Introduction
Animal welfare is increasingly understood as an ethically significant dimension of sustainable food production (United Nations Environment Programme 2019; Coghlan et al. 2021). This is reflected in increasing consumer concern for animal welfare, retailer commitments to meet minimum welfare standards, and government legislation (Alonso et al. 2020; Albalat et al. 2022; Wickens 2022; Wahltinez et al. 2022). Society is increasingly turning attention towards the welfare of aquatic animals in particular, including fish and marine invertebrates (Stien et al. 2020; Albalat et al. 2022; Crump et al. 2022; Wahltinez et al. 2022). It was recently estimated that the most numerous group of animals directly killed for human consumption is shrimp, exceeding even numbers of directly killed fish and chickens (Romero Waldhorn and Autric 2022). This means that the overall animal welfare impact of the global shrimp industry could potentially be very large (Scherer et al. 2018). Wild-caught shrimp can be used for human consumption, production of feed for livestock and farmed fish, and/or other industrial processes such as chitin/chitosan production (Orrego and Salgado 2014).
Broadly speaking, wild-caught shrimp can be divided into three taxonomic groups. These three taxonomic groups are distinguished by important biological characteristics, industry features, and welfare considerations (Comstock 2022a). As such, policies that aim to improve animal welfare (or any other dimension of sustainability) will tend to differ between these three taxonomic groups (Gillett 2008) (
Figure 1).
The penaeids are a family of shrimp within the superfamily Penaeoidea and suborder Dendrobrachiata. These are warm-water (mostly tropical) shrimp, targeted by both industrial trawlers and small-scale fisheries. Penaeids have a relatively large body size (between 2 and 15 shrimp per pound) and a relatively short life cycle (below 3 years) (Gillett 2008).
The carideans are a family of shrimp that constitute the infraorder Caridea in the suborder Pleocyemata. These are cold-water (mostly temperate) shrimp, targeted by both industrial trawlers and small-scale fisheries. Carideans have a small body size (over 100 shrimp per pound) and a longer life cycle (between 3 and 8 years) (Gillett 2008).
The sergestids are a family of shrimp that constitute the family Sergestidae within the suborder Dendrobranchiata. These are warm-water (mostly tropical) shrimp, usually targeted by small-scale fisheries. Sergestid shrimp, especially in the genus Acetes, are particularly important in South-East Asia, East Asia, and East Africa and are commonly used to produce shrimp paste (Gillett 2008; Jagerroos 2016; Ding et al. 2021; Bauer 2023). Sergestids have a very or even microscopic body size and a short lifestyle (below one year) (Gillett 2008).
In this report, we use the word "shrimp" to refer to any crustacean within the suborder Dendrobranchiata or the infraorder Caridea. There are two additional infraorders that are justifiably called "shrimp" (Procarididea and Stenopodidea), but these are not targets for fisheries and less relevant to this report. In this sense, "shrimp" is a paraphyletic group; shrimp in Caridea diverged more recently from crabs and lobsters than they did from shrimp in Dendrobranchiata (De Grave et al. 2009; Wolfe et al. 2019). We view the words "shrimp" and "prawn" as interchangeable (Gillett 2008; Romero Waldhorn and Autric 2022).
Figure 1.
Classification of the main shrimp fisheries in the countries we detail in this report (the top 25 countries by estimated individual shrimp caught).
Figure 1.
Classification of the main shrimp fisheries in the countries we detail in this report (the top 25 countries by estimated individual shrimp caught).
One way to understand the global shrimp fishing industry is as a spectrum. On one extreme, there are commercial trawlers that operate in developed countries (
Figure 2A-B). Examples include the North Sea trawlers that target
Crangon crangon (Innes and Pascoe 2008; Dutch Fish Product Board 2009) and Australia's Northern Prawn Fishery (Farmery et al. 2015). Trawlers can be equipped with one or more beam trawls or otter trawls, and voyages can last weeks or months (Gillett 2008). Fleets may contain a few hundred or even a few dozen vessels (Respondek et al. 2022). These fisheries tend to be relatively wealthy (on a global scale) and technologically sophisticated, with catch often processed and packaged on-board. A trawl may be conducted for several hours; shrimp in the water are exhausted by escape attempts and eventually overrun into the trawl net (Gillett 2008). When finfish enter the net, they may either swim with the net or become exhausted and fall into the codend (Gillett 2008).
After a trawl is conducted for several hours, the net and its contents (the shot) are hauled and released onto the deck. The shot is sorted, which can involve removing bycatch and grading or sizing the shrimp. This step can be entirely manual, though grading may involve a machine (Carnie 2002). Sorted shrimp are washed in clean seawater and occasionally treated with chemicals (e.g. metabisulphite) (Carnie 2002; Gokoglu et al. 2022). On shorter voyages or on trips where the catch can be unloaded intermittently, the shrimp may be stored in cold water (Carnie 2002). On longer trips, the catch can be cooked in boiling water before being packaged and stored in an on-board industrial freezer (Carnie 2002; Innes and Pascoe 2008; Dutch Fish Product Board 2009; Vestergaard et al. 2011; Mann 2018; Gokoglu et al. 2022).
On the other extreme, there are small-scale fishing communities that operate in developing countries, usually close to the shore or in estuaries (
Figure 2C-D). These fisheries tend to be characterised by low levels of technological sophistication and comparatively high levels of poverty. Examples include the Vietnam coastal communities, where shrimp fishing is considered a "last resort" occupation and poverty is high (Tran Thi Phung Ha 2012), and Thailand, where there have been widespread reports of modern slavery aboard fishing vessels (Kadfak et al. 2023). These fisheries tend to be multi-species, and fishers will sell the shrimp alongside other invertebrates and finfish that are also caught (Thanh Viet Nguyen 2011; Cinco et al. 2015; Wong and Yong 2020). These fisheries can be conducted aboard small vessels (non-motorised or with small engines) or by wading into the water from shore. As for gear types, Gillet (2008) lists: stow nets, lift nets, push nets, beach seines, gillnets, barriers across estuaries, net, weirds, and traps. Some of these are passive gear types, such as the stow net, which catch shrimp by using the flow of the current. Small-scale fisheries in developing countries often struggle with enforcing fisheries regulations.
Figure 2.
Shrimp fisheries differ widely around the world. (A) Crangon crangon caught in the industrialised and technologically sophisticated North Sea fishery. (B) Trawlers targeting C. crangon in the adjacent Wash shrimp fishery, England. (C) Shrimp and bycatch aboard a trawler in India's shrimp fishery, which involves both commercial and small-scale fishing. (D) Fishers warm themselves with a fire by their boats as they wait for customers to arrive. Note the debris on the beach. (Credit: A: Hans Hillewaert, CC BY-NC-ND 2.0 licence; B: Ian Simons, CC BY-SA 2.0 licence; C, D: S. Chakrabarti / We Animals Media, Non-Commercial Use licence.).
Figure 2.
Shrimp fisheries differ widely around the world. (A) Crangon crangon caught in the industrialised and technologically sophisticated North Sea fishery. (B) Trawlers targeting C. crangon in the adjacent Wash shrimp fishery, England. (C) Shrimp and bycatch aboard a trawler in India's shrimp fishery, which involves both commercial and small-scale fishing. (D) Fishers warm themselves with a fire by their boats as they wait for customers to arrive. Note the debris on the beach. (Credit: A: Hans Hillewaert, CC BY-NC-ND 2.0 licence; B: Ian Simons, CC BY-SA 2.0 licence; C, D: S. Chakrabarti / We Animals Media, Non-Commercial Use licence.).
Of course, there are many industries that can be positioned between these two extremes, such as small-scale shrimp fisheries in developed countries (e.g. the in-shore pot fishery targeting Palaemon serratus in Ireland (Fahy and Gleeson 1996; Tully 2017)) and large, technologically sophisticated trawlers in developing countries (e.g. industrial freezer-trawlers in Mozambique's shrimp fishery (Torres Coll 2013)). It is self-evident that policies to improve fishing practices need to vary significantly along this spectrum (Gillett 2008; Suuronen et al. 2020).
Much of the academic and policy discussion around shrimp fisheries focus on the issue of bycatch. The rates of bycatch in shrimp trawling can be very high, with estimates exceeding 80% or even 90% by weight in some contexts (Jang et al. 2009; Pillai et al. 2022; James et al. 2023). Animals caught as bycatch can include marine invertebrates, finfish, turtles, marine mammals, and seabirds (Wickliffe and Jodice 2010; Brito 2012). Bycatch may also include juvenile individuals of the target shrimp species. Bycatch may be returned to the water, but mortality is high, including for juvenile shrimp (Temming et al. 2022). Bycatch has been mitigated by technical solutions (e.g. bycatch reduction devices or other net modifications (Gillett 2008; Brito 2012; Mann 2018)) and policy solutions (e.g. moving fisheries away from areas where high rates of bycatch are detected (Gillett 2008; Hvingel and Zimmermann 2023)). Bycatch is a more salient issue in large trawl fisheries, particularly in developed countries; in contrast, small-scale fishers tend to target multiple species and view "bycatch" as a valuable component of their catch (Gillett 2008).
The remainder of this report is structured as follows. Firstly, we summarise the current state of scientific knowledge related to shrimp welfare and recent developments in government policy, industry policy, and technology. Secondly, we give a brief overview of issues relating to human rights and wellbeing in global shrimp fisheries, as any successful animal welfare policy needs to incorporate an understanding of social and economic needs. Then, we critically discuss potential policies that can be leveraged by governments, retailers, and industry to improve the welfare of wild-caught shrimp. Finally, we examine the global shrimp fishing industry from the perspective of catch-by-number, a more relevant measure of animal welfare impact than catch-by-weight. We give an in-depth summary of shrimp fisheries in each of the world's top 25 countries by number of individual shrimp caught, before concluding the report with a discussion of how ethics can advise policy in wild-caught shrimp fisheries.
Are shrimp sentient beings?
The capacity of crustaceans, including shrimp, to feel pain is an emerging area of interest in academic research, and this has already led to government regulations and industry policies that respond to recent findings. While many of these policies have focused on the welfare of farmed crustaceans, there are some cases where wild shrimp welfare is explicitly taken into account.
The leading report on sentience in crustaceans was carried out by Birch et al (2021) at the London School of Economics. This team reviewed more than 300 scientific papers on crustaceans and cephalopods, and their report played a key role in the inclusion of decapod crustaceans and cephalopods within the UK's Animal Welfare (Sentience) Act 2022 (Department for Environment, Food & Rural Affairs 2021). The Birch report provides a framework that can be used to evaluate the evidence of sentience in crustaceans. This framework evaluates taxa against eight criteria considered to be relevant to the capacity of organisms to feel. The authors evaluated caridean shrimp and penaeid shrimp (among other taxa) against these criteria:
1) Possession of nociceptors: "High" confidence that this criterion is satisfied by carideans and penaeids.
2) Possession of integrative brain regions: Very high" confidence for carideans and "low" confidence for penaeids.
3) Connections between nociceptors and integrative brain regions: "Low" confidence for carideans and penaeids.
4) Responses affected by potential local anaesthetics or analgesics: "Medium" confidence for carideans and penaeids.
5) Motivational trade-offs that show a balancing of threat against opportunity for reward: "Low" confidence for carideans and penaeids.
6) Flexible self-protective behaviours in response to injury and threat: "Medium" confidence for carideans and "low" confidence for penaeids.
7) Associative learning that goes beyond habituation and sensitisation: "Low" confidence for carideans and penaeids.
8) Behaviour that shows the animal values local anaesthetics or analgesics when injured: "Very low" confidence for carideans and penaeids.
A key challenge is that there have been relatively few primary studies that provide evidence, whether positive or negative, against these criteria for shrimp (Albalat et al. 2022). As the authors conclude (Birch et al. 2021): "[...] in cases where we are not able to have high or very high confidence that a criterion is satisfied, this is invariably because of a lack of positive evidence, rather than because of clear evidence that the animals fail the criterion."
Nevertheless, the framework of Birch et al. provides a systematic way to evaluate existing studies and identify key knowledge gaps —future primary studies can focus on filling these key gaps for the most numerous commercially important decapods. In addition, the framework leads to hypotheses that can be tested. Comstock (2022a; 2022b) points out that the case for sentience is currently a bit stronger for caridean shrimp than penaeid shrimp and offers a number of testable hypotheses that could examine whether caridean and penaeid shrimp indeed differ in sentience. Mallatt and Feinberg (2022) offer the testable hypothesis that, if decapod crustaceans are indeed sentient, then they may have efficient neural circuitry. Rigorous scientific enquiry leading to verifiable, replicable results can place society in the best position to make informed decisions about protecting the welfare of shrimp and the specific details of those protections (Freeling and Connell 2021; Albalat et al. 2022; Diggles et al. 2023).
Policies to protect shrimp welfare
Governments have responded to the growing body of evidence regarding the likelihood that shrimp can feel pain by implementing legislation to protect shrimp welfare in the following jurisdictions: Austria, Italy, New Zealand, Norway, Switzerland, and New South Wales (Australia) (Morton et al. 2020; Albalat et al. 2022). Victoria (Australia) also has draft legislation currently under consultation that would include protections for decapod crustaceans (Kolovos 2023). In addition, the UK's Animal Welfare (Sentience) Act 2022 empowers an Animal Sentience Committee to make recommendations to the government about the treatment of all sentient animals, which explicitly includes decapod crustaceans. Shrimp welfare legislation is usually part of broader animal welfare acts, which tend to be focused on animals in captivity and often contain explicit exemptions for fisheries.
Some shrimp fishers and industry bodies are taking active steps to seek and apply information that would improve the welfare of shrimp they catch (Albalat et al. 2022). This is exemplified by the Australian Council of Prawn Fisheries, which has received government and industry funding to develop a "Responsible (animal welfare) fishing practice for the Australian Wild Prawn industry" with an expected completion in mid-2024 (Fisheries Research and Development Corporation 2022). This reflects Australia's history of making large investments in shrimp fishing research and management, which also provides useful policy insights that can be applied elsewhere (Gillett 2008).
Such developments are supported by technological advances. In particular, methods for stunning shrimp before slaughter is an active area of academic research (Weineck et al. 2018; Albalat et al. 2022). Research is underway to develop and validate electrical stunning of commercially important shrimp species (University of Stirling 2023). The manufacturer Ace Aquatec has developed an electrical shrimp stunner (Prawn portable A-HSU™) that can be installed on shrimp farms and on wild-catch shrimp fishing vessels (Ace Aquatec 2023). Technological development is also relevant to bycatch reduction, as bycatch reduction devices and net modifications can provide a technological solution to help mitigate bycatch (Gillett 2008). The advance of machine learning and artificial intelligence may also offer useful tools (discussed further below).
Human rights and wellbeing in shrimp fisheries
Before turning to a discussion of potential animal welfare interventions, we believe it is important to recognise that the global shrimp fishing industry is closely related to a number of challenges related to human rights and justice. There is widespread recognition that policies aiming to improve animal welfare and other areas of sustainability must understand these dimensions of human wellbeing (Wrenn and Johnson 2013; Rodriguez 2018; Coghlan et al. 2021; Reisman et al. 2022; Conneely and Coates 2023). Here, we will summarise three key challenges, focusing on shrimp fisheries in developing countries.
The first is poverty in small-scale shrimp fishing communities. For example, small-scale fishing in Vietnam has been called "the occupation of the last resort" and an occupation taken up by the "poorest of the poor" (Tran Thi Phung Ha 2012) (but see (Thorpe et al. 2007)). Shrimp fishers in Vietnam tend to be poorly educated and have little political representation (Tran Thi Phung Ha 2012). Fisheries policies in Vietnam have failed to incorporate the economic needs of these communities, rendering such policies ineffective in achieving many of their goals (Pomeroy et al. 2009; Khan and Khan 2011; Tran Thi Phung Ha 2012). Another example is Pakistan, where poverty and problems with the credit market (alongside other social, economic, and environmental problems) have resulted in "the indebtedness and the more intensive exploitation of fishery resources" (Khan and Khan 2011). In these contexts, women often experience specific harms and risks (Dunaway and Macabuac 2007; Khan and Khan 2011). Clearly, fishing communities need to be incorporated into national strategies that aim to reduce poverty, and any reforms need to be based on a fine-grained understanding of the local context (Pomeroy et al. 2009; Khan and Khan 2011; Tran Thi Phung Ha 2012; Suuronen et al. 2020).
The second is conflict due to resource management and fisheries policy, in some cases even leading to violence. In South-East Asia, demand for shrimp (domestically and overseas) and diminishing catches have frequently led to the development of more intensive fishing methods (e.g. trawling) and the expansion of shrimp aquaculture. Intensive trawlers may directly compete with small-scale fishers for shrimp, while the clearing of mangrove to build shrimp farms can reduce the abundance of wild shrimp available to fisheries (Dunaway and Macabuac 2007; Gillett 2008; Tran Thi Phung Ha 2012; Altamiranoa et al. 2015). Small-scale fishers have been killed during collisions with industrial vessels (Gillett 2008). When large-scale shrimp trawls offload their catch onto local markets, this can cause prices received by small-scale shrimp fishers to decrease (Gillett 2008). Beyond the direct harms, these conflicts can sometimes spill over into social tension, property destruction, and violence (Gillett 2008; James et al. 2023).
The third is the problem of forced labour (modern slavery) (Suuronen et al. 2020). This problem has been particularly salient in Thai fisheries, including trawlers that catch shrimp and trawlers that catch fish to feed to farmed shrimp. The problem of forced labour came to the attention of the international community around 2014, after several high-profile investigations were published in the press (Szep and Grudgings 2013; Hodal and Kelly 2014). People forced to work on Thai fishing vessels experience extremely long hours at sea, regular beatings and injuries. These workers can be kept in chains or even murdered (Hodal and Kelly 2014; Seo 2018). Local police and politicians are often considered "business partners" by traffickers (Hodal and Kelly 2014; Kadfak et al. 2023). Many people forced to work aboard Thai fishing vessels are Rohingya men fleeing ethnic cleansing in neighbouring Myanmar (Sylwester 2014; Ruden 2017). The demand for forced fishing labour is stimulated by both economic and environmental dynamics. Thailand's economic growth has created attractive employment opportunities for Thais but also created strong demand for Thailand's exported shrimp by multinational corporations supplying consumers in wealthy countries (Sylwester 2014; Seo 2018). Additionally, overfishing has driven trawlers further offshore and therefore further out of reach of authorities (Sylwester 2014; Seo 2018). Thailand has made some reforms, such as the strengthening of the legislative framework around forced labour (Stringer et al. 2022). However, the International Labour Organization (2020) concluded that the prevalence of forced labour in Thailand's fisheries had remained relatively steady, at 14% in 2019 compared with 17% in 2013.
Welfare interventions in shrimp fisheries
Animal welfare policies in wild-catch shrimp fisheries will necessarily focus on one of three domains. Firstly, it is possible to improve the welfare of shrimp at capture and slaughter. This can be supported by studies that identify and validate welfare indicators (Albalat et al. 2022; Conneely and Coates 2023). Secondly, it is possible to reduce the number of shrimp or other animals caught. Thirdly, it is possible to strengthen government and regulatory capacity and conduct research to create opportunities for animal welfare reform in the long-term. All three of these domains can be supported by emerging technologies, including machine learning and artificial intelligence. These domains notably contrast with opportunities for improving animal welfare in farmed shrimp, which can also focus on improving welfare conditions during grow-out, breeding, and transport (Albalat et al. 2022).
Improving welfare at capture and slaughter
During the capture of wild shrimp, there are welfare risks at each stage of the process. Each of these stages provides an opportunity for reducing stress and improving welfare.
Trawling, like other harvest methods, can be very stressful for crustaceans (Albalat et al. 2022; Conneely and Coates 2023). Trawling can crush carapaces, cause collisions with other animals, and drive shrimp to exhaustion as they try to escape the net (Gillett 2008; Conneely and Coates 2023). One solution could be to reduce the duration of trawls (Crustacean Compassion). It is common for shrimp trawls to have a duration of 3 to 8 hours (Thong Ba Nguyen 2015; Lira et al. 2021). Conducting more frequent but shorter trawls may minimise the duration for which shrimp experience this stress.
Handling can cause stress (Albalat et al. 2022), and this is particularly true when handling is conducted by hauling shrimp in large trawl nets. Trawl nets may contain so many animals that individual shrimp are crushed by animals above them (Birch et al. 2021; Lewit-Mendes et al. 2022). One solution could be to reduce trawl weights, in line with the suggestion above (Birch et al. 2021; Lewit-Mendes et al. 2022). More frequent trawls with smaller weights may reduce the frequency with which shrimp experience stress and pain from being crushed.
When on board, shrimp may experience oxygen deprivation and/or inappropriate air temperatures (Birch et al. 2021; Conneely and Coates 2023). Shrimp may become stressed and eventually die from asphyxiation, which is typically considered inhumane (Lewit-Mendes et al. 2022; Conneely and Coates 2023). Shrimp may also experience stress if directly exposed to rain (Lavallee et al. 2000; Saraswathy et al. 2021). Suffering from oxygen deprivation may be minimised by optimising handling processes and improving on-board logistics where possible to reduce the amount of time that shrimp spend out of water. Shrimp could plausibly be delivered to recovery tanks rather than directly onto the deck in situations where this is practicable (Conneely and Coates 2023).
