1. Introduction
Acute pulmonary embolism (PE) is a potentially life-threatening disease spanning a wide spectrum of clinical outcomes. PE is classified according to the 30-day risk of mortality based on clinical, imaging and laboratory findings [
1].
Reperfusion therapy with systemic thrombolysis is the treatment of choice in high-risk PE, however only a minority of such patients receive this treatment, due to comorbidities or the risk of major hemorrhagic side effects. Intermediate-risk PE accounts for 45% to 65% of PE, with a short-term mortality around 3% [
2]. Systemic thrombolysis is not generally recommended for this group, as hemorrhagic complications can overweight the benefits. However, patients at higher risk within this group (i.e intermediate-high risk patients) present a short-term mortality around 12%, suggesting the need of a more aggressive treatment [
2].
Minimally invasive reperfusion treatments, such as catheter-directed treatment (CDT) strategies, are currently suggested by ESC guidelines (class IIa, LOE C) for patients at high-risk PE and contraindications for systemic thrombolysis, or patients at intermediate-high risk PE and hemodynamic deterioration while on anticoagulation [
1]. One of these treatments is the ultrasound-assisted thrombolysis (USAT), which allows local infusion of a low-dose thrombolytic agent facilitated by ultrasounds.
Data regarding USAT are promising in terms of safety and efficacy, showing acute improvement of right ventricular (RV) function and a reduction in pulmonary artery systolic pressure (PASP), without major hemorrhagic complications [
3,
4]. There is, however, insufficient data on mid- and long-term outcomes of patients receiving such therapy and a lack of evidence to support widespread adoption of CDT for acute PE. We sought to present retrospective analyses as well as the rationale and design of the prospective USAT IH-PE registry.
2. Materials and Methods
2.1. Trial Design
The USAT IH-PE was a single center retrospective study recently upgraded into a multicenter prospective registry including all consecutive patients hospitalized in 5 centers (ASST Grande Ospedale Metropolitano Niguarda in Milan, IRCCS Ospedale San Raffaele in Milan, Ospedale San Giovanni Bosco in Turin, Spedali Civili in Brescia, Ospedale Sant’Anna in Como) with a diagnosis of intermediate-high and high risk PE treated with Ekosonic® device (Boston Scientific Corporation, Natick, MA, USA). Both retrospective and prospective studies were approved by each local ethical committee and conducted in agreement with the Helsinki II declaration, with written informed consent obtained from all participants.
Our manuscript includes the analysis of retrospective data from 102 patients admitted for acute intermediate-high and high risk PE between March 2018 and July 2023 and treated with EKOSTM at ASST Grande Ospedale Metropolitano Niguarda, prior to the expansion of the retrospective registry. Trial recruitment will be completed across the 5 Italian hospital sites in November 2026. Participants will be followed until the end of June 2027. We plan to report the results by December 2027.
The primary endpoint was the incidence of pulmonary hypertension (PH) at follow up. PH was defined by mean pulmonary artery pressure ≥ 20 mm Hg at rest during right heart catheterization in patients which presented PASP ≥ 40 mmHg at the echocardiographic follow-up. Secondary efficacy endpoints were short- and mid-term changes in echocardiographic parameters of RV function (tricuspid annular plane systolic excursion (TAPSE), RV/left ventricle (LV) ratio, pulmonary artery systolic pressure (PASP), TAPSE/PASP ratio), in-hospital mortality and all-cause mortality. Secondary safety endpoint were procedure-related bleeding according to the Bleeding Academic Research Consortium (BARC) criteria: major bleeding was defined as ≥ IIIa. PE risk was defined according to the current classification provided by ESC Guidelines, dividing patients in low, intermediate-low, intermediate-high and high risk. [
1] For the purpose of the current analysis we included both intermediate-high and high risk patients. Other inclusion criteria were: symptoms onset within the previous 14 days associated or not with deep venous thrombosis (DVT), confirmation of the PE by contrast-enhanced computed tomography with thrombotic occlusion in at least one of the main pulmonary arteries or one of the proximal lower lobe arteries, RV disfunction at echocardiography (defined as at least one of the following: RV/LV ratio > 1, TAPSE ≤ 16mm, TAPSE/PASP < 0.4) and intermediate to high probability of PH (PASP > 40 mm Hg). Exclusion criteria were: age < 18 years old, patients unable to give informed consent, pregnancy, fibrinolytic drugs in the previous 4 days, bleeding diathesis and/or known bleeding disorder, low platelet count (< 100.000/uL), gastrointestinal bleeding in the previous 3 months, active cancer with expected survival < 6 months, advanced chronic kidney disease (CKD, defined as estimated glomerular filtration rate < 30 ml/min/1.73 m
2 or patient on dialysis).
