Submitted:
21 December 2023
Posted:
21 December 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Nutritional composition, bioactives’ content and associated health benefits of watermelon and its y-products
2.1. Nutritional composition, bioactives’ content and associated health benefits of watermelon
| Fruit | By-products | |||||
|---|---|---|---|---|---|---|
| Flesh | Juice | Pulp | Seed | Peel | Rind | |
| Compounds | Quantity g/100g | Quantity g/100g | ||||
| Moisture | 91.45 | 90.1-92.42 | 11.5 | 3.39-8.5 | 8.78 | 5.12-94.62 |
| Ash | 0.25 | 0.1-0.37 | 3.66 | 2.48-4.9 | 5.31 | 0.46-20 |
| Protein | 0.61 | 0.4-0.84 | 3.33 | 17.75-49.7 | 2.88 | 0.63-21 |
| Crude fat | 0.15 | 0.05-0.027 | 0.5 | 13.7-50.5 | 2.33 | 0.08-15 |
| Carbohydrates | 7.55 | 7.55 | 73.35 | 6.06-46.3 | 70.04 | 4.2-80.75 |
| Crude fiber | 0.4 | 0.4-0.7 | 7.66 | 2.1-40.75 | 10.66 | 2.6-23 |
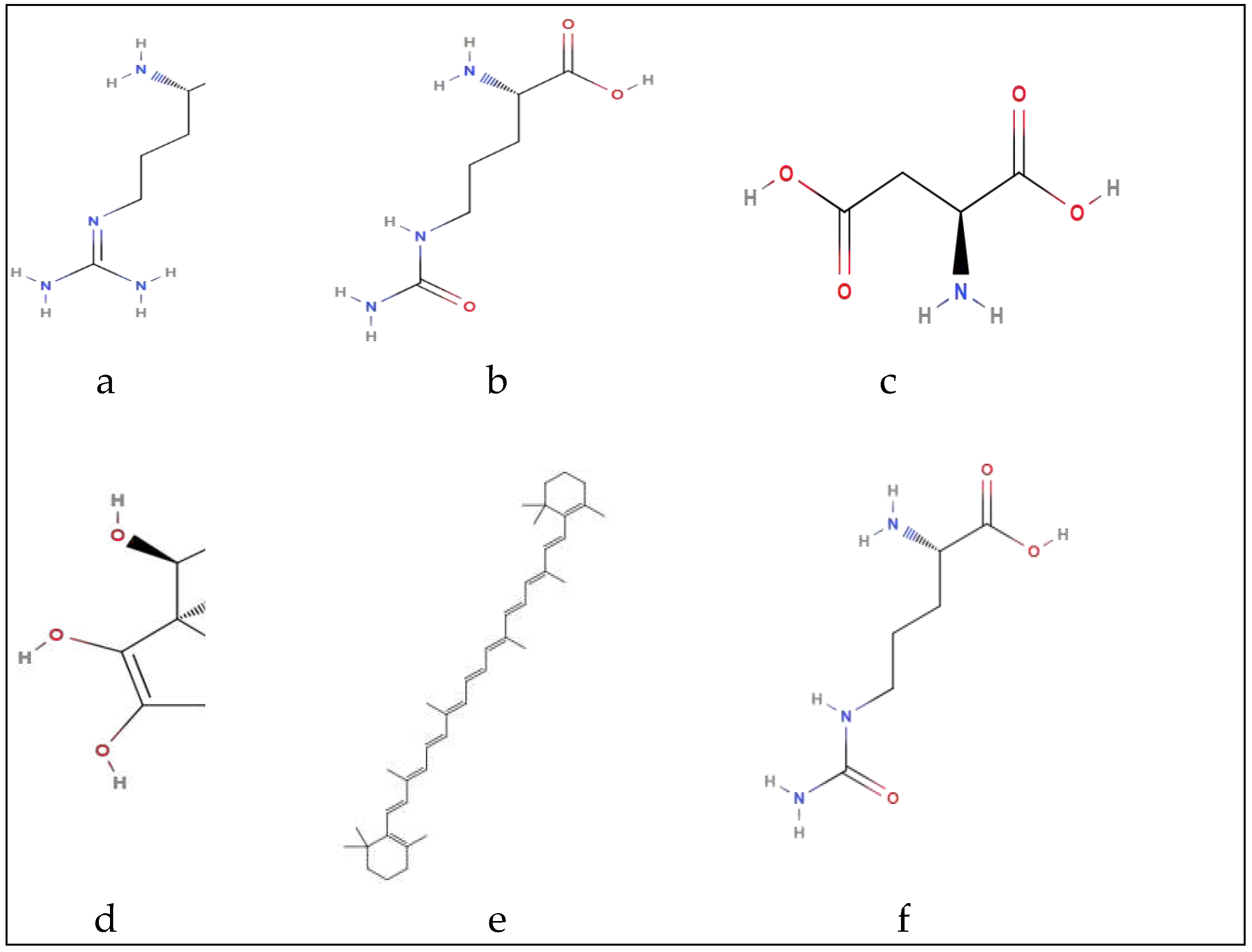

2.2. Nutritional composition, bioactives’ content and associated health benefits of watermelon by-products

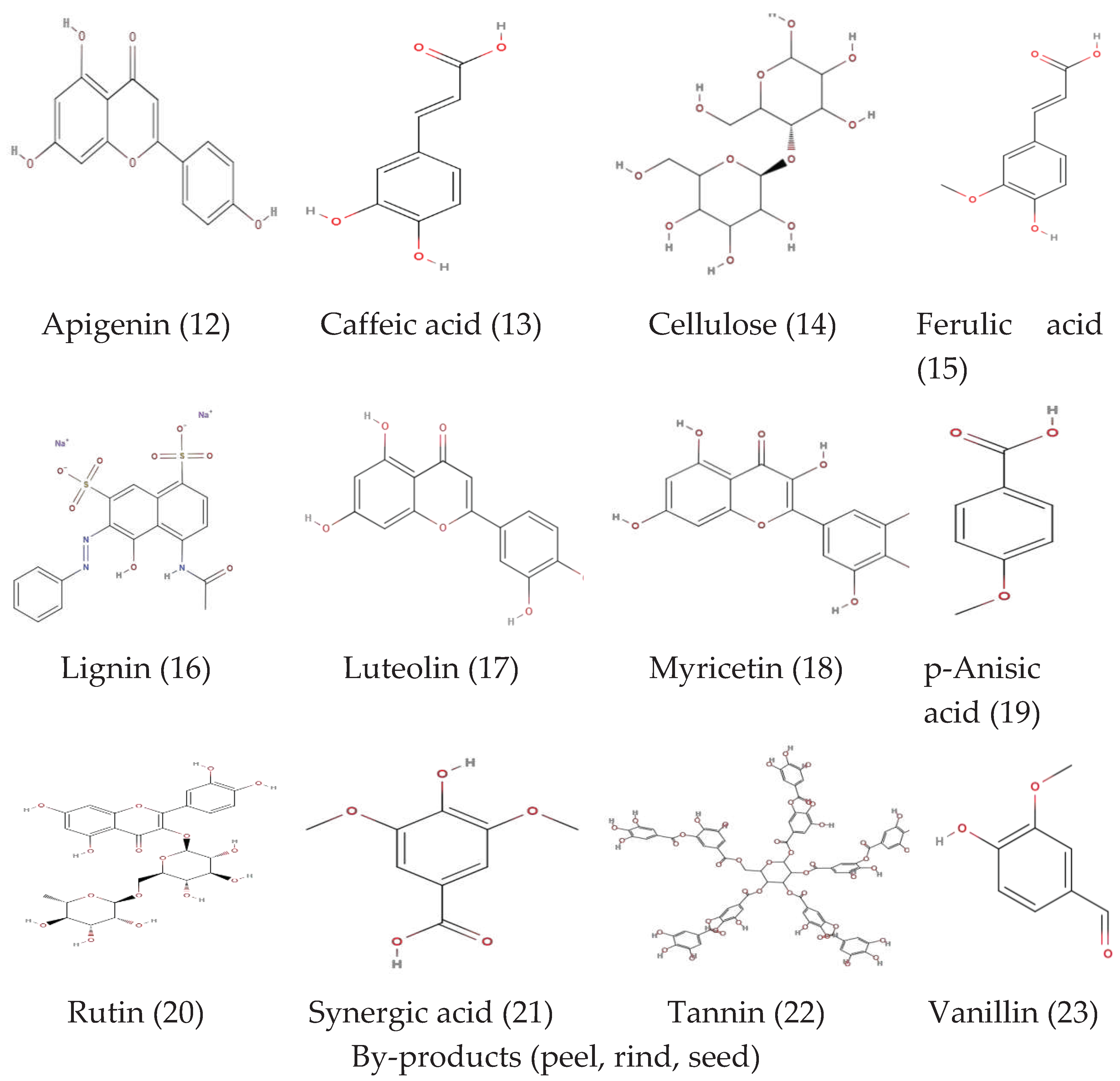
| Parts | Carbohydrates | Anti-nutrients | Vitamins | Minerals | Amino acids | Phenols | Fatty acids |
|---|---|---|---|---|---|---|---|
| WMR | Galactose Xylose Arabinose Glucose Glucoranic acid Mannose Rhamnose Glucuronic acid |
Oxalates Alkaloids Phytates Saponin Tanin Flavonoids Phenols | A B1 B2 B3 B6 C |
Fe Mn P Ca Na Zn Cu K Mg |
Citrulline phenylalanine Valine Leucine Tyrosine Lysine | Gallic acid, Synapic acid, Hydroxycinnamic acid, Quercetin, m-Coumaric acid, Chlorogenic acid, Syringic acid, p-coumaric acid, myricetin, caffeic acid, vanillic acid, 4-hydroxybenzoic acid, p-anisic acid. |
Saturated Myristic acid, Palmitic acid, Margeric acid, Stearic acid, Arachidic acid, Behenic acid, Lignoceric acid. Unsaturated Elaidic acid, Linolenic acid, Linoleic acid, Vaccenic acid Oleic acid, Docosahexaenoic acid, Eicosadienoic acid, eicosenoic acid, hypogeic acid, vaccenic acid, gondoic acid. |
| Seed | Galactose Arabinose Glucose Sucrose Mannose Fructose Xylose |
Oxalates Alkaloids Phytates Saponin Tannin Phenols Flavonoids | A B1 B2 B3 B6 B12 D E K |
Fe Mn P Ca Na Cu Zn Cr K Mg Pb |
Arginine Phenylalanine Lysine Leucine Iso-leucine Aspartic acid Glutamicacid, Serine Valine Cysteine Glycine Histidine Threonine Alanine Proline Methionine |
Gallic acid, Caggeic acid, Syringic acid, Sinapic acid, Rosmarinic acid, Vanillic acid, Prochatechuic acid, Leuteolin, Flavonoids, Flavone, Chlorogenic acid, Apigenin, Phenolic acid, 4-hydroxybenzoic acid, Ferulic acid, Coumaric acid. |
Saturated Myristic acid, Arachidic acid, Pentadecyclic acid, Margeric acid, Behenic acid, Stearic acid, Lignoceric acid. Unsaturated Palmitoleic acid, Gadoleic acid, Linolenic acid, Linoleic acid, Oleic acid, Erucic acid. |
| Peel | Galactose Xylose Arabinose Glucose Glucoranic acid Mannose Rhamnose Glucuronic acid |
Alkaloids Saponin Tanin Flavonoids Phenols | A B1 B2 B3 B6 C |
Fe Mn P Ca Na Zn Cu K Mg |
Citrulline Phenylalanine Valine Leucine Tyrosine Lysine | Gallic acid, Synapic acid, Hydroxycinnamic acid, Quercetin, m-Coumaric acid, chlorogenic acid, syringic acid, p-coumaric acid myricetin caffeic acid vanillic acid 4hydroxybenzoic acid, p-anisic acid. |
Saturated Myristic acid Palmitic acid Margaric acid Stearic acid Arachidic acid Unsaturated Linolenic acid Linoleic acid Oleic acid |
| Pulp | Fructose Sucrose Glucose |
Flavonoids Glycosides Tannins Phenols Saponins Tannins |
A B1 B6 C E |
Ca Fe Mg Zn Na K |
Citrulline Leucine Glutamic acid, Aspartic acid Arginine |
Carotenoids Quercetin, Luteolin Gallic acid Coumarin Aviprin |
Linoleic acid Oleic acid Palmitic acid Stearic acid Linolenic acid |
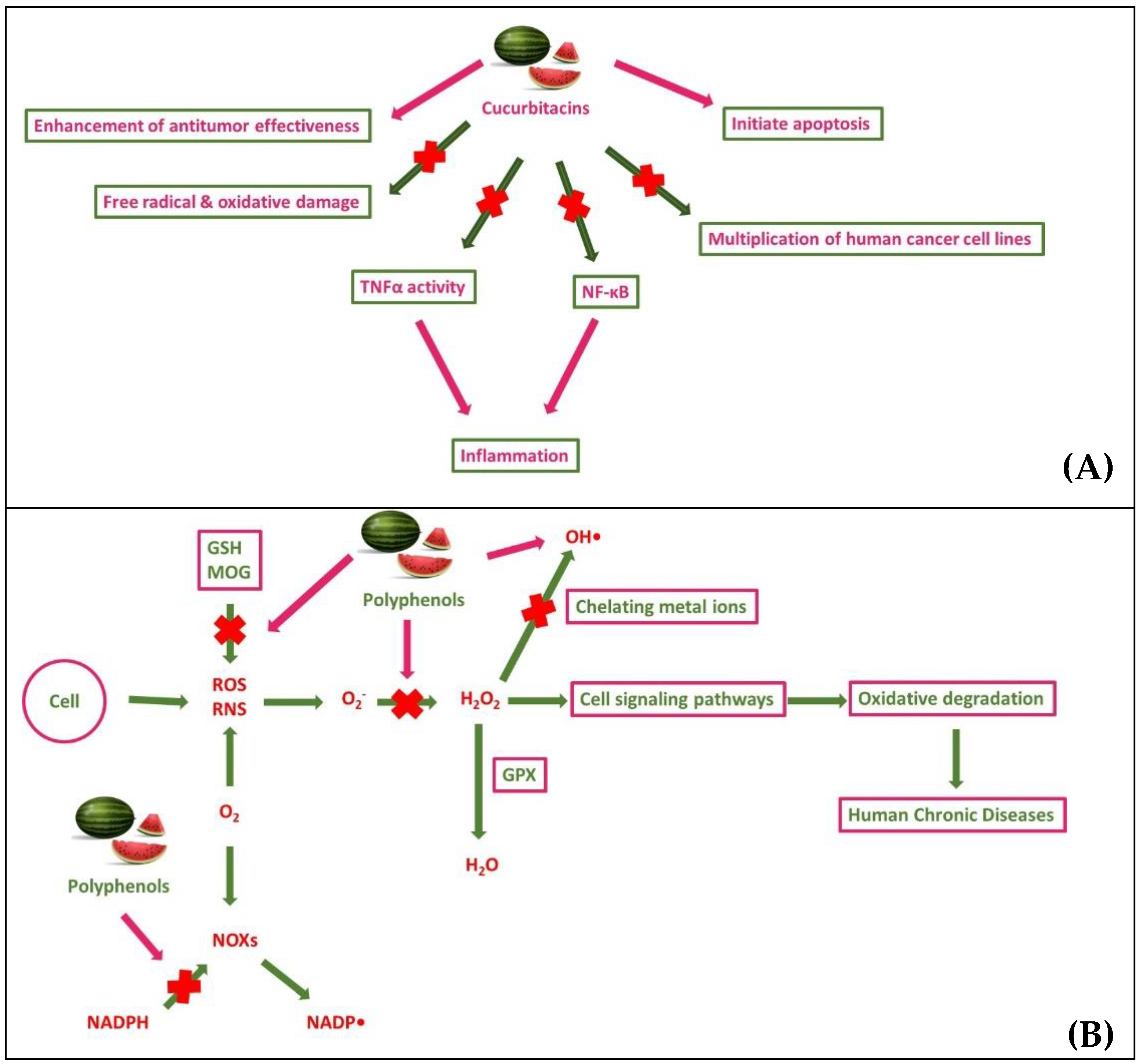
2.3. Molecular-Biochemical-Cellular Mechanisms of Action of of the most Bioactive Compounds present in Watermelon and Its By-Products: A Molecular Perspective
3. Health promoting effects associated with watermelon and its by-products bioactives
3.1. Antioxidant and anti-inflammatory properties and associated health promoting effects
3.2. Cardio-protective health promoting effects
3.3. Anti-cancer health promoting effects
3.4. Anti-diabetic health promoting effects
3.5. Other health promoting effects
3.5.1. Hepatoprotective properties
3.5.1. Antimicrobial properties
3.5.1. Analgesic properties
| Heath Promoting Properties |
Watermelon Part | Compound/ component |
Study type | Specific effects | References |
|---|---|---|---|---|---|
| Antioxidant |
Seed | Aqueous extract | In-vivo | [179] | |
| Seed | Extract | In-vitro | [182] | ||
| Seed | Extract (chloroform, ethyl acetate, and methanol) | In-vitro | [178] | ||
| Seed | Extract | In-vitro | [182] | ||
| Rind& Seed | Extract | In-vivo | [183,191,194,188] | ||
| Peel | Extract | In-vitro | [192] | ||
| Pulp & Seed | Lycopene | In-vitro | [233] | ||
| Seed | Extract | In-vitro | [234] | ||
| Whole watermelon powder | In-vivo | Ingestion | [186] | ||
| Fruit | Bioactive compounds | In-vitro | [10] | ||
|
Cardioprotective |
Pulp | Extract | In-vivo | Anti- atherosclerotic activity |
[174] |
| Seeds | Phytochemicals | In-vivo | Anti- hypertensive |
[40] | |
| Rind | Extract of polysaccharides | In-vivo | Anti- hypertensive |
[14] | |
| Seeds | Extract | In-vivo | Decrease serum Cholesterol | [235] | |
| Pulp | Carotene | In-vitro | Heart health | [236] | |
| Pulp | Lycopene | In-vitro | heart health | [74] | |
| Rind | Phytochemicals |
In-vitro In-vivo |
Hypocholesterolemic effect | [215] | |
| Seed | Extract, |
In-vitro In-vivo |
Hypolipidemic effect anti- inflammatory and antioxidant properties |
[182] [235] |
|
| Seeds | Kernel | In-vivo | Hypolipidemic efficiency | [83] | |
| Whole watermelon powder | In-vivo | Improvement of lipid profiles | [186] | ||
| Rind and peel | Powder | In-vivo | LDL Control anti- inflammatory reduces atherosclerosis |
[174] | |
| Pulp | Juice; L-Citrulline | In-vivo In-vitro | Recovery heart rate and muscle soreness | [201] | |
| Seeds | Extract | In-vivo | Reduction in creatine kinase, triglycerides, LDL, and sodium |
[161] | |
| Flesh, Seeds, Rind | L-citrulline and Arginine | In-vivo | Regulation of blood pressure and vascular health | [237] | |
| Seeds | Extract | In-vivo | Cardioprotective potential | [203] | |
| Seeds | Extract | In-vivo | Cardiovascular benefits, Anti-hypertension, reduce blood pressure | [174,200] | |
| Seed | Sitosterol, campesterol, and stigmasterol in seed oil. | In-vitro | Cardiovascular health | [238,239] | |
| Rind | Citrulline | In-vitro | Cardiovascular health | [75] | |
| Rind | l-citrulline and l-arginine | In-vivo | Cardiovascular health | [240] | |
| Pulp | In-vivo | Cardiovascular Protective | [187,241] | ||
| Pulp | Juice | In-vivo | Control serum lipid profile |
[242] | |
| Antidiabetic |
Flesh, seed, rind | Extract and Juice | In-vivo | [72,93,180,220,243,244] | |
| Rind | Citrulline | In-vitro | [215,216,245,246] |
||
| Seeds | Extract | In-vivo | [218] | ||
| Flesh and Rind | Extract and powder | In-vivo | [213] | ||
| Pulp | Juice | In-vivo | Inhibitory activity against -glucosidase and -amylase that is dosage dependent. | [242] | |
| Pulp | Juice, extract (containing alkaloids, flavonoids, and saponins) | In-vivo | Stimulate insulin release during pancreatic-cell regeneration and hinder intestinal glucose absorption. | [247] | |