The slaughter or death process is a key period of significant welfare risk. There is limited information about when shrimp actually die during commercial harvest, and these details may hinge on the species targeted and the specific practices of a given fleet (Albalat et al. 2022). Shrimp, particularly individuals from small-bodied species such as Acetes japonicus, may die from stress during capture and handling before reaching the air. Alternatively, shrimp may die from asphyxiation when on deck. Shrimp that do not die during these processes may be killed when washed with, or stored in, iced water, or potentially when cooked in boiling water. In the case of boiling water, shrimp would experience intense suffering for up to several minutes (though probably slightly a shorter period for small-bodied shrimp) (Fregin and Bickmeyer 2016). In the case that shrimp do not die until aboard the vessel, electrical stunning appears to be the most promising method to prevent suffering at slaughter (Birch et al. 2021; Lewit-Mendes et al. 2022). To be effective, electrical stunning would need to be implemented properly and consistently, ideally using species-specific stunning parameters and validating the equipment in commercial settings (Birch et al. 2021; Albalat et al. 2022). There is at least one electrical shrimp stunner that is commercially available and can be installed on wild-catch fishing vessels (Ace Aquatec's "Prawn portable A-HSU™") (Ace Aquatec 2023). Due to investment costs (European Commission 2017), it is more plausible to install electrical stunners on trawlers targeting high-value shrimp in developed countries than in other contexts.
There has also been some discussion about the use of ice slurry to slaughter shrimp (Weineck et al. 2018). However, further research is needed to figure out whether ice slurry actually renders shrimp insensible or simply paralyses them (Birch et al. 2021; Lewit-Mendes et al. 2022). Moreover, ice "stunning" would necessitate placing shrimp in complete and sudden contact with consistently ice-cold water (Albalat et al. 2022), but it is common in commercial settings for shrimp to be stored in a container with a layer of ice on top—this would not satisfy the criteria for a humane slaughter method (Lewit-Mendes et al. 2022).
Reducing the number of animals caught
There are a number of technical and policy tools that can be applied to reduce the number of animals caught in shrimp fisheries.
The most well-developed option involves reducing rates of bycatch, which can reduce the number of juvenile shrimp, finfish, and other non-target animals captured by shrimp fisheries. Most commonly, high bycatch rates are reduced by installing bycatch reduction devices (BRD) in trawl nets (Gillett 2008). Other measures to reduce bycatch include: blanket bans on trawling; bans on fishing areas where high bycatch is observed; catch quotas; discard bans; and limits on the shrimp-to-bycatch ratios (Gillett 2008). Even when animals are returned to the water, mortality rates can be high (Temming et al. 2022). It may be possible to optimise on-board processes to return animals to the water more quickly, which could both reduce the duration of stress and improve chances of survival (Cook et al. 2015). Bycatch reduction is more relevant in large-scale trawl fisheries, especially in developed countries. Due to economic incentives and low levels of enforcement, policies to reduce bycatch in small-scale fisheries in developing countries are unlikely to succeed (Gillett 2008; Suuronen et al. 2020). In fact, in these contexts, "bycatch" is often a valuable part of the catch and used for human consumption or as feed for farmed livestock and fish (Gillett 2008).
More speculatively, there may be some latitude for reducing the number of Acetes shrimp captured in small-scale fisheries for shrimp paste production. Depending on the local context, production methods, and market, shrimp paste production could be modified to reduce the shrimp content. Similarly, some consumers may choose to reduce their shrimp intake on the basis of animal welfare (Scherer et al. 2018) or human wellbeing (International Labour Organization 2020).
Regulatory capacity, supply chains, and additional research
Particularly in developing countries with low levels of government capacity, it may be possible to find ways to improve either regulatory capacity or industry practice. As Gillet (2008) points out, many developing countries do succeed in establishing and enforcing shrimp fisheries regulations. In contrast, there are many developing countries that struggle to enforce regulations (see country-by-country analysis below). The enforcement of existing regulations may reduce the number of shrimp caught while also potentially mitigating overfishing and improving human wellbeing (Gillett 2008; Suuronen et al. 2020; Kadfak et al. 2023). Beyond the inherent value of regulatory capacity in helping to mitigate poverty, environmental degradation, and human rights violations (Tran Thi Phung Ha 2012; Sylwester 2014; Seo 2018; Suuronen et al. 2020; Stringer et al. 2022), developing a strong regulatory regime may open up further opportunities for targeted shrimp welfare policies in the coming decades. While regulatory capacity and enforcement obviously requires political will (Cho 2012; Mangar et al. 2023), assistance can come from knowledge sharing, incentives offered by trade partners or multinational corporations, or technology such as monitoring vessels via satellite tracking (Gillett 2008; Alonso and Marschke 2023).
Landing technologies and food safety management could be improved to reduce unnecessary wastage throughout the supply chain. In South-East Asian shrimp fisheries, storing the catch in ice boxes or bags commonly leads to spoilage (Suuronen et al. 2020). When shrimp is landed, it may be in a state of decomposition (Pillai et al. 2014). Historically, lax hygiene standards have caused importing countries to reject or even ban imports of shrimp products (Alam and Pokrant 2009; Geetha et al. 2020). Pollution in the sea or on the beach can also threaten food safety and product quality (Pomeroy et al. 2009). These problems can lead to wastage and reduce the prices that fishers and fish traders obtain for their products. Therefore, improving hygiene practices aboard vessels, during processing, and in the marine environment could lead to more profitable enterprises for fishers and traders while also reducing the number of shrimp caught and wasted.
Transnational governance can include industry, corporate social responsibility, and public-private partnerships around ethical certification standards (Parkes et al. 2010; Main et al. 2014). Certification schemes are increasingly valued by consumers, businesses, and even governments as a legitimate governance tool in fisheries and aquaculture (Washington and Ababouch 2011; Main et al. 2014). For example, patterns of voluntary uptake of Marine Stewardship Council (MSC) certification have grown rapidly. The increase in the number of chain of custody certifications and products sold with the MSC eco-label can create substantial revenue for governance from logo licensing fees (Foley 2013) and can benefit producers (Yaumidin and Zuas 2021). Some shrimp fisheries are certified by private certification schemes. The MSC (2023) lists current certifications for: Pandalus borealis caught by many countries in the North Atlantic and North Pacific; Crangon crangon caught by European countries in the North Sea; Pandalus species caught on the west coast of the United States; Xiphopenaeus kroyeri caught by Guyana and Suriname; and Penaeus, Metapenaeus, Fenneropenaeus, and Haliporoides species caught by the Australian trawler fleet. Through this collaborative framework, transnational governance may help to propel more ethical harvesting practices, where animal welfare, accountability, transparency and stewardship are prioritised.
To date, no private certification schemes have been developed that cover wild-caught shrimp welfare explicitly. Additionally, schemes that have included various farmed shrimp species within their remit, such as the Aquaculture Stewardship Council (ASC) or Best Aquaculture Practices (BAP) focus solely on environmental sustainability and animal health (Aquaculture Stewardship Council 2023a; Best Aquaculture Practices 2023). The concept of “animal welfare” has thus far only been used by these schemes in the context of health and productivity. This trend is beginning to shift as certification schemes recognise the demand from NGOs and, to a lesser extent, consumers, to incorporate actual animal welfare parameters as part of certification scheme standards. Schemes face a number of challenges in doing this, the largest being lack of welfare research on the majority of wild-caught or farmed aquatic animal species. Without primary research to support the identification of best practices for animal welfare, certification schemes may struggle to positively impact animal welfare . Currently, the farm certification scheme ASC is the only major certification scheme in the process of adding a required welfare component to their standards (Aquaculture Stewardship Council 2023b). It is unclear whether its sister organisation, the MSC, will explore similar welfare additions in their wild-caught standards.
Another benefit of increasing primary research in shrimp welfare is that such research can help inform the work of organisations seeking to better quantify the suffering of farmed aquatic animals in order to guide priority areas of impact. For example, there is an ongoing research project that aims to apply the Cumulative Pain Framework to create "Pain-Track" welfare profiles for farmed Litopenaeus vannamei shrimp (Alonso and Schuck-Paim 2021; Alonso and Schuck-Paim 2022). The Cumulative Pain Framework provides one way to quantify the duration and intensity of pain and suffering experienced by shrimp during various points of the production process, and it can be applied to both farmed shrimp and wild-caught shrimp (McKay and McAuliffe 2023).
Lastly, there is a clear need for additional basic and applied research on shrimp sentience and welfare. Additional research would be invaluable in improving our general understanding of shrimp sentience (Birch et al. 2021) and specific knowledge about the welfare of shrimp due to particular industry practices (Albalat et al. 2022). This would benefit both scientific knowledge and the regulatory capacity of governments and certification schemes. Likewise, the scale of the global shrimp industry is still not fully understood. We estimate the number of shrimp killed in the world's shrimp fisheries below. However, precision is hindered by bycatch, highgrading, and unreported catch (Jang et al. 2009; Jacquet et al. 2010; Cho 2012; Pedrozo 2022; Pillai et al. 2022; Temming et al. 2022; James et al. 2023). Understanding how these challenges affect the number of shrimp killed in fisheries would help refine the priority countries and priority industries for improving shrimp welfare in fisheries.
Future technologies and shrimp welfare
Future technologies have the potential to greatly change the landscapes of wild catch fisheries, and therefore affect the lives of many aquatic animals, including shrimps. Using satellite imagery, radiofrequency and infrared frequency detection, Automatic Identification System (AIS), and on-ship cameras, lots of data related to ship activities can be gathered (Longépé et al. 2018; May Petry et al. 2020; Fernandes-Salvador et al. 2022; 张佳泽 et al. 2022; Khokher et al. 2022). It is likely that underwater drones, with machine vision systems installed, can join this list of data collection methods in the future. Machine learning algorithms can be used to identify, estimate, or predict the behaviours of the ships, such as whether they are fishing, transshipping, entering ports, disabling the AIS, dumping catch, or using certain types of gears. This allows much more effective law enforcement to prevent illegal fishing and dumping, including that of shrimp. China and Indonesia are interested in using these kinds of technologies to tackle illegal fishing activities (Agence Française de Développement 2017; He and Zhang 2022).
Artificial intelligence (AI), particularly machine vision systems, can be employed to reduce the number of animals caught and/or killed as bycatch (Fernandes 2022; Poisson et al. 2022). Machine vision systems can be used to analyse bycatch, including species and numbers of animals, on a particular ship, which can assist future planning. There are a few research projects seeking to use AI to identify whether an animal is a targeted species. This enables two actions that could reduce bycatch. Firstly, this can help fishers identify whether a particular fishing ground at a particular point of time mainly consists of target species. Secondly, such systems allow the trawling device to be designed and programmed in a way that releases the non targeted species. Some of these systems can detect shrimp species. Such systems in development or deployment include Smartrawl, Game of Trawls, Scantrol Deep Vision. In the future, it is possible that on-ship cameras and machine vision systems can be installed on ships and used to monitor the treatment of animals during capture.
Shrimp catch-by-number around the world
When it comes to an industry's impact on animal welfare, catch-by-weight is a less informative category than catch-by-number (Mood et al. 2023). A small catch of many tiny individuals might affect more animals than a large catch of many bigger individuals. However, global fisheries catches are almost always reported as catch-by-weight. This is exemplified by Ireland, which is rarely considered a major contributor to the world's shrimp fisheries, but arises in the following analysis as a surprisingly high priority.
Recently, Waldhorn and Autric (2022) estimated mean species weights for each of the world's 92 commercially relevant wild-caught shrimp taxa. This follows the precedent of Mood et al. (2023), who applied a similar method when estimating the number of fish in aquaculture around the world. By combining these estimated mean species weights with global catch data, we can estimate the number of shrimp caught in wild-catch fisheries (Romero Waldhorn and Autric 2022).
Here, we provide estimates of the wild shrimp caught in all of the country's fisheries, down to the scale of species-country combination. This enables us to identify priority countries for shrimp welfare policy.
Naturally, there are a few aspects of this analysis that need to be treated with caution. Firstly, we use FAO catch data. FAO data has some well-known issues. For example, not all countries report their catch accurately, and landed catch may ignore the mortality of discarded bycatch or juveniles (Jacquet et al. 2010; Temming et al. 2022). It may be valuable to improve the accuracy of countries' reported shrimp catches by conducting reconstructions for each shrimp species on a country-by-country basis (e.g. following the example of the Sea Around Us Project (Pauly and Zeller 2016)). Nevertheless, the FAO data provides a useful first approximation, enabling us to identify priority countries for further analysis. Secondly, the estimated mean species weights are reported as ranges, not point estimates (Romero Waldhorn and Autric 2022). We visualise the ranges in our corresponding estimates of numbers of individuals, but we also use the midpoint to provide a more readily interpretable measure of industries' scale. Thirdly, there are some instances in the FAO data where catch is reported in generic statistical categories, rather than species groups (e.g. "Natantia nei", "Penaeus spp"). Waldhorn and Autric (2022) provided weight estimates for these statistical categories, and we note cases where the species composition of those general statistical categories may be relevant.
For each of the world's top 25 countries by estimated catch-by-number, we summarise the number of individual shrimp caught per year and provide an approximate breakdown by taxonomic group. We also provide a short review of the key characteristics of the industry in that country, including fleet size, socio-economic details, and exports. For exports, we use FAO trade data. We make no attempt to convert trade data to individuals, as highly variable meat conversion ratios make this implausible; or to distinguish between wild and farmed shrimp, due to complicated supply chains. However, the raw weight of exports remains a useful metric to convey the magnitude of exports and the major destination countries. Detailed species breakdown for catch-by-number for all countries represented in the FAO data are available in our online repository [
link removed for peer review]. We conducted this analysis in R (Wickham 2016; R Core Team 2020; Becker et al. 2023; Iannone et al. 2023).
Table 1.
The top 25 countries by estimated number of individual shrimp caught. Each country is detailed in this article.
Table 1.
The top 25 countries by estimated number of individual shrimp caught. Each country is detailed in this article.
| Country |
Catch by Weight (t) |
Weight (Percent of World) |
Catch by Number |
Number (Percent of World) |
| China |
1,131,828 |
36.51 % |
2.5e+13 (3.42e+12,4.67e+13) |
66.98 % |
| Malaysia |
106,988 |
3.45 % |
5.56e+12 (1.07e+11,1.1e+13) |
14.9 % |
| Thailand |
47,882 |
1.54 % |
2.29e+12 (4.3e+10,4.53e+12) |
6.14 % |
| South Korea |
33,044 |
1.07 % |
2.08e+12 (2.79e+11,3.89e+12) |
5.57 % |
| Philippines |
38,794 |
1.25 % |
1.24e+12 (2.44e+10,2.45e+12) |
3.32 % |
| Mozambique |
14,631 |
0.47 % |
3.65e+11 (2.12e+10,7.09e+11) |
0.98 % |
| Indonesia |
151,949 |
4.9 % |
1.67e+11 (6.79e+09,3.26e+11) |
0.45 % |
| Netherlands |
17,947 |
0.58 % |
1.01e+11 (2.24e+10,1.79e+11) |
0.27 % |
| Egypt |
8,614 |
0.28 % |
1.01e+11 (4.05e+08,2.01e+11) |
0.27 % |
| Greenland |
110,853 |
3.58 % |
5.94e+10 (7.92e+09,1.11e+11) |
0.16 % |
| Germany |
9,047 |
0.29 % |
5.09e+10 (1.13e+10,9.05e+10) |
0.14 % |
| Brunei |
2,350 |
0.08 % |
4.74e+10 (9.53e+08,9.38e+10) |
0.13 % |
| Canada |
68,580 |
2.21 % |
3.56e+10 (4.8e+09,6.65e+10) |
0.1 % |
| Argentina |
183,975 |
5.94 % |
2.64e+10 (1.94e+10,3.35e+10) |
0.07 % |
| India |
320,102 |
10.33 % |
2.08e+10 (9.2e+09,3.24e+10) |
0.06 % |
| USA |
129,141 |
4.17 % |
2e+10 (9.21e+09,3.08e+10) |
0.05 % |
| Russia |
37,414 |
1.21 % |
1.9e+10 (2.87e+09,3.51e+10) |
0.05 % |
| Vietnam |
153,367 |
4.95 % |
1.52e+10 (7.1e+09,2.32e+10) |
0.04 % |
| Norway |
24,197 |
0.78 % |
1.3e+10 (1.73e+09,2.42e+10) |
0.03 % |
| Japan |
11,621 |
0.37 % |
1.1e+10 (5.34e+08,2.15e+10) |
0.03 % |
| Denmark |
8,225 |
0.27 % |
1.08e+10 (2.11e+09,1.95e+10) |
0.03 % |
| Ireland |
179 |
0.01 % |
6.7e+09 (91500000,1.33e+10) |
0.02 % |
| UK |
1,333 |
0.04 % |
6.7e+09 (1.48e+09,1.19e+10) |
0.02 % |
| Brazil |
38,500 |
1.24 % |
6.35e+09 (3.02e+09,9.67e+09) |
0.02 % |
| Mexico |
85,580 |
2.76 % |
5.68e+09 (3.21e+09,8.16e+09) |
0.02 % |
Key patterns
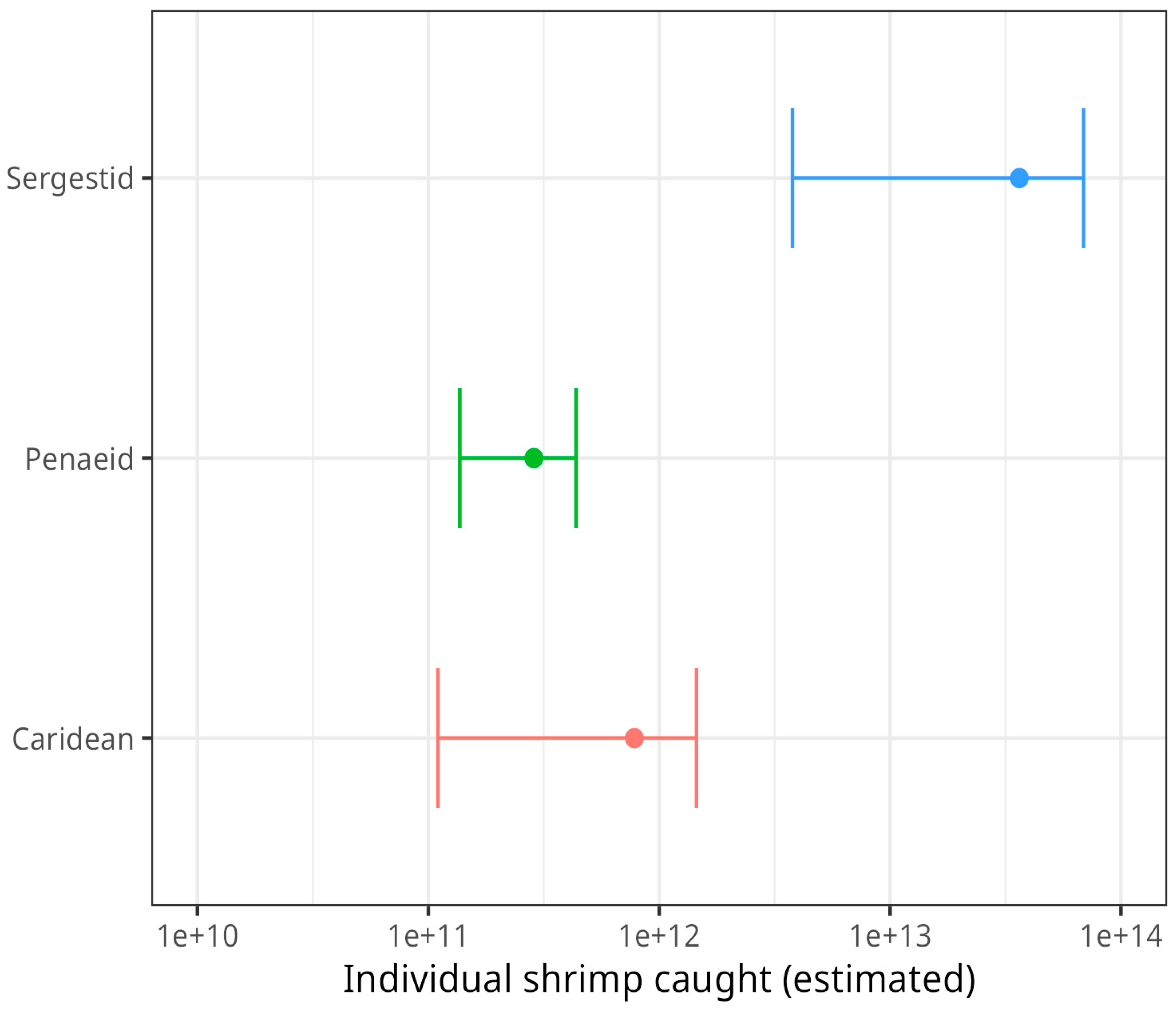
Globally, we estimate that the wild-catch shrimp fishing industry catches 37.4 trillion individual shrimp each year (36.3 trillion sergestids, 781 billion carideans, and 287 billion penaeids) (
Figure 1). This number is almost certainly an underestimate, given well-known, widespread challenges with monitoring, enforcement, and bycatch.
