Introduction
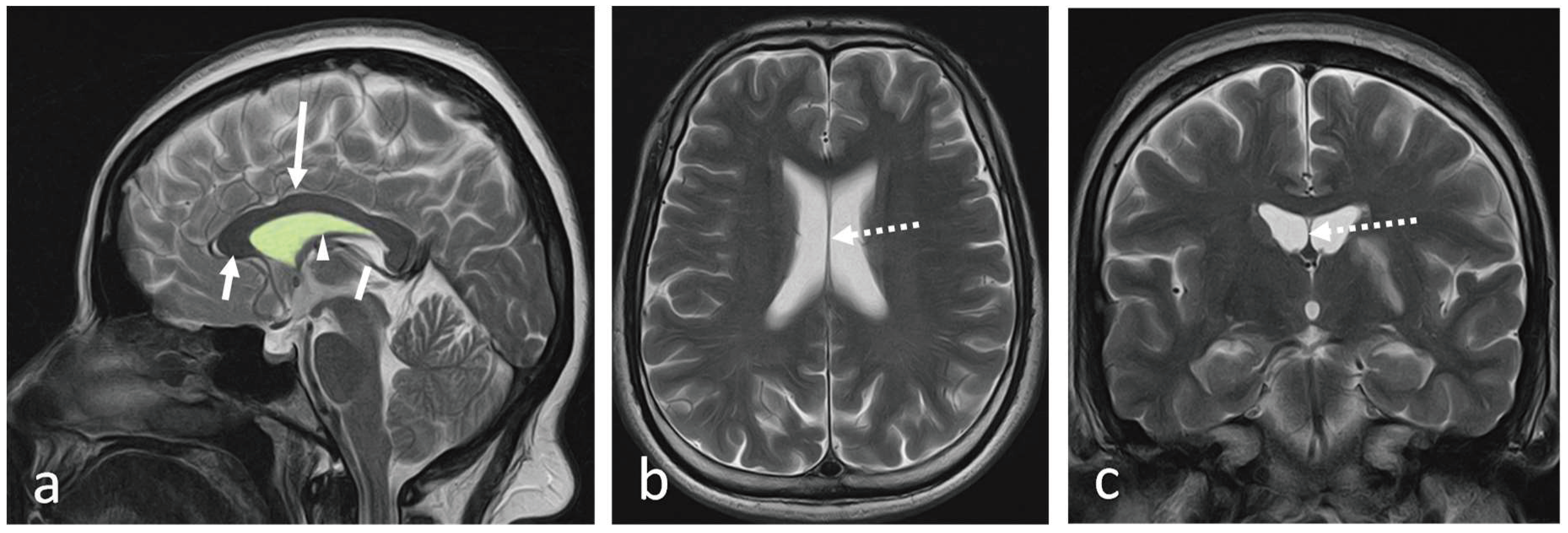
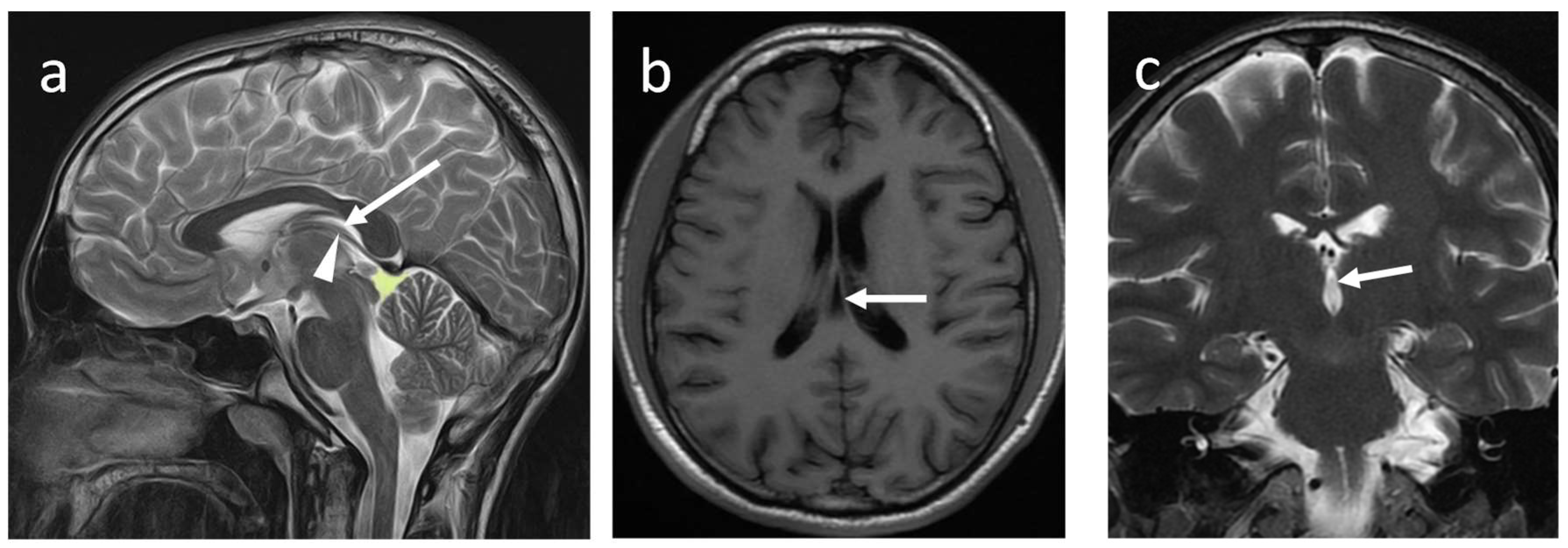
The septum pellucidum (SP) is a thin, translucent, vertical double-membrane sheet of white and grey matter between the anterior horns of the brain's lateral ventricles (1). This triangular membrane extends from the rostrum, genu, and anterior part of the body of the corpus callosum(CC) to the superior surface of the fornix (1)
(Figure 1a-c).
