1. Introduction
Climate change, manifested as an increase in temperature and or episodes of temperature spikes during winter, and insufficient cold exposure during dormancy increases tree vulnerability to spring frosts, and threatens fruit production worldwide [
1,
2]. Freeze damage poses a significant ecological risk to stone fruit crops, and destructive spring frosts are projected to intensify due to global climate change [
3]. Peach (
Prunus persica [L.] Batsch) is a highly economically valuable temperate fruit tree that is widely cultivated in moderate climatic zones throughout the world and, therefore, affected by climate change. Peach trees exhibit a remarkable adaptation to seasonal climatic variations by experiencing a period of dormancy during the winter months [
4]. This evolutionary process involves shedding their leaves and entering a stage of reduced metabolic activity (endo-dormancy). Then, once trees have experienced adequate chilling temperatures, they are released from dormancy (eco-dormancy) and start developing and blooming in response to warm temperatures. Increasingly warmer winters, as well as weather patterns with more severe winter and spring temperature fluctuations, disrupt this normal pattern. Warmer winters can cause trees to bloom prematurely and expose their flowers or fruitlets to lethal freezing temperatures. This scenario has caused disastrous losses of the peach crop in the southeastern U.S. in recent years. Numerous research has focused on assessing the susceptibility of trees, buds, and flowers to freezing events [
5,
6,
7], but the fruitlet freeze tolerance is relatively understudied [
8,
9,
10].
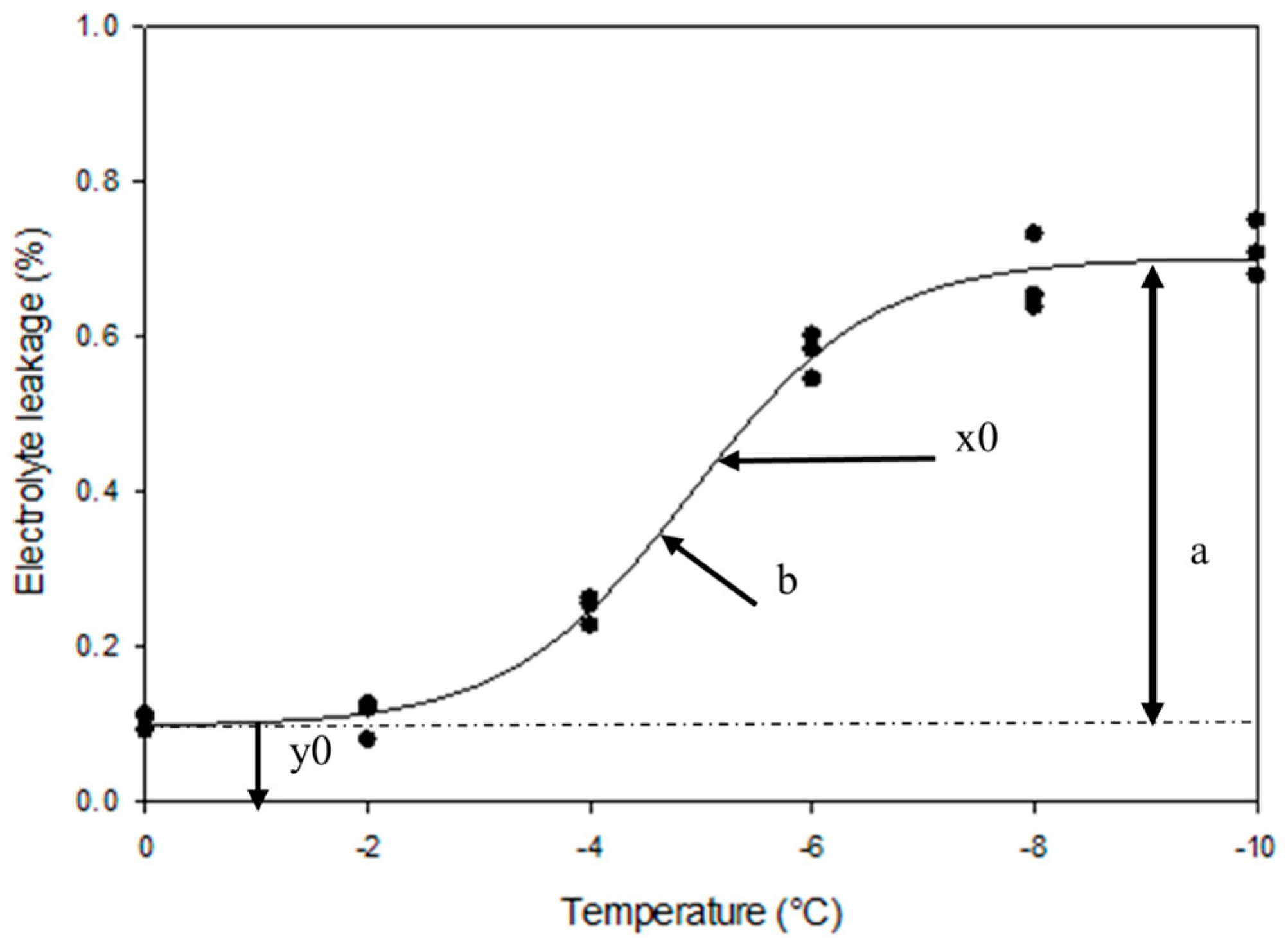
Various methods are available for assessing freeze damage, such as visual evaluation of tissue discoloration, thermal analysis, measure of electrolyte leakage, and triphenyl tetrazolium chloride reduction analysis, the selection and implementation of which mostly depend on the organ or tissue that is being assessed [
7]. Dexter et al. [
11] first noted that freezing temperatures led to the destabilization of cellular membranes and an accelerated release of symplastic solutes from cells, e.g., electrolyte leakage. The electrolyte leakage has evolved into a standard method for assessing the relative quantity of cell damage in many species in reaction to biotic and abiotic stresses, including cold stress [
12,
13,
14,
15,
16]. The electrolyte leakage, and temperature at which 50% of tissue experiences damage (LT50), used to evaluate fruitlet freeze tolerance in selected peach germplasm from the National Clonal Germplasm Repository (NCGR) in Davis, CA, and Clemson University [