The EKOSTM system is implanted with fluoroscopic guidance. For unilateral PE in one main or proximal lobar pulmonary artery, one catheter was placed in the involved vessel. With bilateral PE in the main or proximal lobar pulmonary arteries, two catheters were placed, one in each of the involved vessels. Site of venous access (jugular or femoral) was at the discretion of the treating physician. Over 10 hours, 10 mg of alteplase per catheter are continuously administered with a rate of 1 mg/h. At the same time, an intravenous infusion of unfractioned heparin (UFH) is initiated with target aPTT 50-70 sec. After 10 hours, the therapy is stopped and the EKOSTM catheter removed.
In-hospital and mid-term (3-6 months) outcomes were evaluated from hospital records and follow-up assessments. Follow-up consisted of ambulatory visit with clinical assessment, 12-lead ECG and transthoracic echocardiography. The timing of follow up after discharge is planned considering the echocardiographic probability of pulmonary hypertension (PH), estimated by echocardiogram performed during hospitalization. Such probability may be judged as high, intermediate or low according to ESC PH guidelines [
5]. If the probability was judged low, follow up was performed at 6 months, while in case it was judged intermediate or high, follow up occurred at 3 months with echocardiography and PH ambulatory consultation. In case of persistence of intermediate or high probability of PH at follow-up, a perfusion imaging exam (V/Q scan) and right heart catheterization (RHC) are performed to confirm the diagnosis of chronic thrombo-embolic pulmonary hypertension (CTEPH).
2.2. Statistical Analysis
The distribution of continuous variables was described using median and interquartile range (IQR) while categorical variables were reported as absolute numbers and percentages. The incidence of PH, in-hospital death and mortality during follow-up were computed with 95% confidence intervals.
The distribution of TAPSE, PASP, RV/LV ratio and TAPSE/PASP ratio was compared between the three time points (T0, T24 and T-FU) using the Wilcoxon signed-rank test. The percentage of patients with TAPSE ≤16 mm and/or PASP ≥40 mmHg and/or RV/LV>1 was also calculated for the three time points and compared pairwise using McNemar test.
Statistical analyses were performed using the SPSS software (version 25.0, SPSS Inc., Chicago, IL, USA) and R statistical software version 4.2.2 (R Foundation for Statistical Computing, Vienna, Austria).
3. Results
Between March 2018 and July 2023, 102 patients affected by PE at intermediate-high and high risk were included. The majority of patients (86%, n = 88) had an intermediate-high risk PE. An overview of the patients’ baseline characteristics is provided in
Table 1. Briefly, median age was 66.5 years (IQR 56-74 years), with a female prevalence (56.9%, n=58) and high incidence of active cancer (28.4%, n= 29). Systemic thrombolysis was contraindicated for 19% of patients, of which 14% presented an absolute contraindication.
The majority of patients (n = 77, 76%) presented at CT scan a bilateral involvement of the main pulmonary arteries, 7 patients (7%) had an unilateral involvement of one of the main pulmonary arteries and 15 patients (15%) had a bilateral lobar involvement of the pulmonary arteries.