The ependymal cells of the lateral ventricles cover it on either side. Boundaries and relations of the SP are illustrated in
Table 1.
The SP is a double membrane structure with a potential space in-between. The width of SP varies from 1.5 to 3.0 mm (2). The SP belongs to the limbic system and consists of glial cells, fascicles, several scattered neurons, and veins that connect to the veins of the choroid plexus (1). It connects the hypothalamus to the amygdala, hippocampus, habenula, and the reticular formation of the brain stem, serving as a relay station (3).
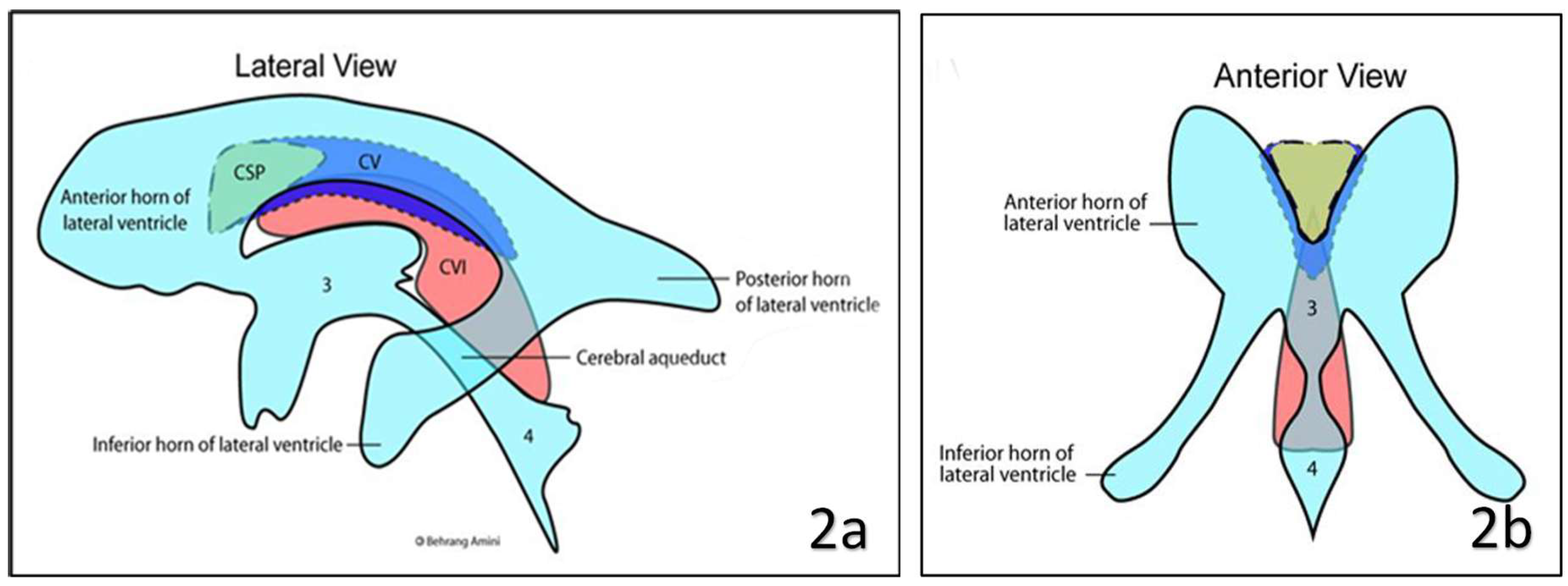
Anatomical variations and clinical significance have been described in the literature. There are three types of variations of SP exits naming cavum septum pellucidum (CSP), cavum vergae (CV) and cavum veli interpositi (CVI)
(Figure 2 and Figure 3). The objective of this article is to describe the gross and radiological anatomy of these variations and the clinical implications of variations.