10] revealed freeze tolerance to temperatures as low as -10 ºC, with majority of accessions exhibiting tolerance within -5 and -6 ºC. The highest tolerance, < -8 ºC, was observed in several cultivars from various breeding programs and released during last five decades (‘White Lady’, ‘Scarletpearl’, ‘Raritan Rose’, ‘Manon’, ‘MA Blake’, ‘Canadian Harmony’, ‘Harrow Diamond’, and ‘Sugar Giant’) suggesting that diversity for this trait in peach germplasm could be explored in breeding. However, the graphical presentation of the LT50 results revealed that the two accessions with similar LT50 values have distinctively different asymmetric sigmoid curve patterns. The authors suggested that further investigation is needed to determine how best to describe the level of freeze damage in the fruitlets. The research highlighted the possibility of harnessing the genetic potential for freeze tolerance in peach breeding to address the effect of changing climate and predicted more frequent late spring frosts on stone fruit production [
17,
18,
19,
20]. These findings suggested that breeding for improved fruitlet freeze tolerance in peaches might be possible.
The breeding goals in peach breeding programs have evolved significantly over the decades [
21,
22]. Initially, the focus was primarily on improving traits related to yield, disease resistance, and adaptability to specific growing regions. However, as consumer preferences and environmental concerns evolved, breeding goals shifted towards quality attributes, such as fruit flavor, appearance, and nutritional content [
23,
24]. Importance of breeding for climate resilience or plasticity became more emphasized with production disruptions caused by climate change. However, focus was on the dormancy related traits, such as lowering chilling requirement and delaying bloom time [
25,
26], and not fruitlet freeze tolerance.
Therefore, we expanded the Melgar et al. [
10] study by evaluating fruitlet freeze tolerance and heritability in modern peach breeding germplasm. Our hypothesis was that observed diversity in peach fruitlets’ ability to tolerate low temperatures is genetically controlled and can be used in breeding to incorporate fruitlet freeze tolerance in newly developed cultivars and provide recommendations to growers. The results of this study provide the foundation for further understanding the genetics behind this trait and support development of molecular tools to enable breeding of new peach cultivars with enhanced freeze tolerance.
