Submitted:
20 December 2023
Posted:
21 December 2023
You are already at the latest version
Abstract
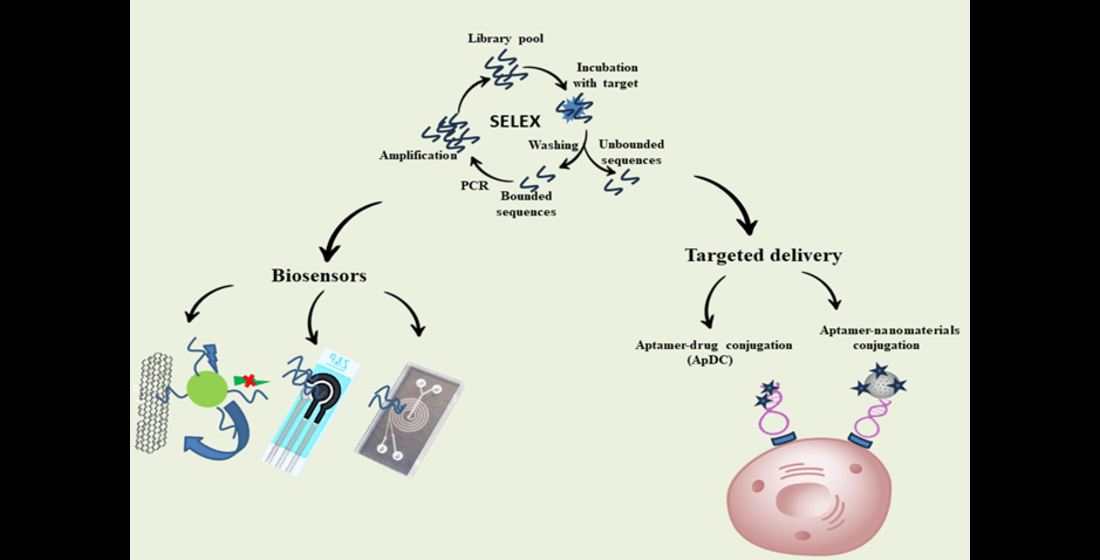
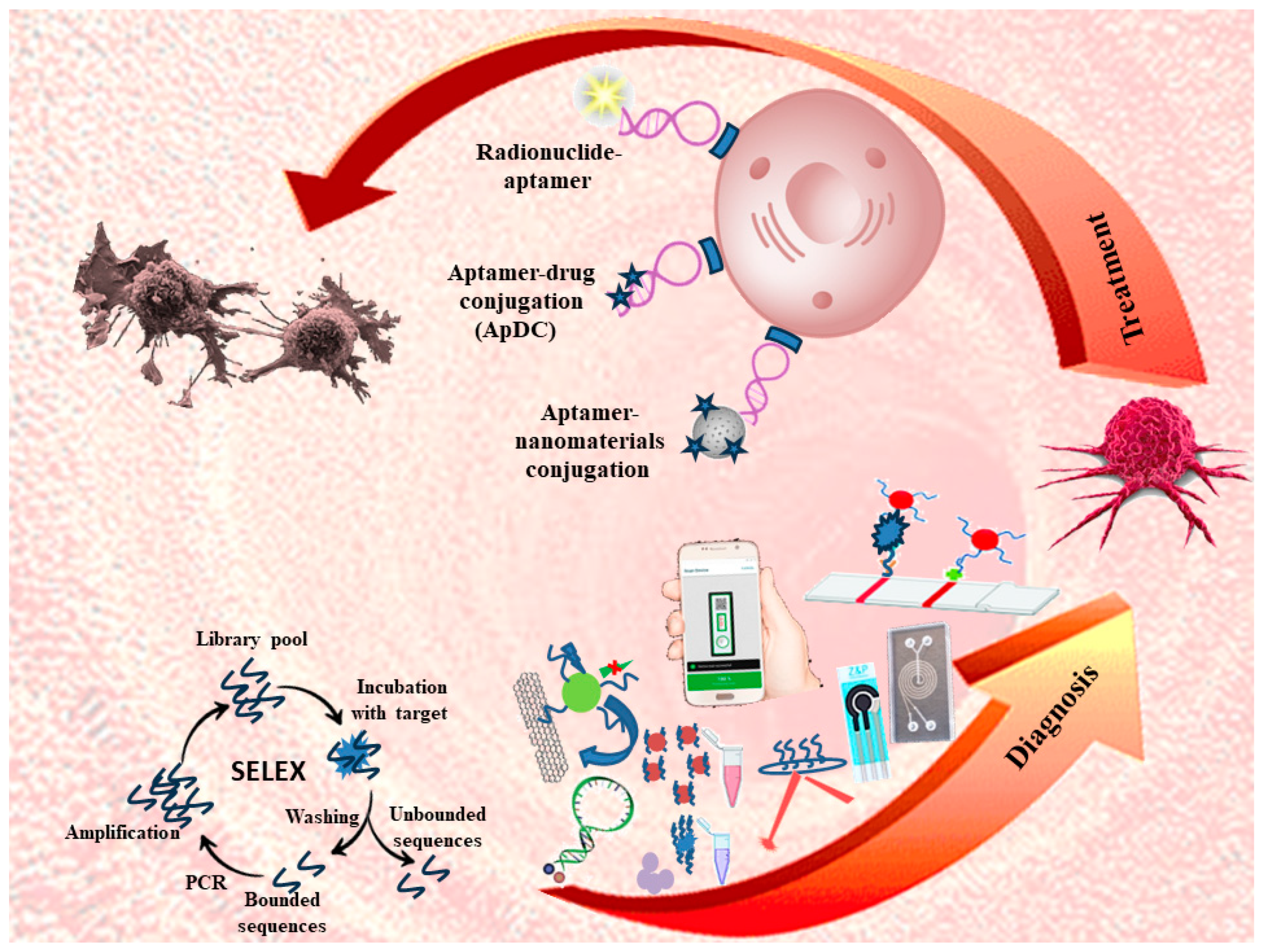
Keywords:
1. Introduction
2. Aptamer-Based Targeted Delivery Systems
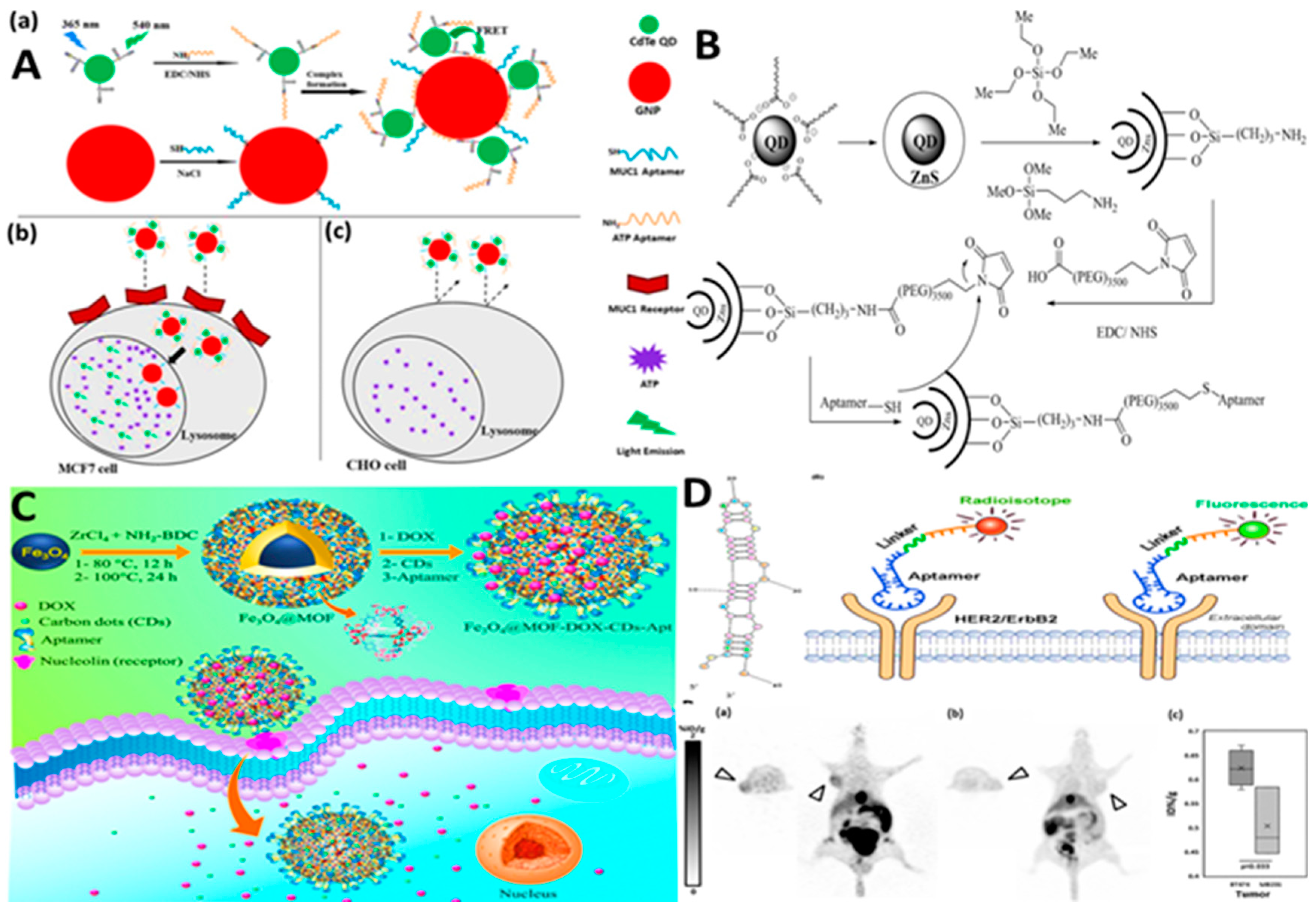
2.1. Aptamer-Nanomaterials Conjugation
2.2. Critical Note
2.3. Aptamer-Drug Conjugation (ApDC)
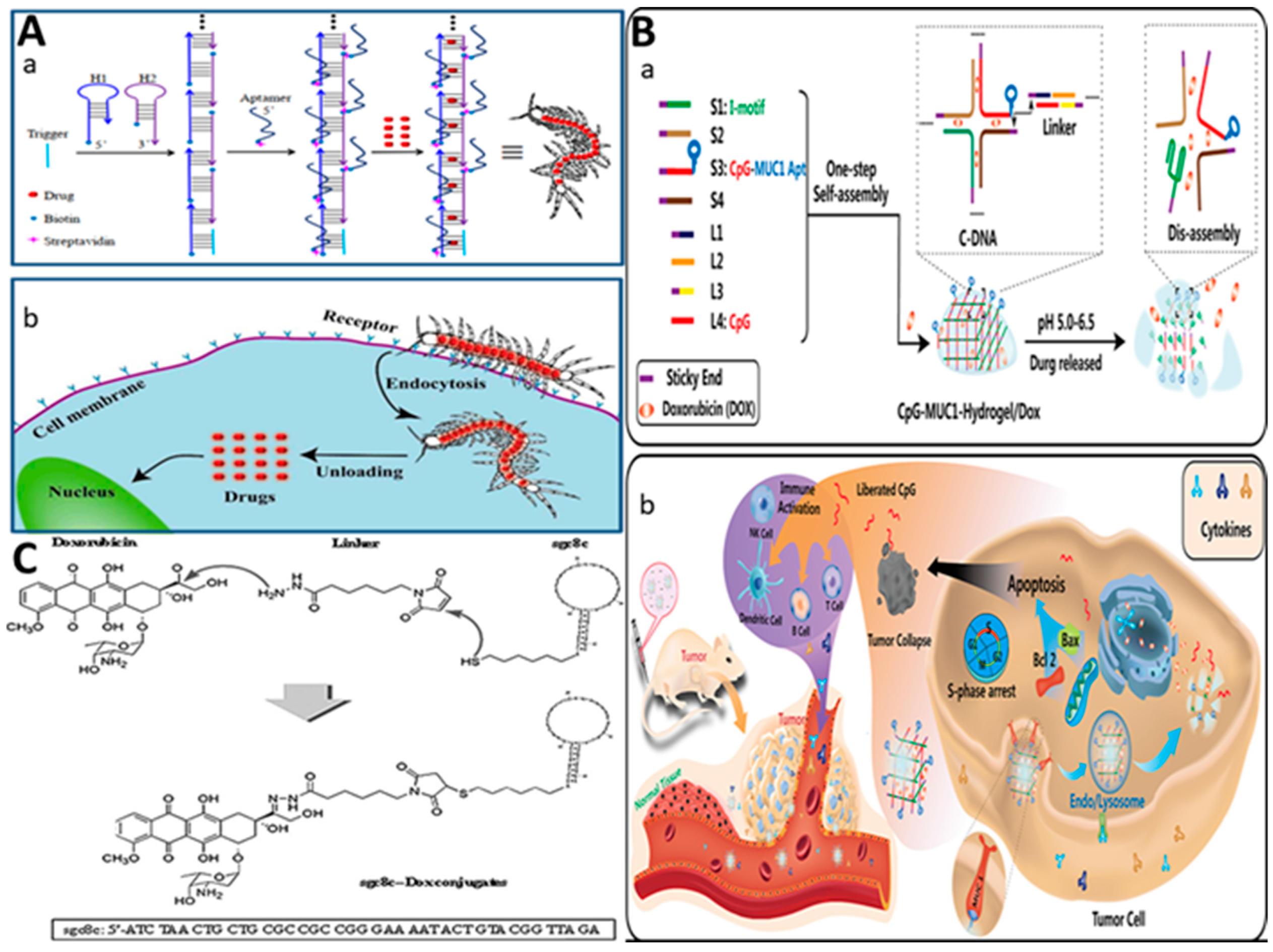
2.3.1. Physical Conjugation (Intercalation)
2.3.2. Covalent Conjugation
2.3.3. Critical Note
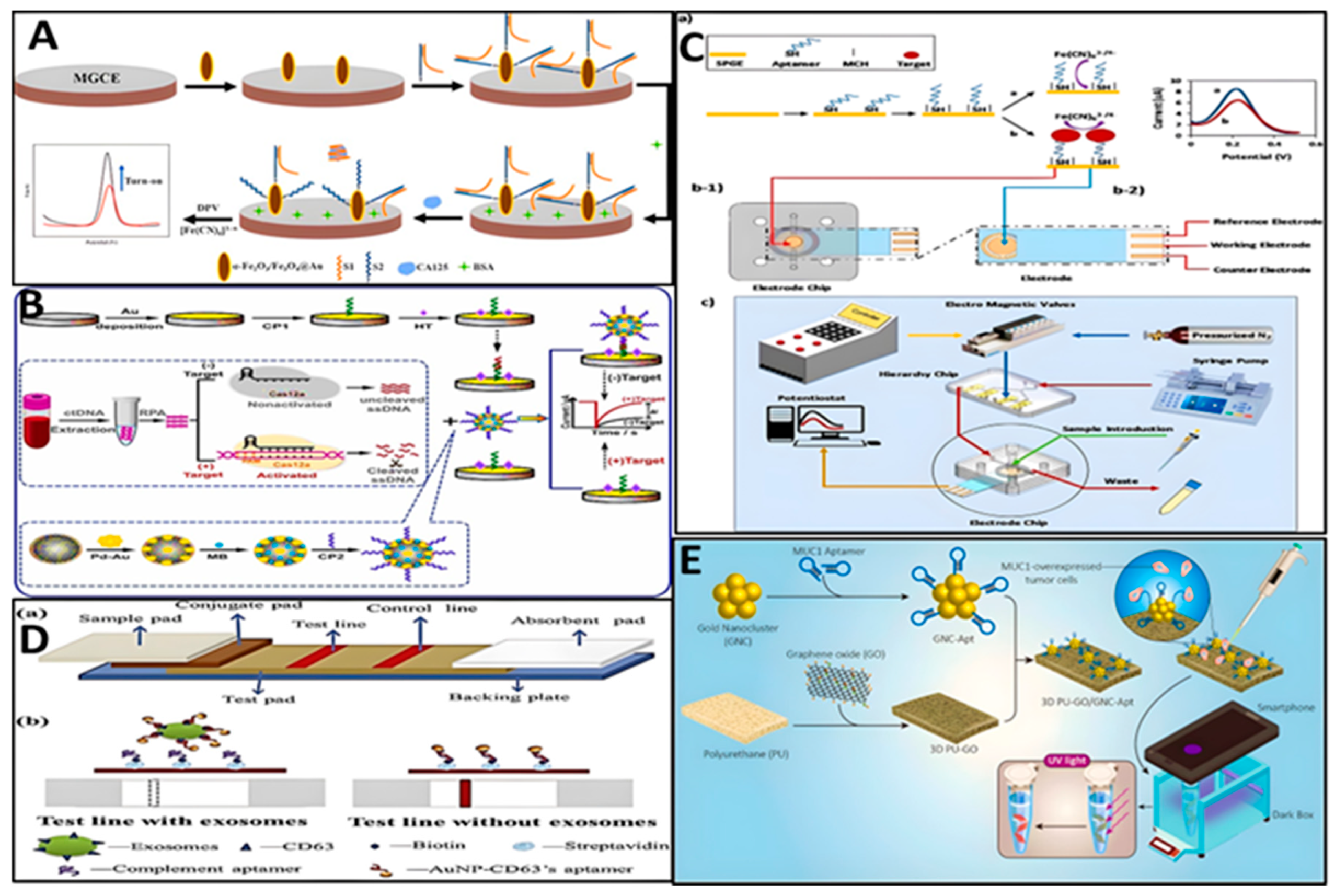
3. Biosensors
4. Critical Note
5. Conclusion and Future Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AuNPs | Gold nanoparticles |
| ApDC | Aptamer-drug conjugates |
| β-CD-PELA | Beta-cyclodextrin-linked poly (ethylene glycol)-b-polylactide block copolymers |
| ctDNA | Circulating tumor DNA |
| COFs | Covalent organic frameworks |
| CNTs | Carbon nanotubes |
| CDs | Carbon dots |
| C-DNA | Cross hybridization formation of DNA |
| CCRF-CEM | Human lymphoblasts T-cell ALL |
| CTCs | Circulating tumor cells |
| CpG ODNs | Cytosine-phosphate-guanine oligonucleotides |
| crRNA | CRISPR RNA |
| CA125 | Cancer antigen 125 |
| DOX | Doxorubicin |
| DNM | Daunomycin |
| DM1 | Maytansine 1 |
| EGFR L858R | Exon 21 mutations in L858R substitution |
| ELISA | Enzyme-linked immunosorbent assay |
| FDA | Food and drug administration |
| 5-FU | 5-fluorouracil |
| FRET | Fluorescence resonance energy transfer, resonance energy transfer |
| GNCs | Gold nanoclusters |
| GO | Graphene oxide |
| HCR | Hybridization chain reaction |
| IDA | Integrin α6β4-specific DNA aptamer |
| LDL-R | Low-density lipoprotein receptor |
| LFA | Lateral flow assay |
| MMAE | Monomethyl auristatin E |
| MAB | Molecular aptamer beacon |
| MOFs | Metal organic frameworks |
| MB | Methylene Blue |
| NSCLC | Non-small cell lung cancer |
| NCHS | National Center for Health Statistics |
| PAM | Protospacer adjacent motif |
| PDGF | Platelet-derived growth factor |
| PTX | Paclitaxel |
| QDs | Quantum dots |
