Introduction
Distant metastases of DTCs are a rare evenience, occurring in 2-13% of all patients [
3]. They can be generally diagnosed at the discovery of tumor or later, decades after the first treatment and are usually diagnosed because of clinical symptoms or suspicious findings at laboratory, as increasing values of thyroglobulin (Tg) and positive imaging [
5]. They can be considered synchronous if discovered within 6 months following thyroid surgery and metachronous when discovered more than six months after thyroidectomy [
4].
Bones are the second most frequent sites of localizations, after lungs, and BM are more frequently observed in FTC than PTC [
6,
7].
Spinal and pelvic bones are more generally involved followed by chest, extremities, shoulder girale and cranio-maxillo-facial bones. The aim of treatment is to reduce high risk skeletal related events (SREs), such as fractures, spinal cord compressions, pain and hypercalcemia which are associated to worse prognosis [
8,
9].
Metastases are generally observed in case of aggressive histological subtypes of PTC as tall-cell, hob nail, solid, diffuse sclerosing and columnar variant and in FC. Risk factors are: vascular invasion, large primary tumors, macroscopic extrathyroidal extension, bulky loco-regional nodal disease [
10].
The risk of persistent/recurrent DTC can be predicted referring to ATA system developed in 2015. In this system the overall estimated risk of recurrences ranges from <1% to>55% and is classified as low risk (≤5%) intermediate risk (6%-20%) or high risk>20% [
5].
Here we report the case of a bone metastatic recurrence of FTC diagnosed 15 years after the first treatment with excellent response to RAI.
This case confirms the need of prolonged follow-up observation in DTC and the use of RAI therapy of first line therapy of BM, in patients with no RAI refractory disease [
8].
Case report
A 72yrs old female patient underwent thyroid surgery in 2002 (57yrs old), for a multinodular goiter with an intermediate nodule at fine needle aspiration biopsy. At pathology diagnosis of FC (maximum diameter 2.5cm) of the right thyroid lobe infiltrating thyroid parenchyma has been made. She was euthyroid, and thyroglobulin auto-antibodies (TgAb) and thyroperoxidase auto-antibodies (TPOAb) were absent. No other relevant morbidities were present.
In 2003 she underwent to RAI therapy with 4025 MBq of 131-I.
The Whole- Body Scan (WBS) after therapeutic dose showed up-take in thyroid bed with no other areas of pathological radioiodine up-take.
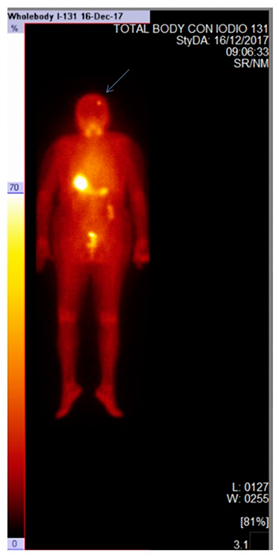
Six months after the treatment a diagnostic RAIU was performed and did not reveal any pathological up-take.
Patient was on L- thyroxine therapy and underwent to annual follow-up, with Tg basal values <0.2ng/ml, TgAb absent and negative imaging up to fifteen years after surgery, when Tg values on L-thyroxine suppressive therapy increased (7 ng/ml, TSH 0.1mUI/L), with ultrasound neck imaging negative for loco- regional disease.18-FDG PET TC total body scanner was then performed. No areas of abnormal FDG up-take were revealed.
For this reason, the patient underwent to RAI with 5550 MBq131-I after rh-TSH.
The WBS after therapy showed an area of abnormal up-take of the skull, in right parietal bone. She had no symptoms due to the lesion.
Twelve months after treatment, basal Tg was 0.1ng/ml with absent TgAb and WBS after 3700MBq of radioiodine administration with rh-TSH treatment did not reveal any area of abnormal uptake.