3. Results
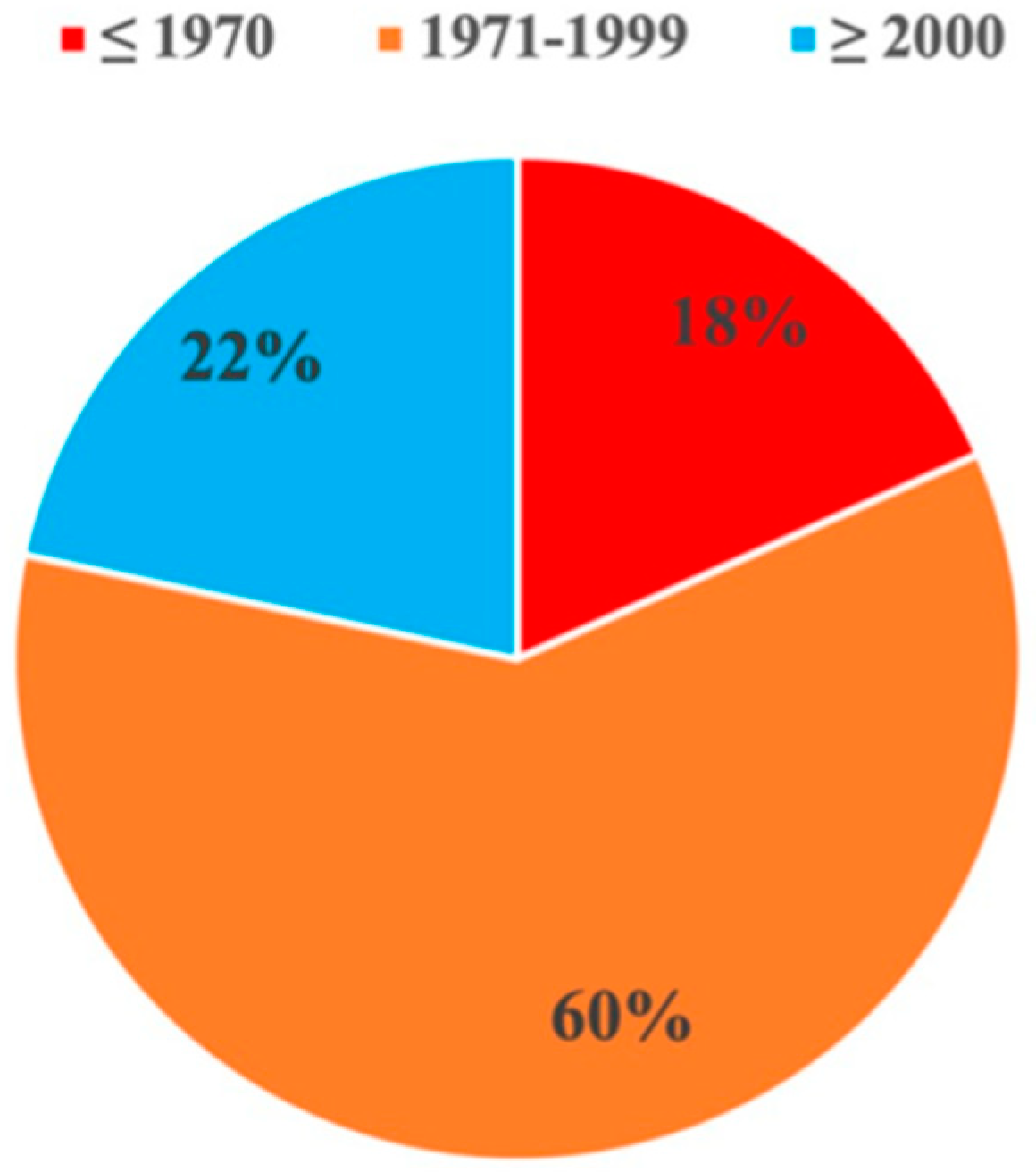
Diverse peach (66) and nectarine (9) germplasm, consisting of cultivars important for the southeast U.S. peach industry (heirlooms and cultivars patented within the last five decades) was evaluated for fruitlet freeze tolerance (
Table S1;
Figure 2). The majority of cultivars were released within the last three decades of the 20
th century (1971-1999). Some of them are still grown in the southeast U.S. and used as standards in regional trials or represent important breeding parents used in peach breeding programs. This germplasm is adapted to or evaluated for suitability in the southeast U.S. with chilling requirement ranging from 500 to over 1,000 CH, majority being in the 600-900 CH range, with bloom time from 62 – 80 JD and estimated minimum heat requirement from 1,362 – 7,039 GDH.