| Rind | Extract | In-vivo | [211,248] | ||
| Rind and Peel | Pomace | In-vivo | [171] | ||
| Gastrointestinal tract diseases | Seed | Seed extract | In-vivo | Anti-ulcer activity | [65] |
| Pulp | Juice | In-vino | Anti-secretary activity | [249] | |
| Pulp | Juice | In-vivo | Anti-secretory effects | [249] | |
| Rind | Hydro-methanolic extracts | In-vivo | Anti-ulcer | [193] | |
| Seeds | Extract | In-vivo | Antiulcer and gastroprotective | [66] | |
| Seed | Seed extract | In-vivo | Anti-ulcer action in pyloric ligated and water immersion stress generated ulcer models. | [65,66] | |
| Seeds | Methanolic extract | In-vivo | Anti-ulcerogenic | [65] | |
| Pulp | Pulp extract | In-vivo | Gastroprotective Laxative Activity in Indomethacin-Induced Ulcer Model | [250] | |
| Pulp | Pulp extract | In-vivo | Loperamide-induced constipation decreases in a dose-dependent manner by laxative activity. | [250] | |
| Pulp | Extract | In-vivo | Laxative Activity | [250] | |
| Pulp | In-vivo | Ameliorate the gastrointestinal discomforts | [171] | ||
| Hepatoprotective | Pulp | Juice | In-vivo | [251] | |
| Seeds | Extract | In-vivo | [252] | ||
| Seed | Seed oil | In-Vivo | Oral, estimate serum hepatic enzyme level | [64] | |
| Seeds | Oil | In-vivo | [64] | ||
| Seeds | Extract | In-vivo | [155] [253] |
||
| Anti-inflammatory | Seed | Seed Oil | In-vivo & In-Vitro | [64] | |
| Seed | Aqueous extract | In-vivo | [179] | ||
| Seeds | Extract | In-vivo | [184] | ||
| Seed | Oil, extract | In-vitro | [254,255,256] | ||
| Rind | Methanolic extract | In-vivo | [184,193] | ||
| Pulp | Juice | In-vivo | α-glucosidase and α-amylase inhibitory action in a dose-dependent manner | [242] | |
| Seed | Seed oil | In-vivo & In-vitro | Carrageenan-induced paw edema in a rat model | [64,257] | |
| Seed | Seed Oil, | In-vitro and In-vivo | [64] | ||
| Whole watermelon powder | In-vivo | Ingestion | [186] | ||
| Pulp | l-citrulline and l-arginine | In-vivo | [258] | ||
| Antimicrobial | Seed | Seed extract | In-vitro | cup-plate and disc diffusion method | [229,230] |
| Rind | Extract | In-vitro | [259] | ||
| Seed | Extract | In-vitro | [99,228,260] | ||
| Seed | Oil, extract | In-vitro | [254,255,256] | ||
| Seed | Seed extract | In-vitro | [94,261] | ||
| Seeds | Extract | In-vitro | [229] | ||
| Pulp | Extract | In-vitro | [67] | ||
| Seeds | Extract | In-vitro | [229] | ||
| Seed | Seed extract | In-vitro | [225] | ||
| Pulp | Juice | In-vivo | [8,224] | ||
| Role in oxidative stress | Pulp | l-citrulline & l-arginine | In-vivo | [258] | |
| Peel | Extract | In-vivo | [231] | ||
| Analgesic and antipyretic | Seed | Aqueous extract | In-vivo | [179] | |
| Rind | Methanolic extract | In-vivo | [184,193] | ||
| Pulp | Lycopene | In-vitro | [74] | ||
| Anticancer | Rind | Extract | In-vivo | [262] | |
| Rind | Extracted polysaccharides | In-vivo | [262] | ||
| Pulp | Extract | In-vivo In-vitro | U Anti- urolithiatic and diuretic activities | [71] | |
| Urinary tract disorders | Fruit | Aqueous extract | In-vivo | U Kidney health | [263] |
| Flesh and Rind | Extract | In-vivo | Uterine Contractility | [264] | |
| Seeds | Extract | In-vivo | Anti-Prostatic Hyperplasia activity | [265] | |
| Miscellaneous Effects | Seeds | Methanolic extract | In-vivo | Nephroprotective effects | [253,266] |
| Seeds | Extract | In-vivo | Neuroprotective | [267,268] | |
| Seeds | Extract | Male Sexual enhancement | [252] | ||
| Seeds | Extract | In-vivo | Weight loss and hematological benefits | [269] |
4. Potential applications of watermelon by-products that improve bio-functionality and health promoting effects of food and cosmetics products
4.1. Applications in bakery and other staple-based products
4.2. Applications in meat-based products
4.3. Applications in dairy products and dairy alternatives
4.4. Applications in other food-based products
4.5. Alternetive applications related to food industry
4.6. Applications in cosmetics
5. Limitations – Future Perspectives
6. Conclusions
Author Contributions
Funding
Acknowledgments
References
- WHO, A. World Health Statistics 2016: Monitoring Health for the SDGs Sustainable Development Goals. World Health Organ. 2016. [Google Scholar]
- Tsoupras, A.B.; Iatrou, C.; Frangia, C.; Demopoulos, C.A. The Implication of Platelet Activating Factor in Cancer Growth and Metastasis: Potent Beneficial Role of PAF-Inhibitors and Antioxidants. Infect. Disord. - Drug TargetsDisorders 2009, 9, 390–399. [Google Scholar] [CrossRef]
- Tsoupras, A.; Lordan, R.; Zabetakis, I. Inflammation, Not Cholesterol, Is a Cause of Chronic Disease. Nutrients 2018, 10. [Google Scholar] [CrossRef] [PubMed]
- Zabetakis, I.; Lordan, R.; Tsoupras, A. Chapter 9 - Nutrition Versus Statins in Primary Prevention: Where Do We Stand Now? In The Impact of Nutrition and Statins on Cardiovascular Diseases; Zabetakis, I., Lordan, R., Tsoupras, A., Eds.; Academic Press, 2019; pp. 289–317 ISBN 978-0-12-813792-5.
- Tsoupras, A. The Anti-Inflammatory and Antithrombotic Properties of Bioactives from Orange, Sanguine and Clementine Juices and from Their Remaining By-Products. Beverages 2022, 8, 39. [Google Scholar] [CrossRef]
- Zabetakis, I.; Lordan, R.; Tsoupras, A.; Ramji, D. Functional Foods and Their Implications for Health Promotion; Academic Press, 2022; ISBN 0-12-823812-7.
- Karasawa, M.M.G.; Mohan, C. Fruits as Prospective Reserves of Bioactive Compounds: A Review. Nat. Prod. Bioprospecting 2018, 8, 335–346. [Google Scholar] [CrossRef] [PubMed]
- Naz, A.; Butt, M.S.; Sultan, M.T.; Qayyum, M.M.N.; Niaz, R.S. Watermelon Lycopene and Allied Health Claims. EXCLI J. 2014, 13, 650. [Google Scholar] [PubMed]
- Kyriacou, M.C.; Leskovar, D.I.; Colla, G.; Rouphael, Y. Watermelon and Melon Fruit Quality: The Genotypic and Agro-Environmental Factors Implicated. Sci. Hortic. 2018, 234, 393–408. [Google Scholar] [CrossRef]
- Tlili, I.; Hdider, C.; Lenucci, M.S.; Ilahy, R.; Jebari, H.; Dalessandro, G. Bioactive Compounds and Antioxidant Activities during Fruit Ripening of Watermelon Cultivars. J. Food Compos. Anal. 2011, 24, 923–928. [Google Scholar] [CrossRef]
- Abourashed, E.A. Bioavailability of Plant-Derived Antioxidants. Antioxidants 2013, 2, 309–325. [Google Scholar] [CrossRef]
- Sa’id, M. A Study in the Variability of Some Nutrient Contents of Watermelon (Citrullus Lanatus) before and after Ripening Consumed within Kano Metropolis, Nigeria. Int. J. Sci. Res. IJSR 2014, 3, 1365–1368. [Google Scholar]
- Jumde, A.; Shukla, R. ; Gousoddin DEVELOPMENT AND CHEMICAL ANALYSIS OF WATERMELON BLENDS WITH BEETROOT JUICE DURING STORAGE. Int. J. Sci. Eng. Technol. 2015, 3, 960–964. [Google Scholar] [CrossRef]
- Romdhane, M.B.; Haddar, A.; Ghazala, I.; Jeddou, K.B.; Helbert, C.B.; Ellouz-Chaabouni, S. Optimization of Polysaccharides Extraction from Watermelon Rinds: Structure, Functional and Biological Activities. Food Chem. 2017, 216, 355–364. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, B.; Haldhar, S.; Maheshwari, S.; Bhargava, R.; Sharma, S. Phytochemicals and Antioxidants in Watermelon (Citrullus Lanatus) Genotypes under Hot Arid Region. 2015.
- Ijah, U.; Auta, H.; Aransiola, S. Microbiological and Some Sensory Attributes of Water Melon Juice and Watermelon-Orange Juice Mix. 2015.
