Submitted:
22 December 2023
Posted:
22 December 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
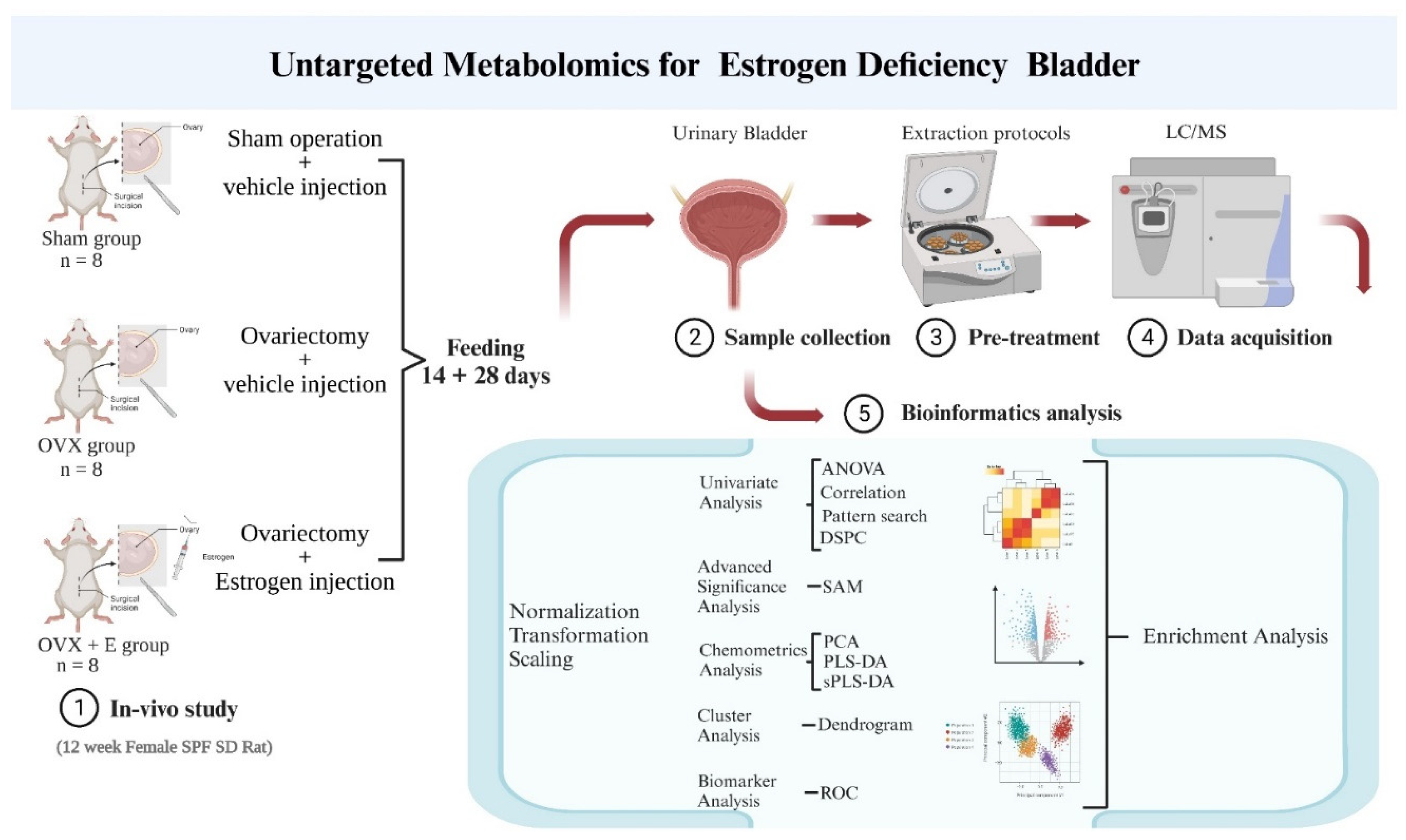