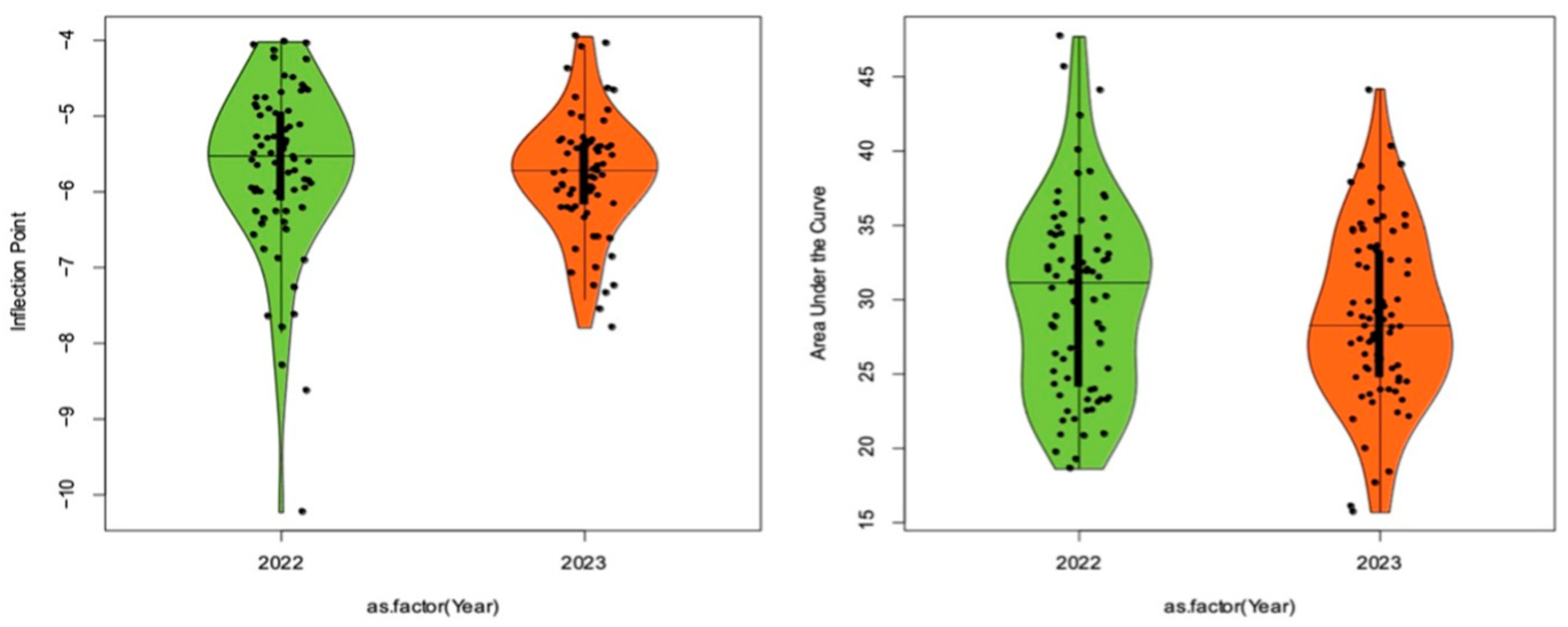
Peach germplasm showed variable fruitlet freeze tolerance, estimated with both inflection point (IP), the temperature at which 50% of the material shows sign of freeze damage, and % of freeze damage, estimated by the area under the sigmoid curve (AUC), in evaluated seasons (
Table 1;
Figure 3).
IP distribution was skewed towards lower tolerance in 2022 and did not exhibit normal distribution (Shapiro Wilk test; W = 0.91135, p-value = 6.484e-05) (
Figure 3 and
Figure S1). However, bimodal normal distribution (Shapiro Wilk test; W = 0.97354, p-value = 0.1385) was observed in IP2023 dataset. Percent of freeze damage, estimated by AUC, was normally distributed in both years with a higher median observed in 2022 than in 2023 season. The AUC distribution was wider, with a higher degree of variability, than the IP distribution.
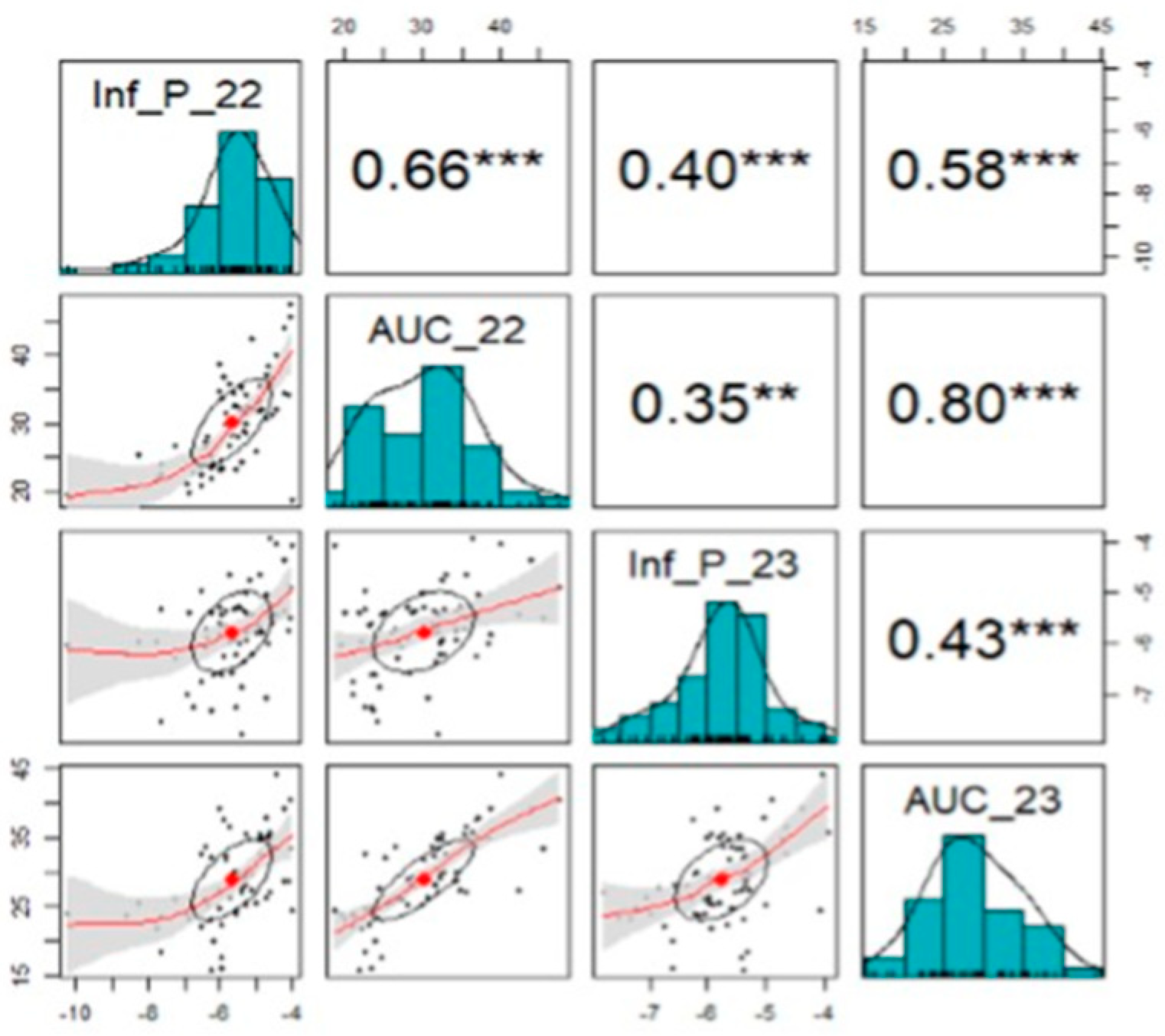
Highly significant positive correlation was observed among all datasets (
Figure 4). The lowest correlation was observed for IP between years (0.40), while the highest correlation was observed between AUC datasets (0.80) and IP2022 and AUC2022 (0.66) (
Figure 4).
IP ranged from -3.94°C (IP2022) to -10.22°C (IP2023), with similar overall average IP (IP_Ave = -5.72ºC) observed in both years (
