Submitted:
21 December 2023
Posted:
21 December 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results and discussion
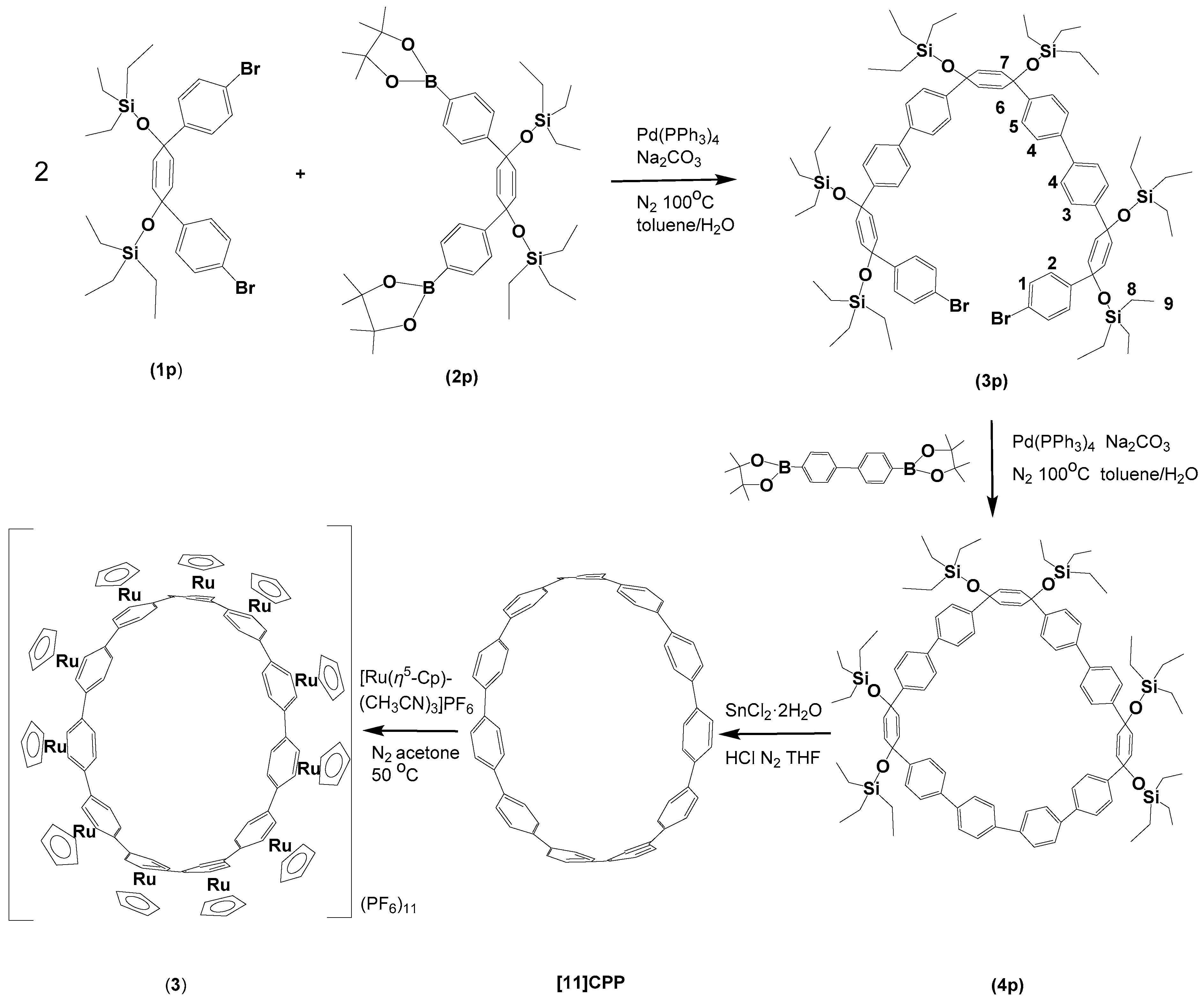
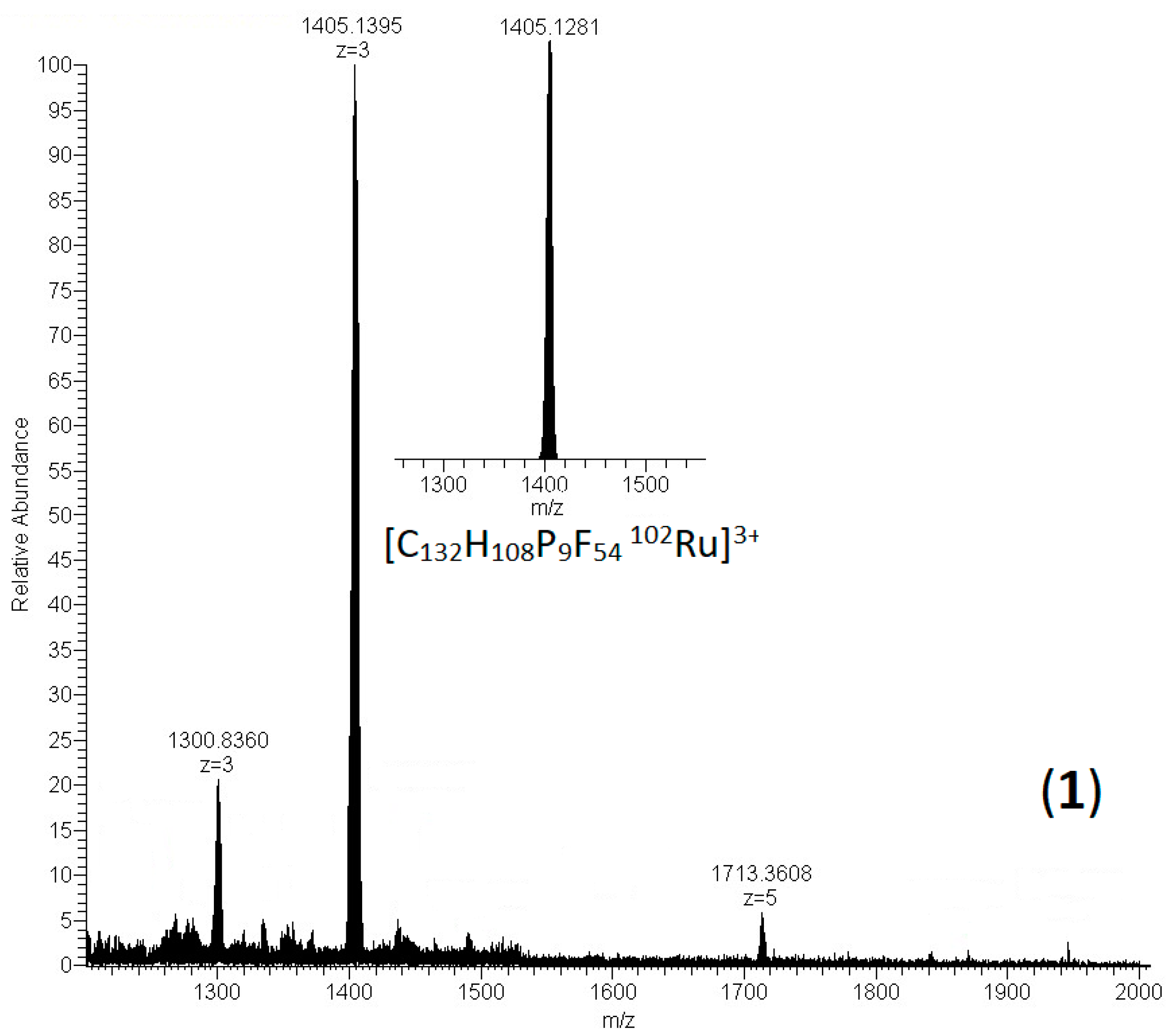
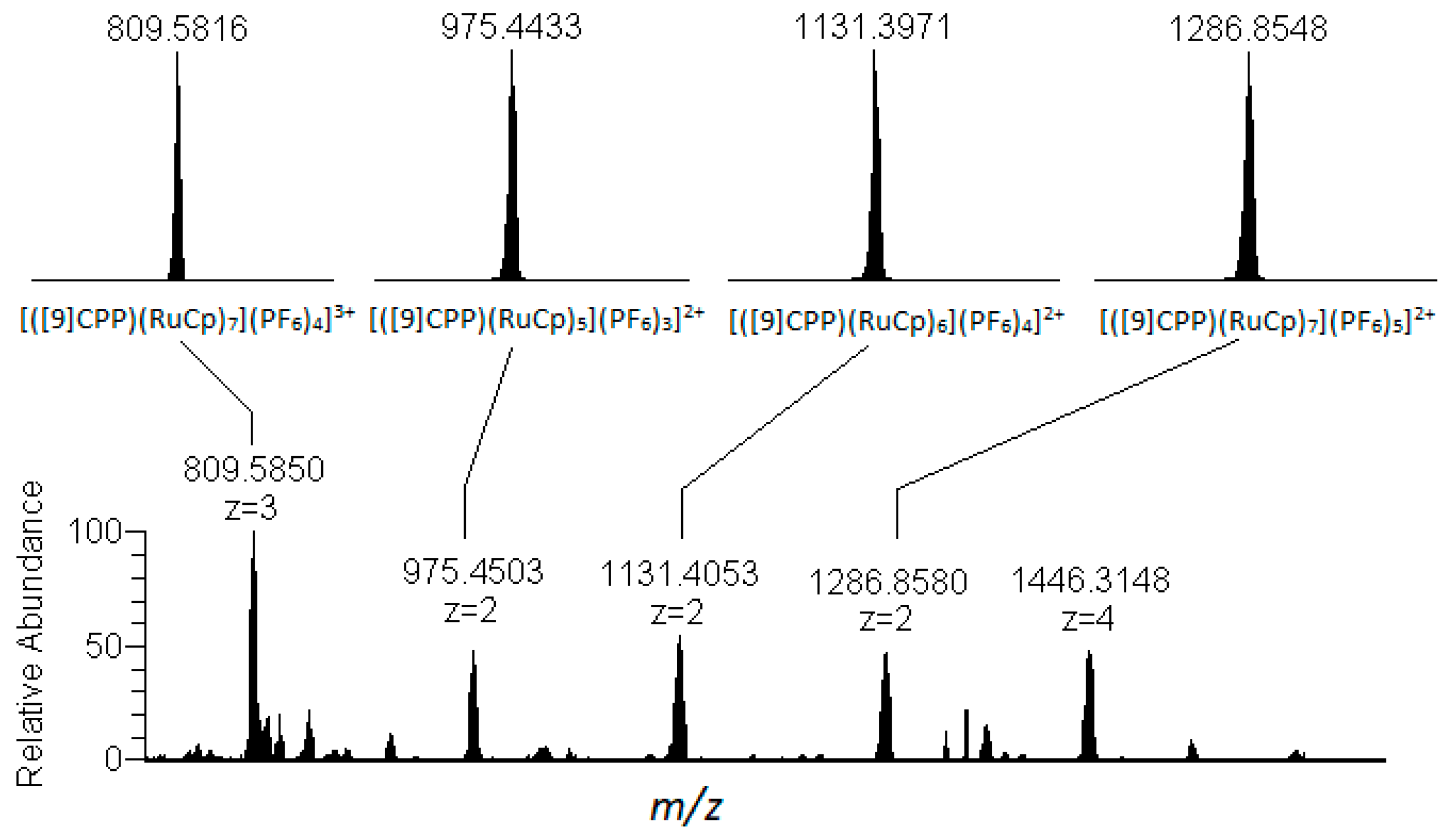
2.1. Synthesis and characterization of [(η6-[12]CPP)[Ru(η5-Cp)]12](PF6)12 and [(η6-[11]CPP)[Ru(η5-Cp)]11](PF6)11
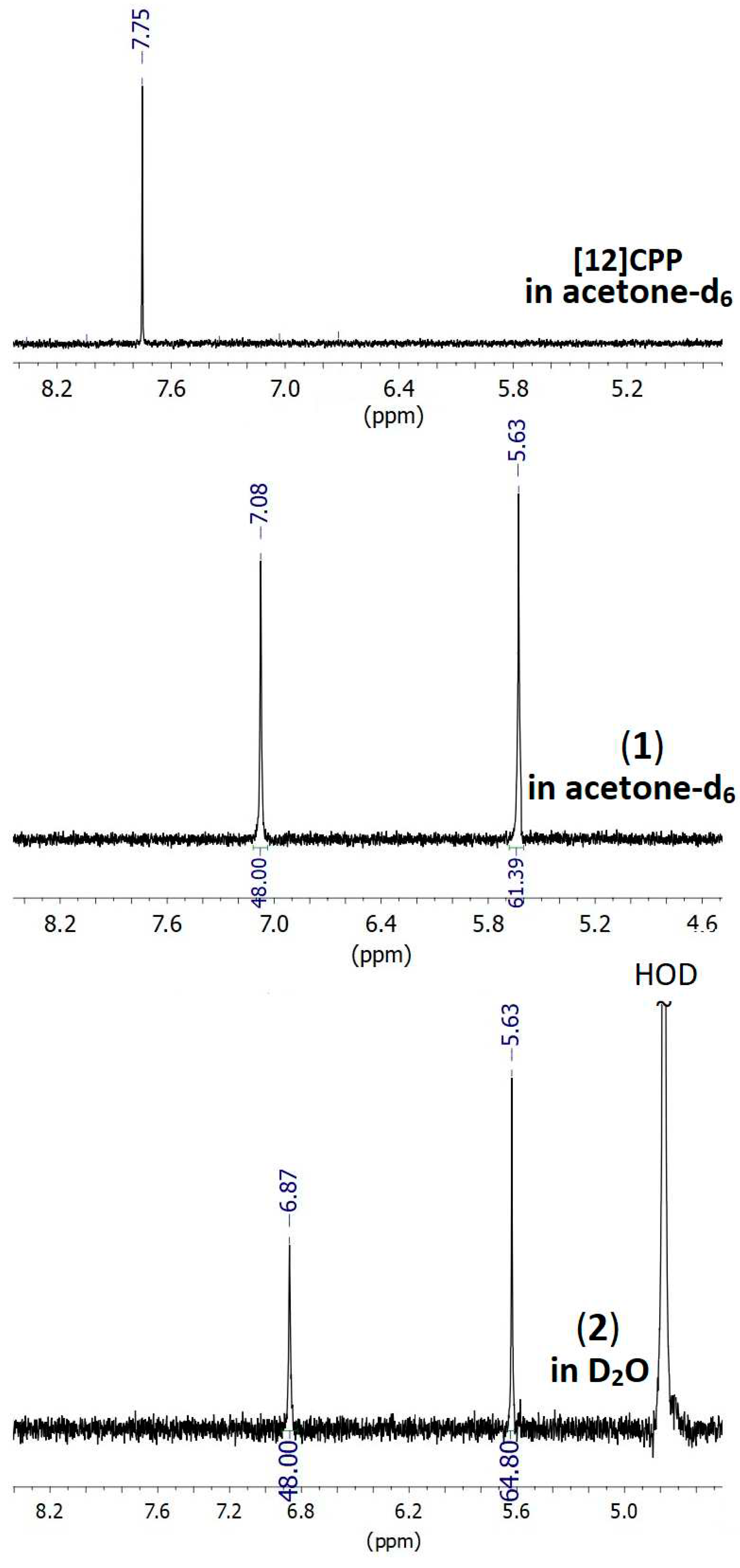
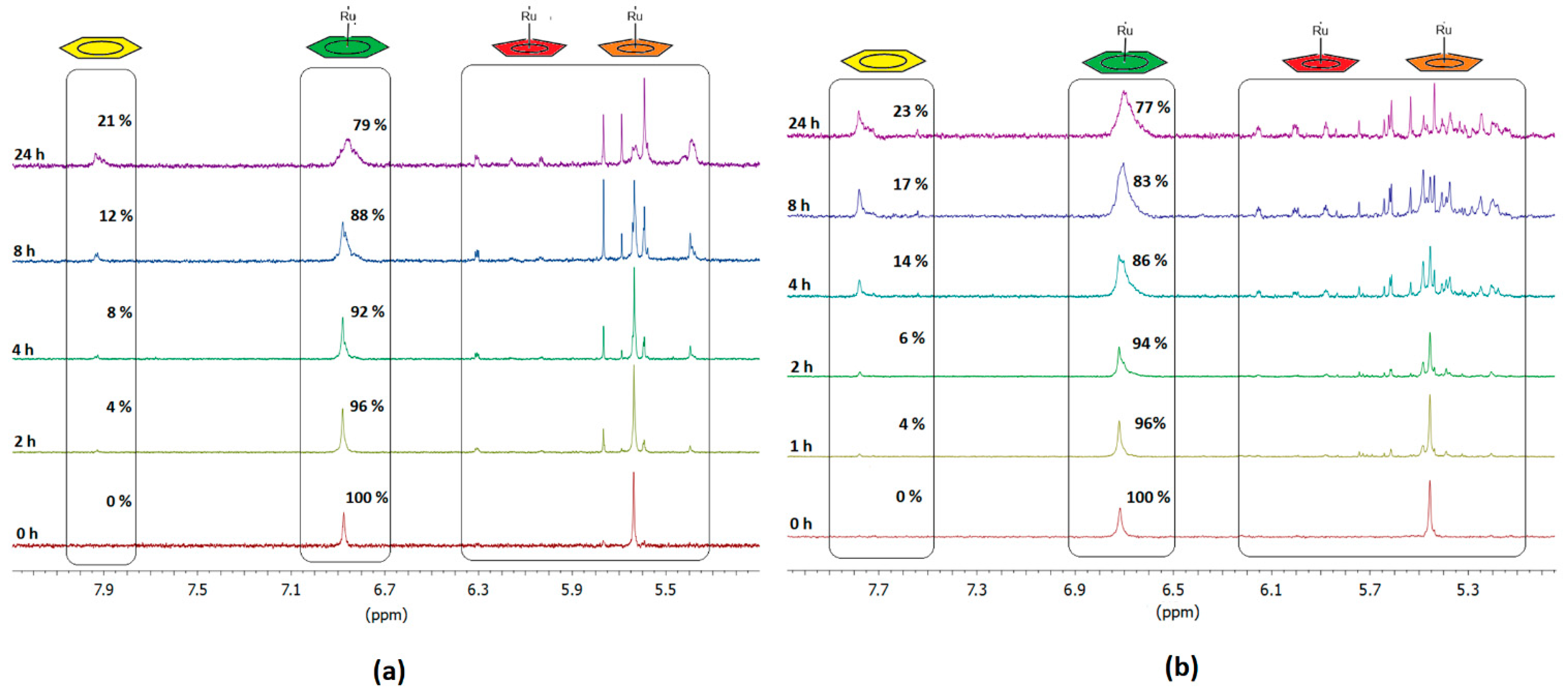
2.2. Stability studies of the complex in aqueous media
2.3. Biological studies
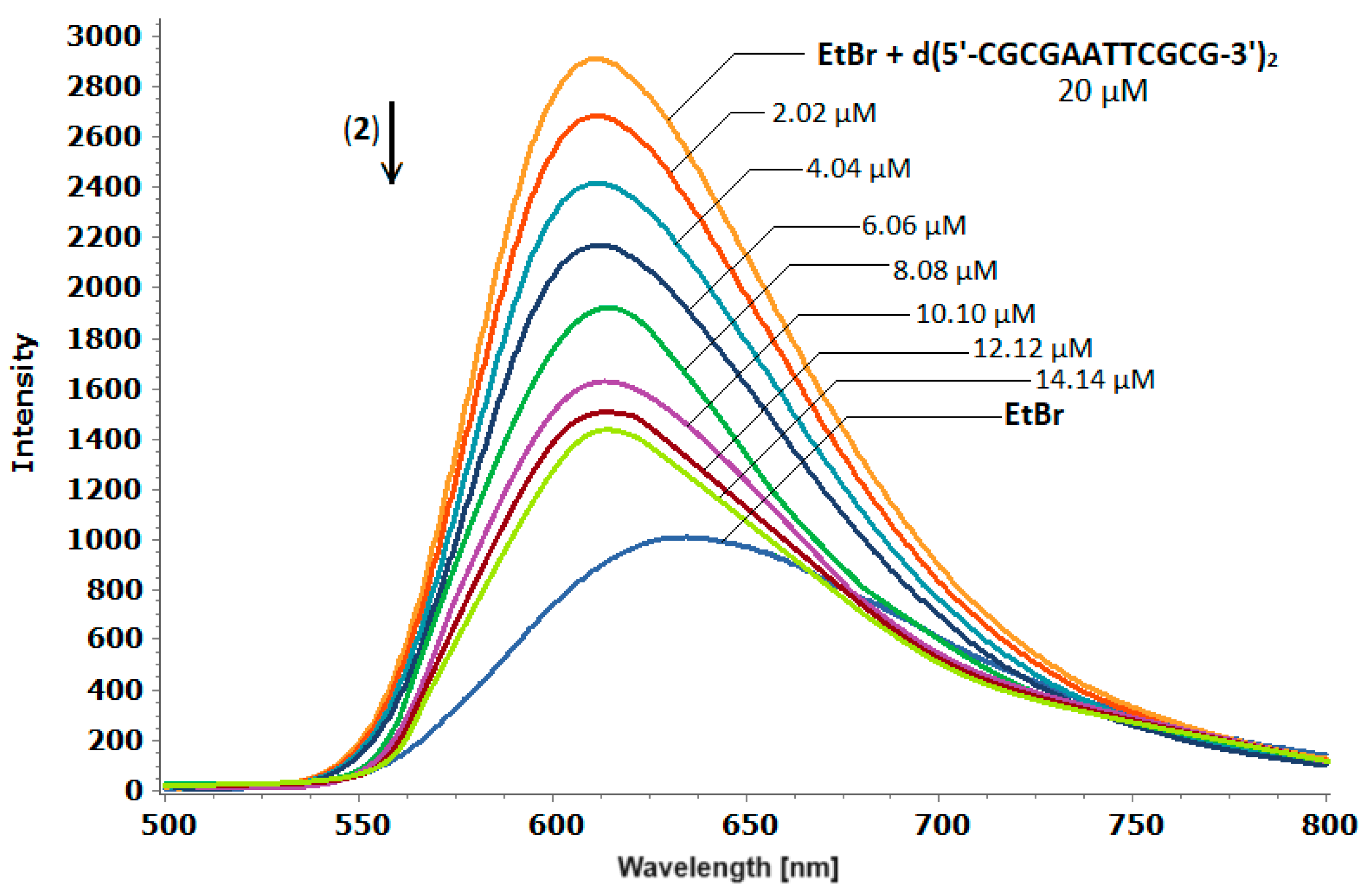
2.3.1. Fluorescence quenching studies of the d(5′-CGCGAATTCGCG-3′)2-ethidium bromide adducts, with (2).
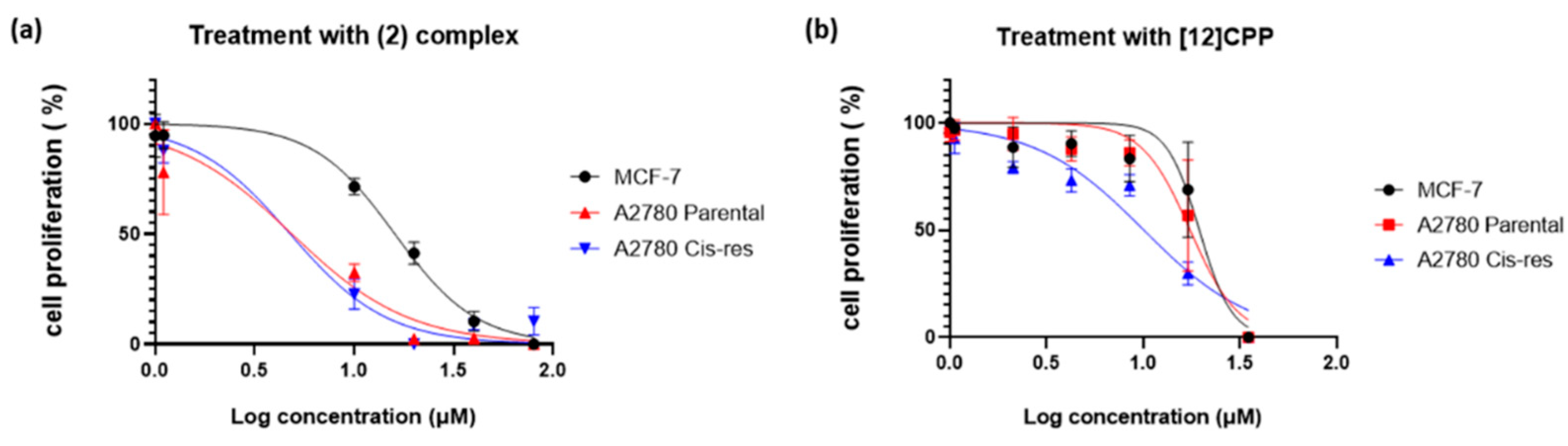
2.3.2. Cytotoxic activity
3. Experimental
