1. Introduction
Annually, approximately 15 million infants are born prematurely around the world [
1]. Although advanced perinatal care has led to a decrease in the mortality rate among preterm infants in recent years, the incidence of developmental morbidity remains notably high [
2]. Long-term follow-up studies conducted on preterm infants have revealed significant developmental disorders such as cerebral palsy, hearing impairment, visual deficits, and growth delays [
3]. According to a recent study, over 25% of neonates born between 28 and 32 weeks of gestation exhibit developmental disorders by the age of 2 [
4]. Neurological care involves employing strategies aimed at averting neuronal cell death. [
5]. These interventions are designed to safeguard the developing brain and mitigate neuron loss following detrimental events while enhancing their function by establishing new communication pathways. The vulnerability of the infant's brain increases as its maturity decreases, making neurological care all the more crucial [
6]. To mitigate these complications, researchers have proposed and applied diverse methods over the past few decades. Many of these approaches involve developmental interventions or care for infants admitted to the Neonatal Intensive Care Unit (NICU). Developmental care methods aim to modify the NICU environment to reduce stress, enhance behavioral organization, improve physiological stability, maintain sleep patterns, and foster neural growth and infant maturation [
7,
8]. Beyond medical care services, there arises a need for diverse physiotherapy techniques aimed at promoting the typical maturation and growth of sensorimotor organization while minimizing risks that could adversely impact the baby's neurodevelopmental progress. These physiotherapy approaches, designed to enhance the baby's neuromuscular development and reduce stress and discomfort, encompass techniques such as protecting natural joint movement, appropriate positioning, massages, fostering holding patterns, timely gravity-resistant exercises, and oral motor training [
9].
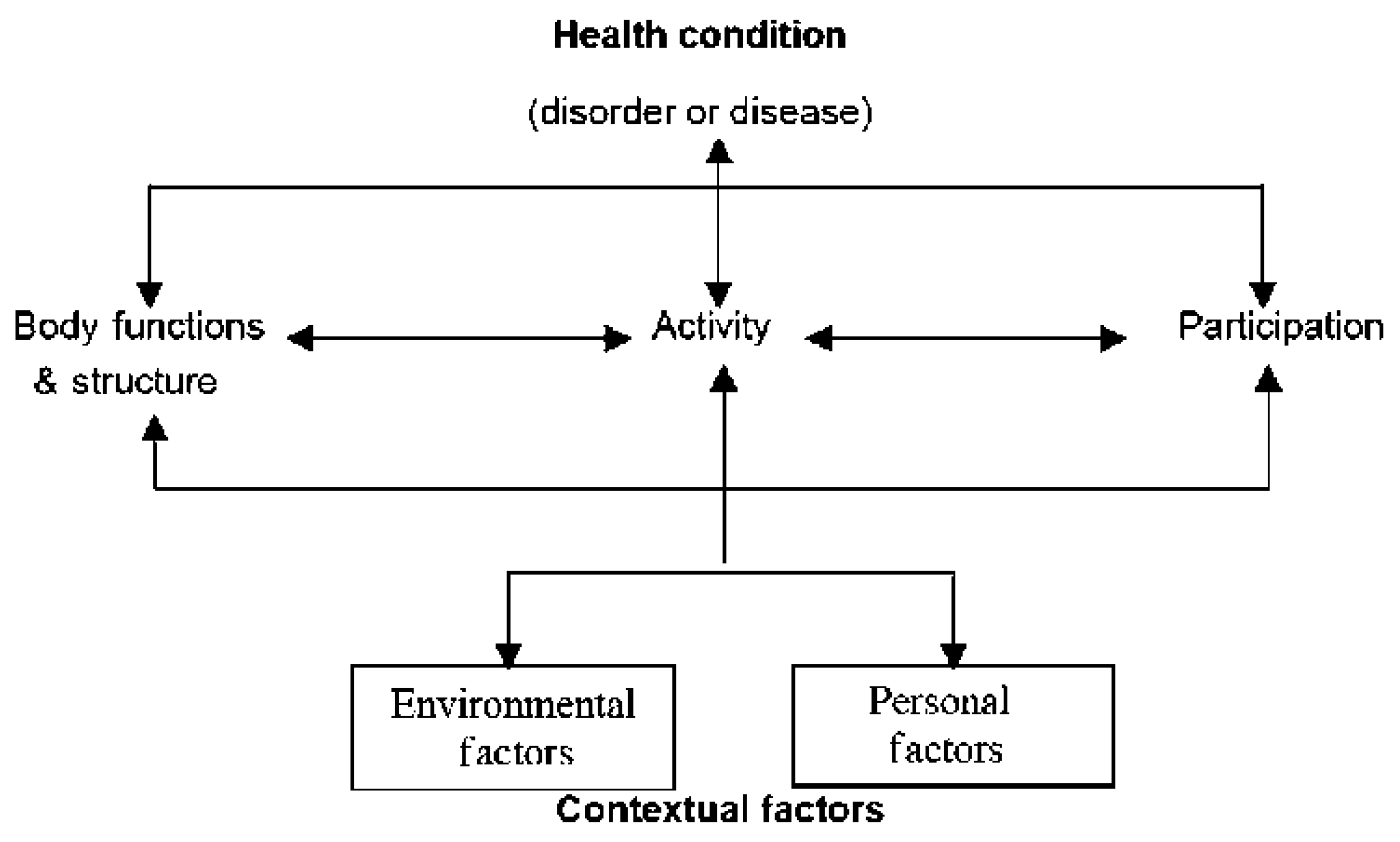