Table 1). Significant differences between IP ranges in the two experimental years were observed, with a wider range (-4.02 to -10.22 ºC) observed in IP2022 and a narrower range (-3.94 to -7.79 ºC) in IP2023 dataset. The highest negative temperature at which fruitlet damage was observed was -3.94ºC, in peach ‘Sweetstar’, in IP2023, and the lowest temperature at which the highest fruitlet tolerance was observed was -10.22ºC in nectarine ‘Arctic Pride’ in IP2022. The remaining accessions fell within the range of freezing temperatures between these two extremes with the majority showing fruitlet tolerance in -4 to -6ºC range in both datasets (
Table S1;
Figure S2 and
Figure S3).
Overall AUC in all peach and nectarine accessions ranged from 15.75% in 2023 to 47.76% in 2022 (
Table 1). AUC was normally distributed (Shapiro Wilk test; W = 0.96973, p-value = 0.06932 in 2022; 0.98839, p-value = 0.7603 in 2023) in both years and showed bimodal distribution (
Figure 2). The AUC interval was similar in the two experimental years, 29.06% and 28.41% in 2022 and 2023, respectively (
Table 1). The lowest AUC was calculated in 2023, with 13.08% less fruitlet damage than the average, while the highest freezing damage was estimated in AUC2022, 17.64% higher than the mean (
Table 1). The lowest estimated damage, 15.75%, was observed in ‘Glory’ in AUC2023, while the highest damage was detected in fruitlets of ‘Julyprince’ in AUC2022. Commercial peach cultivars Julyprince, June Gold, Rich Joy, and Caroking exhibited high degree of susceptibility to freeze tolerance, with AUC values of 47.7, 45.6, 44.1, and 40.1%, respectively (
Table S1). Interestingly, the same cultivars had the lowest IP range, - 4.03 and - 4.45 °C.