3.1. Materials and methods
3.2. Preparation of ligands and complexes
3.3. Stability studies of the complex in aqueous media
3.4. Fluorescence Measurements
3.5. Cell culture
3.6. Cell growth assay
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Segawa, Y.; Fukazawa, A.; Matsuura, S.; Omachi, H.; Yamaguchi, S.; Irle, S.; Itami, K. Combined Experimental and Theoretical Studies on the Photophysical Properties of Cycloparaphenylenes. Org. Biomol. Chem. 2012, 10, 5979. [CrossRef]
- Lu, D.; Zhuang, G.; Jia, H.; Wang, J.; Huang, Q.; Cui, S.; Du, P. A Novel Symmetrically Multifunctionalized Dodecamethoxy-Cycloparaphenylene: Synthesis, Photophysical, and Supramolecular Properties. Org. Chem. Front. 2018, 5, 1446–1451. [CrossRef]
- Darzi, E.R.; Sisto, T.J.; Jasti, R. Selective Syntheses of [7]-[12]Cycloparaphenylenes Using Orthogonal Suzuki-Miyaura Cross-Coupling Reactions. J. Org. Chem. 2012, 77, 6624–6628. [CrossRef]
- Patel, V.K.; Kayahara, E.; Yamago, S. Practical Synthesis of [n]Cycloparaphenylenes (N=5, 7-12) by H2SnCl4-Mediated Aromatization of 1,4-Dihydroxycyclo-2,5-Diene Precursors. Chem. - A Eur. J. 2015, 21, 5742–5749. [CrossRef]
- Kayahara, E.; Cheng, Y.; Yamago, S. Short-Step Synthesis of Large Cycloparaphenylenes. Chem. Lett. 2018, 47, 1108–1111. [CrossRef]
- Kayahara, E.; Sun, L.; Onishi, H.; Suzuki, K.; Fukushima, T.; Sawada, A.; Kaji, H.; Yamago, S. Gram-Scale Syntheses and Conductivities of [10]Cycloparaphenylene and Its Tetraalkoxy Derivatives. J. Am. Chem. Soc. 2017, 139, 18480–18483. [CrossRef]
