1. Introduction
With the changing climate, the global reduction in precipitation has resulted in an increased occurrence of droughts [
1]. Drought stress can significantly influence plant root growth by impeding elongation and branching, ultimately diminishing a plant's capacity to absorb water and nutrients in soils. Furthermore, it can lead to reduced photosynthesis and nutrient accumulation, cellular damage, and, in the end, plant wilting and mortality [
2,
3]. Notably, it has been documented that from 2010 to 2017, drought events were responsible for the demise of approximately 129 million trees in California [
4]. Likewise, severe drought has resulted in widespread mortality of
Pinus tabulaeformis in central and eastern China [
5]. The global impact of drought on plant growth and crop yields is undeniable, and climate-induced and soil-related droughts are projected to intensify further due to ongoing global climate change [
6]. When plants experience drought stress, their phenotypic properties and physiological traits can undergo responses, and the degree to which each property responds in the early stage of drought is closely linked to the drought resistance of the plants [
7]. Consequently, it is imperative to explore strategies aimed at enhancing the drought resistance of plants under the early stage of drought stress.
Pinus massoniana stands as one of the most vital timber resources in southern China, distinguished by its rapid growth, high yield, and multifaceted utility, all while contributing significantly to ecological value [
8]. Nonetheless, during the early stages of afforestation, the survival rate of
P. massoniana seedlings is notably hindered by factors such as inadequate shade and soil dryness [
9]. Therefore, it becomes imperative to enhance the drought resistance of
P. massoniana seedlings.
Ectomycorrhiza (ECM) represents a mutualistic symbiosis formed between ectomycorrhizal fungi (ECMF) and non-lignified absorbing roots [
10]. ECMF can establish symbiotic relationships with plant families such as Pinaceae, Fagaceae, Salicaceae, and Betulaceae [
11,
12,
13,
14]. Under conditions of drought stress, ECM demonstrates the capacity to expand the root absorption zone, thereby enhancing water and nutrient uptake through external hyphae. This expansion results in improved root water retention and notable enhancements in root traits, including increased root biomass, total length, average diameter, and the number of root tips in the host plant. These factors collectively play a crucial role in augmenting drought resistance [
15]. Furthermore, Zou, et al. [
16] have observed that under water-deficient conditions, ECM hyphae can extend into soil gaps, providing additional water resources to mitigate drought effects. Additionally, ECM symbiosis contributes to increased water and nutrient absorption, facilitates photosynthesis, regulates the synthesis of antioxidant enzymes, and facilitates the accumulation of osmotic substances within plants [
17]. Notably, Ahmed, et al. [
18] have reported that ECMF promotes the formation of osmotic adjustment substances, such as plant soluble proteins, thereby maintaining cellular water balance and enhancing plant drought tolerance. In a study by Zhao, et al. [
19], ECMF inoculation was found to elevate antioxidant enzyme activity in
P. sylvestris. These findings collectively underscore the significant role of ECM symbiosis in enhancing plant resilience to drought, a phenomenon that holds paramount importance in the face of increasing environmental challenges. Therefore, the investigation of the influence of the different ECMF species on
P. massoniana drought resistance, and the underlying molecular mechanisms governing is indispensable to the early stages of afforestation of
P. massoniana.
Cenococcum geophlium Fr., a commonly encountered ectomycorrhizal fungus in natural ecosystems, typically exists in soil in mycorrhizal and sclerotial forms, without the formation of spores, and is characterized by a profusion of external hyphae [
20]. This fungal species exhibits a broad spectrum of host compatibility and displays robust adaptability and stress resistance, making it a valuable ally for plant growth across diverse and challenging environments [
21]. Notably,
C. geophlium demonstrates exceptional drought tolerance and is often employed as a prominent candidate in water-scarce forest ecosystems. Research by Coleman, et al. [
22] has illuminated its role in preserving cellular integrity under conditions of water deficit while mitigating the surge in reactive oxygen species (ROS) typically observed during drought stress. Furthermore, recent findings by Li, et al. [
23] underscore the ability of different ecotypes of
C. geophlium to induce the expression of drought-related genes when subjected to drought conditions, thereby enhancing drought resistance. It is important to note that different ecotypes of
C. geophlium exhibit distinct responses to drought, presenting an intriguing avenue for further exploration in this context.
In this study, we undertook the inoculation of P. massoniana seedlings with eight ecotypes of C. geophlium. By meticulously simulating the early stage of drought stress conditions for 7 days, we aimed to investigate the diverse impacts of these ecotypes of C. geophlium on the growth and physiological responses of P. massoniana seedlings when subjected to the early stage of drought stress. Subsequently, we selected mycorrhizal seedlings that performed better (drought-tolerant) and worse (drought-sensitive) under the early stage of drought stress for in-depth RNA sequencing. The comparative assessment of differentially expressed genes in drought-tolerant and drought-sensitive mycorrhizal seedlings under the early stage of drought stress provided valuable insights into the physiological and molecular mechanisms underlying the capacity of various ecotypes of C. geophlium to enhance the drought tolerance of P. massoniana. This research endeavor aims to furnish a robust theoretical foundation for future afforestation efforts involving mycorrhizal seedlings, ultimately contributing to more resilient ecosystems in the face of drought challenges.
2. Materials and Methods
2.1. The mycelial growth of different ecotypes of C. geophilum under drought treatment
Eight ecotypic strains of
C. geophilum (Jacg16, Jacg21, Jacg37, Jacg81, Jacg121, Jacg189, Jacg243, Chcg57) were used in this study. The strains in Japan were isolated by Laboratory of Forest Symbiosis at the Graduate School of Agricultural and Life Sciences, the University of Tokyo, while the strain in China was provided by the International Joint Laboratory of Forest Symbiosis at Fujian Agriculture and Forestry University. The source information of each strain is shown in
Table S1. The mycelia of
C. geophilum strains were pre-cultured in a modified Melin-Norkrans (MMN) agar medium [
24] at 25 ℃ in the dark for 30 days. Subsequently, an agar plug of
C. geophilum with a 7 mm diameter was transferred to the center of a 90 mm petri dish, which was lined with cellophane membrane at the bottom. Each dish contained 10 mL of MMN liquid medium with 0% or 10% polyethylene glycol (PEG-6000, simulated drought) and then cultured in dark at 25 ℃. Three replicates of each strain were conducted for each treatment. After culturing for 30 days, the mycelial growth area of each strain was measured by X-Plan 380dⅢ, Ushikata (Kantum Ushikata Co., LTD., Yokohama, Japan).
2.2. Preparation of mycorrhizal seedlings of P. massoniana
Pinus massoniana seeds were obtained from the Wuyi National Forest Farm in Fujian Province. These seeds were sterilized in 1% sodium hypochlorite (NaCIO, v/v) for 10 min, followed by rinsing with sterile deionized water five times. The sterilized seeds were then sown in sterilized vermiculite and cultured in a controlled environment for 6 weeks (25 ℃, 16 h light cycle). Simultaneously, the eight C. geophilum strains were pre-cultured in MMN agar medium at 25 ℃ in the dark for 45 days.
The substrate used for planting the mycorrhizal seedlings of P. massoniana inoculated by C. geophilum consisted of a mixture of forest soil and red jade soil (1:2, v/v). The forest soil was collected from the Soil and Water Moisturizing Garden of Fujian Agriculture and Forestry University in Fujian Province, China. The mixture was then sterilized by autoclaving at 121 ℃ for 3 h. Six-week-old seedlings of P. massoniana with consistent growth were transplanted into a rectangle rhizobox (23.5×8.5×1.6 cm) and then C. geophlium plugs were inoculated onto the lateral roots, while the roots of non-inoculated seedlings were covered with the agar plugs with the same size containing MMN medium. These seedlings were placed in a growth chamber with a light cycle of 16 h at 25 ℃. Throughout the cultivation period, the seedlings were weekly applied by 0.1% Hoagland nutrient solution. After 45 days of cultivation, the seedlings of P. massoniana with mycorrhizal root rate exceeding 90% were selected for further drought stress experiments.
2.3. Experimental design for drought stress of P. massoniana seedlings
Each of aforementioned seedlings was transplanted into a 50 mL centrifuge tube filled with 40.0 g of a soil mixture (forest soil:red jade soil=1:2, v/v) and then cultivated in a greenhouse under controlled conditions with an average relative humidity of 70% and the illumination duration was set at 16 h at 25 ℃. During the cultivation period, the moisture level in soil was consistently maintained at a ranged of 85%-90% of its field capacity. After 2 months of growth, the seedlings were treated by different drought stress. During the treatment period, each tube with seedling was weighted, and watered daily at 9:00 am to keep the water content in soil at the required level. For the drought treatment, the field capacity in soil was reduced at 30%-35%, while for the non-drought treatments, the field capacity in soil was maintained at 85%-90%. In each treatment, each seedling inoculated by different ecotypic strains of C. geophilum had 15 replicates. Following 7 days of different drought treatments, the seedlings were collected for the further analysis.
2.4. Determination of photosynthetic index
After the 7 days of drought treatments, the gas exchange parameters of P. massoniana needles were measured using a photosynthesis instrument with a red-blue light source (LI-6400XT, Lincoln, NE, USA). The parameters measured included the net photosynthetic rate (Pn), transpiration rate (Tr), intercellular CO2 concentration (Ci), and stomatal conductance (Gs).