The highest fruitlet resilience to freezing temperatures according to average IP (< -7ºC) was observed in nectarines ‘Arctic Pride’ and ‘Summerfire’ and peaches ‘Brightstar’, ‘Sweet September’ and ‘Summerprince’. However, the highest fruitlet resilience to freezing temperatures predicted by average AUC was in four peach cultivars Glory, Early Augustprince, Parade and Redglobe, and four nectarines ‘Actic Blaze’, ‘Silver Gem’, ‘Arctic Pride’ and ‘Juneprincess’.
Broad sense heritability of 0.52 and 0.85, was estimated with IP data and AUC data from both seasons (
Table 1).
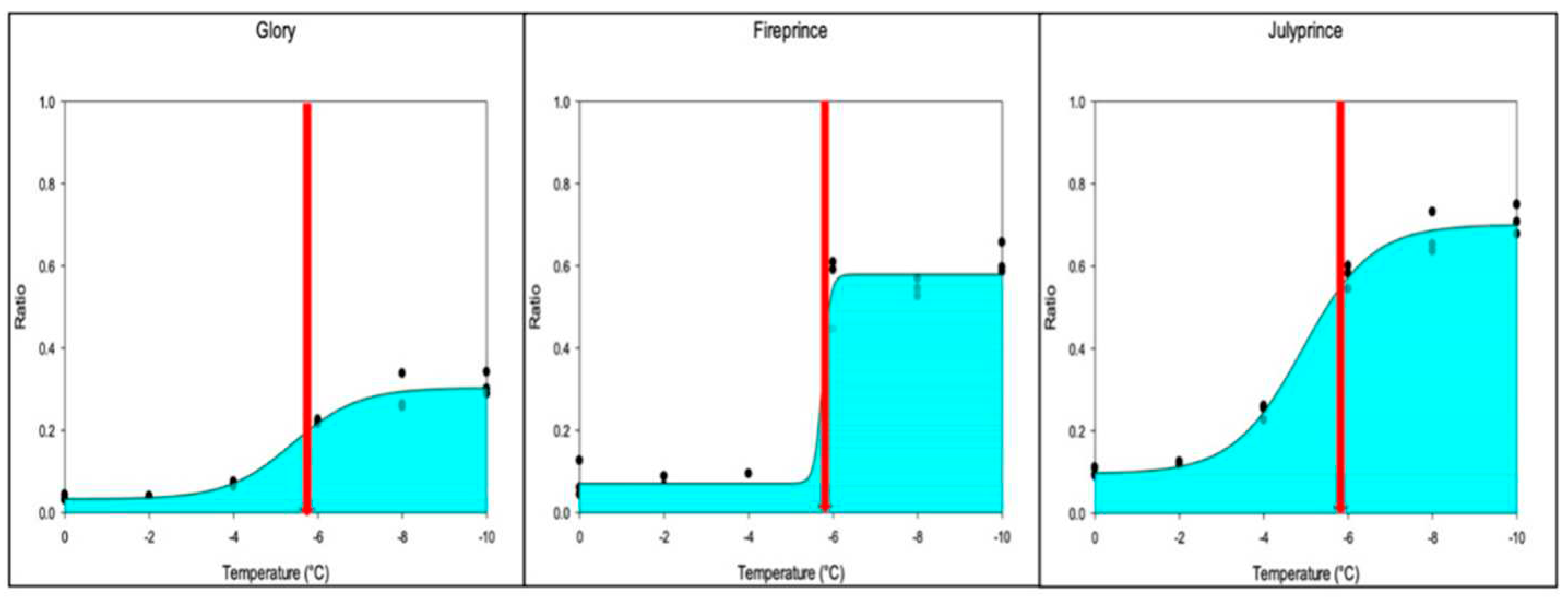
Fruitlet freeze tolerance estimated by IP did not accurately represent the divergence and steepness of the sigmoid curve (
Figure 5). For example, the same IP of approximately -5.9ºC was observed in ‘Glory’, ‘Fireprince’, and ‘Julyprince’, but the actual temperature when fruitlets started to experience damage differed (
Figure 5;
Table S1). Fruitlets of ‘Julyprince’ began to show damage at -2ºC and ‘Glory’ at -4ºC while the fruitlets of ‘Fireprince’ did not show any freeze damage until close to -6 ºC. The AUC observed in the three cultivars ranged from 22% in ‘Glory’, 29% in ‘Fireprince’ to 47% in 'Julyprince', suggesting ‘Glory’ had the highest fruitlet freeze tolerance and ‘Julyprince’ the lowest. However, evaluation of the divergence and steepness of the sigmoid curve showed that the electrolyte leakage in fruitlets of ‘Fireprince’ did not begin until -6ºC which is two degrees lower than that observed for the fruitlets of ‘Glory’.
