1. Introduction
The characteristics of drug substance crystals conclusively influence the drug's bioavailability because they possess various physicochemical properties (e.g., stability, solubility, and dissolution rate). Polymorphism is one of the most critical research matters. The optimal polymorph should be carefully selected and produced for drug development. However, the polymorph transformation can complicate the selection and reproducible production of an optimal polymorph because solvation and hydration of drug substances complicate polymorphism. The Good Manufacturing Practice (GMP), International Council for Harmonization, Q3C [1], and other quality standards regulate that all the organic solvents should be reduced below a certain level in crystals, which complicates the selection of polymorphs. The hydrates can be used as drugs, although the reproducibility of crystallization and the stability of drug substances must be guaranteed, even in the case of hydrated crystals. Crystalline doripenem, a carbapenem, exists as an anhydrate, monohydrate, and trihydrate [2]. Hickey et al. reported, based on Physician’s Desk References (2006), that ~45% of β-lactam compounds on the market (e.g., cephalosporin and penicillin), exist as crystalline hydrates [3].
Furthermore, the transformation from a crystal polymorph to another polymorph complicates polymorphism. The polymorphs may transform through the solvent-mediated and the solid phase transition. The solvent-mediated transformation is a vital research matter in the pharmaceutical industry. The solid-phase transition is also helpful in getting the favorable polymorph not necessarily captured by routine pharmaceutical polymorph screening. A novel polymorph of venlafaxine hydrochloride was discovered at 180-190 °C, and it was more stable than marked drug forms [4]. The solid phase transition is induced by various factors in addition to temperature [5], the presence of defects in a crystal [6–8], the pressure [9], the photochromic reaction by the UV irradiation [10], the solvent vapor [11], etc.
The solid-phase transition is helpful for the crystallization of the substance intolerant of hydrolysis. In our previous work [12], we induced a hydrate crystal of the antibiotic carbapenem CS-023 with a β-lactam structure, the polymorph Form H (CS-023·4H2O), by the solid-phase transition of an ethanol (EtOH)-solvate polymorph, Form A (5/2EtOH ·1/2H2O). The solid-phase transition from an ethanol-solvate crystal into a hydrate crystal avoided the quick hydrolysis of b-lactam compound that is unavoidable in the direct crystallization from an aqueous solution. Form A transformed to another solvate polymorph, Form C (1EtOH·3H2O), by the solvent-mediated transformation under 40-80% (mainly 40-75%) ethanol [13].
This study investigated the solid-phase transition of the carbapenem CS-023 crystal, Form C to Form H. Then, the solid-phase transition mechanism of the solvate crystal to the hydrate crystal was investigated.
2. Materials and Methods
2.1. Materials
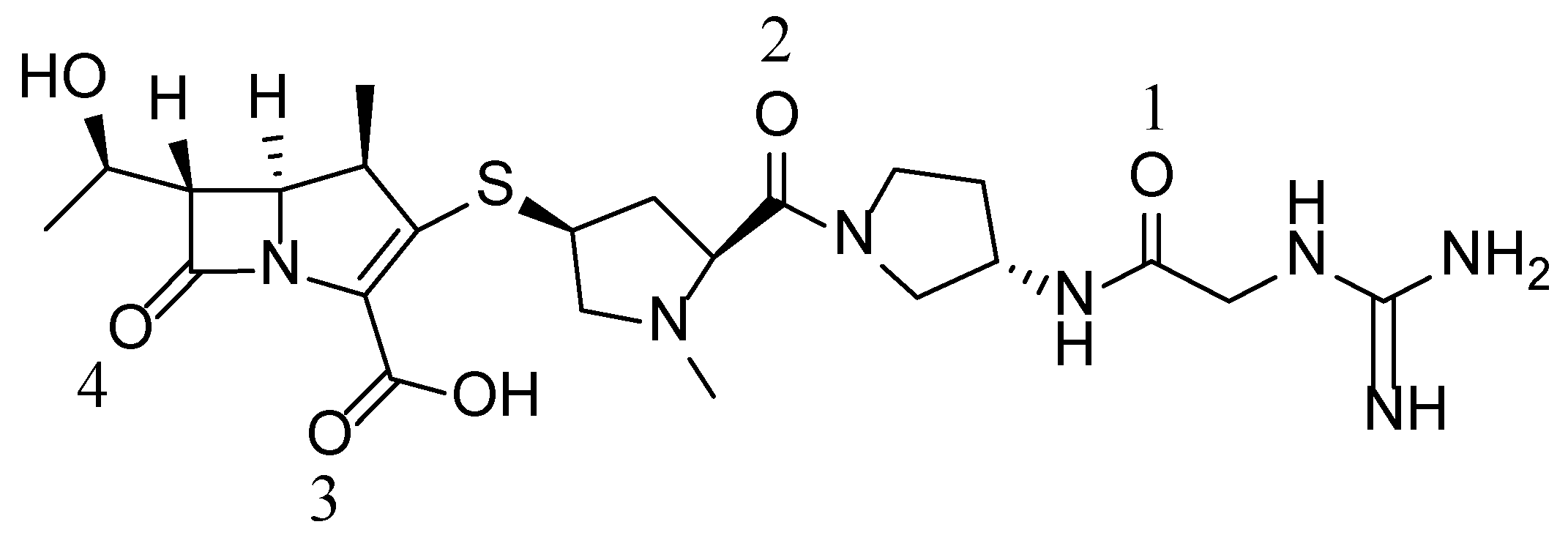
Carbapenem (CS-023), with the chemical structure shown in
Figure 1, was supplied by Daiichi Sankyo Co., Ltd. (Tokyo, Japan) [14,15]. The purity was determined to be 99.5% using a reverse-phase column based on the HPLC analysis and was used without further purification. Analytical reagent-grade ethanol was bought from Wako Pure Chemical Industries (Osaka, Japan). Ultrapure water was prepared in the laboratory.