2.5. Determination of morphological and physiological indicators
The collected seedlings were cleaned with double distilled water, and the excess water was removed with absorbent paper. Subsequently, three seedlings for each treatment were randomly selected to measure the fresh weight of shoots and roots. The freshly weighed seedlings were then dried at 80 ℃ until constant weight, and their dry weight was recorded using a digital scale. The water content in both shoot and root of seedlings was determined by gravimetric method. The remaining fresh seedlings were immediately treated with liquid nitrogen and stored in a refrigerator at -80 ℃ for the determination of osmotic adjustment substances, malondialdehyde (MDA) and antioxidant enzymes. To determinate each index, 0.2 g of fresh shoot or root tissue from each sample were quickly ground into a powder after freezing with liquid nitrogen. The determination of antioxidant enzymes and MDA followed the methods described by Zhang [
25]: Catalase (CAT) activity was assessed using the ultraviolet absorption method, peroxidase (POD) activity was determined by employing guaiacol and hydrogen peroxide as substrates in the reaction and measuring the absorbance at 470 nm, superoxide dismutase (SOD) activity was measured using the nitroblue tetrazolium (NBT) reduction method, and the content of MDA in the seedlings was quantified utilizing the thiobarbituric acid (TBA) method. In addition, the proline (Pro) content was determined at 520 nm, following the procedure outlined by Bates, et al. [
26], while the soluble protein (SP) content was assessed using the Coomassie brilliant blue method as described by Bradford [
27].
2.6. RNA-seq and qRT-PCR analysis
After evaluating the drought tolerance of different ecotypic strains of
C. geophilum and mycorrhizal seedlings of
P. massoniana inoculated with these strains of
C. geophilum, the Jacg121-inoculated (drought-tolerant) and Chcg57-inoculated (drought-sensitive) seedlings were selected for transcriptome analysis. The preparation method and drought stress treatment for these mycorrhizal seedlings remained consistent with the procedures previously described. After 7 days of different drought stress treatment, the shoots and roots of each seedling were frozen in liquid nitrogen and stored at -80 ℃. The frozen shoots and roots were sent to the Biomarker Technologies (Beijing, China) for total mRNA extraction, cDNA library construction, and sequencing. Briefly, the frozen samples were ground into powder by mortar and pestle, and immediately transferred to the lysis extraction buffer. Total mRNA from both the shoots and roots of seedlings was extracted using the RNAsimple total RNA extraction kit provided by Tiangen Biotech (Beijing, China). The cDNA library was constructed, and the sequencing was performed by an Illumina NovaSeq 6000 platform in accordance with standard protocols. The clean reads of sequencing were assembled using Trinity software to obtain an Unigene library [
28]. Differentially expressed genes analysis was performed by DESeq2 software [
29]. The novel genes identified in the enrichment analysis were ruled out in further analysis. Additional annotation and functional analysis of identified DEGs were conducted using Kyoto Encyclopedia of Genes and Genomes (KEGG) and Gene Ontology (GO) databases [
30,
31], as well as PlantGSEA analysis [
32].
To confirm the gene expression levels obtained from the RNA-seq, a real-time quantification polymerase chain reaction (RT-qPCR) analysis was performed. An aliquot of the RNA sample prepared for RNA sequencing were also used for RT-qPCR analysis. The total RNA reverse transcription and RT-qPCR reactions were performed using Novozyme HiScript II Q RT SuperMix for qPCR (+gDNA wiper) Kit and ChamQ Uniweisal SYBR qPCR Master Mix Kit (Vazyme Biotech, Nanjing, China). Gene-specific primers were designed based on the unigene sequence by the tool Premier 5.0 (
http://www.premierbiosoft.com/; accessed on 2 June 2022). The Aquaporin protein gene (AQP) was amplified as an internal reference gene to detect the effectiveness of template preparation. The expression of each gene was confirmed in at least three rounds of independent RT-qPCR reactions.
2.7. Statistical analysis
A principal component analysis (PCA) for comprehensively assessing the drought tolerance of plant was employed to evaluate the drought tolerance of mycorrhizal seedlings of
P. massoniana inoculated by different ecotypic strains of
C. geophilum, as reported by Zou, et al. [
33]. This evaluation was based on parameters including water content, photosynthetic indexes, and various physiological indexes.
The formula for calculating the membership function of comprehensive index of mycorrhizal seedlings inoculated by different ecotypic strains of
C. geophilum is as below:
Comprehensive evaluation of drought tolerant level:
Where CIj was the jth comprehensive index, CImin is the minimum value of the jth comprehensive index, CImax was the maximum value of the jth comprehensive index, and Pj was the contribution index of CIj.
SPSS (Statistical Product and Service Solutions 21.0) was used for principal component analysis, Pearson correlation analysis, Mann-Whitney U test and Student’s t-test. Pictures was drawn using Origin software.
4. Discussion
The current literature on enhancing the drought resistance of
P. massoniana through the use of ECMF has predominantly concentrated on various ECMF species [
34,
35]. However, there has been limited attention dedicated to investigating the impact of different ecotypes within each ECMF species.
Cenococcum geophilum, characterized by abundant genetic diversity, holds ecological significance linked to its genetic variations [
21]. Numerous studies have highlighted the varying resistance levels of different ecotypic strains of
C. geophilum to stresses such as drought, high temperature, heavy metals, and salt [
23,
36,
37,
38]. Nevertheless, existing studies primarily focus on the strain's inherent stress resistance mechanism, with limited exploration of the symbiotic relationship between strains and plants in facing diverse stresses. In this study, we delved into the enhancement and mechanisms of drought resistance in
P. massoniana through the inoculation of eight ecotypes of
C. geophilum, each with distinct drought tolerances during the early stages of drought stress. Notably, in mycelial culture experiments, Jacg243 exhibited the strongest drought resistance, Chcg57 displayed the weakest, and the remaining six ecotypic strains showed no evident growth response to drought stress. Intriguingly, our findings suggest that the inoculation of all eight ecotypes effectively enhanced the drought resistance of
P. massoniana seedlings, regardless of the drought tolerance exhibited by
C. geophilum mycelia.
During the early stage of drought stress, the inoculation of most ecotypes of
C. geophilum increased both shoot and root water contents, photosynthetic levels, and the contents of Pro and SP in
