3.2. Experimental Section
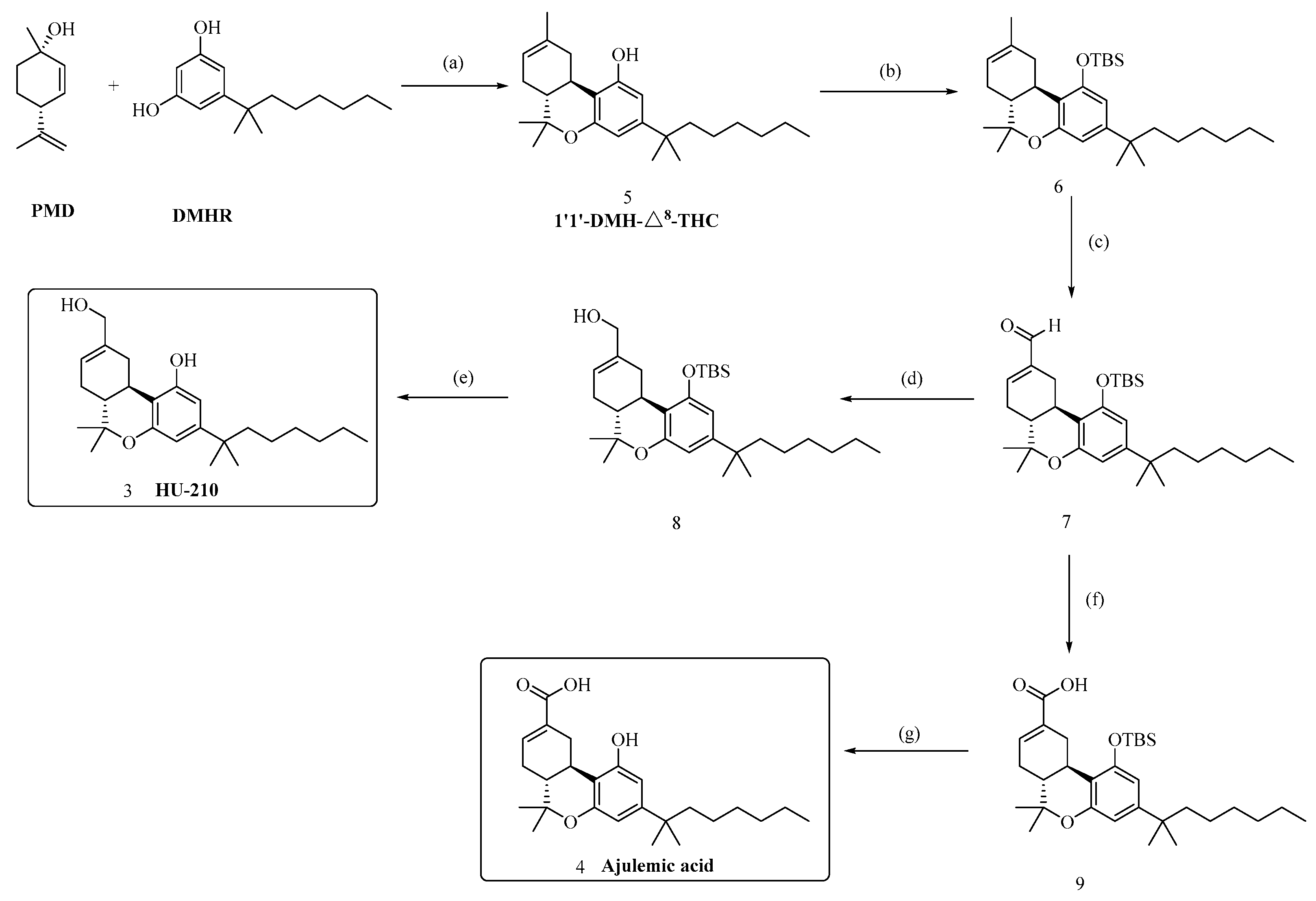
3.2.1. Synthesis of compound (5)
To a 3-neck round bottom flask was charged DMHR (5 g, 21.18 mmol, 1 equiv), p- toluenesulfonic acid (0.728 g, 3.83 mmol, 0.2 equiv) and toluene (150 ml). To this was added PMD (3.54 g, 23.29 mmol, 1.1 equiv) over 1 h, followed by a toluene (8 ml) rinse while maintaining the batch temperature at 15–30 °C. The batch was heated to 70–80 °C under partial vacuum, and a Dean–Stark trap filled with toluene was used to remove water azeotropically. And the reaction was quenched by addition of a saturated solution of NH4Cl (15 ml). and the mixture was extracted with EtOAc (3 × 50 mL). The combined organic layers were washed with brine (20 mL), and dried with Na2SO4.The solvent was removed in vacuum, and the residue was purified by a flash chromatography on silica gel (hexane/ethyl acetate = 50/1) to give 5 (6.45 g, 82% yield) as a yellow oil. Rf = 0.7 (silica gel, EtOAc/hexanes= 1/10). 1H NMR (600 MHz, Chloroform-d) δ 6.40 (d, J = 1.8 Hz, 1H), 6.23 (d, J = 1.8 Hz, 1H), 5.44 (dt, J = 5.0, 1.4 Hz, 1H), 3.20 (dt, J = 17.2, 3.0 Hz, 1H), 2.75 – 2.66 (m, 1H), 2.14 (d, J = 4.1 Hz, 1H), 1.95 – 1.77 (m, 3H), 1.71 (q, J = 1.3 Hz, 3H), 1.50 (ddd, J = 11.3, 6.1, 2.6 Hz, 2H), 1.40 (s, 3H), 1.26 – 1.16 (m, 13H), 1.12 (s, 3H), 1.07 (td, J = 8.9, 8.4, 4.1 Hz, 2H), 0.85 (t, J = 7.1 Hz, 3H). 13C NMR (151 MHz, Chloroform-d) δ 154.65, 154.55, 150.17, 134.90, 119.47, 110.29, 108.17, 105.54, 44.98, 44.61, 37.45, 36.10, 31.93, 31.63, 30.17, 28.88, 28.81, 28.01, 27.75, 24.76, 23.65, 22.82, 18.66, 14.24. IR (film, cm-1): 3384, 2958, 2927, 2856, 1622, 1413, 1034, 965, 838; HRMS (ESI) calcd for C25H38O2 [M+H]+: m/z 371.2945, found: 371.2944.