- Bianchi, G.; Rizzolo, A.; Grassi, M.; Provenzi, L.; Scalzo, R.L. External Maturity Indicators, Carotenoid and Sugar Compositions and Volatile Patterns in ‘Cuoredolce®’and ‘Rugby’Mini-Watermelon (Citrullus Lanatus (Thunb) Matsumura & Nakai) Varieties in Relation of Ripening Degree at Harvest. Postharvest Biol. Technol. 2018, 136, 1–11. [Google Scholar]
- Chidambara Murthy, K.; Vanitha, A.; Mahadeva Swamy, M.; Ravishankar, G. Antioxidant and Antimicrobial Activity of Cissus Quadrangularis L. J. Med. Food 2003, 6, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Sharififar, F.; Dehghn-Nudeh, G.; Mirtajaldini, M. Major Flavonoids with Antioxidant Activity from Teucrium Polium L. Food Chem. 2009, 112, 885–888. [Google Scholar] [CrossRef]
- Suhail, N.; Bilal, N.; Khan, H.; Hasan, S.; Sharma, S.; Khan, F.; Mansoor, T.; Banu, N. Effect of Vitamins C and E on Antioxidant Status of Breast-cancer Patients Undergoing Chemotherapy. J. Clin. Pharm. Ther. 2012, 37, 22–26. [Google Scholar] [CrossRef] [PubMed]
- Mehra, M.; Pasricha, V.; Gupta, R.K. Estimation of Nutritional, Phytochemical and Antioxidant Activity of Seeds of Musk Melon (Cucumis Melo) and Water Melon (Citrullus Lanatus) and Nutritional Analysis of Their Respective Oils. J. Pharmacogn. Phytochem. 2015, 3, 98–102. [Google Scholar]
- Castro-López, C.; Ventura-Sobrevilla, J.M.; González-Hernández, M.D.; Rojas, R.; Ascacio-Valdés, J.A.; Aguilar, C.N.; Martínez-Ávila, G.C. Impact of Extraction Techniques on Antioxidant Capacities and Phytochemical Composition of Polyphenol-Rich Extracts. Food Chem. 2017, 237, 1139–1148. [Google Scholar] [CrossRef] [PubMed]
- Hasanin, M.S.; Hashem, A.H. Eco-Friendly, Economic Fungal Universal Medium from Watermelon Peel Waste. J. Microbiol. Methods 2020, 168, 105802. [Google Scholar] [CrossRef]
- Zamuz, S.; Munekata, P.E.; Gullón, B.; Rocchetti, G.; Montesano, D.; Lorenzo, J.M. Citrullus Lanatus as Source of Bioactive Components: An up-to-Date Review. Trends Food Sci. Technol. 2021, 111, 208–222. [Google Scholar] [CrossRef]
- Johnson, J.; Lennox, J.; Ujong, U.; Odey, M.; Fila, W.; Edem, P.; Dasofunjo, K. Comparative Vitamins Content of Pulp, Seed and Rind of Fresh and Dried Watermelon (Citrullus Lanatus). Int. J. Sci. Technol. 2013, 2, 99–103. [Google Scholar]
- Oberoi, D.P.S.; Sogi, D.S. Utilization of Watermelon Pulp for Lycopene Extraction by Response Surface Methodology. Food Chem. 2017, 232, 316–321. [Google Scholar] [CrossRef] [PubMed]
- Oyeleke, G.; Ojo, A.; Ajao, F.; Adetoro, R. Development and Analysis of Blended Pineapple-Watermelon Ready-to-Drink (RTD) Juice. J. Environ. Sci. Toxicol. Food Technol. 2013, 4, 22–24. [Google Scholar] [CrossRef]
- Al-Sayed, H.M.; Ahmed, A.R. Utilization of Watermelon Rinds and Sharlyn Melon Peels as a Natural Source of Dietary Fiber and Antioxidants in Cake. Ann. Agric. Sci. 2013, 58, 83–95. [Google Scholar] [CrossRef]
- Kiin-Kabari, D.; Akusu, O. Effect of Processing on the Proximate Composition, Functional Properties and Storage Stability of Water Melon (Citrullus Lanatus) Seed Flour. Int J Biotechnol Food Sci 2014, 2, 143–148. [Google Scholar]
- Egbuonu, A.C.C. Comparative Investigation of the Proximate and Functional Properties of Watermelon (Citrullus Lanatus) Rind and Seed. Res. J. Environ. Toxicol. 2015, 9, 160–167. [Google Scholar] [CrossRef]
- Hoque, M.M.; Iqbal, A. Drying of Watermelon Rind and Development of Cakes from Rind Powder. Int. J. Nov. Res. Life Sci. 2015, 2, 14–21. [Google Scholar]
- Badr, S. Quality and Antioxidant Properties of Pan Bread Enriched with Watermelon Rind Powder. Curr. Sci. Int. 2015, 4, 117–126. [Google Scholar]
- Omoboyowa, D.A.; Otuchristian, G.; Danladi, G.J.; Igara, C.; Ngobidi, K.; Okon, M.U.; Agbo, F.A. Evaluation of Chemical Compositions of Citrulus Lanatus Seed and Cocos Nucifera Stem Bark. Afr. J. Food Sci. Technol. 2015, 6, 75–83. [Google Scholar] [CrossRef]
- Tabiri, B.; Agbenorhevi, J.K.; Wireko-Manu, F.D.; Ompouma, E.I. Watermelon Seeds as Food: Nutrient Composition, Phytochemicals and Antioxidant Activity. 2016.
- Romelle, F.D.; Rani, A.; Manohar, R.S. Chemical Composition of Some Selected Fruit Peels. Eur. J. Food Sci. Technol. 2016, 4, 12–21. [Google Scholar]
- Abu-Hiamed, H.A. Research Article Chemical Composition, Flavonoids and $-Sitosterol Contents of Pulp and Rind of Watermelon (Citrullus Lanatus) Fruit. 2017.
- Badr, S.; El-Waseif, M.; Ghaly, M. Effect of Addition Watermelon Rind Powder on Quality Criteria and Microbial Aspects of Beef Burger Patties during Frozen Storage Periods. J. Food Dairy Sci. 2018, 9, 177–187. [Google Scholar] [CrossRef]
- Milala, M.; Luther, A.; Burah, B. Nutritional Comparison of Processed and Unprocessed Citrillus Lanatus (Watermelon) Seeds for Possible Use in Feed Formulation. Am. J. Food Nutr. 2018, 6, 33–36. [Google Scholar] [CrossRef]
- United States Department of Agriculture National Nutrient Database for Standard Reference Release 28. USDA Agric Res Serv 2016.
- Sajjad, S.; Israr, B.; Ali, F.; Pasha, I. INVESTIGATING THE EFFECT OF PHYTOCHEMICALS RICH WATERMELON SEEDS AGAINST HYPERTENSION. Pak. J. Agric. Sci. 2020, 57. [Google Scholar]
- Kausar, T.; Hassan, M.T.; ud Din, G.M. 21. Utilization of Watermelon Seed Flour as Protein Supplement in Cookies. Pure Appl. Biol. PAB 2020, 9, 202–206. [Google Scholar]
- Chakrabarty, N.; Mourin, M.M.; Islam, N.; Haque, A.R.; Akter, S.; Siddique, A.A.; Sarker, M. Assessment of the Potential of Watermelon Rind Powder for the Value Addition of Noodles. J. Biosyst. Eng. 2020, 45, 223–231. [Google Scholar] [CrossRef]
- Sadiq, I.; Saminu, M.; Zainab, L.; Adeleye, A.; Sanni, L.; Dandalma, Z. Proximate Analysis and Phytochemical Screening of Watermelon (Citrullus Lanatus) Pulp, Peels and Seeds. 2021.
- Burton-Freeman, B.; Freeman, M.; Zhang, X.; Sandhu, A.; Edirisinghe, I. Watermelon and L-Citrulline in Cardio-Metabolic Health: Review of the Evidence 2000–2020. Curr. Atheroscler. Rep. 2021, 23, 81. [Google Scholar] [CrossRef] [PubMed]
- Adedeji, T.; Oluwalana, I. Physico-Chemical, Sensory and Microbial Analysis of Wine Produced from Watermelon (Citrullus Lanatus) and Pawpaw (Carica Papaya) Blend. Food Sci. Qual. Manag. 2013, 19, 41–50. [Google Scholar]
- Sivudu, S.N.; Umamahesh, K.; Reddy, O. A Comparative Study on Probiotication of Mixed Watermelon and Tomato Juice by Using Probiotic Strains of Lactobacilli. Int. J. Curr. Microbiol. Appl. Sci. 2014, 3, 977–984. [Google Scholar]
- Soteriou, G.; Kyriacou, M.; Siomos, A.; Gerasopoulos, D. Evolution of Watermelon Fruit Physicochemical and Phytochemical Composition during Ripening as Affected by Grafting. Food Chem. 2014, 165, 282–289. [Google Scholar] [CrossRef]
- Jebir, R.M.; Mustafa, Y.F. Natural Coumarin-Lead Compounds: A Review of Their Medicinal Potentials. Iraqi J. Pharm. 2022, 18, 139–161. [Google Scholar] [CrossRef]
- Okonmah, L. Effects of Different Types of Staking and Their Cost Effectiveness on the Growth, Yield and Yield Components of Cucumber (Cucumis Sativa L.). Int. J. AgriScience 2011, 1, 290–295. [Google Scholar]
- Campbell, I. Macronutrients, Minerals, Vitamins and Energy. Anaesth. Intensive Care Med. 2017, 18, 141–146. [Google Scholar] [CrossRef]
- Shao, P.; Qiu, Q.; Xiao, J.; Zhu, Y.; Sun, P. Chemical Stability and in Vitro Release Properties of β-Carotene in Emulsions Stabilized by Ulva Fasciata Polysaccharide. Int. J. Biol. Macromol. 2017, 102, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Zhao, S.; Lu, X.; He, N.; Zhu, H.; Dou, J.; Liu, W. Comparative Transcriptome Analysis Reveals Key Genes Potentially Related to Soluble Sugar and Organic Acid Accumulation in Watermelon. PloS One 2018, 13, e0190096. [Google Scholar] [CrossRef]
- King, S. Lycopene and Citrulline Contents in Watermelon (Citrullus Lanatus) Fruit with Different Ploidy and Changes during Fruit Development.; 2009; pp. 543–550.
- Perkins-Veazie, P.; Collins, J.K.; Davis, A.R.; Roberts, W. Carotenoid Content of 50 Watermelon Cultivars. J. Agric. Food Chem. 2006, 54, 2593–2597. [Google Scholar] [CrossRef]
- Perkins-Veazie, P.; Davis, A.; Collins, J.K. Watermelon: From Dessert to Functional Food. Isr. J. Plant Sci. 2012, 60, 395–402. [Google Scholar]
- Liu, J.; Guo, S.; He, H.; Zhang, H.; Gong, G.; Ren, Y.; Xu, Y. Dynamic Characteristics of Sugar Accumulation and Related Enzyme Activities in Sweet and Non-Sweet Watermelon Fruits. Acta Physiol. Plant. 2013, 35, 3213–3222. [Google Scholar] [CrossRef]
- Casacchia, T.; Sofo, A.; CRINATOMA, C.; DRĂGĂNESCU, D.; TIȚA, B.; Statti, G.A. NUTRACEUTICAL PROPERTIES AND HEALTH-PROMOTING BIOLOGICAL ACTIVITIES OF FRUITS OF WATERMELON CULTIVARS WITH DIFFERENT ORIGINS. Farmacia 2020, 68. [Google Scholar] [CrossRef]
- Lemos, Á.T.; Ribeiro, A.C.; Fidalgo, L.G.; Delgadillo, I.; Saraiva, J.A. Extension of Raw Watermelon Juice Shelf-Life up to 58 Days by Hyperbaric Storage. Food Chem. 2017, 231, 61–69. [Google Scholar] [CrossRef]
- Kehili, M.; Kammlott, M.; Choura, S.; Zammel, A.; Zetzl, C.; Smirnova, I.; Allouche, N.; Sayadi, S. Supercritical CO2 Extraction and Antioxidant Activity of Lycopene and β-Carotene-Enriched Oleoresin from Tomato (Lycopersicum Esculentum L.) Peels by-Product of a Tunisian Industry. Food Bioprod. Process. 2017, 102, 340–349. [Google Scholar] [CrossRef]
- Leong, L.; Shui, G. An Investigation of Antioxidant Capacity of Fruits in Singapore Markets. Food Chem. 2002, 76, 69–75. [Google Scholar] [CrossRef]
- Lewinsohn, E.; Sitrit, Y.; Bar, E.; Azulay, Y.; Meir, A.; Zamir, D.; Tadmor, Y. Carotenoid Pigmentation Affects the Volatile Composition of Tomato and Watermelon Fruits, as Revealed by Comparative Genetic Analyses. J. Agric. Food Chem. 2005, 53, 3142–3148. [Google Scholar] [CrossRef] [PubMed]
- Charoensiri, R.; Kongkachuichai, R.; Suknicom, S.; Sungpuag, P. Beta-Carotene, Lycopene, and Alpha-Tocopherol Contents of Selected Thai Fruits. Food Chem. 2009, 113, 202–207. [Google Scholar] [CrossRef]
- Rimando, A.M.; Perkins-Veazie, P.M. Determination of Citrulline in Watermelon Rind. J. Chromatogr. A 2005, 1078, 196–200. [Google Scholar] [CrossRef] [PubMed]
- Madhavi, P.; Vakati, K.; Rahman, H. Evaluation of Anti-Inflammatory Activity of Citrullus Lanatus Seed Oil by in-Vivo and in-Vitro Models. Int. Res. J. Pharm. Appl. Sci. 2012, 2, 104–108. [Google Scholar]
- Bhardwaj, A.; Kumar, R.; Dabasa, V.; Alam, N. Evaluation of Anti-Ulcer Activity of Citrullus Lanatus Seed Extract in Wistar Albino Rats. Int. J. Pharm. Pharm. Sci. 2012, 4, 135–139. [Google Scholar]
- Lucky, O.O.; John, U.O.; Kate, I.E.; Peter, O.O.; Jude, O.E. Quantitative Determination, Metal Analysis and Antiulcer Evaluation of Methanol Seeds Extract of Citrullus Lanatus Thunb (Cucurbitaceae) in Rats. Asian Pac. J. Trop. Biomed. 2012, 2, S1261–S1265. [Google Scholar] [CrossRef]
- Hassan, L.E.A.; Sirat, H.M.; Yagi, S.M.A.; Koko, W.S.; Abdelwahab, S.I. In Vitro Antimicrobial Activities of Chloroformic, Hexane and Ethanolic Extracts of Citrullus Lanatus Var. Citroides (Wild Melon). J. Med. Plants Res. 2011, 5, 1338–1344. [Google Scholar]
- Naz, A.; Butt, M.S.; Pasha, I.; Nawaz, H. Antioxidant Indices of Watermelon Juice and Lycopene Extract. Pak. J. Nutr. 2013, 12, 255. [Google Scholar] [CrossRef]
- Kim, C.; Park, M.; Kim, S.; Cho, Y. Antioxidant Capacity and Anti-inflammatory Activity of Lycopene in Watermelon. Int. J. Food Sci. Technol. 2014, 49, 2083–2091. [Google Scholar] [CrossRef]
- Alemika, T.; Ojerinde, O.S.; Samali, A.; Mustapha, B.K.; Gamaniel, K.S. Nutriceutical Potentials of Nigerian Grown Citrullus Lanatus (Watermelon) Seed. J. Pharm. Bioresour. 2017, 14, 253–259. [Google Scholar] [CrossRef]
- Siddiqui, W.A.; Shahzad, M.; Shabbir, A.; Ahmad, A. Evaluation of Anti-Urolithiatic and Diuretic Activities of Watermelon (Citrullus Lanatus) Using in Vivo and in Vitro Experiments. Biomed. Pharmacother. 2018, 97, 1212–1221. [Google Scholar] [CrossRef] [PubMed]
- Jibril, M.M.; Abdul-Hamid, A.; Ghazali, H.M.; Dek, M.S.P.; Ramli, N.S.; Jaafar, A.H.; Karrupan, J.; Mohammed, A.S. Antidiabetic Antioxidant and Phytochemical Profile of Yellow-Fleshed Seeded Watermelon (Citrullus Lanatus) Extracts. J Food Nutr Res 2019, 7, 82–95. [Google Scholar]
- Bailey, S.J.; Blackwell, J.R.; Williams, E.; Vanhatalo, A.; Wylie, L.J.; Winyard, P.G.; Jones, A.M. Two Weeks of Watermelon Juice Supplementation Improves Nitric Oxide Bioavailability but Not Endurance Exercise Performance in Humans. Nitric Oxide 2016, 59, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Rao, A.V.; Agarwal, S. Role of Antioxidant Lycopene in Cancer and Heart Disease. J. Am. Coll. Nutr. 2000, 19, 563–569. [Google Scholar] [CrossRef] [PubMed]
- Romero, M.J.; Platt, D.H.; Caldwell, R.B.; Caldwell, R.W. Therapeutic Use of Citrulline in Cardiovascular Disease. Cardiovasc. Drug Rev. 2006, 24, 275–290. [Google Scholar] [CrossRef] [PubMed]
- Wan, X.; Liu, W.; Yan, Z.; Zhao, S.; He, N.; Liu, P. Changes of the Contents of Functional Substances Including Lycopene, Citrulline and Ascorbic Acid during Watermelon Fruits Development. Sci. Agric. Sin. 2011, 44, 2738–2747. [Google Scholar]
- Tarazona-Díaz, M.P.; Viegas, J.; Moldao-Martins, M.; Aguayo, E. Bioactive Compounds from Flesh and By-product of Fresh-cut Watermelon Cultivars. J. Sci. Food Agric. 2011, 91, 805–812. [Google Scholar] [CrossRef]
- Sultana, B.; Ashraf, R. Watermelon (Citrullus Lanatus) Oil. Fruit Oils Chem. Funct. 2019, 741–756. [Google Scholar]
- Abu-Reidah, I.M.; Arráez-Román, D.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Profiling of Phenolic and Other Polar Constituents from Hydro-Methanolic Extract of Watermelon (Citrullus Lanatus) by Means of Accurate-Mass Spectrometry (HPLC–ESI–QTOF–MS). Food Res. Int. 2013, 51, 354–362. [Google Scholar] [CrossRef]
- Mushtaq, M.; Sultana, B.; Bhatti, H.N.; Asghar, M. RSM Based Optimized Enzyme-Assisted Extraction of Antioxidant Phenolics from Underutilized Watermelon (Citrullus Lanatus Thunb.) Rind. J. Food Sci. Technol. 2015, 52, 5048–5056. [Google Scholar] [CrossRef]
- Jimoh, T.O.; Ademiluyi, A.O.; Oboh, G.; Boligon, A.A. Phenolic Extracts and Amino Acids Content from Cucumeropsis Mannii Naudin and Citrullus Lanatus Inhibit Relevant Enzymes of Erectile Dysfunction in Rat’s Penile Tissue. Biochem. Biophys. Rep. 2017, 12, 5. [Google Scholar] [CrossRef]
- Nkoana, D.K.; Mashilo, J.; Shimelis, H.; Ngwepe, R.M. Nutritional, Phytochemical Compositions and Natural Therapeutic Values of Citron Watermelon (Citrullus Lanatus Var. Citroides): A Review. South Afr. J. Bot. 2022, 145, 65–77. [Google Scholar] [CrossRef]
- Biswas, R.; Ghosal, S.; Chattopadhyay, A.; Datta, S. A Comprehensive Review on Watermelon Seed Oil–An Underutilized Product. IOSR J Pharm 2017, 7, 01–07. [Google Scholar]
- Maoto, M.M.; Beswa, D.; Jideani, A.I. Watermelon as a Potential Fruit Snack. Int. J. Food Prop. 2019, 22, 355–370. [Google Scholar] [CrossRef]
- Banureka, V.; Mahendran, T. Formulation of Wheat-Soybean Biscuits and Their Quality Characteristics. 2009.