Grouping the accessions in tolerance groups (TG) based on IP classified 26.67% of material as least (TG3) and most tolerant (TG1) and 46.67% in the intermediate group (TG2) in 2022 (
Table S1). A similar grouping was observed with IP2023 data with 28% characterized as most tolerant (TG1), 54.6% as intermediate (TG2) and 12% as least tolerant (TG3). While most cultivars and selections maintained the group in both datasets, some discrepancies were observed. The most apparent change was from extreme groups to intermediate, with the most extreme change observed for peach cultivar ‘Sweetstar’ that moved from TG1 in IP2022 to TG3 in IP2023. The most tolerant group based on the IP2022 data consisted of cultivars released in the last century with few heirlooms such as ‘Redrose’ and ‘Parade’ released in 1940 and 1960, respectively, and three newer releases, two nectarines from University of Arkansas, ‘Westbrook’ and ‘Arrington’ released in 2002, and one peach from USDA, Byron, GA, ‘Augustprince’ released in 2006. Interestingly, seven nectarine cultivars, Arctic Belle, Arctic Blaze, Arctic Pride, Arrington, Silver Gem, Summer Fire, and Westbrook, were grouped in the most tolerant group based on IP2022, with five of them (Arctic Belle, Arctic Pride, Silver Gem, Summer Fire, and Westbrook) being classified in the same most tolerant group in 2023. Selections from the CUPBP were mostly grouped in TG2 (75 and 73% in 2022 and 2023, respectively) with SC-12 being the most tolerant in both 2022 and 2023.
Similarly, grouping of peach and nectarine accessions based on the AUC values revealed majority of material in intermediate group TG2 (50 and 53% in AUC2022 and AUC2023, respectively), and 28 and 26% of accessions in most tolerant (TG1), and 22 and 17% in the least tolerant (TG3) group in AUC2022 and AUC2023 datasets, respectively (
Table S1). Most accessions maintained their group regardless of season with few changing from most (‘Arrington’ and SC-8) or least (‘June Gold’, ‘Loring’ and ‘Cresthaven’) tolerant to intermediate. Similar to IP grouping, AUC grouping classified all nine nectarines as most tolerant (TG1) in both years, except ‘Arrington’ which moved to TG2 in 2023 (AUC=27%), with 5% difference in AUC between the two seasons and only 2% over the group threshold. Two CUPBP selections SC-8 and SC-7 were in the most tolerant group in 2022 with SC-7 in 2023 having AUC of 27%, 2% above the threshold for the TG1. The CUPBP selection SC-12 was grouped as intermediate in both AUC2022 and AUC2023 datasets, with 2 and 0.86%, respectively, above the threshold of the TG1.
4. Discussion
We have evaluated fruitlet freeze tolerance in the peach germplasm important to the southeast U.S. peach industry. This research addressed the pressing demand for a standardized phenotyping protocol to assess the freeze tolerance of developing fruitlets, a trait of critical importance to temperate fruit production.
Fruitlet freeze tolerance observed in the peach germplasm evaluated in this study was variable across seasons (2022 and 2023) and traits (IP and AUC), with AUC exhibiting better stability. Commercial peach cultivars Julyprince, June Gold, Rich Joy, and Caroking exhibited high degrees of susceptibility to freeze tolerance, with AUC values of above 40% (
Table S1). Furthermore, these cultivars had the lowest IP range, - 4.03 and - 4.45°C, close to the critical temperature (~ -3ºC) for causing damage to fruitlets [
30]. Cultivars Arctic Pride, Rubyprince, Summerfire, and Sweet September exhibited remarkable tolerance to freezing conditions, as evidenced by their significantly reduced ion leakage in comparison to other cultivars (19-27%) and the temperature at the IP close to -10°C (
Table S1). This suggested that fruitlets of these cultivars have a high tolerance to low temperatures which is in agreement with previous reports [
10].
Lower correlation between IP datasets posed difficulty in comparing data from different studies. However, the values were similar when the data from Melgar et al. [
31] were used to calculate AUC.