3.2.2. Synthesis of compound (6)
To a solution of 5 (5.79 g, 15.65 mmol, 1.0 equiv) in dry DMF (100 mL) at room temperature was added dry imidazole (4.75 g, 69.77 mmol, 4.46 equiv) and TBSCl (7.07 g, 46.9 mmol, 3.0 equiv) and the resultant mixture was stirred at the same temperature for 18 h. The mixture was quenched by addition of a saturated solution of NH4Cl (10 mL), and water (200 ml) was added to the mixture. The mixture was extracted with ethyl acetate (3 x 50 mL). Combined organic layers were washed with brine (25 mL), and dried over Na2SO4. The solvent of the organic phase was concentrated in vacuo, and the residue was purified by a flash column chromatography (hexane/EtOAc: 50/1) to give 6 (7.34 g, 97% yield) as a colorless oil. Rf =0.7 (hexane/EtOAc: 20/1). 1H NMR (600 MHz, Chloroform-d) δ 6.41 (d, J = 1.9 Hz, 1H), 6.35 (d, J = 1.9 Hz, 1H), 5.45 – 5.35 (m, 1H), 3.30 – 3.20 (m, 1H), 2.61 – 2.53 (m, 1H), 2.22 – 2.09 (m, 1H), 1.84 – 1.77 (m, 3H), 1.69 (d, J = 2.0 Hz, 3H), 1.50 (ddd, J = 10.3, 5.5, 1.2 Hz, 2H), 1.38 (s, 3H), 1.21 (d, J = 6.4 Hz, 8H), 1.19 (dd, J = 7.1, 3.5 Hz, 4H), 1.10 (s, 3H), 1.07 – 1.03 (m, 2H), 1.01 (s, 9H), 0.84 (t, J = 7.1 Hz, 3H), 0.26 (s, 3H), 0.13 (s, 3H). 13C NMR (151 MHz, Chloroform-d) δ 154.71, 154.30, 149.40, 135.15, 119.36, 114.19, 109.83, 108.66, 45.34, 44.72, 37.44, 36.15, 32.24, 31.97, 31.74, 30.18, 29.06, 28.81, 28.19, 27.64, 26.13, 24.83, 23.54, 22.80, 18.47, 14.27, 14.23, -3.41, -4.24; HRMS (ESI) calcd for C31H52O2Si [M+H]+: m/z 485.3809, found: 485.3818.
3.2.3. Synthesis of compound (7)
To a stirred solution of 6 (5.74 g, 11.84 mmol, 1.0 equiv) in dry dioxane (200 mL) was added SeO2 (4.6 g, 41.46 mmol, 3.5 equiv) at room temperature, and resultant mixture was shielded from light and stirred at the 110 °C for 1 h. The mixture was quenched by filtration off through a celite pad, which was washed with DCM (50 mL). The combined filtrate was washed with saturated aq Na2S2O8 (3 x 20 mL), and the water phase was extracted with DCM (3 x 50 mL). Combined organic layers were washed with brine (50 mL) and dried over Na2SO4. The organic phase was concentrated in vacuo, and the residue was purified by a flash column chromatography (hexane/EtOAc: 10/1 to 4/1) to give 7 (3.82 g, 65% yield) as yellowish solid, Rf = 0.3. (hexane/EtOAc: 10/1). 1H NMR (600 MHz, Chloroform-d) δ 9.50 (s, 1H), 6.83 – 6.77 (m, 1H), 6.41 (d, J = 1.8 Hz, 1H), 6.37 (d, J = 1.8 Hz, 1H), 3.84 (ddd, J = 17.7, 4.3, 2.0 Hz, 1H), 2.60 – 2.48 (m, 2H), 2.19 – 2.10 (m, 1H), 1.90 (td, J = 11.6, 4.7 Hz, 1H), 1.80 (q, J = 2.1 Hz, 1H), 1.52 – 1.48 (m, 2H), 1.43 (s, 3H), 1.20 (dd, J = 15.9, 4.1 Hz, 13H), 1.13 (s, 3H), 1.04 (t, J = 4.0 Hz, 1H), 0.97 (s, 9H), 0.84 (t, J = 7.0 Hz, 3H), 0.28 (s, 3H), 0.13 (s, 3H). 13C NMR (151 MHz, Chloroform-d) δ 193.42, 154.90, 154.06, 149.91, 148.69, 142.57, 112.86, 109.85, 108.43, 45.33, 44.70, 37.50, 31.95, 31.45, 30.16, 29.45, 28.98, 28.85, 27.69, 27.61, 26.08, 24.81, 22.79, 18.46, 18.31, 14.23, -3.43, -4.12. HRMS (ESI) calcd for C31H50O3Si [M+H]+: m/z 499.3602, found: 499.3615.
