Introduction
Due to a lack of research and scientific validation, herbal medicines are still not widely accepted by the medical community [
1]. Antibiotic resistance is an issue in today’s world because of the widespread use of commercial antibiotics to treat infectious diseases [
2].
The immune system’s natural reaction to harm caused by microbial, chemical, and physical agents is inflammation. Excessive inflammation leads too many acute and chronic human diseases characterizes autoimmune diseases, illnesses of the circulatory system, malignancies, and metabolic and neurological disorders [
3].
An entire class of extremely reactive molecules produced by the metabolism of oxygen is known as reactive oxygen species (ROS). Normally occurring physiological quantities of ROS are necessary for cellular functions. Higher quantities of reactive oxygen species, however, can result in significant cellular and tissue damage and accelerate the development of illnesses like cataract formation, liver injuries and aging. Synthetic and natural antioxidants can both be useful in assisting the body in lessening the oxidative damage caused by reactive oxygen species [
3]
. Senna alata contains chemical components that have been shown to have a variety of pharmacological activities. However, the real effects are yet unknown, thus more research is needed to investigate its medical benefits [
4].
Meantime, some studies reported that generation of reactive oxygen species (ROS) caused inflammation and which has been related to the pathogenesis factors for various disease in patients [
5]. Hence, this complication may be prevented through the scavenge ROS by the free radical scavenging mechanism. Most of bioactive phytoconstituents like; phenolic, alkaloid, and flavonoid compounds curing the endogenous cells and cellular proteins through the free radical scavenging activity [
6,
7,
8]. Since such preventive effects are important to the inflammatory, cytotoxicity, and microbial disease which is also due to oxidative process.
Senna alata is commonly known as candle tree or ringworm brush [
1]. It is locally known as Agasti plant and used in various religious rituals.
Senna alata is a significant flowering plant that is both decorative and medicinal [
9]. Plant leaves are utilized as an astringent, expectorant, vermicide, purgative, and in the treatment of fungal illnesses. Cassia alata leaf extract may have cytotoxic, analgesic, antibacterial, anti-inflammatory, and fungicidal properties [
1,
9].
The search for new antimicrobial active agents obtained by using plant extracts of
Sennaalatahas led to the discovery of many clinically useful drugs, which help to solve the problems of antibiotic resistance exhibited by pathogenic microorganisms [
2].
Senna alata contains anti-inflammatory agents; hence can be a sensible and successful research approach in the hunt for novel anti-inflammatory medications. This plant extracts may led to the concentration of scientific effort on finding reliable and efficient sources of antioxidants which prevents from the cellular and tissue damage causes by oxidative stress [
3].
In the search for new drugs, the search for bioactive plant components from natural sources is always a deciding factor. Research on Senna alata leaves will improve the proper use of this plant in various medical conditions as an alternative treatment plan and will help to find possible therapeutic agents for specific diseases. This study aims to investigate the in vitro antioxidant, antibacterial, anti-inflammatory, thrombolytic, cytotoxic effects, phytochemical profiling and his GC-MS analysis of Senna alata leaf extract.
Material and Methods
Drugs and Chemicals
Methanol, Ethyl acetate, and Hexane were purchased from Merck Life Science Pvt. Ltd., while the Vincristine Sulphate was obtained from the Neon Laboratories, Limited, India. Standard reference drug Diclofenac and Ciprofloxacin were obtained from Saheka India and Arati Drugs India respectively. Ascorbic acid, Gallic acid, Quercetin, DPPH was purchased from Hi-Media, India. Every reagent and chemicals of analytical are used for this research.
Study PLANT Material
Senna alata leaveswere gathered from Chandragiri-06, Kathmandu, Nepal. Plant herbarium was authenticated by taxonomist from National Herbarium and Plant Laboratories, Kathmandu, Nepal. A voucher specimen (Acc. No. 02-1221- 2020) was deposited in the herbarium of Manmohan Memorial Institute of Health Science for future reference.
Plant Extracts Preparation and Extracts Percentage Yield
The leaves of the plants were thoroughly cleaned with fresh water. The leaves were then dried and sliced into little bits and introduced to successive maceration technique in which 3 different solvent were used according to polarity of the solvents. The method involved use of non-polar solvent ethyl acetate and n-hexane and polar solvent methanol for extraction of active constituents from the powdered leaves. For this, a known amount of powdered sample was taken in a beaker and extraction was carried out in hexane, ethyl acetate and then methanol subsequently in increasing order of polarity using maceration process. Finally, the obtained extract was transferred to a stainless steel plate and concentrated at room temperature using table fan. Dried extracts were stored in borosilicate glass vials and subjected to different investigations. Then obtained plant extract was filtered by Whatman No. 1 filter paper. The filtrate was evaporated by rota evaporator under reduced pressure (60 mmHg) at 40
oC and store at 4ºC. The extract yield percentage can be determined using the formula below:
GC-MS Analysis
The chemical components present in the methanolic extract that demonstrated the different biological activities were identified by performing a GC-MS analysis on the extract. The Nepal Academy of Science and Technology in Khumaltar, Lalitpur, conducted the GC-MS. For the GC-MS examination of plant material, GCMS QP2010 (Shimadzu, Kyoto, Japan) equipped with RTx-5MS fused silica capillary column of 30m length X 0.25 mm diameter X 0.25μm film thickness. Helium (>99.99% purity) with 36.2 cm/sec linear velocity was employed as carrier gas. The system was configured using 3.9 ml/min of total flow rate, 0.95 ml/min of column flow and 3.0 ml/min purge flow. The volume of injected sample was 1μl. The injector was set in splitless mode having 280ºC of temperature. Starting at 100ºC, the oven temperature increased to 250ºC at 15ºC/min with a 1-min pause. It then increased to 280ºC at 30ºC/min with a 1-min hold, then it increased once more from 280ºC to 300ºC at 15ºC/min with an 11-minute hold. With solvent cut-off duration of 3.5 minutes, the ion source and interface temperatures were set at 200ºC and 280ºC, respectively. 20 minutes were spent on the mass range scan, which covered 40 to 500 m/z. By comparing the mass spectra of the compounds with information from the NIST 08 mass spectral collection, the compounds were identified.
Qualitative Phytochemical Analysis
The
Senna alata leaves extracts undergo phytochemical analysis for the detection of plant secondary metabolites like alkaloid, flavonoid, tannin, carbohydrate, anthraquinone, saponins and protein [
10,
11].
Quantitative Phytochemical Analysis
Using the Folin-Ciocalteu technique, the total phenolic content of
Sennaalata extracts was determined [
12] using certain adjustments, and the results were expressed as gallic acid (GA) equivalents in milligrams (mg) per gram of dry leaf extract (mg GAE/g).
The aluminum chloride technique was used to determine the total flavonoid content [
13] using a few minor adjustments, and the total flavonoid were expressed as milligrams of quercetin equivalents (QE) per gram of extract from dried leaves (mg QE/g).
DPPH Free Radical Scavenging Assays
The prior methodology was utilized to assess the
Sennaalata leaves extract’s capacity to scavenge DPPH free radicals [
14]. To put it briefly, 2 mL of DPPH solution (60 μM) was added to 2 mL of ethanolic and aqueous extracts at varying concentrations (0.1-100 μg/ml). Next, incubate in the dark at 25
oC for 30 minutes. For the positive control, ascorbic acid (AA) was used. A measurement of absorbance at 517 nm revealed that the reaction combination’s reduced absorbance indicated increased free radical scavenging activity [
15,
16]. IC
50 value of the sample containing plant extracts—that is, the concentration required to scavenge 50% radicals was also determined. Each sample’s free radical inhibition activity was calculated using the following formula:
Where, A
control 517 is the control absorbance A
sample 517 is the plant extract sample absorbance
In Vitro Thrombolytic Activity
For assessing the clot-dissolving activity of
Senna alata, a plant extract, compared to positive (Streptokinase) and negative control (water). Blood samples water collected from 21 healthy volunteers and distribution into pre-weighed micro centrifuge tubes. Following clot formation, serum was extracted, and the weight of the clot was measured.
Senna alata extract, streptokinase, and water were added separately to different tubes. Following 90 minutes incubation, after removing any fluid that had leaked, the tubes were weighed again. The weight difference before and after clot lysis was calculated as a percentage of clot lysis. The experimental setup allowed the evaluation of
Senna alata effectiveness in dissolving blood clots and comparing it to the controls [
17].