- Ubbor, S.; Akobundu, E. Quality Characteristics of Cookies from Composite Flours of Watermelon Seed, Cassava and Wheat. Pak. J. Nutr. 2009, 8, 1097–1102. [Google Scholar] [CrossRef]
- Jiang, G.; Wu, Z.; Ameer, K.; Li, S.; Ramachandraiah, K. Particle Size of Ginseng (Panax Ginseng Meyer) Insoluble Dietary Fiber and Its Effect on Physicochemical Properties and Antioxidant Activities. Appl. Biol. Chem. 2020, 63, 1–10. [Google Scholar] [CrossRef]
- Zhao, C.-C.; Ameer, K.; Eun, J.-B. Effects of Various Drying Conditions and Methods on Drying Kinetics and Retention of Bioactive Compounds in Sliced Persimmon. Lwt 2021, 143, 111149. [Google Scholar] [CrossRef]
- Kulczyński, B.; Gramza-Michałowska, A.; Kobus-Cisowska, J.; Kmiecik, D. The Role of Carotenoids in the Prevention and Treatment of Cardiovascular Disease–Current State of Knowledge. J. Funct. Foods 2017, 38, 45–65. [Google Scholar] [CrossRef]
- Guo, Z.; Ge, X.; Yang, L.; Gou, Q.; Han, L.; Yu, Q. Utilization of Watermelon Peel as a Pectin Source and the Effect of Ultrasound Treatment on Pectin Film Properties. LWT 2021, 147, 111569. [Google Scholar] [CrossRef]
- Morais, D.R.; Rotta, E.M.; Sargi, S.C.; Bonafe, E.G.; Suzuki, R.M.; Souza, N.E.; Matsushita, M.; Visentainer, J.V. Proximate Composition, Mineral Contents and Fatty Acid Composition of the Different Parts and Dried Peels of Tropical Fruits Cultivated in Brazil. J. Braz. Chem. Soc. 2017, 28, 308–318. [Google Scholar] [CrossRef]
- Gladvin, G.; Sudhaakr, G.; Swathi, V.; Santhisri, K. Mineral and Vitamin Compositions Contents in Watermelon Peel (Rind). Int. J. Curr. Microbiol. Appl. Sci. 2017, 5, 129–133. [Google Scholar]
- Rezq, A.A. Antidiabetic Activity and Antioxidant Role of Watermelon (Citrullus Lanatus) Peels in Streptozotocine-Induced Diabetic Rats. Egypt. J. Nutr. 2017, 32, 1–36. [Google Scholar]
- Braide, W.; Odiong, I.; Oranusi, S. Phytochemical and Antibacterial Properties of the Seed of Watermelon (Citrullus Lanatus). Prime J. Microbiol. Res. 2012, 2, 99–104. [Google Scholar]
- Khan, M.A.; Amir, R.M.; Ameer, K.; Rakha, A.; Faiz, F.; Hayat, I.; Nadeem, M.; Ahmed, Z.; Riaz, A.; Ashraf, I. Characterization of Oat Bran β-Glucan with Special Reference to Efficacy Study to Elucidate Its Health Claims for Diabetic Patients. Food Sci. Technol. 2020, 41, 105–112. [Google Scholar] [CrossRef]
- Kaul, P. Nutritional Potential, Bioaccessibility of Minerals and Functionality of Watermelon (Citrullus Vulgaris) Seeds. LWT-Food Sci. Technol. 2011, 44, 1821–1826. [Google Scholar]
- Coburn, L.A.; Gong, X.; Singh, K.; Asim, M.; Scull, B.P.; Allaman, M.M.; Williams, C.S.; Rosen, M.J.; Washington, M.K.; Barry, D.P. L-Arginine Supplementation Improves Responses to Injury and Inflammation in Dextran Sulfate Sodium Colitis. PloS One 2012, 7, e33546. [Google Scholar] [CrossRef]
- Ren, W.; Yin, J.; Wu, M.; Liu, G.; Yang, G.; Xion, Y.; Su, D.; Wu, L.; Li, T.; Chen, S. Serum Amino Acids Profile and the Beneficial Effects of L-Arginine or L-Glutamine Supplementation in Dextran Sulfate Sodium Colitis. PloS One 2014, 9, e88335. [Google Scholar] [CrossRef]
- Sola, A.O.; Temitayo, O.O.; Olufunke, A.; Shittu, F. Chemical Composition, Nutritional Values and Antibacterial Activities of Watermelon Seed (Citrullus Lanatus). Int. J. Biochem. Res. Rev. 2019, 27, 1–9. [Google Scholar] [CrossRef]
- Metcalf, R.L.; Metcalf, R.A.; Rhodes, A. Cucurbitacins as Kairomones for Diabroticite Beetles. Proc. Natl. Acad. Sci. 1980, 77, 3769–3772. [Google Scholar] [CrossRef] [PubMed]
- Panosyan, A.; Nikishchenko, M.; Avetisyan, G. Structure of 22-Deoxocucurbitacins Isolated from Bryonia Alba and Ecbalium Elaterium. Chem. Nat. Compd. 1985, 21, 638–645. [Google Scholar] [CrossRef]
- Elfattah, H.; Zaghloul, A.; Halim, A.; Waight, E. CUCURBITACINS AND STEROIDS FROM CUCUMIS-CALLOSUS (ROTTL) CONG. ACTA Pharm. Jugosl. 1989, 39, 137–141. [Google Scholar]
- Karawya, M.; El-Gengaihi, S.; Selim, M.; Motawe, H.; Ibrahim, N. Major Cucurbitacins of Momordica Balsamina L. Pharmazie 1996, 51, 434–435. [Google Scholar]
- Arisawa, M.; Pezzuto, J.M.; Cordell, G.A.; Farnsworth, N.R.; Kinghorn, A.D. Plant Anticancer Agents XXX: Cucurbitacins from Ipomopsis Aggregata (Polemoniaceae). J. Pharm. Sci. 1984, 73, 411–413. [Google Scholar] [CrossRef]
- Stuppner, H.; Moller, E. Cucurbitacins with Unusual Side Chains from Picrorhiza Kurroa. Phytochemistry 1993, 33, 1139–1145. [Google Scholar] [CrossRef]
- Fuller, R.W.; Cardellina, J.H.; Cragg, G.M.; Boyd, M.R. Cucurbitacins: Differential Cytotoxicity, Dereplication and First Isolation from Gonystylus Keithii. J. Nat. Prod. 1994, 57, 1442–1445. [Google Scholar] [CrossRef]
- Miro, M. Cucurbitacins and Their Pharmacological Effects. Phytother. Res. 1995, 9, 159–168. [Google Scholar] [CrossRef]
- Chen, J.C.; Chiu, M.H.; Nie, R.L.; Cordell, G.A.; Qiu, S.X. Cucurbitacins and Cucurbitane Glycosides: Structures and Biological Activities. Nat. Prod. Rep. 2005, 22. [Google Scholar] [CrossRef]
- Jayaprakasam, B.; Seeram, N.P.; Nair, M.G. Anticancer and Antiinflammatory Activities of Cucurbitacins from Cucurbita Andreana. Cancer Lett. 2003, 189, 11–16. [Google Scholar] [CrossRef]
- Tannin-Spitz, T.; Grossman, S.; Dovrat, S.; Gottlieb, H.E.; Bergman, M. Growth Inhibitory Activity of Cucurbitacin Glucosides Isolated from Citrullus Colocynthis on Human Breast Cancer Cells. Biochem. Pharmacol. 2007, 73, 56–67. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.H.; Iwanski, G.B.; Thoennissen, N.H. Cucurbitacin: Ancient Compound Shedding New Light on Cancer Treatment. Sci. World J. 2010, 10, 413–418. [Google Scholar] [CrossRef] [PubMed]
- Park, C.S.; Lim, H.; Han, K.J.; Baek, S.H.; Sohn, H.O.; Lee, D.W.; Kim, Y.-G.; Yun, H.-Y.; Baek, K.J.; Kwon, N.S. Inhibition of Nitric Oxide Generation by 23, 24-Dihydrocucurbitacin D in Mouse Peritoneal Macrophages. J. Pharmacol. Exp. Ther. 2004, 309, 705–710. [Google Scholar] [CrossRef] [PubMed]
- Escandell, J.M.; Recio, M.-C.; Máñez, S.; Giner, R.-M.; Cerdá-Nicolás, M.; Ríos, J.-L. Dihydrocucurbitacin B, Isolated from Cayaponia Tayuya, Reduces Damage in Adjuvant-Induced Arthritis. Eur. J. Pharmacol. 2006, 532, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Escandell, J.M.; Recio, M.C.; Máñez, S.; Giner, R.M.; Cerdá-Nicolás, M.; Ríos, J.L. Cucurbitacin R Reduces the Inflammation and Bone Damage Associated with Adjuvant Arthritis in Lewis Rats by Suppression of Tumor Necrosis Factor-α in T Lymphocytes and Macrophages. J. Pharmacol. Exp. Ther. 2007, 320, 581–590. [Google Scholar] [CrossRef] [PubMed]
- Escandell, J.M.; Kaler, P.; Recio, M.C.; Sasazuki, T.; Shirasawa, S.; Augenlicht, L.; Ríos, J.-L.; Klampfer, L. Activated kRas Protects Colon Cancer Cells from Cucurbitacin-Induced Apoptosis: The Role of P53 and P21. Biochem. Pharmacol. 2008, 76, 198–207. [Google Scholar] [CrossRef] [PubMed]
- Siqueira Jr, J.M.; Peters, R.R.; Gazola, A.C.; Krepsky, P.B.; Farias, M.R.; Rae, G.A.; de Brum-Fernandes, A.J.; Ribeiro-do-Valle, R.M. Anti-Inflammatory Effects of a Triterpenoid Isolated from Wilbrandia Ebracteata Cogn. Life Sci. 2007, 80, 1382–1387. [Google Scholar] [CrossRef] [PubMed]
- Bartalis, J.; Halaweish, F.T. Relationship between Cucurbitacins Reversed-Phase High-Performance Liquid Chromatography Hydrophobicity Index and Basal Cytotoxicity on HepG2 Cells. J. Chromatogr. B 2005, 818, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Blaskovich, M.A.; Jove, R.; Livingston, S.K.; Coppola, D.; Sebti, S.M. Cucurbitacin Q: A Selective STAT3 Activation Inhibitor with Potent Antitumor Activity. Oncogene 2005, 24, 3236–3245. [Google Scholar] [CrossRef]
- L Rios, J.; Andújar, I.; M Escandell, J.; M Giner, R.; C Recio, M. Cucurbitacins as Inducers of Cell Death and a Rich Source of Potential Anticancer Compounds. Curr. Pharm. Des. 2012, 18, 1663–1676. [Google Scholar]
- Recio, M.C.; Prieto, M.; Bonucelli, M.; Orsi, C.; Máñez, S.; Giner, R.M.; Cerda-Nicolas, M.; Ríos, J.-L. Anti-Inflammatory Activity of Two Cucurbitacins Isolated from Cayaponia Tayuya Roots. Planta Med. 2004, 70, 414–420. [Google Scholar]
- Sadzuka, Y.; Hatakeyama, H.; Daimon, T.; Sonobe, T. Screening of Biochemical Modulator by Tumor Cell Permeability of Doxorubicin. Int. J. Pharm. 2008, 354, 63–69. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, M.; Zhang, H.; Sun, C.; Yang, X.; Deng, Y.; Ji, W. Combined Antitumor Activity of Cucurbitacin B and Docetaxel in Laryngeal Cancer. Eur. J. Pharmacol. 2008, 587, 78–84. [Google Scholar] [CrossRef]
- Agati, G.; Azzarello, E.; Pollastri, S.; Tattini, M. Flavonoids as Antioxidants in Plants: Location and Functional Significance. Plant Sci. 2012, 196, 67–76. [Google Scholar] [CrossRef]
- Mahomoodally, M.F.; Gurib-Fakim, A.; Subratty, A.H. Antimicrobial Activities and Phytochemical Profiles of Endemic Medicinal Plants of Mauritius. Pharm. Biol. 2005, 43, 237–242. [Google Scholar] [CrossRef]
- Pandey, A.K. Anti-Staphylococcal Activity of a Pan-Tropical Aggressive and Obnoxious Weed Parthenium Histerophorus: An in Vitro Study. Natl. Acad. Sci. Lett. 2007, 30, 383–386. [Google Scholar]
- Chiou, A.; Panagopoulou, E.A.; Gatzali, F.; De Marchi, S.; Karathanos, V.T. Anthocyanins Content and Antioxidant Capacity of Corinthian Currants (Vitis Vinifera L., Var. Apyrena). Food Chem. 2014, 146, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Chiou, A. Handbook of Anthocyanins: Food Sources, Chemical Applications and Health Benefits; Nova Publishers, 2015; ISBN 1-63321-795-7.