The International Classification of Functioning, Disability, and Health (ICF) framework and its Children and Youth Version (ICF-CY), as put forth by the World Health Organization (WHO), draw from various perspectives on human development. They embody a biopsychosocial approach that recognizes the interplay between Health Conditions, Contextual Factors (including Environmental and Personal Factors), Body Functions and Structures, as well as Activity and Participation. Even though the ICF-CY was introduced in 2007, following the ICF, it shares an identical framework and concept with the ICF, including the same chapters or first-level categories (
Figure 1). The primary distinction between the two lies in the ICF-CY's incorporation of additional or revised lower-level categories, specifically designed to capture changes associated with growth and development [
10,
11]. One potential clinical application of the ICF-CY is to use it as a framework to describe the impairments in body functions and structures, limitations in activities and participation, and the environmental barriers experienced by children with developmental delays and disabilities. This approach provides a comprehensive view of the child's health condition and the factors affecting their well-being [
12]. We aimed to examine whether the one-month NICU physiotherapy intervention could lead to improved outcomes, employing the ICF biopsychosocial model for assessment.
2. Materials and Methods
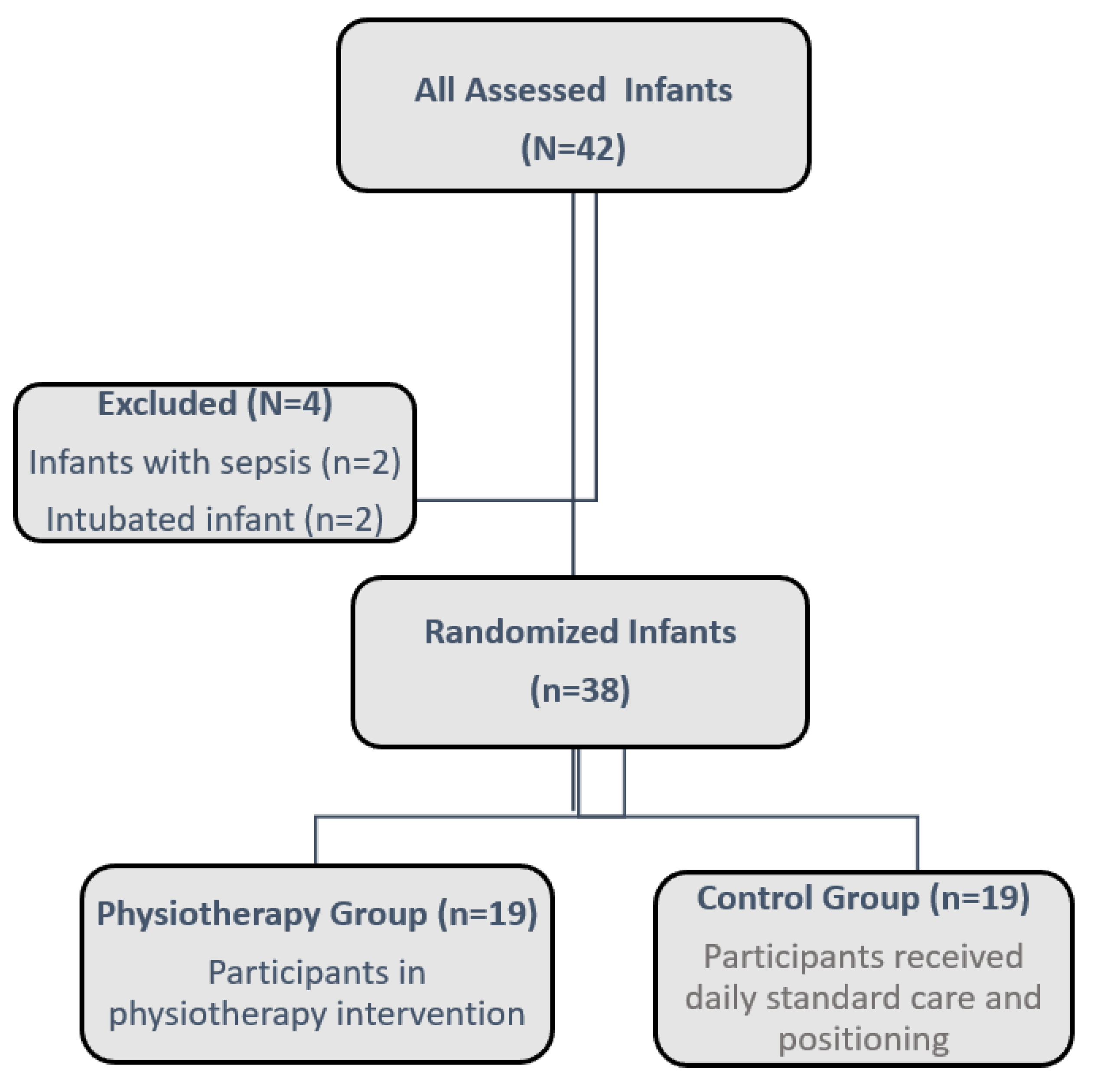
This randomized-controlled study consisted of preterm infants born at 30 weeks of gestational age or earlier who were admitted to the NICU at Ondokuz Mayıs University Health Practice and Research Center from December 2021 to May 2023. Preterm infants followed in the NICU and whose vital signs were stable were assigned to two groups, as physiotherapy group and the control group. The intervention commenced once the infant's vital signs stabilized, around the 31st week of post-conceptional age. A centralized web-based randomization system was employed to facilitate the randomization process. Infants were allocated to either the physiotherapy group or the control group, as illustrated in
Figure 2.
Infants with major congenital abnormalities, invasive mechanical ventilation, sepsis, and NEC were excluded from the study. The Physiotherapy group received 25-30-minute sessions 3 days a week for 1 month including massage of the mouth and swallowing muscles, mobilization exercises in all positions for the whole body, weight-bearing exercises to the body parts, and massage therapy for the extremities. Daily standard care and supine, prone, and side-lying positions were applied to the control group.