- Lovell, T.C.; Colwell, C.E.; Zakharov, L.N.; Jasti, R. Symmetry Breaking and the Turn-on Fluorescence of Small, Highly Strained Carbon Nanohoops. Chem. Sci. 2019, 10, 3786–3790. [CrossRef]
- Darzi, E.R.; Hirst, E.S.; Weber, C.D.; Zakharov, L.N.; Lonergan, M.C.; Jasti, R. Synthesis, Properties, and Design Principles of Donor-Acceptor Nanohoops. ACS Cent. Sci. 2015, 1, 335–342. [CrossRef]
- Kuwabara, T.; Orii, J.; Segawa, Y.; Itami, K. Curved Oligophenylenes as Donors in Shape-Persistent Donor-Acceptor Macrocycles with Solvatofluorochromic Properties. Angew. Chemie - Int. Ed. 2015, 54, 9646–9649. [CrossRef]
- Tang, H.; Gu, Z.; Li, C.; Li, Z.; Wu, W.; Jiang, X. Nanoscale Vesicles Assembled from Non-Planar Cyclic Molecules for Efficient Cell Penetration. Biomater. Sci. 2019, 7, 2552–2558. [CrossRef]
- Lovell, T.C.; Bolton, S.G.; Kenison, J.P.; Shangguan, J.; Otteson, C.E.; Civitci, F.; Nan, X.; Pluth, M.D.; Jasti, R. Subcellular Targeted Nanohoop for One- and Two-Photon Live Cell Imaging. ACS Nano 2021, 15, 15285–15293. [CrossRef]
- White, B.M.; Zhao, Y.; Kawashima, T.E.; Branchaud, B.P.; Pluth, M.D.; Jasti, R. Expanding the Chemical Space of Biocompatible Fluorophores: Nanohoops in Cells. ACS Cent. Sci. 2018, 4, 1173–1178. [CrossRef]
- Dale, L.D.; Tocher, J.H.; Dyson, T.M.; Edwards, D.I.; Tocher, D.A. Studies on DNA Damage and Induction of SOS Repair by Novel Multifunctional Bioreducible Compounds. II. A Metronidazole Adduct of a Ruthenium-Arene Compound. Anticancer. Drug Des. 1992, 7, 3–14.