- Panagopoulou, E.A.; Chiou, A.; Bismpikis, M.; Mouraka, P.; Mangiorou, E.; Karathanos, V.T. Dried Fruits: Phytochemicals and Their Fate during in Vitro Digestion. Int. J. Food Sci. Technol. 2021, 56, 4506–4515. [Google Scholar] [CrossRef]
- Leopoldini, M.; Russo, N.; Chiodo, S.; Toscano, M. Iron Chelation by the Powerful Antioxidant Flavonoid Quercetin. J. Agric. Food Chem. 2006, 54, 6343–6351. [Google Scholar] [CrossRef]
- Dixon, R.; Dey, P.; Lamb, C. Phytoalexins: Enzymology and Molecular Biology. Adv. Enzymol. Relat. Areas Mol. Biol. 1983, 55, 69. [Google Scholar]
- Kumar, S.; Gupta, A.; Pandey, A.K. Calotropis Procera Root Extract Has the Capability to Combat Free Radical Mediated Damage. Int. Sch. Res. Not. 2013, 2013. [Google Scholar]
- Heim, K.E.; Tagliaferro, A.R.; Bobilya, D.J. Flavonoid Antioxidants: Chemistry, Metabolism and Structure-Activity Relationships. J. Nutr. Biochem. 2002, 13, 572–584. [Google Scholar] [CrossRef]
- Mishra, A.; Sharma, A.K.; Kumar, S.; Saxena, A.K.; Pandey, A.K. Bauhinia Variegata Leaf Extracts Exhibit Considerable Antibacterial, Antioxidant, and Anticancer Activities. BioMed Res. Int. 2013, 2013. [Google Scholar] [CrossRef]
- Hollman, P.C.; Bijsman, M.N.; Van Gameren, Y.; Cnossen, E.P.; De Vries, J.H.; Katan, M.B. The Sugar Moiety Is a Major Determinant of the Absorption of Dietary Flavonoid Glycosides in Man. Free Radic. Res. 1999, 31, 569–573. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B. Antioxidant Defence Mechanisms: From the Beginning to the End (of the Beginning). Free Radic. Res. 1999, 31, 261–272. [Google Scholar] [CrossRef]
- BROWN, E.J.; Khodr, H.; HIDER, C.R.; RICE-EVANS, C.A. Structural Dependence of Flavonoid Interactions with Cu2+ Ions: Implications for Their Antioxidant Properties. Biochem. J. 1998, 330, 1173–1178. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Pandey, A. Antioxidant, Lipo-Protective and Antibacterial Activities of Phytoconstituents Present in Solanum Xanthocarpum Root. Int. Rev. Biophys. Chem. 2012, 3, 42–47. [Google Scholar]
- Kerry, N.L.; Abbey, M. Red Wine and Fractionated Phenolic Compounds Prepared from Red Wine Inhibit Low Density Lipoprotein Oxidation in Vitro. Atherosclerosis 1997, 135, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Van Acker, S.; van den Berg, D.; Tromp, M. Structural Aspects of Antioxidant Activity of Flavanoids. 1996.
- Choleva, M.; Boulougouri, V.; Panara, A.; Panagopoulou, E.; Chiou, A.; Thomaidis, N.S.; Antonopoulou, S.; Fragopoulou, E. Evaluation of Anti-Platelet Activity of Grape Pomace Extracts. Food Funct. 2019, 10, 8069–8080. [Google Scholar] [CrossRef] [PubMed]
- Pannala, A.S.; Chan, T.S.; O’Brien, P.J.; Rice-Evans, C.A. Flavonoid B-Ring Chemistry and Antioxidant Activity: Fast Reaction Kinetics. Biochem. Biophys. Res. Commun. 2001, 282, 1161–1168. [Google Scholar] [CrossRef]
- Tsoupras, A.; Ni, V.L.J.; O’Mahony, É.; Karali, M. Wine-Making;“with One Stone Two Birds”? A Holistic Review of the Bio-Functional Compounds, Applications and Health Benefits of Wine and Wineries’ By-Products. 2023.
- Tsoupras, A.; Moran, D.; Byrne, T.; Ryan, J.; Barrett, L.; Traas, C.; Zabetakis, I. Anti-Inflammatory and Anti-Platelet Properties of Lipid Bioactives from Apple Cider by-Products. Molecules 2021, 26, 2869. [Google Scholar] [CrossRef] [PubMed]
- Tsoupras, A.B.; Fragopoulou, E.; Nomikos, T.; Iatrou, C.; Antonopoulou, S.; Demopoulos, C.A. Characterization of the de Novo Biosynthetic Enzyme of Platelet Activating Factor, DDT-Insensitive Cholinephosphotransferase, of Human Mesangial Cells. Mediators Inflamm. 2007, 2007. [Google Scholar] [CrossRef] [PubMed]
- Vlachogianni, I.C.; Fragopoulou, E.; Stamatakis, G.M.; Kostakis, I.K.; Antonopoulou, S. Platelet Activating Factor (PAF) Biosynthesis Is Inhibited by Phenolic Compounds in U-937 Cells under Inflammatory Conditions. Prostaglandins Other Lipid Mediat. 2015, 121, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Aderiye, B.; David, O.; Fagbohun, E.; Faleye, J.; Olajide, O. Immunomodulatory and Phytomedicinal Properties of Watermelon Juice and Pulp (Citrullus Lanatus Linn): A Review. GSC Biol. Pharm. Sci. 2020, 11, 153–165. [Google Scholar] [CrossRef]
- Dahan, K.; Fennal, M.; Kumar, N.B. Lycopene in the Prevention of Prostate Cancer. J. Soc. Integr. Oncol. 2008, 6. [Google Scholar]
- Erdman Jr, J.W.; Ford, N.A.; Lindshield, B.L. Are the Health Attributes of Lycopene Related to Its Antioxidant Function? Arch. Biochem. Biophys. 2009, 483, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Alim-un-Nisa, J.A.; Firdous, S.; Saeed, M.; Hina, S.; Ejaz, N. Nutritional Aspects and Acceptability of Watermelon Juice Syrup. Pak J Food Sci 2012, 22, 32–35. [Google Scholar]
- Elumalai, M.; Karthika, B.; Usha, V. Lycopene-Role in Cancer Prevention. Int J Pharma Bio Sci 2013, 4, 371–378. [Google Scholar]
- Hayashi, T.; Juliet, P.A.; Matsui-Hirai, H.; Miyazaki, A.; Fukatsu, A.; Funami, J.; Iguchi, A.; Ignarro, L.J. L-Citrulline and l-Arginine Supplementation Retards the Progression of High-Cholesterol-Diet-Induced Atherosclerosis in Rabbits. Proc. Natl. Acad. Sci. 2005, 102, 13681–13686. [Google Scholar] [CrossRef]
- Aguirre, V.; Uchida, T.; Yenush, L.; Davis, R.; White, M.F. The C-Jun NH2-Terminal Kinase Promotes Insulin Resistance during Association with Insulin Receptor Substrate-1 and Phosphorylation of Ser307. J. Biol. Chem. 2000, 275, 9047–9054. [Google Scholar] [CrossRef]
- Ilahy, R.; Tlili, I.; Siddiqui, M.W.; Hdider, C.; Lenucci, M.S. Inside and beyond Color: Comparative Overview of Functional Quality of Tomato and Watermelon Fruits. Front. Plant Sci. 2019, 10, 769. [Google Scholar] [CrossRef] [PubMed]
- Hong, M.Y.; Beidler, J.; Hooshmand, S.; Figueroa, A.; Kern, M. Watermelon and L-Arginine Consumption Improve Serum Lipid Profile and Reduce Inflammation and Oxidative Stress by Altering Gene Expression in Rats Fed an Atherogenic Diet. Nutr. Res. 2018, 58, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Bazabang, S.A.; Monday, N.; Adebisi, S.S.; Makena, W.; Iliya, I.A. Hepatoprotective Effects of Aqueous Extract of Watermelon (Citrullus Lanatus) Seeds on Ethanol-Induced Oxidative Damage in Wister Rats. Sub-Sahar. Afr. J. Med. 2018, 5, 129. [Google Scholar]
- DeWeese, R.S.; Ohri-Vachaspati, P. Cost of Children’s Healthy vs Unhealthy Snacks Does Not Differ at Convenience Stores. J. Nutr. Educ. Behav. 2017, 49, 241–243. [Google Scholar] [CrossRef] [PubMed]
- Milind, P.; Kulwant, S. Musk Melon Is Eat-Must Melon. IRJP 2011, 2, 52–57. [Google Scholar]
- Stahl, W.; Sies, H. Lycopene: A Biologically Important Carotenoid for Humans? Arch. Biochem. Biophys. 1996, 336, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Maguer, M.L. Lycopene in Tomatoes: Chemical and Physical Properties Affected by Food Processing. Crit. Rev. Food Sci. Nutr. 2000, 40, 1–42. [Google Scholar] [CrossRef] [PubMed]
- Holzapfel, N.P.; Holzapfel, B.M.; Champ, S.; Feldthusen, J.; Clements, J.; Hutmacher, D.W. The Potential Role of Lycopene for the Prevention and Therapy of Prostate Cancer: From Molecular Mechanisms to Clinical Evidence. Int. J. Mol. Sci. 2013, 14, 14620–14646. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, A.; Babaye, H.; Ya’u, M.; Babagana, K.; Abubakar, S.; Ahmad, J. Nigerian Citrullus Lanatus Fruit and Seed Juice Reduces Cardiovascular Diseases Modifiable Risk Biomarkers in Normal Experimental Rats. J Hypertens Manag 2018, 4, 1–7. [Google Scholar]
- Nadeem, M.; Navida, M.; Ameer, K.; Iqbal, A.; Malik, F.; Nadeem, M.A.; Fatima, H.; Ahmed, A.; Din, A. A Comprehensive Review on the Watermelon Phytochemical Profile and Their Bioactive and Therapeutic Effects. Korean J. Food Preserv. 2022, 29, 546–576. [Google Scholar] [CrossRef]
- Saini, R.K.; Nile, S.H.; Park, S.W. Carotenoids from Fruits and Vegetables: Chemistry, Analysis, Occurrence, Bioavailability and Biological Activities. Food Res. Int. 2015, 76, 735–750. [Google Scholar] [CrossRef] [PubMed]
- Rai, C.; Rai, P.; Majumdar, G.; De, S.; DasGupta, S. Mechanism of Permeate Flux Decline during Microfiltration of Watermelon (Citrullus Lanatus) Juice. Food Bioprocess Technol. 2010, 3, 545–553. [Google Scholar] [CrossRef]
- Montesano, D.; Fallarino, F.; Cossignani, L.; Bosi, A.; Simonetti, M.S.; Puccetti, P.; Damiani, P. Innovative Extraction Procedure for Obtaining High Pure Lycopene from Tomato. Eur. Food Res. Technol. 2008, 226, 327–335. [Google Scholar] [CrossRef]
- Story, E.N.; Kopec, R.E.; Schwartz, S.J.; Harris, G.K. An Update on the Health Effects of Tomato Lycopene. Annu. Rev. Food Sci. Technol. 2010, 1, 189–210. [Google Scholar] [CrossRef] [PubMed]
- Fattore, M.; Montesano, D.; Pagano, E.; Teta, R.; Borrelli, F.; Mangoni, A.; Seccia, S.; Albrizio, S. Carotenoid and Flavonoid Profile and Antioxidant Activity in “Pomodorino Vesuviano” Tomatoes. J. Food Compos. Anal. 2016, 53, 61–68. [Google Scholar] [CrossRef]
- Suwanaruang, T. Analyzing Lycopene Content in Fruits. Agric. Agric. Sci. Procedia 2016, 11, 46–48. [Google Scholar] [CrossRef]
- Manivannan, A.; Lee, E.-S.; Han, K.; Lee, H.-E.; Kim, D.-S. Versatile Nutraceutical Potentials of Watermelon—A Modest Fruit Loaded with Pharmaceutically Valuable Phytochemicals. Molecules 2020, 25, 5258. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, C.; Saxena, V.; Dutta, S. Novel Thermal and Non-Thermal Processing of Watermelon Juice. Trends Food Sci. Technol. 2019, 93, 234–243. [Google Scholar] [CrossRef]
- Wu, G.; Collins, J.K.; Perkins-Veazie, P.; Siddiq, M.; Dolan, K.D.; Kelly, K.A.; Heaps, C.L.; Meininger, C.J. Dietary Supplementation with Watermelon Pomace Juice Enhances Arginine Availability and Ameliorates the Metabolic Syndrome in Zucker Diabetic Fatty Rats. J. Nutr. 2007, 137, 2680–2685. [Google Scholar] [CrossRef]
- Collins, J.K.; Wu, G.; Perkins-Veazie, P.; Spears, K.; Claypool, P.L.; Baker, R.A.; Clevidence, B.A. Watermelon Consumption Increases Plasma Arginine Concentrations in Adults. Nutrition 2007, 23, 261–266. [Google Scholar] [CrossRef]
- El-Razek, F.; Sadeek, E.A. Dietary Supplementation with Watermelon (Citrullus Ianatus) Juice Enhances Arginine Availability and Modifies Hyperglycemia, Hyperlipidemia, and Oxidative Stress in Diabetic Rats. Aust J Basic Appl Sci 2011, 5, 1284–1295. [Google Scholar]
- Poduri, A.; Rateri, D.L.; Saha, S.K.; Saha, S.; Daugherty, A. Citrullus Lanatus ‘Sentinel’(Watermelon) Extract Reduces Atherosclerosis in LDL Receptor-Deficient Mice. J. Nutr. Biochem. 2013, 24, 882–886. [Google Scholar] [CrossRef]
- Deshmukh, A.S.; Long, Y.; de Castro Barbosa, T.; Karlsson, H.; Glund, S.; Zavadoski, W.; Gibbs, E.; Koistinen, H.; Wallberg-Henriksson, H.; Zierath, J.R. Nitric Oxide Increases Cyclic GMP Levels, AMP-Activated Protein Kinase (AMPK) A1-Specific Activity and Glucose Transport in Human Skeletal Muscle. Diabetologia 2010, 53, 1142–1150. [Google Scholar] [CrossRef] [PubMed]
- Lira, V.A.; Soltow, Q.A.; Long, J.H.; Betters, J.L.; Sellman, J.E.; Criswell, D.S. Nitric Oxide Increases GLUT4 Expression and Regulates AMPK Signaling in Skeletal Muscle. Am. J. Physiol.-Endocrinol. Metab. 2007, 293, E1062–E1068. [Google Scholar] [CrossRef]
- Rains, J.L.; Jain, S.K. Oxidative Stress, Insulin Signaling, and Diabetes. Free Radic. Biol. Med. 2011, 50, 567–575. [Google Scholar] [CrossRef] [PubMed]