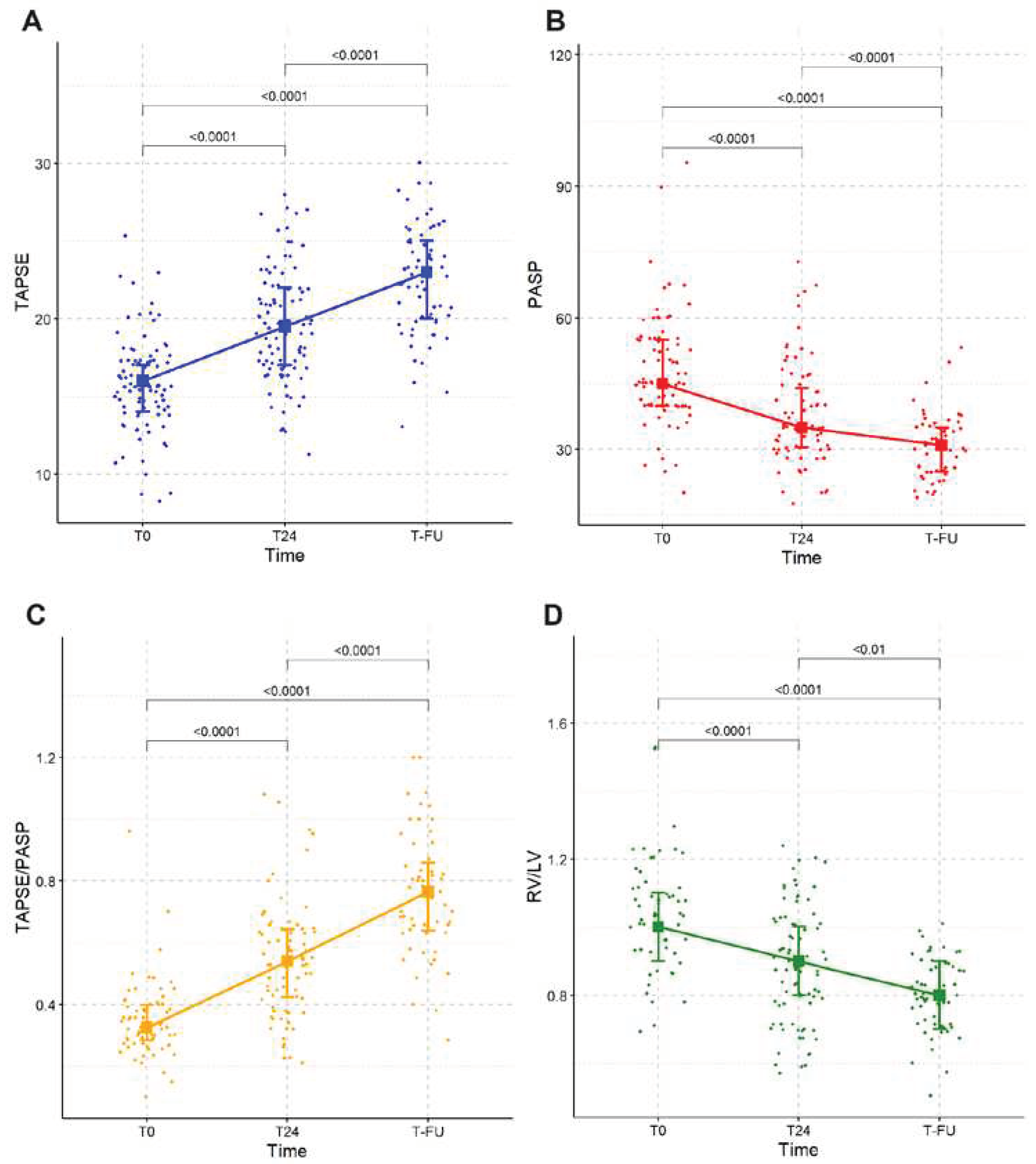
The echocardiographic evaluation at the time of PE diagnosis showed signs of RV impairment, such as median TAPSE 16 mm [IQR 14, 17], median RV/LV ratio 1.0 [IQR 0.9, 1.1], median TAPSE/PASP ratio 0.32 [IQR 0.28, 0.4] and a median PASP of 45 mmHg [IQR 40, 55].