All procedures were conducted according to the principles outlined in the Declaration of Helsinki. Additionally, all protocols were approved by the Ethics Committee of Ondokuz Mayis University (2021/608). The data were collected after informed parental consent and written informed consent was gathered from all participants.
Infants were included in intervention programs after around the first month of life in which there were major health problems. After obtaining the demographic information of the infants in the first evaluation, the baby's vital signs were recorded; in the second evaluation phase, after one month of physiotherapy, the infant's vital signs, motor skills, and feeding skills were evaluated. We made the same evaluation procedure for the control group. After the vital signs were stable of the infants in the control group we waited for one month and made evaluations for each baby. In the control group, the infants did not receive physiotherapy and only got daily standard care and positioning.
Sociodemographic data of the infants, including sex, gestational age, postnatal age, Apgar score, birth weight, mode of delivery, maternal birth information, and parental information such as age, occupation, and educational status, were documented. Vital signs, encompassing oxygen saturation, blood pressure, and heart rate, were also recorded. Changes in the baby's height and weight were monitored, and their growth and development were closely tracked and saved.
Test of Infant Motor Performance (TIMP)
The TIMP (Test of Infant Motor Performance) is capable of detecting changes in motor development at two-week intervals, spanning from 34 weeks Post Menstrual Age to five months of Corrected Age (CA) [
13,
14]. The TIMP evaluated various aspects of movement and postural control, including assessments in prone, supine, supported sitting and standing positions [
14].
The Dubowitz neurological assessment of the preterm and full-term infant- Dubowitz
The Dubowitz Scale for Neurological Assessment of Preterm and Full-term Infants ([
15]) is a scale that has been standardized for preterm infants as well as full-term infants, whose item validity and reliability have been demonstrated and is frequently used in the neurological examination of newborn infants [
16,
17].
Preterm Oral Feeding Readiness Assessment Scale (POFRAS)
POFRAS, developed by Fujinaga et al. [
18], is a tool designed to assess readiness for oral feeding in preterm infants.