3.2.4. Synthesis of compound (8)
To a solution of 7 (2.80 g, 5.62 mmol, 1.0 equiv) in MeOH (30 mL) was added NaBH4 (256 mg, 6.74 mmol, 1.2 equiv) at 0 °C in one portion, and the resultant mixture was then stirred at 0 °C for 50 min. The reaction was quenched by addition of an aqueous solution of NH4Cl (10 mL), and the MeOH in resultant mixture was removed under vacuum, and the resultant residue was extracted with ethyl acetate (3 x 10 mL). The combined organic extracts were washed with brine (10 mL), and dried over Na2SO4. The solvent of the extract was concentrated under vacuum, and the residue was purified by a flash column chromatography (hexane/EtOAc: 10/1 to 4/1) to give 8 (2.5 g, 89% yield) as yellowish solid, Rf = 0.5. (hexane/EtOAc: 5/1). 1H NMR (600 MHz, Chloroform-d) δ 6.42 (d, J = 1.9 Hz, 1H), 6.35 (d, J = 1.9 Hz, 1H), 5.76 – 5.71 (m, 1H), 4.08 – 3.99 (m, 2H), 3.41 – 3.32 (m, 1H), 2.59 (td, J = 11.0, 4.5 Hz, 1H), 2.27 – 2.15 (m, 1H), 1.95 – 1.77 (m, 3H), 1.52 – 1.47 (m, 2H), 1.39 (s, 3H), 1.25 – 1.19 (m, 9H), 1.18 (q, J = 3.5 Hz, 3H), 1.11 (s, 3H), 1.07 – 1.02 (m, 2H), 0.99 (s, 9H), 0.84 (t, J = 7.1 Hz, 3H), 0.25 (s, 3H), 0.13 (s, 3H). 13C NMR (151 MHz, Chloroform-d) δ 154.75, 154.23, 149.57, 138.57, 120.42, 113.78, 109.85, 108.64, 76.42, 66.91, 45.55, 44.70, 37.45, 32.00, 31.95, 30.16, 29.03, 28.80, 27.85, 27.62, 26.13, 24.82, 22.79, 18.45, 18.40, 14.23, -3.43, -4.17. HRMS (ESI) calcd for C31H52O3Si [M+H]+: m/z 501.3759, found: 501.3744.
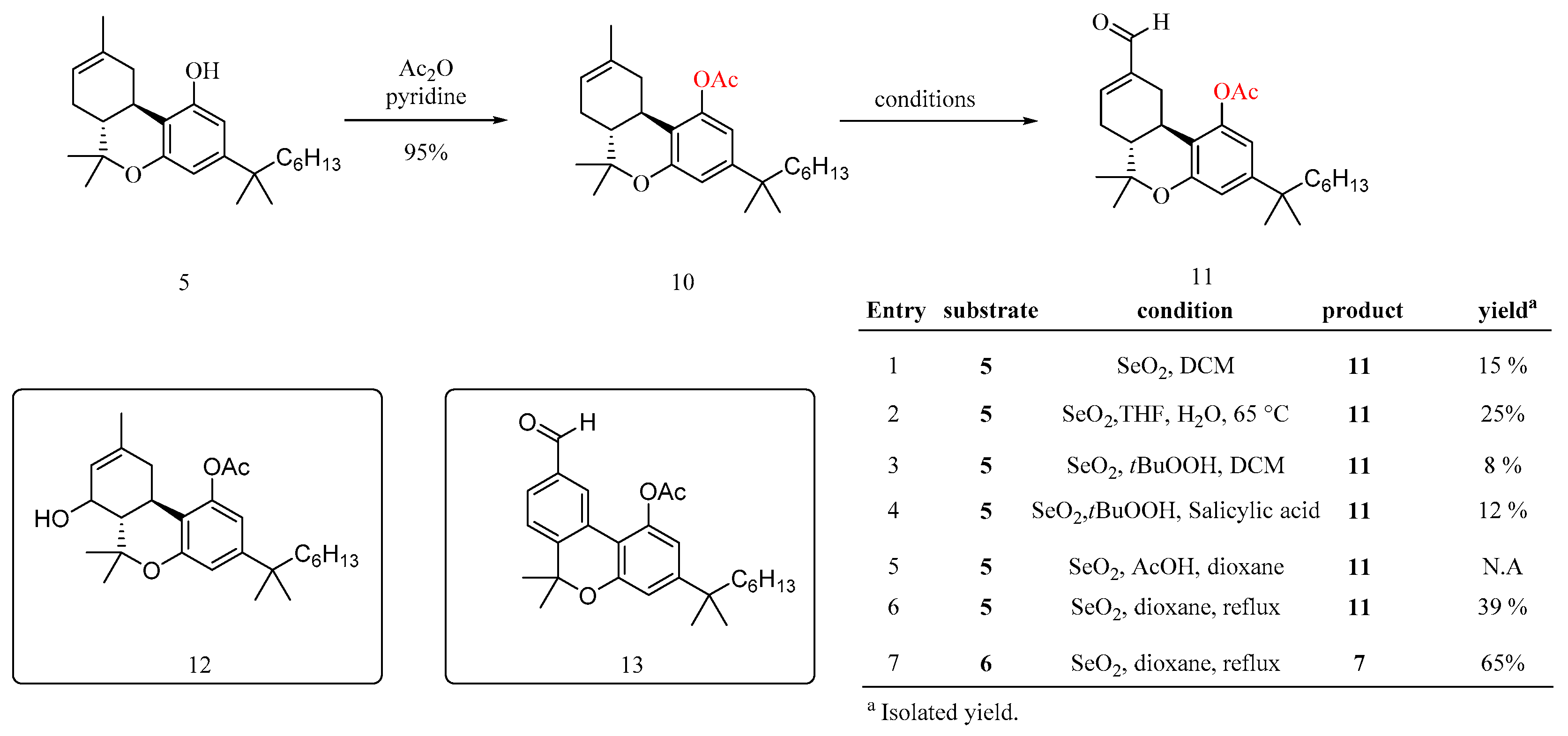
3.2.5. Synthesis of compound (10)
To a solution of 5 (5.6 g, 13.5 mmol, 1.0 equiv) in pyridine (100 mL) was added Ac2O (5.11 ml, 6.74 mmol, 4 equiv) at 0 °C in one portion, and the resultant mixture was then stirred at room temperature for 4 h. The mixture was then added with water (100 mL) and the resultant residue was extracted with ethyl acetate (3 x 30 mL). The