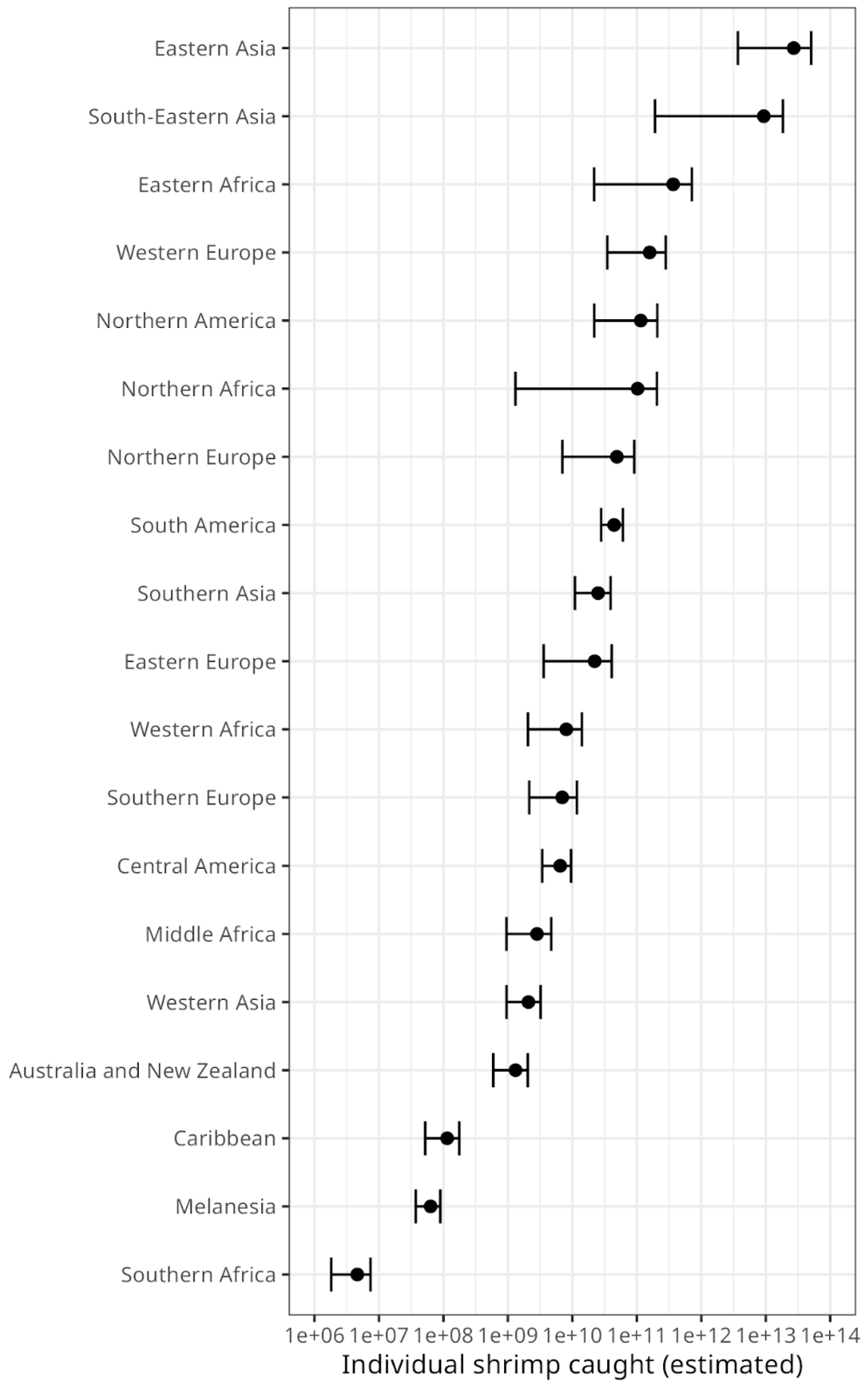
Many more shrimp are caught in East and South-East Asia than any other geographical region (
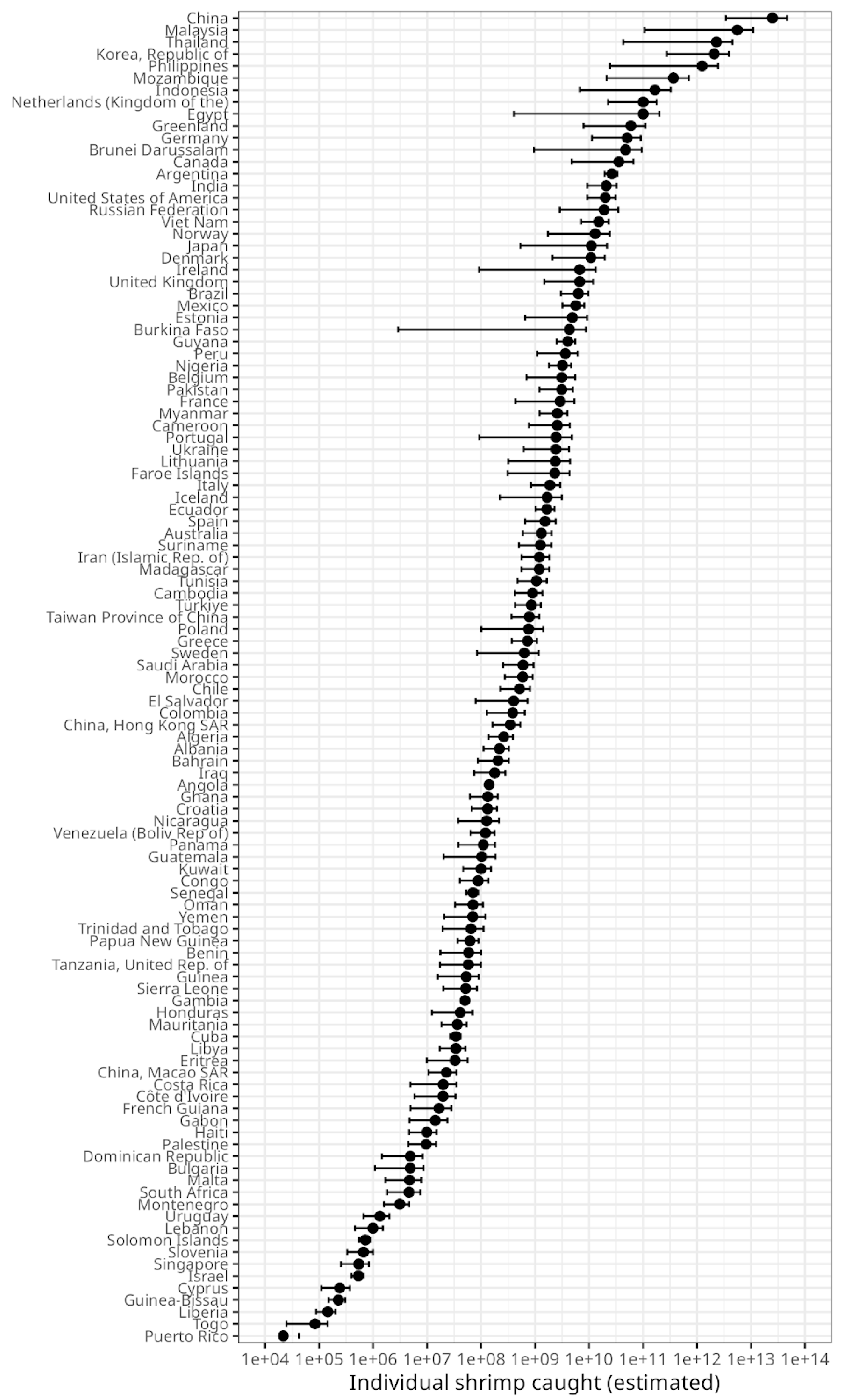
Figure 3). The world's top five countries by catch-by-number are from East or South-East Asia (
Figure 4). China alone accounts for 67% of the world's shrimp catch, with Malaysia accounting for an additional 15% (
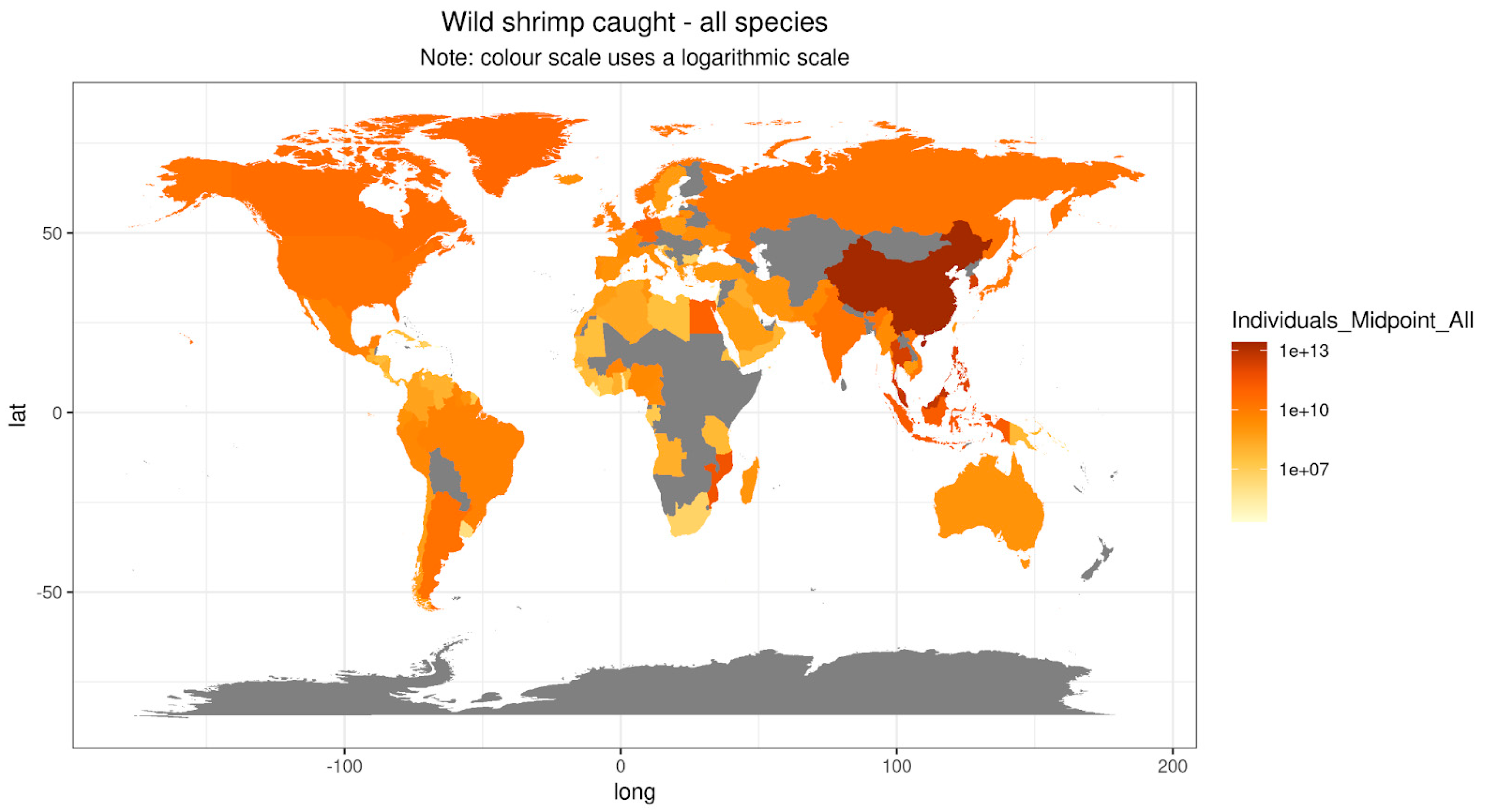
Table 1). Asia's dominance is clearly apparent on a map showing each country's shrimp catch (
Figure 5). Nevertheless, the world's top 25 countries contain many from Europe, Africa, and the Americas (
Table 1).
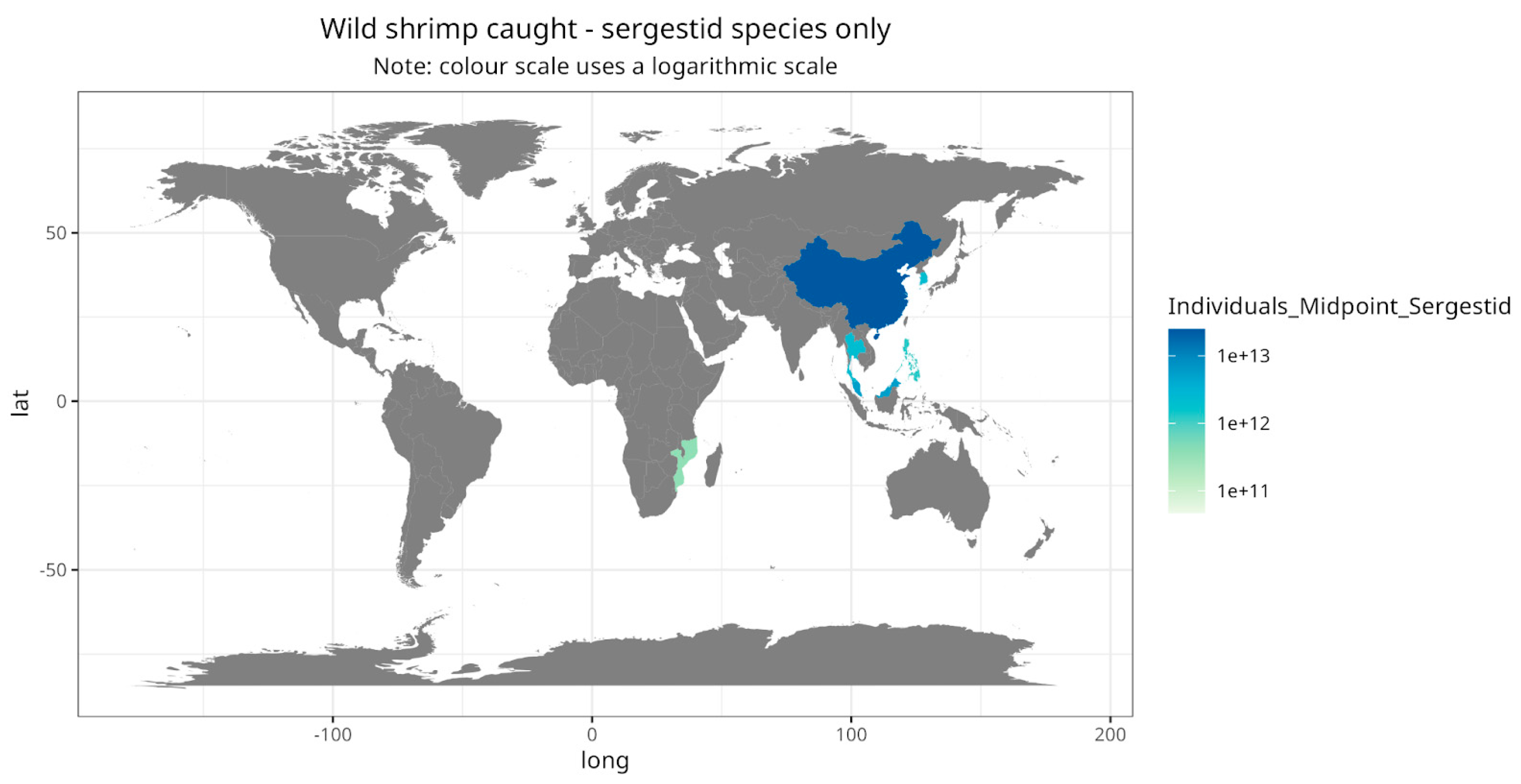
Some nuanced trends are revealed by considering shrimp clades (taxonomic groups). The vast majority of wild-caught shrimp are sergestid shrimp, in particular
Acetes japonicus (
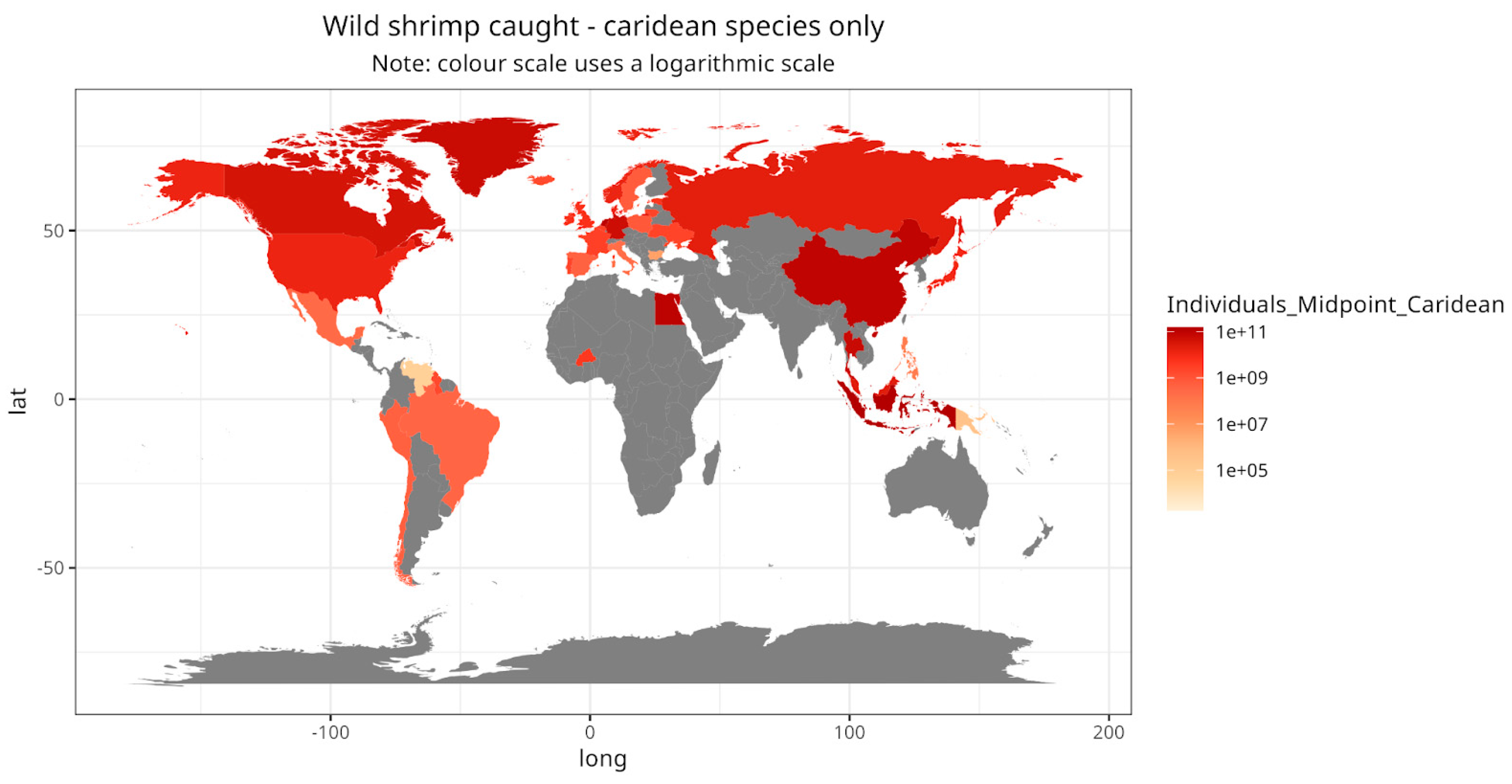
Figure 6) (Romero Waldhorn and Autric 2022). However, sergestid fisheries are only reported for Asia and Africa. In contrast, Europe and the Americas are important for caridean and penaeid fisheries (
Figure 7,
Figure 8 and
Figure 9).
Figure 3.
Estimated number of individual shrimp caught, by geographical region. Error bars show the confidence range, given the ranges for the estimated mean weight by species as calculated by Waldhorn and Autric (2022). Note that the axis is logarithmic.
Figure 3.
Estimated number of individual shrimp caught, by geographical region. Error bars show the confidence range, given the ranges for the estimated mean weight by species as calculated by Waldhorn and Autric (2022). Note that the axis is logarithmic.
Figure 4.
Estimated number of individual shrimp caught, by country. Error bars show the confidence range, given the ranges for the estimated mean weight by species as calculated by Waldhorn and Autric (2022). Note that the axis is logarithmic.
Figure 4.
Estimated number of individual shrimp caught, by country. Error bars show the confidence range, given the ranges for the estimated mean weight by species as calculated by Waldhorn and Autric (2022). Note that the axis is logarithmic.
Figure 5.
Map visualising the estimated number of individual shrimp caught, by country and including all three shrimp clades.
Figure 5.
Map visualising the estimated number of individual shrimp caught, by country and including all three shrimp clades.
Figure 6.
Estimated number of individual shrimp caught, by clade. Error bars show the confidence range, given the ranges for the estimated mean weight by species as calculated by Waldhorn and Autric (2022). Note that the axis is logarithmic.
Figure 6.
Estimated number of individual shrimp caught, by clade. Error bars show the confidence range, given the ranges for the estimated mean weight by species as calculated by Waldhorn and Autric (2022). Note that the axis is logarithmic.
Figure 7.
Estimated number of individual shrimp caught, by continent and clade. Error bars show the confidence range, given the ranges for the estimated mean weight by species as calculated by Waldhorn and Autric (2022). Note that the axis is logarithmic.
Figure 7.
Estimated number of individual shrimp caught, by continent and clade. Error bars show the confidence range, given the ranges for the estimated mean weight by species as calculated by Waldhorn and Autric (2022). Note that the axis is logarithmic.
Figure 8.
Map visualising the estimated number of individual sergestid shrimp caught, by country.
Figure 8.
Map visualising the estimated number of individual sergestid shrimp caught, by country.
Figure 9.
Map visualising the estimated number of individual caridean shrimp caught, by country.
Figure 9.
Map visualising the estimated number of individual caridean shrimp caught, by country.
Figure 10.
Map visualising the estimated number of individual penaeid shrimp caught, by country.
Figure 10.
Map visualising the estimated number of individual penaeid shrimp caught, by country.
East Asia
East Asia has the highest shrimp catch by number of any world region. The three most important countries in East Asia, by estimated number of shrimp caught, are China, the Republic of Korea, and Japan. China in particular accounts for a full two-thirds of the estimated number of individual shrimp caught worldwide. Each of these three countries has a distinct shrimp fishing industry.