| Ramos | Human B-cell Burkitt’s lymphoma |
| RT-PCR | Real time polymerase chain reaction |
| ROS | Reactive oxygen species |
| SELEX | Systematic evolution of ligands by exponential enrichment |
| SPION | Superparamagnetic iron oxide nanoparticles |
| TMPyP | 5, 10, 15, 20-tetra (phenyl-4-N-methyl-4-pyridyl) |
| TDNp | Tetrahedral DNA nanoprobe |
| UCNP | Up conversion nanoparticles |
References
- Zhang: P.-L.; Wang, Z.-K.; Chen, Q.-Y.; Du, X.; Gao, J., Biocompatible G-Quadruplex/BODIPY assembly for cancer cell imaging and the attenuation of mitochondria. Bioorganic & Medicinal Chemistry Letters 2019, 29, 1943–1947ia.
- Kong, H. Y.; Byun, J., Nucleic acid aptamers: New methods for selection, stabilization, and application in biomedical science. Biomolecules & therapeutics 2013, 21, 423.
- Bavi, R.; Hang, Z.; Banerjee, P.; Aquib, M.; Jadhao, M.; Rane, N.; Bavi, S.; Bhosale, R.; Kodam, K.; Jeon, B.-H., Doxorubicin-conjugated innovative 16-mer DNA aptamer-based Annexin A1 targeted anti-cancer drug delivery. Molecular Therapy-Nucleic Acids 2020, 21, 1074–1086.
- Lan, J.; Wu, X.; Luo, L.; Liu, J.; Yang, L.; Wang, F. Fluorescent Ag clusters conjugated with anterior gradient-2 antigen aptamer for specific detection of cancer cells. Talanta 2019, 197, 86–91. [Google Scholar] [CrossRef]
- You, X.; Gopinath, S. C.; Lakshmipriya, T.; Li, D. High-affinity detection of alpha-synuclein by aptamer-gold conjugates on an amine-modified dielectric surface. Journal of analytical methods in chemistry 2019, 2019. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Deng, Z.; Wang, D.; He, J.; Zhang, D.; Tan, Y.; Peng, T.; Wang, X.-Q.; Tan, W. Conjugating aptamer and mitomycin C with reductant-responsive linker leading to synergistically enhanced anticancer effect. Journal of the American Chemical Society 2020, 142, 2532–2540. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Lai, B. S.; Juhas, M. Recent advances in aptamer discovery and applications. Molecules 2019, 24, 941. [Google Scholar] [CrossRef] [PubMed]