combined organic extracts were washed with H2O (3 x 10 mL) and brine (10 mL), and dried over Na2SO4. The solvent of the extract was concentrated under vacuum, and the residue was purified by a flash column chromatography (hexane/EtOAc: 10/1) to give 10 (5.93 g, 95% yield) as yellowish solid, Rf = 0.7. (hexane/EtOAc: 10/1). 1H NMR (600 MHz, Chloroform-d) δ 6.69 (d, J = 2.2 Hz, 1H), 6.52 (d, J = 2.2 Hz, 1H), 5.48 – 5.40 (m, 1H), 2.77 – 2.69 (m, 1H), 2.61 (td, J = 11.0, 5.0 Hz, 1H), 2.30 (s, 3H), 2.17 – 2.11 (m, 1H), 1.97 – 1.89 (m, 1H), 1.83 – 1.77 (m, 2H), 1.70 (s, 3H), 1.52 (dd, J = 8.0, 4.5 Hz, 2H), 1.39 (s, 3H), 1.21 (dd, J = 26.3, 3.2 Hz, 12H), 1.12 (s, 3H), 1.08 (ddd, J = 11.0, 5.2, 2.7 Hz, 2H), 0.85 (t, J = 7.0 Hz, 3H). 13C NMR (151 MHz, Chloroform-d) δ 169.06, 154.31, 150.27, 149.73, 134.01, 119.89, 115.83, 113.19, 112.27, 44.76, 44.57, 37.59, 36.20, 31.88, 31.86, 30.11, 28.75, 28.67, 27.85, 27.60, 24.68, 23.70, 22.80, 21.44, 18.68, 14.23. HRMS (ESI) calcd for C27H40O3 [M+H]+: m/z 413.6215, found: 413.6214.
3.2.6. Synthesis of compound (11)
To a 100-ml, 3-neck round bottom flask was charged compound 10 (1.23 g, 2.98 mmol, 1 equiv), Selenium dioxide (378 mg, 3.41 mmol, 1.25 equiv), tetrahydrofuran (12.2 ml, 4.3 equiv) and water (0.57 ml, 0.2 equiv). The reactor was heated to 55–65 °C, for 23.5 h. The mixture was quenched by filtration off through a celite pad, which was washed with DCM (20 mL). The combined filtrate was washed with saturated aq Na2S2O8 (3 x 10 mL), and the water phase was extracted with DCM (3 x 12 mL). Combined organic layers were washed with brine (15 mL) and dried over Na2SO4. The organic phase was concentrated in vacuo, and the residue was purified by a flash column chromatography (hexane/EtOAc: 10/1 to 4/1) to give 11 (0.32 g, 25% yield) as yellowish solid, Rf = 0.3. (hexane/EtOAc: 10/1), And also to give two byproducts 12 and 13.