China
In 2020, China caught 1.1 million tonnes of shrimp, equating to 37% of the world catch by weight. This equates to roughly 25 trillion individuals, equating to 67% of the world catch by number. Almost all of these individuals were sergestid shrimp (specifically Acetes japonicus), though there were also sizable catches of penaeids (48% of the world's penaeid catch by number) and carideans (11% of the world's caridean catch by number). China's marine fisheries can be divided into two categories. The first category is the "Four Seas" accounting for China's coastline (Bohai Sea, Yellow Sea, East China Sea, and South China Sea). Bottom trawling is particularly intense in the East and South China Seas. The second category is distant waters (Zhang and Vincent 2020). Zhang and Vincent (2020) estimate that there are around 30,000 trawlers in China (total power around 5 million kW) and over 1,000 trawlers operated by China in distant waters (total power around 1 million kW). The mesh size of the codend is often between 20 - 30 mm, which results in high catches of immature fish and shrimp (Wang et al. 2022). One estimate placed the discard ratio at around 62% (Wang et al. 2022).
Many of China's vessels, including the distant water fleet, are involved in IUU fishing (Pedrozo 2022). Some cases have been identified that appear to involve large quantities of shrimp from other countries, such as Ecuador, entering the grey market or informal trade in China without format documentation (European Parliament 2022). In 2021, China exported a reported 181,000 tonnes of shrimp and crustacean products. The major importing jurisdictions were Japan (19%), Hong Kong (11%), the Republic of Korea (9%), USA (9%), Chile (8%), Spain (8%), and Taiwan (7%) (FAO 2023).
Republic of Korea
In 2020, the Republic of Korea caught 33,000 tonnes of shrimp, equating to around 1.1% of the world catch by weight. This equates to roughly 2 trillion individuals, equating to 5.6% of the world catch by number. Almost all of these individuals were sergestid shrimp (specifically Acetes japonicus), with a small minority of penaeid shrimp also. Acetes japonicus is mostly caught in the Yellow Sea, with a smaller but sizable contribution from the East China Sea. Coastal beam trawls are most common in the Yellow Sea, with only a handful operating in the East China Sea or the Sea of Japan (Yoon et al. 2014). The Republic of Korea also operates a distant water fishing fleet (Tickler et al. 2018). Overall, the majority of the Republic of Korea's catch is industrial, with an important minority of the catch coming from small-scale fishers (Shon et al. 2014). On average, between 1990 and 2011, around 453 of the Republic of Korea's 44,000 coastal fishing vessels were beam trawlers (Yoon et al. 2014).
The Republic of Korea has a sizable small-scale fishery, mostly consisting of motorised boats below a few tons (Taberna 2019). Many small-scale fishers use stow nets or operate in multiple fisheries, suggesting that small-scale fishers may be important contributors to the shrimp catch (Taberna 2019). There are some unregulated small-scale otter trawlers (Shon et al. 2014). The rate of discarded catches has been estimated at around 50% (Park et al. 2007). In 2021, the Republic of Korea exported 54,500 tonnes of shrimp and crustacean products. The major importing jurisdictions were Vietnam (52%), Indonesia (24%), and Thailand (12%) (FAO 2023).
Japan
In 2020, Japan caught 11,600 tonnes of shrimp, equating to around 0.375% of the world catch by weight. This equates to roughly 11 billion individuals, equating to 0.029% of the world catch by number. However, this estimate should be considered as imprecise, as the shrimp catch reported by Japan to the FAO are not identified to species level. Shrimp is not considered a major fishery by the Japanese government (Popescu and Ogushi 2013). Nevertheless, Japan probably catches mostly caridean shrimp belonging to the genera Pandalus and Crangon (Hayashi and Kim 1999; Maeda and Nishiuchi 1999; Yamaguchi et al. 2011). For example, Pandalus eous accounts for about a quarter of Japan's shrimp catch by weight (Yamaguchi et al. 2014). Shrimp fishing in Japan involves beam trawls and shrimp pots (Maeda and Nishiuchi 1999). About 5% of Japan's shrimp catch by weight comes from inland fisheries (Popescu and Ogushi 2013). In 2021, Japan exported 1,200 tonnes of shrimp and crustacean products. The major importing jurisdictions were USA (32%), Vietnam (22%), China (15%), Indonesia (10%), and Hong Kong (9%) (FAO 2023).
South and South-East Asia
Shrimp fisheries in south-east Asia typically consist of both commercial and small-scale fleets. Small-scale fishers often target multiple species and use most of what they catch, including small fish and invertebrates that would be unmarketable in other parts of the world. The shrimp catch in many south-east Asian countries is dominated by the sergestid Acetes japonicus, landed fresh and used to make shrimp paste (Bauer 2023). South-east Asia's shrimp fisheries face declining catches due to overexploitation, land use change, and other environmental challenges. Governments often have poor capacity to monitor and regulate the fisheries. Technology varies, but it is common for the catch (or at least the most valuable components) to be stored on-board in bags or boxes with some ice (Suuronen et al. 2020).
Malaysia
In 2020, Malaysia caught 107,000 tonnes of shrimp, equating to around 3.5% of the world catch by weight. This equates to roughly 5.6 trillion individuals, equating to 15% of the world catch by number. Over 99% of the individuals were sergestid shrimp (Acetes species (Stephenie et al. 2021)), with small contributions from the other two clades (including Penaeus merguiensis (Jagerroos 2016)). Sergestid shrimp caught in Malaysia are used to make shrimp paste (Jagerroos 2016). The catch is dominated by the states of Perak and Selangor, on the west coast of Peninsular Malaysia, though other states on the Peninsula and on Borneo contribute significant amounts (Stephenie et al. 2021). Shrimp are caught almost entirely by trawl nets and bag nets (Stephenie et al. 2021). Commercial fishing vessels must operate beyond 5 nm from shore, and commercial vessels greater than 40 gross register tonnes (GRT) must operate beyond 12 nm from shore (Ahmad et al. 2003). There are many traditional vessels, averaging 5 GRT (Ahmad et al. 2003). These vessels are permitted to operate within 5 nm of shore and generally target shrimp with trammel nets (Ahmad et al. 2003). There is a small-scale trawl fishery targeting multiple species (Wong and Yong 2020).
Shrimp catch rates have declined, motivating the development of the offshore fleet (bin Hashim and Kathamuthu 2005; Wong and Yong 2020). Malaysia experiences IUU fishing due to a lack of financial resources and regulatory capacity. This IUU fishing is sometimes carried out by foreign vessels, such as from Thailand (Wong and Yong 2020). Malaysia's small-scale fisheries heavily depend on migrant workers (Wong and Yong 2020). In 2021, Malaysia exported 51,000 tonnes of shrimp and crustacean products. The major importing jurisdictions were China (27%), Singapore (19%), Thailand (16%), Taiwan (10%), and the Republic of Korea (9%) (FAO 2023).
Thailand
In 2020, Thailand caught 48,000 tonnes of shrimp, equating to around 1.6% of the world catch by weight. This equates to roughly 2.3 trillion individuals, equating to 6% of the world catch by number. Over 97% of the individuals were sergestid shrimp (unspecified Sergestidae but including Acetes shrimp (Boonchuwongse and Dechboon 2003)), with small contributions from the other two clades. The Gulf of Thailand accounts for 70% of Thailand's marine catch, with the remainder in the Andaman Sea, though only a minority of the catch consists of shrimp (Janekitkosol et al. 2003). Fisheries are concentrated in the south of the country in both the Gulf and the Andaman Sea (Janekitkosol et al. 2003). Shrimp trawls are conducted along this southern coast, and shrimp gillnets are used along the length of the coast (Boonchuwongse and Dechboon 2003). The cod-end mesh size is often between 12 and 25 mm (Janekitkosol et al. 2003), though the government has expressed interest in trialling larger mesh sizes (Phoonsawat et al. 2016). Commercial shrimp trawlers use otter trawls, beam trawls, and paired trawls, with vessels typically being between 10 and 20 metres long (Boonchuwongse and Dechboon 2003). Small-scale fishers use a variety of gear, including shrimp gillnets and shrimp trawlers (Boonchuwongse and Dechboon 2003). Small-scale fishers tend to remain within 5 km of the shore (Janekitkosol et al. 2003). The number of registered vessels has increased recently, even while total marine production has gradually decreased (Sampantamit et al. 2019).
Thailand has been the subject of a high-profile scandal involving the widespread use of forced labour and other human rights abuses (see above), with forced labourers accounting for around 14% of the country's labour aboard fishing vessels (Seo 2018; International Labour Organization 2020; Suuronen et al. 2020; Stringer et al. 2022). Many vessels are unregistered (Hodal and Kelly 2014). In 2021, Thailand exported 160,000 tonnes of shrimp and crustacean products, which appears to include the vast majority of the wild shrimp catch (Sylwester 2014). The major importing jurisdictions were USA (27%), Japan (22%), China (15%), Myanmar (7%), and the Republic of Korea (6%) (FAO 2023). The USA and the EU (which imports a small volume of Thailand's shrimp and a higher volume of other seafood products) have exercised their economic influence to encourage the Thai government to introduce labour reforms (Stringer et al. 2022; Kadfak et al. 2023).
Philippines
In 2020, the Philippines caught 39,000 tonnes of shrimp, equating to around 1.3% of the world catch by weight. This equates to roughly 1.2 trillion individuals, equating to 3.3% of the world catch by number. Over 99% of the individuals were sergestid shrimp (unspecified Sergestidae but probably consisting of Acetes shrimp (Gay et al. 2022)), with small contributions from the other two clades (including Penaeus monodon and Metapenaeus ensis (Altamiranoa et al. 2015)). The most important locations for trawlers are Samar Sea, Visayan Sea, San Miguel Bay, Lingayen Gulf, Ragay Gulf, Carigara Bay, Guimaras Strait and Manila Bay (Ramiscal et al. 2015). The Philippines has a commercial shrimp trawl sector and a small-scale shrimp sector (Ramiscal et al. 2015). Commercial trawlers range from 60 to 370 kW and usually exceed 12 metres in length. Commercial fishing trips can last up to a week, though trips of just a couple of days when close to fishing ports (Ramiscal et al. 2015). There were 398 registered trawlers in 2007, though many commercial trawlers operate without being registered or licensed (Ramiscal et al. 2015). Small-scale trawlers average around 7 kW and are operated by a crew of one or two fishers (Ramiscal et al. 2015). Acetes shrimp are also caught with filter nets that are manually used by small-scale fishers and involve wading into the water (Gay et al. 2022). Small-scale fishers typically use containers that can hold between 5 and 60 kg of raw Acetes shrimp at a time (Gay et al. 2022).
Many of the country's shrimp fisheries have declined in catch and product quality due to overexploitation and the destruction of mangrove habitat (in many cases to build aquaculture ponds) (Altamiranoa et al. 2015). The destruction of ecosystems means that many people can no longer support themselves and their family by fishing, forcing those people to either migrate or switch to a different form of employment (Dunaway and Macabuac 2007). Some regions are experimenting with stock enhancement to support the restoration of wild shrimp populations (Altamirano et al. 2016). There has been some interest in expanding fisheries in the deep sea where there is an abundance of caridean shrimp (Pandalidae), though this has been limited by the capacity and knowledge of local fishers (Nepomuceno et al. 2013; Cruz et al. 2014). Many trawl fishing areas have officially closed, but trawlers have frequently continued to fish in these areas (Ramiscal et al. 2015). In 2021, the Philippines exported 5,400 tonnes of shrimp and crustacean products. The major importing jurisdictions were Japan (37%), Hong Kong (15%), USA (14%), the Republic of Korea (9%), and China (8%) (FAO 2023).
Indonesia
In 2020, Indonesia caught 152,000 tonnes of shrimp, equating to around 4.9% of the world catch by weight. This equates to roughly 167 billion individuals, equating to 0.45% of the world catch by number. Over 90% of the individuals were caridean shrimp (unspecified Palaemonidae, though including freshwater species (Gillett 2008)), with small contributions from a variety of penaeid species (including Metapenaeus monoceros, M. ensis, M. elegans, and Penaeus merguiensis (Gillett 2008)). There may be an important Acetes industry that is absent from reported data (Ali et al. 2019; Helmi et al. 2022). Both freshwater and marine species are major contributors to the catch by number. There is a significant small-scale fishery, though small-scale fishers often target multiple species. The small-scale fishery is focused on western Indonesia, especially North Java (Gillett 2008).
Indonesia banned trawl fisheries in 1980 in all areas except for the Arafura Sea (Gillett 2008; Af-idati and Lee 2009). The trawl ban was motivated by competition for shrimp and conflict between trawlers and small-scale fishers (Muawanah and Kasim 2021). The ban caused around 25,000 fishers to lose their employment, though there was a short-term reduction in violence and social tension (Gillett 2008). Large-scale shrimp trawling was, until 2015, focused on the Arafura Sea in the south-east of Indonesia (Gillett 2008). In 2005, there were a couple of hundred commercial shrimp trawlers fishing in and adjacent to the Arafura Sea (Gillett 2008). In 2015, trawling was banned nation-wide, after which trawling in the Arafura Sea has gradually decreased (Endroyono 2015; Muawanah and Kasim 2021). The number of trawlers landing has declined since the ban was implemented. Some fishers have switched to other types of fishing gears with lower capacities. Small-scale catches have been improving (Endroyono 2015). Nevertheless, there are still some "trawl-like" gears in use (Kasim 2019). This ban has not always been enforced successfully, and many fishers simply continue trawling after changing the terminology of their trawl gear (Gillett 2008). Enforcement in Indonesia's fisheries is weak. There is a large amount of illegal trawling by Indonesian and foreign vessels (Gillett 2008). In 2021, Indonesia exported 256,000 tonnes of shrimp and crustacean products. Almost all of this was imported by USA (71%) and Japan (14%) (FAO 2023).
Vietnam
In 2020, Vietnam caught 153,000 tonnes of shrimp, equating to around 4.9% of the world catch by weight. This equates to an estimated 15 billion individuals, equating to 0.041% of the world catch by number. However, Vietnam's shrimp catch is reported to the FAO data (and is therefore listed in our dataset) as unspecified Natantia, meaning that the estimated number of individuals is imprecise. Dinh et al. (2010) identify the following penaeid species in Vietnam's fisheries: Haliporoides sibogae, Metapenaeus affinis, Metapenaeus brevicornis, Metapenaeus tenuipes, Mierspenaeopsis cultrirostris, Parapenaeopsis gracilima, and Kishinouyepenaeopsis maxillipedo. As in other south-east Asian countries, sergestid species like Acetes japonicus are used to produce shrimp paste (Ung et al. 2021; Stefanny and Pamungkaningtyas 2023). This indicates that Acetes japonicus may constitute much or most of Vietnam's shrimp catch, though we cannot be sure.
Vietnam's marine fisheries are concentrated near the shore and are predominantly small-scale (Dinh et al. 2010; Tran Thi Phung Ha 2012). Fishing occurs along the length of Vietnam's coast, with the southern regions being particularly important (Pomeroy et al. 2009). Estuaries in the Mekong Delta are major shrimp fishing grounds (Dinh et al. 2010; Tran Thi Phung Ha 2012). 82% of Vietnam's marine catch comes from near-shore vessels (Tran et al. 2020). Fishers use a variety of gears and target multiple species (Tran Thi Phung Ha 2012). Vietnam's marine fisheries have around 100,000 fishing vessels and 550,000 full-time fishers (Pomeroy et al. 2009; Tran et al. 2020). Around 70% of Vietnam's mechanised vessels have an engine power below 70 kW (Tran et al. 2020). Vessels are often wooden, with ice tanks in which to store the catch (Tran Thi Phung Ha 2012; Thong Ba Nguyen 2015). Shrimp trawlers may make trips lasting around a week (Thong Ba Nguyen 2015). It is common to use codends with small mesh sizes, and bycatch reduction devices are not used (Thong Ba Nguyen 2015). Some fishers use explosives and chemical substances (Pomeroy et al. 2009).
Vietnam's small-scale fisheries are closely linked with poverty in coastal communities (Pomeroy et al. 2009; Tran Thi Phung Ha 2012). Fishing is considered a "last resort" occupation, and small-scale fishers are "the poorest of the poor" (Tran Thi Phung Ha 2012). The education levels of small-scale fishers (and people in coastal communities more broadly) is very low, making it difficult for people to find better employment (Pomeroy et al. 2009; Tran Thi Phung Ha 2012). Policies often fail to take into account widespread poverty, and fishers have very little political power (Pomeroy et al. 2009; Tran Thi Phung Ha 2012). Catches have declined significantly over the past couple of decades due to intense overexploitation and destruction of habitat (including for conversion into shrimp aquaculture ponds) (Tran Thi Phung Ha 2012; Armitage and Marschke 2013). One fisher expressed this poignantly (Tran Thi Phung Ha 2012): "In the evening, there are as many fishing boat lights as stars in the sky. Fishing brings me strength and health; I can fish for many more decades, but I am afraid that there will be nothing to catch by then." The export of Vietnamese shrimp to the US, Japan, and the EU is a critical contributor to Vietnam's export earnings, though this export would also involve farmed shrimp (Tran Thi Phung Ha 2012). Catch hygiene management is often poor, with quality of marine catches often hindered by pollution (Pomeroy et al. 2009). Government policies to manage Vietnam's marine fisheries have been generally ineffective (Pomeroy et al. 2009; Tran Thi Phung Ha 2012). The government has a low capacity to monitor fish catches and stocks, create policies, and enforce regulation (Pomeroy et al. 2009; Tran Thi Phung Ha 2012; Thong Ba Nguyen 2015). Non-compliance with fisheries regulations is routine (Tran Thi Phung Ha 2012; Nguyen et al. 2021). We suspect that the reported catch is an underestimate, as data collection is not always commonplace (Thong Ba Nguyen 2015).
In 2021, Vietnam exported 411,000 tonnes of shrimp and crustacean products. The major importing jurisdictions were the USA (27%), Japan (15%), the Republic of Korea (10%), China (8%), and the UK (6%) (FAO 2023).
Brunei Darussalam
In 2020, Brunei caught 2,350 tonnes of shrimp, equating to around 0.076% of the world catch by weight. This equates to roughly 47 billion individuals, equating to 0.127% of the world catch by number. Over 99% of this catch is composed of sergestid shrimp (unspecified Sergestidae), with small contributions from the other two clades. Brunei's marine fisheries are divided into a commercial fishery, which operates beyond 3 nm from the shore, and a small-scale fishery, which operates within 3 nm (Cinco et al. 2015). The small-scale fishery catches around 60% of the total marine catch (Cinco et al. 2015). There are around 20 commercial trawlers (Cinco et al. 2015). Small-scale fishers target multiple species and travel up to 40 km from the shore, using vessels of 20 to 30 kW. Small-scale fishers use a variety of gear, including trammel nets, gill nets, and drift nets (Cinco et al. 2015). Shrimp only constitute a small proportion of the total catch of both the commercial and small-scale fishers (Ebil 2013; Cinco et al. 2015). There were around 3,000 licensed vessels in 2008 (Gin 2015). In 2021, Brunei exported 4,000 tonnes of shrimp and crustacean products. The major importing jurisdictions were Taiwan (35%), Japan (35%), Singapore (13%), Australia (9%), and China (6%) (FAO 2023).
India
In 2020, India caught 320,102 tonnes of shrimp, equating to around 10% of the world catch by weight. This equates to roughly 21 billion individuals, equating to 0.056% of the world catch by number. This catch was entirely composed of penaeid shrimp, with sizable contributions from three categories: unspecified Natantia, Penaeus monodon, and unspecified Penaeus. India's trawl fisheries are split about equally between the west and east coasts (Madhu 2018; Dineshbabu et al. 2022). The major fishing harbours on the west coast are Veraval, Mumbai, Karwar, Mangalore, Calicut, Cochin, Sakthikulangara, and on the east coast are Chennai and Visakhapatnam (Dineshbabu et al. 2022). Together, these harbours account for around a third of India's trawl landings, and half of India's multi-day trawl landings (Dineshbabu et al. 2022). Trawl fisheries account for around half of India's marine catch (Dineshbabu et al. 2022). There are around 35,000 trawlers across all of India's marine fisheries (Madhu 2018). Commercial trawlers conduct trips that last up to 13 days (Dineshbabu et al. 2022). Small-scale fishers catch prawns using hand trawls and cast-nets (Dineshbabu et al. 2022).
India struggles with enforcing fisheries regulations. Fishers often use larger boats, higher capacity engines, and smaller cod-end mesh sizes than are permitted (Divipala et al. 2019; Mangar et al. 2023). Some areas of the country have been experiencing diminishing shrimp catches (Dineshbabu et al. 2022). This has driven some Indian trawlers to conduct illegal fishing in Sri Lankan waters, risking being shot by the Sri Lankan Navy (Mangar et al. 2023). Vessel owners who support India's ruling political party often accrue benefits like lax enforcement and lower charges (Mangar et al. 2023). When India's shrimp exports are rejected by trade partners (e.g. USA, EU, Japan), the reason is often due to a failure to comply with microbiological safety standards though these exports involve farmed shrimp too (Geetha et al. 2020). In 2021, India exported 741,000 tonnes of shrimp and crustacean products. The major importing jurisdictions were the USA (47%), China (17%), Japan (6%), and Vietnam (6%) (FAO 2023).
Africa
Africa has two main shrimp-catching countries that are identified by our dataset, though these countries are quite distinct. Mozambique catches an estimated 1% of the world's sergestid shrimp by number, while Egypt catches around 13% of the world's caridean shrimp by number. Importantly, other coastal nations in Africa may have a catch of sergestid shrimp, used to make shrimp paste, that is not reported to the FAO (Kensley 1971; Omori 1975; Zafar and Alam 1997). It is plausible that some of these other coastal countries could have a shrimp catch, by number, that is indeed in the world's top 25 countries.