3.1. Outcomes
3.1.1. The Primary Endpoint
For 32 patients, no echocardiographic follow-up data could be obtained after discharge, and they were excluded from the analysis. This resulted in a total of 70 patients who were analysed for the primary outcome. Only 2 patients had a PASP ≥ 40 mmHg at the echocardiographic evaluation and, of those, in only 1 patient the diagnosis of PH was confirmed by right heart catheterization. Hence, the risk of incidence of PH was 1.43% (CI 95%, 0.036-7.7) and rate of incidence 3.31 (CI 95%, 0.08-17.42) x 100 year-person.
3.1.2. Secondary Efficacy Endpoint
The changes in echocardiographic parameters of RV function are summarized in
Table 2. At 24 hours median TAPSE was 19.5 mm [IQR 17, 22], median PASP 35 [IQR 30.5, 44], median RV/LV ratio 0.9 [IQR 0.8, 1.0] and median TAPSE/PASP 0.54 [IQR 0.42, 0.64]. The median time of echocardiographic follow up was 144 days from discharge [IQR 90, 207]. At follow-up, median TAPSE was 23 [IQR 20, 25], median PASP 31 [IQR 25, 35], median RV/LV ratio 0.8 [IQR 0.7, 0.9] and median TAPSE/PASP ratio 0.76 [IQR 0.64, 0.86].
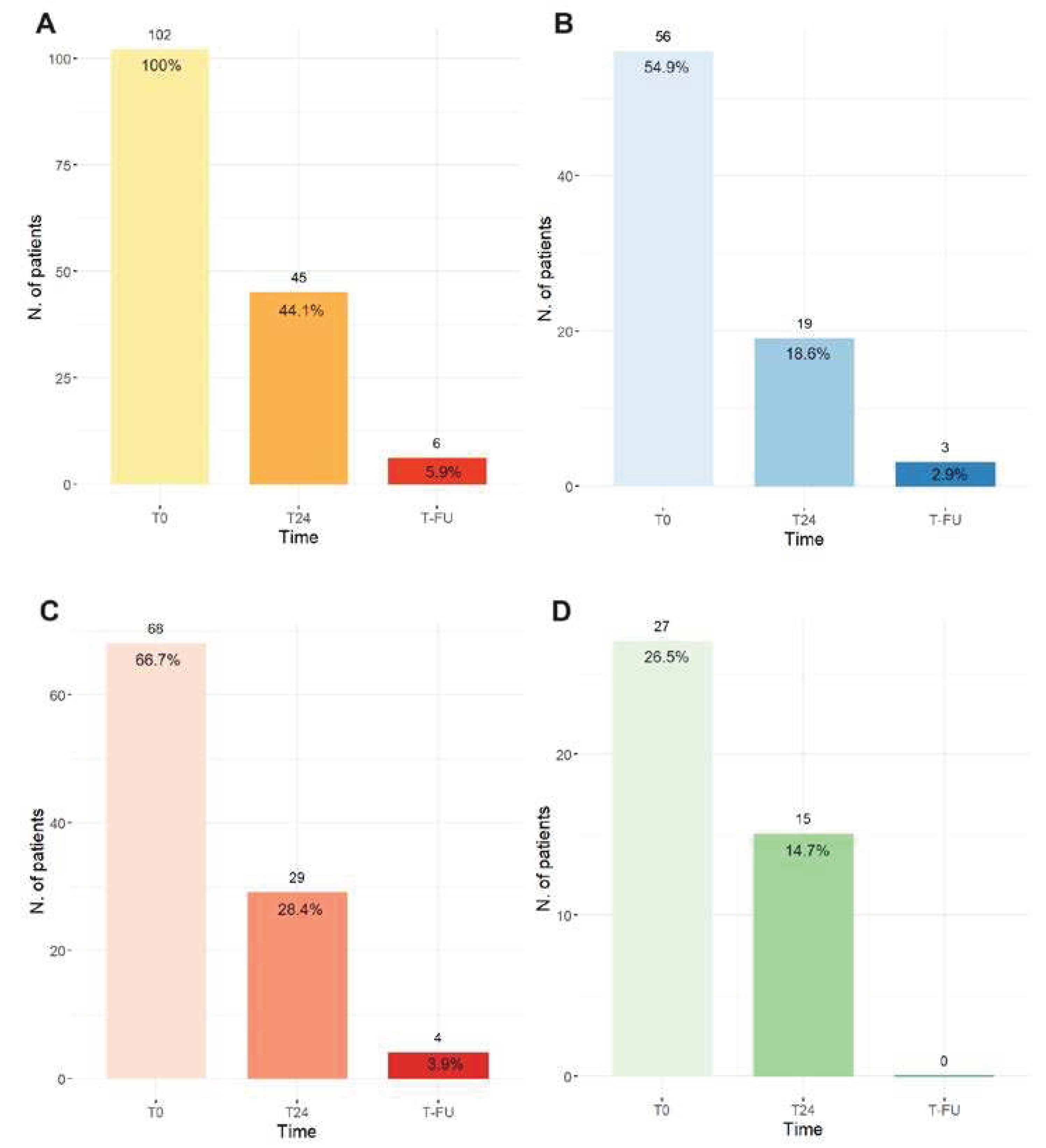
At the first echocardiographic assessment 24 hours after the treatment (T24), 44.1% of patients showed signs of RV failure (TAPSE ≤16 mm and/or PASP ≥40 mmHg and/or RV/LV>1) while at follow they were only 5.9% (
Figure 1 panel A).