Compound 11, 1H NMR (600 MHz, Chloroform-d) δ 9.50 (s, 1H), 6.90 – 6.81 (m, 1H), 6.70 (d, J = 1.7 Hz, 1H), 6.56 (d, J = 2.0 Hz, 1H), 3.43 – 3.34 (m, 1H), 2.57 – 2.54 (m, 1H), 2.30 (s, 3H), 2.16 – 2.10 (m, 1H), 1.91 (td, J = 11.5, 4.5 Hz, 2H), 1.53 – 1.49 (m, 2H), 1.44 (s, 3H), 1.23 (d, J = 2.9 Hz, 8H), 1.19 (t, J = 4.0 Hz, 5H), 1.16 (s, 3H), 1.07 (dq, J = 9.1, 3.7, 3.3 Hz, 2H), 0.84 (d, J = 7.3 Hz, 3H).13C NMR (151 MHz, Chloroform-d) δ 193.43, 169.28, 154.03, 150.83, 149.79, 148.81, 141.65, 114.59, 113.21, 112.67, 44.72, 44.54, 37.66, 31.87, 31.07, 30.09, 29.12, 28.75, 28.63, 27.55, 27.11, 24.67, 22.80, 21.46, 18.55, 14.26, 14.22. HRMS (ESI) calcd for C27H38O4 [M+H]+: m/z 427.2843, found: 427.2838.
Compound 12, 1H NMR (600 MHz, Chloroform-d) δ 6.99 (s, 1H), 6.43 (d, J = 1.9 Hz, 1H), 6.38 (d, J = 2.2 Hz, 1H), 5.68 (dt, J = 5.2, 1.7 Hz, 1H), 5.64 – 5.59 (m, 1H), 3.04 – 2.96 (m, 1H), 2.24 (s, 3H), 2.23 – 2.17 (m, 1H), 2.10 (td, J = 11.3, 3.8 Hz, 1H), 1.97 – 1.90 (m, 1H), 1.52 – 1.48 (m, 2H), 1.40 (s, 3H), 1.23 (s, 3H), 1.20 (s, 12H), 1.08 – 1.04 (m, 2H), 1.02 (s, 3H), 0.84 (d, J = 7.2 Hz, 3H). 13C NMR (151 MHz, Chloroform-d) δ 169.69, 155.11, 151.56, 134.68, 125.64, 108.70, 107.42, 107.09, 79.86, 75.64, 46.69, 44.43, 37.49, 37.35, 31.87, 30.10, 28.93, 28.80, 28.72, 27.55, 24.67, 22.77, 21.19, 19.19, 18.34, 14.22. HRMS (ESI) calcd for C27H40O4 [M+H]+: m/z 429.2999, found: 429.3000.
Compound 14, 1H NMR (600 MHz, Chloroform-d) δ 6.47 (d, J = 2.2 Hz, 1H), 6.33 (d, J = 2.2 Hz, 1H), 5.73 (d, J = 6.1 Hz, 1H), 4.39 – 4.32 (m, 1H), 2.80 (dd, J = 11.8, 8.5 Hz, 1H), 2.22 – 2.13 (m, 1H), 1.97 – 1.89 (m, 1H), 1.89 – 1.82 (m, 1H), 1.79 (d, J = 2.1 Hz, 3H), 1.51 (ddd, J = 11.3, 6.0, 2.4 Hz, 2H), 1.38 (s, 3H), 1.27 – 1.16 (m, 13H), 1.09 (s, 3H), 1.08 – 1.05 (m, 2H), 0.85 (t, J = 7.0 Hz, 3H). 13C NMR (151 MHz, Chloroform-d) δ 155.92, 154.69, 151.21, 134.70, 125.84, 108.23, 107.83, 107.60, 77.51, 77.37, 75.21, 44.89, 44.49, 40.95, 37.39, 31.95, 30.18, 28.78, 27.96, 27.87, 24.77, 22.83, 19.28, 18.64, 14.25. HRMS (ESI) calcd for C25H38O3 [M+H]+: m/z 387.2893, found: 387.2899.
Compound 15, 1H NMR (600 MHz, Chloroform-d) δ 9.94 (s, 1H), 8.57 (d, J = 1.4 Hz, 1H), 7.23 (d, J = 1.5 Hz, 1H), 6.56 (d, J = 1.7 Hz, 1H), 6.43 (d, J = 1.8 Hz, 1H), 5.97 (d, J = 5.0 Hz, 1H), 5.61 (d, J = 3.1 Hz, 1H), 1.76 (s, 6H), 1.55 (dd, J = 8.0, 4.3 Hz, 2H), 1.26 (s, 6H), 1.20 (q, J = 4.9, 4.0 Hz, 6H), 1.09 – 1.06 (m, 2H), 0.84 (s, 3H). HRMS (ESI) calcd for C25H32O4 [M+H]+: m/z 381.2424, found: 381.2428.