Cavum septum pellucidum (CSP)
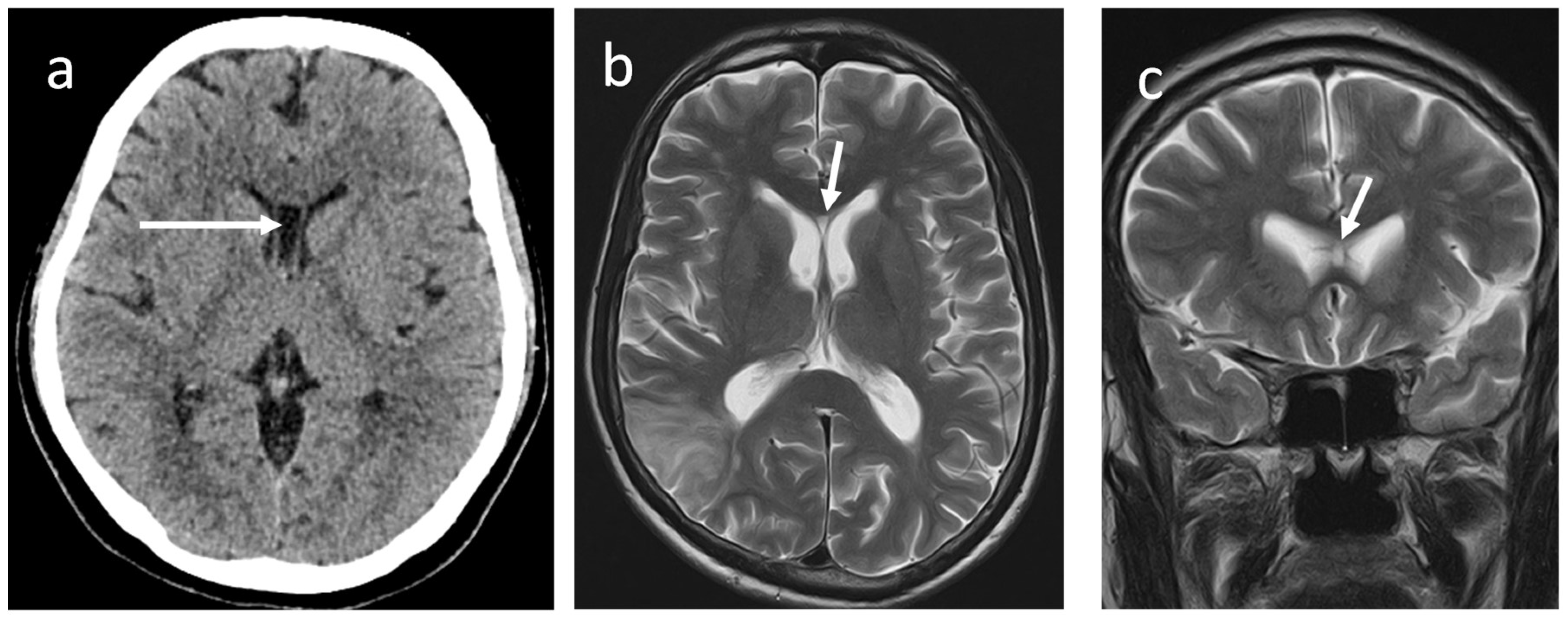
The development of the SP begins early intrauterine life with the development of the fetal brain. During the first trimester of pregnancy, the SP arises due to the folding and growth of the neural tissue in the midline. By the latter part of the first trimester or beginning of the second trimester of pregnancy, it consists of two parallel sheets which project downward from the roof of the developing brain to form a fluid-filled pocket-like closed vertically oriented compartment called CSP. The CSP becomes visible ultrasonographically from the second trimester and obliterates between 36 and 40 weeks of gestation. Based on cranial ultrasonography, Mott
et al. demonstrated that CSP is detected in all normal infants under 36 weeks of gestational age. They further described the prevalence of CSP at 36, 38, and 40 weeks of gestation, in about 69%, 54%, and 36% of foetuses, respectively (4).Two leaflets gradually fuse each other, starting anteriorly and progressing posteriorly to obliterate the cavity, resulting in a fully formed septum pellucidum. This fusion occasionally delays up to 24 months after birth and in some individuals, it may persist throughout adult life. These midline intracranial cysts are more common than expected due to frequent detection using CT and MRI brains up to 72 % in healthy adults (5)
(Figure 3a-c).The boundaries of CSP are described in
Table 2 (6).
In sagittal and coronal sections, it is triangular, and the base is at the corpus callosum. This cavity does not communicate with the lateral ventricles, which are located on either side of it. The CSP contains a concentration of cerebrospinal fluid that filters through the septal laminae. This fluid is reabsorbed by capillaries and veins of the septal leaflets (7).
Cavum vergae (CV)
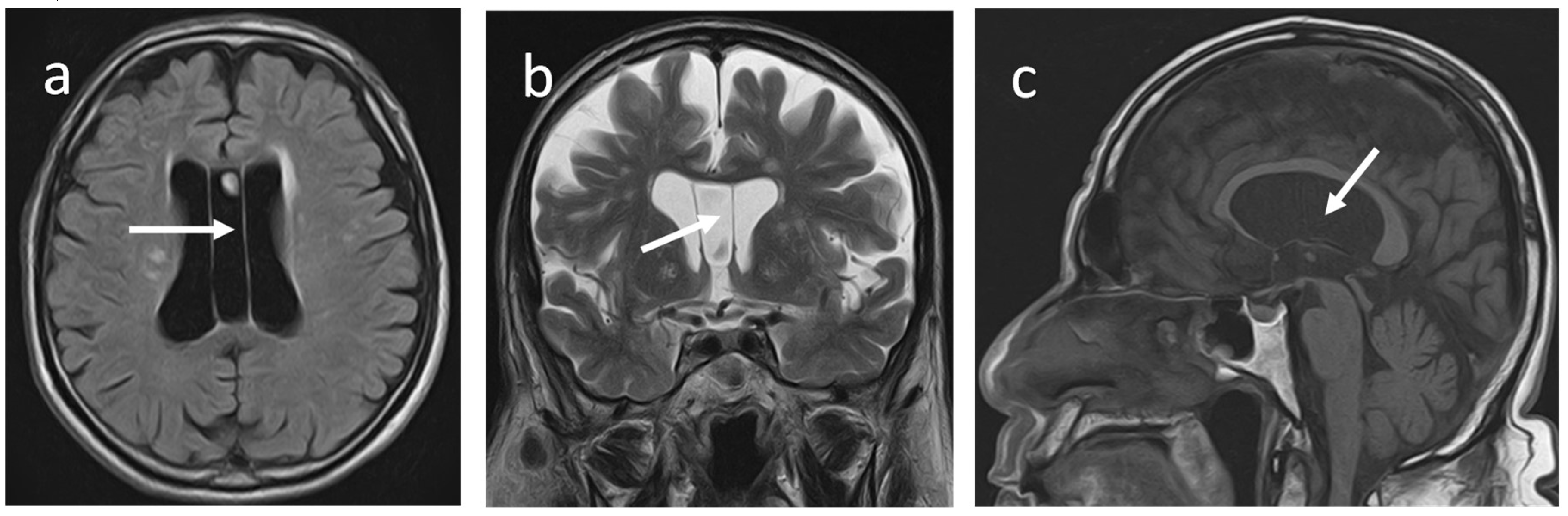
An Italian anatomist and psychiatrist first described this fluid-filled midline space (Andrea Verga: 1811 -1895), also known as a Verga´s ventricle. The CV is a horizontal cleft between the commisura fornicis (or psalterium) and the corpus callosum when the two commissural plates fail to fuse completely during foetal development (6). It is also considered a posterior extension of the CSP. According to some authors, CV does not occur without CSP, and vice versa it exists. (7).