Where, Clot weight = W
2-W
1
W1 = weight of tube alone, W2 = weight of clot containing tube, W3 = final weight of tube with test
Brine Shrimp Lethality Bioassay
In this experiment, the lethality test for brine shrimp was utilized to assess the cytotoxic potential of a plant extract [
18]. Six different concentrations of the extract were tested, ranging from 800 μg/ml, 400 μg/ml, 200 μg/ml, 100 μg/ml, 50 μg/ml. After a 24 hour exposure, the number of surviving shrimp was recorded. Larvae showing no movement were considered dead. Negative control using Dimethyl sulfoxide and a Vincristine sulfate as reference standard were included. To make sure famine was not the cause of the observed death, comparisons were made with the control group.
The toxicity of the plant exracts was determined by calculating the median lethal concentration (LC
50) using probit analysis, as described by Finney (Singleton & Rossi, 1965). The Brine Shrimp lethality bioassay offers several advantages, including its rapidity, low cost, simplicity, and the ability to use a large number of organisms for statistical validation. It also requires minimal sample volume (2-20 mg or less) and does not necessitate animal serum, which is typically needed for other cytotoxicity assays.
Antibacterial Activity
Evaluation of Antibacterial Activity
To evaluate antibacterial activity, the agar well method was employed. In the method test, organisms were gathered, isolated as pure cultures, and standardized using the 0.5 M Mac-Farland standard [
19].
Microorganism Culture
It was decided that selectable microorganisms may be used for the investigation of antibacterial characteristics. The ATCC culture was obtained from the MMIHS laboratory, while the clinical isolates were obtained from the Natural Product Research Laboratory, Thapathali, Kathmandu. The creatures being assessed were:
Gram positive: Staphylococcus aureus (ATCC 6538P), Bacillus subtilis (ATCC6051)
Gram negative: E.coli (ATCC 8739), Klebsiella pneumoniae (ATCC700603)
Mueller Hilton Agar (MHA) petri plates that had just been made were used to cultivate the obtained bacteria. The organism was allowed to develop in peptone water broth for the purpose of standardization prior to the test. Before being injected into the petri plates, the organisms in peptone water broth were cultured for 4-5 hours.
Standard Preparation
The antimicrobial evaluation standard employed in the experiment consisted of (320, 160, 80, 40) μg/ml of a solution of Gentamycin and Azithromycin dissolved in 1% DMSO.
Sample Preparation
Different concentration of Normal extract solution (320, 160, 80, 40) μg/ml was dissolved in DMSO solution.
Test Procedure
The bore well diffusion method was employed for the test. According to the manufacturer’s directions, MHA agar was made. The agar was prepared and 15 minutes of autoclaving at 15 pounds of pressure. In a sterile laminar hood, sterile petri dishes were filled with agar. The sun could set. For the test method, bores were prepared using an 8mm well borer. Using a sanitized swab stick, the bacteria were extracted from the peptone water broth and swabbed in the petri plates. Using a micropipette with sterile tips, 100 g/L of the extracts were collected. For 5 different concentrations of the test extracts, 5 holes were punched into each plate. Standard and blank underwent a similar process. Following a 24-hour incubation period, the zones of inhibition were identified on the plates.
Statistical Analysis
The data was shown as mean ± standard deviation. The gallic acid and quercetin standard calibration curve was draw for estimation of phenolic and flavonoid content using Microsoft Excel’s regression line equation, 2007. The table and picture presented the results. The statistical analysis was carried out using SPSS version 16.
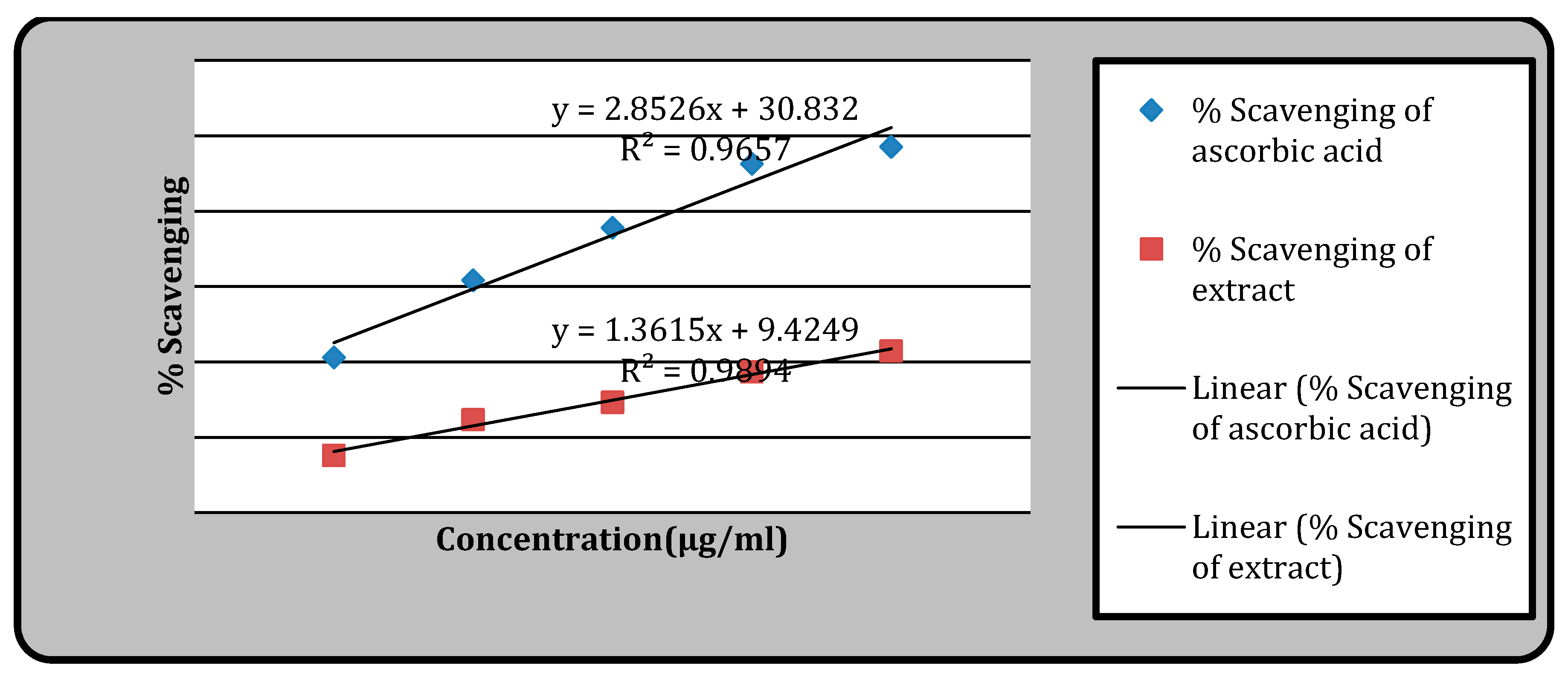
Figure 1.
Antioxidant activity by using DPPH.
Figure 1.
Antioxidant activity by using DPPH.
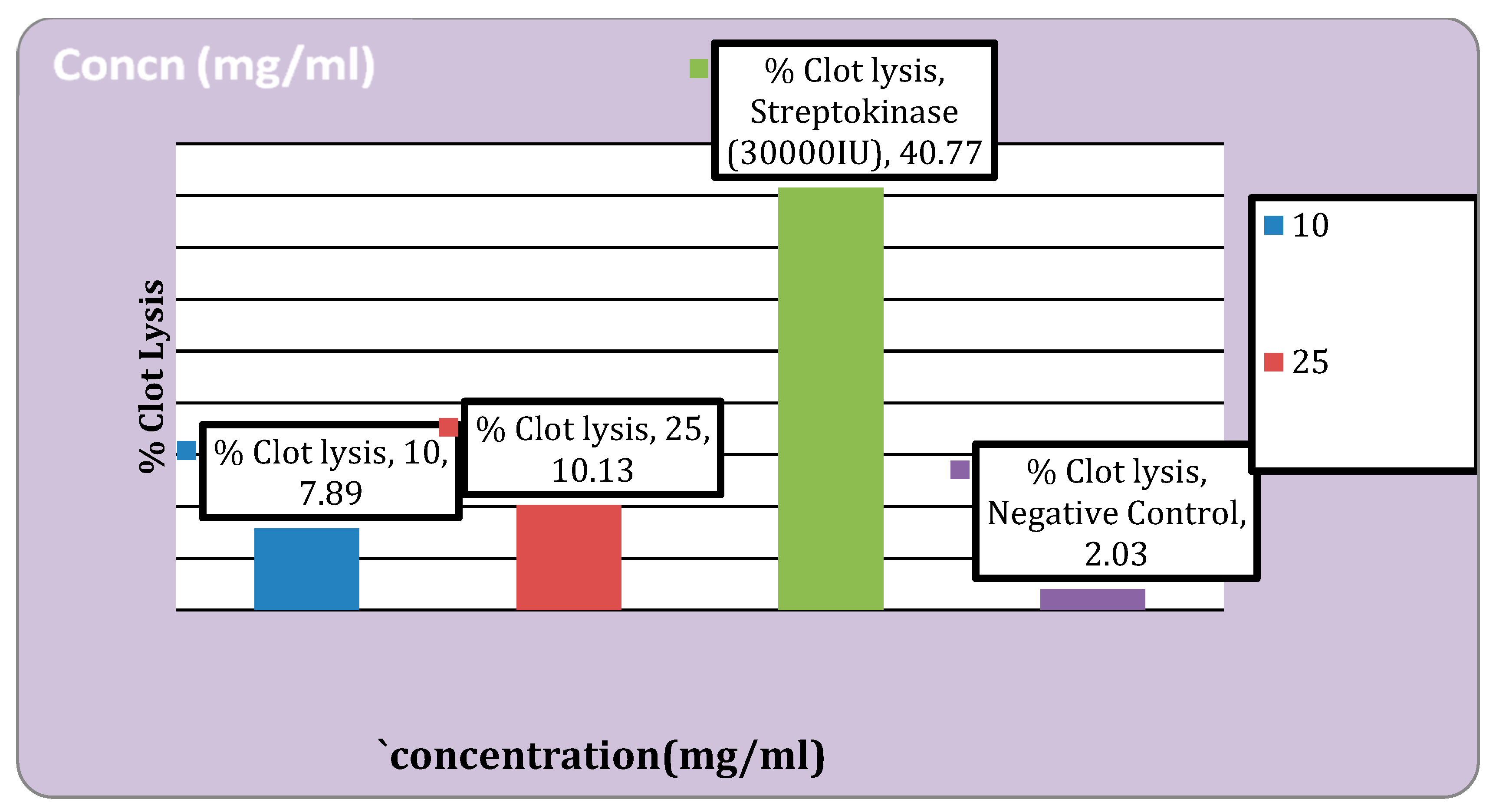
Figure 2.