2.2. Crystallization
Form C was recovered from 30-75% ethanol solution by poor solvent crystallization at a constant temperature of 25 °C. The crystals were filtered with a 1.0 mm-cellulose filter (ADVANTEC No.5). Then, crystals were washed with 96 v/v% ethanol solvent of three double volumes of crystals under 50% RH at 25°C and dried at 25°C and 1.6 kPa. The PXRD pattern, TG-analysis, and the water emission and absorption isotherm confirmed the crystals as Form C (CS-023·1EtOH·3H2O) [13]. Form C was the product of the solvent-mediated transformation of Form A (5/2EtOH·1/2H2O [13]. Form A has been obtained from an 80% ethanol solution by poor solvent crystallization. Namely, we had failed to get Form C from an 80% ethanol solution. It is due to the slow solvent-mediated transformation of Form A to Form C. In the present work, Form C recovered from a 75% ethanol solution was used for the starting material of the solid-phase transition of Form C to Form H.
2.3. Phase Transition on Instrumental Analysis
The phase transition experiment used a powder X-ray diffraction (PXRD) device with an adjustable temperature and humidity. The PXRD patterns were derived using BrukerD8 Advance (Bruker AXS GmbH,) with Cu K_ radiation (1.5418 Å) at 40 kV and 40 mA. The patterns were recorded between 2° and 42°, with a step 0.02°. A domed hot-stage DHS900 (Anton Paar, Graz, Austria) was attached for the in situ temperature and humidity diffraction measurements. Humidity was controlled using SRG-1R (SHINEI,) at 25 °C.
2.4. Phase Transition by Absorption and Emission of Water and Ethanol
Vapor absorption and emission isotherms of crystalline materials were determined using DVS advantage-1 (Surface Measurement Systems Ltd., London, UK) in the relative humidity range of 0–95% at intervals of 5%. The equilibrium criterion for each step is that the weight change should be less than 0.001% for 5 min.
2.5. Determination of Crystal Structure by Single-Crystal X-Ray Diffraction
Single crystal structure analysis was performed on crystals that could grow to a size available for structure analysis. Single-crystal X-ray diffraction were collected using a Graphite Monochromator (Rigaku/MSC Mercury CCD, Japan) with Mo K_ radiation (0.7107Å) at 150 K. All structures were solved by direct methods using SIR97 [16] as implemented in the program package CrystalClear (Rigaku, 2001). Refinement was carried out using the full-matrix least-squares method with the SHELXL-97 program [17]. All non-hydrogen atoms were refined anisotropically. The hydrogen atoms were located using the difference Fourier methods. Hydrogen atoms were refined in the riding mode.
3. Results and Discussion
3.1. The Solid-Phase Transition of Form C to Form H
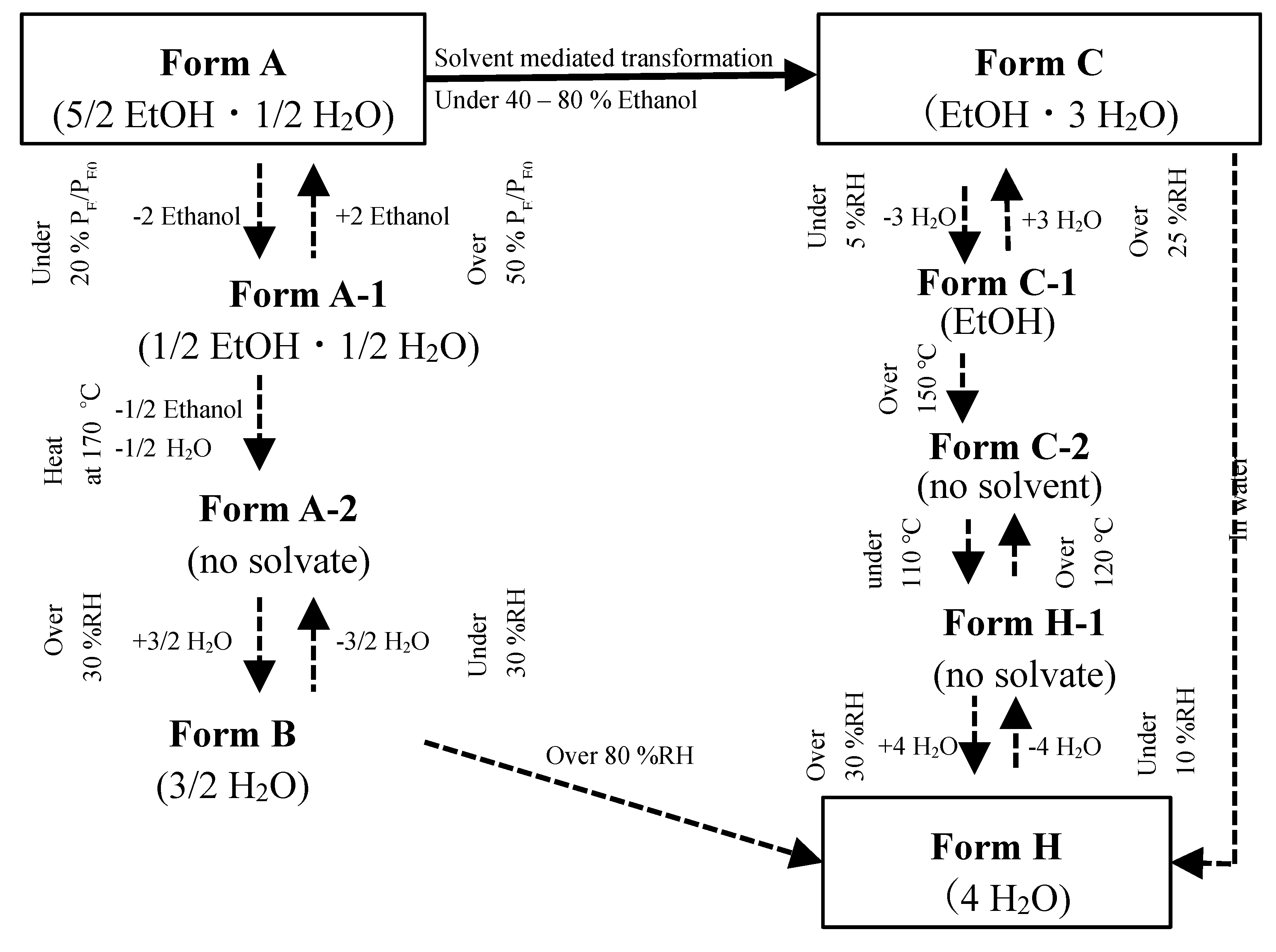
The direct immersion of Form C in water is an easy way to get the hydrate crystal Form H. However, it has a risk of hydrolysis of dissolved CS-023 molecules. Then, at present, we attempted the solid-phase transition of Form C to Form H by putting Form C under humidity and temperature control.