CV has the following boundaries: anteriorly; the anterior limb of the fornix, superiorly; the body of the corpus callosum, posteriorly; the splenium of the corpus callosum, inferiorly; the hippocampal commissure (5).They demonstrated a concurrent prevalence of around 21.1% for CSP and CV in healthy adults using an MRI brain study and isolated CV in 4 % of subjects. Nakano
et al. found that the average prevalence of CV alone in 1,050 children was 0.4% using CT brains (8). However, Schwidde
et al. did not find CV alone in any of the 1,032 CT brains they examined(9). When associated with CSP, the CV is collectively known as the cavum septum pellucidum et vergae (CSPV)
(Figure 4a-c).
Cavum velum interpositum (CVI)
The velum interpositum space is a triangular shape midline potential space, and when this space is expanded, it is called CVI
(Figure 5a-c).
This CSF-filled space is an anterior continuation of the quadrigeminal plate cistern. The CVI is located between two layers of the tela choroidea. The inferior layer of the tela choroidea attaches to the roof of the third ventricle, and the superior layer attaches to the inferior column of the fornix and the hippocampal commissure (psalterium). Anteriorly, tela choroidea fuses at the level of the foramen of Monro and forms the apex of triangular CVI. Its base or posterior end remains open and communicates with the quadrigeminal cistern, superior to the pineal gland (10,11,12). CVI is closely related to the internal cerebral veins and the posterior medial choroidal artery. A layer of ependymal cells does not line this space or contribute to CSF production. Therefore, it is not considered a part of the cerebral ventricular system (10).
The CVI disappears between the last two months of pregnancy and up to the 3rd year of postnatal life. Using ultrasonography, Chen et al. demonstrated a prevalence of CVI of 21 % in preterm infants (12). Another study performed at Yas women's Hospital, Teheran found the prevalence of CVI in the second trimester was 18–23%, and this was nearly similar to its prevalence in infancy (13).It is rarely seen in adults; however, based on MRI brains, Oktem et al. demonstrated a prevalence of 1% among adults aged between 18 and 80 years (3). A CT examination conducted on 442 adults revealed that the occurrence rate of CVI was 7.24% (6).
Radiological Features of Anatomical Variations of Septum Pellucidum
Sonographic imaging, Magnetic Resonance (MR) and Computed Tomography (CT) have been utilised as imaging techniques to identify SP and its variations.
Ultrasonic evaluation of the brain
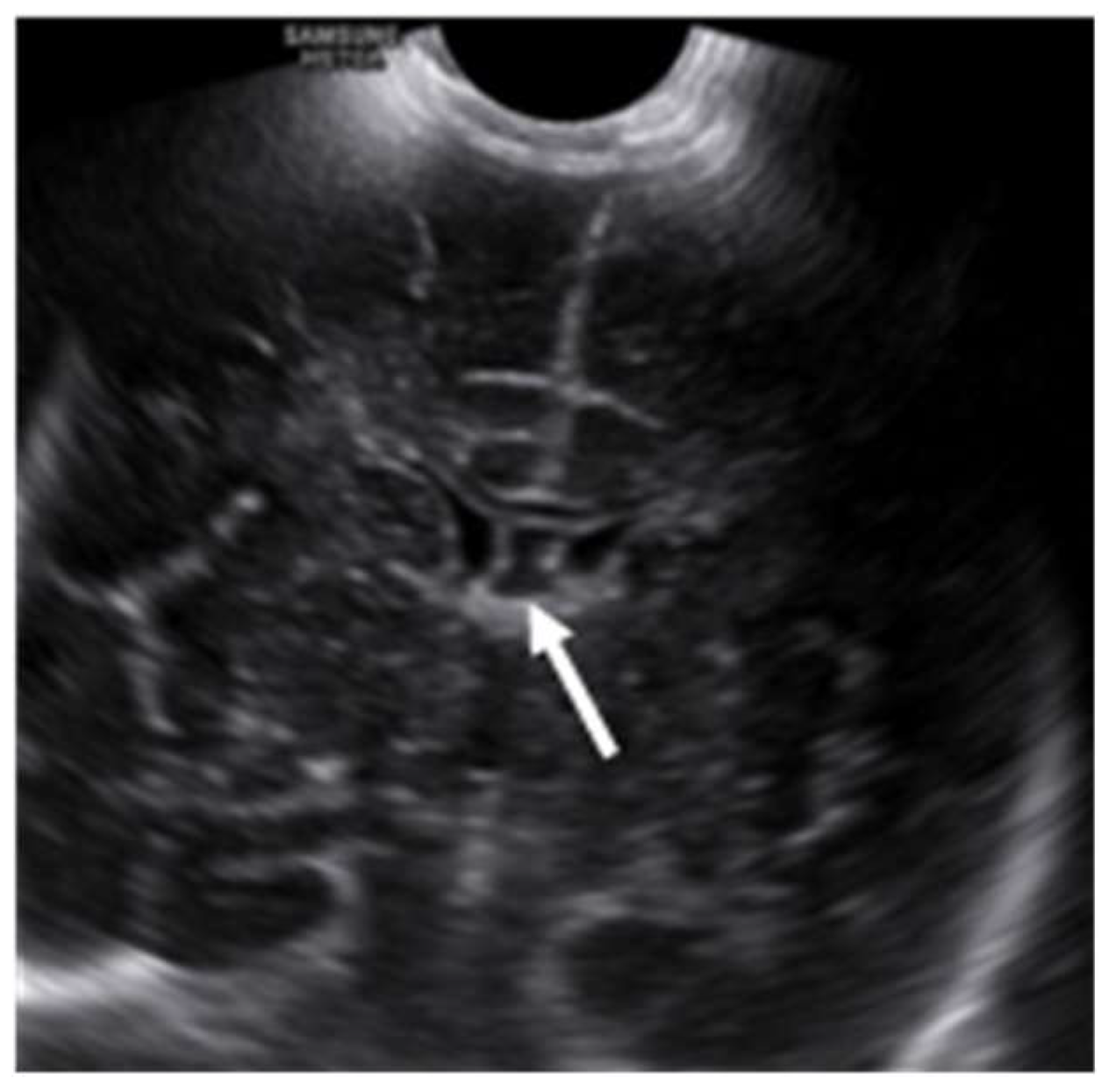
Ultrasonography (USG) is an extremely useful tool in the evaluation of foetal and infants’ brain during the latter two-thirds of the pregnancy to the first year of post-natal life. It is a cost-effective, non-invasive, easily accessible bedside test with a real-time imaging facility and a lack of ionizing radiation. In the post-natal period, SP and its variations are evaluated with axial and sagittal images through the anterior fontanelle with a symmetrical calvarium. Ultrasonically, the SP appears as a midline hyperechoic line inferior to the corpus callosum, separating the anterior horns of the lateral ventricles. The CSP appears as a fluid-filled box between the paired septa, which separates the CSP from the frontal horns of the lateral ventricles