Thrombolytic activity of the extract.
Figure 2.
Thrombolytic activity of the extract.
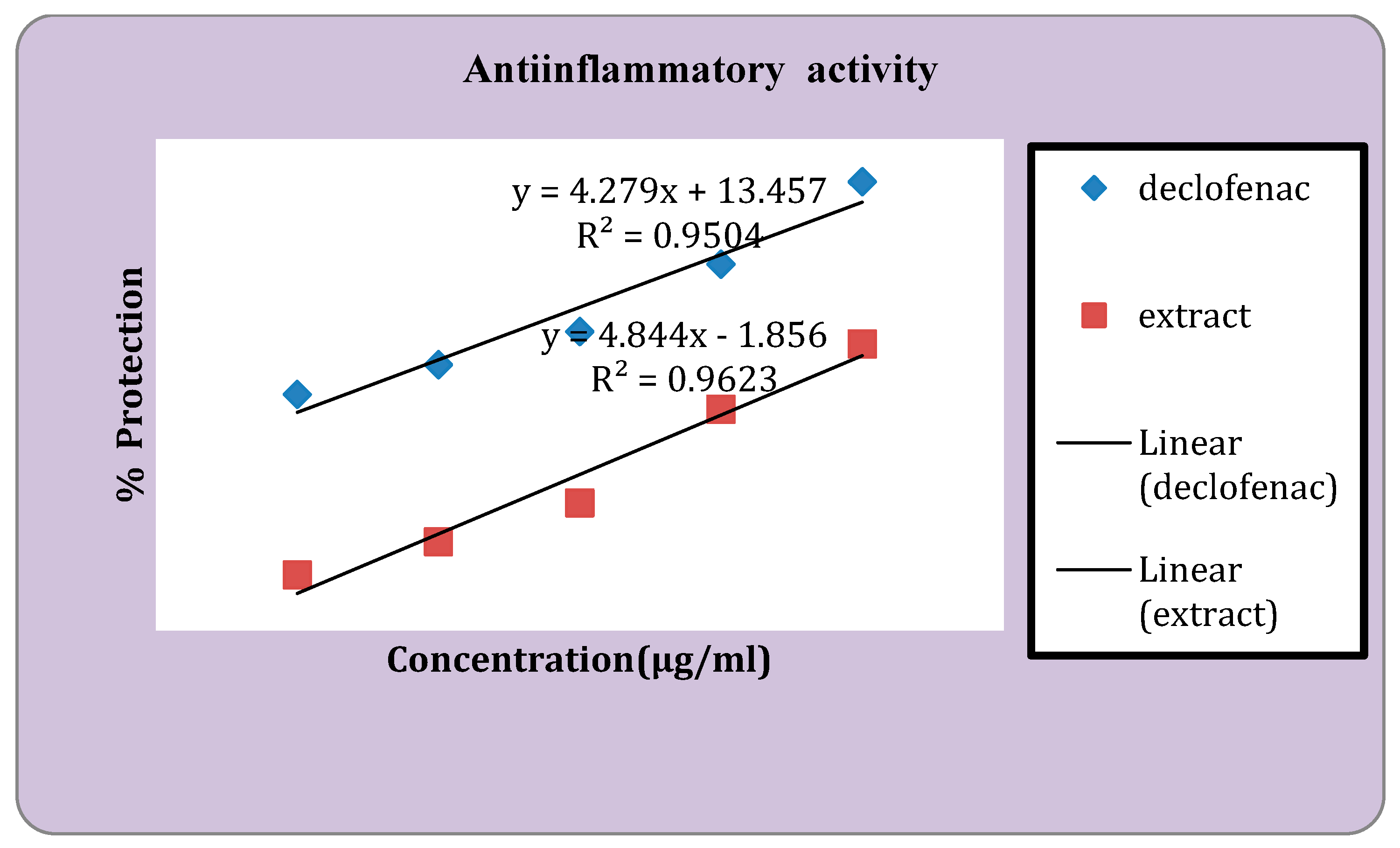
Figure 3.
Anti-inflammatory activity of standard and the extract.
Figure 3.
Anti-inflammatory activity of standard and the extract.