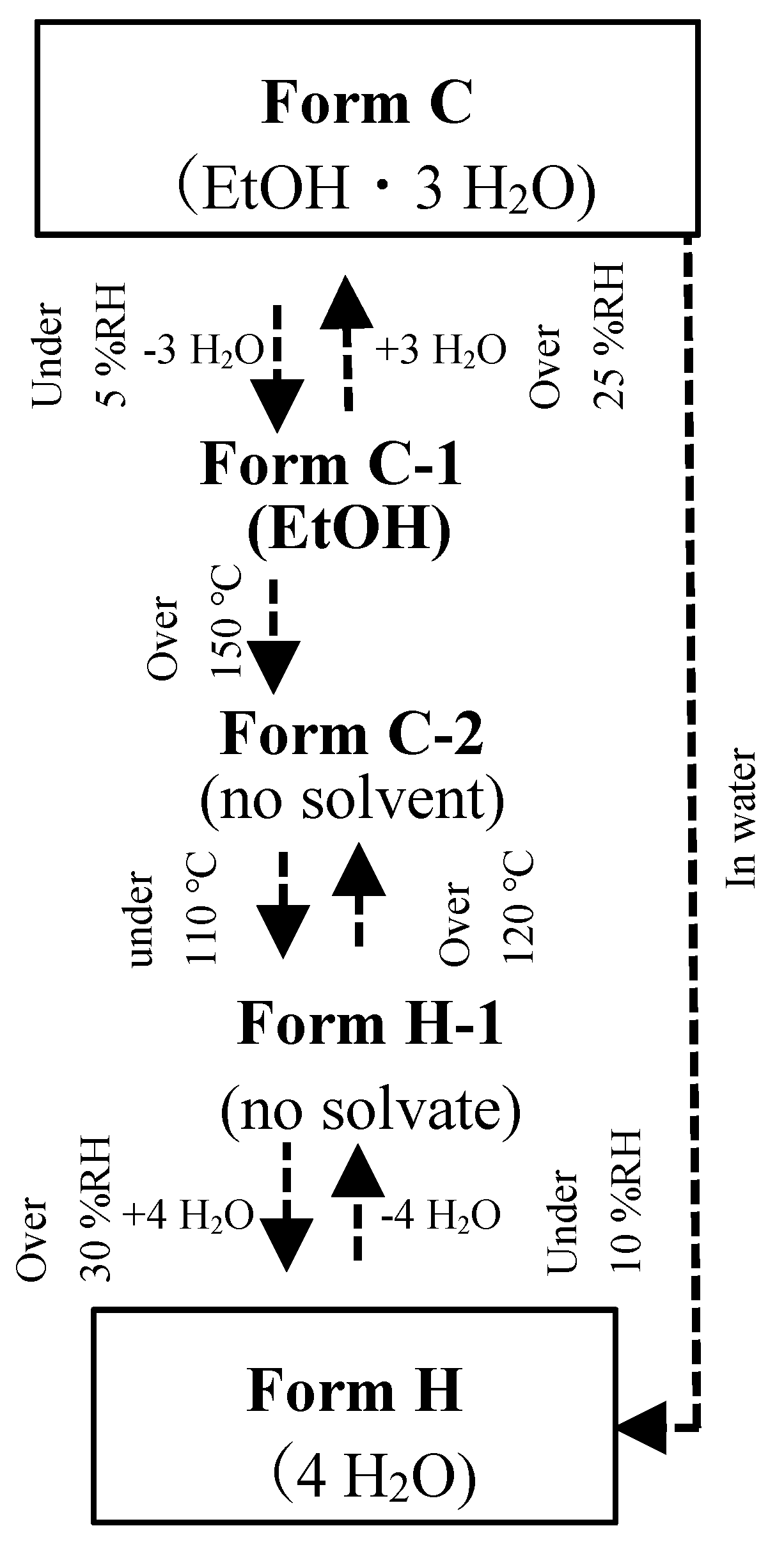
Figure 2 presents the diagram of the solid-phase transition of Form C (CS-023·1EtOH·3H
2O) to Form H (4H
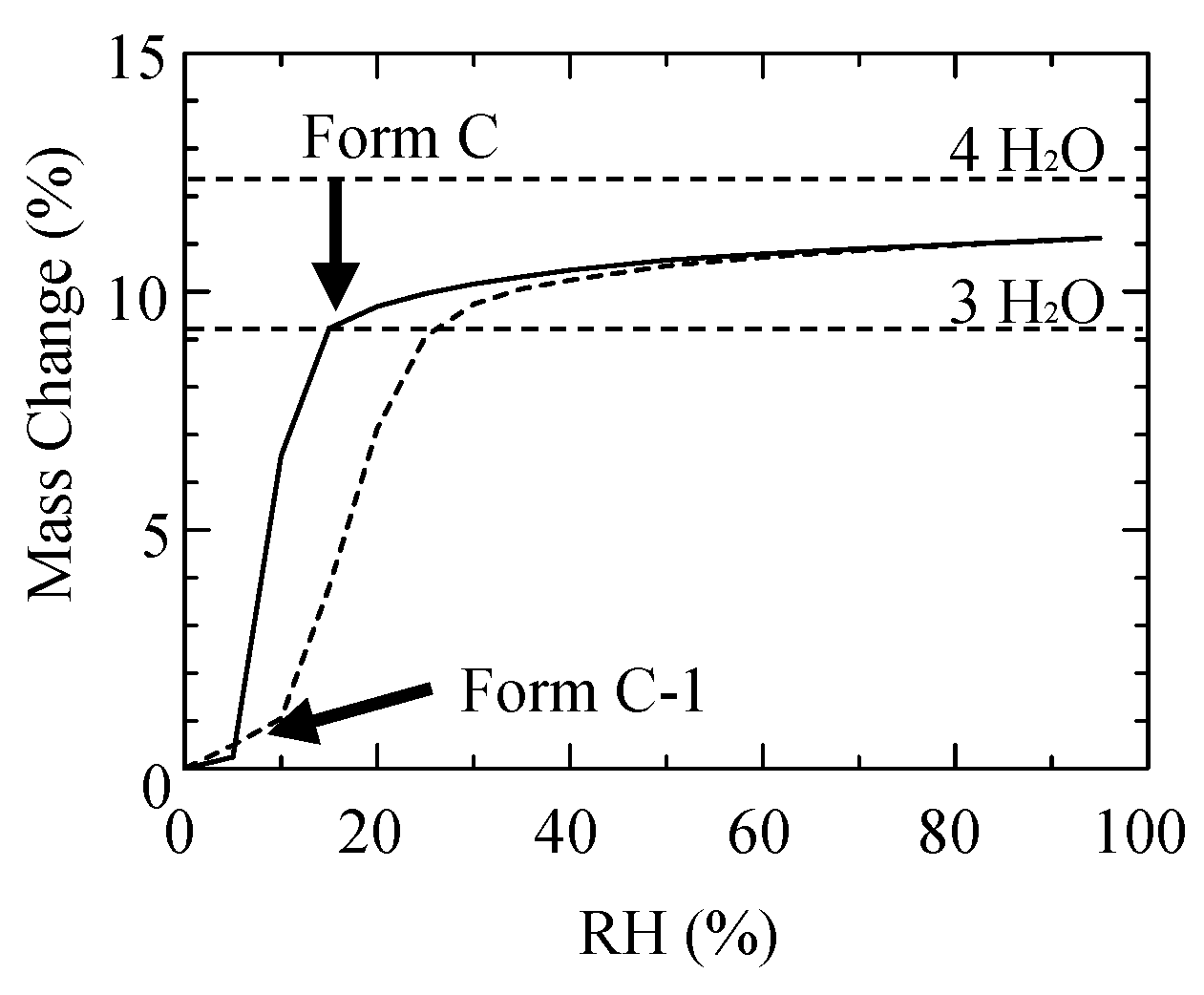
2O). The dotted arrow directly tied to Form H is a result shown in the previous paper [13]. When Form C was placed under 5%RH, it lost 3H