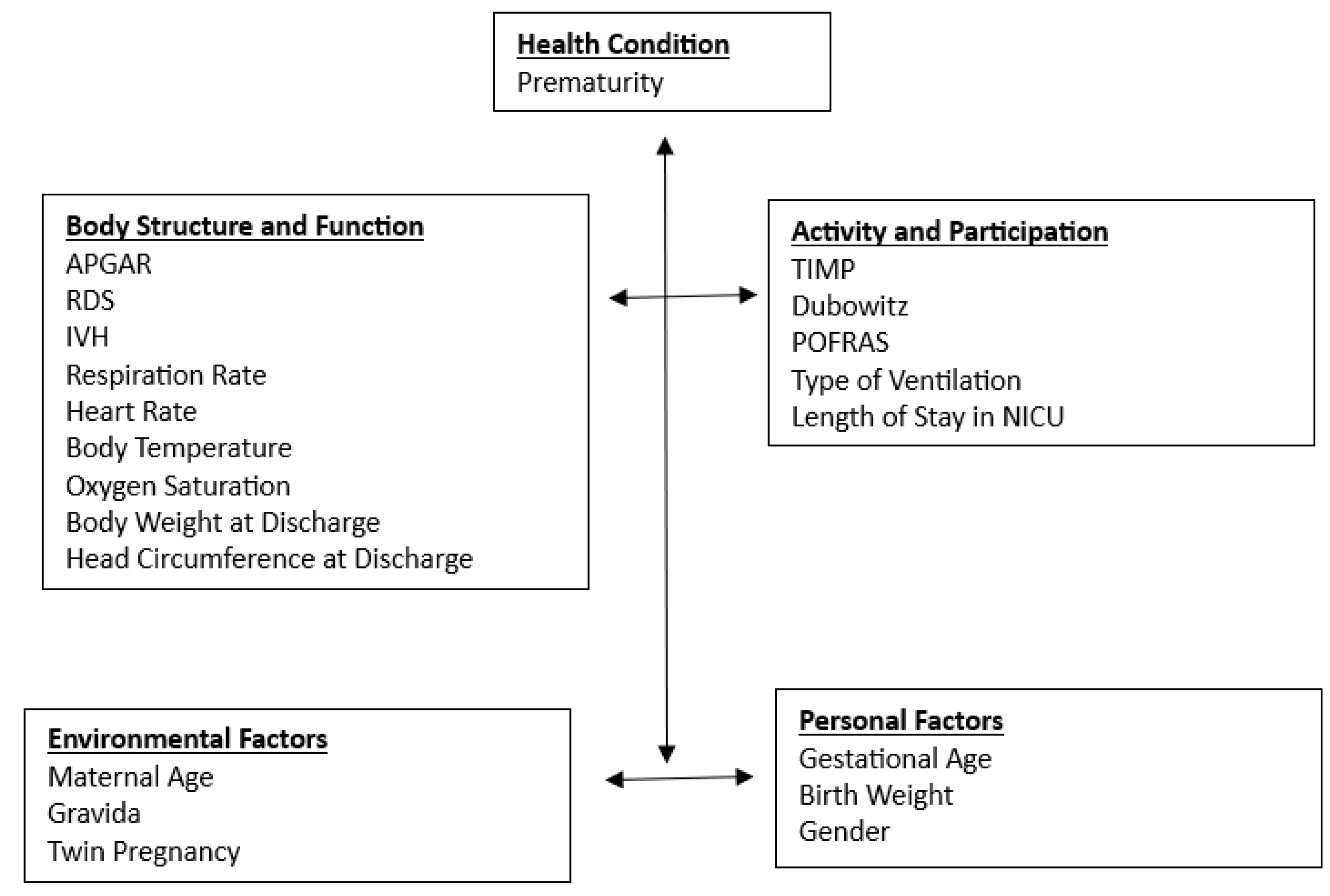
An outcome model based on the ICF-CY framework
Among the components of the ICF-CY, Activity and Participation is considered the most important outcome for young children ([
19]). Young children’s general development was assessed with a comprehensive developmental test, designed to capture the ICF-CY component of Activity and Participation ([
20]). In our study, we have used Dubowitz, TIMP, and POFRAS to identify the Activity and Participation of the Infants. Health Condition, Body Function and Structures, Environmental Factors, and Personal Factors were identified as the multiple predictors of children’s developmental outcomes [
19]. For measuring Health Condition in the NICU, premature birth was identified as one of the indexes. For Body Functions and Structures, many studies have demonstrated the impact of movement-related factors on developmental outcomes, such as sitting balance, muscle power function, muscle tone function, seeing function hearing functions, and attention [
21]. In our study, we used birth weight, and gender for personal factors, RDS, IVH for body functions and structures, maternal age, and twin pregnancy for environmental factors (
Figure 3).
Sample Size Calculation
The sample size was calculated using PASS 2005 software (NCSS, Kaysville, UT, USA), found that 15 subjects were required for one group to achieve 90% power with a 5% type 1 error. To account for a potential 20% dropout rate, we recruited 19 subjects for each group, aiming to maintain 90% power in the study.