3.2.7. Synthesis of compound (3)
To a solution of 8 (2.20 g, 4.40 mmol, 1.0 equiv) in THF (60 mL) was added tetrabutylammonium fluoride (1M in THF, 4.84 mL, 1.1 equiv), and the resultant mixture was stirred at room temperature for 2 h. The reaction was quenched with saturated. aq. NH4Cl (10 mL), and the resultant mixture was extracted with Et2O (3 x 10 mL). The organic layers were sequentially washed with water (5 mL) and then with brine (5 mL), and dried over anhydrous MgSO4. The solvent of the extract was removed under vacuum, the residue was subjected to column chromatography on silica gel (hexane/EtOAc: 2/1) to afford 3 (1.57 mg, 93%) as a white solid. Rf = 0.4 (hexane/EtOAc: 2/1). 1H NMR (600 MHz, Chloroform-d) δ 6.39 (d, J = 1.8 Hz, 1H), 6.24 (d, J = 1.9 Hz, 1H), 5.75 (dd, J = 4.0, 2.4 Hz, 1H), 4.07 (q, J = 12.7 Hz, 2H), 3.43 (dd, J = 15.9, 4.5 Hz, 1H), 2.71 (d, J = 4.6 Hz, 1H), 2.22 (s, 1H), 1.92 – 1.80 (m, 3H), 1.49 (ddd, J = 11.3, 6.1, 2.7 Hz, 2H), 1.40 (s, 3H), 1.28 – 1.14 (m, 13H), 1.11 (s, 3H), 1.09 – 1.02 (m, 2H), 0.85 (t, J = 7.1 Hz, 3H). 13C NMR (151 MHz, Chloroform-d) δ 154.78, 154.50, 150.30, 138.33, 121.98, 110.03, 107.94, 105.75, 67.22, 45.12, 44.61, 37.42, 31.94, 31.50, 31.44, 30.19, 28.86, 28.79, 27.80, 27.71, 24.76, 22.83, 18.53, 14.24. IR (film, cm-1): 3413, 3223, 2924, 1624, 1580, 1415, 1186, 991, 842; HRMS (ESI) calcd for C25H38O3 [M+H]+: m/z 387.2894, found: 387.2891.
3.2.8. Synthesis of compound (9)
To a mixture of aldehyde 7 (2.69 g, 5.39 mmol, 1.0 equiv), NaH2PO4·2H2O (2.59 g, 648 mmol, 4.0 equiv), and 2-methyl-2-butene (3.79 g, 53.9 mmol, 10.0 equiv) in t-BuOH (100 mL) and H2O (25 mL) was added NaClO2 (1.95 g, 648 mmol, 4.0 equiv) at 0 °C. After being stirred for 1 h at that temperature, the mixture was extracted with EtOAc (3 × 40 mL) and H2O (15 mL). The organic layer was separated, dried over Na2SO4, and concentrated to give the residue, which was purified by column chromatography on silica gel (hexane/EtOAc: 20/1) to afford 9 (2.50 g, 90%) as a yellowish oil. Rf = 0.4 (hexane/EtOAc: 10/1). 1H NMR (600 MHz, Chloroform-d) δ 7.19 – 7.14 (m, 1H), 6.44 (d, J = 1.8 Hz, 1H), 6.40 (d, J = 1.9 Hz, 1H), 3.90 (ddd, J = 17.9, 4.4, 2.1 Hz, 1H), 2.57 (td, J = 11.2, 4.3 Hz, 1H), 2.50 – 2.40 (m, 1H), 2.04 (ddt, J = 16.4, 11.9, 2.4 Hz, 1H), 1.97 – 1.90 (m, 1H), 1.86 (td, J = 11.7, 4.6 Hz, 1H), 1.54 – 1.51 (m, 2H), 1.42 (s, 3H), 1.22 (dd, J = 13.7, 5.9 Hz, 13H), 1.13 (s, 3H), 1.10 – 1.04 (m, 2H), 1.01 (s, 9H), 0.86 (t, J = 7.0 Hz, 3H), 0.30 (s, 3H), 0.16 (s, 3H). 13C NMR (151 MHz, Chloroform-d) δ 172.77, 154.84, 154.13, 149.74, 140.24, 130.98, 112.99, 109.81, 108.50, 75.98, 44.69, 44.53, 37.45, 31.93, 31.79, 30.15, 30.06, 29.01, 28.88, 28.85, 27.57, 26.07, 24.80, 22.77, 18.46, 18.26, 14.21, -3.44, -4.11. HRMS (ESI) calcd for C31H50O4Si [M+H]+: m/z 515.3551, found: 515.3559.