Figure 1 (panel B, C and D) shows singularly the number of patients with TAPSE≤16 mm, PASP≥40 mmHg and RV/LV>1.
3.1.3. Mortality
Four patients died during the index hospitalization, resulting in an in-hospital mortality of 3.9% (CI 95%, 1.08-9.74). Ten patients died during follow up (11%), none for complications related to PE but mainly due to neoplastic disorders (n =7) or unknown cause (n=3). The incidence of all-cause mortality during follow-up was 11% (CI 95%, 5.4-19.2).
3.1.4. Secondary Safety Endpoint
The majority of patients (88%) had no bleeding complications. Of patients presenting any bleeding, only 4.9% were classified as major bleeding (BARC ≥ IIIa), with no reported intracranial or fatal bleeding.
4. Discussion
The main findings of our study can be summarized as follows: i) incidence of PH following USAT is low, ii) echocardiographic parameters of RV function improve both at short- (24 hours) and mid-term (3-6 months) follow-up, (iii) USAT is a safe procedure with low rate of bleeding events. Baseline characteristics of our population are comparable with the existing literature regarding this subject, in terms of age, female sex prevalence and BMI [
3,
4,
6,
7].
The main studies in literature concerning CDT focused primarily on short-term outcomes of efficacy (i.e improvement in RV function, defined with echocardiography) and safety. Indeed, a recent systematic review [
2] which aimed at including randomized controlled trials (RCTs) of CDT for the treatment of high-risk and intermediate risk acute PE, identified only one RCT in this specific setting [
6]. Moreover, as pointed out by the authors, the majority of trials investigated surrogate echocardiographic outcomes without considering relevant clinical outcomes. Therefore, the purpose of our study was to attempt to fill the gap by investigating a relevant primary outcome such as the development of PH and extending all analyses to mid-term follow up (3-6 months). Several RCTs on this topic are ongoing, among which the STRATIFY and the HI-PEITHO trials will specifically provide information regarding the incidence of PH at follow-up. However, only the HI-PEITHO trial requires confirmation of PH at RHC after having found elevated PAPS at echocardiography, as recommended by the ESC guidelines [
5]. In our study, the incidence of PH, defined as well by the confirmation at RHC of elevated PASP at echocardiography, was low (1.43%), with only one of the two patients with PASP ≥ 40 mmHg having the diagnosis confirmed at RHC.
When we considered echocardiographic parameters of RV function, all improved after the USAT treatment, both at 24 hours and at 3-6 months follow up. Among all considered parameters, RV to pulmonary arterial coupling, expressed as TAPSE/PASP ratio, deserves special considerations, given its validated prognostic role in several settings such as heart failure (HF) and PH [
8,
9,
10,
11]; by measuring the adaptation of the RV to its afterload, this parameter may help to detect pending RV failure. However, to the best of our knowledge, TAPSE/PASP ratio in patients undergoing CDT has not been evaluated to date. In a retrospective analysis of 627 patients, Lyhne et al found that a TAPSE/PASP ratio < 0.4 was an independent predictor for mortality in patients with PE [
12]. In our study, we found a median TAPSE/PASP ratio during the acute phase of 0.32 (0.28-0.4, p<0.0001) which improved, 24 hours after the end of USAT, up to a median of 0.54 (0.42-0.64, p<0.0001) and, at follow up, up to a median of 0.76 [0.64-0.86, p<0.0001], a value close to the validated normal range in literature (0.8–1.8) [
13,
14,
15].