2.1. Animal Model
2.2. Weighing and Storage of Bladder Samples
2.3. Radioimmunoassay Test
2.4. Hematoxylin-eosin (HE) Staining
2.5. Bladder Sample Preparation in Metabolomics
2.6. Metabolomics Measurement
2.7. Metabolomics Data Processing
2.8. Bioinformatics Analysis
3. Results
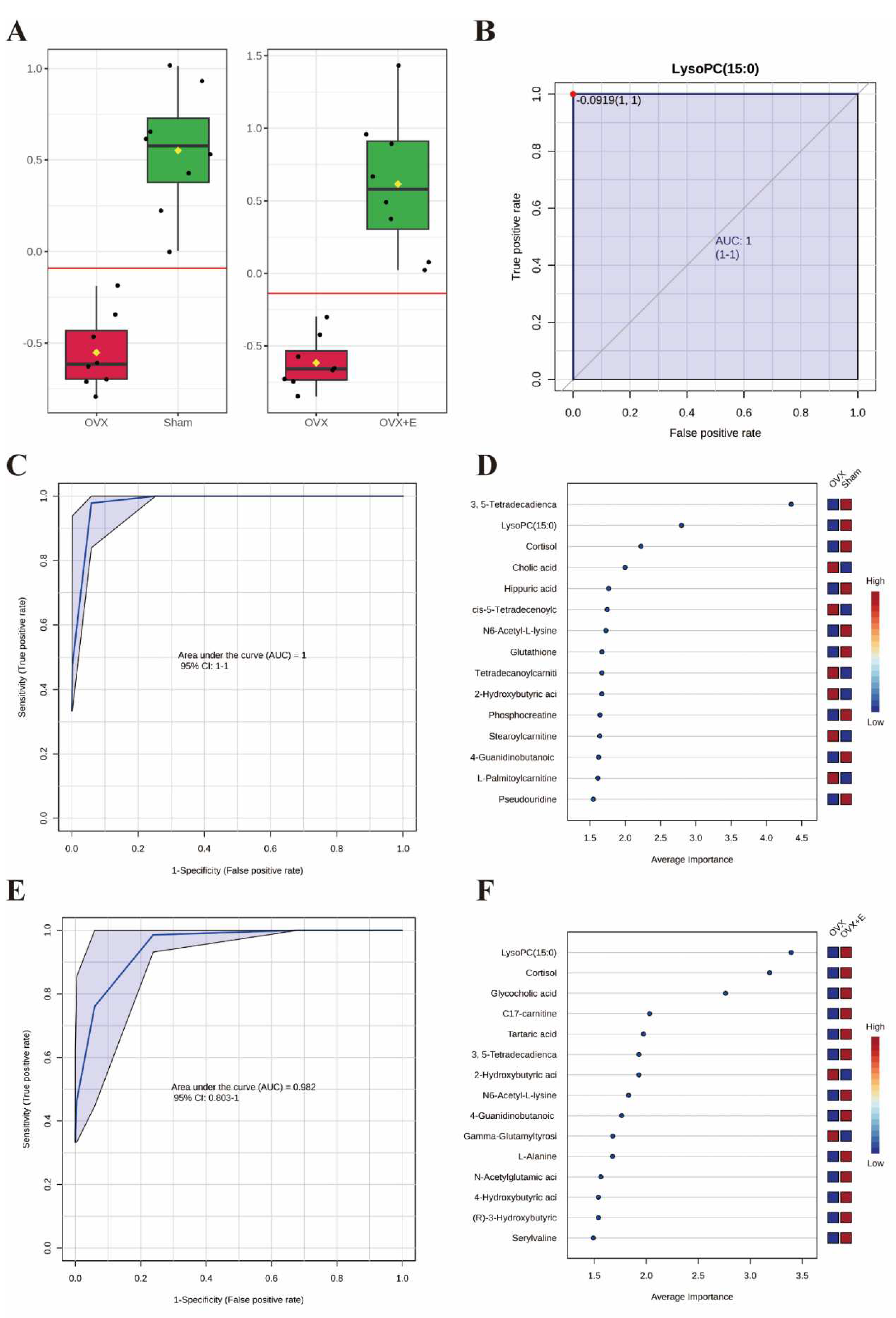
3.1. Establishment of Estrogen Deprivation Animal Models
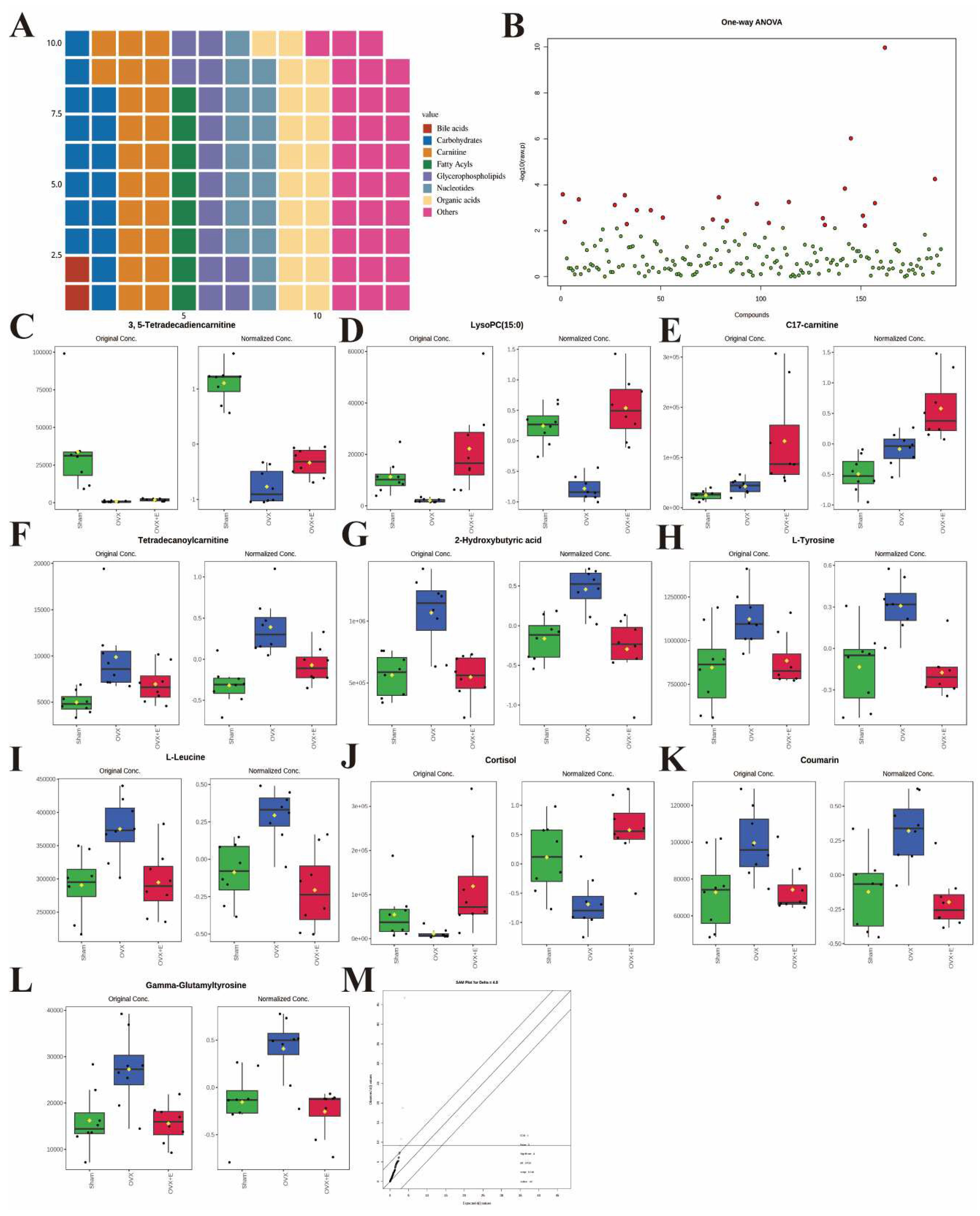