P. massoniana seedlings. These responses align with previous studies emphasizing the importance of maintaining high water content, enhancing photosynthetic activity, and accumulating osmotic regulators for plant drought resistance [
39]. Photosynthetic indexes not only affect the water potential of stomatal guard cells, induce water absorption or water loss, regulate leaves stomatal opening and closing, but also carry out photosynthesis through gas exchange between internal and external environment to ensure energy supply [
40]. As the main osmotic regulators of plants, Pro and SP are hydrophilic organic solvents, which can stabilize protein structure protect macromolecular substances and are positively correlated with drought tolerance of plants [
41]. Wang, et al. [
42] showed that inoculation with ECMF increased the water content, photosynthetic rate and accumulation of osmotic adjustment substances in
P. tabulaeformis under drought stress. Rasouli, et al. [
43] also reported that inoculation with mycorrhizal fungi can help
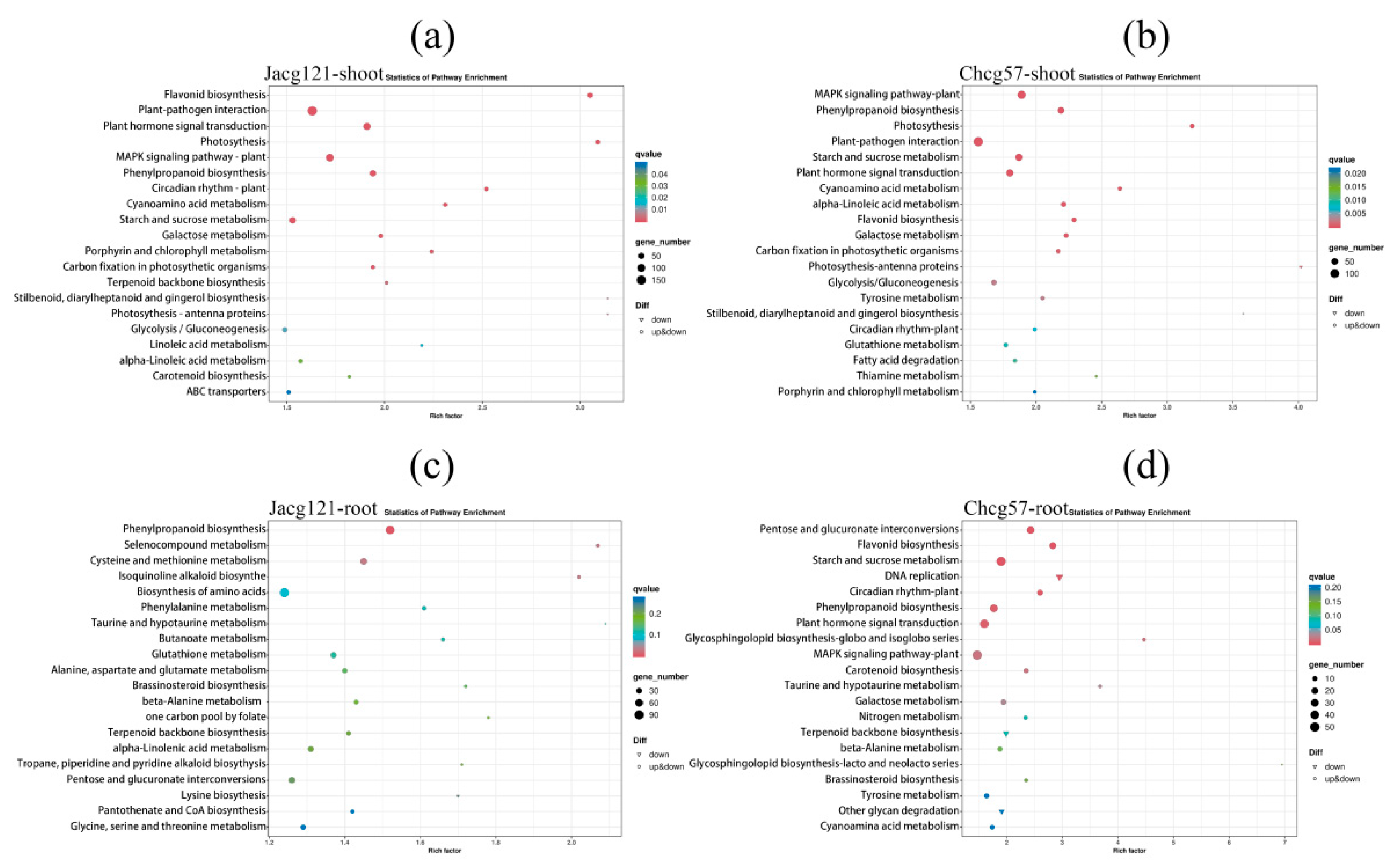
Satureja hortensis resist drought stress by increasing its water content, photosynthetic levels, and accumulation of osmotic regulators. KEGG pathway enrichment analysis highlighted the significance of pathways such as phenylpropanoid biosynthesis, photosynthesis, and starch and sucrose metabolism in both drought-tolerant and drought-sensitive mycorrhizal seedlings. As an important phenolic compound, the synthesis and accumulation of phenylpropanoids can enhance the thickness of the needle cell wall and reduce the loss of water transpiration to resist the negative effects caused by drought stress [
44]. Feng, et al. [
45] have shown that genes such as
DHNs,
LEA,
Annexin D2, and
NAC in the phenylpropanoid biosynthesis pathway play an important role in protecting plant cell membrane permeability. These results further demonstrated that inoculation with
C. geophilum could help
P. massoniana resist drought stress by regulating energy supply and reducing water loss.
Plants have evolved complex defense mechanism to resist external stress, which can effectively remove the accumulation of ROS and reduce the damage caused by membrane lipid peroxidation to plant [
46]. Antioxidant enzymes, as an important protective enzyme in plants, play an important role in plant stress resistance, by eliminating excessive ROS, reducing cell membrane damage, and protecting cell membranes integrity [
47]. Our results showed that the activities of POD and SOD in
P. massoniana seedlings, inoculated with
C. geophilum were generally higher compared to non-inoculation during the early stage of drought stress, while the inoculation seedlings exhibited lower MDA content, indicating that
C. geophilum inoculation can help
P. massoniana to resist drought stress by reducing cell damage and scavenging ROS. Alvarez, et al. [
48] have reported that ECMF inoculation can influence the activity of ROS scavenging enzyme, thereby regulating host resistance to stress. [
19], Zhao, et al. [
49] also found that ECMF inoculation could promote SOD activity and reduce MDA content in
P. sylvestris, which was consistent with our research results. Pan, et al. [
50] also suggested that plants exhibiting lower MDA content under stress had stronger drought tolerance. The results of transcriptome showed that the DEGs in
P. massoniana seedlings inoculated with
C. geophilum during early stage of drought stress, were significantly enriched in pathways such as flavonoid biosynthesis, plant hormone signal transduction, and MAPK signaling pathway-plant. Flavonoids are mainly derived from the phenylpropanoid pathway, which can enhance the antioxidant activity of plants and help plants resist various biotic and abiotic stresses [
51]. Moreover, Hodaei, et al. [
52] have shown that drought stress can lead to a significant up-regulation of flavonoid metabolism-related genes in
Chrysanthemum morifolium, and a significant increase in flavonoid compounds, thereby improving the antioxidant capacity of
C. morifolium. The MAPK signaling pathway is an important tool for plants to respond to abiotic and biotic stress, with ROS accumulation serving as an activator of this pathway [
53]. Liu, et al. [
54] found that GhMAPKKK49 in
Gossypium hirsutum was induced by abscisic acid (ABA) and ROS and involved in ROS and ABA-mediated responses to various abiotic stresses. Zhu, et al. [
55] reported that up-regulation of StMAPK11 in
Solanum tuberosum under drought conditions can promote the activity of antioxidant enzymes, thus improving drought resistance. In our study, the CAT activity of
P. massoniana seedlings inoculated with most ecotypic strains of
C. geophilum was significantly lower than that of non-inoculation, possibly due to the competitive relationship between CAT and POD on the substrate H
2O
2 [
56]. However, our results showed that the POD activity in
P. massoniana seedlings inoculated with
C. geophilum was higher than that of non-inoculation, indicating that
C. geophilum mainly decomposed H
2O
2 by increasing POD activity of
P. massoniana.
In this study, we found that the drought resistance of mycorrhizal seedlings did not correlate with the inherent drought resistance of the
C. geophilum strain itself. Jacg121, despite showing no growth response to drought in mycelium culture, exhibited the strongest drought resistance when forming symbionts with
P. massoniana. Conversely, the mycorrhizal seedlings of
P. massoniana inoculated with Jacg243 of the strongest drought resistance in mycelium culture,
demonstrated comparatively weak drought resistance. Under the same water condition, the biomass of
P. massoniana seedlings inoculated with Jacg121 was significantly higher than that of Jacg243, especially the fresh and dry weight of roots. This implies that the establishment of a symbiotic relationship with Jacg121 significantly increased the root absorption area, enhancing the seedlings' moisture absorption capacity. Rasouli, et al. [
43] have already reported that ECMF can expand the root absorption area by establishing a symbiotic relationship, improve the water status and nutrient metabolism of host plants, and avoid or slow down the drought stress to plants. This emphasizes the importance of promoting root growth to help plants overcome drought stress [
57,
58]. Based on our findings, it is suggested to cultivate drought-resistant ECM seedlings using strains that not only exhibit drought resistance but also possess the ability to significantly enhance plant growth in mycorrhizal afforestation in drought areas.
Author Contributions
Conceptualization, C.L. and S.Z.; Methodology, X.Z.; Software, X.Z.; Validation, X.Z., C.L. and S.Z.; Formal analysis, X.Z.; Investigation, X.Z., J.Z., J.H., M.L., N.M. and Q.G.; Resources, X.Z., M.L. and C.L.; Data curation, X.Z., C.L. and S.Z.; Writing—original draft, X.Z.; Writing—Review & Editing, N.M.; Q.G.; C.L. and S.Z., Visualization, X.Z.; Supervision, C.L. and S.Z.; Project administration, C.L. and S.Z.; Funding acquisition, C.L. and S.Z. All authors have read and agreed to the published version of the manuscript.