- >Morris, R.E.; Aird, R.E.; del Socorro Murdoch, P.; Chen, H.; Cummings, J.; Hughes, N.D.; Parsons, S.; Parkin, A.; Boyd, G.; Jodrell, D.I.; et al. Inhibition of Cancer Cell Growth by Ruthenium(II) Arene Complexes. J. Med. Chem. 2001, 44, 3616–3621. [CrossRef]
- Tsolis, T.; Manos, M.J.; Karkabounas, S.; Zelovitis, I.; Garoufis, A. Synthesis, X-Ray Structure Determination, Cytotoxicity and Interactions with 9-Methylguanine, of Ruthenium(II) H6-Arene Complexes. J. Organomet. Chem. 2014, 768, 1–9. [CrossRef]
- Tsolis, T.; Nikolaou, N.; Ypsilantis, K.; Kougioumtzi, A.; Kordias, D.; Magklara, A.; Garoufis, A. Synthesis, Characterization, Interactions with 9-MeG and Cytotoxic Activity of Heterobimetallic RuII-PtII Complexes Bridged with 2, 2′-Bipyrimidine. J. Inorg. Biochem. 2021, 219, 111435. [CrossRef]
- Tsolis, T.; Ypsilantis, K.; Kourtellaris, A.; Garoufis, A. Synthesis, Characterization and Interactions with 9-Methylguanine of Ruthenium(II) H6-Arene Complexes with Aromatic Diimines. Polyhedron 2018, 149, 45–53. [CrossRef]
- Tsolis, T.; Papavasileiou, K.D.; Divanis, S.A.; Melissas, V.S.; Garoufis, A. How Half Sandwich Ruthenium Compounds Interact with DNA While Not Being Hydrolyzed; A Comparative Study. J. Inorg. Biochem. 2016, 160, 12–23. [CrossRef]
- Loughrey, B.T.; Healy, P.C.; Parsons, P.G.; Williams, M.L. Selective Cytotoxic Ru(II) Arene Cp* Complex Salts [R-PhRuCp*]+X- for X = BF4-, PF6-, and BPh4-. Inorg. Chem. 2008, 47, 8589–8591. [CrossRef]
- Loughrey, B.T.; Williams, M.L.; Carruthers, T.J.; Parsons, P.G.; Healy, P.C. Synthesis, Structure, and Selective Cytotoxicity of Organometallic Cp *RuII O-Alkyl-N-Phenylcarbamate Sandwich Complexes. Aust. J. Chem. 2010, 63, 245–251. [CrossRef]
- Loughrey, B.T.; Cunning, B. V.; Healy, P.C.; Brown, C.L.; Parsons, P.G.; Williams, M.L. Selective, Cytotoxic Organoruthenium(II) Full-Sandwich Complexes: A Structural, Computational and in Vitro Biological Study. Chem. - An Asian J. 2012, 7, 112–121. [CrossRef]
- Perekalin, D.S.; Molotkov, A.P.; Nelyubina, Y. V.; Anisimova, N.Y.; Kudinov, A.R. Synthesis of Amino Acid Esters of the Ruthenium Naphthalene Complex [(C5Me4CH2OH)Ru(C10H8)]+. Inorganica Chim. Acta 2014, 409, 390–393. [CrossRef]
- Perekalin, D.S.; Novikov, V. V.; Pavlov, A.A.; Ivanov, I.A.; Anisimova, N.Y.; Kopylov, A.N.; Volkov, D.S.; Seregina, I.F.; Bolshov, M.A.; Kudinov, A.R. Selective Ruthenium Labeling of the Tryptophan Residue in the Bee Venom Peptide Melittin. Chem. - A Eur. J. 2015, 21, 4923–4925. [CrossRef]
- Bihari, Z.; Vultos, F.; Fernandes, C.; Gano, L.; Santos, I.; Correia, J.D.G.; Buglyó, P. Synthesis, Characterization and Biological Evaluation of a 67Ga-Labeled (H6-Tyr)Ru(H5-Cp) Peptide Complex with the HAV Motif. J. Inorg. Biochem. 2016, 160, 189–197. [CrossRef]
- Gelle, D.; Lamač, M.; Mach, K.; Šimková, L.; Gyepes, R.; Sommerová, L.; Martišová, A.; Bartošík, M.; Vaculovič, T.; Kanický, V.; et al. Enhanced Intracellular Accumulation and Cytotoxicity of Ferrocene-Ruthenium Arene Conjugates. Chempluschem 2020, 85, 1034–1043. [CrossRef]