3.2. Characteristics of Bladder Metabolites associated with Estrogen deprivation
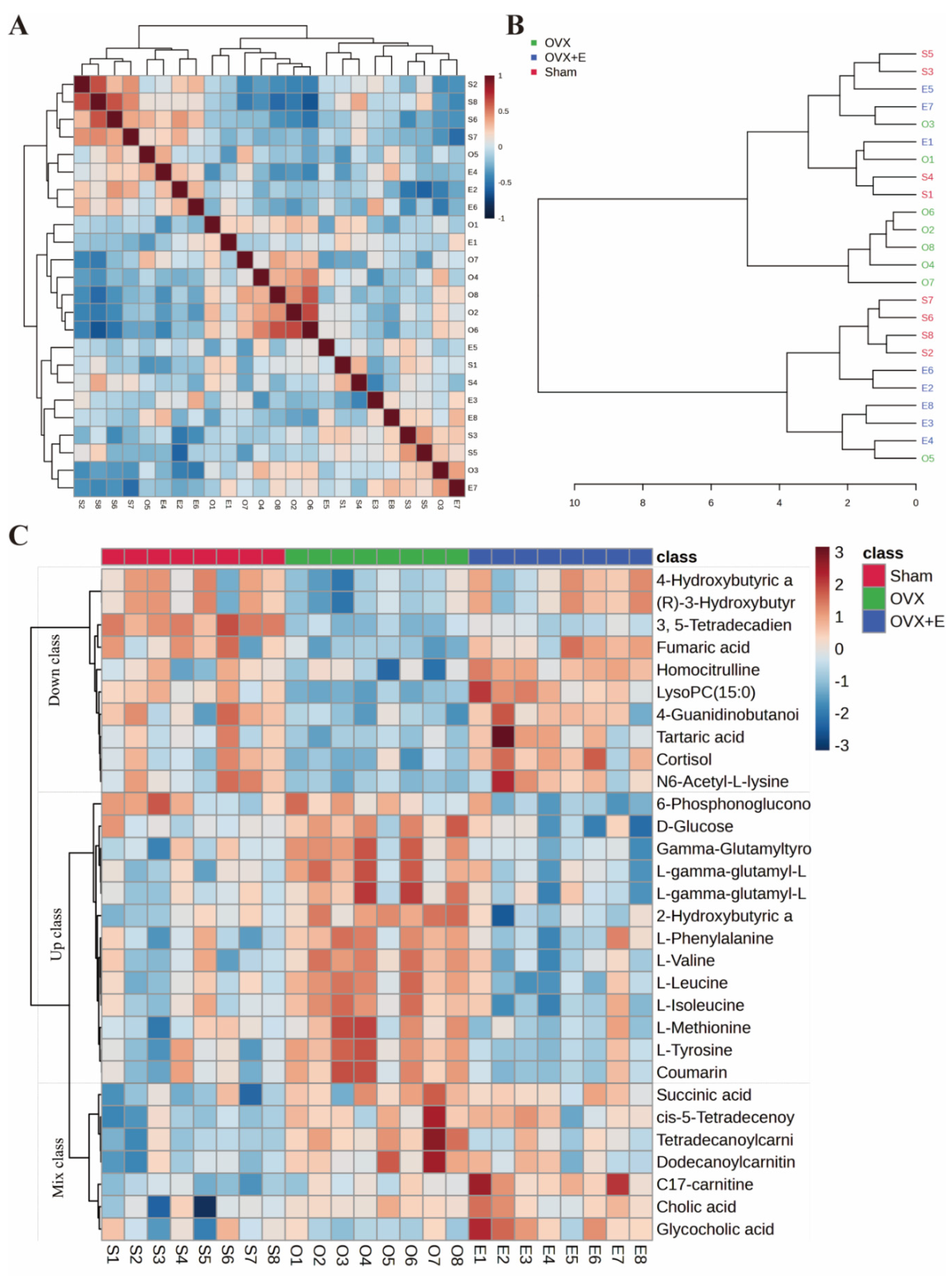
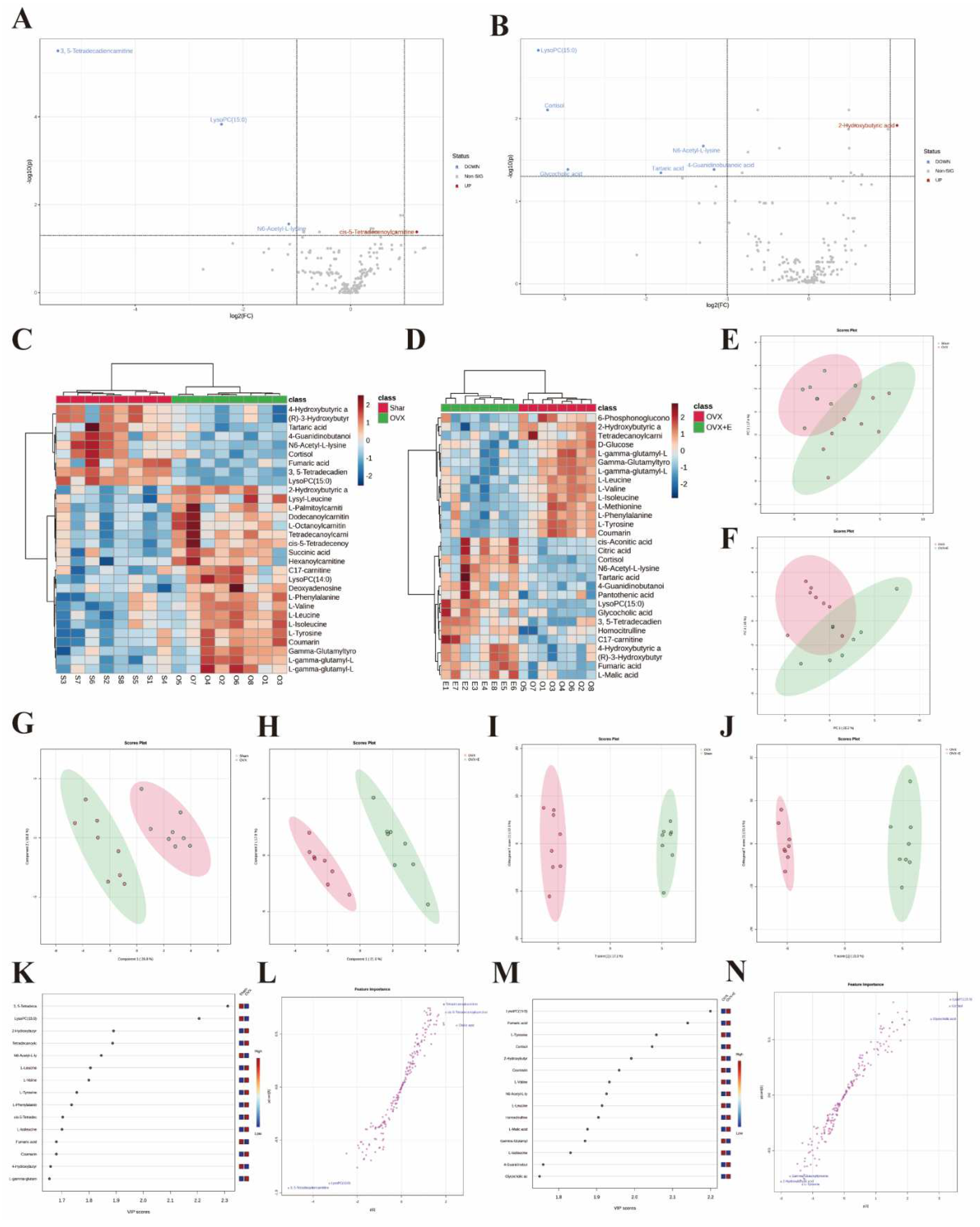