(Figure 6).
Winter et al. described the normal CSP on sonographic imaging as a fluid-filled low echogenic box between the echogenic paired septa, which separates the CSP from the frontal horns of the lateral ventricles (14). Jou et al. have shown that the width of CSP ranged from 2.0 mm to 10.0 mm with a mean width of 5.50 ± 1.48 mm after studying about 608 consecutive fetuses between 19 and 42 weeks of age (15).
The CSPV is generally rectangular, with a posterior border extending past the midpoint of the brain (16). Tao et al. investigated 322 uncomplicated pregnancies from 25 to 39 weeks of gestation and showed that the width of CSPV ranged from 5.1mm to 9 mm with mean width is 6.7 ± 1.0 mm (16). The CVI may appear as an anechoic cystic lesion inferior to the splenium of the corpus callosum and above the internal cerebral veins in the pineal region on midsagittal neonatal ultrasound. It shows the characteristic shape of an inverted helmet with a mean anteroposterior diameter ranging from 3 mm to 10 mm with an average of 5.6mm (12).
CT/MRI evaluation of the brain
The MR/CT findings of the SP and its variations are straightforward and represented by the presence of a fluid-filled cavity between the two leaflets of the SP. Therefore, this will follow CSF signal intensity in all the MRI sequences. This is characterised by low signal intensity on T1W, high signal intensity on T2W and suppression of signal in FLAIR sequences. The rapid advancement of in utero magnetic resonance (iuMR) imaging techniques has increased the prenatal detection of abnormalities in the corpus callosum, such as the absence of the corpus callosum, a narrow or wide corpus callosum, and abnormal shape of the corpus callosum (17).After conducting a study on 200 iuMR in foetuses ranging from 18 to 37 weeks of gestational age, Javis et al. concluded that the CSPV length constantly increased throughout pregnancy. This increase likely implies the rapid expansion of the cerebral hemispheres during the second and third trimesters. On the other hand, the width and volume of the CSPV peaked between 29 and 31 weeks of gestational age and subsequently decreased during the later stages of pregnancy. They further stated that the width of the cavum septi pellucidi is larger than the width of the cavum vergae at each stage of gestation. Born et al. studied three age groups involving 151 healthy subjects. The study revealed that the length of the SP was measured to be 28 ± 6.9 mm in children, 35 ± 7.3 mm in young adults, and 37 ± 5.0 mm in elderly adults (mean ± SD) (18).Tsutsumi et al. applied the Coronal Interference Steady State (CISS) sequence, a gradient-echo MR imaging technique, to precisely delineate a wide range of pathologies. This method proves valuable when standard MR imaging sequences fail to provide adequate anatomical details and enhanced sensitivity in diagnosing conditions such as CSP, CV, and CVI (19).
The CV should be differentiated from the Cavum Veli Interpositi at the roof of the third ventricle. The two can be readily distinguished on sagittal MR/CT images: the Cavum Veli Interpositi lies inferior to the fornices whilst the CV lies superiorly; it is characteristically triangular with a broad base dorsally that converges anteriorly at the interventricular foramina (6).