- Matsui, K.; Segawa, Y.; Itami, K. Synthesis and Properties of Cycloparaphenylene-2,5-Pyridylidene: A Nitrogen-Containing Carbon Nanoring. Org. Lett. 2012, 14, 1888–1891. [CrossRef]
- Van Raden, J.M.; Louie, S.; Zakharov, L.N.; Jasti, R. 2,2′-Bipyridyl-Embedded Cycloparaphenylenes as a General Strategy To Investigate Nanohoop-Based Coordination Complexes. J. Am. Chem. Soc. 2017, 139, 2936–2939. [CrossRef]
- Heras Ojea, M.J.; Van Raden, J.M.; Louie, S.; Collins, R.; Pividori, D.; Cirera, J.; Meyer, K.; Jasti, R.; Layfield, R.A. Spin-Crossover Properties of an Iron(II) Coordination Nanohoop. Angew. Chemie - Int. Ed. 2021, 60, 3515–3518. [CrossRef]
- Kubota, N.; Segawa, Y.; Itami, K. H6-Cycloparaphenylene Transition Metal Complexes: Synthesis, Structure, Photophysical Properties, and Application to the Selective Monofunctionalization of Cycloparaphenylenes. J. Am. Chem. Soc. 2015, 137, 1356–1361. [CrossRef]
- Kayahara, E.; Patel, V.K.; Mercier, A.; Kündig, E.P.; Yamago, S. Regioselective Synthesis and Characterization of Multinuclear Convex-Bound Ruthenium-[n]Cycloparaphenylene ( n =5 and 6) Complexes. Angew. Chemie Int. Ed. 2016, 55, 302–306. [CrossRef]
- Ypsilantis, K.; Tsolis, T.; Garoufis, A. Interactions of (H5-CpRu)-[12]Cycloparaphenylene Full-Sandwich Complexes with 9-Methylguanine. Inorg. Chem. Commun. 2021, 134, 108992. [CrossRef]
- Bocekova-Gajdošíkova, E.; Epik, B.; Chou, J.; Akiyama, K.; Fukui, N.; Guénée, L.; Kündig, E.P. Microwave-Assisted Synthesis and Transformations of Cationic CpRu(II)(Naphthalene) and CpRu(II)(Naphthoquinone) Complexes. Helv. Chim. Acta 2019, 102, 1–10. [CrossRef]
- Evans, P.J.; Darzi, E.R.; Jasti, R. Efficient Room-Temperature Synthesis of a Highly Strained Carbon Nanohoop Fragment of Buckminsterfullerene. Nat. Chem. 2014, 6, 404–408. [CrossRef]
- Amaya, T.; Hirao, T. Chemistry of Sumanene. Chem. Rec. 2015, 15, 310–321. [CrossRef]
- Xia, A.; Selegue, J.P.; Carrillo, A.; Brock, C.P. Stereochemical Inversion of a Coordinated , Curved Hydrocarbon : Syntheses and Structures of Exo - and Department of Chemistry , Uni V Ersity of Kentucky Recei V Ed December 6 , 1999 Such as Strained Cycloalkenes , 1 - 3 in Which the inside and Outside Su. J. Am. Chem. Soc. 2000, 122, 3973–3974.
- Kamieth, M.; Klärner, F.G.; Diederich, F. Modeling the Supramolecular Properties of Aliphatic-Aromatic Hydrocarbons with Convex-Concave Topology. Angew. Chemie - Int. Ed. 1998, 37, 3303–3306. [CrossRef]
- Ramana, M.M.V.; Betkar, R.; Nimkar, A.; Ranade, P.; Mundhe, B.; Pardeshi, S. In Vitro DNA Binding Studies of Antiretroviral Drug Nelfinavir Using Ethidium Bromide as Fluorescence Probe. J. Photochem. Photobiol. B Biol. 2015, 151, 194–200. [CrossRef]
- Bittman, R. Studies of the Binding of Ethidium Bromide to Transfer Ribonucleic Acid: Absorption, Fluorescence, Ultracentrifugation and Kinetic Investigations. J. Mol. Biol. 1969, 46, 251–268. [CrossRef]