- Singh Gill, N.; Sood, S.; Muthuraman, A.; Bali, M.; Dev Sharma, P. Evaluation of Antioxidant and Anti-Ulcerative Potential of Citrullus Lanatus Seed Extract in Rats. Lat. Am. J. Pharm. 2011, 30. [Google Scholar]
- Gill, N.; Bansal, R.; Manju, G.; Shailja, S.; Arunachalam, M.; Manoj, B. Evaluation of Antioxidant, Anti-Inflammatory and Analgesic Potential of Citrullus Lanatus Seed Extract in Rodent Model. Internet J. Nutr. Wellness 2010, 9. [Google Scholar]
- Adebayo, A.O.; Alozie, I.; somtochi Olivia, C.-O. Evaluating the Influence of Citrullus Lanatus Seed Extracts on Electrolytes, Urea and Creatinine in Streptozotocin Induced Diabetic Albino Rats. 2663-1040 2018, 2, 87–94. [Google Scholar]
- Deshmukh, C.D.; Jain, A.; Tambe, M.S. Phytochemical and Pharmacological Profile of Citrullus Lanatus (THUNB). Biolife 2015, 3, 483–488. [Google Scholar]
- Rahman, H.; Manjula, K.; Anoosha, T.; Nagaveni, K.; Eswaraiah, M.C.; Bardalai, D. In-Vitro Antioxidant Activity of Citrullus Lanatus Seed Extracts. Asian J Pharm Clin Res 2013, 6, 152–157. [Google Scholar]
- Ikpeme, E.; Udensi, O.; Ekerette, E.; Okon, U. Potential of Ginger (Zingiber Officinale) Rhizome and Watermelon (Citrullus Lanatus) Seeds in Mitigating Aspartame-Induced Oxidative Stress in Rat Model. Res J Med Plant 2016, 10, 55–66. [Google Scholar] [CrossRef]
- Wahid, S.; Khan, R.A.; Feroz, Z.; Ikram, R. Analgesic, Anti-Inflammatory and Toxic Effects of Ethanol Extracts of Cucumis Melo and Citrullus Lanatus Seeds. Pak. J. Pharm. Sci. 2020, 33. [Google Scholar]
- Petchsomrit, A.; McDermott, M.I.; Chanroj, S.; Choksawangkarn, W. Watermelon Seeds and Peels: Fatty Acid Composition and Cosmeceutical Potential. OCL 2020, 27, 54. [Google Scholar] [CrossRef]
- Hong, M.Y.; Hartig, N.; Kaufman, K.; Hooshmand, S.; Figueroa, A.; Kern, M. Watermelon Consumption Improves Inflammation and Antioxidant Capacity in Rats Fed an Atherogenic Diet. Nutr. Res. 2015, 35, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Connolly, M.; Lum, T.; Marx, A.; Hooshmand, S.; Kern, M.; Liu, C.; Hong, M.Y. Effect of Fresh Watermelon Consumption on Risk Factors for Cardiovascular Disease in Overweight and Obese Adults (P06-102-19). Curr. Dev. Nutr. 2019, 3, nzz031-P06. [Google Scholar] [CrossRef]
- Wen, C.; Zhang, J.; Feng, Y.; Duan, Y.; Ma, H.; Zhang, H. Purification and Identification of Novel Antioxidant Peptides from Watermelon Seed Protein Hydrolysates and Their Cytoprotective Effects on H2O2-Induced Oxidative Stress. Food Chem. 2020, 327, 127059. [Google Scholar] [CrossRef] [PubMed]
- Dash, P.; Rath, G.; Ghosh, G. In Vivo Antioxidant Potential of Protein Hydrolysates of Some Cucurbitaceae Seed. J. Drug Deliv. Ther. 2020, 10, 128–132. [Google Scholar] [CrossRef]
- Wen, C.; Zhang, J.; Zhang, H.; Duan, Y.; Ma, H. Effects of Divergent Ultrasound Pretreatment on the Structure of Watermelon Seed Protein and the Antioxidant Activity of Its Hydrolysates. Food Chem. 2019, 299, 125165. [Google Scholar] [CrossRef] [PubMed]
- Sonawane, S.K.; Arya, S.S. Citrullus Lanatus Protein Hydrolysate Optimization for Antioxidant Potential. J. Food Meas. Charact. 2017, 11, 1834–1843. [Google Scholar] [CrossRef]
- Asghar, M.N.; Shahzad, M.T.; Nadeem, I.; Ashraf, C.M. Phytochemical and in Vitro Total Antioxidant Capacity Analyses of Peel Extracts of Different Cultivars of Cucumis Melo and Citrullus Lanatus. Pharm. Biol. 2013, 51, 226–232. [Google Scholar] [CrossRef]
- Kolawole, T.; Ojeka, S.; Dapper, D. Anti-Diabetic Effects of the Methanolic Extract of the Rind of Citrullus Lanatus (Watermelon) in Alloxan Induced Diabetes in Male Albino Wistar Rats. J. Med. Med. Sci. 2016, 7, 023–029. [Google Scholar]
- Kolawole, A.; Dapper, V.; Eziuzo, C. Effects of the Methanolic Extract of the Rind of Citrullus Lanatus (Watermelon) on Some Erythrocyte Parameters and Indices of Oxidative Status in Phenylhydrazine-Treated Male Wistar Rats. J. Afr. Assoc. Physiol. Sci. 2017, 5, 22–28. [Google Scholar]
- Alamsyah, N.; Djamil, R.; Rahmat, D. Antioxidant Activity of Combination Banana Peel (Musa Paradisiaca) and Watermelon Rind (Citrullus Vulgaris) Extract in Lotion Dosage Form. Asian J Pharm Clin Res 2016, 9, 300–304. [Google Scholar] [CrossRef]
- Figueroa, A.; Wong, A.; Kalfon, R. Effects of Watermelon Supplementation on Aortic Hemodynamic Responses to the Cold Pressor Test in Obese Hypertensive Adults. Am. J. Hypertens. 2014, 27, 899–906. [Google Scholar] [CrossRef] [PubMed]
- Sultan, S.; D’Souza, A.; Zabetakis, I.; Lordan, R.; Tsoupras, A.; Kavanagh, E.P.; Hynes, N. Chapter 6 - Statins: Rationale, Mode of Action, and Side Effects. In The Impact of Nutrition and Statins on Cardiovascular Diseases; Zabetakis, I., Lordan, R., Tsoupras, A., Eds.; Academic Press, 2019; pp. 171–200 ISBN 978-0-12-813792-5.
- Figueroa, A.; Sanchez-Gonzalez, M.A.; Wong, A.; Arjmandi, B.H. Watermelon Extract Supplementation Reduces Ankle Blood Pressure and Carotid Augmentation Index in Obese Adults with Prehypertension or Hypertension. Am. J. Hypertens. 2012, 25, 640–643. [Google Scholar] [CrossRef] [PubMed]
- Ellis, A.C.; Dudenbostel, T.; Crowe-White, K. Watermelon Juice: A Novel Functional Food to Increase Circulating Lycopene in Older Adult Women. Plant Foods Hum. Nutr. 2019, 74, 200–203. [Google Scholar] [CrossRef] [PubMed]
- Khalaf, D.; Krüger, M.; Wehland, M.; Infanger, M.; Grimm, D. The Effects of Oral L-Arginine and L-Citrulline Supplementation on Blood Pressure. Nutrients 2019, 11, 1679. [Google Scholar] [CrossRef] [PubMed]
- Tarazona-Díaz, M.P.; Alacid, F.; Carrasco, M.; Martínez, I.; Aguayo, E. Watermelon Juice: Potential Functional Drink for Sore Muscle Relief in Athletes. J. Agric. Food Chem. 2013, 61, 7522–7528. [Google Scholar] [CrossRef] [PubMed]
- Jorge, N.; da Silva, A.C.; Malacrida, C.R. Physicochemical Characterisation and Radical-Scavenging Activity of Cucurbitaceae Seed Oils. Nat. Prod. Res. 2015, 29, 2313–2317. [Google Scholar] [CrossRef]
- Karikpo, C.; Bartimaeus, E.; Holy, B. Evaluation of the Cardioprotective Effect of Citrullus Lanatus (Watermelon) Seeds in Streptozotocin Induced Diabetic Albino Rats. Asian J. Biochem. Genet. Mol. Biol. 2019, 1, 1–6. [Google Scholar] [CrossRef]
- Gajowik, A.; Dobrzynska, M. Lycopene-Antioxidant with Radioprotective and Anticancer Properties. A Review. Rocz. Państw. Zakładu Hig. 2014, 65. [Google Scholar]
- Nahum, A.; Hirsch, K.; Danilenko, M.; Watts, C.K.; Prall, O.W.; Levy, J.; Sharoni, Y. Lycopene Inhibition of Cell Cycle Progression in Breast and Endometrial Cancer Cells Is Associated with Reduction in Cyclin D Levels and Retention of p27Kip1 in the Cyclin E–Cdk2 Complexes. Oncogene 2001, 20, 3428–3436. [Google Scholar] [CrossRef] [PubMed]
- Butt, M.S.; Sultan, M.T. Selected Functional Foods for Potential in Disease Treatment and Their Regulatory Issues. Int. J. Food Prop. 2013, 16, 397–415. [Google Scholar] [CrossRef]
- Barkur, S.; Bankapur, A.; Chidangil, S.; Mathur, D. Effect of Infrared Light on Live Blood Cells: Role of β-Carotene. J. Photochem. Photobiol. B 2017, 171, 104–116. [Google Scholar] [CrossRef] [PubMed]
- Fadimu, G.J.; Ghafoor, K.; Babiker, E.E.; Al-Juhaimi, F.; Abdulraheem, R.A.; Adenekan, M.K. Ultrasound-Assisted Process for Optimal Recovery of Phenolic Compounds from Watermelon (Citrullus Lanatus) Seed and Peel. J. Food Meas. Charact. 2020, 14, 1784–1793. [Google Scholar] [CrossRef]
- Patel, S.; Rauf, A. Edible Seeds from Cucurbitaceae Family as Potential Functional Foods: Immense Promises, Few Concerns. Biomed. Pharmacother. 2017, 91, 330–337. [Google Scholar] [CrossRef]
- Simpson, R.; Morris, G.A. The Anti-Diabetic Potential of Polysaccharides Extracted from Members of the Cucurbit Family: A Review. Bioact. Carbohydr. Diet. Fibre 2014, 3, 106–114. [Google Scholar] [CrossRef]
- Oseni, O.; Odesanmi, O.; Oladele, F. Antioxidative and Antidiabetic Activities of Watermelon (Citrullus Lanatus) Juice on Oxidative Stress in Alloxan-Induced Diabetic Male Wistar Albino Rats. Niger. Med. J. J. Niger. Med. Assoc. 2015, 56, 272. [Google Scholar] [CrossRef] [PubMed]
- Vincellette, C.M.; Losso, J.; Early, K.; Spielmann, G.; Irving, B.A.; Allerton, T.D. Supplemental Watermelon Juice Attenuates Acute Hyperglycemia-Induced Macro-and Microvascular Dysfunction in Healthy Adults. J. Nutr. 2021, 151, 3450–3458. [Google Scholar] [CrossRef]
- Ahn, J.; Choi, W.; Kim, S.; Ha, T. Anti-Diabetic Effect of Watermelon (Citrullus Vulgaris Schrad) on Streptozotocin-Induced Diabetic Mice. Food Sci. Biotechnol. 2011, 20, 251–254. [Google Scholar] [CrossRef]
- Zia, S.; Khan, M.R.; Aadil, R.M.; Shahid, M. Development and Storage Stability of Value-added Watermelon Fruit Butter by Incorporating Watermelon Rind Byproduct. J. Food Process. Preserv. 2022, 46, e17031. [Google Scholar] [CrossRef]
- Abu-Hiamed, H. Hypocholesterolemic Effects of Watermelon Fruit Rind on Rats. Nutr. Food Sci. 2018, 48, 836–845. [Google Scholar] [CrossRef]
- Owo, J.; Beresford, S. Blood Sugar Lowering Potentials of Aqueous and Ethanol Extracts of the Mixture of Rinds of Citrullus Vulgaris Schrad (Watermelon) and Chrysophyllum Albidum G.(Udara) Fruits on Alloxan-Induced Diabetic Wistar Rats. J. Pharm. Res. Int. 2020, 32, 86–90. [Google Scholar]
- Parmar, H.S.; Kar, A. Possible Amelioration of Atherogenic Diet Induced Dyslipidemia, Hypothyroidism and Hyperglycemia by the Peel Extracts of Mangifera Indica, Cucumis Melo and Citrullus Vulgaris Fruits in Rats. Biofactors 2008, 33, 13–24. [Google Scholar] [CrossRef]
- Omigie, I.; Agoreyo, F. Effects of Watermelon (Citrullus Lanatus) Seed on Blood Glucose and Electrolyte Parameters in Diabetic Wistar Rats. J. Appl. Sci. Environ. Manag. 2014, 18, 231–233. [Google Scholar] [CrossRef]
- Belemkar, S.; Shendge, P.N. Toxicity Profiling of the Ethanolic Extract of Citrullus Lanatus Seed in Rats: Behavioral, Biochemical and Histopathological Aspects. Biosci. Rep. 2021, 41, BSR20202345. [Google Scholar] [CrossRef] [PubMed]
- Arise, R.O.; Yekeen, A.A.; Ekun, O.E. In Vitro Antioxidant and α-Amylase Inhibitory Properties of Watermelon Seed Protein Hydrolysates. Environ. Exp. Biol. 2016, 14. [Google Scholar] [CrossRef]
- Montesano, D.; Rocchetti, G.; Putnik, P.; Lucini, L. Bioactive Profile of Pumpkin: An Overview on Terpenoids and Their Health-Promoting Properties. Curr. Opin. Food Sci. 2018, 22, 81–87. [Google Scholar] [CrossRef]
- Liang, J.; Zhang, X.; Yuan, J.; Zhang, H.; Liu, D.; Hao, J.; Ji, W.; Wu, X.; Chen, D. Cucurbitacin B Inhibits the Migration and Invasion of Breast Cancer Cells by Altering the Biomechanical Properties of Cells. Phytother. Res. 2019, 33, 618–630. [Google Scholar] [CrossRef]
- Kaushik, U.; Aeri, V.; Mir, S.R. Cucurbitacins–an Insight into Medicinal Leads from Nature. Pharmacogn. Rev. 2015, 9, 12. [Google Scholar]
- Oyenihi, O.R.; Afolabi, B.A.; Oyenihi, A.B.; Ogunmokun, O.J.; Oguntibeju, O.O. Hepato-and Neuro-Protective Effects of Watermelon Juice on Acute Ethanol-Induced Oxidative Stress in Rats. Toxicol. Rep. 2016, 3, 288–294. [Google Scholar] [CrossRef] [PubMed]
- Sathya, J.; Shoba, F.G. Assessment of Antimicrobial Efficacy of Citrullus Lanatus Methanolic Seed Extract. J Chem Pharm Res 2014, 6, 640–643. [Google Scholar]
- Dash, P.; Ghosh, G. Fractionation, Amino Acid Profiles, Antimicrobial and Free Radical Scavenging Activities of Citrullus Lanatus Seed Protein. Nat. Prod. Res. 2017, 31, 2945–2947. [Google Scholar] [CrossRef] [PubMed]