Pathological Associations
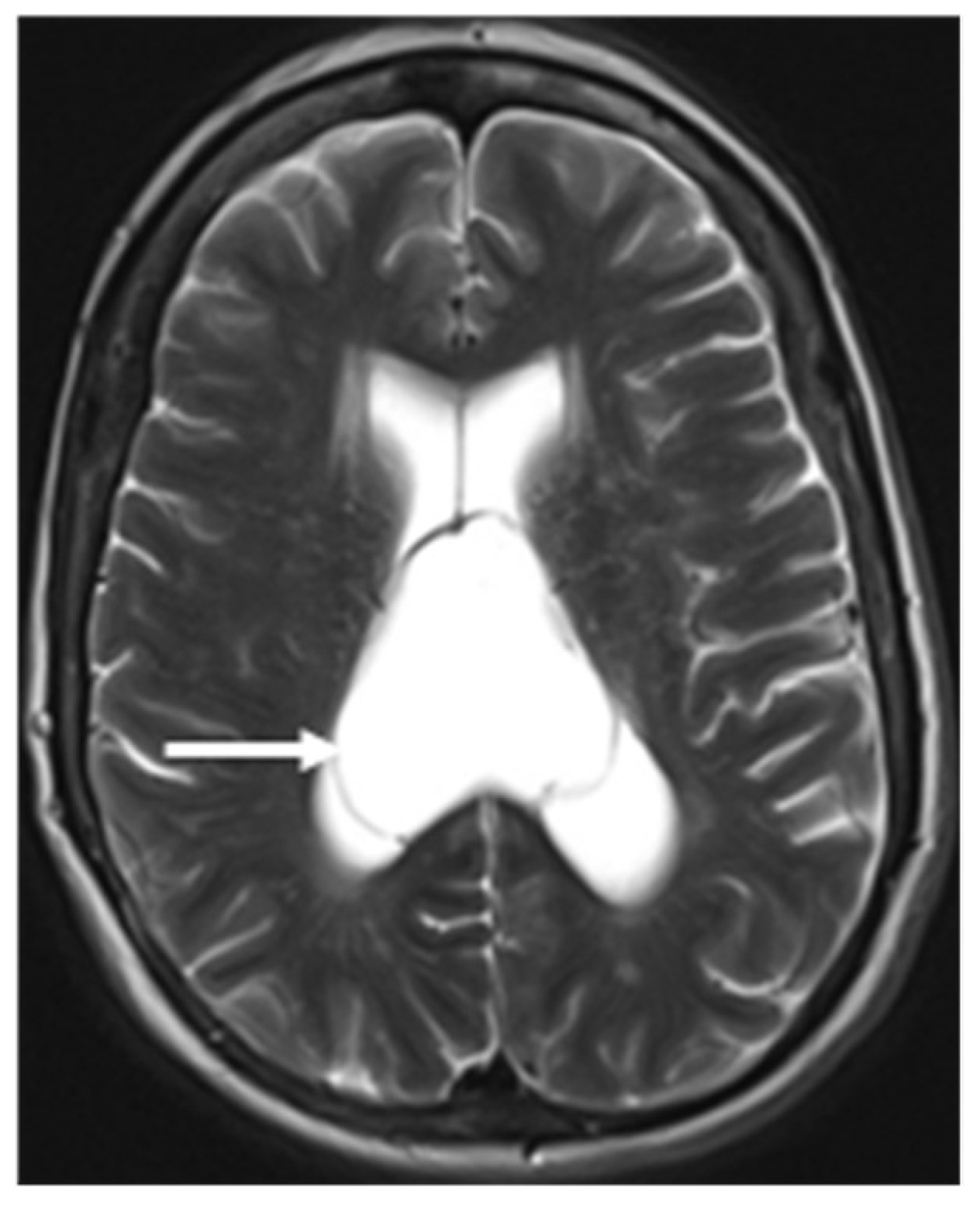
A congenital absent SP can be due to a primary developmental failure or a secondary disruption (
Figure 7).