2O, as shown in the emission isotherm,
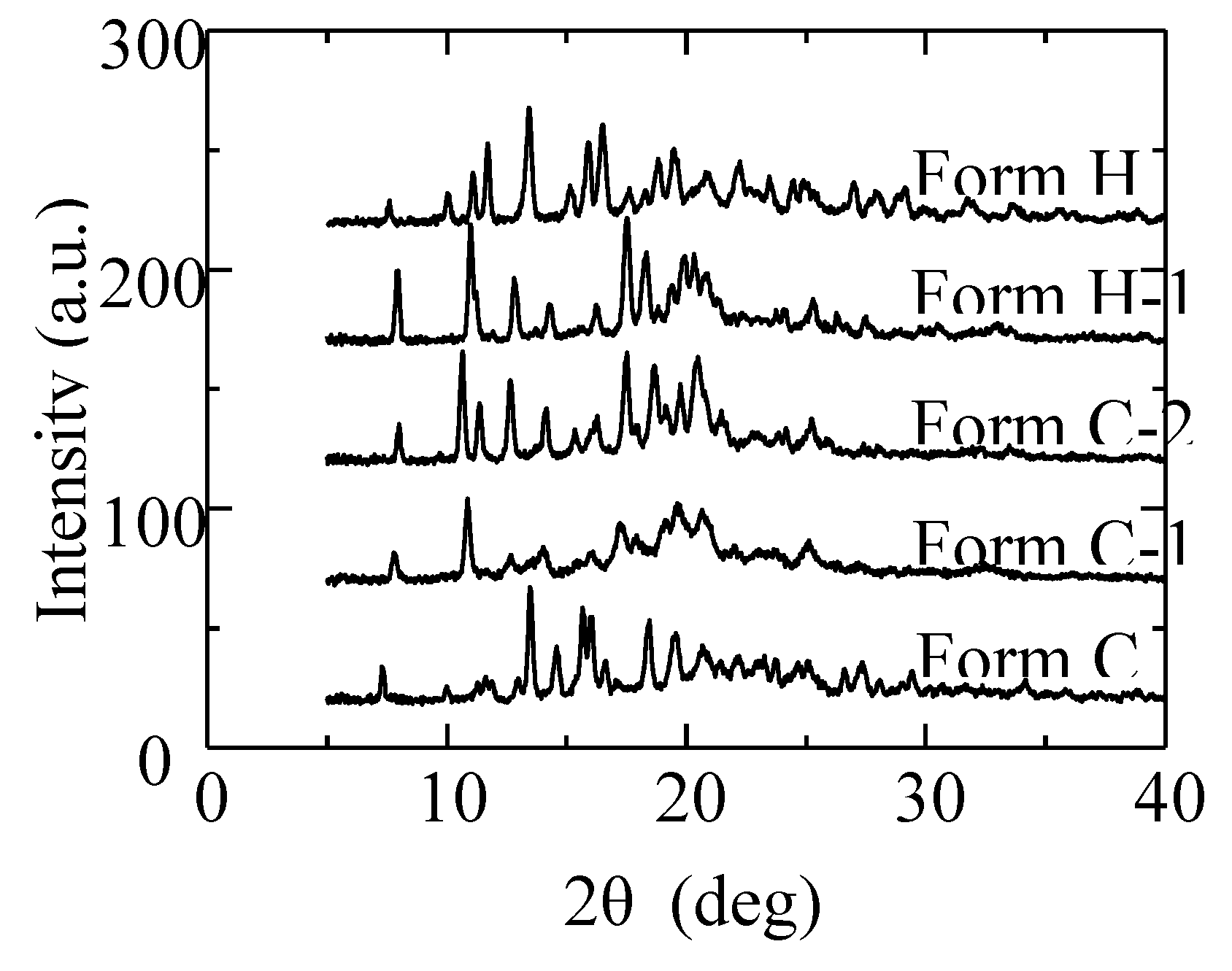
Figure 3. The XRD pattern of the dehydrated solid presented in
Figure 4 showed that the dry solid holds the crystalline state. Then, the crystal was named Form C-1 (CS-023·EtOH). Form C-1 returned to Form C under the higher than 25% RH.