The outcomes of this study indicate that fruitlet freeze tolerance in peach germplasm shows a diverse range of responses, with IP values ranging from -3.94°C to -10.22°C. Furthermore, the accessions exhibited varying degrees of vulnerability to freeze tolerance, with AUC values ranging from 16% to 48%. While both approaches were effective in distinguishing differences in fruitlet freeze tolerance, it is worth noting that the IP value was most variable and vulnerable to methodological error. The observed difference in IP values could be attributed to variation in fruitlet sampling due to slight differences in fruitlet size and or timing between sampling and analysis that could affect healing of the scar after fruitlet detachment from the branch, thus, increasing electrolyte leakage and skewing the results. Another important point is the steepness of the curve. When the slope is highly inclined, most fruits are damaged simultaneously. In opposition, on a gentler slope, some fruits may be damaged sooner, while others may be affected later. The timing of damage to specific fruits on a less steep slope can also be crucial in determining overall crop success. The difference of just one degree in tolerance in peach production could mean having the peach crop or not, so it is important to further describe the fruitlet freeze tolerance. Even though the AUC was obtained from sigmoid curve developed using IP data, this study showed that AUC approach reduces data variability and, therefore, might be more adequate for predicting cultivar’s response to freezing temperatures. This is also supported by the tolerance group assignment, as less group assignment mismatches were observed for AUC than IP values. Furthermore, the mismatches observed in the AUC group assignment were on the borderline of the TG threshold.
Interestingly, nectarine fruitlets showed high tolerance in both seasons suggesting that the nectarine fruitlet morphology or absence of pubescence might influence fruitlets susceptibility/tolerance to freezing temperatures. Single gene mutation from pubescent (
G-) to glabrous (
gg) skin on chromosome 5 is the difference between the peach and nectarine phenotype [
32,
33]. Other subtle differences in the flesh density and texture between peach and nectarine are suggested and speculated to be attributed to the pleiotropic effect of this single gene mutation, but the research documenting them is lacking. Increased tolerance to freezing temperatures in nectarines observed in this study might be explained by the morphological characteristics of the fruit tissues, e.g., lower water content in the nectarine fruit tissue than in peach [
34]. Formation of both intercellular or intracellular ice crystals can lead to cell death. However, grape study on freeze damage during spring suggested that damage was caused by the formation of intercellular rather than intra-cellular ice [
35]. Generation of a water vapor gradient between the interior and exterior of cells directly contributes to intercellular ice crystal formation. Furthermore, cells become dehydrated as water moves from the interior to the exterior of the cell and accumulates on intercellular ice crystals, causing a loss of turgor [
36]. To confirm if this contributed to the higher tolerance predicted in nectarine fruitlets further studies are needed.
Absence of fuzz or trichomes might be another reason for nectarine fruitlets exhibiting higher tolerance to low temperatures. However, studies mostly focused on the trichome morphology and differences between peach and nectarine but never on other aspects of fruit morphology [
37]. It would be interesting to explore this line of thought and determine the cause of higher tolerance to freeze in nectarine fruitlets in laboratory experiments, especially since our field observations do not support this difference between peach and nectarine fruitlet tolerance to freeze nor it was reported in the literature [
38]. The lab data suggests significant differences between peach and nectarines, thus, expanding the fruitlet freeze evaluation to include more nectarine cultivars might help explain observations made here. In addition, it is important to emphasize that electrolyte leakage method, used in this study, is only a way to compare the accessions for their tolerance to low temperatures and do not necessarily reflect temperatures in field. Field freeze tolerance data for the same material will be needed to validate the accuracy of the lab prediction.
However, the ability to characterize peach and nectarine cultivars for potential of their fruitlets to tolerate low temperature in spring and assign them to tolerance groups outweighs the shortcomings of this assessment. Tolerance group assignment, based on either the IP or AUC, could be valuable information for advising growers about cultivars’ young fruit ability to tolerate low temperatures. This information can be included when choosing the cultivars to plant in areas more prone to freezing, or consulted for arranging freeze protection (e.g., wind machines, irrigation) especially when limited resources to reduce the impact of late spring (radiation) frosts are available. Change in just a few degrees could mean a difference between full production or a total crop loss. Thus, results of this study may provide crucial piece of information on climate resilience in peach and nectarine cultivars for growers and county agents that contributes to minimizing possible economic damage due to low spring temperatures. This information can also be useful to breeders that are developing climate resilience as an important trait.
The observed variation of IP among cultivars suggested that both IP and AUC would be beneficial for estimating fruitlet freeze tolerance through multiple conditions or time. Increased frequency in occurrence of spring frosts all over the world is putting emphasis on adding this trait in breeding efforts for climate resilience. It is crucial to conduct more in-depth investigations into the influence of climate characteristics of plants. The peach crop losses the southeast U.S. industry endured in the last two decades, indicate that satisfaction of chill requirement and increasing heat requirement to delay bloom are no longer enough to ensure sustainability of peach production in the changing climate. Thus, fruitlet freeze tolerance should be included in the suite of traits when breeding for climate resilience. Furthermore, broad sense heritability estimated in this study supports genetic control of the fruitlet freeze tolerance in peach and nectarine germplasm and potential for improvement of this trait via breeding. This is the first extensive study into the peach fruitlet freeze tolerance that lays foundation for further investigation into the genetic control of this trait.