- Ng, E. W.; Shima, D. T.; Calias, P.; Cunningham Jr, E. T.; Guyer, D. R.; Adamis, A. P. Pegaptanib, a targeted anti-VEGF aptamer for ocular vascular disease. Nature reviews drug discovery 2006, 5, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R. L.; Miller, K. D.; Fuchs, H. E.; Jemal, A. Cancer statistics, 2021. Ca Cancer J Clin 2021, 71, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Stein, C. A.; Castanotto, D. FDA-approved oligonucleotide therapies in 2017. Molecular Therapy 2017, 25, 1069–1075. [Google Scholar] [CrossRef]
- Tsuchikama, K.; An, Z., Antibody-drug conjugates: recent advances in conjugation and linker chemistries. Protein & cell 2018, 9, 33–46.
- Mohammadinejad, A.; Taghdisi, S. M.; Es' haghi, Z.; Abnous, K.; Mohajeri, S. A. Targeted imaging of breast cancer cells using two different kinds of aptamers-functionalized nanoparticles. European Journal of Pharmaceutical Sciences 2019, 134, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, M.; Salmasi, Z.; Hashemi, M.; Mosaffa, F.; Abnous, K.; Ramezani, M. Single-walled carbon nanotubes functionalized with aptamer and piperazine–polyethylenimine derivative for targeted siRNA delivery into breast cancer cells. International journal of pharmaceutics 2015, 485, 50–60. [Google Scholar] [CrossRef]
- Akbarzadeh, M.; Babaei, M.; Abnous, K.; Taghdisi, S. M.; Peivandi, M. T.; Ramezani, M.; Alibolandi, M. Hybrid silica-coated Gd-Zn-Cu-In-S/ZnS bimodal quantum dots as an epithelial cell adhesion molecule targeted drug delivery and imaging system. International journal of pharmaceutics 2019, 570, 118645. [Google Scholar] [CrossRef]
- Yang, Y.; Zhao, W.; Tan, W.; Lai, Z.; Fang, D.; Jiang, L.; Zuo, C.; Yang, N.; Lai, Y. An efficient cell-targeting drug delivery system based on aptamer-modified mesoporous silica nanoparticles. Nanoscale Research Letters 2019, 14, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yu, Y.; Ji, Q.; Qiu, L., Targeted delivery of anticancer drugs by aptamer AS1411 mediated Pluronic F127/cyclodextrin-linked polymer composite micelles. Nanomedicine: Nanotechnology, Biology and Medicine 2015, 11, 175–184.