- Sonawane, S.K.; Bhagwat, A.N.; Arya, S. Limonia Acidissima and Citrullus Lanatus Fruit Seeds: Antimicrobial, Thermal, Structural, Functional and Protein Identification Study. Food Biosci. 2018, 26, 8–14. [Google Scholar] [CrossRef]
- Hameed, B.; Ali, Q.; Hafeez, M.; Malik, A. Antibacterial and Antifungal Activity of Fruit, Seed and Root Extracts of Citrullus Colocynthis Plant. Biol. Clin. Sci. Res. J. 2020, 2020. [Google Scholar] [CrossRef]
- Loiy, E.; Hassan, A.; Hasnah, M.; Ahmed, Y.; Asking, M.; Koko, W.; Siddig, A. In Vitro Anti-Microbial Activities of Chloroformic, Hexane & Ethanolic Extracts of C. Lanatus Var. Citroides. J Med Plants Res 2011, 5, 1338–1344. [Google Scholar]
- Adunola, A.; Chidimma, A.L.; Olatunde, D.S.; Peter, O.A. Antibacterial Activity of Watermelon (Citrullus Lanatus) Seed against Selected Microorganisms. Afr. J. Biotechnol. 2015, 14, 1224–1229. [Google Scholar]
- Kumari, A.; Rao, J.; Kumari, J.; Sharma, N.; Jain, P.; Dave, V.; Sharma, S. Analgesic Activity of Aqueous Extract of Citrullus Lanatus Peels. Adv. Pharmacol. Pharm. 2013, 1, 135–138. [Google Scholar] [CrossRef]
- Akshaya, M.; Sasikala, S.; Nithyalakshmi, V.; Meenakshi, N.; Dhivya Kiruthika, K.; Pavithra, M. Effect of Pre-Treatments on the Phytochemical Composition of Watermelon (Citrullus Lanatus) Rind. Int. Food Res. J. 2018, 25. [Google Scholar]
- Rajasree, R.; Sibi, P.; Francis, F.; William, H. Phytochemicals of Cucurbitaceae Family—A Review. Int. J. Pharmacogn. Phytochem. Res. 2016, 8, 113–123. [Google Scholar]
- Yadav, S.; Bhargav, M.; Ramya, R.; Nowfal, H. In-Vitro Antioxidant and Immunomodulatory Activity of Citrullus Lanatus Seed. Int. J. Eng. Sci. Res. Technol. 2016, 5, 679–685. [Google Scholar]
- Messaoudi, S.; Tebibel, S.; Beladjila, A.K.; Touhami, F.K.; Kabouche, Z. Anti-Hyperlipidemic, Anti-Inflammatory and Antioxidant Activities of Citrullus Lanatus. World J. Environ. Biosci. 2019, 8, 100–106. [Google Scholar]
- Tomes, M.; Johnson, K.; Hess, M. The Carotene Pigment Content of Certain Red Fleshed Watermelons.; 1963; Vol. 82, pp. 460–464.
- Fan, J.; Park, E.; Zhang, L.; Edirisinghe, I.; Burton-Freeman, B.; Sandhu, A.K. Pharmacokinetic Parameters of Watermelon (Rind, Flesh, and Seeds) Bioactive Components in Human Plasma: A Pilot Study to Investigate the Relationship to Endothelial Function. J. Agric. Food Chem. 2020, 68, 7393–7403. [Google Scholar] [CrossRef] [PubMed]
- Rezig, L.; Chouaibi, M.; Meddeb, W.; Msaada, K.; Hamdi, S. Chemical Composition and Bioactive Compounds of Cucurbitaceae Seeds: Potential Sources for New Trends of Plant Oils. Process Saf. Environ. Prot. 2019, 127, 73–81. [Google Scholar] [CrossRef]
- Oelschlägel, S.; Menzel, C.; Speer, K. Phytosterols and Steryl Esters in Diverse Cucurbita, Cucumis and Citrullus Seed Oils. Lipid Technol. 2012, 24, 181–184. [Google Scholar] [CrossRef]
- Suliburska, J.; Bogdanski, P.; Krejpcio, Z.; Pupek-Musialik, D.; Jablecka, A. The Effects of L-Arginine, Alone and Combined with Vitamin C, on Mineral Status in Relation to Its Antidiabetic, Anti-Inflammatory, and Antioxidant Properties in Male Rats on a High-Fat Diet. Biol. Trace Elem. Res. 2014, 157, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Shanely, R.A.; Zwetsloot, J.J.; Jurrissen, T.J.; Hannan, L.C.; Zwetsloot, K.A.; Needle, A.R.; Bishop, A.E.; Wu, G.; Perkins-Veazie, P. Daily Watermelon Consumption Decreases Plasma sVCAM-1 Levels in Overweight and Obese Postmenopausal Women. Nutr. Res. 2020, 76, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Ajiboye, B.O.; Shonibare, M.T.; Oyinloye, B.E. Antidiabetic Activity of Watermelon (Citrullus Lanatus) Juice in Alloxan-Induced Diabetic Rats. J. Diabetes Metab. Disord. 2020, 19, 343–352. [Google Scholar] [CrossRef] [PubMed]
- Onyeso, G.; Nkpaa, K.; Omenihu, S. Co-Administration of Caffeine and Hydromethanolic Fraction of Citrullus Lanatus Seeds Improved Testicular Functions in Alloxan-Induced Diabetic Male Wistar Rats. Asian Pac. J. Reprod. 2016, 5, 105–110. [Google Scholar] [CrossRef]
- Sorour, H.; Selim, M.; Almoselhy, L.; Gouda, S. Ameliorative Effect of Watermelon Rind Ingestion on the Pancreas of Diabetic Female Albino Rat (Histological, Immunohistochemical and Morphometric Study). Egypt. J. Histol. 2019, 42, 10–22. [Google Scholar]
- Azizi, S.; Mahdavi, R.; Vaghef-Mehrabany, E.; Maleki, V.; Karamzad, N.; Ebrahimi-Mameghani, M. Potential Roles of Citrulline and Watermelon Extract on Metabolic and Inflammatory Variables in Diabetes Mellitus, Current Evidence and Future Directions: A Systematic Review. Clin. Exp. Pharmacol. Physiol. 2020, 47, 187–198. [Google Scholar] [CrossRef] [PubMed]
- Becraft, A.R.; Sturm, M.L.; Mendez, R.L.; Park, S.H.; Lee, S.I.; Shay, N.F. Intake of Watermelon or Its Byproducts Alters Glucose Metabolism, the Microbiome, and Hepatic Proinflammatory Metabolites in High-Fat–Fed Male C57BL/6 J Mice. J. Nutr. 2020, 150, 434–442. [Google Scholar] [CrossRef] [PubMed]
- Gad-Elkareem, M.A.; Abdelgadir, E.H.; Badawy, O.M.; Kadri, A. Potential Antidiabetic Effect of Ethanolic and Aqueous-Ethanolic Extracts of Ricinus Communis Leaves on Streptozotocin-Induced Diabetes in Rats. PeerJ 2019, 7, e6441. [Google Scholar] [CrossRef] [PubMed]
- Okechukwu, H.; Ihentuge, C.; Ugochukwu, C.; Anibeze, C. Histological Changes in the Pancreas of Streptozotocin Induced Diabetic Rats Fed with Rind of Citrullus Lanatus. FASEB J. 2015, 29, 544–548. [Google Scholar] [CrossRef]
- Oluwole, F.S.; Balogun, M.E.; Temitope, A.G. Antisecretory Effects of Watermelon (Citrullus Lanatus) Juice in Male Albino Rats. Annu. Res. Rev. Biol. 2013, 358–366. [Google Scholar]
- Sharma, S.; Sarvesh, P.; Dwivedi, J.; Amita, T. First Report on Laxative Activity of Citrullus Lanatus. Pharmacologyonline 2011, 2, 790–797. [Google Scholar]
- Altaş, S.; Kızıl, G.; Kızıl, M.; Ketani, A.; Haris, P.I. Protective Effect of Diyarbakır Watermelon Juice on Carbon Tetrachloride-Induced Toxicity in Rats. Food Chem. Toxicol. 2011, 49, 2433–2438. [Google Scholar] [CrossRef] [PubMed]
- Zhan, Y.-Y.; Wang, J.-H.; Tian, X.; Feng, S.-X.; Xue, L.; Tian, L.-P. Protective Effects of Seed Melon Extract on CCl4-Induced Hepatic Fibrosis in Mice. J. Ethnopharmacol. 2016, 193, 531–537. [Google Scholar] [CrossRef] [PubMed]
- Ogbeifun, H.; Peters, D.; Monanu, M. Ameliorative Effect of Citrullus Lanatus (Water Melon) Seeds on Alloxan Induced Hepato and Nephro Toxicity. Asian J. Adv. Res. Rep. 2020, 9, 1–10. [Google Scholar] [CrossRef]
- Iswariya, S.; Uma, T. Evaluation of in Vitro Anti-Inflammatory and Antimicrobial Activity of Aqueous and Methanolic Seed Extracts of Citrullus Lanatus. Int J Pharm Pharm Sci 2017, 9, 29–33. [Google Scholar]
- Gabriel, A.; Igwemmar, N.; Sadam, A.; Babalola, S. Characterization of Seed Oil from Citrullus Lanatus (Watermelon). Direct Res J Public Health Env. Technol 2018, 3, 34–40. [Google Scholar]
- Athar, S.; Ghazi, A.; Chourasiya, O.; Karadbhajne, V.Y. „Watermelon Seed Oil: Its Extraction, Analytical Studies, Modification and Utilization in Cosmetic Industries‟. Int. Res. J. Eng. 2020. [Google Scholar]
- Deng, J.; Wang, S.; Guo, L.; Fan, L. Anti-Inflammatory and Analgesic Effects of Extract from Roots and Leaves of Citrullus Lanatus. Chin Herb Med 2010, 2, 231–235. [Google Scholar]
- Alam, M.A.; Kauter, K.; Withers, K.; Sernia, C.; Brown, L. Chronic L-Arginine Treatment Improves Metabolic, Cardiovascular and Liver Complications in Diet-Induced Obesity in Rats. Food Funct. 2013, 4, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Soliman, W. Fabrication of Electrospun Nanofibers Made of Watermelon Peel Extract and PVA and Investigating Their Antioxidant and Antibacterial Activities. 2019.
- Hassan, R.; Amira, E.; Odeyemi, O.; Omorinoye, J. Phytochemicals and Antimicrobial Potential of Citrullus Vulgaris Seed and Pod Extracts. Int. J. Innov. Res. Adv. Stud. 2020, 7, 103–106. [Google Scholar]
- Thirunavukkarasu, P.; Ramanathan, T.; Ravichandran, N.; Ramkumar, L. Screening of Anti-Microbial Effect in Watermelon (Citrullus Sp.). J. Biol. Sci. 2010, 10, 682–685. [Google Scholar] [CrossRef]
- Dammak, M.I.; Salem, Y.B.; Belaid, A.; Mansour, H.B.; Hammami, S.; Le Cerf, D.; Majdoub, H. Partial Characterization and Antitumor Activity of a Polysaccharide Isolated from Watermelon Rinds. Int. J. Biol. Macromol. 2019, 136, 632–641. [Google Scholar] [CrossRef] [PubMed]
- Oyewo, O.; Onyije, F.; Akintunde, O.; Ashamu, E. Effects of Aqueous Extract of Citrullus Lanatus on the Histology of the Kidney of Adult Wistar Rats. World Appl. Sci. J. 2012, 17, 1178–1181. [Google Scholar]
- Munglue, P.; Eumkep, G.; Wray, S.; Kupittayanant, S. The Effects of Watermelon (Citrullus Lanatus) Extracts and L-Citrulline on Rat Uterine Contractility. Reprod. Sci. 2013, 20, 437–448. [Google Scholar] [CrossRef]
- Olamide, A.A.; Olayemi, O.O.; Demetrius, O.O.; Olatoye, O.J.; Kehinde, A.A. Effects of Methanolic Extract of Citrullus Lanatus Seed on Experimentally Induced Prostatic Hyperplasia. Eur. J. Med. Plants 2011, 1, 171–179. [Google Scholar] [CrossRef]
- Omotoso, O.; Osadiaye, A. The Regenerative Efficacy of Aqueous and Methanolic Extracts of Watermelon, Citrullus Lanatus Seeds on Physiological Saline and Acetaminophen-Induced Kidney Damage in Female Albino Rats. Br. J. Multidiscip. Adv. Stud. 2018, 2, 98–108. [Google Scholar]
- Owoeye, O.; Akinbami, R.; Thomas, M. Neuroprotective Potential of Citrullus Lanatus Seed Extract and Vitamin E against Mercury Chloride Intoxication in Male Rat Brain. Afr. J. Biomed. Res. 2018, 21, 43–49. [Google Scholar]
- Finbarrs, E.; Chinedu, F.; Ojo, O.P. Effect of Ethanolic Seed Extract of Citrus Lanatus (Watermelon) on Immunoreactivity of Hippocampal Neurofilament of Adult Wistar Rats. Eras J. Med. Res. 2018, 5, 1–5. [Google Scholar] [CrossRef]
- Adedeji, G.; Bamidele, O.; Ogunbiyi, A. Haematological and Biochemical Properties of Methanolic Extract of Citrullus Lanatus Seeds. Br. J. Pharm. Res. 2017, 15, 1–10. [Google Scholar] [CrossRef]
- Ferreira, J.; Rodriguez-Amaya, D. Degradation of Lycopene and Β-carotene in Model Systems and in Lyophilized Guava during Ambient Storage: Kinetics, Structure, and Matrix Effects. J. Food Sci. 2008, 73, C589–C594. [Google Scholar] [CrossRef] [PubMed]
- Shivapour, M.; Yousefi, S.; Ardabili, S.M.S.; Weisany, W. Optimization and Quality Attributes of Novel Toast Breads Developed Based on the Antistaling Watermelon Rind Powder. J. Agric. Food Res. 2020, 2, 100073. [Google Scholar] [CrossRef]
- Imoisi, C.; Iyasele, J.; Michael, U.; Imhontu, E. The Effects of Watermelon Rind Flour on the Functional and Proximate Properties of Wheat Bread. J. Chem. Soc. Niger. 2020, 45. [Google Scholar] [CrossRef]
- Anang, D.A.; Pobee, R.A.; Antwi, E.; Obeng, E.M.; Atter, A.; Ayittey, F.K.; Boateng, J.T. Nutritional, Microbial and Sensory Attributes of Bread Fortified with Defatted Watermelon Seed Flour. Int. J. Food Sci. Technol. 2018, 53, 1468–1475. [Google Scholar] [CrossRef]
- Adegunwa, M.; Oloyede, I.; Adebanjo, L.; Alamu, E. Quality Attribute of Plantain (Musa Paradisiaca) Sponge-Cake Supplemented with Watermelon (Citrullus Lanatus) Rind Flour. Cogent Food Agric. 2019, 5, 1631582. [Google Scholar] [CrossRef]
- ASHOKA, S.; BEGUM, S.S.; RAY, B.R.M. Effect of Watermelon (Citrullus Lanatus) Rind Flour Incorporation on Nutritional and Organoleptic Attributes of Cakes. 2021.