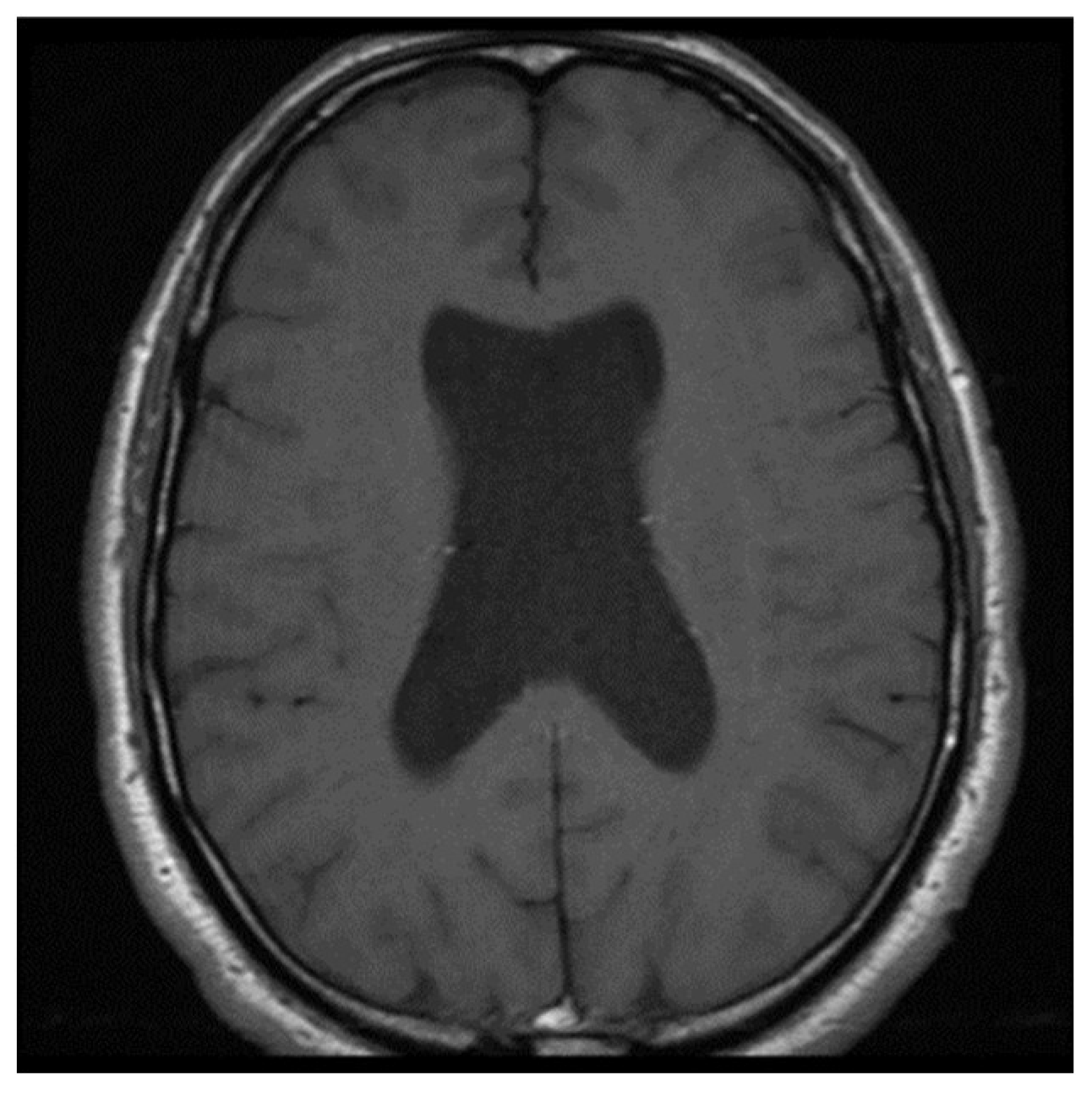
Barkovich
et al. demonstrated in a case series that absence of septum pellucidum (ASP) was an indicator of additional brain anomalies such as septooptic dysplasia (SOD), schizencephaly, holoprosencephaly, agenesis of the corpus callosum, porencephaly/hydranencephaly, basilar encephaloceles, Chiari II malformation and aqueductal stenosis (19). Associations with Alexander disease, tuberous sclerosis, phakomatosis, pinealoma, trisomy 21 are also seen (20).When the transverse diameter of CSP is more than 10 mm and shows the lateral bulging of the septal leaflets, it is referred to as a cyst of the CSP
(Figure 8).
However, if no symptoms are associated with this finding, it is considered an anatomical variation (21). A large CSP cyst obstructing the foramen of Monro has been reported as causing symptomatic obstructive hydrocephalus (22).Furthermore, larger CSP cysts can be linked to acute headaches and multiple cranial nerve palsies (23). A meta-analysis conducted by Wang et al. found that individuals with mental disorders had a significantly higher occurrence of CSP of any size compared to healthy comparison subjects. However, no significant difference in the prevalence of CSP was observed between patients with schizophrenia spectrum and mood disorders and psychiatrically healthy comparison subjects (24). Boxers exhibit a larger CSP and a more posterior extent than individuals in the control group due to abrupt elevations in intracranial pressure, which cause the CSF to pass through minor defects in the septal leaflets. This results in an enlargement in the size and or extent of the CSP (25).
Conclusion
The SP(SP) and its variations, namely the cavum SP(CSP), cavum vergae (CV), and cavum veli interpositi (CVI), are in the midline of the brain. These variants are linked to a range of structural and functional abnormalities in the brain. The anatomy and variations of the SP can be detected using pre and post-natal imaging techniques, such as ultrasound, MRI, and CT scans. Knowledge about its normal formation, gross and radiological anatomy, and pathological spectrum is essential when managing these pathological conditions.
Author Contributions
SRS formulated the concept, designed the review, conducted the literature review, and wrote the manuscript. CKP conducted the literature review and wrote the manuscript. All authors reviewed the manuscript.
Funding
This review received no external funding.
Availability of Data and Materials
Acquired DICOM medical images during the current review are available from the corresponding author upon reasonable request. The subject's identifying information was removed in radiology images.
Acknowledgements
The author acknowledges the Management and Radiographers of Leesons Hospital, Ragama, Sri Lanka.
Conflicts of Interest
The authors declare no conflict of interest.
Ethical Approval
Ethical approval was not considered, as our work is a narrative review. However, we have secured informed consent to publish radiological images from each patient (Image numbers:
Figure 1 a-c,
Figure 3a-c,
Figure 4 a-c,
Figure 5a-c,
Figure 6). For
Figure 2a,
Figure 2b,
Figure 7, and
Figure 8, image credit has been provided, and references are mentioned at the end of the image caption. Additionally, we have intentionally removed identifying information from the radiology images. Consent was obtained in the local language, and fully completed consent forms are available upon request.
References
- Sarwar M. Review Article The Septum Pellucidum: Normal and Abnormal. Am J Neuroradiol. 1989; 10, 989–1005.