- Li, X.; Wu, X.; Yang, H.; Li, L.; Ye, Z.; Rao, Y., A nuclear targeted Dox-aptamer loaded liposome delivery platform for the circumvention of drug resistance in breast cancer. Biomedicine & Pharmacotherapy 2019, 117, 109072.
- Zhao, J.; Liu, P.; Ma, J.; Li, D.; Yang, H.; Chen, W.; Jiang, Y. Enhancement of radiosensitization by silver nanoparticles functionalized with polyethylene glycol and aptamer As1411 for glioma irradiation therapy. International journal of nanomedicine 2019, 9483–9496. [Google Scholar] [CrossRef]
- Sun, X.; Liu, B.; Chen, X.; Lin, H.; Peng, Y.; Li, Y.; Zheng, H.; Xu, Y.; Ou, X.; Yan, S. Aptamer-assisted superparamagnetic iron oxide nanoparticles as multifunctional drug delivery platform for chemo-photodynamic combination therapy. Journal of Materials Science: Materials in Medicine 2019, 30, 1–15. [Google Scholar]
- Alijani, H.; Noori, A.; Faridi, N.; Bathaie, S. Z.; Mousavi, M. F. Aptamer-functionalized Fe3O4@ MOF nanocarrier for targeted drug delivery and fluorescence imaging of the triple-negative MDA-MB-231 breast cancer cells. Journal of Solid State Chemistry 2020, 292, 121680. [Google Scholar] [CrossRef]
- Lin, H.-C.; Li, W.-T.; Madanayake, T. W.; Tao, C.; Niu, Q.; Yan, S.-Q.; Gao, B.-A.; Ping, Z. Aptamer-guided upconversion nanoplatform for targeted drug delivery and near-infrared light-triggered photodynamic therapy. Journal of Biomaterials Applications 2020, 34, 875–888. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.; Yang, C.-S.; Huang, D.-M. Aptamer-conjugated DNA icosahedral nanoparticles as a carrier of doxorubicin for cancer therapy. ACS nano 2011, 5, 6156–6163. [Google Scholar] [PubMed]
- Liu, J.; Wei, T.; Zhao, J.; Huang, Y.; Deng, H.; Kumar, A.; Wang, C.; Liang, Z.; Ma, X.; Liang, X.-J. Multifunctional aptamer-based nanoparticles for targeted drug delivery to circumvent cancer resistance. Biomaterials 2016, 91, 44–56. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.; Huang, K.-W.; Reebye, V.; Spalding, D.; Przytycka, T. M.; Wang, Y.; Swiderski, P.; Li, L.; Armstrong, B.; Reccia, I., Aptamer-drug conjugates of active metabolites of nucleoside analogs and cytotoxic agents inhibit pancreatic tumor cell growth. Molecular Therapy-Nucleic Acids 2017, 6, 80–88.
- Huang, Y. F.; Shangguan, D.; Liu, H.; Phillips, J. A.; Zhang, X.; Chen, Y.; Tan, W. Molecular assembly of an aptamer–drug conjugate for targeted drug delivery to tumor cells. ChemBioChem 2009, 10, 862–868. [Google Scholar] [CrossRef]
- Li, W.; Yang, X.; He, L.; Wang, K.; Wang, Q.; Huang, J.; Liu, J.; Wu, B.; Xu, C., Self-assembled DNA nanocentipede as multivalent drug carrier for targeted delivery. ACS applied materials & interfaces 2016, 8, 25733–25740.
- Wei, H.; Zhao, Z.; Wang, Y.; Zou, J.; Lin, Q.; Duan, Y., One-step self-assembly of multifunctional DNA nanohydrogels: an enhanced and harmless strategy for guiding combined antitumor therapy. ACS applied materials & interfaces 2019, 11, 46479–46489.
- Wang, T.; Rahimizadeh, K.; Veedu, R. N., Development of a novel DNA oligonucleotide targeting low-density lipoprotein receptor. Molecular Therapy-Nucleic Acids 2020, 19, 190–198.
- Li, F.; Lu, J.; Liu, J.; Liang, C.; Wang, M.; Wang, L.; Li, D.; Yao, H.; Zhang, Q.; Wen, J. A water-soluble nucleolin aptamer-paclitaxel conjugate for tumor-specific targeting in ovarian cancer. Nature communications 2017, 8, 1390. [Google Scholar] [PubMed]
- Jeong, H. Y.; Kim, H.; Lee, M.; Hong, J.; Lee, J. H.; Kim, J.; Choi, M. J.; Park, Y. S.; Kim, S.-C. Development of HER2-specific aptamer-drug conjugate for breast cancer therapy. International journal of molecular sciences 2020, 21, 9764. [Google Scholar]
- Thiel, K. W.; Hernandez, L. I.; Dassie, J. P.; Thiel, W. H.; Liu, X.; Stockdale, K. R.; Rothman, A. M.; Hernandez, F. J.; McNamara, J. O.; Giangrande, P. H. Delivery of chemo-sensitizing siRNAs to HER2+-breast cancer cells using RNA aptamers. Nucleic acids research 2012, 40, 6319–6337. [Google Scholar] [CrossRef]
- Kim, H. J.; Park, J. Y.; Lee, T. S.; Song, I. H.; Cho, Y. L.; Chae, J. R.; Kang, H.; Lim, J. H.; Lee, J. H.; Kang, W. J. PET imaging of HER2 expression with an 18F-fluoride labeled aptamer. PLoS One 2019, 14, e0211047. [Google Scholar] [CrossRef] [PubMed]
- Alshaer, W.; Hillaireau, H.; Fattal, E. Aptamer-guided nanomedicines for anticancer drug delivery. Advanced drug delivery reviews 2018, 134, 122–137. [Google Scholar] [CrossRef] [PubMed]
- Serrano, C. M.; Freeman, R.; Godbe, J.; Lewis, J. A.; Stupp, S. I. DNA-peptide amphiphile nanofibers enhance aptamer function. ACS applied bio materials 2019, 2, 2955–2963. [Google Scholar] [CrossRef] [PubMed]
- Vazquez-Gonzalez, M.; Willner, I., Aptamer-functionalized micro-and nanocarriers for controlled release. ACS Applied Materials & Interfaces 2021, 13, 9520–9541.
- Bai, J.; Luo, Y.; Wang, X.; Li, S.; Luo, M.; Yin, M.; Zuo, Y.; Li, G.; Yao, J.; Yang, H. A protein-independent fluorescent RNA aptamer reporter system for plant genetic engineering. Nature Communications 2020, 11, 3847. [Google Scholar] [CrossRef] [PubMed]
- Nooranian, S.; Mohammadinejad, A.; Mohajeri, T.; Aleyaghoob, G.; Kazemi Oskuee, R. Biosensors based on aptamer-conjugated gold nanoparticles: a review. Biotechnology and Applied Biochemistry 2022, 69, 1517–1534. [Google Scholar] [CrossRef] [PubMed]
- Debnath, S. K.; Srivastava, R. Drug delivery with carbon-based nanomaterials as versatile nanocarriers: progress and prospects. Frontiers in Nanotechnology 2021, 3, 644564. [Google Scholar] [CrossRef]
- Nair, A.; Haponiuk, J. T.; Thomas, S.; Gopi, S., Natural carbon-based quantum dots and their applications in drug delivery: A review. Biomedicine & Pharmacotherapy 2020, 132, 110834.