- Ho, L.-H.; Paet, M.; Suhaimi, M.A. The Physicochemical and Sensory Attributes of Watermelon Rind Flour Incorporated Steamed Cupcake. J. Agrobiotechnology 2018, 9, 31–42. [Google Scholar]
- HASSAN, N.S.; MAHMOUD, A.H.; EL-KHOLAN, E.A. Quality Enhancing of Cake Using White Watermelon Rinds. Egypt. J. Agric. Res. 2017, 95, 1653–1667. [Google Scholar] [CrossRef]
- Naknaen, P.; Itthisoponkul, T.; Sondee, A.; Angsombat, N. Utilization of Watermelon Rind Waste as a Potential Source of Dietary Fiber to Improve Health Promoting Properties and Reduce Glycemic Index for Cookie Making. Food Sci. Biotechnol. 2016, 25, 415–424. [Google Scholar] [CrossRef]
- Olaitan, N.; Eke, M.; Agudu, S. Effect of Watermelon (Citrullus Lanatus) Rind Flour Supplementation on the Quality of Wheat Based Cookies. Int J Eng Sci 2017, 6, 59–66. [Google Scholar]
- Omobolanle, O.; Oluwatoyin, A.; Michael, I.; Adefunke, B.; Olamiji, A. Evaluation of Potentials of Some Selected Seeds’ Flours as Partial Substitute for Wheat in Cookies Production. Intl J Sci Eng Res 2017, 8, 739–752. [Google Scholar]
- Ogo, A.; Ajekwe, D.; Enenche, D.; Obochi, G. Quality Evaluation of Novel Biscuits Made from Wheat Supplemented with Watermelon Rinds and Orange Pomace Flour Blends. Food Nutr. Sci. 2021, 12, 332–341. [Google Scholar] [CrossRef]
- Peter-Ikechukwu, A.; Omeire, G.; Kabuo, N.; Eluchie, C.; Amandikwa, C.; Odoemenam, G. Production and Evaluation of Biscuits Made from Wheat Flour and Toasted Watermelon Seed Meal as Fat Substitute. J Food Res 2018, 7, 112. [Google Scholar] [CrossRef]
- Franca, E. CHEMICAL PROPERTIES OF WATERMELON SEED AND THE UTILIZATION OF DEHULLED SEED IN COOKIES PRODUCTION. Carpathian J. Food Sci. Technol. 2017, 9. [Google Scholar]
- Ho, L.-H.; Che Dahri, N. Effect of Watermelon Rind Powder on Physicochemical, Textural, and Sensory Properties of Wet Yellow Noodles. CyTA-J. Food 2016, 14, 465–472. [Google Scholar] [CrossRef]
- Kumar, P.; Mehta, N.; Malav, O.; Kumar Chatli, M.; Rathour, M.; Kumar Verma, A. Antioxidant and Antimicrobial Efficacy of Watermelon Rind Extract (WMRE) in Aerobically Packaged Pork Patties Stored under Refrigeration Temperature (4±1 C). J. Food Process. Preserv. 2018, 42. [Google Scholar] [CrossRef]
- Shahein, M.R.; Atwaa, E.S.H.; El-Zahar, K.M.; Elmaadawy, A.A.; Hijazy, H.H.A.; Sitohy, M.Z.; Albrakati, A.; Elmahallawy, E.K. Remedial Action of Yoghurt Enriched with Watermelon Seed Milk on Renal Injured Hyperuricemic Rats. Fermentation 2022, 8, 41. [Google Scholar] [CrossRef]
- Qayyum, A.; Huma, N.; Sameen, A.; Siddiq, A.; Munir, M. I Mpact of Watermelon Seed Flour on the Physico-chemical and Sensory Characteristics of Ice Cream. J. Food Process. Preserv. 2017, 41, e13297. [Google Scholar] [CrossRef]
- Bisla, G.; Archana, P.V.; Sharma, S. Development of Ice Creams from Soybean Milk & Watermelon Seeds Milk and Evaluation of Their Acceptability and Nourishing Potential. Adv Appl Sci Res 2012, 3, 371–376. [Google Scholar]
- Nasir, H.; Allai, F.; Gull, A.; Ganaie, T.; Azad, Z. Effect of Pre-Treatments on Desirable Qualities Attributes of Watermelon Rind Based Candy. J. Postharvest Technol. 2020, 8, 38–52. [Google Scholar]
- Thapa, P.; Pahari, A.; Thagunna, B.; Kusma, R. Preparation and Quality Evaluation of Sugar and Honey-Based Watermelon Rind (By-Product) Candies.
- Dhakal, D.; Pradhananga, M.L. Utilization of Watermelon Rind (Byproduct) in Preparation of Candy and Its Quality Evaluation. Int. J. Multidiscip. Pap. 2017, 2, 1–6. [Google Scholar]
- Hani, M.N.F.; Zahidah, W.; Saniah, K.; Irwani, H. Effects of Drying on the Physical Characteristics of Dehydrated Watermelon Rind Candies. J Trop Agric Fd Sc 2014, 42, 115–123. [Google Scholar]
- Islam, M. Value Addition to Watermelon Rind through Jam Preparation. 2017.
- Petkowicz, C.; Vriesmann, L.; Williams, P. Pectins from Food Waste: Extraction, Characterization and Properties of Watermelon Rind Pectin. Food Hydrocoll. 2017, 65, 57–67. [Google Scholar] [CrossRef]
- Prabha, H.P.; Deva Pavithra, B.; Ishwariya, S.; Jashwanthi, P.; Shendhura Devi, M. Valorization of Watermelon Rind as Dietary Chips Fortified with Composite Flour. Int. J. Creat. Res. Thoughts 2021, 9, 591–596. [Google Scholar]
- Evanuarini, H.; Amertaningtyas, D.; Utama, D. Viscosity, Fat Content, Total Acidity, and Antioxidant Capacity of Reduced-Fat Mayonnaise Made with Watermelon (Citrullus Lanatus) Rind Flour as Stabilizer.; IOP Publishing, 2021; Vol. 788, p. 012100.
- Saavedra, G.; Maternal, J.A.E.; Comahig, M.C.; Pimentel, J. Nutrient Analysis and Shelf Life Study of Watermelon Rind Sports Drink.
- Kumar, V.; Jain, S.K.; Amitabh, A.; Chavan, S.M. Effect of Ohmic Heating on Physicochemical, Bioactive Compounds, and Shelf Life of Watermelon Flesh-rind Drinks. J. Food Process Eng. 2022, 45, e13818. [Google Scholar] [CrossRef]
- Gunawan, W.B.; Basoeki, L.E.A.S. Incorporating Watermelon Rind into Jackfruit Seed Drink: A Functional Drink Rich in Antioxidant and Phenolic Compounds. EDUFORTECH 2022, 7. [Google Scholar] [CrossRef]
- Todhanakasem, T.; Jaiprayat, C.; Sroysuwan, T.; Suksermsakul, S.; Suwapanich, R.; Maleenont, K.K.; Koombhongse, P.; Young, B.M. Active Thermoplastic Starch Film with Watermelon Rind Extract for Future Biodegradable Food Packaging. Polymers 2022, 14, 3232. [Google Scholar] [CrossRef]
- Łopusiewicz, Ł.; Drozłowska, E.; Trocer, P.; Kostek, M.; Śliwiński, M.; Henriques, M.H.; Bartkowiak, A.; Sobolewski, P. Whey Protein Concentrate/Isolate Biofunctional Films Modified with Melanin from Watermelon (Citrullus Lanatus) Seeds. Materials 2020, 13, 3876. [Google Scholar] [CrossRef]
- Łopusiewicz, Ł.; Macieja, S.; Śliwiński, M.; Bartkowiak, A.; Roy, S.; Sobolewski, P. Alginate Biofunctional Films Modified with Melanin from Watermelon Seeds and Zinc Oxide/Silver Nanoparticles. Materials 2022, 15, 2381. [Google Scholar] [CrossRef]
- Wang, F.; Xie, C.; Ye, R.; Tang, H.; Jiang, L.; Liu, Y. Development of Active Packaging with Chitosan, Guar Gum and Watermelon Rind Extract: Characterization, Application and Performance Improvement Mechanism. Int. J. Biol. Macromol. 2023, 227, 711–725. [Google Scholar] [CrossRef]
- Han, H.-S.; Song, K.B. Antioxidant Properties of Watermelon (Citrullus Lanatus) Rind Pectin Films Containing Kiwifruit (Actinidia Chinensis) Peel Extract and Their Application as Chicken Thigh Packaging. Food Packag. Shelf Life 2021, 28, 100636. [Google Scholar] [CrossRef]
- Guo, Z.; Ge, X.; Li, W.; Yang, L.; Han, L.; Yu, Q. Active-Intelligent Film Based on Pectin from Watermelon Peel Containing Beetroot Extract to Monitor the Freshness of Packaged Chilled Beef. Food Hydrocoll. 2021, 119, 106751. [Google Scholar] [CrossRef]
- Xie, Q.; Liu, X.; Zhang, Y.; Liu, G. Development and Characterization of a New Potato Starch/Watermelon Peel Pectin Composite Film Loaded with TiO2 Nanoparticles and Microencapsulated Lycium Barbarum Leaf Flavonoids and Its Use in the Tan Mutton Packaging. Int. J. Biol. Macromol. 2023, 252, 126532. [Google Scholar] [CrossRef]
- Colivet, J.; Garcia, V.A. dos S.; Lourenço, R.V.; Yoshida, C.M.P.; Oliveira, A.L. de; Vanin, F.M.; Carvalho, R.A. de Characterization of Films Produced with Cross-Linked Cassava Starch and Emulsions of Watermelon Seed Oils. Foods 2022, 11, 3803. [Google Scholar] [CrossRef]
- Guo, Z.; Wu, S.; Lin, J.; Zheng, H.; Lei, H.; Yu, Q.; Jiang, W. Active Film Preparation Using Pectin and Polyphenols of Watermelon Peel and Its Applications for Super-Chilled Storage of Chilled Mutton. Food Chem. 2023, 417, 135838. [Google Scholar] [CrossRef]
- Guo, Z.; Zuo, H.; Ling, H.; Yu, Q.; Gou, Q.; Yang, L. A Novel Colorimetric Indicator Film Based on Watermelon Peel Pectin and Anthocyanins from Purple Cabbage for Monitoring Mutton Freshness. Food Chem. 2022, 383, 131915. [Google Scholar] [CrossRef]
- Kelm, G.R.; Wickett, R.R. The Role of Fatty Acids in Cosmetic Technology. In Fatty Acids; Elsevier, 2017; pp. 385–404.
- Omoniyi, S. Nutrient and Anti-Nutritional Composition of Watermelon (Citrullus Lanatus) Seed: A Review. FUW Trends Sci. Technol. 2020, 5, 048–051. [Google Scholar]
- Baeeri, M.; Sarkhail, P.; Hashemi, G.; Marefatoddin, R.; Shahabi, Z. Data Showing the Optimal Conditions of Pre-Extraction and Extraction of Citrullus Lanatus (Watermelon) White Rind to Increase the Amount of Bioactive Compounds, DPPH Radical Scavenging and Anti-Tyrosinase Activity. Data Brief 2018, 20, 1683–1685. [Google Scholar] [CrossRef] [PubMed]
- Simamora, F.S.; Janice, J.; Amir, W.P. Effectiveness Test of White Skin Extract Cream from Red Watermelon (Citrullus Lanatus) on Increasing Elasticity, Sebum and Hydration in White Mice (Mus Musculus) Skin. Int. J. Health Pharm. IJHP 2023, 3, 322–330. [Google Scholar] [CrossRef]
- Patra, J.K.; Das, G.; Baek, K.-H. Phyto-Mediated Biosynthesis of Silver Nanoparticles Using the Rind Extract of Watermelon (Citrullus Lanatus) under Photo-Catalyzed Condition and Investigation of Its Antibacterial, Anticandidal and Antioxidant Efficacy. J. Photochem. Photobiol. B 2016, 161, 200–210. [Google Scholar] [CrossRef] [PubMed]
- Cefali, L.C.; Ataide, J.A.; Donegá, R.T.; Godoy, K. de M.S.; de AraÃojo, F.G.; Mazzola, P.G. Development of Emulsion Containing Watermelon Extract for Skin Aging. J. Med. Plants Res. 2017, 11, 749–754. [Google Scholar]
- Kamble, C.; Kamble, V. Formulation and Evaluation of Herbal Lipstick Using Lycopene Extracted from Citrullus Lanatus. Research & Reviews: A Journal of Pharmaceutical Science. 2021; 12 (3): 50–55p. Formul. Eval. Herb. Lipstick Kamble Al STM J. 2021, 2.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