Patient is now alive, and she is on L-thyroxine treatment (475mcg/ week) with persistence of excellent response to bone metastasis treatment (TSH 1.5mUI/L, Tg 0.0 ng/mL, TgAb 10UI/L)
Discussion
FTC accounted for about 10% of incidence of thyroid cancer, mainly with hematogenous metastases and prone to distant metastasis. It has higher invasiveness, metastasis rate and mortality than PTC [
11] and occurs more likely in women over 50 yrs old [
12].
Risk stratification after the initial therapy and the long-term outcome of patients with TC has been advocated for by most clinicians to predict the risk of persistent/recurrent disease.
The stratification of the risk for DTC is a dynamic process and the assignment to the risk class is revised during the follow-up, depending on the response to the initial treatment and the evolution of the disease. The outcomes after therapy of DTC vary from excellent response to structural incomplete response. Excellent response to therapy is defined as stimulated Tg <1 ng/mL, suppressed Tg <0.1 ng/mL, and no evidence of the disease by imaging [
13]. Biochemical incomplete response is defined as high Tg or rising TgAb following treatment without structural evidence of disease [
14]. DTC patients with biochemical incomplete response still have a good long-term survival rate, whereas a structural incomplete response to initial therapy was associated with significantly worse clinical outcome [
15]. Structural incomplete response to therapy, defined as having anatomical evidence of disease regardless of the Tg level and the status of TgAb, has worse clinical outcomes [
16]. Older age, male gender, multifocality, mediastinal or lateral lymph node involvement, and class III extent of the disease have been identified as strong predictors of incomplete response to therapy [
17]. In this case diagnosed before 2015, the initial risk class assignment could has been low risk category, with excellent response respectively based on ATA risk stratification (2015) and response to first treatment classification in ESMO guidelines (2019) [
5,
17].
Bone localization is generally associated to the osteolytic tumors, which are characterized by an increased production of IL-6 and IL-1 and receptor activator of nuclear factor B ligand (RANKL) (osteoprotegerin ligand). They increase osteoclastic activity and bone resorption, predisposing the bone microenvironment to the colonization by metastatic cells [
1,
10,
18]. For these reasons treatment with anti-resorptive drugs as bisphosphonates and denosumab is highly recommended in patients with diffuse and /or symptomatic BM from RAI -refractory DTC [
19] although data of efficacy and safety of bone active drugs in this clinical setting are scanty and limited to primary prevention [
20,
21]
BM treatment is always challenging because it can control the symptoms, but complete remission is a rare event.
If the lesions are RAI avid, as in this case, RAI therapy can control the disease and alleviate and delay symptom [
6,
7,
8,
9]. Loco-regional treatments may allow longer progression free intervals and cure in patients with oligometastatic disease. Surgery followed by external beam radio therapy (EBRT) is associated with the best out-comes at least for limb localizations [
19], but the the improvement of overall survival was reported in patients with no more than five metastases and in patients younger than 45 years old [
22,
23].
If surgery is not feasible, bone lesions are associated with high risk of fractures and pain should be treated with EBRT and/or interventional treatment radiology techniques, such as cementoplasty and thermoablation [
24,
25,
26]. Its disappearance is a rare event. Because of the close relationship between SREs and mortality an early treatment of BM from DTCs is mandatory to prevent it.
In our case, because of the sudden increase of Tg level, as high as 12 ng/ml on L-thyroxine treatment, an FDG PET-CT scan had performed to exclude any RAI refractory metastatic disease, and it did not show any areas of abnormal FDG up-take, nor suspect lesions on CT scan. Therefore, we decided to submit patient to radio iodine therapy with
131-I after rh-TSH stimulation with evidence of a radio iodine up-taking area at right parietal bone to be considered as a bone lesion. Twelve months after treatment, the decrease of Tg value from 12ng before treatment to 0.1 ng/ml and a second RAI treatment with no up-taking areas at WBS confirmed the response. The excellent response of the up-taking area of the skull to radioiodine, in this case, could be probably explained due to a low heterogeneity of tumor cells and low grade of dedifferentiation, as expected from the absence of areas of abnormal FDG up-take at 18-FDG-PET-CT scan [
3]. This is a rare evenience in a metachronous bone metastasis. Infact metachronous BM generally appear to have a more biological and clinical aggressive behavior with higher risk of SREs as compared to synchronous, at least in patients underwent post-surgical RAI ablation as first disease treatment. As in this case, metachronous BM may have been expression of a neoplastic progression rather than a delayed diagnosis [
8].