- Griffiths PD, Batty R, Reeves MJ, Connolly DJA. Imaging the corpus callosum, septum pellucidum and fornix in children: Normal anatomy and variations of normality. Neuroradiology 2009, 51, 337–45. [CrossRef]
- Oktem H, Dilli A, Kurkcuoglu A, Pelin C. Prevalence of Septum Pellucidum Variations: A Retrospective Study. OALib 2018, 05, 1–9.
- Mott SH, Bodensteiner JB, Allan WC. The Cavum Septi Pellucidi in Term and Preterm Newborn Infants. J Child Neurol 1992, 7, 35–8. [CrossRef]
- Born CM, Meisenzahl EM, Frodl T, Pfluger T, Reiser M, Möller HJ, et al. The septum pellucidum and its variants: An MRI study. Eur Arch Psychiatry Clin Neurosci 2004, 254, 295–302.
- Saba L, Anzidei M, Raz E, Suri J, Piga M, Grassi R, et al. MR and CT of Brain’s Cava. J Neuroimaging 2013, 23, 326–35.
- Oteruelo, FT. On the cavum septi pellucidi and the cavum Vergae. Anat Anz 1986, 162, 271–278. [Google Scholar]
- Nakano, S., Hojo, H., Kataoka, K., & Yamasaki, S. Age Related Incidence of Cavum Septi Pellucidi and Cavum Vergae on CT Scans of Pediatric Patients. Journal of Computer Assisted Tomography 1981, 5, 348–349.
- Schwidde, JT. Incidence of cavum septi pellucidi and cavum Vergae in 1,032 human brains. AMA Arch Neurol Psychiatry 1952, 67, 625–632. [Google Scholar] [CrossRef]
- Giussani C, Fiori L, Trezza A, Riva M, Sganzerla EP. Cavum veli interpositi: Just an anatomical variant or a potentially symptomatic CSF compartmentalization? Pediatr Neurosurg 2012, 47, 364–8.
- Tubbs RS, Krishnamurthy S, Verma K, Shoja MM, Loukas M, Mortazavi MM, et al. Cavum velum interpositum, cavum septum pellucidum, and cavum vergae: A review. Child’s Nerv Syst 2011, 27, 1927–30. [CrossRef]
- Chen CY, Chen FH, Lee CC, Lee KW, Hsiao HS. Sonographic characteristics of the cavum velum interpositum. Am J Neuroradiol 1998, 19, 1631–5.
- Moradi B, Rahmani M, Kia K, Kazemi MA, Tahmasebpour AR. Cavum velum interpositum cysts in normal and anomalous fetuses in second trimester of pregnancy: Comparison of its size and prevalence. Taiwan J Obstet Gynecol [Internet] 2019, 58, 814–9. [CrossRef]
- Winter TC, Kennedy AM, Byrne J, Woodward PJ. The cavum septi pellucidi: Why is it important? J Ultrasound Med 2010, 29, 427–44.
- Jou HJ, Shyu MK, Wu SC, Chen SM, Su CH, Hsieh FJ. Ultrasound measurement of the fetal cavum septi pellucidi. Ultrasound Obstet Gynecol 1998, 12, 419–21. [CrossRef]
- Tao G, Lu G, Zhan X, Li J, Cheng L, Lee K, et al. Sonographic appearance of the cavum septum pellucidum et vergae in normal fetuses in the second and third trimesters of pregnancy. J Clin Ultrasound 2013, 41, 525–31. [CrossRef]
- Cooper S, Katorza E, Berkenstadt M, Hoffmann C, Achiron R, Bar-Yosef O. Prenatal abnormal width of the cavum septum pellucidum – MRI features and neurodevelopmental outcome. J Matern Neonatal Med 2018, 31, 3043–50. [CrossRef]
- Jarvis D, Griffiths PD. Normal appearances and dimensions of the foetal cavum septi pellucidi and vergae on in utero MR imaging. Neuroradiology 2020, 62, 617–27. [CrossRef]
- Tsutsumi S, Ishii H, Ono H, Yasumoto Y. Visualization of the cavum septi pellucidi, cavum Vergae, and cavum veli interpositi using magnetic resonance imaging. Surg Radiol Anat [Internet] 2018, 40, 159–64. [CrossRef]
- Barkovich, A., & Norman, D. (). Absence of the septum pellucidum: a useful sign in the diagnosis of congenital brain malformations. American Journal of Roentgenology 1989, 152, 353–360.
- Siala S, Homen D, Smith B, Guimaraes C. Imaging of the septum pellucidum: normal, variants and pathology. Br J Radiol. 2023, 1–11.
- Shtaya A, Hettige S. Cavum Septum Pellucidum Causing Obstructive Hydrocephalus in a Toddler. Pediatr Neurosurg 2019, 54, 416–8. [CrossRef] [PubMed]
- Oliveira Ferreira DBC. Medeiros JW, Cwajg E, Ferreira-Pinto PHC, Carvalho LU, Nigri F. Cavum septum pellucidum and vergae cyst: A symptomatic case with intracranial hypertension and multiple nerve involvement. Surg Neurol Int. 2022, 13, 564. [Google Scholar] [CrossRef] [PubMed]
- Wang LX, Li P, He H, Guo F, Tian P, Li C, et al. The prevalence of cavum septum pellucidum in mental disorders revealed by MRI: A meta-analysis. J Neuropsychiatry Clin Neurosci 2020, 32, 175–84. [CrossRef]
- Aviv RI, Tomlinson G, Kendall B, Thakkar C, Valentine A. Cavum Septi Pellucidi in Boxers. Can Assoc Radiol J [Internet] 2010, 61, 29–32.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).