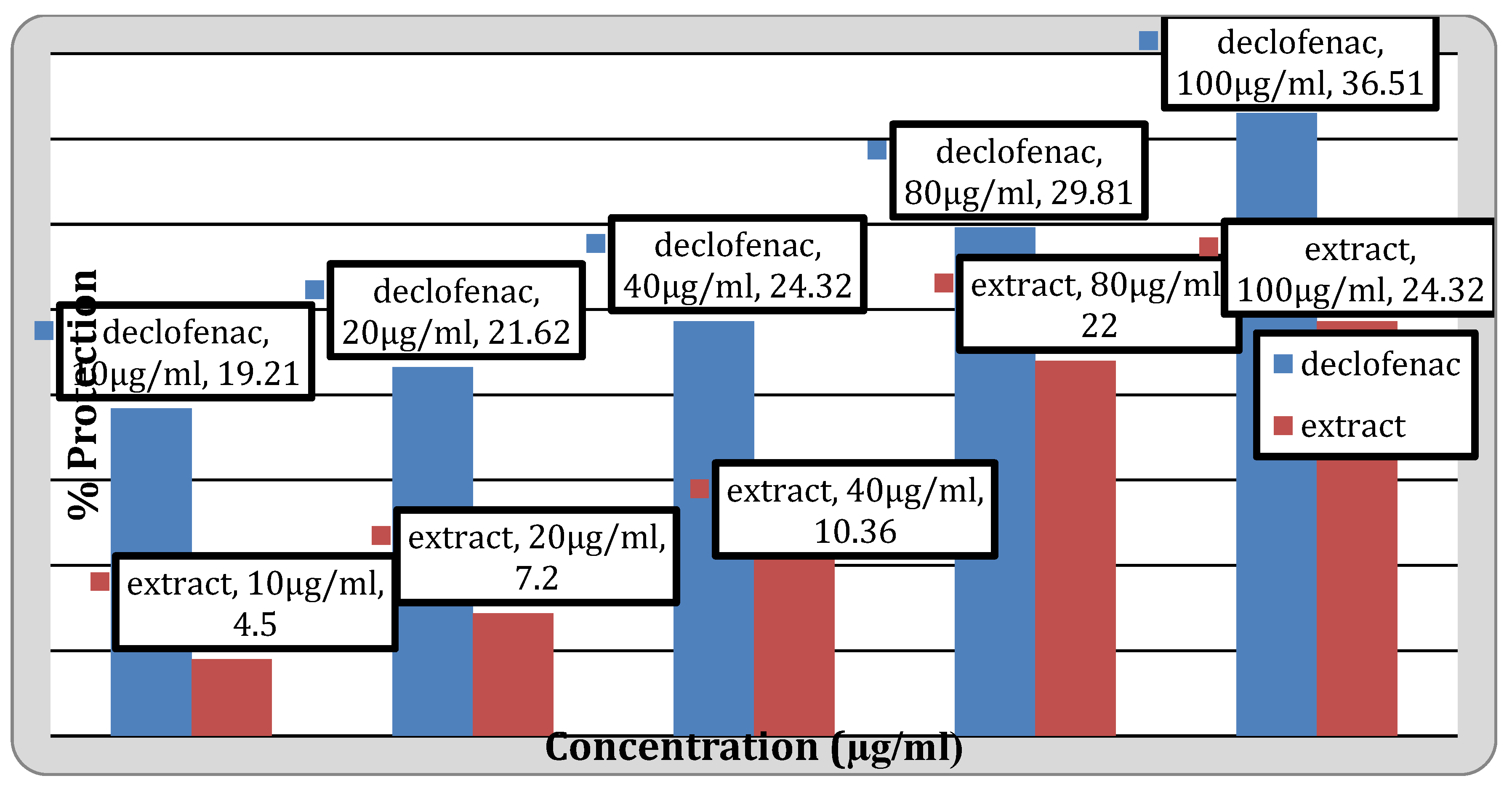
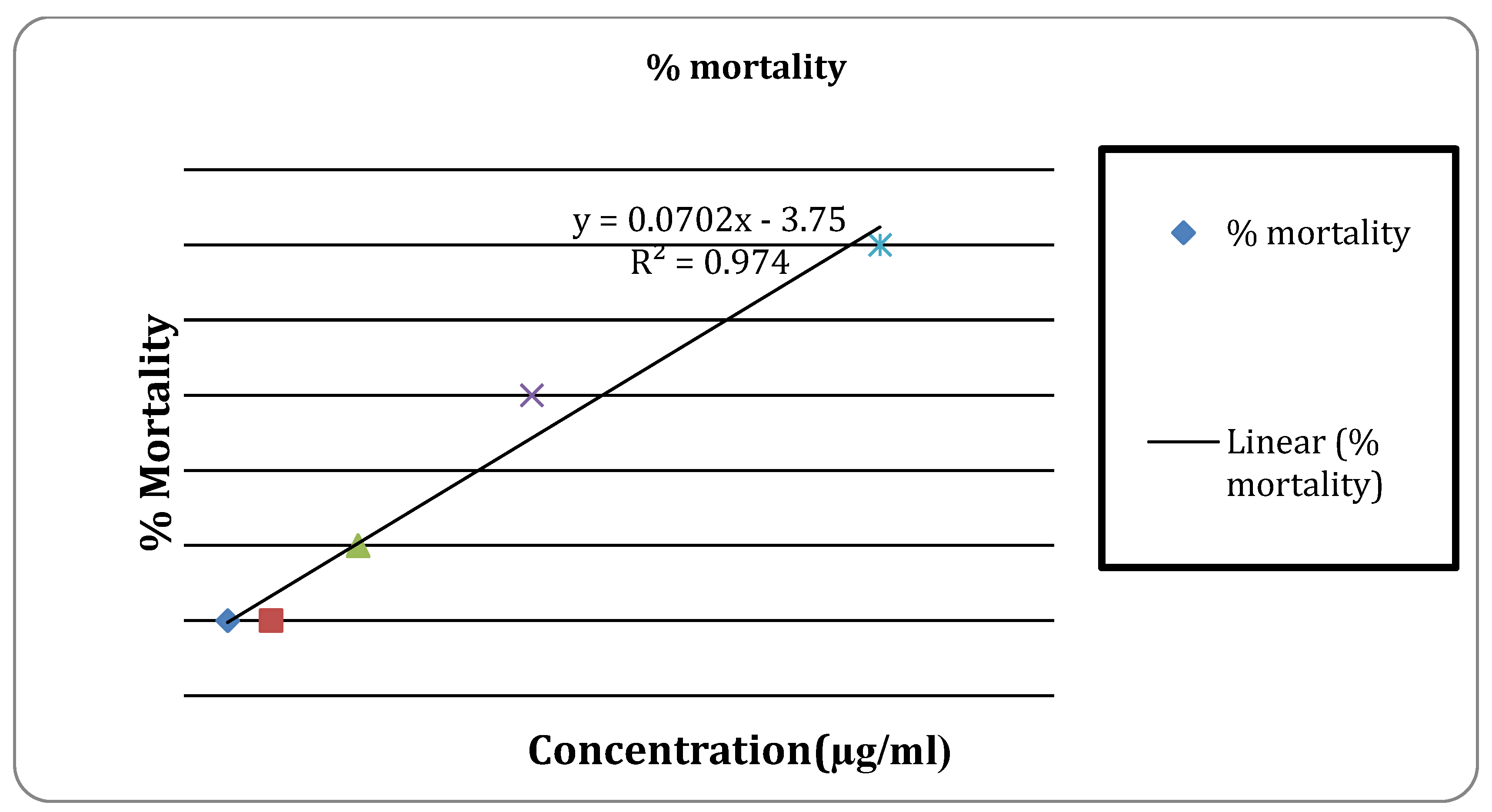
Figure 4.
Anti-inflammatory activity of the extract.
Figure 4.
Anti-inflammatory activity of the extract.
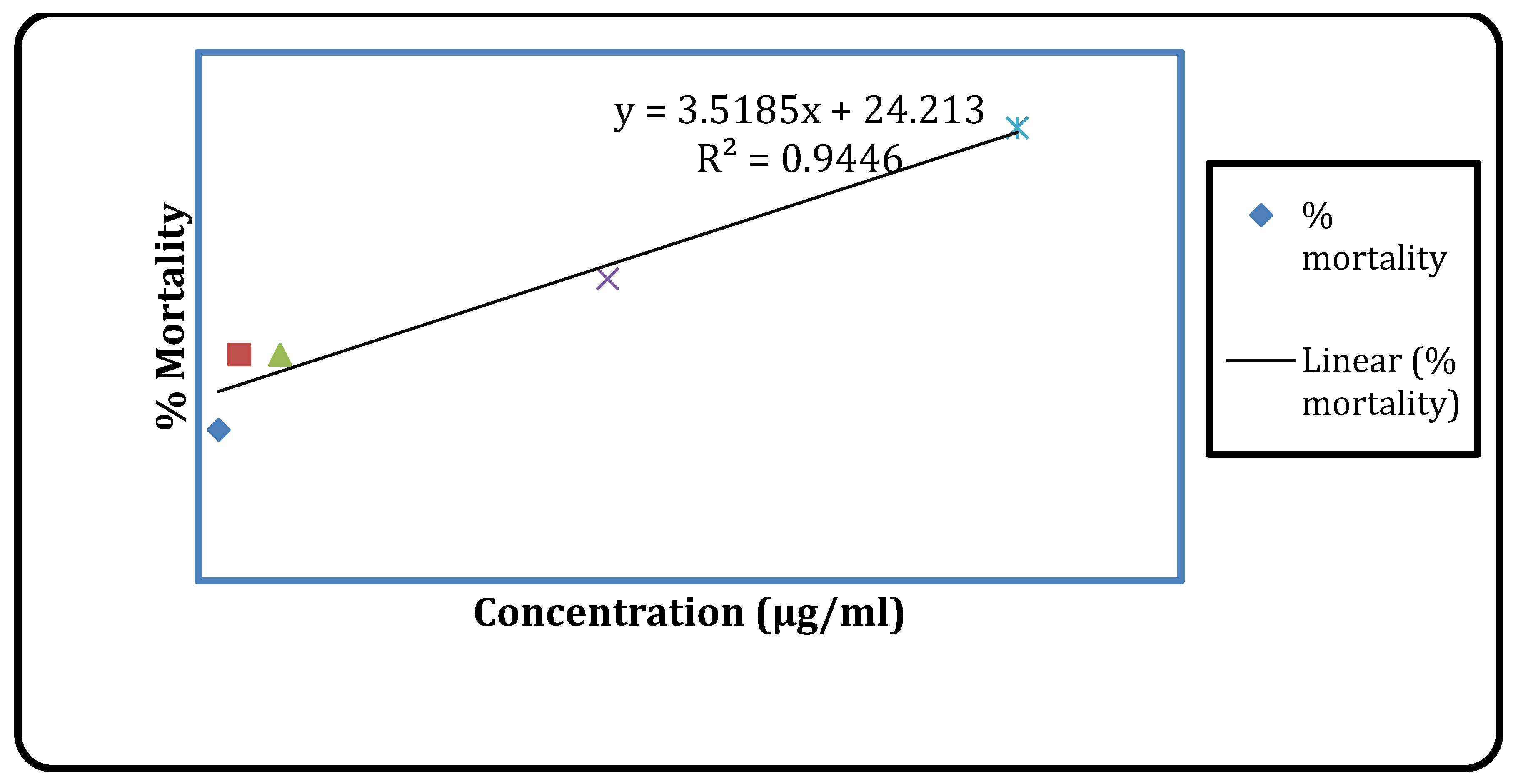
Figure 5.