Then, we adopted the thermal transition at a high temperature. When Form C-1 was heated to 150°C, it changed to Form C-2 by releasing one crystalline ethanol. The XRD pattern change is presented in
Figure 4. When the temperature was raised to 180°C for confirmation, the same XRD pattern as Form C-2 was observed (Data not shown here). Furthermore, by cooling down Form C-2 under 110°C, it changed to Form H-1. Form H-1 returned to Form C-2 over the temperature of 120°C. Form H-1 transforms to Form H, as reported in the previous paper [12].
3.2. The relationship of CS-023 Polymorphs, Form A, Form C, and Form H
We summarize the relationship of polymorphs of CS-023 by combining the diagram of the solid-phase transition of Form C to Form H with the diagram of the solid-phase transition of Form A to Form H [12] in
Figure 5. We will explain the value of the transition map in
Figure 5. We have obtained a hydrate polymorph of the antibiotic carbapenem CS-023, Form H (CS-023·4H
2O) by the cooling crystallization from an aqueous solution [12]. However, the cooling crystallization from the aqueous solution had a potential problem. Namely, the CS-023 dissolved in water was prone to the hydrolysis. Then, we got a solvate polymorph, Form A (CS-023·5/2EtOH) 1/2H
2O), by the poor solvent crystallization using ethanol as a poor solvent at 25°C [12]. Form A was recovered from an 80% ethanol solution that directly added pure ethanol into the aqueous CS-023 synthesis reaction solution. Form A transformed Form H through the solid-phase transition. Form H was obtained through another solid-phase transition route reported in the present work, from Form C (CS-023·1EtOH·3H
2O) to Form H.
Figure 5 shows that, in this case, it is possible to select the solid-phase transition of solvate crystals to anhydrous or hydrate crystals in the crystallization of water-unstable substances.
3.3. The Comparison of the Crystal Structure between Form A, Form C, and Form H
Table 1 presents Form A, C, and H's crystallographic data. The data for Form A and Form C have been reported in the previous paper [13]. The structures of Form C and Form H are similar except for the solvation. The similarity in lattice structure suggests that the solid-phase transition of Form C into Form H is easy because it seems possible only by going in and out of solvent. On the other hand, the structures of Form A and Form H are distinguishably different. However, it can be reconfirmed that Form A also transformed into the same hydrate polymorph, Form H. Then, it was investigated how Form A transformed into Form H.
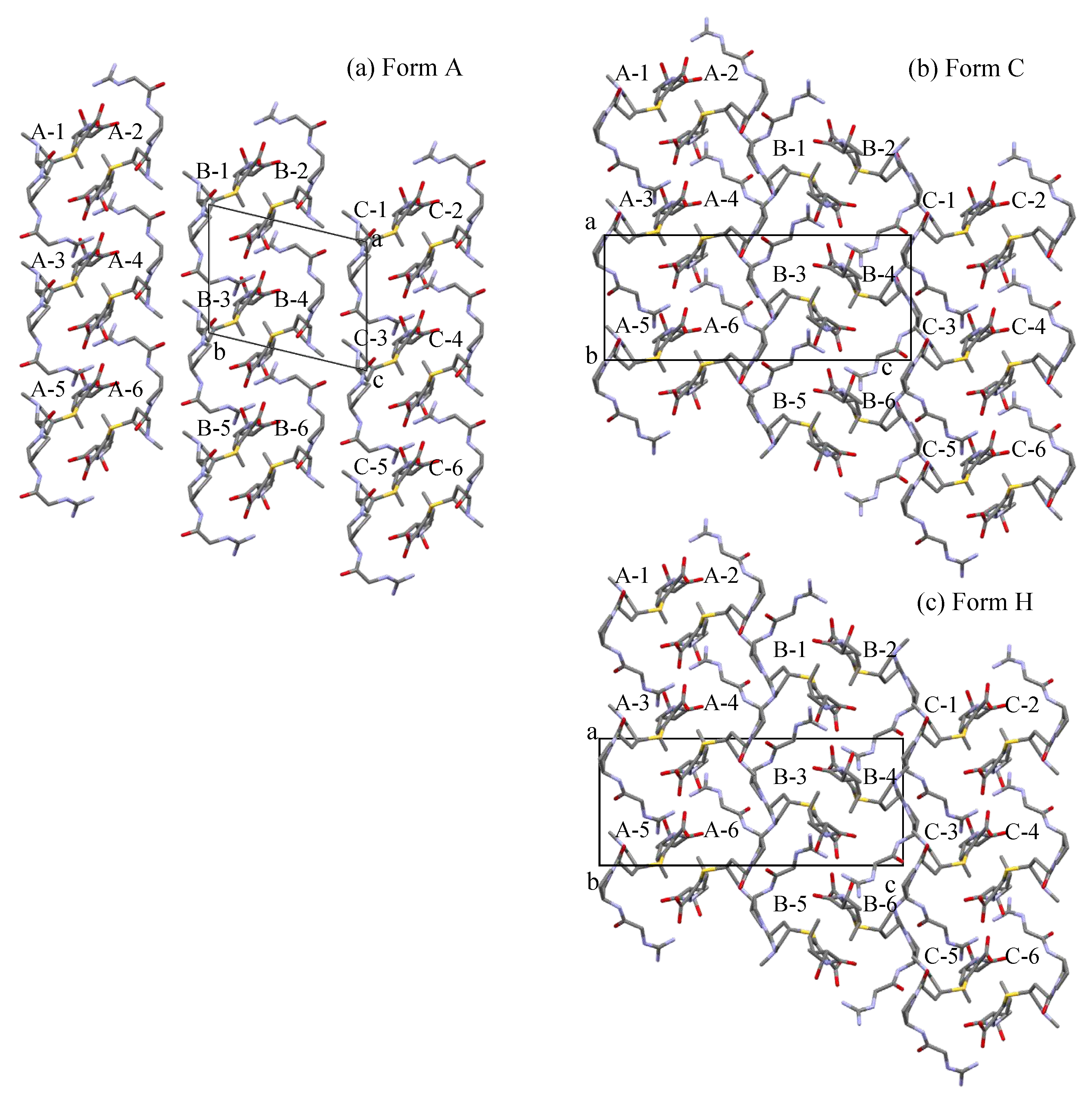
The three polymorphs are composed of similar helical chain structures as follows.