- Mohammadinejad, A.; Es' haghi, Z.; Abnous, K.; Mohajeri, S. A. Tandem determination of mitoxantrone and ribonucleic acid using mercaptosuccinic acid-capped CdTe quantum dots. Journal of Luminescence 2017, 190, 254–260. [Google Scholar] [CrossRef]
- Croissant, J. G.; Butler, K. S.; Zink, J. I.; Brinker, C. J. Synthetic amorphous silica nanoparticles: toxicity, biomedical and environmental implications. Nature Reviews Materials 2020, 5, 886–909. [Google Scholar] [CrossRef]
- Ghosh, B.; Biswas, S. Polymeric micelles in cancer therapy: State of the art. Journal of Controlled Release 2021, 332, 127–147. [Google Scholar] [CrossRef]
- Malam, Y.; Loizidou, M.; Seifalian, A. M. Liposomes and nanoparticles: nanosized vehicles for drug delivery in cancer. Trends in pharmacological sciences 2009, 30, 592–599. [Google Scholar] [CrossRef]
- Vangijzegem, T.; Stanicki, D.; Laurent, S. Magnetic iron oxide nanoparticles for drug delivery: applications and characteristics. Expert opinion on drug delivery 2019, 16, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Deng, K.; Hou, Z.; Li, X.; Li, C.; Zhang, Y.; Deng, X.; Cheng, Z.; Lin, J. Aptamer-mediated up-conversion core/MOF shell nanocomposites for targeted drug delivery and cell imaging. Scientific reports 2015, 5, 7851. [Google Scholar] [CrossRef] [PubMed]
- Abdelhamid, H. N. Nanoparticle assisted laser desorption/ionization mass spectrometry for small molecule analytes. Microchimica Acta 2018, 185, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Soontornworajit, B.; Zhou, J.; Shaw, M. T.; Fan, T.-H.; Wang, Y. Hydrogel functionalization with DNA aptamers for sustained PDGF-BB release. Chemical communications 2010, 46, 1857–1859. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.-Y.; Chen, L.-Q.; Sun, W.; Du, H.-H.; Dong, S.; Ahmed, A. M. Q.; Cao, D.; Cui, J.-H.; Zhang, Y.; Cao, Q.-R. Collagenase IV and clusterin-modified polycaprolactone-polyethylene glycol nanoparticles for penetrating dense tumor tissues. Theranostics 2021, 11, 906. [Google Scholar] [CrossRef] [PubMed]
- Patil, Y. P.; Jadhav, S. Novel methods for liposome preparation. Chemistry and physics of lipids 2014, 177, 8–18. [Google Scholar] [CrossRef]
- Gao, J.; Gu, H.; Xu, B. Multifunctional magnetic nanoparticles: design, synthesis, and biomedical applications. Accounts of chemical research 2009, 42, 1097–1107. [Google Scholar] [CrossRef]
- Azzouz, A.; Goud, K. Y.; Raza, N.; Ballesteros, E.; Lee, S.-E.; Hong, J.; Deep, A.; Kim, K.-H. Nanomaterial-based electrochemical sensors for the detection of neurochemicals in biological matrices. TrAC Trends in Analytical Chemistry 2019, 110, 15–34. [Google Scholar] [CrossRef]
- Wen, M.; Li, G.; Liu, H.; Chen, J.; An, T.; Yamashita, H., Metal–organic framework-based nanomaterials for adsorption and photocatalytic degradation of gaseous pollutants: recent progress and challenges. Environmental Science: Nano 2019, 6, 1006–1025.
- Mehtab, T.; Yasin, G.; Arif, M.; Shakeel, M.; Korai, R. M.; Nadeem, M.; Muhammad, N.; Lu, X. Metal-organic frameworks for energy storage devices: batteries and supercapacitors. Journal of Energy Storage 2019, 21, 632–646. [Google Scholar] [CrossRef]
- Yan, Y.; Li, C.; Wu, Y.; Gao, J.; Zhang, Q. From isolated Ti-oxo clusters to infinite Ti-oxo chains and sheets: recent advances in photoactive Ti-based MOFs. Journal of Materials Chemistry A 2020, 8, 15245–15270. [Google Scholar] [CrossRef]
- Wang, S.; Chen, Y.; Wang, S.; Li, P.; Mirkin, C. A.; Farha, O. K. DNA-functionalized metal–organic framework nanoparticles for intracellular delivery of proteins. Journal of the American Chemical Society 2019, 141, 2215–2219. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Liu, Q.; Feng, W.; Sun, Y.; Li, F. Upconversion luminescent materials: advances and applications. Chemical reviews 2015, 115, 395–465. [Google Scholar] [CrossRef]
- Mohammadinejad, A.; Abnous, K.; Nameghi, M. A.; Yahyazadeh, R.; Hamrah, S.; Senobari, F.; Mohajeri, S. A., Application of green-synthesized carbon dots for imaging of cancerous cell lines and detection of anthraquinone drugs using silica-coated CdTe quantum dots-based ratiometric fluorescence sensor. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy 2023, 288, 122200.
- Yang, Q.; Zhao, C.; Zhao, J.; Ye, Y. Synthesis and singlet oxygen activities of near infrared photosensitizers by conjugation with upconversion nanoparticles. Optical Materials Express 2017, 7, 913–923. [Google Scholar] [CrossRef]
- Lim, C.-K.; Heo, J.; Shin, S.; Jeong, K.; Seo, Y. H.; Jang, W.-D.; Park, C. R.; Park, S. Y.; Kim, S.; Kwon, I. C. Nanophotosensitizers toward advanced photodynamic therapy of Cancer. Cancer letters 2013, 334, 176–187. [Google Scholar] [CrossRef] [PubMed]
- Yano, S.; Hirohara, S.; Obata, M.; Hagiya, Y.; Ogura, S.-i.; Ikeda, A.; Kataoka, H.; Tanaka, M.; Joh, T., Current states and future views in photodynamic therapy. Journal of Photochemistry and Photobiology C: Photochemistry Reviews 2011, 12, 46–67.
- Del Rosal, B.; Jaque, D. Upconversion nanoparticles for in vivo applications: limitations and future perspectives. Methods and Applications in Fluorescence 2019, 7, 022001. [Google Scholar] [CrossRef] [PubMed]
- Institute, N. C. Radiation Therapy Side Effects. https://www.cancer.gov/about-cancer/treatment/types/radiation-therapy/side-effects.
- Mohammadinejad, A.; Oskuee, R. K.; Eivazzadeh-Keihan, R.; Rezayi, M.; Baradaran, B.; Maleki, A.; Hashemzaei, M.; Mokhtarzadeh, A.; de la Guardia, M. Development of biosensors for detection of alpha-fetoprotein: As a major biomarker for hepatocellular carcinoma. TrAC Trends in Analytical Chemistry 2020, 130, 115961. [Google Scholar] [CrossRef]
- Schneider-Futschik, E. K.; Reyes-Ortega, F. Advantages and disadvantages of using magnetic nanoparticles for the treatment of complicated ocular disorders. Pharmaceutics 2021, 13, 1157. [Google Scholar] [CrossRef]
- Gu, K.; Meng, F. In Former research and recent advances of metal-organic frameworks (MOF) for anti-cancer drug delivery, Journal of Physics: Conference Series, IOP Publishing: 2021; p 012021.
- Hu, R.; Zhang, X.; Zhao, Z.; Zhu, G.; Chen, T.; Fu, T.; Tan, W. DNA nanoflowers for multiplexed cellular imaging and traceable targeted drug delivery. Angewandte Chemie 2014, 126, 5931–5936. [Google Scholar] [CrossRef]
- Huang, F.; You, M.; Chen, T.; Zhu, G.; Liang, H.; Tan, W. Self-assembled hybrid nanoparticles for targeted co-delivery of two drugs into cancer cells. Chemical Communications 2014, 50, 3103–3105. [Google Scholar] [CrossRef]
- Chen, X.; Zhou, L.; Wang, J.; Jiang, G.; Cheng, H.; Pei, R. The Study of the Interaction between Doxorubicin and Single-Stranded DNA. ChemistrySelect 2016, 1, 3823–3828. [Google Scholar] [CrossRef]
- Richards, A. D.; Rodger, A. Synthetic metallomolecules as agents for the control of DNA structure. Chemical Society Reviews 2007, 36, 471–483. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Chen, X. Aptamer-based targeted therapy. Advanced drug delivery reviews 2018, 134, 65–78. [Google Scholar] [CrossRef] [PubMed]
- Keefe, A. D.; Pai, S.; Ellington, A. Aptamers as therapeutics. Nature reviews Drug discovery 2010, 9, 537–550. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Niu, G.; Chen, X. Aptamer–drug conjugates. Bioconjugate chemistry 2015, 26, 2186–2197. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Lifson, M. A.; Inci, F.; Liang, L.-G.; Sheng, Y.-F.; Demirci, U. Advances in addressing technical challenges of point-of-care diagnostics in resource-limited settings. Expert review of molecular diagnostics 2016, 16, 449–459. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-H.; Seo, J.-M.; Shin, K.-J.; Yang, S.-G. Design and clinical developments of aptamer-drug conjugates for targeted cancer therapy. Biomaterials Research 2021, 25, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Testing.com Point-of-Care Testing. https://www.testing.com/articles/point-of-care-testing/.