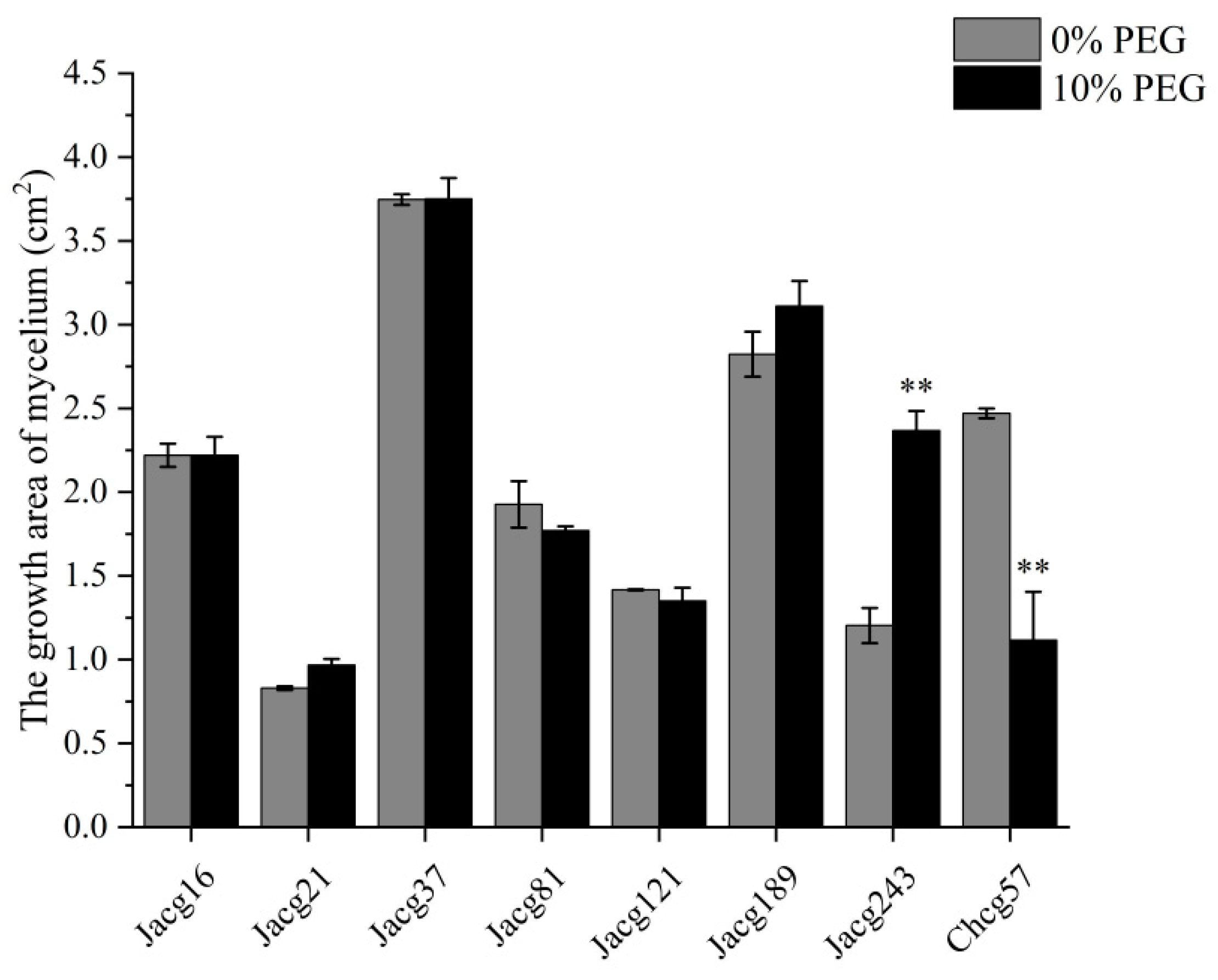
Figure 1.
The mycelial growth of eight ecotypes of Cenococcum geophilum under drought and non-drought treatments. Data and bars are shown as mean and ± SE of the replicates, respectively (n=3). The statistically significant difference between 0% PEG and 10% PEG treatments is tested by Student’s t-test (** P<0.01).
Figure 1.
The mycelial growth of eight ecotypes of Cenococcum geophilum under drought and non-drought treatments. Data and bars are shown as mean and ± SE of the replicates, respectively (n=3). The statistically significant difference between 0% PEG and 10% PEG treatments is tested by Student’s t-test (** P<0.01).
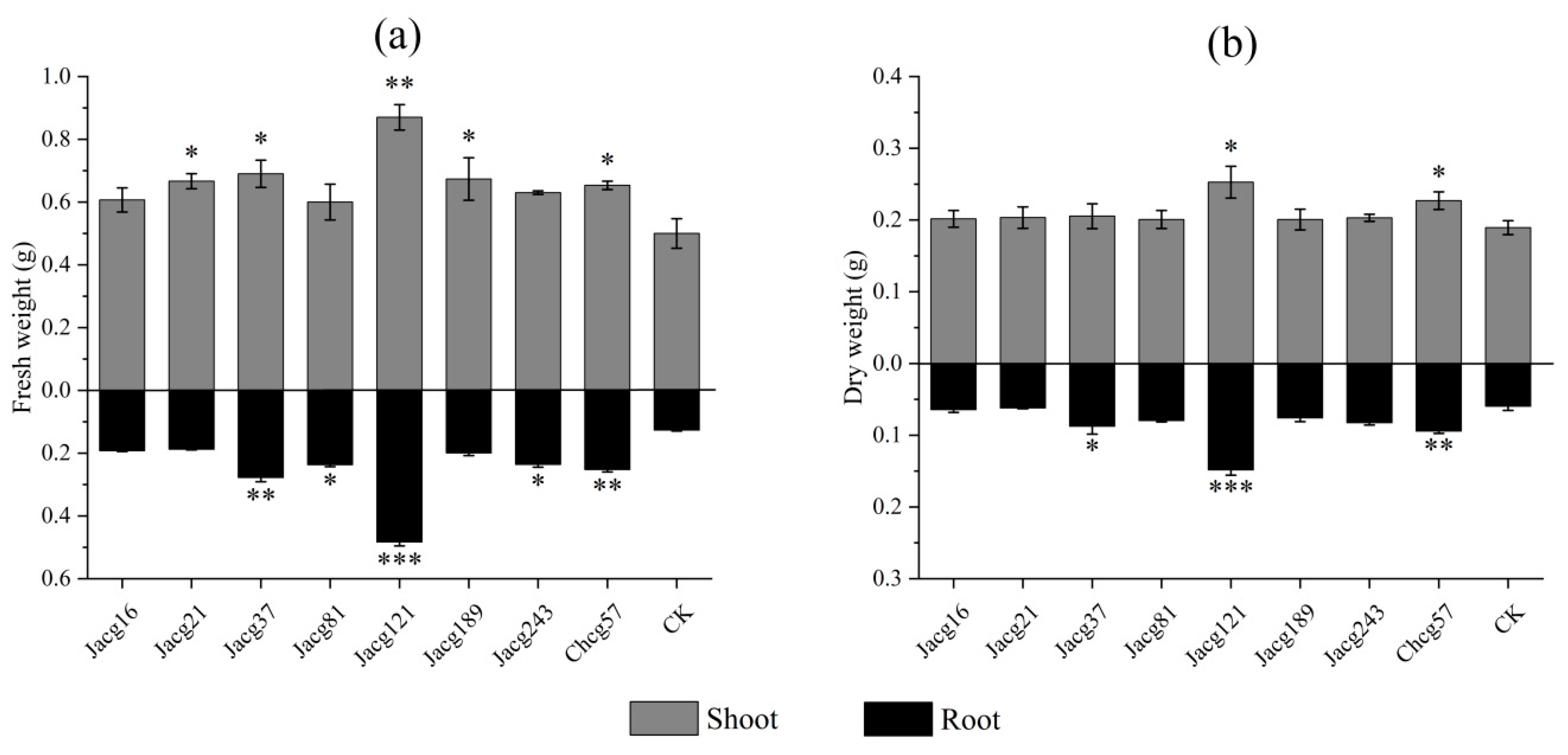
Figure 2.
Effects of different Cenococcum geophilum ecotypes inoculation on (a) fresh weights and (b) dry weights of Pinus massoniana seedlings. Data and bars are shown as mean and ± SE of the replicates, respectively (n=3). The statistically significant difference between different Cenococcum geophilum ecotypes inoculation and non-inoculation treatments is tested by Mann-Whitney U test (* P<0.05; ** P<0.01; *** P<0.001). CK, non-inoculation.
Figure 2.
Effects of different Cenococcum geophilum ecotypes inoculation on (a) fresh weights and (b) dry weights of Pinus massoniana seedlings. Data and bars are shown as mean and ± SE of the replicates, respectively (n=3). The statistically significant difference between different Cenococcum geophilum ecotypes inoculation and non-inoculation treatments is tested by Mann-Whitney U test (* P<0.05; ** P<0.01; *** P<0.001). CK, non-inoculation.
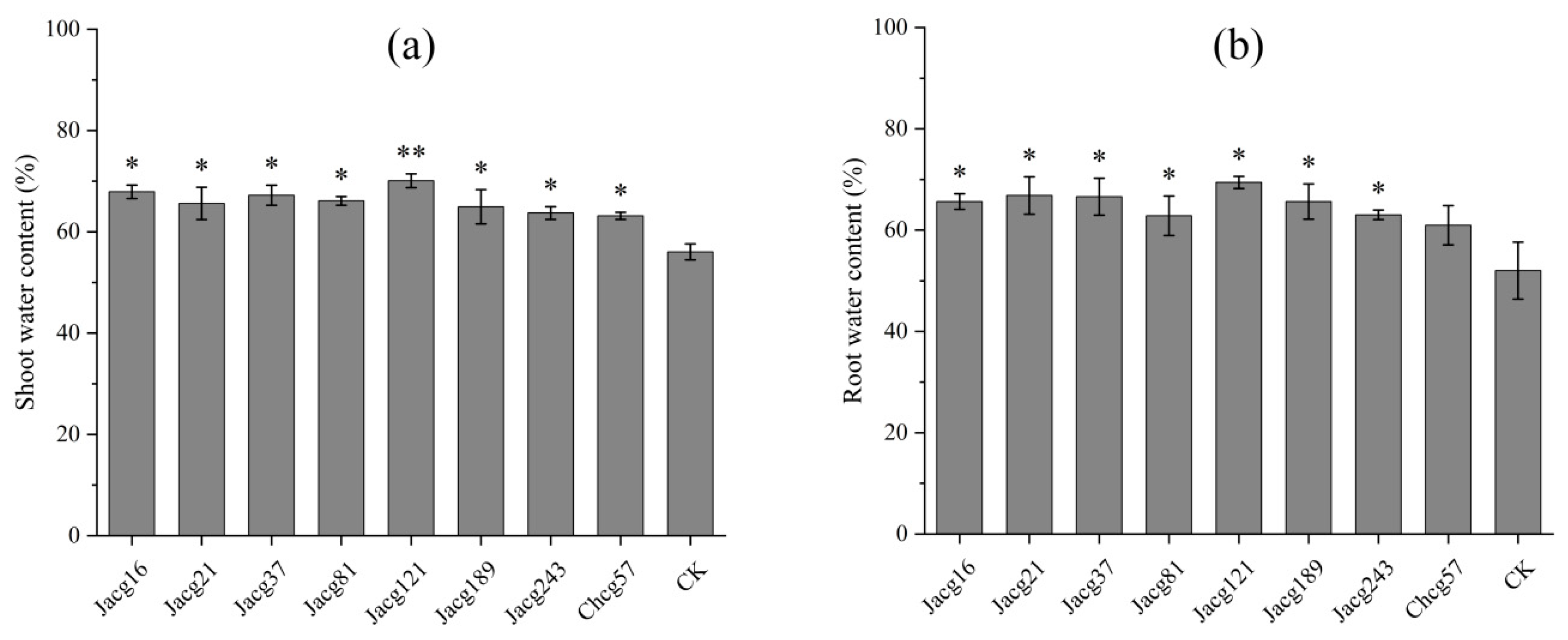
Figure 3.