Cytotoxic activity of vincristine sulphate.
Figure 5.
Cytotoxic activity of vincristine sulphate.
Figure 6.
Cytotoxic activity of the extract.
Figure 6.
Cytotoxic activity of the extract.
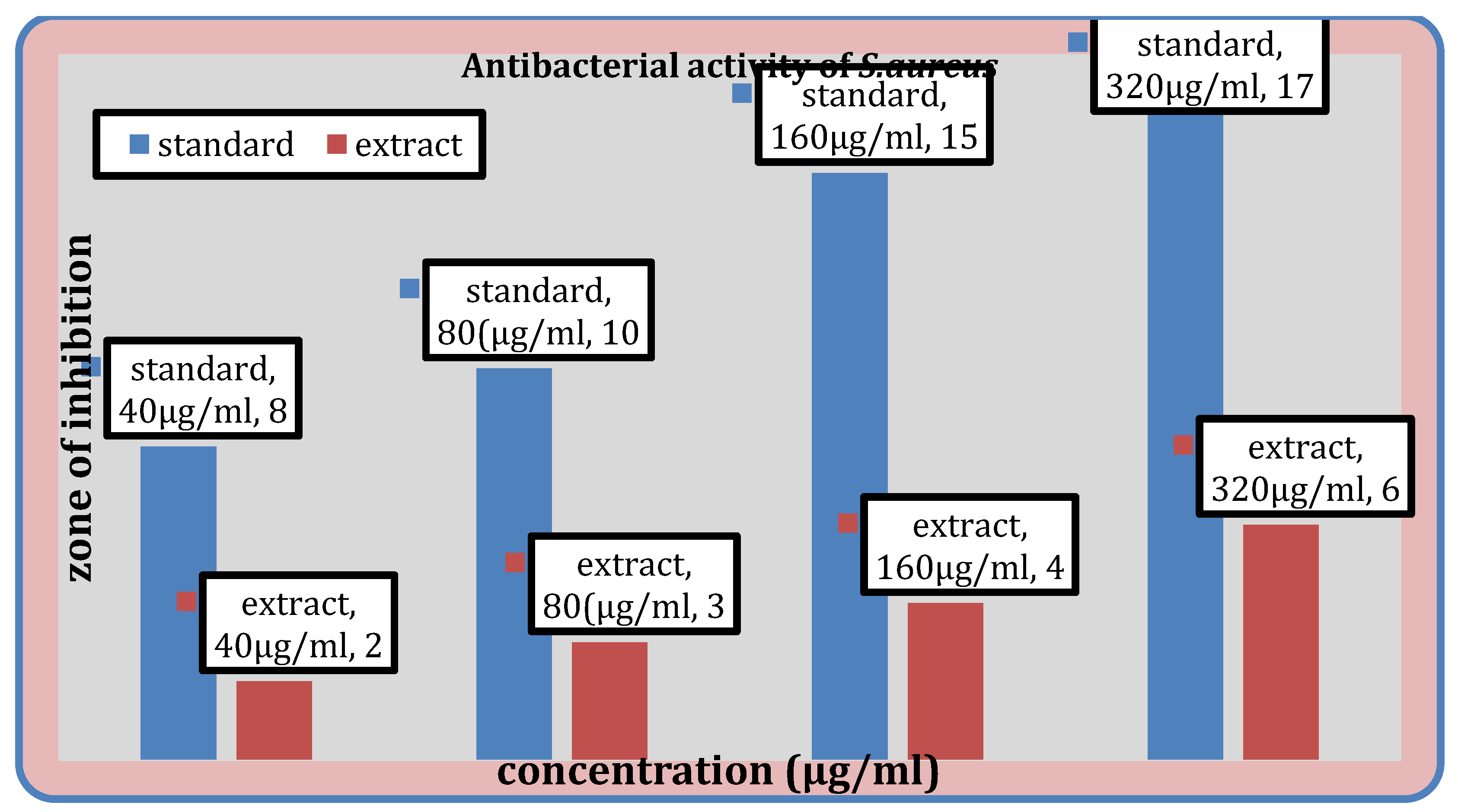
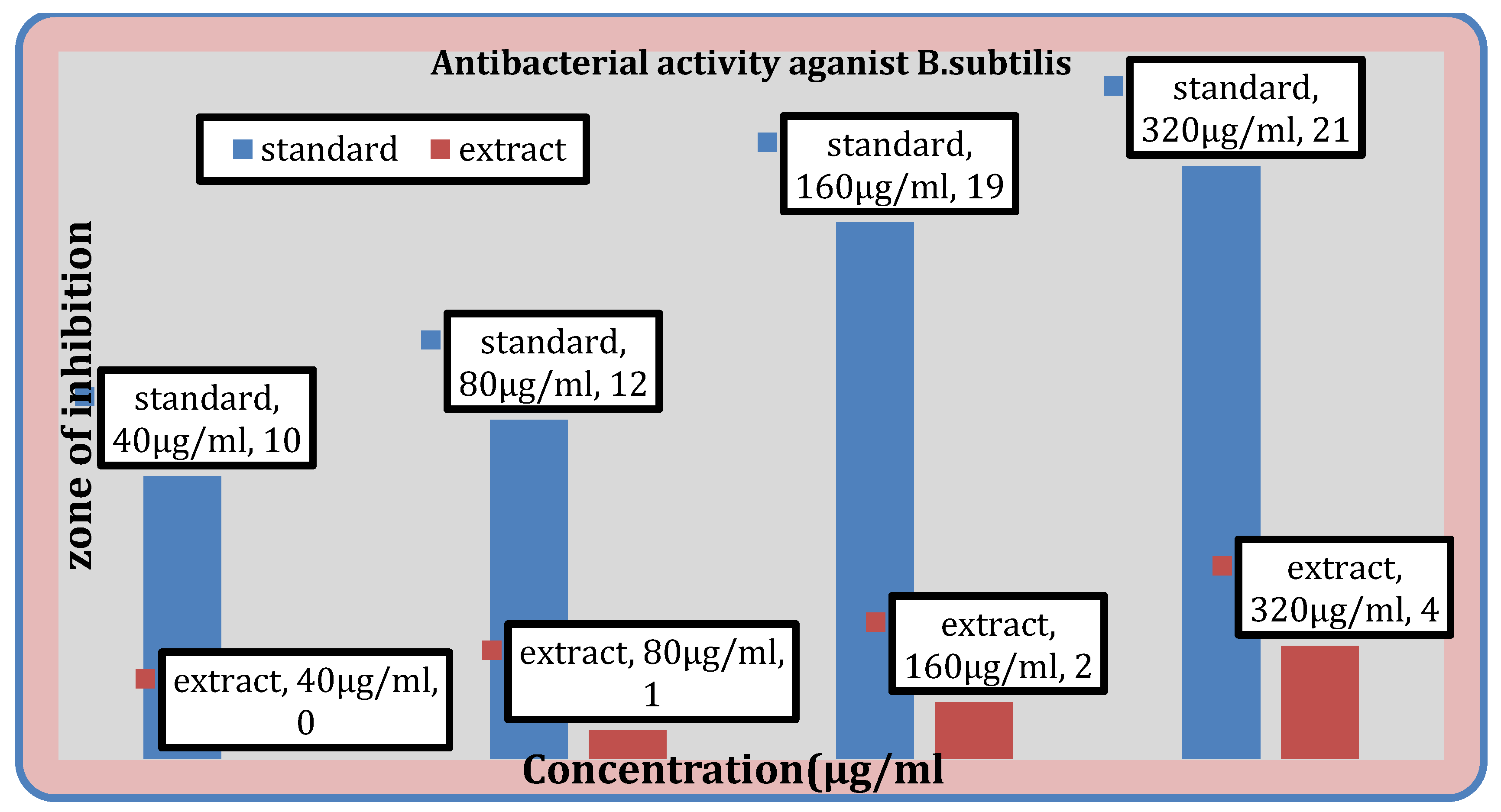
Figure 7.
Antibacterial activity of the extract against S. aureus, B. subtilis.
Figure 7.
Antibacterial activity of the extract against S. aureus, B. subtilis.