In a recent 2022 meta-analysis [
16] of 65,589 patients with intermediate and high-risk PE treated with CDT, in-hospital mortality was 6.4%, reduced by half if compared to a previous meta-analysis published in 2009 (in-hospital mortality 13.6%) [
17]. The authors explained such reduction by the improvement in CDT techniques, the increased experience and the establishment of pulmonary embolism response teams (PERTs) for risk stratification and appropriate patient selection for CDT. In our study, in-hospital mortality was 3.9% (CI 95%, 1.08-9.74) and such even lower event rate could be due to the smaller sample size and the specific focus on USAT. During follow up, 10 patients (11%) died, none for complications related to PE but mainly due to neoplastic disorders or unknown cause. The risk of all-cause mortality was 11% (CI 95%, 5.4-19.2).
Regarding the safety endpoint, the majority of patients (88%) had no bleeding complications. Of patients presenting bleeding complications, only 4.9% were classified as major bleeding (BARC ≥ IIIa), with no reported intracranial or fatal bleeding. In the SEATTLE II study [
3], the rate major bleeding events was twice higher (10%), probably due to the different classification of bleeding events (GUSTO classification instead of BARC) and an higher prevalence of high risk PE (21% vs 14%).
One aspect of particular interest in our study is the relatively high prevalence of active cancer, which was present in about 1/3 of our population. As is it known from literature [
18], in cancer patients PE is the second cause of death after death due to the cancer itself. In this specific subset of PE patients, the best therapeutic strategy is not standardized yet, because of the coexisting increased risk of bleeding and recurrent thrombosis. Moreover, Weeda et al. [
19] and Shalaby et al. [
20] found that cancer patients are less likely to receive thrombolysis as compared to the non-cancer ones (OR = 0.55, 95% CI (0.48–0.64), and OR = 0.68, 95% CI (0.64–0.72), particularly in case of metastatic disease, probably due to a general overestimation of the bleeding risk. Unfortunately, no data on the incidence of bleeding or mortality was reported in these two studies. The underuse of systemic thrombolysis has consequently led to a less accurate estimation of the risk of bleeding in cancer patients who undergo thrombolysis.
A small number of cancer patients were included in the studies of CDT, and the isolated outcomes of this specific subgroup were not assessed [
3,
4,
6]. Therefore, in cancer patients, indications of CDT, as well as data on efficacy and safety are lacking. Considering that almost 1/3 of our population is represented by cancer patients, the positive results in terms of low incidence of PH, favorable efficacy and favorable safety, constitute important data if applied to a poorly represented population in other studies of CDT.
The main limitations of our study include its mainly retrospective nature, as well as the absence of a control arm. Moreover, all echocardiographic parameters were collected independently by each center without involving external validation by a core lab.
5. Conclusions
The ultrasound assisted CDT with EKOS system in patients with intermediate-high and high risk PE is associated with low incidence of PH at follow up, improvement in echocardiographic parameters of RV function and a safe profile. The relatively high percentage of cancer patients included in our study, generally underrepresented in the studies conducted in this field, provide important information regarding efficacy and safety in this specific subset of patients. This study adds valuable information about relevant clinical and mid-term outcomes. Data from the ongoing prospective registry as well as the upcoming results from RCTs will better define the role of CDT in patients at intermediate-high and high risk PE and the effects on clinically relevant outcomes.