Effects of different Cenococcum geophilum ecotypes inoculation on the (a) shoot and (b) root water contents of Pinus massoniana seedlings after 7 days of drought stress treatment (field capacity 30%-35%). Data and bars are shown as mean and ± SE of the replicates, respectively (n=3). The statistically significant difference between different C. geophilum ecotypes inoculation and non-inoculation treatments is tested by Mann-Whitney U test (* P<0.05; ** P<0.01). CK, non-inoculated.
Figure 3.
Effects of different Cenococcum geophilum ecotypes inoculation on the (a) shoot and (b) root water contents of Pinus massoniana seedlings after 7 days of drought stress treatment (field capacity 30%-35%). Data and bars are shown as mean and ± SE of the replicates, respectively (n=3). The statistically significant difference between different C. geophilum ecotypes inoculation and non-inoculation treatments is tested by Mann-Whitney U test (* P<0.05; ** P<0.01). CK, non-inoculated.
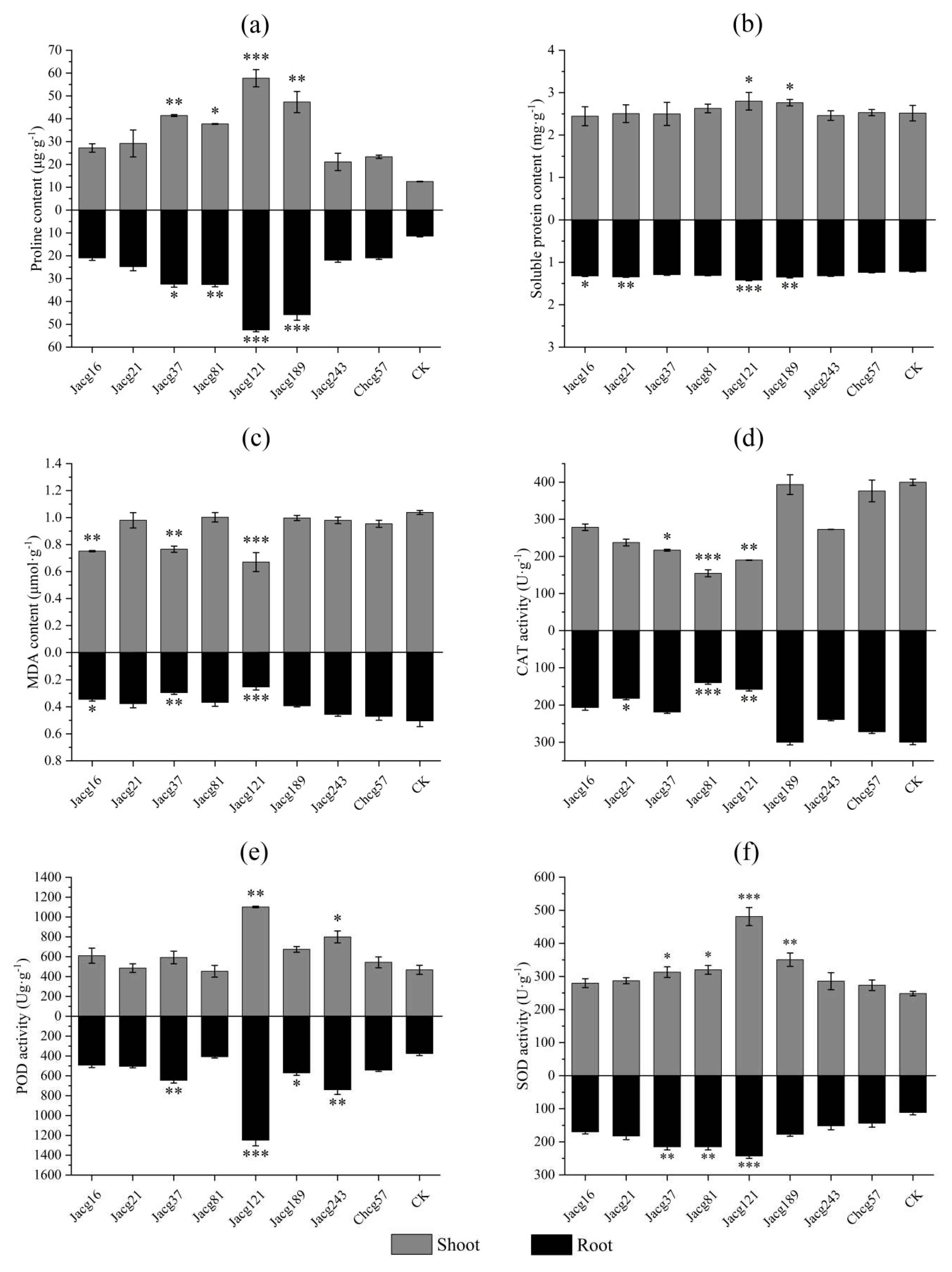
Figure 4.
Effects of different Cenococcum geophilum ecotypes inoculation on the (a) proline content, (b) soluble protein content, (c) malondialdehyde (MDA) content, (d) catalase (CAT) activities, (e) peroxidase (POD) activities and (f) superoxide dismutase (SOD) activities of Pinus massoniana seedlings after 7 days of drought stress treatment (field capacity 30%-35%). Data and bars are shown as mean and ±SE of the replicates, respectively (n=3). The statistically significant difference between different C. geophilum ecotypes inoculation and non-inoculation treatments is tested by Mann-Whitney U test (* P<0.05; ** P<0.01; *** P<0.001). CK, non-inoculation.
Figure 4.
Effects of different Cenococcum geophilum ecotypes inoculation on the (a) proline content, (b) soluble protein content, (c) malondialdehyde (MDA) content, (d) catalase (CAT) activities, (e) peroxidase (POD) activities and (f) superoxide dismutase (SOD) activities of Pinus massoniana seedlings after 7 days of drought stress treatment (field capacity 30%-35%). Data and bars are shown as mean and ±SE of the replicates, respectively (n=3). The statistically significant difference between different C. geophilum ecotypes inoculation and non-inoculation treatments is tested by Mann-Whitney U test (* P<0.05; ** P<0.01; *** P<0.001). CK, non-inoculation.