Conclusion
The real issue of this case is to understand why 15 years after excellent response, the patient had a bone metastasis. Probably some circulating tumor cells gained a metastatic potential through new mutations without impairing radioiodine up-taking ability.
Moreover because of the persistence of undetectable thyroglobulin for a long time, the patient has been considered as having an excellent response to the first treatment before this diagnosis, although this observation remarks the need of a long- life follow-up with Tg and TgAb dosage, particularly in FTC.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org.
Informed Consent Statement
The patient gave informed consent.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Nervo A, Ragni A, Retta F et al Bone metastases from differentiated thyroid carcinoma: current knowledge and open issues. J Endocrinol Invest (2021)44((3):403-419. [CrossRef]
- Iniguez-Ariza NM, Bible KC, ClarkeBL Bone metastases in thyroid cancer. J Bone Oncol (2020)21:100282. [CrossRef]
- Muresan MM, Olivier P, Leclere J, Sirveaux F, Brunaud L, Klein M, Zarnegar R, Weryha G.Bone metastases from differentiated thyroid carcinoma. Endocrin. Relat. Cancer (2008) 15: 37-49. [CrossRef]
- Jannin A, Lamartina L, Moutarde C, Djennauoi M, Lion G, Chevalier B, Vantyghem MC, Deschamp F, Hadoux J, Boudin E, Schlumberger M, Leboulleux S, Do Cao C.Bone metastases from differentiated thyroid carcinoma: heterogeneous tumor response to radioactive Iodine therapy and overall survival. European Journal of Nuclear Medicine and Molecular Imaging (2022) 49: 2401-2413. [CrossRef]
- Haugen BR, Alexander EK, Bible KC et al. American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid(2016); 26(1): 1-133. [CrossRef]
- Durante C, Haddy N, Baudin E et al. Long-term outcome of 444 patients with distant metastases from papillary and follicular thyroid: benefits and limits of radio iodine therapy. J Clin Endocrinol Metabol (2006) 91(8): 2892-2899. [CrossRef]
- Osorio M, Moubabyed SP, Su H, Urken ML. Systematic review of site distribution of bone metastases in differentiated thyroid cancer.Head and Neck(2017), 39(4): 812-818. [CrossRef]
- Mazziotti G, Formenti AM, Pavarotti MB te al. Real life management and out-come of thyroid carcinoma -related bone metastases: result from a nationwide multi center experience.Endocrine (2018) 59(1): 90-101. [CrossRef]
- Choksi P, Papaleontiou M, Guo C et al. Skeletal complications and mortality in thyroid cancer: a population-based study. J Clin Endocrinol Metab (2017); 102(4): 1254-1260. [CrossRef]
- G.D. Roodman, Mechanisms of bone metastasis.N. Engl. J. Med (2004). 350, 1655–1664. [CrossRef]
- Paschkle R., Lincke T, Muller SSP, Kreisler MC, Dralle H, Fasnscth M. The treatment of well differentiated thyroid carcinoma. Dtsch Arztebl INT (2015)112(26): 452-8. [CrossRef]
- Lee J, Soh EY. Differentiated thyroid carcinoma presenting with distant metastasis at initial diagnosis clinical outcomes and prognostic factors. Ann Surg (2010) 251(1): 114-9.