Mozambique
In 2020, Mozambique caught 14,600 tonnes of shrimp, equating to around 0.472% of the world catch by weight. This equates to roughly 365 billion individuals, equating to 0.977% of the world catch by number. Over 99% of this catch was composed of sergestid shrimp (specifically Acetes erythraeus), with the remainder composed of penaeid shrimp (unspecified Penaeus). However, these reported catches consist of only a small proportion of Mozambique's true catch (Jacquet et al. 2010). Most industrial and semi-industrial vessels are based in Beira (Sofala Bank), in the centre of Mozambique's coastline (Alex Benkenstein 2013; Torres Coll 2013). There were 14 semi-industrial and 50 industrial vessels in 2011 (Torres Coll 2013). Small-scale fishers operate along the entire length of the coastline (Alex Benkenstein 2013). Small-scale fishers have exclusive use of the waters within 3 nm of the shoreline, and industrial vessels are electronically monitored (A. Benkenstein 2013).
Mozambique's industrial and semi-industrial sectors primarily target shrimp, while the small-scale sector targets multiple species (A. Benkenstein 2013). The semi-industrial fishery consists of vessels between 10 and 20 m, and the industrial fishery consists of all vessels exceeding 20 m (A. Benkenstein 2013). Industrial vessels have the capacity to freeze the catch on board, while semi-industrial vessels without this ability cannot remain on the sea overnight (Torres Coll 2013). The small-scale fishery involves trawling near the beach, Chicocota nets, and mosquito nets (Manhice et al. 2023). All sectors target the same shrimp species and stocks, but at different life stages (Manhice et al. 2023). Like in other countries, small-scale fishing in Mozambique is a physically demanding activity, and fishers express pride in their occupation (Manhice et al. 2023). Due to a lack of government capacity, Mozambique severely underreports the small-scale catch (Jacquet et al. 2010). Since the country's small-scale fishery accounts for around 80% of total marine catch, this means that Mozambique's catch by weight (across all species) may be over six times greater than the reported catch (Jacquet et al. 2010; Alex Benkenstein 2013). Mozambique's fisheries exports are dominated by shrimp produced by the semi-industrial and industrial fisheries (Alex Benkenstein 2013). Mozambique struggles with enforcement (Manhice et al. 2023). In particular, the country experiences a large amount of illegal fishing in estuaries. This involves using passive fine-mesh nets, often created from discarded commercial nets (A. Benkenstein 2013). In 2021, Mozambique exported 4,700 tonnes of shrimp and crustacean products. Almost all of this was exported by Spain (63%), Portugal (25%), and China (8%) (FAO 2023).
Egypt
In 2020, Egypt caught 8,600 tonnes of shrimp, equating to around 0.278% of the world catch by weight. This equates to roughly 100 billion individuals, equating to 0.270% of the world catch by number. Over 99% of this catch was apparently composed of caridean shrimp, though Egypt does not report its shrimp catch to FAO at the species level. Egypt is home to both penaeid and caridean shrimp species (Cumberlidge 2009; Abdulraheem et al. 2021). Considering all aquatic fisheries, Egypt's catch by weight is highest in its lakes, followed by roughly equal catches in the Mediterranean and the Red Sea (Mehanna 2022). Trawling along the Mediterranean coast focuses on the central region between Alexandria and Port Said (Alsayes et al. 2010). This includes artisanal trawling (El-Bokhty and El-Aiatt 2014). There were 1,098 trawlers in the Egyptian Mediterranean Sea in 2012 (Lucchetti et al. 2016). Trawlers in the Gulf of Suez (Red Sea) target penaeid shrimp, and there were 79 trawlers in 2012. Trawlers in the Gulf of Suez are typically between 20 and 30 metres in length with engines ranging from 150 to 450 kw. The cod-end mesh size is around 180 mm. There are around 13,000 sailing boats reported in Egypt's lakes and 10,000 wooden boats in the Nile, though these numbers may be half the true values (Mehanna 2022). In total, Egypt has around 13,600 licensed fishers, as well as over 20,000 unlicensed fishers who operate entirely in the informal market (Lucchetti et al. 2016). Egypt's marine and inland catches have severely declined due to exploitation (Alsayes et al. 2010; Mehanna 2022). The ratio of bycatch-to-shrimp in marine trawlers reaches 15:1 by weight (El-Ganainy and Yassien 2012). In 2021, Egypt exported 1,800 tonnes of shrimp and crustacean products. The major importing jurisdictions were Tunisia (37%), Italy (28%), USA (17%), China (10%), and Sweden (4%) (FAO 2023).
Europe
Europe (which we here define to include Russia and Greenland) accounts for nine countries out of the world's top 25 countries by number of shrimp caught. We categorise Russia in the European fisheries due to the close management ties with Norway, and we include Greenland as it is politically part of Europe. These nine countries can be divided into three categories: a fishery targeting Crangon crangon in the North Sea (Netherlands, Germany, Denmark, and the UK); a fishery targeting Pandalus borealis in the northern Atlantic and Pacific oceans (Norway, Russia, Greenland, and Denmark); and the surprising case of Ireland.
Ireland has a very small shrimp catch by weight, where small-scale fishers use set pots to catch Palaemon serratus. Despite the small catch by weight, the Ireland shrimp fishery accounts for a surprisingly high number of individual shrimps, illustrating the value of considering catch by number alongside catch by weight. With the exception of Ireland and a small fishery in Wales, Europe's main shrimp-fishing countries have negligible small-scale shrimp fisheries. Rather, Europe's shrimp fisheries involve large, commercial vessels that usually cook, freeze, and/or package the shrimp on-board.
All of the countries in the North Sea shrimp fishery follow a single management plan, which specifies a minimum mesh size of 20 mm (Goti-Aralucea et al. 2021). Therefore, these countries share very similar fishing practices (Goti-Aralucea et al. 2021). Fishers return large amounts of undersized or otherwise unmarketable shrimp to the sea (Innes and Pascoe 2008; Dutch Fish Product Board 2009). The mortality of these shrimp after release may be very high, even reaching 40% (Temming et al. 2022).
Netherlands
In 2020, the Netherlands caught 18,000 tonnes of shrimp, equating to around 0.579% of the world catch by weight. This equates to roughly 100 billion individuals, equating to 0.270% of the world catch by number. The catch was entirely composed of the caridean species C. crangon. The Netherlands is part of the North Sea shrimp fishery. The Netherlands had 60 full-year shrimp fishers in 2009, as well as 155 fishers who fish for shrimp during only part of the year (Dutch Fish Product Board 2009). Vessels, which are typically around 20 metres in length, generally carry two trawl nets and have engine power up to 220 kW (Dutch Fish Product Board 2009; Turenhout et al. 2016; Verschueren et al. 2019). Shrimp are cooked on-board (Dutch Fish Product Board 2009). The Dutch shrimp fishery is mostly unregulated (Tulp et al. 2016). The fishery had previously used pulse (electric) trawlers, though this practice was controversial and recently banned by the EU (Le Manach et al. 2019; Foo Yun Chee 2021). In 2021, the Netherlands exported 97,000 tonnes of shrimp and crustacean products. The major importing jurisdictions were Germany (28%), Morocco (21%), Belgium (13%), France (11%), and Italy (5%) (FAO 2023).
Germany
In 2020, Germany caught 9,000 tonnes of shrimp, equating to around 0.292% of the world catch by weight. This equates to roughly 50 billion individuals, equating to 0.136% of the world catch by number. The catch was entirely composed of the caridean species C. crangon. Germany is part of the North Sea shrimp fishery. Germany has around 200 shrimp trawlers, though the vessels are ageing and new investment is scarce (Döring et al. 2020; Goti-Aralucea et al. 2021). The bulk of vessels are between 12 and 14 metres long (Goti-Aralucea et al. 2021). Most vessels are owner-operated (Goti-Aralucea et al. 2021). In 2021, Germany exported 48,000 tonnes of shrimp and crustacean products. The major importing jurisdictions were the Netherlands (22%), Denmark (11%), Austria (9%), France (9%), and Poland (7%) (FAO 2023).
Denmark
In 2020, Denmark caught 8,200 tonnes of shrimp, equating to around 0.265% of the world catch by weight. This equates to roughly 11 billion individuals, equating to 0.029% of the world catch by number. The catch was almost entirely composed of the caridean species, with about two-thirds composed of C. crangon and the remaining third mostly Pandalus borealis. The C. crangon catch comes from Denmark's participation in the North Sea fishery. In contrast, the Pandalus borealis catch comes from the Norwegian channel (Eigaard and Munch-Petersen 2010) and more closely resembles the fisheries for Pandalus borealis by Norway, Russia, and Greenland. Denmark's C. crangon and Pandalus borealis fisheries are single-species fisheries, separate from each other (Skov et al. 2020). Denmark's C. crangon fleet had 28 vessels in 2010 (Respondek et al. 2022). Denmark's Pandalus borealis fishery had 9 vessels in 2021, with an average engine power between 300 and 500 kW (Eigaard and Munch-Petersen 2010; Engø 2021). At least one such vessel cooks shrimp on-board and packs them into chilled cartons (Engø 2021). These vessels use a codend mesh size of 35 mm and experience low bycatch (Eigaard and Munch-Petersen 2010). In 2021, Denmark exported 127,000 tonnes of shrimp and crustacean products. The major importing jurisdictions were China (15%), Russia (13%), Sweden (13%), Norway (9%), and Germany (8%) (FAO 2023).
United Kingdom
In 2020, the United Kingdom caught 1,300 tonnes of shrimp, equating to around 0.043% of the world catch by weight. This equates to roughly 6.7 billion individuals, equating to 0.018% of the world catch by number. The catch was entirely composed of caridean species, with over 98% of the catch being C. crangon with the remainder from Pandalus borealis and Pandalus serratus. The UK's catch of C. crangon comes from the North Sea shrimp fishery. The UK had 35 active vessels in the North Sea shrimp fishery in 2006 (Catchpole et al. 2008). These vessels ranged from 10 to 20 metres in length, with an engine power between 50 and 230 kW (Catchpole et al. 2008). Crews consist of around three people (Innes and Pascoe 2008). Shrimp are cooked on deck. In contrast, the UK's small catch of Pandalus serratus comes from an artisanal, static-gear fishery in Cardigan Bay, Wales (Emmerson et al. 2017). In 2021, the UK exported 7,500 tonnes of shrimp and crustacean products. The major importing jurisdictions were the Netherlands (38%), Denmark (32%), Ireland (8%), France (4%), and Germany (3%) (FAO 2023).
Norway
In 2020, Norway caught 24,200 tonnes of shrimp, equating to around 0.781% of the world catch by weight. This equates to roughly 13 billion individuals, equating to 0.035% of the world catch by number. The catch was almost entirely composed of the caridean species Pandalus borealis, with a tiny contribution from C. crangon. Norway's shrimp fisheries fish from three stocks. In 2004, the offshore stock had 90 licences; the coastal south stock, 174; and the coastal north stock, 78 (Gillett 2008). The vast majority of shrimp catch by weight came from the offshore stock (Gillett 2008). The number of vessels fishing the offshore stock declined around 2010, but has since returned to the mid-2000s number (Hvingel and Zimmermann 2023). The coastal stocks are fished by small wooden or steel trawlers between 10 and 20 metres in length, while the offshore stock is fished by large steel trawlers up to 70 metres in length and with an average power of around 3,700 kW (Gillett 2008; Hvingel and Zimmermann 2023). Vessels use single or twin trawls (Gillett 2008). Around half of the offshore vessels also operate in finfish fisheries (Gillett 2008). Offshore vessels freeze their catch on-board, while coastal vessels land the catch fresh (Gillett 2008). In 2021, Norway exported 49,000 tonnes of shrimp and crustacean products. Almost all of this was imported by Uruguay (87%) and Iceland (6%) (FAO 2023).
Russia
In 2020, Russia caught 37,400 tonnes of shrimp, equating to around 1.2% of the world catch by weight. This equates to roughly 19 billion individuals, equating to 0.051% of the world catch by number. The catch was almost entirely composed of caridean species, with over 99% of the catch composed of Pandalus borealis, Pandalus goniurus, and Pandalus hypsinotus. Major shrimp fishing areas in Russian waters include the Barents Sea and the northern coasts of the Okhotsk Sea and the Japan Sea (Mikhailova 2012; Sokolov et al. 2020). Commercial vessels are often 50 metres long and exceed 1,000 kW in power (Mikhailova 2012). The minimum mesh size is 35 mm (Hvingel and Zimmermann 2023). Russia has a long history of cooperating closely with Norway in the management and scientific monitoring of their shared fisheries (Hammer and Hoel 2012; Gornova 2016). Russia's shrimp fishery in the Barents Sea is exploited at a relatively low level (Gornova 2016). When excessive bycatch of shrimp is detected, the corresponding fishing area is closed temporarily (Hvingel and Zimmermann 2023). In 2021, Russia exported 18,000 tonnes of shrimp and crustacean products. The identity of the importing countries was not reported (FAO 2023).
Greenland
In 2020, Greenland caught 111,000 tonnes of shrimp, equating to around 3.6% of the world catch by weight. This equates to roughly 59 billion individuals, equating to 0.159% of the world catch by number. The catch was almost entirely composed of the caridean species Pandalus borealis. Greenland's shrimp fishery is divided into a coastal fleet, which has exclusive access to shrimp within 3 nm from the coast, and an offshore fleet (Mann 2018). The coastal fleet has around 20 vessels, most of which are smaller than 75 GRT (Mann 2018). The offshore fleet has around eight vessels (Mann 2018). Vessels use otter trawls with a minimum mesh size of 40 mm (Mann 2018). It is not permitted to discard small shrimp, though small finfish are often discarded (Mann 2018). The offshore fleet is permitted to process up to 75% of its catch at sea. The majority of the processed catch is cooked, though several thousand tonnes is processed as the raw "Japan" or "Italian" shrimp products (Vestergaard et al. 2011; Mann 2018). The remaining 25% of the catch must be landed unprocessed at one of four processing plants on the west coast, a regulation aimed to support the labour market (Vestergaard et al. 2011; Mann 2018). The coastal fleet lands its catch unprocessed (Mann 2018). In 2021, Greeland exported 73,000 tonnes of shrimp and crustacean products. The identity of the importing countries was not reported for most of this export (FAO 2023).
Ireland
In 2020, Ireland caught 179 tonnes of shrimp, equating to around 0.006% of the world catch by weight. This equates to roughly 6.7 billion individuals, equating to 0.018% of the world catch by number. The catch was entirely composed of caridean species, with over 95% of the catch being the Palaemonidae species Palaemon serratus (Fahy and Gleeson 1996; Tully 2017). The catch of Palaemon serratus comes from a small-scale in-shore fishery in the southwest of Ireland (Fahy and Gleeson 1996). These shrimp are caught using baited pots (Tully 2017). The catch by weight is only around 200 tonnes per year (Fahy and Gleeson 1996). Despite this small weight, this catch consists of a surprisingly high number of individual shrimp. The number estimated in our dataset may be an overestimate. The reason is that in the FAO catch data, this species is recorded as unspecified Palaemonidae; that statistical group was assigned an estimated mean weight between 0.01 and 4 grams by Waldhorn and Autric (2022). The average weight of Palaemon serratus specifically is closer to, or slightly above, the upper end of that range (Fahy and Gleeson 1996). Even so, the true number of shrimp caught annually in Ireland would be around 3.5 billion individuals. Unusually for Europe, this shrimp fishery is small-scale. There are several hundred vessels, generally below 8 metres in length (Tully 2017). Together, these vessels set over 50,000 pots each year. The bulk of the fishing occurs on the west coast (Tully 2017). In 2021, Ireland exported 1,500 tonnes of shrimp and crustacean products. The major importing jurisdictions were Italy (50%), France (18%), Spain (15%), and the UK (12%) (FAO 2023).
Americas
The Americas have a diverse shrimp catch, ranging from cold-water caridean fisheries in the far north to warm-water penaeid fisheries further south. Canada has the highest number of estimated individuals out of the American countries, and these individuals come from Canada's cold-water Pandalus borealis fishery. Likewise, the USA has a cold-water fleet targeting Pandalus species in the north. But the USA also has a southern fleet that targets penaeid shrimp, which is more typical of the Latin American countries. Mexico and Brazil operate penaeid trawlers. These are sequential fisheries that involve both artisanal and industrial fleets. In contrast, Argentina is much more focused on the industrial catch of Pleoticus muelleri, though there is still a small artisanal fleet.
Canada
In 2020, Canada caught 68,600 tonnes of shrimp, equating to around 2.2% of the world catch by weight. This equates to roughly 36 billion individuals, equating to 0.095% of the world catch by number. Almost all of the catch was composed of caridean species (Pandalus borealis and unspecified Pandalus species). The fishery is concentrated off Canada's east coast and is divided into an offshore and inshore fleet (Araya-Schmidt 2022). The offshore fleet consists of around 10 vessels, which range between 50 and 75 metres in overall length and operate from Newfoundland and Nova Scotia (Foley 2012; Araya-Schmidt 2022). Trips on these vessels can last up to 75 days, and shrimp are cooked, frozen and packaged on-board (Araya-Schmidt 2022). Vessel operators prefer to use twin trawling, but vessels can switch to single trawling if environmental conditions are poor (Araya-Schmidt 2022). The minimum mesh size is 40 mm. The inshore fleet consists of over 300 vessels. These vessels range from 14 to 20 metres in length and target shrimp as well as other species. Provincial legislation requires inshore vessels to land their catch before it is cooked, similar to the situation in Greenland (Foley 2012).
United States of America
In 2020, the United States of America caught 129,000 tonnes of shrimp, equating to around 4.2% of the world catch by weight. This equates to roughly 20 billion individuals, equating to 0.053% of the world catch by number. The majority of the catch was composed of caridean species (Pandalus jordani and other Pandalus species), with a sizable minority composed of penaeid species (mainly Penaeus setiferus, Penaeus aztecus, and Penaeus duorarum). This corresponds to a division between the fishery for cold-water shrimp in the country's northeast and northwest and the fishery for warm-water shrimp off the southeast Atlantic coast and in the Gulf of Mexico (Gillett 2008). The cold-water fishery is concentrated in the Pacific states of Oregon and Washington, with only a small catch by weight in the Atlantic waters. The state with the highest catch, Oregon, has averaged only 64 vessels since the year 2000 (Hannah et al. 2018). The cold-water fishery mostly uses otter trawls, though there are some beam trawls and traps in Alaska. The warm-water fishery mainly uses otter trawls (Gillett 2008). There are over 20,000 shrimp vessels in the USA's waters of the Gulf of Mexico and southeast Atlantic (Gillett 2008; Purcell et al. 2017). This fishery consists of both small, inshore vessels and larger, offshore vessels (Gillett 2008). Discard rates range between 60 and 80% by weight (Gillett 2008). Commercial fishing is one of the most dangerous occupations in the USA, and the Gulf of Mexico shrimp fishery has a rate of fatalities (from vessel disasters, falls overboard, and on-board injuries) higher even than the average for US commercial fisheries (Lincoln and Lucas 2010). In 2021, the USA exported 16,000 tonnes of shrimp and crustacean products. The major importing jurisdictions were Denmark (20%), the Netherlands (19%), India (12%), Mexico (10%), and Canada (8%) (FAO 2023).
Mexico
In 2020, Mexico caught 85,600 tonnes of shrimp, equating to around 2.8% of the world catch by weight. This equates to roughly 5.7 billion individuals, equating to 0.015% of the world catch by number. Almost all of the catch was composed of a variety of penaeid species from the Penaeus genus, with a small contribution from caridean species (unspecified Macrobrachium). The penaeid catch can be distinguished into three broad species assemblages, each from a distinct geographical location. On the country's northern Pacific coast, the catch is dominated by Penaeus californiensis and Penaeus stylirostris. On the southern Pacific coast, the catch is dominated by Penaeus vannamei. On the Gulf of Mexico, the catch is dominated by Penaeus aztecus, Xiphopenaeus kroyeri, and Penaeus setiferus (Avilés-Polanco et al. 2023). Around 70% of the catch by weight comes from Mexico's Pacific coast, with the remainder from the Gulf of Mexico (Avilés-Polanco et al. 2023).
The fleet is sequential. Small-scale vessels use cast nets to target juvenile shrimp in estuaries and bays, while the industrial marine trawlers focus on adult shrimp (Avilés-Polanco et al. 2023). The small-scale fleet has around 90,000 vessels, and the industrial fleet around 1,400 (Avilés-Polanco et al. 2023). Industrial vessels range between 18 and 25 metres in length and 180 to 460 kW in power (Dubay et al. 2010). Industrial vessels make trips that last up to 28 days, and the shrimp is processed and frozen on board (Dubay et al. 2010). In 2021, Mexico exported 24,000 tonnes of shrimp and crustacean products. Almost all of this was imported by the USA (84%), Japan (7%), and China (6%) (FAO 2023).
Brazil
In 2020, Brazil caught 38,500 tonnes of shrimp, equating to around 1.2% of the world catch by weight. This equates to roughly 6.4 billion individuals, equating to 0.017% of the world catch by number. Almost all of the catch was composed of a variety of penaeid species (Xiphopenaeus kroyeri, Artemesia longinaris, and Penaeus species) with a small contribution from caridean species (unspecified Macrobrachium). Like in Mexico, the shrimp fishery in Brazil is divided into an artisanal inshore fleet and an off-shore industrial fleet. The inshore fleet uses cast nets and trawl nets to catch shrimp in estuaries and other in-shore locations, while the off-shore fleet focuses on marine trawls (Musiello-Fernandes et al. 2017; Camargo 2020). The inshore and off-shore fleets each account for a roughly equal share of marine catch by weight, across all invertebrate and finfish species (Vasconcellos et al. 2011). The most important region for shrimp fishing is the south-east, and 60% of the prawn catch is landed at Santos or Guarujä (Simões et al. 2017). Fishing further north is mostly small-scale (Vasconcellos et al. 2011).