Author Contributions
All the authors have made a meaningful contribution to this research paper. C.C., conceptualization, formal analysis, methodology, and writing original draft; N.C., conceptualization and methodology; M.I., conceptualization and supervision; M.A., conceptualization and supervision; G.V., conceptualization and investigation; I.B., conceptualization and methodology; L.V., conceptualization and investigation; C.T., conceptualization and investigation; F.M., conceptualization and methodology; R.G., conceptualization and methodology; L.O., conceptualization and methodology; G.C., conceptualization and supervision; A.R., conceptualization and supervision; F.M., conceptualization and methodology; G.DN., conceptualization and supervision; M.G., conceptualization and supervision; G.B., conceptualization and supervision; D.S., conceptualization and supervision; D.B., methodology and formal analysis; A.G., conceptualization and supervision; A.C., conceptualization and supervision; M.M., conceptualization and supervision; F.O., conceptualization, methodology and supervision A.S., supervision and writing—review and editing.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Ethics Committee of every Hospital involved.
Informed Consent Statement
Written informed consent has been obtained from the patients to publish this paper.
Acknowledgments
The authors are sincerely grateful to all the patients enrolled in this study form their willingness to participate and to provide all relevant information to support the analysis. We also thank the Fondazione Casiraghi for the support of the study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Konstantinides, S.V.; Meyer, G.; Becattini, C.; Bueno, H.; Geersing, G.J.; Harjola, V.P.; et al. 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur. Heart J. 2020, 41, 543–603. [Google Scholar] [CrossRef] [PubMed]
- Harvey, J.J.; Huang, S.; Uberoi, R. Catheter-directed therapies for the treatment of high risk (massive) and intermediate risk (submassive) acute pulmonary embolism. Cochrane Database Syst Rev. 2022, 2022, CD013083. [Google Scholar]
- Piazza, G.; Hohlfelder, B.; Jaff, M.R.; Ouriel, K.; Engelhardt, T.C.; Sterling, K.M.; et al. A Prospective, Single-Arm, Multicenter Trial of Ultrasound-Facilitated, Catheter-Directed, Low-Dose Fibrinolysis for Acute Massive and Submassive Pulmonary Embolism: The SEATTLE II Study. JACC Cardiovasc. Interv. 2015, 8, 1382–1392. [Google Scholar] [CrossRef] [PubMed]
- Kuo, W.T.; Banerjee, A.; Kim, P.S.; DeMarco, F.J.; Levy, J.R.; Facchini, F.R.; et al. Pulmonary Embolism Response to Fragmentation, Embolectomy, and Catheter Thrombolysis (PERFECT): Initial Results From a Prospective Multicenter Registry. Chest 2015, 148, 667–673. [Google Scholar] [CrossRef] [PubMed]
- Humbert, M.; Kovacs, G.; Hoeper, M.M.; Badagliacca, R.; Berger, R.M.F.; Brida, M.; et al. 2022 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur. Heart J. 2022, 43, 3618–3731. [Google Scholar] [CrossRef] [PubMed]
- Kucher, N.; Boekstegers, P.; Müller, O.J.; Kupatt, C.; Beyer-Westendorf, J.; Heitzer, T.; et al. Randomized, Controlled Trial of Ultrasound-Assisted Catheter-Directed Thrombolysis for Acute Intermediate-Risk Pulmonary Embolism. Circulation 2014, 129, 479–486. [Google Scholar] [CrossRef] [PubMed]
- Tapson, V.F.; Sterling, K.; Jones, N.; Elder, M.; Tripathy, U.; Brower, J.; et al. A Randomized Trial of the Optimum Duration of Acoustic Pulse Thrombolysis Procedure in Acute Intermediate-Risk Pulmonary Embolism: The OPTALYSE PE Trial. JACC Cardiovasc. Interv. 2018, 11, 1401–1410. [Google Scholar] [CrossRef] [PubMed]