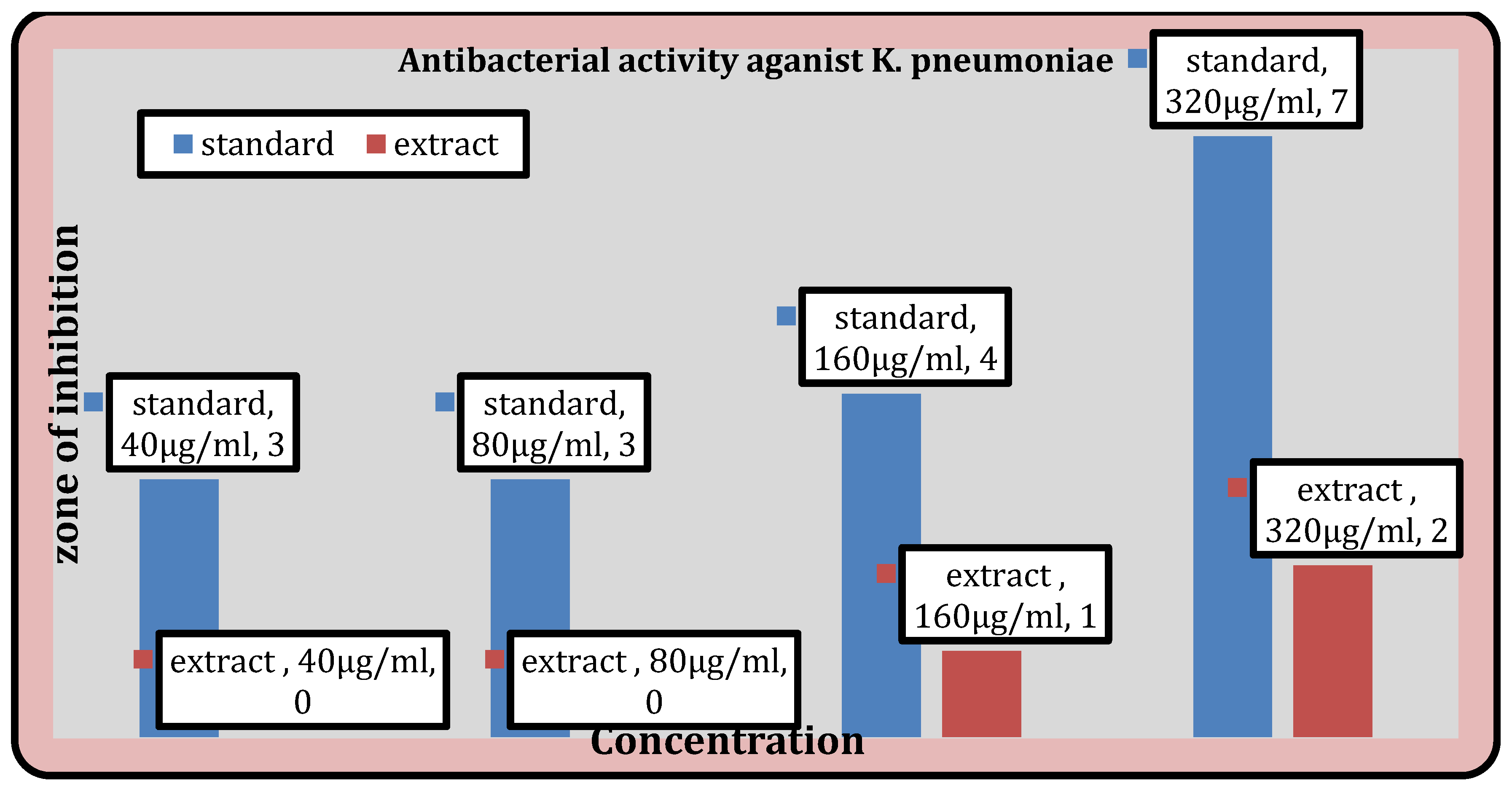
Figure 8.
Antibacterial activity of the extract against K. pneumoniae.
Figure 8.
Antibacterial activity of the extract against K. pneumoniae.
Results
Qualitative Phytochemical Analysis
Numerous phytoconstituents, including tannin, flavonoids, saponin, carbohydrates, terpenoids, and cardiac glycosides, were found in methanol extracts using qualitative phytochemical screening; these results are shown in
Table 1.
Table 1.
Phytochemical screening of water and ethanol extract of Sennaalataleaves.
Table 1.
Phytochemical screening of water and ethanol extract of Sennaalataleaves.
| Phytochemical constituents |
Specific tests |
Result |
| Methanol extract |
|---|
| Alkaloid |
Mayer’s test |
- |
| Wagner test |
- |
| Hager’s test |
- |
| Dragendroff’s test |
- |
| Carbohydrate |
Molish’s test |
+ |
| Benedict’s test |
+ |
| Glycoside |
Modified Borntrager test (Anthraquinones) |
- |
| Killer killiani test (Cardiac glycoside) |
+ |
| Saponin |
Foam test |
+ |
| Phenol |
Ferric chloride test |
+ |
| Flavonoid |
Alkaline reagent test |
+ |
| Shinoda test |
+ |
| Zn-HCl test |
+ |
| Tannin |
Gelatin test |
+ |
| Ferric Chloride test |
+ |
| Terpenoid |
Salkowaski test |
- |
| Copper acetate test |
- |
| +: Presence, -: Absence |
Table 2.
GC –MS analysis.
Table 2.
GC –MS analysis.
| S.N |
Name of Compound |
Molecular formula |
Reported Activity |
|
| 1 |
3-Methylmannoside |
C7H14O6 |
Plant growth regulator/ regulate plant growth by modulating glycoconjugation to lectins in plants |
| 2 |
Neophytadiene |
C20H38 |
Antimicrobial, additive for liquid cigarette |
| 3 |
Squalene |
C30H50 |
Antioxidant |
| 4 |
Campesterol |
C28H48O |
Anticancer, Antimicrobial, anti-inflammatory |
| 5 |
Stigmasterol |
C29H48O |
Anticancer, Antiinflammatory |
| 6 |
Alpha.Tocospiro |
C29H50O4 |
Cytotoxicity against human A549 cells by SRB assay. |
| Antimicrobacterial activity against Mycobacterium tuberculosis H37Rv |
| |
Table 3.
Extraction yield (%) of three solvents of Sennaalata leaves, total phenolic and flavonoids content.
Table 3.
Extraction yield (%) of three solvents of Sennaalata leaves, total phenolic and flavonoids content.
| Extract |
Extraction yield (%) |
Phenols (mg GAE/g dry extract weight) |
Flavonoids (mg QE/g dry extract weight) |
| Methanol extract |
5.92 |
46.36±4.5 |
480.4±3.055 |
| Hexane extract |
1.54 |
44.89±4.49 |
476.17±4.33 |
| Ethyl acetate |
0.78 |
40.66±0.36 |
435.77±4.81 |
| Values calculated from the mean of three times experiment and represented as mean ± S.D |
Table 4.
IC50 value and DPPH free radical scavenging activity of both Sennaalata extracts at varying concentrations.
Table 4.
IC50 value and DPPH free radical scavenging activity of both Sennaalata extracts at varying concentrations.
| Extract/ Standard |
% activity of DPPH scavenging |
|
IC50 µg/mL |
| 5 µg/mL |
10 µg/mL |
15 µg/mL |
20 µg/mL |
25 µg/mL |
| Methanol extract |
15.13±0.0093 |
24.65±0.0063 |
29.28±0.0094 |
37.33±0.0290 |
42.83±0.0049 |
29.81 |
| Ascorbic acid |
41.19±0.0090 |
61.69±0.0012 |
75.59±0.0050 |
92.56±0.0050 |
97.07±0.0080 |
6.12 |
| |
Values calculated from the mean of three times experiment and represented as mean ± standard deviation (n=3). |
Table 5.
Percentage clot lysis of extract.
Table 5.
Percentage clot lysis of extract.
| S.N |
Concentration(mg/ml) |
% clot lysis |
| Extract 1 |
10 |
7.89 |
| Extract 2 |
25 |
10.13 |
| Streptokinase |
30,000 I.U |
40.77 |
| Negative control |
D/W |
2.03 |
| |
|
|
Table 6.
Percentage protection and percentage hemolysis of extract and standard.
Table 6.
Percentage protection and percentage hemolysis of extract and standard.
| Concentration(μg/ml) |
Percentage Protection |
% hemolysis |
| Diclofenac |
Extract |
Diclofenac |
Extract |
| 10 |
19.21 |
4.5 |
80.79 |
95.46 |
| 20 |
21.62 |
7.4 |
78.38 |
92.75 |
| 40 |
24.32 |
10.32 |
75.68 |
89.62 |
| 80 |
29.81 |
18.16 |
70.19 |
77.90 |
| 100 |
36.51 |
23.32 |
63.49 |
75.17 |
Table 7.
EC50 values for Diclofenac and extract.
Table 7.
EC50 values for Diclofenac and extract.
| Name |
EC50 |
| Diclofenac sodium |
8.54 |
| Extract |
9.93 |
Table 8.
Percentage mortality by standard Vincristine Sulphate.
Table 8.