Statistical Analysis
The test results were presented as mean ± standard deviation, median, and minimum-maximum values. To decide on the appropriate statistical methods for comparing the study groups, the homogeneity (Levene's Test) and normality (Shapiro-Wilk) tests were used. If the groups were normally distributed and exhibited homogeneous variances, comparisons between two groups were conducted using the Student's t-test, while comparisons within dependent groups were done using the Paired t-test. However, some variables did not meet the parametric test assumptions, so comparisons between two independent groups were performed using the Mann-Whitney U test, and comparisons within dependent groups were conducted using the Wilcoxon test. For categorical data analysis, Fischer's Exact Test and Chi-square test were employed. When the number of cases was expected to be less than 20% of cells for inclusion in the analysis, the "Monte Carlo Simulation Method" was used to determine the values. All statistical analyses were carried out using SPSS software (IBM Corp. Released 2017. IBM SPSS Statistics for Windows, Version 25.0 Armonk, NY: IBM Corp.). A p-value of < 0.05 was considered statistically significant.
3. Results
Thirty-eight infants (19 infants in the physiotherapy group and 19 infants in the control group) were enrolled in the study. Mean gestational age and birth weights were 29.03±1.26 weeks, 1299.15± 318.98 grams, respectively. There were no statistical differences between the two groups in terms of gestational age, birth weight, gender, 1st and 5th-minute Apgar scores, maternal age, gravida, multiparity, respiratory distress syndrome, and intraventricular hemorrhage (p>0.05) (
Table 1). The results are presented as the domain of ICF in the tables.
The starting time for the interventions of the infants for both groups, length of stay in the NICU, Respiration rate, Heart Rate, Body temperature, Oxygen saturation, Body weight on the evaluation day, Body Weight at Discharge, and Head circumference at discharge were similar between the physiotherapy and control group (p>0.05) (
Table 2). The TIMP, Dubowitz Optimal Score, Type of Non-Invasive Ventilation, TIMP Range, and POFRAS scores were statistically significant in the physiotherapy group rather than the control group (p<0.05) (
Table 2).
4. Discussion
This study represents one of the initial clinical trials aimed at examining the effectiveness of a one-month physiotherapy intervention for early preterm infants in the NICU who are at risk of neuromotor delay, within the context of the ICF framework.
Our objective was to implement the intervention within the NICU to capitalize on the considerable neuroplasticity that exists during early infancy [
22,
23], although this may be influenced by infant medical stability and length of stay. A recent systematic review, which assessed motor development interventions for preterm infants, whether initiated during their hospital stay or afterward, concluded that interventions emphasizing the infants' active movements in various positions proved to be the most effective in improving motor skills from birth up to 24 months CA [
24]. Although the impact waned as time passed, at 3 months CA, motor-specific interventions exhibited a substantial and noteworthy effect size on motor skills. The majority of these interventions encompassed developmental assistance for the infant as well as guidance and education for parents. Furthermore, there are parallels in both the activities and the underlying theoretical framework between the previous and the current intervention. Our findings showed beneficial outcomes in the motor and feeding skills of the preterm infants. This indicates that there exists a potential timeframe in which providing optimal support for adaptive development is most effective. Infants who received the intervention demonstrated positive improvements in developmental assessment and imaging metrics, thereby endorsing the findings of previous literature which propose that early physical therapy interventions may be advantageous for overall development [
25,
26,
27]. These findings justify the need for additional research involving larger study cohorts to systematically evaluate the differential effects of an intervention as compared to standard care.
In contrast to other intervention studies focusing on specific populations of high-risk infants [
25,
28,
29,
30,
31], we recruited infants with high-risk levels because of their very early prematurity that all infants were born at 30th or under 30 weeks of gestational age. All the infants had nearly the same medical condition and were at the same risk of neuromotor delay [
32]. This transdiagnostic approach enabled us to pinpoint all the infants who derived benefits from early therapeutic intervention.
The research conducted by Girolami and Campbell [
33] revealed that the intervention had no adverse effects on weight gain, and it did not result in an increase in apnea or heart rate changes during the intervention. Our study also has similar results between the groups in terms of length of hospital stay, heart rate, body temperature, oxygen saturation, body weight, and head circumference at discharge. We can conclude that such vital signs are the infants’ body functions and structure due to the child's medical factors and are not affected by physiotherapy intervention.
The majority of physiotherapy studies conducted in the NICU involved training parents to administer the intervention, which resulted in improved scores for infants. Additionally, research indicates that maternal interventions, such as baby massage and skin-to-skin care in the NICU, have positive effects on developmental outcomes [
34,
35,
36]. It has been suggested that optimizing parent-child interactions and the infant's environment can be protective and supportive of the infant's development and competence [
35,
37,
38]. In contrast to these previous studies, our study exclusively involved physiotherapists administering the intervention, with a focus on demonstrating the outcomes within the ICF framework. Future research could involve the inclusion of the infants' families to explore the effects within the ICF framework.