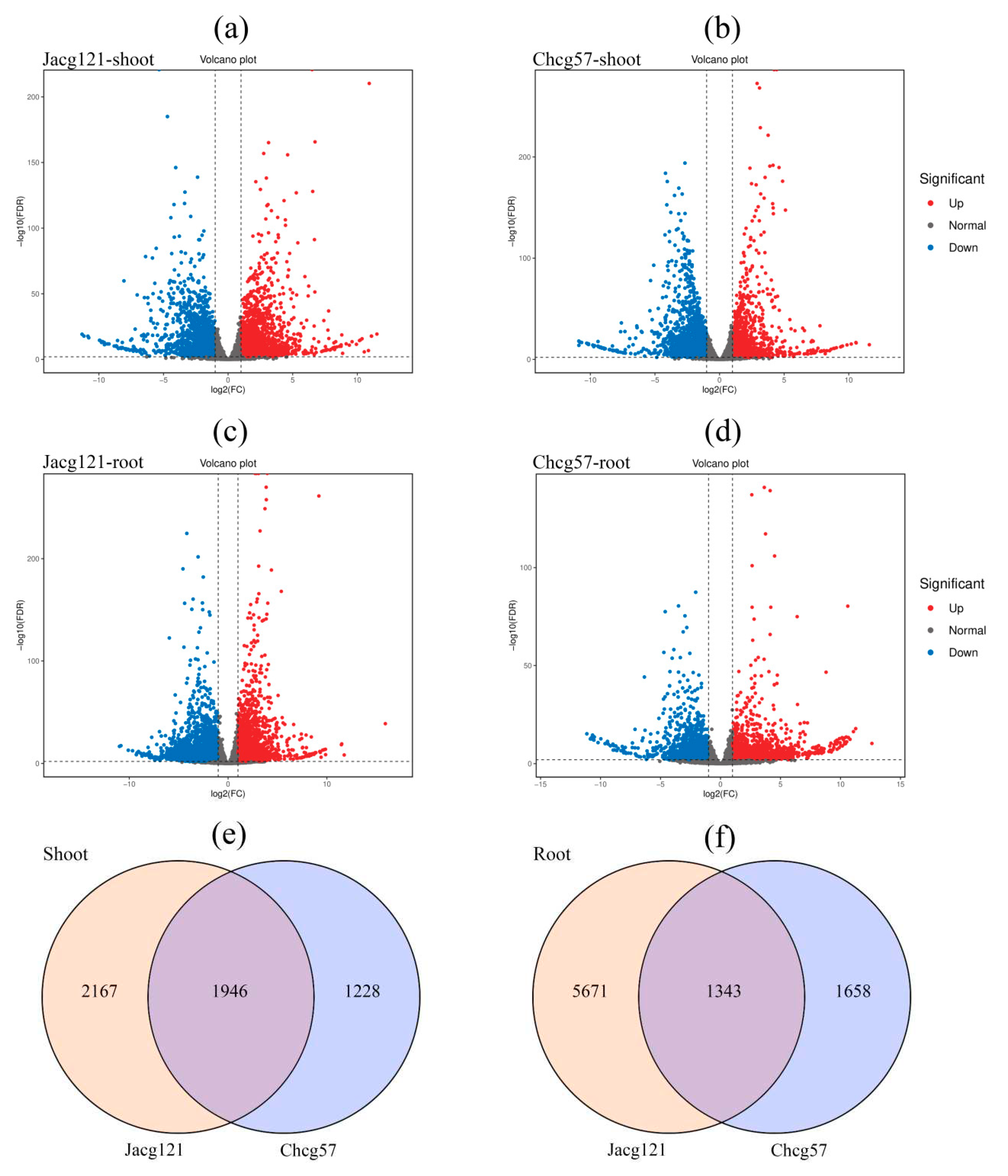
Figure 5.
Volcano (a-d) and Wenn (e, f) maps of differentially expressed genes (DEGs) analysis of drought-tolerant (Jacg121) and drought-sensitive (Chcg57) seedlings of Pinus massoniana inoculated by Cenococcum geophilum after 7 days of drought (field capacity 30%-35%) and well-watered (field capacity 85%-90%) treatments. Red spot indicates significant up-regulated genes after drought treatment; blue spot indicates significantly down-regulated genes; and gray spot indicates no-change genes. |log2Foldchange| ≥ 2 are used as the screening criteria of DEGs.
Figure 5.
Volcano (a-d) and Wenn (e, f) maps of differentially expressed genes (DEGs) analysis of drought-tolerant (Jacg121) and drought-sensitive (Chcg57) seedlings of Pinus massoniana inoculated by Cenococcum geophilum after 7 days of drought (field capacity 30%-35%) and well-watered (field capacity 85%-90%) treatments. Red spot indicates significant up-regulated genes after drought treatment; blue spot indicates significantly down-regulated genes; and gray spot indicates no-change genes. |log2Foldchange| ≥ 2 are used as the screening criteria of DEGs.
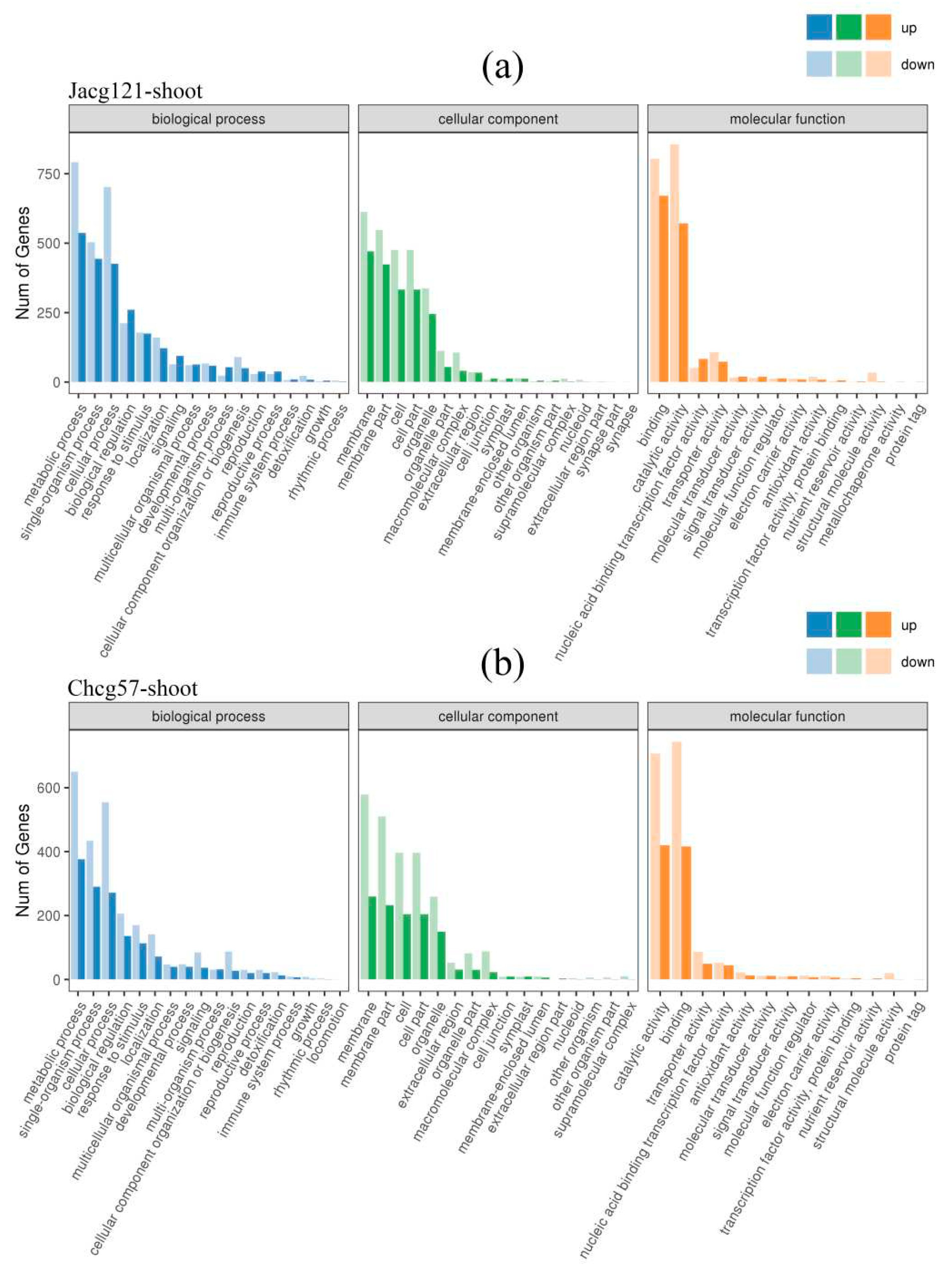
Figure 6.
Gene ontology (GO) enrichment analysis of the differentially expressed genes (DEGs) in shoots of drought-tolerant (Jacg121) (a) and drought-sensitive (Chcg57) (b) seedlings of Pinus massoniana inoculated by Cenococcum geophilum after 7 days of drought (field capacity 30%-35%) and well-watered (field capacity 85%-90%) treatments. The abscissa is the GO classification, and the ordinate is the number of genes.
Figure 6.
Gene ontology (GO) enrichment analysis of the differentially expressed genes (DEGs) in shoots of drought-tolerant (Jacg121) (a) and drought-sensitive (Chcg57) (b) seedlings of Pinus massoniana inoculated by Cenococcum geophilum after 7 days of drought (field capacity 30%-35%) and well-watered (field capacity 85%-90%) treatments. The abscissa is the GO classification, and the ordinate is the number of genes.