Industrial vessels are often over 15 metres long, with engine power ranging from 70 to 300 kW, and make trips that can last several weeks (Ricardo-Pezzuto and Mastella-Benincà 2015; Camargo 2020). Some vessels are equipped with freezers, but many store the shrimp on crushed ice (Ricardo-Pezzuto and Mastella-Benincà 2015). Industrial vessels appear to number in the vicinity of one thousand (Ricardo-Pezzuto and Mastella-Benincà 2015; Port et al. 2016). Artisanal vessels are often wooden and around 10 metres long with engines and two or three crew members (Vasconcellos et al. 2011). Artisanal fishing often involves family or community labour in catching, processing, and selling the shrimp (Vasconcellos et al. 2011; Lopes and Begossi 2011). There is no precise data, though the number of artisanal shrimp vessels probably numbers in the thousands or more (which would be consistent with Mexico). Fishers sort the catch and return low-value animals to the water, but these animals are often dead before or shortly after being returned to the sea (Camargo 2020). In 2021, Brazil exported 590 tonnes of shrimp and crustacean products. The major importing jurisdictions were Japan (37%), the United Arab Emirates (19%), Malaysia (14%), USA (13%), and Vietnam (8%) (FAO 2023).
Argentina
In 2020, Argentina caught 184,000 tonnes of shrimp, equating to around 5.9% of the world catch by weight. This equates to roughly 26 billion individuals, equating to 0.071% of the world catch by number. The catch was entirely composed of penaeid species, with over 99% coming from Pleoticus muelleri. Most of Argentina's shrimp catch comes from the south coast, in Patagonia (De la Garza et al. 2017; Cervellini and Pierini 2021). A small amount of catch comes from artisanal vessels along the north coast, in Buenos Aires Province (Elías et al. 2011; Cervellini and Pierini 2021). There were around 140 vessels in Patagonia's industrial shrimp fleet in 2011 (Glembocki et al. 2015). On the larger end of this range are around 80 large industrial vessels equipped with on-board freezers, and these vessels account for over three-quarters of Argentina's shrimp catch by volume (Góngora and González-Zevallos 2012; De la Garza et al. 2017). These large vessels make trips lasting up to 40 days and have an average engine power around 700 kW (González-Zevallos et al. 2011). Smaller shrimp and bycatch species are discarded into the sea (González-Zevallos et al. 2011). The remaining 60 or so industrial vessels are smaller, ranging between 11 and 31 metres in length (Glembocki et al. 2015). These smaller industrial vessels land the catch unprocessed (Glembocki et al. 2015). In 2021, Argentina exported 166,000 tonnes of shrimp and crustacean products. The major importing jurisdictions were Spain (38%), Italy (15%), China (8%), Japan (6%), and Peru (6%) (FAO 2023).
Future directions for ethics and policy
In this report, we have examined the global shrimp fishing industry in terms of common practices and the number of individual shrimp caught. What does this analysis mean for the ethics and policy of humanity's food systems?
There is widespread agreement, both among the general public in many countries and cultures, and among philosophers and others writing on ethics, that we should seek to avoid inflicting pain on animals, and that if we cannot completely avoid it, we should seek to reduce it. That view is shared by utilitarians, such as Peter Singer (1975; 2023); advocates of animal rights like Tom Regan (1983); of social contract ethics like Mark Rowlands (1998); of feminist philosophy like Carol Adams (1990), Alice Crary and Lori Gruen (Gruen 2011; Crary and Gruen 2022); Kantians like Christine Korsgaard (2018); those working within the capabilities approach like Martha Nussbaum (2022); Christian ethicists such as Andrew Linzey (1987) and Charles Camosy (2013); and Buddhists like Shih Chao-hwei (Singer and Shih Chao-hwei 2023). Many of these thinkers, and the authors of this report, hold the principle of equal consideration of similar interests, irrespective of the race, sex, or species of the being whose interests are affected (Singer 1975; Singer 2023). In accordance with that fundamental ethical principle, pain of similar intensity and duration should be treated as equally significant, and equally to be avoided, whether it is experienced by a human being or a nonhuman animal. In applying this principle, whether among humans only, or between humans and nonhuman animals, we will often be uncertain about whether pains or pleasures experienced by different individuals are of similar intensity and duration. This uncertainty is obviously even greater when we are considering beings as different from us as shrimp (Diggles et al. 2023).
When considering the shrimp industry, it needs to be acknowledged that, while it is probable that at least some groups of shrimp (particularly the carideans) are sentient, there is less research about other groups (Birch et al. 2021; Comstock 2022a; Comstock 2022b). Hence there is considerable uncertainty about how many of the shrimp killed for human consumption are sentient, and of course we do not know what the intensity of their pain might be. When we are uncertain about the outcomes of the various policies or actions open to us, we can choose the option or policy that has the highest expected value – that is, we consider the value achieved by each option, discounted by the odds against our achieving it. On the other hand, the scale on which pain occurs obviously matters. If pain is bad, then a similar amount of pain experienced by 100 people is, other things being equal, 100 times worse than pain experienced by one person.
When we apply these considerations to the shrimp industry, the fact that the wild-caught fishing industry kills 37.4 trillion individual shrimp by our underestimate (36.3 trillion sergestids, 781 billion carideans, and 287 billion penaeids;
Figure 1) means that policies to reduce the suffering of shrimp are justified and ethically important. This is true even if the probability of all of these individuals being capable of feeling pain is low (arbitrarily, perhaps 1%) and if we arbitrarily assume that, should they be capable of feeling pain, severe pain experienced by a shrimp is only one-hundredth as bad for them as severe pain is for a human. We obtain that ethical assessment of shrimp welfare policies because 1% of 37.4 trillion is 374 billion, and dividing that future again by 100 gives us 3.74 billion. In other words, the pain experienced by shrimp in being killed for human consumption is equivalent, in its significance, to 3.74 billion humans – almost half the world’s human population - experiencing severe pain.
The air of exactitude given by this figure is, of course, misleading, because it is based on arbitrary assumptions about the probability that all shrimp are sentient, and, if they are sentient, on what their conscious experiences are like. It would be easy to argue that the assumptions we have made are orders of magnitude too high or too low. Even if we were to say that there is only a 0.1% probability that all shrimp are sentient, and that the most severe pain they can experience is only one-thousandth as bad as severe pain for a human, the expected value of eliminating that suffering is still equivalent, in expected value, to eliminating the severe suffering of 37.4 million humans – more than the human population of Australia, New Zealand and Ireland, combined. We should also remember that this is an annual figure – the same amount of suffering is experienced every year. We could also obtain a more nuanced estimate if we varied the probability of sentience according to the different orders of shrimp killed, and then included in our calculation the number of individuals killed in each of those orders, but we will not further complicate the picture here.
Conclusion
Scientific and technological advances and societal awareness can place society in the best position to improve the key dimension of a sustainable food production that is animal welfare (Coghlan et al. 2021). In this report, we have provided a review of shrimp fisheries around the world, and we have identified key research and policy priorities.
These priorities are centred on the world's top 25 countries by number of shrimp caught. Together, these countries range a considerable spectrum in geography and socio-economic conditions. On the other end of the spectrum, we observe concentrated, technologically sophisticated fisheries in comparatively wealthy countries. Prime examples are the North Sea fishery for Crangon crangon (the Netherlands, Germany, Denmark, the UK); the North Atlantic and Pacific caridean fisheries (Russia, Greenland, Canada, and the USA); and the penaeid fisheries in the Americas (especially USA and Argentina).
On the other end of the spectrum, there are small-scale fisheries in developing countries. These fisheries tend to target penaeid and sergestid species, but are often content to take many different invertebrate and finfish species in practice. These include the fisheries in Malaysia, Thailand, the Philippines, Indonesia, Brunei Darussalam, India, and Mozambique.
Many fisheries are situated along this spectrum, such as the sequential fisheries in Mexico and Brazil that involve substantial participation from both industrial and small-scale fishers. Ireland is a developed country that nevertheless has an entirely small-scale, in-shore shrimp fishery. China and the Republic of Korea are also complex examples, having significant levels of industrial trawling alongside artisanal fishing communities.
Drawing upon this spectrum of technological and socio-economic conditions, we make the following recommendations for emerging policy and future research that aims to advance wild-caught shrimp welfare:
In large-scale trawl fisheries, particularly from developed countries, animal welfare can be improved by installing electrical stunning equipment; continuing existing work on bycatch reduction; and optimising on-board processes to reduce the stress endured by shrimp during capture and slaughter (Gillett 2008; Birch et al. 2021; Albalat et al. 2022; Conneely and Coates 2023).
In small-scale fisheries, particularly in developing countries, improving government and regulatory capacity may open up opportunities for more specific shrimp welfare policies in the coming decades. This is beyond the benefits that well-supported regulatory regimes can have for mitigating poverty, addressing overfishing, resolving human rights violations, and reducing the number of shrimp caught (Tran Thi Phung Ha 2012; Sylwester 2014; Seo 2018; Suuronen et al. 2020; Stringer et al. 2022). Industries in developing countries can also be supported by improving landing technology and hygiene practices throughout the supply chain—this can help fishers to obtain higher prices and reduce the number of shrimp caught and wasted (Alam and Pokrant 2009; Geetha et al. 2020).
Additional research can help to improve our general understanding of shrimp sentience (Birch et al. 2021); develop greater specific knowledge about the welfare of shrimp due to particular industry practices (Albalat et al. 2022); and refine our estimates of shrimp killed due to bycatch, highgrading, and unreported catch (Jang et al. 2009; Jacquet et al. 2010; Cho 2012; Pedrozo 2022; Pillai et al. 2022; Temming et al. 2022; James et al. 2023).
To meet contemporary challenges facing global shrimp fisheries, including impacts on animal welfare and human beings, optimism emerges as a potent force when alliances are formed between governments, multinational retailers, industry players, consumers, and scientists. Optimism is key to binding these diverse stakeholders because it encourages a shared belief in the possibility of positive change (McAfee et al. 2019). Governments can set the stage for ethical practices through policy initiatives and international cooperation, while multinational retailers and industries play a central role in influencing supply chains and fishery standards. Simultaneously, consumers can be empowered with knowledge and a sense of responsibility to create a demand for ethically sourced products (Cummins 2004). Using this interconnectedness, we anticipate innovative solutions that enhance the well-being of animals in the food production system, particularly advancement in stress reduction. These technological advancements with societal awareness are driven by the power of collective action that is centred on the use of optimism for positive change.
Author Contributions
Conceptualization: RR. Formal analysis: RR. Writing - original draft: RR, SDC, SMD, YFT, PS. Writing - review & editing RR, SDC, SMD, YFT, PS. Visualisation: RR.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors. Animal Ask is funded by Open Philanthropy and Effective Altruism Funds, and Shrimp Welfare Project is funded by those foundations and Animal Charity Evaluators. These funders played no role in the study conceptualization; study design; collection, analysis and interpretation of data; writing of the paper; or decision to publish.
Disclosure Statement
The institutions of two of the authors (RR and SMD) have an interest in applying the results of any scientific study on animal welfare, though none of the authors or institutions are committed to any particular outcome of any piece of research. The authors have no other conflicts of interest to declare.
Data Availability Statement
Acknowledgements
We are grateful to the members of our academic networks who readily shared information about ongoing research projects.
References
- Abdulraheem S, Ibrahim G, Ahmed M. 2021. Identification and taxonomic study of shrimps in bardawil lagoon, north Sinai, Egypt. Sinai Journal of Applied Sciences. 0(0):0–0. [CrossRef]
- Ace Aquatec. 2023. Prawn portable A-HSUTM. Ace Aquatec [Internet]. [accessed 2023 Nov 13]. https://aceaquatec.com/aquaculture-products/harvest/Prawn-Humane-Stunner-Universal.
- Adams, C. 1990. The Sexual Politics of Meat. New York: Continuum.
- Af-idati N, Lee S-G. 2009. Management measures of shrimp trawling fishery in Arafura Sea, Indonesia: A challenge. Journal of Coastal Development. 12(2):56–63.
- Agence Française de Développement. 2017. Indonesia: The sea and its resources under strict surveillance. Agence Française de Développement [Internet]. [accessed 2023 Nov 27]. https://www.afd.fr/en/actualites/grand-angle/indonesia-sea-and-its-resources-under-strict-surveillance.
- Ahmad AT, Salim K, Chee PE, Isa MM, Lim CF. 2003. An overview of the socioeconomic status of fisheries in Malaysia. In: Silvestre G, Garces L, Stobutzki I, Ahmed M, Valmonte-Santos RA, Luna C, Lachica-Aliño L, Munro P, Christensen V, Pauly D, editors. Assessment, Management and Future Directions for Coastal Fisheries in Asian Countries WorldFish Center Conference Proceedings. [place unknown]: digitalarchive.worldfishcenter.org; p. 517–542.
- Alam SMN, Pokrant B. 2009. RE-ORGANIZING THE SHRIMP SUPPLY CHAIN: AFTERMATH OF THE 1997 EUROPEAN UNION IMPORT BAN ON THE BANGLADESH SHRIMP. Aquacult Econ Manage. 13(1):53–69. [CrossRef]
- Albalat A, Zacarias S, Coates CJ, Neil DM, Planellas SR. 2022. Welfare in Farmed Decapod Crustaceans, With Particular Reference to Penaeus vannamei. Frontiers in Marine Science [Internet]. 9. [CrossRef]
- Ali M, Yunianta, Aulanni’am, Kusnadi J. 2019. Characteristic of acan: a traditional shrimp paste of Maduranese, Indonesia. IOP Conf Ser: Earth Environ Sci. 230(1):012048.
- Alonso G, Marschke M. 2023. Blue boats in deep waters: how aspects of IUU policy impact Vietnamese fish workers. Marit Stud. 22(2):14. [CrossRef]
- Alonso ME, González-Montaña JR, Lomillos JM. 2020. Consumers’ Concerns and Perceptions of Farm Animal Welfare. Animals (Basel) [Internet]. 10(3). [CrossRef]
- Alonso WJ, Schuck-Paim C. 2021. Pain-Track: a time-series approach for the description and analysis of the burden of pain. BMC Res Notes. 14(1):229. [CrossRef]
- Alonso WJ, Schuck-Paim C. 2022. Cumulative Pain: An evidence-based, easily interpretable and interspecific metric of welfare loss. Preprints [Internet]. [CrossRef]
- Alsayes A, Fattouh S, Enin SA. 2010. Development of trawl and shrimp fisheries at the Egyptian Mediterranean area. World Journal of Fish and Marine Sciences. 2(1):1–7.
- Altamiranoa J, Kurokura H, Salayo N, Baticados D, Suyo JG, Ishikawa S. 2015. Community-based shrimp stock enhancement for coastal socio-ecological restoration in the Philippines. In: International Workshop on Resource Enhancement and Sustainable Aquaculture Practices in Southeast Asia 2014. [place unknown]: repository.seafdec.org.ph; p. 159–167.
- Altamirano JP, Salayo ND, Kurokura H, Fushimi H, Ishikawa S. 2016. Aquaculture-based restoration and stock enhancement of tiger shrimps in the Philippines. In: Consolidating the Strategies for Fishery Resources Enhancement in Southeast Asia Proceedings of the Symposium on Strategy for Fisheries Resources Enhancement in the Southeast Asian Region, Pattaya, Thailand, 27-30 July 2015. [place unknown]: Training Department, Southeast Asian Fisheries Development Center; p. 166–170.
- Aquaculture Stewardship Council. 2023a. ASC Shrimp Standard v1.2.1. Aquaculture Stewardship Council [Internet]. [accessed 2023 Nov 27]. https://asc-aqua.org/wp-content/uploads/2023/07/ASC-Shrimp-Standard_v1.2.1.pdf.
- Aquaculture Stewardship Council. 2023b. Shrimp Health and Welfare - Tentative Indicators. Aquaculture Stewardship Council [Internet]. [accessed 2023 Nov 27]. https://asc-aqua.org/wp-content/uploads/2023/08/PC-Sept-Oct-2023-Shrimp-Health-and-Welfare-Draft-Indicators-EN.pdf.
- Araya-Schmidt, T. 2022. Reducing bottom trawl seabed impacts and bycatch in the Eastern Canada offshore Northern shrimp (Pandalus borealis) fishery [PhD] [Internet]. [place unknown]: Memorial University of Newfoundland; [accessed 2023 Nov 6]. https://research.library.mun.ca/15626/.
- Armitage D, Marschke M. 2013. Assessing the future of small-scale fishery systems in coastal Vietnam and the implications for policy. Environ Sci Policy. 27:184–194. [CrossRef]
- Avilés-Polanco G, Almendarez-Hernández MA, Beltrán-Morales LF, Aranceta-Garza F. 2023. Cost Efficiencies of the Shrimp Fishery in Mexico: A Stochastic Frontier Analysis. Fishes. 8(9):472. [CrossRef]
- Bauer, RT. 2023. Fisheries and Aquaculture. In: Bauer RT, editor. Shrimps: Their Diversity, Intriguing Adaptations and Varied Lifestyles. Cham: Springer International Publishing; p. 583–655.
- Becker RA, Wilks AR, Brownrigg R, Minka TP, Deckmyn A. 2023. maps: Draw Geographical Maps. Version 3.4.1.1 [Internet]. https://CRAN.R-project.org/package=maps.
- Benkenstein, A. 2013. Small-scale fisheries in a modernising economy: opportunities and challenges in Mozambique [Internet]. [place unknown]: Governance of Africa’s Resources Programme, South African Institute of International Affairs. https://www.africaportal.org/publications/small-scale-fisheries-in-a-modernising-economy-opportunities-and-challenges-in-mozambique/.
- Benkenstein, A. 2013. Small-scale fisheries in Mozambique [Internet]. [place unknown]: Governance of Africa’s Resources Programme, South African Institute of International Affairs. https://www.africaportal.org/publications/small-scale-fisheries-in-mozambique/.
- Best Aquaculture Practices. 2023. BAP Farm Standard Issue 3.1. Best Aquaculture Practices [Internet]. [accessed 2023 Nov 27]. https://www.bapcertification.org/Downloadables/pdf/GSA%20-%20Farm%20Standard%20-%20Issue%203.1%20-%2007-February-2023.pdf.
- Birch J, Burn C, Schnell A, Browning H, Crump A. 2021. Review of the Evidence of Sentience in Cephalopod Molluscs and Decapod Crustaceans [Internet]. [place unknown]: LSE Consulting, London School of Economics and Political Science; [accessed 2023 Jun 5]. https://www.lse.ac.uk/news/news-assets/pdfs/2021/sentience-in-cephalopod-molluscs-and-decapod-crustaceans-final-report-november-2021.pdf.
- Boonchuwongse P, Dechboon W. 2003. Socioeconomic Assessment of Marine Fisheries of Thailand. In: Silvestre G, Garces L, Stobutzki I, Ahmed M, Valmonte-Santos RA, Luna C, Lachica-Aliño L, Munro P, Christensen V, Pauly D, editors. Assessment, Management and Future Directions for Coastal Fisheries in Asian Countries WorldFish Center Conference Proceedings 67. [place unknown]: WorldFish; p. 577–628.
- Brito, A. 2012. An interview-based assessment of the incidental capture and mortality of sea turtles in Mozambique’s Sofala Bank commercial shrimp fishery [Internet]. [place unknown]: Instituto Nacional de Investigação Pesqueira. https://aquadocs.org/handle/1834/5142.
- Camargo, TR. 2020. An overview of trawling and its bycatch in Brazil [Internet]. Valenti WC, editor. [place unknown]: Centro de Aquicultura da Unesp. https://repositorio.unesp.br/bitstream/handle/11449/192721/camargo_tr_dr_jabo_int.pdf?sequence=4#page=26.
- Camosy, C. 2013. For Love of Animals: Christian Ethics, Consistent Action. Cincinnati: Franciscan Media.
- Carnie, G. 2002. Handling prawns at sea: A guide for prawn trawler crew at level 1 [Internet]. [place unknown]: Australian Prawn Industry Association. https://www.frdc.com.au/sites/default/files/products/A%20Guide%20for%20Prawn%20Trawler%20Crew%20at%20Level%201.pdf.
- Catchpole TL, Revill AS, Innes J, Pascoe S. 2008. Evaluating the efficacy of technical measures: a case study of selection device legislation in the UK Crangon crangon (brown shrimp) fishery. ICES J Mar Sci. 65(2):267–275.
- Cervellini PM, Pierini JO. 2021. Shrimps and Prawns. In: Fiori SM, Pratolongo PD, editors. The Bahía Blanca Estuary: Ecology and Biodiversity. Cham: Springer International Publishing; p. 253–272.
- Cho D-O. 2012. Eliminating illegal bottom trawl fishing in the coastal waters of Korea. Mar Policy. 36(2):321–326. [CrossRef]
- Cinco EA, Teh LCL, Zylich K, Pauly D. 2015. Reconstructing the marine and estuarine fisheries of Brunei Darussalam, 1950 to 2010 [Internet]. [accessed 2023 Nov 6]. https://www.seaaroundus.org/doc/publications/wp/2015/Cinco-et-al-Brunei.pdf.
- Coghlan S, Coghlan BJ, Capon A, Singer P. 2021. A bolder One Health: expanding the moral circle to optimize health for all. One Health Outlook. 3(1):21. [CrossRef]
- Comstock, G. 2022a. Pain in Pleocyemata, but not in Dendrobranchiata? Animal Sentience. 7(32):13.
- Comstock, G. 2022b. The Shrimp Hypothesis. In: Interspecies Welfare Comparison. London School of Economics.
- Conneely E-A, Coates CJ. 2023. Meta-analytic assessment of physiological markers for decapod crustacean welfare. Fish Fish [Internet]. [CrossRef]
- Cook KV, Lennox RJ, Hinch SG, Cooke SJ. 2015. FISH Out of WATER: How Much Air is Too Much? Fisheries. 40(9):452–461. [CrossRef]
- Crary A, Gruen L. 2022. Animal Crisis. Cambridge: Polity Press.