- Feng, D.; Ren, M.; Miao, Y.; Liao, Z.; Zhang, T.; Chen, S.; Ye, K.; Zhang, P.; Ma, X.; Ni, J. Dual selective sensor for exosomes in serum using magnetic imprinted polymer isolation sandwiched with aptamer/graphene oxide based FRET fluorescent ignition. Biosensors and Bioelectronics 2022, 207, 114112. [Google Scholar] [CrossRef] [PubMed]
- Yue, X.; Qiao, Y.; Gu, D.; Wu, Z.; Zhao, W.; Li, X.; Yin, Y.; Zhao, W.; Kong, D.; Xi, R., Reliable FRET-ON imaging of telomerase in living cells by a tetrahedral DNA nanoprobe integrated with structure-switchable molecular beacon. Sensors and Actuators B: Chemical 2020, 312, 127943.
- Hasan, M. R.; Sharma, P.; Pilloton, R.; Khanuja, M.; Narang, J., Colorimetric biosensor for the naked-eye detection of ovarian cancer biomarker PDGF using citrate modified gold nanoparticles. Biosensors and Bioelectronics: X 2022, 11, 100142.
- Shahbazlou, S. V.; Vandghanooni, S.; Dabirmanesh, B.; Eskandani, M.; Hasannia, S. Biotinylated aptamer-based SPR biosensor for detection of CA125 antigen. Microchemical Journal 2023, 194, 109276. [Google Scholar] [CrossRef]
- Ni, Y.; Ouyang, H.; Yu, L.; Ling, C.; Zhu, Z.; He, A.; Liu, R. Label-free electrochemical aptasensor based on magnetic α-Fe2O3/Fe3O4 heterogeneous hollow nanorods for the detection of cancer antigen 125. Bioelectrochemistry 2022, 148, 108255. [Google Scholar] [CrossRef]
- Liu, F.; Peng, J.; Lei, Y.-M.; Liu, R.-S.; Jin, L.; Liang, H.; Liu, H.-F.; Ma, S.-Y.; Zhang, X.-H.; Zhang, Y.-P., Electrochemical detection of ctDNA mutation in non-small cell lung cancer based on CRISPR/Cas12a system. Sensors and Actuators B: Chemical 2022, 362, 131807.
- Khaksari, S.; Ameri, A. R.; Taghdisi, S. M.; Sabet, M.; Bami, S. M. J. G.; Abnous, K.; Shaegh, S. A. M. A microfluidic electrochemical aptasensor for highly sensitive and selective detection of A549 cells as integrin α6β4-containing cell model via IDA aptamers. Talanta 2023, 252, 123781. [Google Scholar] [CrossRef]
- Yu, Q.; Zhao, Q.; Wang, S.; Zhao, S.; Zhang, S.; Yin, Y.; Dong, Y. Development of a lateral flow aptamer assay strip for facile identification of theranostic exosomes isolated from human lung carcinoma cells. Analytical biochemistry 2020, 594, 113591. [Google Scholar] [CrossRef]
- Sanati, A.; Esmaeili, Y.; Khavani, M.; Bidram, E.; Rahimi, A.; Dabiri, A.; Rafienia, M.; Jolfaie, N. A.; Mofrad, M. R.; Javanmard, S. H. Smartphone-assisted lab-in-a-tube device using gold nanocluster-based aptasensor for detection of MUC1-overexpressed tumor cells. Analytica Chimica Acta 2023, 1252, 341017. [Google Scholar] [CrossRef] [PubMed]
- Moutsiopoulou, A.; Broyles, D.; Dikici, E.; Daunert, S.; Deo, S. K. Molecular aptamer beacons and their applications in sensing, imaging, and diagnostics. Small 2019, 15, 1902248. [Google Scholar] [CrossRef]
- Liu, Z.; Zhao, J.; Zhang, R.; Han, G.; Zhang, C.; Liu, B.; Zhang, Z.; Han, M.-Y.; Gao, X. Cross-platform cancer cell identification using telomerase-specific spherical nucleic acids. ACS nano 2018, 12, 3629–3637. [Google Scholar] [CrossRef]
- Sharma, P.; Panchal, A.; Yadav, N.; Narang, J. Analytical techniques for the detection of glycated haemoglobin underlining the sensors. International journal of biological macromolecules 2020, 155, 685–696. [Google Scholar] [CrossRef] [PubMed]
- Hasanzadeh, M.; Shadjou, N.; Eskandani, M.; de la Guardia, M.; Omidinia, E. Electrochemical nano-immunosensing of effective cardiac biomarkers for acute myocardial infarction. TrAC Trends in Analytical Chemistry 2013, 49, 20–30. [Google Scholar] [CrossRef]
- Zhong, Q.; Ding, H.; Gao, B.; He, Z.; Gu, Z. Advances of microfluidics in biomedical engineering. Advanced materials technologies 2019, 4, 1800663. [Google Scholar] [CrossRef]
- Mohammadinejad, A.; Aleyaghoob, G.; Ertas, Y. N. Nanomaterials in Lateral Flow Assay. In Functionalized Smart Nanomaterials for Point-of-Care Testing, Springer: 2023; pp 49-81.
- Khandan-Nasab, N.; Askarian, S.; Mohammadinejad, A.; Aghaee-Bakhtiari, S. H.; Mohajeri, T.; Oskuee, R. K. Biosensors, microfluidics systems and lateral flow assays for circulating microRNA detection: A review. Analytical Biochemistry 2021, 633, 114406. [Google Scholar] [CrossRef]
- ul ain Zahra, Q.; Mohsan, S. A. H.; Shahzad, F.; Qamar, M.; Qiu, B.; Luo, Z.; Zaidi, S. A. Progress in smartphone-enabled aptasensors. Biosensors and Bioelectronics 2022, 215, 114509. [Google Scholar]
- Burnett, J. C.; Rossi, J. J., RNA-based therapeutics: current progress and future prospects. Chemistry & biology 2012, 19, 60–71.