Pan et al. [
12] created an ICF-CY code set for early retardation and disability for the first team assessment for infants under three years of age. They created a code set of 82 ICF-CY categories to describe the functional status of infants with developmental delays younger than three years of age. Of these, 28 included activities and participation. The category distribution in activities and participation reflected the fact that major functional changes and development during infancy are related to learning, communication, and mobility. These categories are also the areas in which general difficulties are addressed for infants with developmental delays [
39,
40]. Hwang et al. [
21] evaluated 122 infants at birth, 4 months, 6 months, and 2.5 years of age. They reported that among the components of ICF-CY, activities and participation were considered the most important outcomes for children. Fonseca et al. [
41] evaluated 35 premature infants at 38 weeks and 12 months corrected. Similar to our study, they evaluated gestational week, CA, and gender as personal factors, activities, and participation with Ages and Stages Questionnaire-3 which evaluates motor functions, family-related conditions, and physiotherapy as environmental factors.
The most important limitation of our study is that we could not follow up with the babies. Since our NICU is a large center that consists of level 4 to level 2 and accepts many out-of-town babies, most of the babies did not come for follow-up. Future studies must follow the development of the babies.
5. Conclusions
In conclusion, touching, targeted handling, facilitation, and weight bearing to the body parts during exercises while the infants were in the NICU may have offered infants an enhanced understanding of the amount of physical stimulation. Ultimately, this may have enabled the physiotherapists to give infants a more stimulating environment in the NICU, perhaps continuing to pursue developmental activities throughout the first two years of the infant's life. In addition, the realities of the country and the psychosocial aspects that concern families should also be taken into consideration when evaluating infant development. Beyond clinical conditions, the environment also has a great influence on child development [
41]. Our most important goal should be to minimize the functional limitation of neuromotor loss of life in high-risk premature infants in clinics, by performing a holistic physiotherapy approach to the baby, and by evaluating the biopsychosocial development of the baby to provide an independent, productive, enjoyable, quality in their life. For this, it is important to create an approach that includes adequate time, appropriate and adequate team, communication, information exchange, and social solidarity between team members (health team- education team).
Author Contributions
For research articles with several authors, a short paragraph specifying their individual contributions must be provided. The following statements should be used “Conceptualization, N.C.B., S.T. and M.A.A.; methodology, N.C.B., S.T. and M.A.A.; investigation, N.C.B., S.T. and M.A.A.; writing—original draft preparation, N.C.B., S.T. and M.A.A.; writing—review and editing, N.C.B., S.T. and M.A.A.; visualization, S.T. and M.A.A..; supervision, S.T. and M.A.A.; project administration, S.T. and M.A.A. All authors have read and agreed to the published version of the manuscript.”
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board (or Ethics Committee) of Ondokuz Mayis University Ethics Board (protocol code 2021/608) for studies involving humans.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available in article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Liu L, Oza S, Hogan D, Chu Y, Perin J, Zhu J, Lawn JE, Cousens S, Mathers C, Black RE. Global, regional, and national causes of under-5 mortality in 2000-15: an updated systematic analysis with implications for the Sustainable Development Goals. Lancet. 2016 Dec 17;388(10063):3027-3035. 3027. [CrossRef]
- Soleimani F, Zaheri F, Abdi F. Long-term neurodevelopmental outcomes after preterm birth. Iran Red Crescent Med J. 2014 Jun;16(6):e17965. [CrossRef]
- Saigal S, Doyle LW. An overview of mortality and sequelae of preterm birth from infancy to adulthood. Lancet. 2008 Jan 19;371(9608):261-9. [CrossRef]
- Johnston KM, Gooch K, Korol E, Vo P, Eyawo O, Bradt P, Levy A. The economic burden of prematurity in Canada. BMC Pediatr. 2014 Apr 5;14:93. [CrossRef]
- Graven SN, Browne JV. Sensory development in the fetus, neonate, and infant: introduction and overview. Newborn Infant Nurs Rev, 2008;8(4):169–72. [CrossRef]
- Altimier, LB. Neuroprotective core measure 1: the healing NICU environment. Newborn Infant Nurs Rev, 2015;15(3):91–6. [CrossRef]
- Altimier L, Phillips R. The neonatal integrative developmental care model: advanced clinical applications of the seven Core measures for Neuroprotective family-centered developmental care. Newborn Infant Nurs Rev. [CrossRef]
- Sizun J, Westrup B. Early developmental care for preterm neonates: a call for more research. Arch Dis Child Fetal Neonatal Ed. 2004 Sep;89(5):F384-8. PMID: 15321953; PMCID: PMC1721761. 10.1136/adc.2002.025114. [CrossRef]
- Balci N, Kaya Kara Ö. Bebeklik Döneminde Fizyoterapi Gerektiren Durumlar. In: Riskli Bebeklerde Fizyoterapi ve Rehabilitasyon. Ankara: Hipokrat Yayıncılık, 2019, pp: 141–143.