- Crump A, Browning H, Schnell AK, Burn C, Birch J. 2022. Invertebrate sentience and sustainable seafood. Nat Food. 3(11):884–886. [CrossRef]
- Crustacean Compassion. Sea-to-Plate: The welfare journey of decapod crustaceans [Internet]. [place unknown]. https://www.crustaceancompassion.org/_files/ugd/46ca59_b9636b2becde4d9590188b01e118247e.pdf.
- Cruz WSD, Ramiscal RV, Asuncion RP, Sapul ES, Mojena M, Gonzales G, Dela Cruz WS, Rafael V, Ramiscal RP, Asuncion ES. 2014. Efficiency of deep sea traps in catching Pandalid shrimps along continental slopes and seamounts in Northeast and West Philippine Sea. Int J Fish Aquat Stud. 1(5):170–175.
- Cumberlidge, N. 2009. Freshwater Crabs and Shrimps (Crustacea: Decapoda) of the Nile Basin. In: Dumont HJ, editor. The Nile: Origin, Environments, Limnology and Human Use. Dordrecht: Springer Netherlands; p. 547–561.
- Cummins, A. 2004. The Marine Stewardship Council: A multi-stakeholder approach to sustainable fishing. Corp Soc Responsibility Environ Manage. 11(2):85–94. [CrossRef]
- De Grave S, Pentcheff ND, Ahyong ST, Chan T-Y, Crandall KA, Dworschak PC, Felder DL, Feldmann RM, Fransen CHJM, Goulding LYD, et al. 2009. A classification of living and fossil genera of decapod crustaceans. Raffles Bull Zool.:8358.
- De la Garza J, Moriondo Danovaro P, Fernández M, Ravalli C, Souto V, Waessle J. 2017. An overview of the argentine red shrimp (Pleoticus muelleri, Decapoda, Solenoceridae) fishery in Argentina: biology, fishing, management and ecological interactions [Internet]. [place unknown]: Instituto Nacional de Investigación y Desarrollo Pesquero. https://aquadocs.org/handle/1834/15133.
- Department for Environment, Food & Rural Affairs. 2021. News story: Lobsters, octopus and crabs recognised as sentient beings. UK Government [Internet]. [accessed 2023 Nov 27]. https://www.gov.uk/government/news/lobsters-octopus-and-crabs-recognised-as-sentient-beings.
- Diggles BK, Arlinghaus R, Browman HI, Cooke SJ, Cooper RL, Cowx IG, Derby CD, Derbyshire SW, Hart PJB, Jones B, et al. 2023. Reasons to be skeptical about sentience and pain in fishes and aquatic invertebrates. Rev Fish Sci Aquac.:1–24. [CrossRef]
- Dineshbabu AP, Sarada PT, Josileen J. 2022. Saga of marine prawn fishery of Karnataka. Mar Fish Inf Serv Tech Ext Ser. 253:7–14.
- Ding Q, Shan X, Jin X, Gorfine H. 2021. A multidimensional analysis of marine capture fisheries in China’s coastal provinces. Fish Sci. 87(3):297–309. [CrossRef]
- Dinh TD, Moreau J, Van MV, Phuong NT, Toan VT. 2010. Population dynamics of shrimps in littoral marine waters of the Mekong Delta, south of Viet Nam. Pak J Biol Sci. 13(14):683–690. [CrossRef]
- Divipala I, Ghosh S, Dineshbabu AP, Raju SS, Muktha M, Edward L, Behera PR, Manas HM, Jasmin F, Rao TN, et al. 2019. Trawl Fisheries in Andhra Pradesh: Facts and fishers insight. Mar Fish Inf Serv Tech Ext Ser.(241):25–26.
- Döring R, Berkenhagen J, Hentsch S, Kraus G. 2020. Small-Scale Fisheries in Germany: A Disappearing Profession? In: Pascual-Fernández JJ, Pita C, Bavinck M, editors. Small-Scale Fisheries in Europe: Status, Resilience and Governance. Cham: Springer International Publishing; p. 483–502.
- Dubay K, Tokuoka S, Gereffi G. 2010. A value chain analysis of the Sinaloa, Mexico shrimp fishery [Internet]. [place unknown]: Center on Globalization, Governance & Competitiveness Duke University. http://www.cggc.duke.edu/.
- Dunaway WA, Macabuac MC. 2007. “The Shrimp Eat Better than We Do”: Philippine Subsistence Fishing Households Sacrificed for the Global Food Chain. Rev Fed Am Health Syst. 30(4):313–337.
- Dutch Fish Product Board. 2009. Fishfacts: North Sea brown shrimp [Internet]. [place unknown]. https://www.eastern-ifca.gov.uk/wp-content/uploads/2016/04/Fishfacts_North_Sea_shrimp.pdf.
- Ebil, S. 2013. Assessment of demersal fishery resources in Brunei Darussalam [PhD] [Internet]. [place unknown]: University of Warwick. https://wrap.warwick.ac.uk/57704/1/WRAP_THESIS_Ebil_2013.pdf.
- Eigaard OR, Munch-Petersen S. 2010. Influence of fleet renewal and trawl development on landings per unit effort of the Danish northern shrimp (Pandalus borealis) fishery. ICES J Mar Sci. 68(1):26–31. [CrossRef]
- El-Bokhty EA, El-Aiatt A. 2014. Trends in artisanal fisheries of Bardawil lagoon with reference to shrimp bottom trawling (kalsa). Egypt J Aquat Biol Fish. 18(4):95–107.
- El-Ganainy AA, Yassien MH. 2012. The population biology of penaeid prawns in the Gulf of Suez, Red Sea, Egypt. Mar Biol Res. 8(4):405–411. [CrossRef]
- Elías I, Carozza C, Di Giácomo EE, Isla MS, Orensanz JM (lobo), Parma AM, Pereiro RC, Perier MR, Perrotta RG, Ré ME, Ruarte C. 2011. Coastal fisheries of Argentina. In: Salas S, Chuenpagdee R, Charles A, Seijo JC, editors. Coastal fisheries of Latin America and the Caribbean: FAO Fisheries and Aquaculture Technical Paper 544. [place unknown]: notablesdelaciencia.conicet.gov.ar; p. 13–48.
- Emmerson JA, Haig JA, Robson G, Hinz H, Le Vay L, Kaiser MJ. 2017. Size-selective fishing of Palaemon serratus (Decapoda, Palaemonidae) in Wales, UK: implications of sexual dimorphism and reproductive biology for fisheries management and conservation. J Mar Biol Assoc U K. 97(6):1223–1232. [CrossRef]
- Endroyono. 2015. Overview of the trawl fisheries socio-economic conditions in Indonesia after the second trawl ban. Socio-economics of trawl fisheries in Southeast Asia and Papua New Guinea. 26:3.
- Engø, T. 2021. Modernising Denmark’s shrimp fleet [Internet]. [accessed 2023 Nov 10]. https://mag.hookandnet.com/2021/05/11/2021-05northsea/content.html.
- European Commission. 2017. Welfare of farmed fish: Common practices during transport and at slaughter [Internet]. [place unknown]: European Commission. https://publications.europa.eu/resource/cellar/facddd32-cda6-11e7-a5d5-01aa75ed71a1.0001.01/DOC_1.
- European Parliament. 2022. Role and impact of China on world fisheries and aquaculture. [place unknown]: Policy Department for Structural and Cohesion Policies, Directorate-General for Internal Policies.
- Fahy E, Gleeson P. 1996. The commercial exploitation of shrimp Palaemon serratus (Pennant) in Ireland [Internet]. [place unknown]: The Marine Institute; [accessed 2023 Nov 6]. http://oar.marine.ie/handle/10793/794.
- FAO. 2023. Global aquatic trade - By partner country Quantity (2019 - 2021) [Internet]. [accessed 2023 Nov 9]. https://www.fao.org/fishery/statistics-query/en/trade_partners/trade_partners_quantity.
- Farmery A, Gardner C, Green BS, Jennings S, Watson R. 2015. Life cycle assessment of wild capture prawns: expanding sustainability considerations in the Australian Northern Prawn Fishery. J Clean Prod. 87:96–104. [CrossRef]
- Fernandes, PG.2022. Smartrawl: a system to eliminate discards and bycatch in fisheries. In: MASTS: Annual Science Meeting [Internet]. [place unknown]. https://masts.ac.uk/wp-content/uploads/2022/11/General-Science-4-Session-abstracts-FINAL.pdf.
- Fernandes-Salvador JA, Goienetxea I, Ibaibarriaga L. 2022. Research for PECH Committee: Artificial Intelligence and the fisheries sector [Internet]. https://policycommons.net/artifacts/2445802/research-for-pech-committee/3467524/.
- Fisheries Research and Development Corporation. 2022. Responsible (animal welfare) fishing practice for the Australian Wild Prawn industry. FRDC [Internet]. [accessed 2023 Nov 13]. https://www.frdc.com.au/project/2022-064.
- Foley, P.2012. The political economy of marine stewardship council certification: Processors and access in Newfoundland and Labrador’s inshore shrimp industry. J Agrar Chang. 12(2-3):436–457. [CrossRef]
- Foley, P. 2013. National Government Responses to Marine Stewardship Council (MSC) Fisheries Certification: Insights from Atlantic Canada. New Political Economy. 18(2):284–307. [CrossRef]
- Foo Yun Chee. 2021. Dutch lose court fight against EU electric pulse fishing ban. Reuters [Internet]. [accessed 2023 Nov 10]. https://www.reuters.com/business/environment/dutch-lose-court-fight-against-eu-electric-pulse-fishing-ban-2021-04-15/.
- Freeling BS, Connell SD. 2021. Animal Minds, Social Change, and the Future of Fisheries Science. Frontiers in Marine Science [Internet]. 8. [CrossRef]
- Fregin T, Bickmeyer U. 2016. Electrophysiological Investigation of Different Methods of Anesthesia in Lobster and Crayfish. PLoS One. 11(9):e0162894. [CrossRef]
- Gay DB, Ramos MN, Ferrer AJG. 2022. Value chain of sergestid shrimp (Acetes spp.) caught in Banate Bay, Iloilo Province, Philippines. Marit Stud. 22(1):1. [CrossRef]
- Geetha R, Ravisankar T, Patil PK, Avunje S, Vinoth S, Sairam CV, Vijayan KK. 2020. Trends, causes, and indices of import rejections in international shrimp trade with special reference to India: a 15-year longitudinal analysis. Aquac Int. 28(3):1341–1369. [CrossRef]
- Gillett, R.2008. Global study of shrimp fisheries. FAO Fish Tech Pap. 475:25–29.
- Gin, OK. 2015. Brunei - History, Islam, Society and Contemporary Issues. [place unknown]: Routledge.
- Glembocki NG, Williams GN, Góngora ME, Gagliardini DA, Orensanz JM (lobo). 2015. Synoptic oceanography of San Jorge Gulf (Argentina): A template for Patagonian red shrimp (Pleoticus muelleri) spatial dynamics. J Sea Res. 95:22–35. [CrossRef]
- Gokoglu N, Gumus B, Ceylan A, Gokoglu M. 2022. Storage in ice incorporated antimelanotic agent and its effects on melanosis and quality of giant red shrimp (Aristaeomorpha foliacea). Food Bioscience. 46:101599. [CrossRef]
- Góngora ME, González-Zevallos D. 2012. Characterization of the main fisheries in San Jorge Gulf, Patagonia, Argentina. Latin American Journal of Aquaculture Research. 40(1):1–11. [CrossRef]
- González-Zevallos D, Yorio P, Svagelj WS. 2011. Seabird attendance and incidental mortality at shrimp fisheries in Golfo San Jorge, Argentina. Mar Ecol Prog Ser. 432:125–135. [CrossRef]
- Gornova, AM. 2016. Review of fishing in the Arctic waters. Arctic.(25):171. [CrossRef]
- Goti-Aralucea L, Berkenhagen J, Sulanke E, Döring R. 2021. Efficiency vs resilience: The rise and fall of the German brown shrimp fishery in times of COVID 19. Mar Policy. 133:104675. [CrossRef]
- Gruen, L. 2011. Ethics and Animals. Cambridge: Cambridge University Press.
- Hammer M, Hoel AH. 2012. The Development of Scientific Cooperation under the Norway–Russia Fisheries Regime in the Barents Sea. Arctic Review on Law and Politics. 3(2):244–274. [CrossRef]
- Hannah RW, Jones SA, Groth SD. 2018. Fishery Management Plan for Oregon’s Trawl Fishery for Ocean Shrimp (Pandalus jordani) [Internet]. [place unknown]: Oregon Department of Fish and Wildlife; [accessed 2023 Nov 10]. https://dfw.state.or.us/MRP/shellfish/commercial/shrimp/docs/Oregon%20Pink%20Shrimp%20Fishery%20Management%20Plan%20March2018.pdf.
- bin Hashim M, Kathamuthu S. 2005. Shrimp farming in Malaysia. In: Sulit VT, Aldon MET, Tendencia IT, Ortiz AMJ, Alayon SB, Ledesma AS, editors. Regional Technical Consultation on the Aquaculture of P vannamei and Other Exotic Shrimps in Southeast Asia. Tigbauan, Iloilo, Philippines: Aquaculture Department Southeast Asian Fisheries Development Center; p. 50–56.
- Hayashi K-I, Kim JN. 1999. Revision of the East Asian species of Crangon (Decapoda: Caridea: Crangonidae). Crustac Res. 28:62–103. [CrossRef]
- He J, Zhang X. 2022. China Revamping Decades-Old Fisheries Law to Combat Illegal, Unreported, and Unregulated Fishing: Stimulating the Intersection of Law, Technology, and Markets. Frontiers in Ecology and Evolution [Internet]. 10. [CrossRef]
- Helmi H, Astuti DI, Dungani R, Aditiawati P. 2022. A comparative study on quality of fermented shrimp paste (terasi) of pelagic shrimp from different locations in Indonesia. SQUALEN Bull Mar Fish Postharvest Biotechnol. 17(1):23–34. [CrossRef]
- Hodal K, Kelly C. 2014. Trafficked into slavery on Thai trawlers to catch food for prawns. Guardian [Internet]. http://policeprostitutionandpolitics.org/pdfs_all/Trafficking_All%20/Non%20sex%20work%20related%20trafficking/2014%20Trafficked%20into%20slavery%20on%20Thai%20trawlers%20to%20catch%20food%20for%20prawns%20%7C%20Global-development%20%7C%20The%20Guardian.pdf.
- Hvingel C, Zimmermann F. 2023. Barents Sea Shrimp-stock assessment report 2022/23 [Internet]. [place unknown]: IMR-PINRO. https://imr.brage.unit.no/imr-xmlui/handle/11250/3063086.
- Iannone R, Cheng J, Schloerke B, Hughes E, Lauer A, Seo J. 2023. gt: Easily Create Presentation-Ready Display Tables. R package version 0.10.0 [Internet]. https://CRAN.R-project.org/package=gt.
- Innes J, Pascoe S. 2008. Productivity impacts of veil nets on UK Crangon vessels. J Agric Econ. 59(3):574–588. [CrossRef]
- International Labour Organization. 2020. Endline research findings on fishers and seafood workers in Thailand [Internet]. [place unknown]: ILO Country Office for Thailand, Cambodia and Lao People’s Democratic Republic. https://www.ilo.org/asia/publications/WCMS_738042/lang--en/index.htm.
- Jacquet J, Fox H, Motta H, Ngusaru A, Zeller D. 2010. Few data but many fish: marine small-scale fisheries catches for Mozambique and Tanzania. Afr J Mar Sci. 32(2):197–206. [CrossRef]
- Jagerroos, S. 2016. Assessment of living resources in the straits of Malacca, Malaysia: Case study. J Aquac Mar Biol. 4(1):00070. [CrossRef]
- James MA, Gozzer-Wuest R, Mendo T, Gomez I, Grillo-Núñez J, Mendo J. 2023. To ignore or mitigate – Economic implications of an illegal artisanal trawl fishery in northern Peru. Mar Policy. 158(105865):105865. [CrossRef]
- Janekitkosol W, Somchanakij H, Eiamsa-ard M, Supongpan M. 2003. Strategic Review of the Fishery Situation in Thailand. In: Silvestre G, Garces L, Stobutzki I, Ahmed M, Valmonte-Santos RA, Luna C, Lachica-Aliño L, Munro P, Christensen V, Pauly D, editors. Assessment, Management and Future Directions for Coastal Fisheries in Asian Countries WorldFish Center Conference Proceedings 67. [place unknown]: WorldFish; p. 915–956.
- Jang CS, Cho YH, Lim CR, Kim BY. 2009. An analysis on catch of the shrimp beam trawl fishery in Korea coastal sea. Journal of the Korean Society of Fisheries Technology. 45(1):1–13. [CrossRef]
- Kadfak A, Wilhelm M, Oskarsson P. 2023. Thai labour NGOs during the “modern slavery” reforms: NGO transitions in a post-aid world. Dev Change. 54(3):570–600. [CrossRef]
- Kasim, K. 2019. Fishery Performance Indicators (FPIs) and Production Analysis: What Works Before and After the Ban of Cantrang Trawl Fishing in the Java Sea–Indonesia [Master] [Internet]. Anderson CM, Hilborn R, editors. [place unknown]: University of Washington. https://digital.lib.washington.edu/researchworks/handle/1773/45195.
- Kensley, BF. 1971. The family Sergestidae in the waters around southern Africa (Crustacea, Decapoda, Natantia). Ann S Afr Mus [Internet]. 57(10). https://repository.si.edu/bitstream/handle/10088/9963/iz_1971_Kensley_The_family_Sergestidae_around_southern_Africa.pdf.
- Khan SR, Khan SR. 2011. Fishery degradation in Pakistan: a poverty–environment nexus? Rev Can Etudes Dev. 32(1):32–47. [CrossRef]
- Khokher MR, Little LR, Tuck GN, Smith DV, Qiao M, Devine C, O’Neill H, Pogonoski JJ, Arangio R, Wang D. 2022. Early lessons in deploying cameras and artificial intelligence technology for fisheries catch monitoring: where machine learning meets commercial fishing. Can J Fish Aquat Sci. 79(2):257–266. [CrossRef]
- Kolovos, B. 2023. Animals to be recognised as sentient beings under proposed Victorian cruelty laws. The Guardian [Internet]. [accessed 2023 Nov 14]. https://www.theguardian.com/australia-news/2023/nov/14/animals-sentient-beings-victorian-cruelty-laws.
- Korsgaard, C. 2018. Fellow Creatures. Oxford: Oxford University Press.
- Lavallee J, Spangler E, Hammell KL. 2000. Analytical assessment of handling, fishing practices, and transportation risk factors on lobster (Homarus americanus) health in Prince Edward Island, Canada. J Shellfish Res [Internet]. https://islandscholar.ca/islandora/object/ir%3Air-batch6-3253/datastream/PDF/view.
- Le Manach F, Bisiaux L, Villasante S, Nouvian C. 2019. Public subsidies have supported the development of electric trawling in Europe. Mar Policy. 104:225–231. [CrossRef]
- Lewit-Mendes L, Saugh S, Boddy A. 2022. Shrimp Welfare Report: Factors Affecting Shrimp Welfare in Aquaculture [Internet]. [place unknown]: Shrimp Welfare Project. https://www.shrimpwelfareproject.org/shrimp-welfare-report.
- Lincoln JM, Lucas DL. 2010. Occupational Fatalities in the United States Commercial Fishing Industry, 2000–2009. J Agromedicine. 15(4):343–350. [CrossRef]
- Linzey, A. 1987. Christianity and the Rights of Animals. London: SPCK Publishing.
- Lira AS, Lucena-Frédou F, Le Loc’h F. 2021. How the fishing effort control and environmental changes affect the sustainability of a tropical shrimp small scale fishery. Fish Res. 235:105824. [CrossRef]
- Longépé N, Hajduch G, Ardianto R, Joux R de, Nhunfat B, Marzuki MI, Fablet R, Hermawan I, Germain O, Subki BA, et al. 2018. Completing fishing monitoring with spaceborne Vessel Detection System (VDS) and Automatic Identification System (AIS) to assess illegal fishing in Indonesia. Mar Pollut Bull. 131(Pt B):33–39. [CrossRef]
- Lopes PFM, Begossi A. 2011. Decision-making processes by small-scale fishermen on the southeast coast of Brazil. Fish Manag Ecol. 18(5):400–410. [CrossRef]
- Lucchetti A, Kholeif SEA, Mahmoud HH, Notti E. 2016. Towards sustainable fisheries management in emerging markets: An overview of properties, gaps and opportunities in Egypt. Mar Policy. 72:1–10. [CrossRef]
- Madhu, VR. 2018. A Review of Trawl Selectivity Studies carried out along Indian Coast. Fish Technol Soc Fish Technol. 55:1–18.
- Maeda K, Nishiuchi S. 1999. Vertical distribution of the Pacific pink shrimp, Pandalus eous Makarov, in Ishikari Bay, Sea of Japan [Internet]. [place unknown]: Hokkaido Fisheries Experimental Station. http://www.hro.or.jp/list/fisheries/marine/att/hokoku_55.PDF#page=191.
- Main DCJ, Mullan S, Atkinson C, Cooper M, Wrathall JHM, Blokhuis HJ. 2014. Best practice framework for animal welfare certification schemes. Trends Food Sci Technol. 37(2):127–136. [CrossRef]
- Mallatt J, Feinberg TE. 2022. Decapod sentience: Promising framework and evidence. Animal Sentience. 7(32):24. [CrossRef]
- Mangar RS, Foster SJ, Vincent ACJ. 2023. Understanding the fishers to change the fishery in the bottom trawl industry in India [Internet]. [place unknown]: Fisheries Centre Research Report. https://open.library.ubc.ca/soa/cIRcle/collections/52383/items/1.0435884.