- Mi, J.; Liu, Y.; Rabbani, Z. N.; Yang, Z.; Urban, J. H.; Sullenger, B. A.; Clary, B. M. In vivo selection of tumor-targeting RNA motifs. Nature chemical biology 2010, 6, 22–24. [Google Scholar] [CrossRef]
- He, C.; Hu, Y.; Yin, L.; Tang, C.; Yin, C. Effects of particle size and surface charge on cellular uptake and biodistribution of polymeric nanoparticles. Biomaterials 2010, 31, 3657–3666. [Google Scholar] [CrossRef] [PubMed]
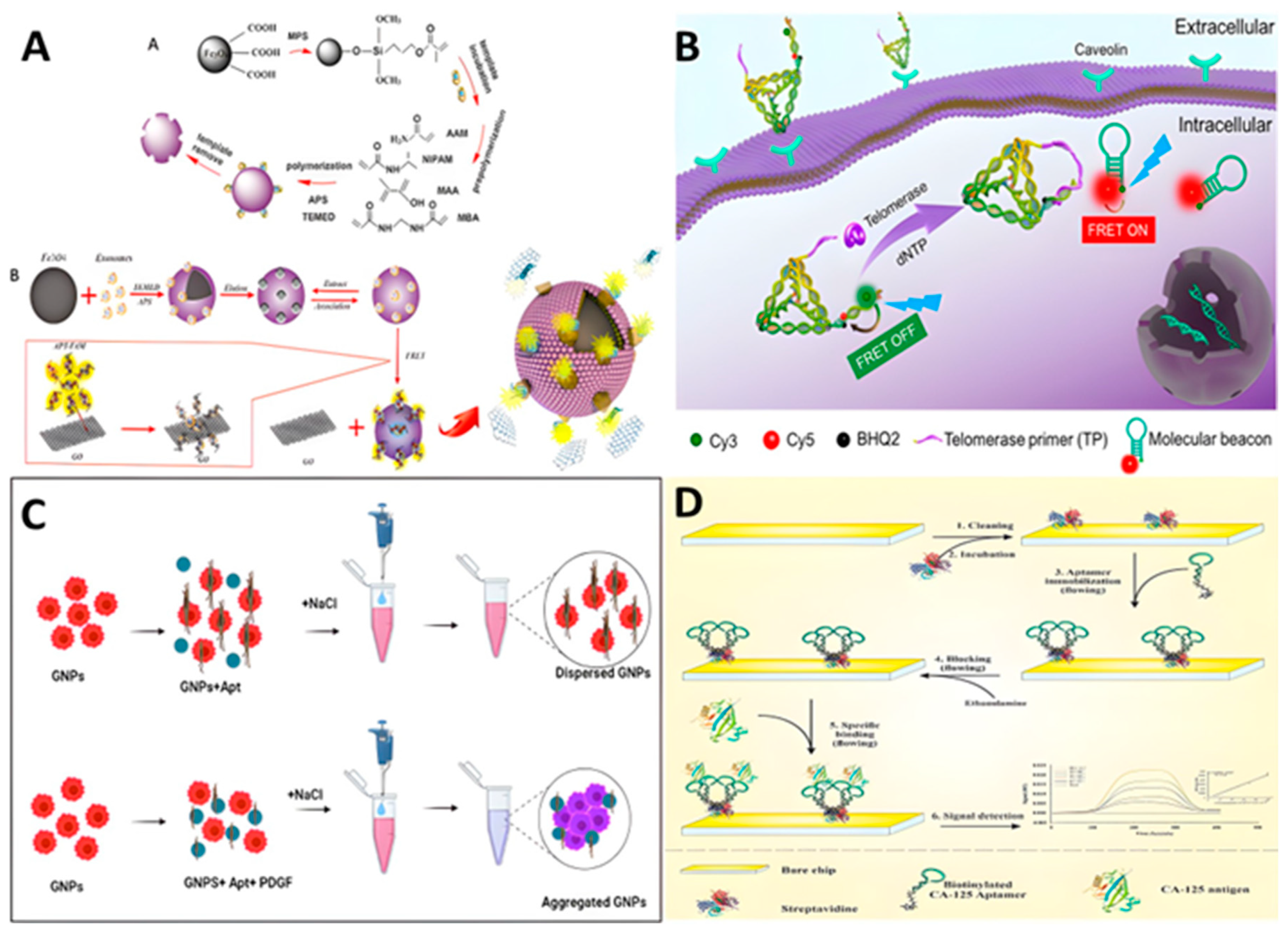
| Method of delivery | Sequences of aptamer (5’ to 3’) | Cell or animal | Marker | Therapeutic agent | Ref. |
|---|---|---|---|---|---|
| Nanocomplex of ATP aptamer/QDs and MUC-1 aptamer/ AuNPs | ATP-aptamer: NH2-AACCTGGGGGAGTATTGCGGAGGAAGGTMUC1-aptamer: 5′-SH-GAAGTGAAAATGACAGAACACAACA-3′ | MCF-7 | Muc-1, ATP | - | [12] |
| EpCAM aptamer-conjugated SWNT/piperazine–polyethylenimine | EpCAM aptamer: GCG ACU GGU UAC CCG GUC G SiRNA: GGAUGUUCAAGAUCCCAUGCAGCTC |
MCF-7 | EpCAM | siRNA for suppressing BCL9l | [13] |
| silica coated-Gd-Zn-Cu-In-S/ZnS QDs/PEG/EpCAM DNA | EpCAM aptamer: CAC TAC AGA GGT TGC GTC TGT CCC ACG TTG TCA TGG GGG GTT GGC CTG | 4T1, MCF-7 | EpCAM | DOX | [14] |
| Sgc8 aptamer-modified silica nanoparticles system | Sgc8 aptamer: ATCTAACTGCCGCCGCGGGAAAATGTACGGTTA G(T)10-COOH | CCRF-CEM human acute T lymphocyte leukemia | protein tyrosine kinase-7 (PTK-7) | DOX | [15] |
| As141 aptamer conjugated-pluronic F127 /beta-cyclodextrin-linked poly (ethylene glycol)-b-polylactide block copolymers (β-CD-PELA) | As141 aptamer: TTGGTGGTGGTGGTTGTGGTGGTGGTGG | MCF-7, female BALB/c nude mice |
Nucleolin | DOX | [16] |
| Encapsulation of aptamer-DOX in liposome | Aptamer AS1411: GGT GGT GGT GGT TGT GGT GGT GGT GGT T |
Human breast tumor MCF-7/Adr cells | nucleolin | DOX | [17] |
| irradiation therapy using AgNPs functionalized with PEG and As141 aptamer | As1411 aptamer: (CH2)6-NH2-GGTGGTGGTGGTTGTGGTGGTG GTGG | C6 glioma, C6 glioma-bearing mice |
nucleolin | - | [18] |
| Photodynamic therapy by aptamer-conjugated superparamagnetic iron oxide nanoparticles (SPION) loaded by daunomycin (DNM) and 5, 10, 15, 20-tetra (phenyl-4-N-methyl-4-pyridyl) (TMPyP) | As1411 and DNM aptamer: 5′-NH2-GGG GGG GGT TGT CCC CCC CCT TTT TTG GTG GTG GTG GTT GTG GTG GTG GTG G |
C26, A549 | nucleolin | DNM, TMPyP | [19] |
| Fe3O4@ UiO-66-NH2 MOF/DOX/CDs/AS1411 aptamer | As1411 aptamer: GGTGGTGGTGGTTGTGGTGGTG GTGG | MDA-MB-231 | nucleolin | DOX | [20] |
| photosensitizer protoporphyrin IX/ AS1411/NaYF4:Yb, Er nanocluster (UCNP) | As1411 aptamer: NH2–GG TGGTGGTGG TTG TGG TGGTGG TGG |
MCF7, Hella | nucleolin | photosensitizer protoporphyrin IX produced ROS | [21] |
| assembling five DNA strands to form five-point-star motif | strand I: ATAGTGAGTCGTATTAATTAACCCTCACTAAAAAGGATCCGGATCCTT strand II: TTTAGTGAGGGTTAATCATACGATTTAGGTGAAAGGATCCGGATCCTT strand III: TCACCTAAATCGTATGGGAGCTCTGCTTATATAAGGATCCGGATCCTT strand IV: ATATAAGCAGAGCTCCTAGAAGGCACAGTCGAAAGGATCCGGATCCTT strand V: TCGACTGTGCCTTCTATAATACGACTCACTATAAGGATCCGGATCCTT |
MCF7 | MUC1 | DOX | [22] |
| Assembling two strands contains a DNA aptamer with G-quadruplex and double-stranded DNA | CCCCCCCCCCTGTTGGGGGGGGGGTTTTTTTTTGGTGGTGGTGGTTGTGGTGGTGGTGG | MCF7 | MUC1 | DOX | [23] |
| Conjugation of aptamer P19 to gemcitabine, 5-fluorouracil (5-FU), monomethyl auristatin E (MMAE) and derivative of maytansine 1 (DM1) | GGGAGACAAGAAUAAACGCUCAAUGGCGAAUGCCCGCCUAAUAGGGCGUUAUGACUUGUUGAGUUCGACAGGAGGCUCACAACAGGC | PANC-1 AsPC-1 | cells | Gemcitabine 5-FU MMAE DM1 |
[24] |
| Covalently binding sgc8ca aptamer- doxorubicin | ATC TAA CTG CTG CGC CGC CGG GAA AAT ACT GTA CGG TTA GA | Human T-cell ALL (CCRF-CEM), human B-cell Burkitt’s lymphoma (Ramos) | kinase 7 (PTK7) | DOX | [25] |
| self-assembly of biotinylated hairpin DNAs followed by streptavidin-aptamers (Zy1) conjugation |
H1: Biotin-CGT CGT GCA GCA GCA GCA GCA GCA ACG GCT TGC TGC TGC TGC TGC TGC H2: Biotin-TGC TGC TGC TGC TGC TGC ACG ACG GCA GCA GCA GCA GCA GCA AGC CGT Trigger: TGC TGC TGC TGC TGC TGC ACG ACG Zy1: ACG CGC GCG CGC ATA GCG CGC TGA GCT GAA GAT CGT ACC GTG AGC GCG T(T)10- streptavidin |
SMMC-7721 | cell | DOX | [26] |
| self-assembly of 8 sequences of S1−S4 DNA and linker L1−L4 DNA to form nanohydrogels: -unmethylated cytosine-phosphate-guanine oligonucleotides (CpG ODNs) -DNA nanohydrogels (CpG-MUC1-hydrogel) - I-motif cytosine (C)-rich single-stranded DNA |