- Waring, M.J. Complex Formation between Ethidium Bromide and Nucleic Acids. J. Mol. Biol. 1965, 13, 269–282. [CrossRef]
- Lepecq, J.-B.; Paoletti, C. A Fluorescent Complex between Ethidium Bromide and Nucleic Acids. J. Mol. Biol. 1967, 27, 87–106. [CrossRef]
- Galindo-Murillo, R.; Cheatham, T.E. Ethidium Bromide Interactions with DNA: An Exploration of a Classic DNA–Ligand Complex with Unbiased Molecular Dynamics Simulations. Nucleic Acids Res. 2021, 49, 3735–3747. [CrossRef]
- Rouzina, I.; Bloomfield, V.A. DNA Bending by Small, Mobile Multivalent Cations. Biophys. J. 1998, 74, 3152–3164. [CrossRef]
- Brukner, I.; Susic, S.; Dlakic, M.; Savic, A.; Pongor, S. Physiological Concentration of Magnesium Ions Induces a Strong Macroscopic Curvature in GGGCCC-Containing DNA. J. Mol. Biol. 1994, 236, 26–32. [CrossRef]
- Porschke, D. Structure and Dynamics of Double Helices in Solution: Modes of DNA Bending. J. Biomol. Struct. Dyn. 1986, 4, 373–389. [CrossRef]
- Goodsell, D.S.; Kopka, M.L.; Cascio, D.; Dickerson, R.E. Crystal Structure of CATGGCCATG and Its Implications for A-Tract Bending Models. Proc. Natl. Acad. Sci. 1993, 90, 2930–2934. [CrossRef]
- Marquet, R.; Houssier, C. Different Binding Modes of Spermine to A-T and G-C Base Pairs Modulate the Bending and Stiffening of the DNA Double Helix. J. Biomol. Struct. Dyn. 1988, 6, 235–246. [CrossRef]
- Duguid, J.; Bloomfield, V.A.; Benevides, J.; Thomas, G.J. Raman Spectroscopy of DNA-Metal Complexes. I. Interactions and Conformational Effects of the Divalent Cations: Mg, Ca, Sr, Ba, Mn, Co, Ni, Cu, Pd, and Cd. Biophys. J. 1993, 65, 1916–1928. [CrossRef]
- Rykowski, S.; Gurda-Woźna, D.; Orlicka-Płocka, M.; Fedoruk-Wyszomirska, A.; Giel-Pietraszuk, M.; Wyszko, E.; Kowalczyk, A.; Stączek, P.; Biniek-Antosiak, K.; Rypniewski, W.; Olejniczak, W.; Agnieszka, B. Design of DNA Intercalators Based on 4-Carboranyl-1,8-Naphthalimides: Investigation of Their DNA-Binding Ability and Anticancer Activity. Int. J. Mol. Sci. 2022, 23, 4598. [CrossRef]
- Kayahara, E.; Patel, V.K.; Xia, J.; Jasti, R.; Yamago, S. Selective and Gram-Scale Synthesis of [6]Cycloparaphenylene. Synlett 2015, 26, 1615–1619. [CrossRef]
- Yamago, S.; Watanabe, Y.; Iwamoto, T. Synthesis of [8]Cycloparaphenylene from a Square-Shaped Tetranuclear Platinum Complex. Angew. Chemie Int. Ed. 2010, 49, 757–759. [CrossRef]
- Rehman, S.U.; Yaseen, Z.; Husain, M.A.; Sarwar, T.; Ishqi, H.M.; Tabish, M. Interaction of 6 Mercaptopurine with Calf Thymus DNA - Deciphering the Binding Mode and Photoinduced DNA Damage. PLoS One 2014, 9, 1–11. [CrossRef]
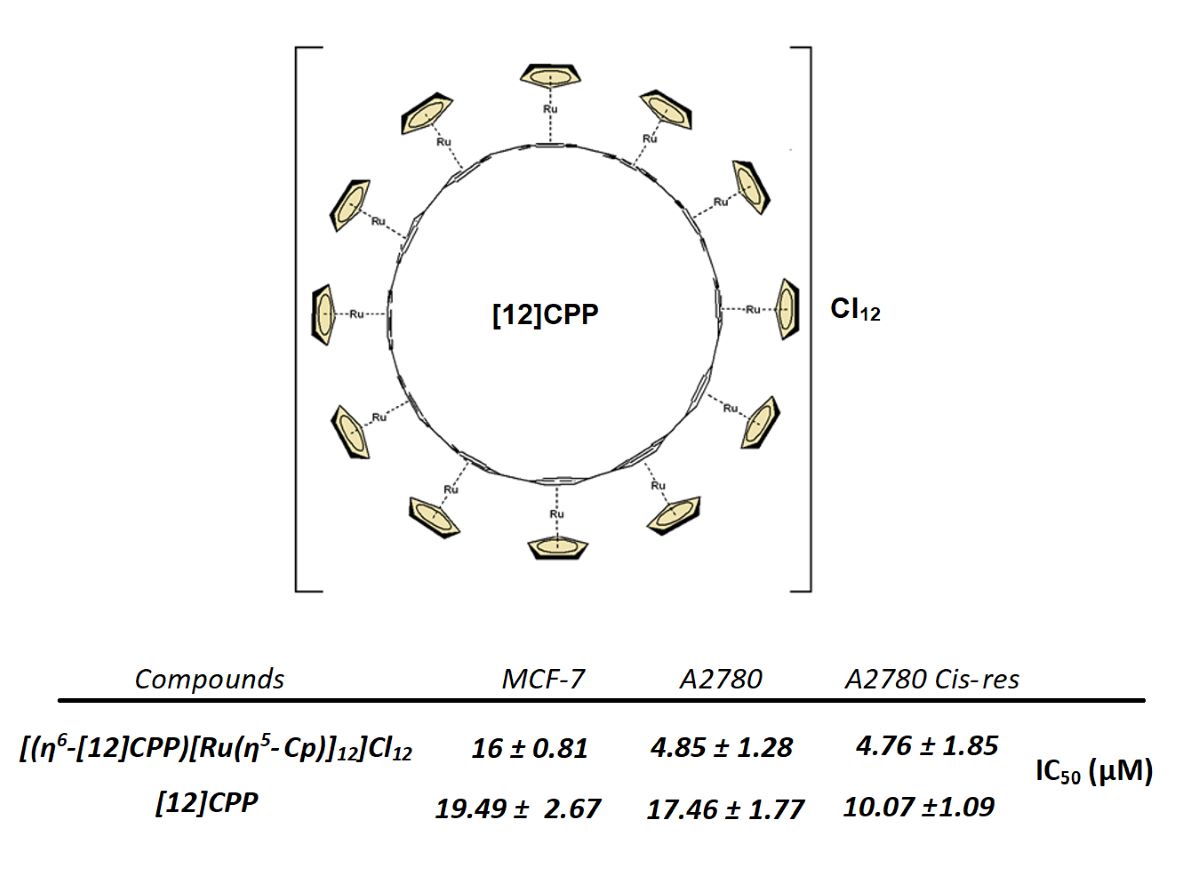
| Compounds | MCF-7 | A2780 | A2780 Cis-res |
| (2) | 16 ± 0.81 | 4.85 ± 1.28 | 4.76 ± 1.85 |
| [12]CPP | 19.49 ± 2.67 | 17.46 ± 1.77 | 10.07 ± 1.09 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