Figure 6 presents the mutual relationship between 3 helix chains (named A-, B-, and C-chains) composed of the three polymorphs: (a) Form A, (b)Form C, and (c) Form H. However, solvent molecules, namely water and ethanol, are omitted here for the simple look of the three polymorph structures. Our eyes are on the ab-plane of the Form A crystal lattice (the panel (a)) and the ac-plane in Form C and Form H’s crystal lattice (the panels (b) and (c)). Each molecule in each helix is named a number from 1 to 6. In Form A, the three-helix chains point in the same direction.
On the other hand, in Form C and Form H, the three-helix chains alternate. Namely, the mutual relationship between the neighboring helix chains in Form A differs from those in Form C and Form H. This observation also suggests that the solid-phase transition of Form C into Form H is easy because the CS-023 molecule does not need to move as much.
3.4. The Mechanism of the Solid-Phase Transition of Form A into Form H
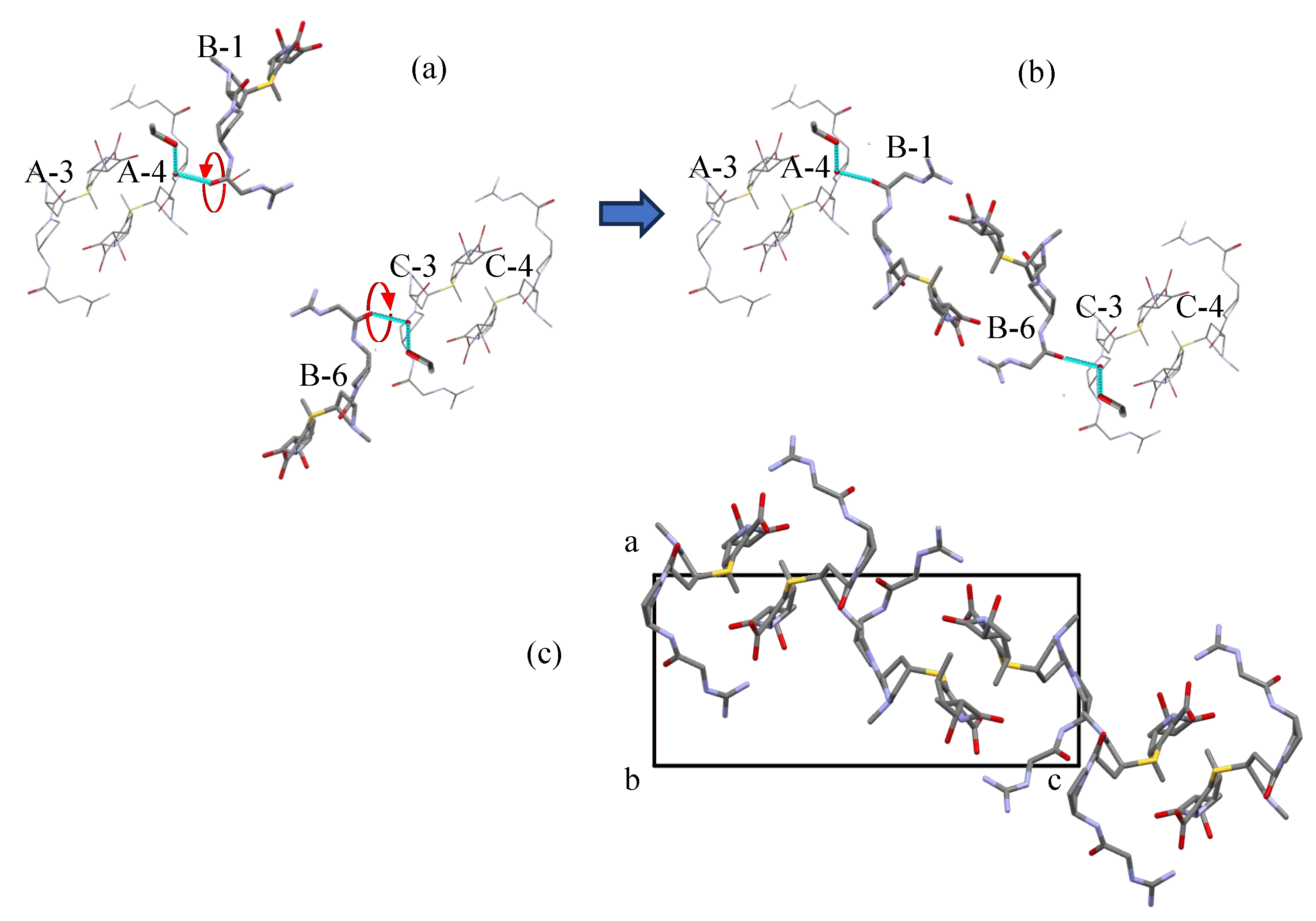
Figure 6 shows that the mutual relationship between the neighboring helix chains of Form A must change to transition Form A into Form H.
Figure 7a presents the imaginary 180°-rotation of two CS-023 molecules (B-1 and B-6 in Form A). Each molecule rotates inversely. The rotation results in the relationship illustrated in
Figure 7b. It seems similar to the structure of Form H shown in
Figure 7c, except for a slight difference in the conformation of the CS-023 molecule. The rotated B-1 and B-6 molecules in Form A correspond to the B-3 and B-4 molecules in Form H (
Figure 6c). The newly formed mutual relationship between the helix chains A, B, and C should induce the new stable conformation of each molecule: it should be Form H.
A question remains in the above explanation about the solid-phase transition of Form A into Form H: how did the rotation of molecules start in a solid crystal? Some polymorphic transition phenomena were interpreted as a rearrangement of weak intermolecular interaction caused by a slight molecular movement inside the crystal lattice [19,20]. The 180°-rotation of a molecule in the crystal lattice shown in
Figure 7 is not a little movement of a molecule. We discuss the role of water molecules that Form B (CS-023·3/2H
2O) excessively absorbs at high humidity.