3.2.9. Synthesis of compound (4)
To a solution of 9 (1.4 g, 2.72 mmol, 1.0 equiv) in THF (40 mL) was added tetrabutylammonium fluoride (1M in THF, 3.0 mL, 1.1 equiv), and the resultant mixture was stirred at room temperature for 2 h. The reaction was quenched with saturated. aq. NH4Cl (10 mL), and the resultant mixture was extracted with Et2O (3 x 10 mL). The organic layers were sequentially washed with water (5 mL) and then with brine (5 mL), and dried over anhydrous MgSO4. The solvent of the extract was removed under vacuum, the residue was subjected to column chromatography on silica gel (hexane/EtOAc: 10/1) to afford 4 (1.0 g, 92%) as a white solid. Rf = 0.6 (hexane/EtOAc: 10/1). 1H NMR (600 MHz, Chloroform-d) δ 7.17 (dt, J = 5.1, 2.3 Hz, 1H), 6.40 (d, J = 1.8 Hz, 1H), 6.24 (d, J = 1.8 Hz, 1H), 3.84 (ddd, J = 17.8, 4.8, 2.3 Hz, 1H), 2.68 (td, J = 11.3, 4.6 Hz, 1H), 2.50 – 2.38 (m, 1H), 2.10 – 1.95 (m, 2H), 1.85 (td, J = 11.7, 4.6 Hz, 1H), 1.50 (ddd, J = 8.3, 6.2, 3.5 Hz, 2H), 1.42 (s, 3H), 1.26 – 1.20 (m, 9H), 1.18 (d, J = 4.0 Hz, 3H), 1.14 (s, 3H), 1.09 – 1.01 (m, 2H), 0.85 (t, J = 7.1 Hz, 3H). 13C NMR (151 MHz, Chloroform-d) δ 172.30, 154.64, 154.48, 150.56, 140.54, 130.58, 109.23, 108.05, 105.72, 44.61, 44.14, 37.48, 31.92, 31.17, 30.16, 29.89, 28.91, 28.78, 27.68, 24.75, 22.82, 18.46, 14.24. IR (film, cm-1): 3384, 2958, 2927, 2856, 1622, 1412, 1185, 1032, 965, 838; HRMS (ESI) calcd for C25H36O4 [M+H]+: m/z 401.2686, found: 401.2675.
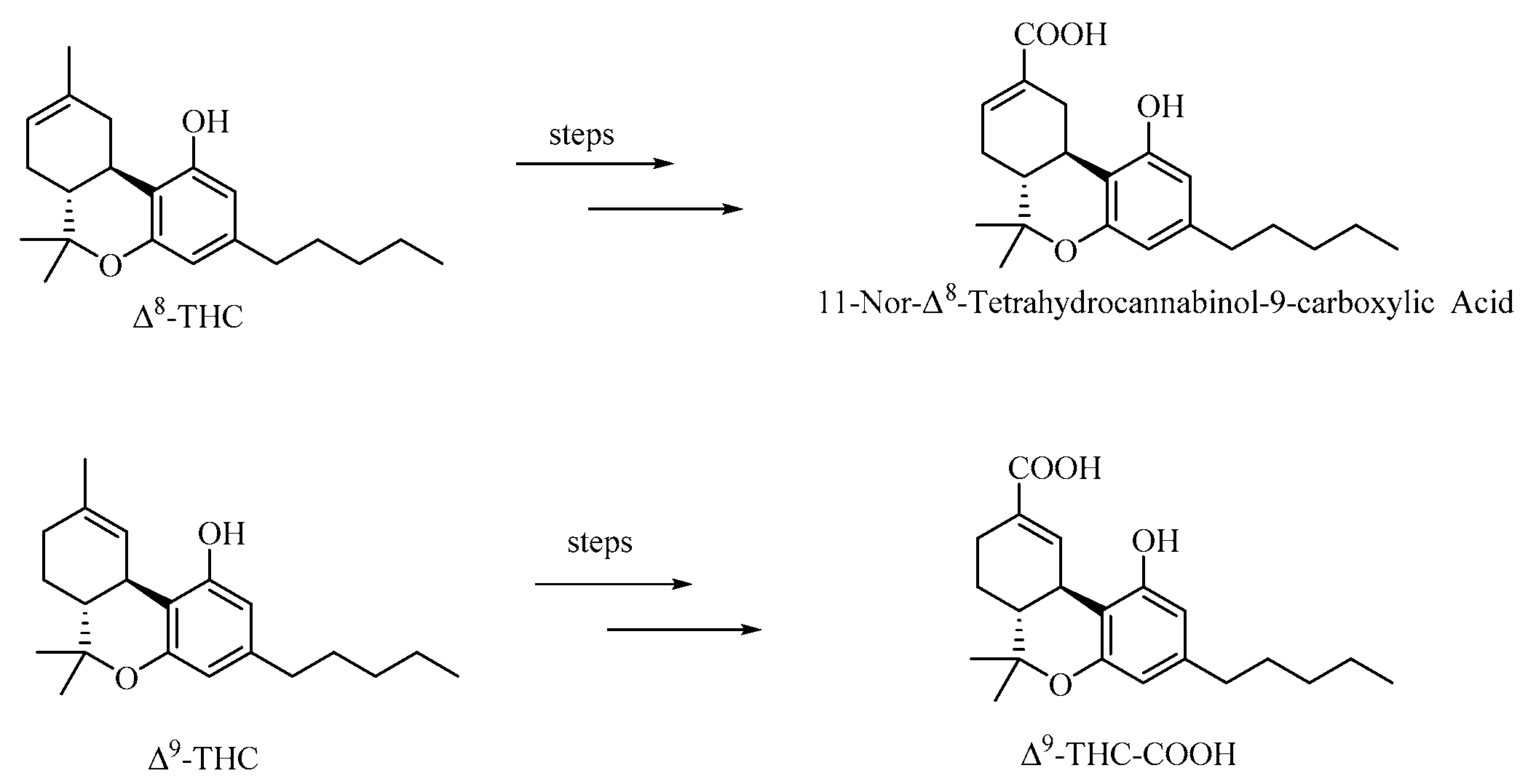
Δ8 -THC, 1H NMR (600 MHz, Chloroform-d) δ 6.29 (d, J = 1.6 Hz, 1H), 6.11 (d, J = 1.8 Hz, 1H), 5.44 (dd, J = 4.8, 2.6 Hz, 1H), 4.66 (s, 1H), 3.24 – 3.16 (m, 1H), 2.70 (d, J = 4.8 Hz, 1H), 2.45 (td, J = 7.7, 5.2 Hz, 2H), 2.18 – 2.11 (m, 1H), 1.89 – 1.78 (m, 3H), 1.71 (s, 3H), 1.60 – 1.54 (m, 2H), 1.38 (s, 3H), 1.34 – 1.29 (m, 4H), 1.11 (s, 3H), 0.89 (t, J = 7.0 Hz, 3H). 13C NMR (151 MHz, Chloroform-d) δ 155.00, 154.88, 142.87, 134.90, 119.47, 110.63, 110.26, 107.75, 45.00, 36.14, 35.57, 31.70, 30.75, 28.02, 27.71, 23.64, 22.69, 18.64, 14.17. HRMS (ESI) calcd for C21H30O2 [M+H]+: m/z 315.2319, found: 315.2330.
