob. Agents Chemother. 40, 443–447. [CrossRef]
- Ghelardi, E., Celandroni, F., Gueye, S.A., et al., 2014. Potential of Ergosterol synthesis inhibitors to cause resistance or cross-resistance in Trichophyton rubrum. Antimicrob. Agents Chemother. 58, 2825–2829. [CrossRef]
- Hryncewicz-Gwóźdź, A., Kalinowska, K., Plomer-Niezgoda, E., et al., 2013. Increase in resistance to fluconazole and itraconazole in Trichophyton rubrum clinical isolates by sequential passages in vitro under drug pressure. Mycopathologia 176, 49–55. [CrossRef]
- Singh, A., Kalinowska, K., Plomer-Niezgoda, E., et al., 2013. Increase in resistance to fluconazole and itraconazole in Trichophyton rubrum clinical isolates by sequential passages in vitro under drug pressure. Mycopathologia 176, 49–55. [CrossRef]
- McTaggart LR, Cabrera A, Cronin K, Kus JV. Antifungal Susceptibility of Clinical Yeast Isolates from a Large Canadian Reference Laboratory and Application of Whole-Genome Sequence Analysis to Elucidate Mechanisms of Acquired Resistance. Antimicrob Agents Chemother. 2020 Aug 20;64(9):e00402-20. [CrossRef]
- Fattahi A, Shirvani F, Ayatollahi A, et al. Multidrug-resistant Trichophyton mentagrophytes genotype VIII in an Iranian family with generalized dermatophytosis: report of four cases and review of literature. Int J Dermatol. 2021;60(6): 686–692. [CrossRef]
- Suß A, Uhrlaß S, Ludes A, et al. [Extensive tinea corporis € due to a terbinafine-resistant Trichophyton mentagrophytes isolate of the Indian genotype in a young infant from Bahrain in Germany]. Hautarzt. 2019;70(11):888–896.
- Das S, De A, Saha R, et al. The current Indian epidemic of dermatophytosis: a study on causative agents and sensitivity patterns. Indian J Dermatol. 2020;65(2):118–122. [CrossRef]
- Marquez L, Quave CL. Prevalence, and therapeutic challenges of fungal drug resistance: role for plants in drug discovery. Antibiotics (Basel). 2020;9(4):150. [cited 2021 Apr 19] Available from: pmc/articles/PMC7235788. [CrossRef]
- Frías-De-León MG, Hernández-Castro R, Conde-Cuevas E, García-Coronel IH, Vázquez-Aceituno VA, Soriano-Ursúa MA, Farfán-García ED, Ocharán-Hernández E, Rodríguez-Cerdeira C, Arenas R, Robledo-Cayetano M, Ramírez-Lozada T, Meza-Meneses P, Pinto-Almazán R, Martínez-Herrera E. Candida glabrata Antifungal Resistance and Virulence Factors, a Perfect Pathogenic Combination. Pharmaceutics. 2021 Sep 22;13(10):1529.
- Forsberg K, Woodworth K, Walters M, et al. Candida auris: the recent emergence of a multidrug-resistant fungal pathogen. Med Mycol. 2019;57(1):1–12. [CrossRef]
- Dong J, Liang G, Zheng H, et al. In Vitro activity of ravuconazole against Candida auris and vaginal candida isolates. Mycoses. 2021; 64:651–655. [CrossRef]
- Alexander BD, Perfect JR. Antifungal resistance trends towards the year 2000. Implications for therapy and new approaches. Drugs 1997; 54: 657–78. [CrossRef]
- Andes D, Stamsted T, Conklin R. Pharmacodynamics of amphotericin B in a neutropenic-mouse disseminated-candidiasis model. Antimicrob Agents Chemother 2001; 45: 922–26. [CrossRef]
- Louie A, Drusano GL, Banerjee P, et al. Pharmacodynamics of fluconazole in a murine model of systemic candidiasis. Antimicrob Agents Chemother 1998; 42: 1105–09. [CrossRef]
- Polak A. The past, present and future of antimycotic combination therapy. Mycoses 1999; 42: 355–70. [CrossRef]
- Francis P, Walsh TJ. Evolving role of flucytosine in immunocompromised patients: new insights into safety, pharmacokinetics, and antifungal therapy. Clin Infect Dis 1992; 15: 1003–18. [CrossRef]
- Groll AH, Piscitelli SC, Walsh TJ. Clinical pharmacology of systemic antifungal agents: a comprehensive review of agents in clinical use, current investigational compounds, and putative targets for antifungal drug development. Adv Pharmacol 1998; 44: 343–500.
- Sheehan DJ, Hitchcock CA, Sibley CM. Current and emerging azole antifungal agents. Clin Microbiol Rev 1999; 12: 40–79. [CrossRef]
- Neely MN, Ghannoum MA. The exciting future of antifungal therapy. Eur J Clin Microbiol Infect Dis 2000; 19: 897–914.
- Kontoyiannis DP. A clinical perspective for the management of invasive fungal infections: focus on IDSA guidelines. Infectious Diseases Society of America. Pharmacotherapy 2001; 21: 175S–87S. [CrossRef]
- Gupta AK, Venkataraman M, Renaud Hj Summerbell R, et al. The increasing problem of treatment-resistant fungal infections: a call for antifungal stewardship programs. Int J Dermatol. 2021. [CrossRef]
- Chen E, Ghannoum M, Elewski BE. Treatment-resistant tinea corporis, a potential public health issue. Br J Dermatol. 2021;184(1):164–165.
- Kumar S, Kaur A, Kaur S. Autoimplantation therapy in extensive and recalcitrant dermatophytosis: a case series. J Clin Aesthet Dermatol. 2021;14(1):34–37.
- Abastabar M, Hosseini T, Valadan R, et al. Novel point mutations in cyp51A and cyp51B genes associated with itraconazole and posaconazole resistance in Aspergillus clavatus isolates. Microb Drug Resist. 2019;25(5):652–662. [CrossRef]
- Hivary S, Fatahinia M, Halvaeezadeh M, et al. Luliconazole, a new antifungal, vs. amphotericin B, voriconazole, posaconazole and caspofungin against clinical and environmental Aspergillus nigri complex. bioRxiv. 2019.
- Beardsley J, Halliday CL, Chen SC-A, et al. Responding to the emergence of antifungal drug resistance: perspectives from the bench and the bedside. Future Microbiol. 2018; 13:1175–1191. [CrossRef]
- Yamaguchi H. Potential of ravuconazole and its prodrugs as the new oral therapeutics for onychomycosis. Med Mycol J. 2016;57(4): E93– E110. [CrossRef]
- Kano R, Hiruma J, Yokota M, et al. In Vitro ravuconazole susceptibility of anthropophilic dermatophyte strains isolated from Japanese patients. Jpn J Infect Dis. 2020;73(3): 250–252. [CrossRef]
- Mazzantini D, Celandroni F, Calvigioni M, et al. In Vitro resistance and evolution of resistance to tavaborole in Trichophyton rubrum. Antimicrob Agents Chemother. 2021; 65(4): e02324–20. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).