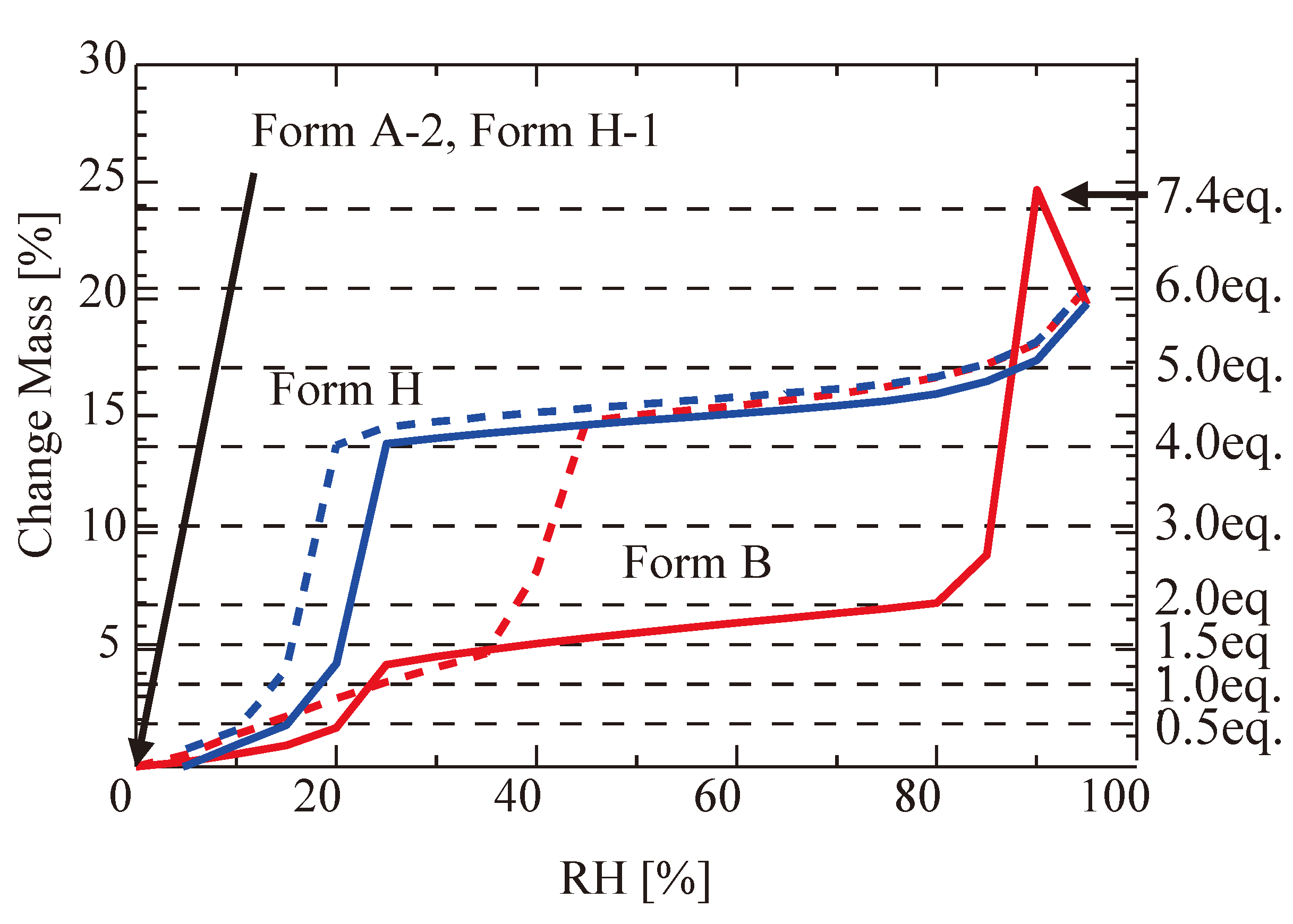
Figure 8 presents the water-absorption isotherm curve of Form A-2 (no solvent) to Form H via Form B (the solid red curve) and the water-emission curve of Form H into Form H-1 (the solid blue curve). In
Figure 8, the water-absorption isotherm curve of Form H-1 (no solvent) to Form H (the dotted red curve) and the water-emission curve of Form H into Form H-1 (the dotted blue curve) are also presented. Here, it should be noted that Form A-2 absorbed water over a 4- or 5-equivalent level. The absorption reached a 7.4-equivalent level at high humidity and then dropped to a 6-equivalent level. After that, the level gradually decreased. This observation means that Form A-2 absorbed too much water over Form H’s level. The loosening of the helix chain of Form A-2 must cause this water-absorption excess. We suppose that the loosening was caused by the movement of the CS-023 molecules in Form B, namely by the rotation of the molecules, as shown in
Figure 7. We suppose the rotation started after forming Form B, namely after the 1.5 equivalent water was absorbed in Form A-2. The hydrogen bond formed between the carbonyl group of the CS-023 molecule (No. 2 in
Figure 1) and one water molecule in Form B played an essential role as the rotation axis.
4. Conclusions
The solid-phase transition of Form C (CS-023·EtOH·3H2O) into Form H (CS-023·4H2O) was performed. At first, Form C-1 (CS-023·EtOH) was obtained by placing Form C under less than 5% RH. Then, by heating Form C-1 to 150-180°C, the solvate-free polymorph, Form C-2, was obtained. Form C-2 was transformed into Form H-1 by cooling it down to 110°C. Finally, Form H-1 changed to Form H as described in the previous paper [12]. As a result, the hydrate polymorph was recovered without exposing Form C to bulk water, with no risk of the hydrolysis of CS-023’s b-lactam structure. This is a merit of adopting the solid-phase transition for the polymorph change.
Figure 5 shows that two solvate polymorphs, Form A and Form C, were transformed into the same hydrate polymorph. The three polymorphs, Form A, H, and C, were composed of the helical-chain structures, respectively. However, there was an incomprehensible affair in the solid-phase transition among the three polymorphs. Namely, Form A comprised a left-handed helix. On the other hand, Form C and Form H’s helix chains were in a left- and right-handed helix com-plex. In the case of the transition of Form A to Form H, the helicity change had to be involved. The solid-phase transition of Form A into Form H suggested the switch of helicity in the solid. We attempted to explain the helicity change in solid phase transition. As a result, we understood that the over-absorption of water of no solvent polymorph, Form A-2, induced from Form A, plays a vital role in the helicity change.