S1(I-motif): TCAACACTAATCCCCAATCCCAATCCCAATCCCAAACG A S2: TCAACACTAATCCGTTTGGGATTGGGACAAAACGACGAA S3(CpG-MUC1): TCAACACTAATCCCCAATCGTCGTTTTGTCGTTTTGTCGTT-S-S GCAGTTGATCCTTTGGATACCCTGG S4: TCAACACTAATCAAAACGACAAAACGATTGGGATTGGGA L1: GATTAGTGTTGAAACGACA-S-S-AAACG L2: ACAAAA-S-S-CGACGAGCCCTCCCCC L3: GATTAGTGTTGAGGGGGAGGGC L4 (CpG): TCGTCGTTTTGTCGTTTTGTCGTT |
MCF-7, A549, HepG-2, Female BALB/c (nu/nu) athymic nude mice (5−6 wk) |
MUC1 | DOX | [27] |
| antimiR-21 DNAzyme linked to the aptamer of low-density lipoprotein receptor (LDL-R) | TCA ACA GGC TAG CTA CAA CGA CAG TCT GAT AAG CTA TTTTTA GGA CAG GAC CAC ACC CAG CGC GGT CGG CGG GTG GGC GGG GGG AGA ACG AGG TAG GG | Huh-7, MDA-MB-231 | LDL-R | antimiR-21 DNAzyme | [28] |
| Covalently bonding paclitaxel (PTX) to the nucleolin AS1411 aptamer (NucA) through dipeptide bond | TTGGTGGTGGTGGTTGTGGTGGTGGTGG | SKOV3 (ATCC HTB-77), OVCAR3 (ATCC HTB-161) | Nucleolin and cathepsin | PTX | [29] |
| HER2- aptamer -conjugated to the mertansine (DM1) | AGC CGCGAG GGG AGG GAU AGG GUA GGG CGC GGC U | BT-474, MDA-MB-231 MCF-7, A549, mouse xenografts with BT-474 breast cancer |
HER2 | DM1 | [30] |
| conjugation of HER2 aptamer to siRNAs targeting Bcl-2 | GGGAGGACGAUGCGGCGAUGCUUACGUGCACGCGCCAGACGACUCGCCCGAGCUGUCACAGAGGGGCUACUU | N202.1A | HER2 | siRNAs targeting Bcl-2 | [31] |
| 18F-fluoride- HER2 aptamer | TCCTGGCATGTTCGATGGAGGCCTTTGATTACAGCCCAGA | tumor-bearing mice | HER2 | 18F-fluoride- HER2 aptamer | [32] |
| Method | Sequence | Biomarker | Linear range | LOD | Ref. |
|---|---|---|---|---|---|
| Exosomes on the molecularly imprinted polymer (MIP)-coated Fe3O4 release the aptamer-FAM from GO | CD63: FAM-CACCCCACCTCGCTCCCGTGACACTAATGCTA MUC1: FAM-CAGCCTGCACTCTAACGCAGTTGATCCTTTGGATAGCCTGGGTTAGA |
CD63, MUC1 | 1.19 × 10-6 - 4.76 × 10−5 mol/L |
2.27 × 10−6 mol/L |
[76] |
| tetrahedral DNA (L1–L4) hybridized with MAB | L1: ACATTCCTAAGTCTGAAACATTACAGCTTGCTACACGAGAAGA GCCGCCATAGTA L2: TCAACTGCCTGGTGATAAAACGACACTACGTGGGAATCTACTA TGGCGGCTCTTCTTTTTAATCCGTCGAGCAGAGTT L3: TATCACCAGGCAGTTGACAGTGTAGCAAGCTGTAATAGATGCG AGGGTCCAATACTTTTT/iBHQ2dT/TGCACCCTAACCCTAACCCT L4: TTCAGACTTAGGAATGTGCTTCCCACGTAGTGTCGTTTGTATTG GACCCTCGCAT MB: Cy3-AACGATTAGGGTTAGGGTCGTT-Cy5 |
telomerase | 50 - 2000 HeLa cells | 35 HeLa cells | [77] |
| Prevention of AuNPs aggregation by aptamer | CCGATCTCTCCCACTCTCTCCAACTCACAGGCTACGGCACGTAGAGCATCACCATGATCCTGTGGGTGTGTTGTTGATGGATCGGATCATCATGGTGAT | platelet-derived growth factor (PDGF) | 0.01–10 μg/ml | 0.01 μg/ml | [78] |
| Gold chip modified with CA125 aptamer through streptavidin–biotin | CTC ACT ATA GGG AGA CAA GAA TAA ACG CTC AA-biotin | Mucin 16 (MUC16) or cancer antigen 125 (CA125) | 10–100 U/mL |
0.01 U/mL |
[79] |
| Magnetic glass carbon electrode (MGCE)/α-Fe2O3/Fe3O4/Au/complementary strand/aptamer | Complementary strand: SH-TTTTTTTTTTTTTTTTTTTTCCCTATAGTGAG Aptamer: CTCACTATAGGGAGACAAGAATAAACGCTCAA |
cancer antigen 125 (CA125) | 5–125 U/mL | 2.99 U/mL | [80] |
| CRISPR/Cas12a + RPA + electrochemistry (Modified electrode with complementary 1 (CP1) and MB /Fe3O4@COF/PdAu modified with complementary 2 (CP2)) | crRNA template: AAGTACCCAGCAGTTTGGCCCGCCATCTACACTTAGTAGAAATTCCtatagtgagtcgtattag CP1: SH-ACACTTGAAGTGTATTTCCTAAATA CP2: AATTGCAAGTATGTAGAAGTTCACA-SH Synthetic target: ATGTCAAGATCACAGATTTTGGGCTGGCCAAACTGCTGGGTGCG |
ctDNA EGFR L858R | 10 aM –100 pM | 3.3 aM | [81] |
| Microfluidic system incorporated with screen-printed gold electrode was modified with integrin α6β4-specific aptamer (IDA) | GCCTGTTGTGAGCCTCCTAACCGTGCGTATTCGTACTGGAACTGATATCGATGTCCCCATGCTTATTCTTGTCTCCC–SH | α6β4 integrin on A549 cells |
50–5 × 105 cells/mL | 14 cells/mL | [82] |
| Lateral flow assay (LFA) with streptavidin (SA)-biotin-CD63 aptamer on T-line | CD63 aptamer: 5′-GTGGGGTGGACGAGGGCACGTGATTACGTA-3′ complement aptamer: CACCCCACCTCGCTCCCGTGACACTAATGCTA -Biotin |
CD63 on the non-small cell lung cancer (NSCLC) | - | 6.4 × 109 particles/mL | [83] |
| Smartphone control of emission from gold nanoclusters (GNCs)-aptamer as emitter and polyurethane (PU) - coated with GO as quencher | SH-CCCCCCGATCCTTTGGATA | Mucin 1 (MUC1) | 250–20,000 cells /mL | 221 cells /mL |
[84] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