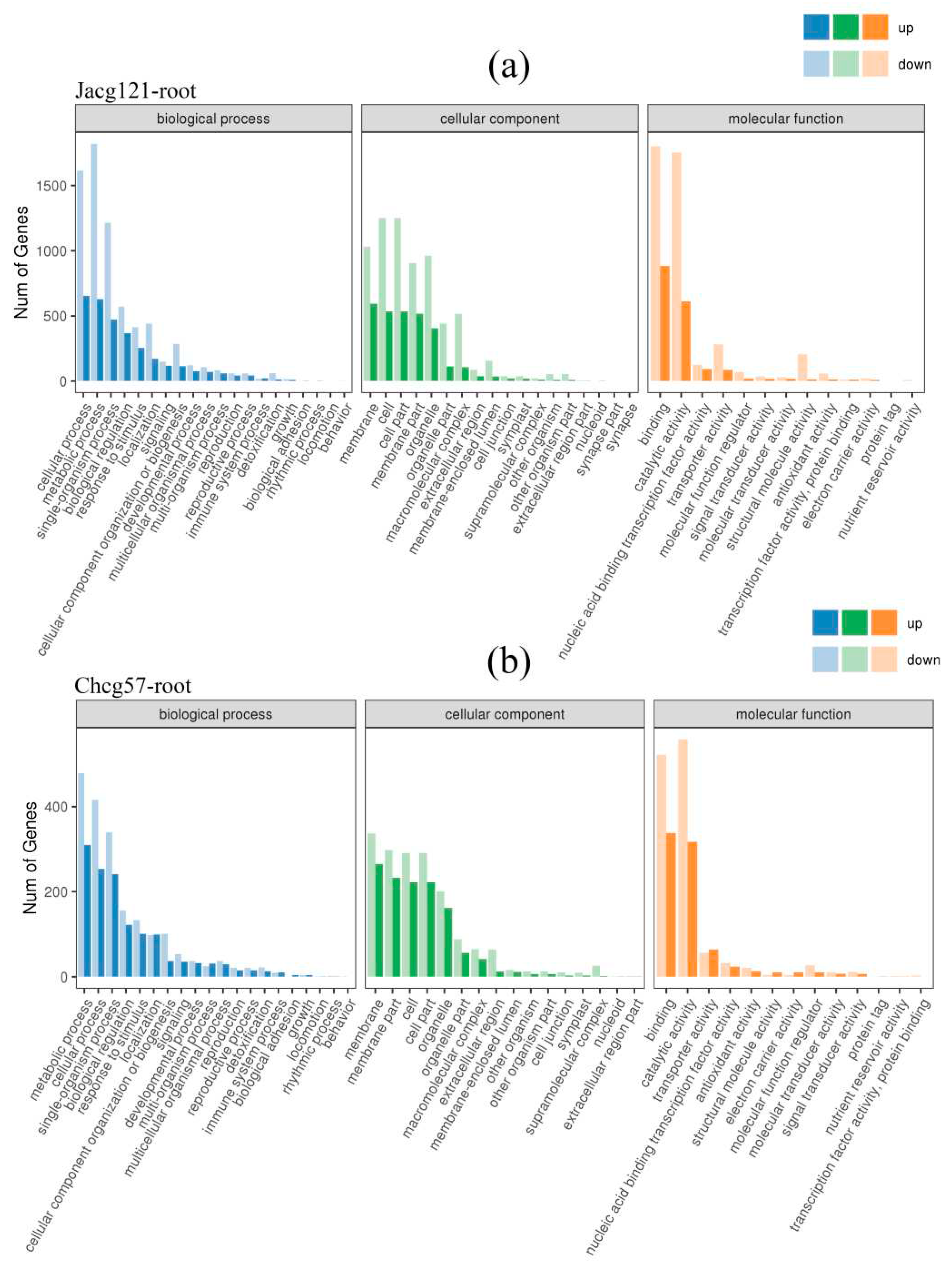
Figure 7.
Gene ontology (GO) enrichment analysis of the differentially expressed genes (DEGs) in roots of drought-tolerant (Jacg121) (a) and drought-sensitive (Chcg57) (b) seedlings of Pinus massoniana inoculated by Cenococcum geophilum after 7 days of drought (field capacity 30%-35%) and well-watered (field capacity 85%-90%) treatments. The abscissa is the GO classification, and the ordinate is the number of genes.
Figure 7.
Gene ontology (GO) enrichment analysis of the differentially expressed genes (DEGs) in roots of drought-tolerant (Jacg121) (a) and drought-sensitive (Chcg57) (b) seedlings of Pinus massoniana inoculated by Cenococcum geophilum after 7 days of drought (field capacity 30%-35%) and well-watered (field capacity 85%-90%) treatments. The abscissa is the GO classification, and the ordinate is the number of genes.
Figure 8.
The Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analysis of differentially expressed genes (DEGs) in shoots and roots of drought-tolerant (Jacg121) (a, c) and drought-sensitive (Chcg57) (b, d) seedlings of Pinus massoniana inoculated by Cenococcum geophilum after 7 days of drought (field capacity 30%-35%) and well-watered (field capacity 85%-90%) treatments. The size of the symbol represents the numbers of DEGs involved in the corresponding pathway.
Figure 8.
The Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analysis of differentially expressed genes (DEGs) in shoots and roots of drought-tolerant (Jacg121) (a, c) and drought-sensitive (Chcg57) (b, d) seedlings of Pinus massoniana inoculated by Cenococcum geophilum after 7 days of drought (field capacity 30%-35%) and well-watered (field capacity 85%-90%) treatments. The size of the symbol represents the numbers of DEGs involved in the corresponding pathway.
Figure 9.
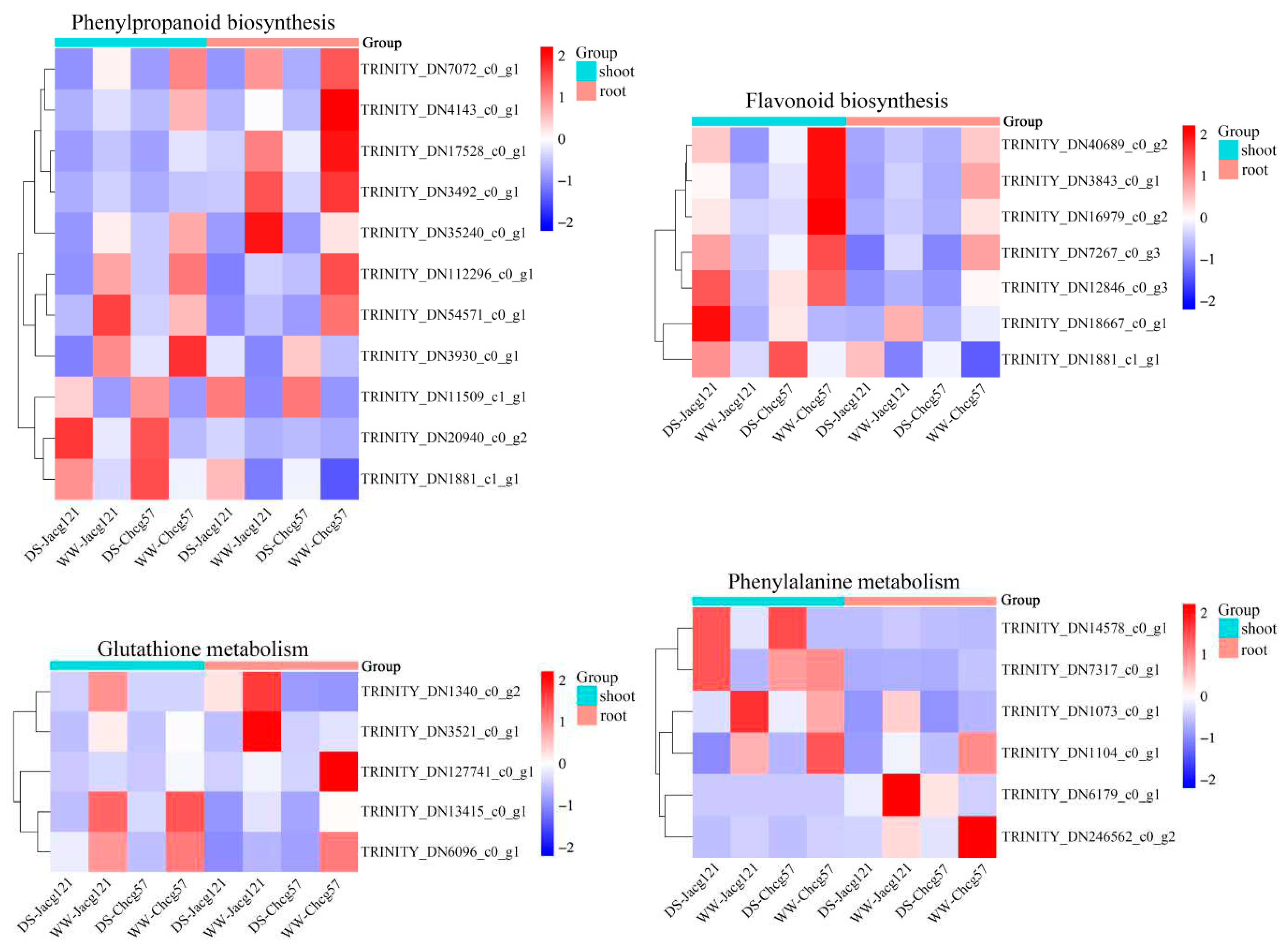
The heatmaps of part up-regulated or down-regulated differentially expressed genes (DEGs) in different pathways of drought-tolerant (Jacg121) and drought-sensitive (Chcg57) seedlings of Pinus massoniana inoculated by Cenococcum geophilum after 7 days of drought (field capacity 30%-35%) and well-watered (field capacity 85%-90%) treatments. The Y- and X-axes represent the DEGs and different samples, respectively. The different colors of the heatmaps, ranging from blue over white to red, represent scaled expression levels of genes with [log2 (FPKM + 1)] across different samples. DS, drought stress; WW, well-watered.
Figure 9.