Percentage mortality by standard Vincristine Sulphate.
| Concn (μg/ml) |
% mortality |
LC50 value |
| 0.25 |
20 |
7.32 |
| 0.5 |
30 |
|
| 1 |
30 |
|
| 5 |
40 |
|
| 10 |
60 |
|
Table 9.
Percentage mortality of brine shrimp by extract.
Table 9.
Percentage mortality of brine shrimp by extract.
| Concentration (μg/ml) |
% Mortality |
LC50 (μg/ml) |
| 50 |
0 |
767.85 |
| 100 |
0 |
|
| 200 |
10 |
|
| 400 |
30 |
|
| 800 |
50 |
|
Table 10.
Antibacterial activity.
Table 10.
Antibacterial activity.
| Sample |
Concn (μg/ml) |
Inhibition zones of antibacterial screening (mm) |
| S. aureus |
B. subtilis |
K. pneumoniae |
E. coli |
| Azithromycin |
40 |
5 |
10 |
- |
- |
| 80 |
7 |
12 |
- |
- |
| 160 |
12 |
19 |
- |
- |
| 320 |
14 |
21 |
- |
- |
| Gentamycin |
40 |
- |
- |
3 |
8 |
| 80 |
- |
- |
3 |
9 |
| 160 |
- |
- |
4 |
13 |
| 320 |
- |
- |
7 |
15 |
| Extract |
40 |
2 |
- |
- |
- |
| 80 |
3 |
1 |
- |
- |
| 160 |
4 |
2 |
1 |
- |
| 320 |
6 |
4 |
2 |
- |
Quantitative Phytochemical Analysis
The methanol leaf extracts of
Senna alata exhibited the highest percentage of extraction yield (5.92%). Highest phenol content was observed in methanol extract (46.36±4.5) mg GAE/gm dry extract weight while highest flavonoid content was found in methanol extract (480.4±3.055) mg QE/gm dry extract weight (
Table 3).
DPPH Radical Scavenging Activity
Compared to conventional ascorbic acid, the methanol extracts of
Senna alata leaves (IC
50 value 29.81 µg/mL) demonstrated good DPPH free radical scavenging action (
Table 4).
Thrombolytic Activity
This study found that the percentage of clot lysis was 7.89% at 10 mg/ml and 10.13 % at 25 mg/ml. In a similar vein, clot lysis with conventional streptokinase was found to be 40.77%.
Anti-Inflammatory Activity
Human red blood cell (HRBC) membrane stabilizing method assessed the in vivo anti-inflammatory properties of
Senna alata extracts. As a standard, Diclofenac Sodium was used. The results obtained by studying in vivo anti-inflammatory activity are tabulated in
Table 6.
Cytotoxic Activity
Vincristine sulfate had an LC50 value of 7.32 (μg/ml), while Senna alata’s methanolic extract had an LC50 value of 767.85 (μg/ml).
Antibacterial Activity
Table 10 displays the methanolic extract’s antibacterial activity. While the extract does not demonstrate activity against
E. coli, it does demonstrate activity against
S. aureus,
B. subtilis, and
K. pneumoniae.
Discussion
It is widely accepted that natural products are the most valuable source of lead compounds for innovative drug development in the pharmaceutical industry. Bioactive plant components are used as drug candidates or drug substitutes to treat various human diseases [
20]. The selection of
Senna alata for this communication was based on its limited scientific research, traditional and ethnomedicinal uses.
In vitro antioxidant,
antibacterial, in vitro anti-inflammatory, thrombolytic, cytotoxic activities, phytochemical profiling and
Senna alata leafmethanolic extract was subjected to GC-MS analysis.
The percentage yield was found maximum in methanol i.e. 5.92 %. In the previous study, methanol extract of
Senna alata wasfoundthat 8.32% [
21]. This was mostly impacted by various cultivation circumstances, including climate, plant location, and harvest times. The solvent’s polarity has an impact on the phytochemicals that are recovered in the extracts.
The phytochemical analysis of
Senna alata extracts showed the occurrence of a variety of chemical components that may have different pharmacological effects, such as flavonoids, carbohydrates, tannins, saponins, and cardiac glycoside. An earlier investigation found that the
Senna alata plant’s root and leaf extracts have antimicrobial properties was done in Nigeria revealed that saponins, alkaloids, flavonoids, anthraquinone, tannins, phenols and glycosides are present in
Senna alata [
18]. Because they contain phenols and flavonoids, plant secondary metabolites have anti-inflammatory and antioxidant properties. They also have a favorable correlation with as antibacterial, cytotoxicity, and anti-inflammatory activity [
18].
The total phenolic content was determined using Folin-Ciocalteu’s technique in using standard agent as gallic acid. The leaves of
Senna alata had the highest quantity of total phenols (42.76±2.13 mg GAE/g dry extract weight) in the methanol extract. Using quercetin as a reference, the total flavonoid concentration was determined using the aluminum chloride colorimetric test. Of the two methanol extracts with flavonoid content, this one has a high concentration of flavonoids (34.97±2.86 mg QE/g dry extract weight). It demonstrates how important a role the solvent system plays in the solubility of various chemical components. It has been demonstrated that higher polarity solvents remove phenolic chemicals from the entire plant more effectively than lower polarity solvents [
22]. This result agrees with the previous study [
23]. Similar previous study was performed on methanol extract of this plant showed that 41.6±0.41 mg GAE/g and 31.9±0.63 QE/g of dried extract [
21].
The various samples’ DPPH radical scavenging ability was tested at various doses (at 5, 10, 15, 20, and 25 µg/ml) methanol extract revealed the concentration-dependent radical scavenging activity. Ascorbic acid’s IC
50 value in the DPPH scavenging method was 6.62 (μg/ml), while plant extract’s IC
50 value was 29.81 (μg/ml). Previous study conducted by J.Sujatha, S.Asokan showed that the IC
50 value was 24.56 µg/ml [
24]. According to this study, the IC
50value decreased as the phenolic and flavonoid concentration increased. The plant samples’ antioxidant activity could be attributed to the presence of these chemical ingredients [
25]. It has been demonstrated that plants with flavonoid and phenolic compounds have the ability to scavenge free radicals in living things [
24]. Plant metabolites known for their phenolic and flavonoid components are widely distributed and exhibit a variety of pharmacological properties, including antibacterial, antioxidant, hepatoprotective, antidiabetic, and antimutagenic properties [
26,
27]. The majority of compounds classified as antioxidants are derived from plants as secondary metabolites, such as phenolic compounds (flavonoids, phenolic acids, tocopherols, etc.) [
25]. Because they can scavenge reactive oxygen species such as superoxide free radicals, singlet oxygen, and hydroxyl radicals, phenolic compounds have the potential to be antioxidants [
28]. The many functional hydroxyl groups found in flavonoids mediate their antioxidant action by scavenging dangerous free radicals and chelating metal ions to prevent the generation of dangerous radicals that damage vital biomolecules. Lipid peroxidation is oxidative stress’s most frequent side effect. Through a variety of mechanisms, flavonoids play a significant role in lipid peroxidation against oxidative damage [
29].
This study showed that, lethal concentration (LC
50 value) for the
Sennaalata leaves extract was found to be 767.85 μg/ml and highest mortality percentage was 30 % at concentration of 800 μg/ml. In the earlier research carried out by M.A. Awal et al, it was found that the toxicity effect of ethanolic leaf and seed extract of
Cassia alata and found promising activity, rated that LC
50 value of 4.31μg/ml for seed and 5.29μg/ml for leaf [
18]. The phytochemicals present in plant such as alkaloids, flavonoids are believed to have anticancer activity which can inhibit either initiation or progression of the tumors. The absent of alkaloids may be causes for relatively lower value of cytotoxicity activities.
In the anti-inflammatory activity, the percentage protection at 100μg/ml was found to be 36.51% and 23.32% for standard drug Diclofenac sodium and extract respectively. It was discovered that the thrombolytic activity at a concentration of 10 mg/ml was 7.89% and at 25 mg/ml it was 10.13 %. In the earlier investigation carried out by Adnan Mannan et al. extract of cassia seed showed 37.92% clot lysis when the amount of 100µl/ml [
17].
The ability of test substance to inhibit the growth is confirmed by the appearance of the zone of inhibition. The largest zone of inhibition was found to be against
Streptococcus aureus i.e. 6mm and for
Bacillus subtilis it was found to be 4 mm. Also the extract didn’t showed any action against the E.
coli and for
Klebsiella pneumoniae, it was found to be 2mm. In the previous study conducted by AA.Ogunjobi and M.A Abiala, the methanol extracts of
Senna alatapowder inhibited the growth of
Staphylococcus aureus, and
Bacillus subtilis with inhibition zone diameter of 15 mm and 12mm respectively [
30].