- World Health Organization. International Classification of Functioning, Disability and Health: ICF. Geneva: World Health Organization; 2001.
- World Health Organization. International Classification of Functioning, Disability and Health: Children & Youth Version (ICF-CY). Geneva: World Health Organization; 2007.
- Pan YL, Hwang AW, Simeonsson RJ, Lu L, Liao HF. ICF-CY code set for infants with early delay and disabilities (EDD Code Set) for interdisciplinary assessment: a global experts survey. Disabil Rehabil. 2015;37(12):1044-54. Epub 2014 Aug 21. [CrossRef]
- Campbell SK, Hedeker D. Validity of the Test of Infant Motor Performance for discriminating among infants with varying risk for poor motor outcome. J Pediatr. 2001 Oct;139(4):546-51. [CrossRef]
- Campbell SK, Levy P, Zawacki L, Liao PJ. Population-based age standards for interpreting results on the test of motor infant performance. Pediatr Phys Ther. 2006 Summer;18(2):119-25. [CrossRef]
- Mercuri E, Guzzetta A, Laroche S, Ricci D, vanhaastert I, Simpson A, Luciano R, Bleakley C, Frisone MF, Haataja L, Tortorolo G, Guzzetta F, de Vries L, Cowan F, Dubowitz L. Neurologic examination of preterm infants at term age: comparison with term infants. J Pediatr. 2003 Jun;142(6):647-55. [CrossRef]
- Mercuri E, Ricci D, Pane M, Baranello G. The neurological examination of the newborn baby. Early Hum Dev. 2005 Dec;81(12):947-56. [CrossRef]
- Dubowitz L, Mercuri E, Dubowitz V. An optimality score for the neurologic examination of the term newborn. J Pediatr. 1998 Sep;133(3):406-16. [CrossRef]
- Fujinaga CI, de Moraes SA, Zamberlan-Amorim NE, Castral TC, de Almeida e Silva A, Scochi CG. Clinical validation of the Preterm Oral Feeding Readiness Assessment Scale. Rev Lat Am Enfermagem. 2013 Jan-Feb;21 Spec No:140-5. English, Portuguese. Erratum in: Rev Lat Am Enfermagem. 2014 Oct;22(5):883. [CrossRef]
-
19. Bartlett DJ, Palisano RJ. A multivariate model of determinants of motor change for children with cerebral palsy. Phys Ther. [CrossRef] [PubMed]
- Simeonsson RJ, Leonardi M, Lollar D, Bjorck-Akesson E, Hollenweger J, Martinuzzi A. Applying the International Classification of Functioning, Disability and Health (ICF) to measure childhood disability. Disabil Rehabil. 2003 Jun 3-17;25(11-12):602-10. [CrossRef]
- Hwang AW, Liao HF, Chen PC, Hsieh WS, Simeonsson RJ, Weng LJ, Su YN. Applying the ICF-CY framework to examine biological and environmental factors in early childhood development. J Formos Med Assoc, 2014 May;113(5):303-12. Epub 2012 May 16. [CrossRef]
- Eyre, JA. Corticospinal tract development and its plasticity after perinatal injury. Neurosci Biobehav Rev, 2007;31(8):1136-49. Epub 2007 Jun 6. [CrossRef]
- Martin JH, Chakrabarty S, Friel KM. Harnessing activity-dependent plasticity to repair the damaged corticospinal tract in an animal model of cerebral palsy. Dev Med Child Neurol. 2011 Sep;53 Suppl 4(Suppl 4):9-13. PMID: 21950387; PMCID: PMC3187875. [CrossRef]
- Hughes AJ, Redsell SA, Glazebrook C. Motor Development Interventions for Preterm Infants: A Systematic Review and Meta-analysis. Pediatrics. 2016 Oct;138(4):e20160147. Pediatrics, 0160. [CrossRef]
- Finlayson F, Olsen J, Dusing SC, Guzzetta A, Eeles A, Spittle A. Supporting Play, Exploration, and Early Development Intervention (SPEEDI) for preterm infants: A feasibility randomised controlled trial in an Australian context. Early Hum Dev, 2020 Dec;151:105172. Epub 2020 Sep 1. [CrossRef]
- Dusing SC, Tripathi T, Marcinowski EC, Thacker LR, Brown LF, Hendricks-Muñoz KD. Supporting play exploration and early developmental intervention versus usual care to enhance development outcomes during the transition from the neonatal intensive care unit to home: a pilot randomized controlled trial. BMC Pediatr, 2018 Feb 9;18(1):46. PMID: 29426320; PMCID: PMC5809115. [CrossRef]