- Tello, K.; Dalmer, A.; Axmann, J.; Vanderpool, R.; Ghofrani, H.A.; Naeije, R.; et al. Reserve of Right Ventricular-Arterial Coupling in the Setting of Chronic Overload. Circ. Heart Fail. 2019, 12, e005512. [Google Scholar] [CrossRef]
- Vanderpool, R.R.; Pinsky, M.R.; hc, D.; Naeije, R.; Deible, C.; Kosaraju, V.; et al. Right Ventricular-Pulmonary Arterial Coupling Predicts Outcome in Patients Referred for Pulmonary Hypertension. Heart 2015, 101, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Guazzi, M.; Bandera, F.; Pelissero, G.; Castelvecchio, S.; Menicanti, L.; Ghio, S.; et al. Tricuspid annular plane systolic excursion and pulmonary arterial systolic pressure relationship in heart failure: an index of right ventricular contractile function and prognosis. Am. J. Physiol. Heart Circ. Physiol. 2013, 305, H1373–H1381. [Google Scholar] [CrossRef] [PubMed]
- Tua, L.; Mandurino-Mirizzi, A.; Colombo, C.; Morici, N.; Magrini, G.; Nava, S.; et al. The impact of transcatheter edge-to-edge repair on right ventricle-pulmonary artery coupling in patients with functional mitral regurgitation. Eur. J. Clin. Invest. 2023, 53, e13869. [Google Scholar] [CrossRef] [PubMed]
- Lyhne, M.D.; Kabrhel, C.; Giordano, N.; Andersen, A.; Nielsen-Kudsk, J.E.; Zheng, H.; et al. The echocardiographic ratio tricuspid annular plane systolic excursion/pulmonary arterial systolic pressure predicts short-term adverse outcomes in acute pulmonary embolism. Eur. Heart J. -Cardiovasc. Imaging 2021, 22, 285–294. [Google Scholar] [CrossRef] [PubMed]
- Forton, K.; Motoji, Y.; Caravita, S.; Faoro, V.; Naeije, R. Exercise stress echocardiography of the pulmonary circulation and right ventricular-arterial coupling in healthy adolescents. Eur. Heart J. Cardiovasc. Imaging 2021, 22, 688–694. [Google Scholar] [CrossRef] [PubMed]
- Wolsk, E.; Bakkestrøm, R.; Kristensen, C.B.; Aagaard Myhr, K.; Thomsen, J.H.; Balling, L.; et al. Right Ventricular and Pulmonary Vascular Function are Influenced by Age and Volume Expansion in Healthy Humans. J. Card Fail. 2019, 25, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, F.; Rudski, L.G.; Vriz, O.; Gargani, L.; Afilalo, J.; D’Andrea, A.; et al. Physiologic correlates of tricuspid annular plane systolic excursion in 1168 healthy subjects. Int. J. Cardiol. 2016, 223, 736–743. [Google Scholar] [CrossRef] [PubMed]
- Pietrasik, A.; Gąsecka, A.; Szarpak, Ł.; Pruc, M.; Kopiec, T.; Darocha, S.; et al. Catheter-Based Therapies Decrease Mortality in Patients with Intermediate and High-Risk Pulmonary Embolism: Evidence From Meta-Analysis of 65,589 Patients. Front. Cardiovasc Med. 2022, 9, 861307. [Google Scholar] [CrossRef] [PubMed]
- Kuo, W.T.; Gould, M.K.; Louie, J.D.; Rosenberg, J.K.; Sze, D.Y.; Hofmann, L.V. Catheter-directed therapy for the treatment of massive pulmonary embolism: systematic review and meta-analysis of modern techniques. J. Vasc. Interv. Radiol. 2009, 20, 1431–1440. [Google Scholar] [CrossRef] [PubMed]
- Poenou, G.; Dumitru Dumitru, T.; Lafaie, L.; Mismetti, V.; Ayoub, E.; Duvillard, C.; et al. Pulmonary Embolism in the Cancer Associated Thrombosis Landscape. J. Clin. Med. 2022, 11, 5650. [Google Scholar] [CrossRef] [PubMed]
- Weeda, E.R.; Hakamiun, K.M.; Leschorn, H.X.; Tran, E. Comorbid cancer and use of thrombolysis in acute pulmonary embolism. J. Thromb. Thrombolysis 2019, 47, 324–327. [Google Scholar] [CrossRef]
- Shalaby, K.; Kahn, A.; Silver, E.S.; Kim, M.J.; Balakumaran, K.; Kim, A.S. Outcomes of acute pulmonary embolism in hospitalized patients with cancer. BMC Pulm. Med. 2022, 22, 11. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).