- Cano-Palomares A, Castells I, Capel I, Bella MR, Barcons S, Serrano A et al. Response to initial therapy of differentiated thyroid cancer predicts the long-termoutcome better than classical risk stratification systems. Int J Endocrinol (2014)014. 2014. [CrossRef]
- Steinschneider M, Pitaro J, koren S, Mizrakli Y, Benbessat C, Muallem Kalmovich L. Differentiated thyroid cancer with biochemical incomplete response: clinico-pathological charactheristics and long term disease outcomes Cancers (2021)13(21): 5422. [CrossRef]
- AhnJ SongE, Kim WG, Kim WB, Shong YK et al. Long term clinical outcomes of papillary thyroid carcinoma patients with incomplete response. Endocrine (2020) 67(3): 623-9.
- Visman F, Tala h, Grewal R, Tuttle RM. In differentited thyroid cancer, an incomplete structural response to therapy is associated with significantly worse clinical outcomes than onlyan incomplete thyroglobuline response. Thyroid (2011) 21(12): 1317-22. [CrossRef]
- Alzahrani AS, Moria Y, Muktar N, Aljamei H, Mazi S, Albalawi L et al. Course and predictive factors of incomplete response to therapy in low and intermediate -risk thyroid cancer. J Endocrin Soc (2021) 5(1): bvaa178.
- Filetti S, Durante C, Harti D, Leboulleux S, Locati LD, Newbold K, Papotti MG and Berruti A on behalf of the ESMO Guidelines Committee. Thyroid cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Annals of Oncology (2019) 30: 1856-1883.
- Cooper DS, Dherety GM, Haugen BR, Kloos RT, Lee SL, Mandel SJ, Mazzaferri EL, McIver B, Pacini F, Schlumberger M, Sherman SI, Steward DL, Tuttle RM, Revised American Thyroid Association Managment Guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid (2009)19,1116-1214. [CrossRef]
- Orita Y, Sugitani I, Toda K, Manabe J, Fujimoto y Thyroid Zoledronic acid in the treatment of bone metastasisi from differentiated thyroid carcinoma. Thyroid (2001) 21: 31-35. [CrossRef]
- Lipton A, FizaziK, Stopeck AT, Henry DH, Smith MR, Shore N, Martin M, Vadhan-Raj S, Brown JE, Richardson GE SaaD F, Yardley DA, Zhou K, Balakumaran A, Braun A. Effect of denosumab versus zoledronic acid in preventing skeletal related events in patients with bone metastases by baseline characteristics. Eur J Carncer (2016)53: 75-83. [CrossRef]
- Bernier MO, Leenhardt L, Hoang C, Aurengo A, Mary JY, Menegaux F, Enkaua E, Turpin G, Chiras J, Saillant G, Hejblum G Survival and therapeutic modalities in patients with bone metastases of differentiated thyroid carcinoma. J. Clin. Endocrinol Metab (2001)86: 1568-1573. [CrossRef]
- Zettinig G, Fueger Bj, Passler C, Kaserer K, Pirich C, Dudczak R, Niederle B. Long-term follow-up of patients with bone metastases from differentiatd thyroid carcinoma-surgery or conventional therapy? Clin Endocrinol (2002) 56: 377-382. [CrossRef]
- Kim Ho, Shin Ho, Lyun Oy, Kim SW, Parker KW et al. Prediction of follicular thyroid carcinoma associated with distant metastasis in the pre- operative and postoperative model. Head Neck (2019) 41(8):2507-13. [CrossRef]
- Drost L, Ganesh V, Wan BA et al. Efficacy of post-operative radiation treatment of bone metastases in the extremities. (2017) Radiotherapy Oncol (2017); 124 (1): 45-48. [CrossRef]
- Deschamps F, Farouil G, de Baere T. Percutaneous ablation of bone tumors. Diagn Interv Imaging (2014): 95 (7-8):659-663.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