- Khurana S, Kane AE, Brown SE, Tarver T, Dusing SC. Effect of neonatal therapy on the motor, cognitive, and behavioral development of infants born preterm: a systematic review. Dev Med Child Neurol, 2020 102 Jun;62(6):684-692. Epub 2020 Feb 19. PMID: 32077096; PMCID: PMC7920849. [CrossRef]
- Øberg GK, Handegård BH, Campbell SK, Ustad T, Fjørtoft T, Kaaresen PI, Girolami GL. Two-year motor outcomes associated with the dose of NICU based physical therapy: The Noppi RCT. Early Hum Dev. 2022 Nov;174:105680. Early Hum Dev. [CrossRef]
- Morgan C, Novak I, Dale RC, Guzzetta A, Badawi N. GAME (Goals—Activity—Motor Enrichment):Protocol of a single blind randomised controlled trial of motor training, parent education and environmental enrichment for infants at high risk of cerebral palsy. BMC Neurol. 2014; 14:203. [CrossRef]
- Lima CRG, de Abreu RWF, Verderio BN, Brugnaro BH, dos Santos MM, dos Santos AN, et al. Early intervention involving specific task-environment-participation (step) protocol for infants at risk: A feasibility study. Phys Occup Ther Pedi. 2022:1–18. PMID: 36329671. [CrossRef]
- Dusing SC, Harbourne RT, Hsu LY, Koziol NA, Kretch K, Sargent B, et al. The SIT-PT Trial Protocol: A dose-matched randomized clinical trial comparing 2 physical therapist interventions for infants and toddlers with cerebral palsy. Phys Ther. 2022; 102(7). [CrossRef]
- Caesar R, Boyd RN, Colditz P, Cioni G, Ware RS, Salthouse K, et al. Early prediction of typical outcome and mild developmental delay for prioritisation of service delivery for very preterm and very low birthweight infants: a study protocol. Bmj Open. 2016; 6(7). [CrossRef]
- Girolami, GL, Campbell, SK. Efficacy of a Neuro-Developmental Treatment Program to Improve Motor Control in Infants Born Prematurely. Pediatric Physical Therapy 1994, 6: 175–184.
-
34. Lai M, D'Acunto G, Guzzetta A, Finnigan S, Ngenda N, Ware RS, Boyd RN, Colditz PB. Infant massage and brain maturation measured using EEG: A randomised controlled trial. Early Hum Dev, Epub 2022 Jul 22. [CrossRef] [PubMed]
- Welch MG, Myers MM, Grieve PG, Isler JR, Fifer WP, Sahni R, Hofer MA, Austin J, Ludwig RJ, Stark RI; FNI Trial Group. Electroencephalographic activity of preterm infants is increased by Family Nurture Intervention: a randomized controlled trial in the NICU. Clin Neurophysiol. 2014 Apr;125(4):675-684. [CrossRef]
- Welch MG, Stark RI, Grieve PG, Ludwig RJ, Isler JR, Barone JL, Myers MM. Family nurture intervention in preterm infants increases early development of cortical activity and independence of regional power trajectories. Acta Paediatr. 2017 Dec;106(12):1952-1960. [CrossRef]
- Landsem IP, Handegård BH, Ulvund SE, Tunby J, Kaaresen PI, Rønning JA. Does An Early Intervention Influence Behavioral Development Until Age 9 in Children Born Prematurely? Child Dev. 2015 Jul;86(4):1063-1079. Epub 2015 Apr 15. [CrossRef]
- Treyvaud K, Anderson VA, Howard K, Bear M, Hunt RW, Doyle LW, Inder TE, Woodward L, Anderson PJ. Parenting behavior is associated with the early neurobehavioral development of very preterm children. Pediatrics. 2009 Feb;123(2):555-61. [CrossRef]
- Petersen MC, Kube DA, Palmer FB. Classification of developmental delays. Semin Pediatr Neurol. 1998 Mar;5(1):2-14. [CrossRef]
- Chi CS. Evaluation of the child with developmental delay. Acta Paediatr Taiwan. 2005 Jul-Aug;46(4):191. PMID: 16381330.
- DA Fonseca Filho GG, Lopes AC, Bezerra RB, de M Candido A, Arrais N, Pereira SA, Lindquist AR. Assessment of child development in premature babies based on the ICF biopsychosocial model. Eur J Phys Rehabil Med. 2021 Aug;57(4):585-592. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).