The heatmaps of part up-regulated or down-regulated differentially expressed genes (DEGs) in different pathways of drought-tolerant (Jacg121) and drought-sensitive (Chcg57) seedlings of Pinus massoniana inoculated by Cenococcum geophilum after 7 days of drought (field capacity 30%-35%) and well-watered (field capacity 85%-90%) treatments. The Y- and X-axes represent the DEGs and different samples, respectively. The different colors of the heatmaps, ranging from blue over white to red, represent scaled expression levels of genes with [log2 (FPKM + 1)] across different samples. DS, drought stress; WW, well-watered.
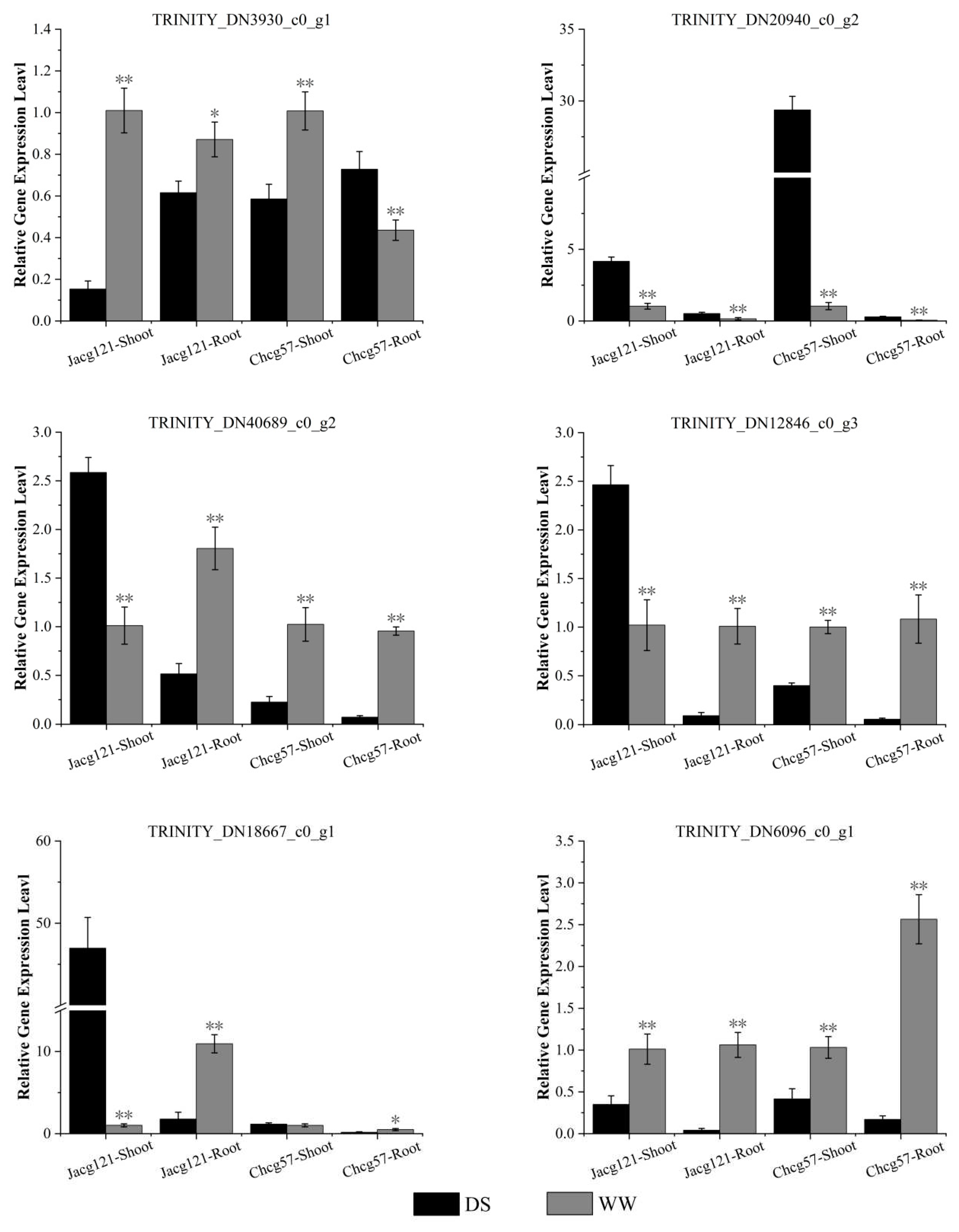
Figure 10.
The relative expression levels of six candidate genes in drought-tolerant (Jacg121) and drought-sensitive (Chcg57) seedlings of Pinus massoniana inoculated by Cenococcum geophilum were analyzed by RT-qPCR after 7 days of drought (field capacity 30%-35%) and well-watered (field capacity 85%-90%) treatments. The relative expression changes of candidate genes in different treatments were calculated by 2- △△Ct method and expressed as mean and ± SE (n=3). The statistically significant difference between drought and well-watered treatments is tested by Student’ s t-test (* P<0.05; ** P<0.01). WW, well-watered; DS, drought stress.
Figure 10.
The relative expression levels of six candidate genes in drought-tolerant (Jacg121) and drought-sensitive (Chcg57) seedlings of Pinus massoniana inoculated by Cenococcum geophilum were analyzed by RT-qPCR after 7 days of drought (field capacity 30%-35%) and well-watered (field capacity 85%-90%) treatments. The relative expression changes of candidate genes in different treatments were calculated by 2- △△Ct method and expressed as mean and ± SE (n=3). The statistically significant difference between drought and well-watered treatments is tested by Student’ s t-test (* P<0.05; ** P<0.01). WW, well-watered; DS, drought stress.
Table 1.
Effects of inoculation with different Cenococcum geophilum ecotypes on photosynthetic parameters of Pinus massoniana seedlings after 7 days of drought stress treatment (field capacity 30%-35%).
Table 1.
Effects of inoculation with different Cenococcum geophilum ecotypes on photosynthetic parameters of Pinus massoniana seedlings after 7 days of drought stress treatment (field capacity 30%-35%).
| ID |
Pn
(μmol·m-2·s-1) |
Gs
(mol·m-2·s-1) |
Ci
(μmol·m-2·s-1) |
Tr
(mmol·m-2·s-1) |
| Jacg16 |
1.57±0.06 |
0.023±0.001 |
322.17±4.37*** |
0.95±0.01 |
| Jacg21 |
2.45±0.32* |
0.019±0.004 |
214.01±3.12 |
1.00±0.02 |
| Jacg37 |
3.22±0.03** |
0.036±0.001*** |
280.97±1.73* |
1.72±0.01*** |
| Jacg81 |
3.53±0.08*** |
0.026±0.007* |
207.72±8.04 |
1.16±0.03* |
| Jacg121 |
3.58±0.12*** |
0.046±0.003*** |
303.57±4.77** |
2.25±0.01*** |
| Jacg189 |
1.93±0.02 |
0.035±0.003** |
327.84±0.94*** |
1.59±0.01** |
| Jacg243 |
1.47±0.04 |
0.020±0.001 |
261.70±3.00 |
0.98±0.01 |
| Chcg57 |
2.30±0.08 |
0.024±0.007 |
280.65±1.61* |
1.20±0.03* |
| CK |
1.08±0.01 |
0.008±0.001 |
161.93±3.55 |
0.93±0.01 |
Table 2.
The value of comprehensive index (CI), index weight (Wj), µ(Xj), D value (comprehensive evaluation of drought tolerance) and comprehensive evaluation in Pinus massoniana seedlings inoculated by different Cenococcum geophilum ecotypes after 7 days of drought stress (field capacity 30%-35%).
Table 2.
The value of comprehensive index (CI), index weight (Wj), µ(Xj), D value (comprehensive evaluation of drought tolerance) and comprehensive evaluation in Pinus massoniana seedlings inoculated by different Cenococcum geophilum ecotypes after 7 days of drought stress (field capacity 30%-35%).
| Code |
CI1
|
CI2
|
CI3
|
μ(X1)
|
μ(X2)
|
μ(X3)
|
D |
Comprehensive evaluation* |
| Jacg16 |
-0.083 |
-0.382 |
-1.990 |
0.437 |
0.354 |
0.000 |
0.386 |
6 |
| Jacg21 |
-0.118 |
-1.057 |
-0.437 |
0.427 |
0.154 |
0.450 |
0.391 |
5 |
| Jacg37 |
0.555 |
-0.492 |
-0.107 |
0.610 |
0.321 |
0.545 |
0.564 |
3 |
| Jacg81 |
0.188 |
-1.574 |
1.465 |
0.510 |
0.000 |
1.000 |
0.483 |
4 |
| Jacg121 |
1.991 |
0.511 |
0.417 |
1.000 |
0.619 |
0.697 |
0.919 |
1 |
| Jacg189 |
0.319 |
1.792 |
0.337 |
0.546 |
1.000 |
0.673 |
0.621 |
2 |
| Jacg243 |
-0.454 |
0.230 |
-0.627 |
0.336 |
0.536 |
0.394 |
0.369 |
7 |
| Chcg57 |
-0.704 |
0.580 |
-0.060 |
0.268 |
0.640 |
0.559 |
0.347 |
8 |
| CK |
-1.693 |
0.393 |
1.002 |
0.000 |
0.584 |
0.866 |
0.160 |
9 |
| Index weight (wj) |
|
|
|
0.770 |
0.140 |
0.09 |
|
|