- Manhice HC de P, Pedersen JST, Dias LF, Santos FD. 2023. Unlocking the Hidden Dynamics of Artisanal Fishing Practices: A Key to Managing Sustainable Coastal Ecosystems in Mozambique. Available at SSRN [Internet]. [accessed 2023 Nov 6]. [CrossRef]
- Mann, M. 2018. Fishing for shrimps in Greenland: Comparing fleets and an alternative fishing method by using life cycle assessment [Bachelor of Science]. Ziegler F, André C, editors. [place unknown]: University of Gothenburg.
- Marine Stewardship Council. 2023. Track a Fishery. Marine Stewardship Council [Internet]. [accessed 2023 Nov 13]. https://fisheries.msc.org/en/fisheries/.
- May Petry L, Soares A, Bogorny V, Brandoli B, Matwin S. 2020. Challenges in Vessel Behavior and Anomaly Detection: From Classical Machine Learning to Deep Learning. In: Advances in Artificial Intelligence. [place unknown]: Springer International Publishing; p. 401–407.
- McAfee D, Doubleday ZA, Geiger N, Connell SD. 2019. Everyone Loves a Success Story: Optimism Inspires Conservation Engagement. Bioscience. 69(4):274–281. [CrossRef]
- McKay H, McAuliffe W. 2023. Impact of welfare issues on farmed penaeid shrimp. RPubs [Internet]. https://rpubs.com/hannah_/penaeidshrimpwelfareissues.
- Mehanna, SF. 2022. Egyptian marine fisheries and its sustainability. In: Galanakis CM, editor. Sustainable Fish Production and Processing. San Diego, CA: Elsevier; p. 111–140.
- Mikhailova, OG. 2012. Coastal monitoring the state of pink shrimp Pandalus borealis population on West Kamchatka. North Pacific Marine Science Organization PICES-2012 Program and Abstracts.:62.
- Mood A, Lara E, Boyland NK, Brooke P. 2023. Estimating global numbers of farmed fishes killed for food annually from 1990 to 2019. Anim Welf. 32:e12. [CrossRef]
- Morton R, Hebart ML, Ankeny RA, Whittaker AL. 2020. Assessing the Uniformity in Australian Animal Protection Law: A Statutory Comparison. Animals (Basel) [Internet]. 11(1). [CrossRef]
- Muawanah U, Kasim K. 2021. Technical Efficiency of the Shrimp Trawl Fishery in Aru and the Arafura Sea, the Eeastern Part of Indonesia. Journal of Business, Economics and Environmental Studies. 11(2):5–13.
- Musiello-Fernandes J, Zappes CA, Hostim-Silva M. 2017. Small-scale shrimp fisheries on the Brazilian coast: Stakeholders perceptions of the closed season and integrated management. Ocean Coast Manag. 148:89–96. [CrossRef]
- Nepomuceno LT, Asuncion RP, Ramiscal RV. 2013. Analyzing the catch rate and species diversity index of deep-water shrimps for sustainable fisheries development and management: Philippine perspective. In: [place unknown]: Secretariat, Southeast Asian Fisheries Development Center.
- Nguyen KQ, Do MD, Phan HT, Nguyen LT, Van To P, Nghiep Ke Vu, Tran PD. 2021. Catch composition and codend selectivity of inshore trawl fishery with the legal minimum mesh size. Regional Studies in Marine Science. 47:101977. [CrossRef]
- Nussbaum, M. 2022. Justice for Animals. New York: Simon and Schuster.
- Omori, M. 1975. The systematics, biogeography, and fishery of epipelagic shrimps of the genus Acetes (Crustacea, Decapoda, Sergestidae). Bulletin of the Ocean Research Institute, University of Toyko [Internet]. [accessed 2023 Nov 10] 7. https://citeseerx.ist.psu.edu/document?repid=rep1&type=pdf&doi=7cf2484fd4e70687c1a84b310ceb3307be052dac.
- Orrego CE, Salgado N. 2014. Green logistics in chitin/chitosan industry. In: Alzate CAC, Castro WAS, editors. Green Supply Chains: applications in agroindustries. Manizales, Colombia: Universidad Nacional de Colombia.
- Parkes G, Young JA, Walmsley SF, Abel R, Harman J, Horvat P, Lem A, MacFarlane A, Mens M, Nolan C. 2010. Behind the Signs—A Global Review of Fish Sustainability Information Schemes. Rev Fish Sci. 18(4):344–356. [CrossRef]
- Park H-H, Jeong E-C, Bae B-S, Yang Y-S, Hwang S-J, Park J-H, Kim Y-S, Lee S-I, Choi S-H. 2007. Fishing investigation and species composition of the catches caught by a bottom trawl in the deep East Sea. J Korean Soc Fish Technol. 43(3):183–191. [CrossRef]
- Pauly D, Zeller D. 2016. Global Atlas of Marine Fisheries: A Critical Appraisal of Catches and Ecosystem Impacts. [place unknown]: Island Press.
- Pedrozo R (pete). 2022. China’s IUU fishing fleet: Pariah of the world’s oceans. Int Law Stud. 99(1):10.
- Phoonsawat R, Auksonphaob U, Cheuamankong T, Panjarat S. 2016. Gulf of Thailand trawl experiments using 4.0 cm codend mesh [Internet]. [place unknown]: Department of Fisheries, Thailand; [accessed 2023 Nov 10]. http://repository.seafdec.or.th/handle/20.500.12067/1709.
- Pillai SL, Kizhakudan SJ, Radhakrishnan EV, Thirumilu P. 2014. Crustacean bycatch from trawl fishery along north Tamil Nadu coast. Indian J Fish. 61(2):7–13.
- Pillai SL, Sarada PT, Josileen J, Baby PK, Ragesh N, Sreesanth L, Sathianandan TV, Kuriakose S, Augustine SK. 2022. Saga of inshore prawn fishery of Kerala. Mar Fish Inf Serv Tech Ext Ser. 253:21–25.
- Poisson F, Budan P, Coudray S, Gilman E, Kojima T, Musyl M, Takagi T. 2022. New technologies to improve bycatch mitigation in industrial tuna fisheries. Fish Fish. 23(3):545–563. [CrossRef]
- Pomeroy R, Thi Nguyen KA, Thong HX. 2009. Small-scale marine fisheries policy in Vietnam. Mar Policy. 33(2):419–428. [CrossRef]
- Popescu I, Ogushi T. 2013. Fisheries in Japan [Internet]. [place unknown]: European Parliament. https://www.europarl.europa.eu/RegData/etudes/note/join/2014/529044/IPOL-PECH_NT(2014)529044_EN.pdf.
- Port D, Perez JAA, de Menezes JT. 2016. The evolution of the industrial trawl fishery footprint off southeastern and southern Brazil. Lat Am J Aquat Res. 44(5):908–925. [CrossRef]
- Purcell KM, Craig JK, Nance JM, Smith MD, Bennear LS. 2017. Fleet behavior is responsive to a large-scale environmental disturbance: Hypoxia effects on the spatial dynamics of the northern Gulf of Mexico shrimp fishery. PLoS One. 12(8):e0183032. [CrossRef]
- Ramiscal RV, Dickson JO, Lamarca NSJ, Hilario EV, Romero RO. 2015. Socio-economi study of trawl fisheries in Samar Sea, Philippines [Internet]. [place unknown]: Bureau of Fisheries and Aquatic Resources; [accessed 2023 Nov 6]. http://repository.seafdec.or.th/handle/20.500.12067/1039.
- R Core Team. 2020. R: A Language and Environment for Statistical Computing [Internet]. https://www.R-project.org/.
- Regan, T. 1983. The Case for Animal Rights. Berkeley and Los Angeles: University of California Press.
- Reisman A, Brown A, Reisman A. 2022. Voices of the Movement: Toward an Equitable Farmed Animal Protection Movement. [place unknown]: EBDI and Encompass.
- Respondek G, Günther C, Beier U, Bleeker K, Pedersen EM, Schulze T, Temming A. 2022. Connectivity of local sub-stocks of Crangon crangon in the North Sea and the risk of local recruitment overfishing. J Sea Res. 181:102173. [CrossRef]
- Ricardo-Pezzuto P, Mastella-Benincà E. 2015. Challenges in licensing the industrial double-rig trawl fisheries in Brazil. Lat Am J Aquat Res. 43(3):495–513. [CrossRef]
- Rodriguez, S. 2018. Food Justice: A Primer. [place unknown]: Sanctuary Publishers.
- Romero Waldhorn D, Autric E. 2022. Shrimp: The animals most commonly used and killed for food production [Internet]. [CrossRef]
- Rowlands, M. 1998. Animal Rights. New York: St Martin’s Press.
- Ruden SAD. 2017. Slaves Are Catching Our Shrimp. Commonweal. 144(13):8–9.
- Sampantamit T, Noranarttragoon P, Lachat C, Goethals P. 2019. Evolution of Fish and Shellfish Supplies Originating from Wild Fisheries in Thailand Between 1995 and 2015. Sustain Sci Pract Policy. 11(24):7198. [CrossRef]
- Saraswathy R, Muralidhar M, Balasubramanian CP, Rajesh R, Sukumaran S, Kumararaja P, Dayal JS, Avunje S, Nagavel A, Vijayan KK. 2021. Osmo-ionic regulation in whiteleg shrimp, Penaeus vannamei, exposed to climate change-induced low salinities. Aquac Res. 52(2):771–782. [CrossRef]
- Scherer L, Tomasik B, Rueda O, Pfister S. 2018. Framework for integrating animal welfare into life cycle sustainability assessment. Int J Life Cycle Assess. 23(7):1476–1490. [CrossRef]
- Seo, HJ. 2018. Trapped at sea: Blood, sweat, and tears of Thailand’s fishing industry. Harvard Int Rev. 39(2):44–47.
- Shon S, Harper S, Zeller D. 2014. Fisheries Centre. Fisheries Centre [Internet]. https://www.flyamritsar.com/doc/publications/wp/2014/Shon-et-al-South-Korea.pdf.
- Simões SM, Heckler GS, Costa RC. 2017. Reproductive period and recruitment of Penaeoidea shrimp on the southeastern Brazilian coast: implications for the closed season. Crustaceana. 90(7-10):1177–1192. [CrossRef]
- Singer, P. 1975. Animal Liberation: A New Ethics for Our Treatment of Animals. New York: New York Review.
- Singer, P. 2023. Animal Liberation Now. [place unknown]: Random House.
- Singer P, Shih Chao-hwei. 2023. The Buddhist and the Ethicist. Boulder, CO: Shambhala Publications.
- Skov C, Berg S, Eigaard OR, Jessen TK, Skov PV. 2020. Danish fisheries and aquaculture: Past, present, and future. Fisheries. 45(1):33–41. [CrossRef]
- Sokolov K, Tyukina O, Martynova D. 2020. Contemporary issues of commercial invertebrates’ harvesting in the Russian sector of the Barents Sea. KnE Life Sci.:547–557. [CrossRef]
- Stefanny, Pamungkaningtyas FH. 2023. Shrimp paste: different processing and microbial composition across Southeast Asia. IOP Conf Ser Earth Environ Sci. 1169(1):012089. [CrossRef]
- Stephenie DK, Chen CA, Hassan R, Mustafa S, Shapawi R, Halid NFA. 2021. Analysis of past 26 years landing data to understand the status of Acetes spp. populations in Malaysia. IOP Conf Ser Earth Environ Sci. 718(1):012060. [CrossRef]
- Stien LH, Bracke M, Noble C, Kristiansen TS. 2020. Assessing Fish Welfare in Aquaculture. In: Kristiansen TS, Fernö A, Pavlidis MA, van de Vis H, editors. The Welfare of Fish. Cham: Springer International Publishing; p. 303–321.
- Stringer C, Burmester B, Michailova S. 2022. Modern slavery and the governance of labor exploitation in the Thai fishing industry. J Clean Prod. 371:133645. [CrossRef]
- Suuronen P, Pitcher CR, McConnaughey RA, Kaiser MJ, Hiddink JG, Hilborn R. 2020. A Path to a Sustainable Trawl Fishery in Southeast Asia. Reviews in Fisheries Science & Aquaculture. 28(4):499–517. [CrossRef]
- Sylwester, JG. 2014. Fishers of men: the neglected effects of environmental depletion on labor trafficking in the Thai fishing industry. Pac Rim L & Pol’y J [Internet]. https://heinonline.org/hol-cgi-bin/get_pdf.cgi?handle=hein.journals/pacrimlp23§ion=16.
- Szep J, Grudgings S. 2013. Authorities implicated in Rohingya smuggling networks. Reuters (July 17, 2013) Available at http://www pulitzer org/files/2014/international-reporting/reuters/01reuters2014 pdf [Internet]. https://www.pulitzer.org/files/2014/international-reporting/reuters/01reuters2014.pdf.
- Taberna A, Jr. 2019. A Comparative Study on Small-scale Fisheries between South Korea and Philippines [Internet]. https://repository.pknu.ac.kr:8443/handle/2021.oak/23050.
- Temming A, Bönisch A, Hagen W, Brenneken C, Dänhardt A. 2022. Unexpected high discard mortalities of juvenile brown shrimp (Crangon crangon) in the North Sea shrimp fishery. Fish Res. 252:106354. [CrossRef]
- Thanh Viet Nguyen. 2011. Sustainable Management of Shrimp Trawl Fishery in Tonkin Gulf, Vietnam. Applied Economics Journal. 18(2):65–81. [CrossRef]
- Thong Ba Nguyen. 2015. Study on trawl fishery Socio-economics and supply chains in Kien Giang, Viet Nam [Internet]. [place unknown]: Training Department, Southeast Asian Fisheries Development Center; [accessed 2023 Nov 6]. http://repository.seafdec.or.th/handle/20.500.12067/1450.
- Thorpe A, Andrew NL, Allison EH. 2007. Fisheries and poverty reduction. CAB Reviews: Perspectives in Agriculture, Veterinary Science, Nutrition and Natural Resources [Internet]. 2007-2(85). [CrossRef]
- Tickler D, Meeuwig JJ, Palomares M-L, Pauly D, Zeller D. 2018. Far from home: Distance patterns of global fishing fleets. Sci Adv. 4(8):eaar3279. [CrossRef]
- Torres Coll, RJ. 2013. Research and management of competing small-scale and industrial fisheries: a modeling study of the shrimp fisheries in Mozambique [Masters] [Internet]. [place unknown]: Arctic University of Norway. https://munin.uit.no/handle/10037/5914.
- Tran DD, Cao HV, Dinh QM. 2020. An assessment of fisheries resources in the coastal water of the Mekong Delta, Vietnam. Aquarium Sci Conserv. 13(6):3683–3693.
- Tran Thi Phung, Ha. 2012. Resilience and livelihood dynamics of shrimp farmers and fishers in the Mekong Delta, Vietnam [Doctoral] [Internet]. Visser LE, van Dijk JWM, Le XuanSinh, editors. [place unknown]: Wageningen University. https://search.proquest.com/openview/696220f3e29120759fcc41aea7e36748/1?pq-origsite=gscholar&cbl=2026366&diss=y.
- Tully, O. 2017. Atlas of commercial fisheries for shellfish around Ireland [Internet]. [place unknown]: Fisheries Ecosystem Advisory Services, Marine Institute. http://oar.marine.ie/handle/10793/1243.
- Tulp I, Chen C, Haslob H, Schulte K, Siegel V, Steenbergen J, Temming A, Hufnagl M. 2016. Annual brown shrimp (Crangon crangon) biomass production in Northwestern Europe contrasted to annual landings. ICES J Mar Sci. 73(10):2539–2551. [CrossRef]
- Turenhout MNJ, Zaalmink BW, Strietman WJ. 2016. Pulse trawling in the Netherlands: economic and spatial impact study [Internet]. [place unknown]: Wageningen Economic Research; [accessed 2023 Nov 6]. https://library.wur.nl/WebQuery/wurpubs/fulltext/396469.
- Ung TD, Tran TYN, Nguyen NL, Phan DA, Tan LV. 2021. Biochemical Characteristics of small shrimp (Acetes japonicus) of varied sizes collected in Ben Tre Province, Vietnam. IOP Conf Ser: Mater Sci Eng. 1092(1):012080. [CrossRef]
- United Nations Environment Programme. 2019. Why is Animal Welfare Important for Sustainable Consumption and Production? [Internet]. [place unknown]: United Nations. https://www.unep.org/resources/perspective-series/issue-no-34-why-animal-welfare-important-sustainable-consumption-and.
- University of Stirling. 2023. OP Validation of operational indicators of consciousness in tropical prawns (Penaeus vannamei). University of Stirling [Internet]. [accessed 2023 Nov 13]. https://www.stir.ac.uk/research/hub/contract/1869801.
- Vasconcellos M, Diegues AC, Kalikoski DC. 2011. Coastal fisheries of Brazil [Internet]. [place unknown]: FAO Fisheries and Aquaculture. https://www.researchgate.net/profile/Regla-Somoza/publication/230838843_Coastal_Fisheries_of_Latin_America_and_The_Caribbean/data/0fcfd505224d1a8f89000000/i1926e.pdf#page=85.
- Verschueren B, Lenoir H, Soetaert M, Polet H. 2019. Revealing the by-catch reducing potential of pulse trawls in the brown shrimp (crangon crangon) fishery. Fish Res. 211:191–203. [CrossRef]
- Vestergaard N, Stoyanova KA, Wagner C. 2011. Cost–benefit analysis of the Greenland offshore shrimp fishery. Acta Agriculturae Scandinavica, Section C — Food Economics. 8(1):35–47. [CrossRef]
- Wahltinez SJ, Stacy NI, Hadfield CA, Harms CA, Lewbart GA, Newton AL, Nunamaker EA. 2022. Perspective: Opportunities for advancing aquatic invertebrate welfare. Front Vet Sci. 9:973376. [CrossRef]
- Wang Z, Tang H, Xu L, Zhang J. 2022. A review on fishing gear in China: Selectivity and application. Aquaculture and Fisheries. 7(4):345–358. [CrossRef]
- Washington S, Ababouch L. 2011. Private standards and certification in fisheries and aquaculture. [place unknown]: FAO.
- Weineck K, Ray AJ, Fleckenstein LJ, Medley M, Dzubuk N, Piana E, Cooper RL. 2018. Physiological Changes as a Measure of Crustacean Welfare under Different Standardized Stunning Techniques: Cooling and Electroshock. Animals (Basel) [Internet]. 8(9). [CrossRef]
- Wickens, S. 2022. Review of the evidence of sentience in cephalopod molluscs and decapod crustaceans. Anim Welf. 31(1):155–156. [CrossRef]
- Wickham, H. 2016. ggplot2: Elegant Graphics for Data Analysis [Internet]. https://ggplot2.tidyverse.org.
- Wickliffe LC, Jodice PGR. 2010. Seabird attendance at shrimp trawlers in nearshore waters of South Carolina. Mar Ornithol. 38:31–39.
- Wolfe JM, Breinholt JW, Crandall KA, Lemmon AR, Lemmon EM, Timm LE, Siddall ME, Bracken-Grissom HD. 2019. A phylogenomic framework, evolutionary timeline and genomic resources for comparative studies of decapod crustaceans. Proc Biol Sci. 286(1901):20190079. [CrossRef]
- Wong HS, Yong CC. 2020. Fisheries regulation: A review of the literature on input controls, the ecosystem, and enforcement in the Straits of Malacca of Malaysia. Fish Res. 230:105682. [CrossRef]
- Wrenn CL, Johnson R. 2013. A Critique of Single-issue Campaigning and the Importance of Comprehensive Abolitionist Vegan Advocacy. Food, Culture & Society. 16(4):651–668. [CrossRef]
- Yamaguchi H, Goto Y, Hoshino N, Miyashita K. 2014. Growth and age composition of northern shrimp Pandalus eous estimated by multiple length frequency analysis. Fish Sci. 80(4):665–678. [CrossRef]
- Yamaguchi H, Nishiuchi S, Takayanagi S, Miyashita K. 2011. Shrimp-pot mesh selectivity for northern shrimp Pandalus eous and the effect on commercial catch of increasing the mesh size of the shrimp pot, off western Hokkaido, the Sea of Japan. Nippon Suisan Gakkaishi. 77(5):809–821. [CrossRef]
- Yaumidin UK, Zuas O. 2021. ECO-LABELING AND INTERNATIONAL TRADE AGREEMENTS: THE CASE OF MARINE STEWARDSHIP COUNCIL CERTIFICATION FOR INDONESIA’S SHRIMP …. Buletin Ilmiah Litbang Perdagangan [Internet]. http://jurnal.kemendag.go.id/bilp/article/view/539. [CrossRef]
- Yoon SC, Jeong YK, Zhang CI, Yang JH, Choi KH, Lee DW. 2014. Characteristics of Korean coastal fisheries. Korean Journal of Fisheries and Aquatic Sciences. 47(6):1037–1052.
- Zafar M, Alam. 1997. Occurrence and abundance of Acetes shrimps in the Kutubdia channel of Bangladesh coastal water. Bangladesh J Fish Res. 1(2):91–96.
- Zhang X, Vincent ACJ. 2020. Reconstructing fishing capacity and landings of China’s bottom trawl fisheries (1950-2018). Institute for the Oceans and Fisheries, University of British Columbia.
- 张佳泽, 张胜茂, 王书献, 杨昱皞, 戴阳, 熊瑛. 2022. 基于 3-2D 融和模型的毛虾捕捞渔船行为识别. South China Fisheries Science [Internet]. 18(4). http://www.aquaticjournal.com/fileSCKX/journal/article/nfsckx/2022/4/PDF/20210263-J.pdf.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).