The current investigation of Senna alata revealed the existence of increased levels of phenolic and flavonoid components together with strong antioxidant and anti-inflammatory activity, thrombolytic activity, cytotoxic activity and antimicrobial activity. Given that it may be a likely contender for the development of a novel oral therapeutic agent.
Conclusions
In conclusion, high extract yields was observed when Senna alata leaves were extracted with methanol, resulting in a large amount of flavonoids and phenolic compounds with significant antioxidant activity. From the GC-MS analysis, it can be concluded that Senna alata has the highest area ratio of 3-Methylmannoside.This study suggests that methanol extract from Senna alata leaves has potential anti-inflammatory, thrombolytic, cytotoxic, and antibacterial effects. Ultimately, this study supports additional scientific confirmations, investigations, and research to determine the potential therapeutic benefits of this medicinal plant in suppressing microorganisms, inflammatory diseases, and harmful cell proliferation-related diseases.
Author Contributions
DK and BR conceived and designed the experiments. DK, BP, PJ, AA performed the experiments. BP, DPK, BR and SP analyzed the data. DK, PJ, BP and AA wrote the manuscript. BP, DPK, BR and SP reviewed the manuscript. SP and BP critically revised and provided intellectual input. DPK and BR supervised the project.
Funding
The author(s) received no specific funding for this work.
Data Availability Statement
All relevant data is in the paper and if any query regarding the finding of this study is obtainable from the corresponding author upon request.
Acknowledgments
The authors are thankful to the Department of Pharmacy, Manmohan Memorial Institute of Health Sciences, Kathmandu, Nepal for providing the research facilities and the laboratory facilities throughout the research.
Conflicts of Interest
The authors declare no conflict of interest.
Approval of the research protocol by an Institutional Reviewer Board and the approval number
Ethical clearance certificate was acquired from the Institute Review Committee of the Department of Pharmacy, Manmohan Memorial Institute of Health Sciences, Kathmandu indicating that the study was carried out with utmost meticulousness and void of any potential threat to human or the environment (Approval No:MMIHS-BP-2018).
Informed Consent
The informed consent of the volunteering human blood donor was duly solicited before their participation. Appropriate permission to research on the study plant was duly solicited from the local legislations.
References
- Ibrahim D, Osman H. Antimicrobial activity of Cassia alata from Malaysia. J Ethnopharmacol. 1995;45(3):151-6. [CrossRef]
- Parveen A, Parveen B, Parveen R, Ahmad S. Challenges and guidelines for clinical trial of herbal drugs. J Pharm Bioallied Sci. 2015;7(4):329-33. [CrossRef]
- Sagnia B, Fedeli D, Casetti R, Montesano C, Falcioni G, Colizzi V. Antioxidant and anti-inflammatory activities of extracts from Cassia alata, Eleusine indica, Eremomastax speciosa, Carica papaya and Polyscias fulva medicinal plants collected in Cameroon. PLoS ONE. 2014;9(8). [CrossRef]
- Carmona F, Pereira AMS. Herbal medicines: old and new concepts, truths and misunderstandings. Revista Brasileira de Farmacognosia. 2013;23(2):379-85. [CrossRef]
- Dembinska-Kiec A, Mykkänen O, Kiec-Wilk B, Mykkänen H. Antioxidant phytochemicals against type 2 diabetes. British Journal of Nutrition. 2008;99(E-S1):ES109-ES17. [CrossRef]
- Rodrigues HG, Diniz YS, Faine LA, Galhardi CM, Burneiko RC, Almeida JA, et al. Antioxidant effect of saponin: potential action of a soybean flavonoid on glucose tolerance and risk factors for atherosclerosis. Int J Food Sci Nutr. 2005;56(2):79-85. [CrossRef]
- Abou El-Soud NH, Khalil M, Hussein J, Oraby F, Farrag AH. Antidiabetic effects of fenugreek alkaliod extract in streptozotocin induced hyperglycemic rats. J Appl Sci Res. 2007;3(10):1073-83.
- Zhang YJ, Gan RY, Li S, Zhou Y, Li AN, Xu DP, et al. Antioxidant Phytochemicals for the Prevention and Treatment of Chronic Diseases. Molecules. 2015;20(12):21138-56. [CrossRef]
- Timothy S, Wazis C, Adati R, Maspalma I. Antifungal activity of aqueous and ethanolic leaf extracts of Cassia alata Linn. Journal of Applied Pharmaceutical Science. 2012;2(7):182-5.
- Evans, WC. Trease & Evans Pharmacognosy (15Th Edition): Elsevier (A Divisionof Reed Elsevier India Pvt. Limited); 2002.
- Yadav M, Chatterji S, Gupta SK, Watal G. Preliminary phytochemical screening of six medicinal plants used in traditional medicine. Int J Pharm Pharm Sci. 2014;6(5):539-42.
- Singleton VL, Rossi JA. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. American Journal of Enology and Viticulture. 1965;16(3):144. [CrossRef]
- Arvouet-Grand A, Vennat B, Pourrat A, Legret P. [Standardization of propolis extract and identification of principal constituents]. J Pharm Belg. 1994;49(6):462-8.
- Molyneux, P. The use of the stable free radical diphenylpicrylhydrazyl (DPPH) for estimating antioxidant activity. Songklanakarin J sci technol. 2004;26(2):211-9. [CrossRef]
- Shodehinde SA, Oboh G. Antioxidant properties of aqueous extracts of unripe Musa paradisiaca on sodium nitroprusside induced lipid peroxidation in rat pancreas in vitro. Asian Pac J Trop Biomed. 2013;3(6):449-57. [CrossRef]
- Blois, MS. Antioxidant determinations by the use of a stable free radical. Nature. 1958;181(4617):1199-200.
- Mannan A, Kawser MJ, Ahmed AA, Islam NN, Alam SM, Emon MAEK, et al. Assessment of antibacterial, thrombolytic and cytotoxic potential of Cassia alata seed oil. Journal of Applied Pharmaceutical Science. 2011(Issue):56-9.
- Awal M, Nahar A, Hossain MS, Bari M, Rahman M, Haque M. Brine shrimp toxicity of leaf and seed extracts of Cassia alata Linn. and their antibacterial potency. J Med Sci. 2004;4(3):188-93.
- Sedighinia F, Afshar AS, Asili J, Ghazvini K. Antibacterial activity of Glycyrrhiza glabra against oral pathogens: an in vitro study. Avicenna journal of phytomedicine. 2012;2(3):118.
- Kumar S, Pandey AK. Chemistry and biological activities of flavonoids: an overview. ScientificWorldJournal. 2013;29(162750). [CrossRef]
- Pamulaparthi A, Prathap VR, Banala M, Nanna RS. Total Phenolic, Flavonoid contents and Antioxidant assays in leaf extracts of Senna alata (L.) Roxb. Journal of Pharmaceutical Sciences and Research. 2016;8(9):981.
- Galanakis C, Goulas V, Tsakona S, Manganaris G, Gekas V. A knowledge base for the recovery of natural phenols with different solvents. Int J Food Prop. 2013;16:382-96. [CrossRef]
- Russo D, Valentão P, Andrade PB, Fernandez EC, Milella L. Evaluation of Antioxidant, Antidiabetic and Anticholinesterase Activities of Smallanthus sonchifolius Landraces and Correlation with Their Phytochemical Profiles. Int J Mol Sci. 2015;16(8):17696-718. [CrossRef]
- Aklima J, Mojumder S, Sikdar D. Total phenolic content, reducing power, antioxidative and anti-amylase activities of five Bangladeshi fruits. International Food Research Journal. 2014;21(1):119.
- Yadav R, Agrawal M. Phytochemical Analysis of Some Medicinal Plants. Journal of Phytology. 2011;3(12):10-4.
- Kumar S, Mishra A, Pandey AK. Antioxidant mediated protective effect of Parthenium hysterophorus against oxidative damage using in vitro models. BMC Complementary and Alternative Medicine. 2013;13(1):120. [CrossRef]
- Singh R, Singh S, Kumar S, Arora S. Evaluation of antioxidant potential of ethyl acetate extract/fractions of Acacia auriculiformis A. Cunn. Food Chem Toxicol. 2007;45(7):1216-23. [CrossRef]
- Mishra A, Kumar S, Pandey AK. Scientific validation of the medicinal efficacy of Tinospora cordifolia. ScientificWorldJournal. 2013;23(292934). [CrossRef]
- Kumar S, Pandey A. Antioxidant, lipo-protective and antibacterial activities of phytoconstituents present in Solanum xanthocarpum root. International Review of Biophysical Chemistry. 2012;3(3):42-7.
- Ogunjobi A, Abiala M. Antimicrobial activity of Senna alata and Phyllanthus amarus. Global journal of pharmacology. 2013;7(2):198-202. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).