11-Nor-Δ8-Tetrahydrocannabinol-9-carboxylic Acid, 1H NMR (600 MHz, Methanol-d4) δ 7.03 (dd, J = 5.2, 2.6 Hz, 1H), 6.18 (d, J = 1.6 Hz, 1H), 6.10 (d, J = 1.6 Hz, 1H), 3.87 (ddd, J = 17.7, 4.5, 2.4 Hz, 1H), 2.60 (td, J = 11.2, 4.4 Hz, 1H), 2.43 (d, J = 8.6 Hz, 1H), 2.40 (d, J = 7.7 Hz, 2H), 2.10 – 1.96 (m, 1H), 1.85 – 1.72 (m, 2H), 1.59 – 1.53 (m, 2H), 1.37 (s, 3H), 1.36 – 1.28 (m, 4H), 1.09 (s, 3H), 0.91 (t, J = 7.1 Hz, 3H). 13C NMR (151 MHz, Methanol-d4) δ 170.90, 157.83, 155.76, 143.73, 139.41, 132.43, 110.98, 109.75, 108.54, 77.00, 46.06, 36.62, 32.80, 32.66, 32.05, 31.52, 29.55, 27.91, 23.60, 18.43, 14.40. HRMS (ESI) calcd for C21H28O4 [M+H]+: m/z 345.2060, found: 345.2056.
Δ9 -THC, 1H NMR (600 MHz, Chloroform-d) δ 6.33 – 6.30 (m, 1H), 6.28 (d, J = 1.6 Hz, 1H), 6.15 (d, J = 1.6 Hz, 1H), 4.80 (s, 1H), 3.21 (dt, J = 10.9, 2.6 Hz, 1H), 2.44 (td, J = 7.5, 3.5 Hz, 2H), 2.21 – 2.14 (m, 2H), 1.95 – 1.89 (m, 1H), 1.70 (d, J = 2.0 Hz, 1H), 1.69 (dq, J = 2.3, 1.0 Hz, 3H), 1.59 – 1.54 (m, 2H), 1.42 (s, 3H), 1.41 (s, 1H), 1.33 – 1.28 (m, 4H), 1.10 (s, 3H), 0.90 – 0.87 (m, 3H). 13C NMR (151 MHz, Chloroform-d) δ 154.91, 154.30, 142.97, 134.56, 123.85, 110.23, 109.17, 107.68, 77.37, 45.93, 35.61, 33.70, 31.65, 31.30, 30.79, 27.71, 25.15, 23.51, 22.68, 19.41, 14.16. HRMS (ESI) calcd for C21H30O2 [M+H]+: m/z 315.2319, found: 315.2320.
Δ9-THC-COOH,1H NMR (400 MHz, Methanol-d4) δ 8.05 (d, J = 2.2 Hz, 1H), 6.20 (s, 1H), 6.11 (s, 1H), 3.35 (d, J = 3.4 Hz, 1H), 2.53 (dd, J = 18.5, 6.8 Hz, 1H), 2.42 (t, J = 7.6 Hz, 3H), 2.04 (dd, J = 12.8, 7.3 Hz, 1H), 1.69 – 1.51 (m, 3H), 1.41 (s, 4H), 1.32 (ddd, J = 12.7, 10.0, 5.6 Hz, 4H), 1.09 (s, 3H), 0.90 (t, J = 6.9 Hz, 3H). 13C NMR (151 MHz, Methanol-d4) δ 171.52, 157.20, 155.95, 144.78, 144.07, 130.08, 109.84, 108.39, 108.19, 77.92, 46.15, 36.64, 35.95, 32.65, 32.06, 27.92, 26.66, 25.50, 23.61, 19.25, 14.40. HRMS (ESI) calcd for C21H28O4 [M+H]+: m/z 345.2060, found: 345.2059.