Acknowledgments
The authors would like to thank Daiichi Sankyo Co., Ltd for supplying CS-023.
References
-
https://www.pmda.go.jp/int-activities/int-harmony/ich/0043.html. (accessed on 18 July 2021).
- Valentina, C.; Norberto, M.; Giovanni, P. Crystal Chemistry of the Antibiotic Doripenem. J. Pharm. Sci. 2014, 103, 3641–3647. [Google Scholar] [CrossRef]
- Hickey, M.B.; Peterson, M.L.; Manas, E.S.; Alvarez, J.; Haeffner, F.; Almarsson, Ö. Hydrates and Solid-State Reactivity, A Survey of β-Lactam Antibiotics. J. Pharm. Sci. 2007, 96, 1090–1099. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Bhatt, P. M.; Nangia, A.; Kruger, G. J. Stable polymorph of venlafaxine hydrochloride by solid-to-solid phase transition at high temperature. Crystal growth & design 2007, 7, 476–480. [Google Scholar] [CrossRef]
- Long, S.; Zhang, M.; Zhou, P.; Yu, F.; Parkin, S.; Li, T. Tautomeric polymorphism of 4-hydroxy nicotinic acid. Crystal Growth & Design 2016, 16, 2573–2580. [Google Scholar] [CrossRef]
- Shi, G.; Li, S.; Shi, P.; Gong, J.; Zhang, M.; Tang, W. Distinct pathways of solid-to-solid phase transitions induced by defects: the case of dl-methionine. IUCrJ 2021, 8, 584–594. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Suzuki, M.; Yang, Y.; Yoshikawa, I.; Yin, Q.; Houjou, H. Seed-triggered solid-to-solid transformation between color polymorphs: striking differences between quasi-isomorphous crystals of dichloro-substituted salicylideneaniline regioisomers. CrystEngComm 2020, 22, 4903–4913. [Google Scholar] [CrossRef]
- Li, M.; Yue, Z.; Chen, Y.; Tong, H.; Tanaka, H.; Tan, P. Revealing thermally-activated nucleation pathways of diffusionless solid-to-solid transition. Nature Communications 2021, 12, 4042. [Google Scholar] [CrossRef] [PubMed]
- Patyk-Kaźmierczak, E.; Kaźmierczak, M. Hydrate vs anhydrate under a pressure-(De) stabilizing effect of the presence of water in solid forms of sulfamethoxazole. Crystal Growth & Design 2021, 21, 6879–6888. [Google Scholar] [CrossRef]
- Kitagawa, D.; Kawasaki, K.; Tanaka, R.; Kobatake, S. Mechanical behavior of molecular crystals induced by a combination of photochromic reaction and reversible single-crystal-to-single-crystal phase transition. Chemistry of Materials 2017, 29, 7524–7532. [Google Scholar] [CrossRef]
- Hariharan, P. S.; Pan, C.; Karthikeyan, S.; Xie, D.; Shinohara, A.; Yang, C.; Wang, L.; Anthony, S. P. Solvent vapor induced rare single-crystal-to-single-crystal transformation of stimuli-responsive fluorophore: Solid state fluorescence tuning, switching and role of molecular conformation and substituents. Dyes and Pigments 2020, 174, 108067. [Google Scholar] [CrossRef]
- Matsuura, S.; Igarashi, K.; Azuma, M.; Ooshima, H. Polymorphic Crystallization Design to Prevent the Degradation of the b-Lactam Structure of a Carbapenem. Crystals 2021, 11, 931–944. [Google Scholar] [CrossRef]
- Matsuura, S.; Igarashi, K.; Azuma, M.; Ooshima, H. The Identification and Characterization of a New Carbapenem CS-023 Solvate Polymorph Achieved by the Appropriate Crystal Washing and Drying. J. of Chemical Engineering of Japan 2023, 51, 2215225. [Google Scholar] [CrossRef]
- Altomare, A.; Burla, M. C.; Camalli, M.; Cascarano, G. L.; Giacovazzo, C.; Guagliardi, A.; Moliterni, A. G. G.; Polidori, G.; Spagna, R. a new tool for crystal structure determination and refinement. Appl. Cryst. 1999, 32, 115–119. [Google Scholar] [CrossRef]
- Sheldrick, G. M.; Schneider, T. R. [16] SHELXL: High-resolution refinement. Methods in enzymology Academic Press. 1997, 277, 319–343. [Google Scholar] [CrossRef] [PubMed]
- Kawamoto, I.; Shimoji, Y.; Kanno, O.; Kojima, K.; Ishikawa, K.; Matsuyama, E.; Ashida, Y.; Shibayama, T.; Fukuoka, T.; Ohya, S. Synthesis and structure-activity relationships of novel parenteral carbapenems, CS-023 (R-115685) and related compounds containing an amidine moiety. J. Antibiot. 2003, 56, 565–579. [Google Scholar] [CrossRef] [PubMed]
- Shibayama, T.; Sugiyama, D.; Kamiyama, E.; Tokui, T.; Hirota, T.; Ikeda, T. Characterization of CS-023 (RO4908463), a Novel Parenteral Carbapenem Antibiotic, and Meropenem as Substrates. Drug Metab. Pharmacokinet. 2007, 22, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, D.; Tamura, R.; Lepp, Z.; Takahashi, H.; Ushio, T. Mechanism of a New Type of Solvent-Assisted Solid-to-Solid Polymorphic Transition Causing Preferential Enrichment: Prominent Influence of C (sp2) H---O Interaction on the Control of a Crystal Structure. Crystal growth & design 2003, 3, 973–979. [Google Scholar] [CrossRef]
- Horiguchi, M.; Okuhara, S.; Shimano, E.; Fujimoto, D.; Takahashi, H.; Tsue, H.; Tamura, R. Mechanistic flexibility of solvent-assisted solid-to-solid polymorphic transition causing preferential enrichment: Significant contribution of π/π and CH/π interactions as well as hydrogen bonds. Crystal growth & design 2007, 7, 1643–1652. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).