3.3. Overall analysis of bladder differential metabolites related to estrogen deprivation
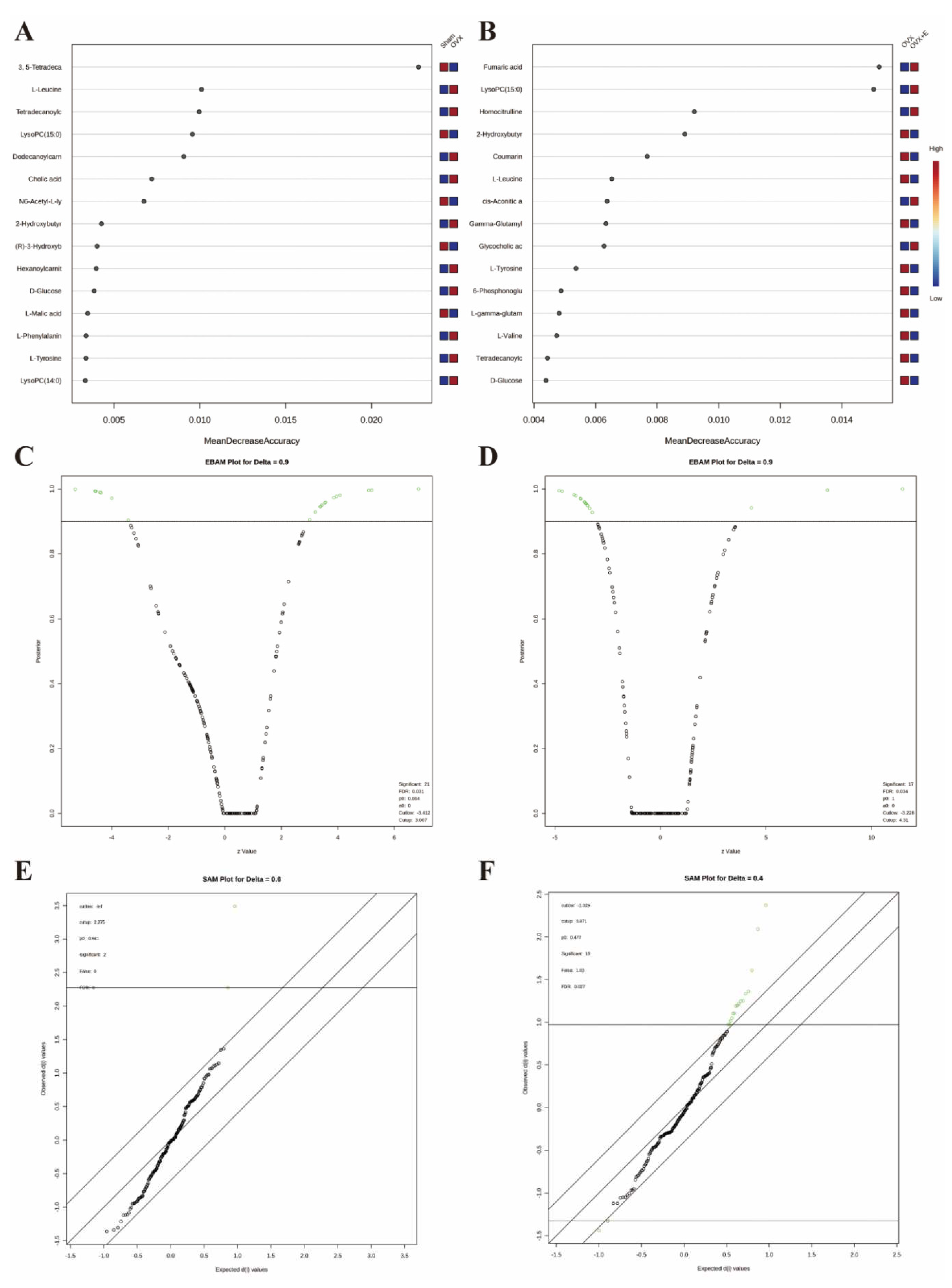
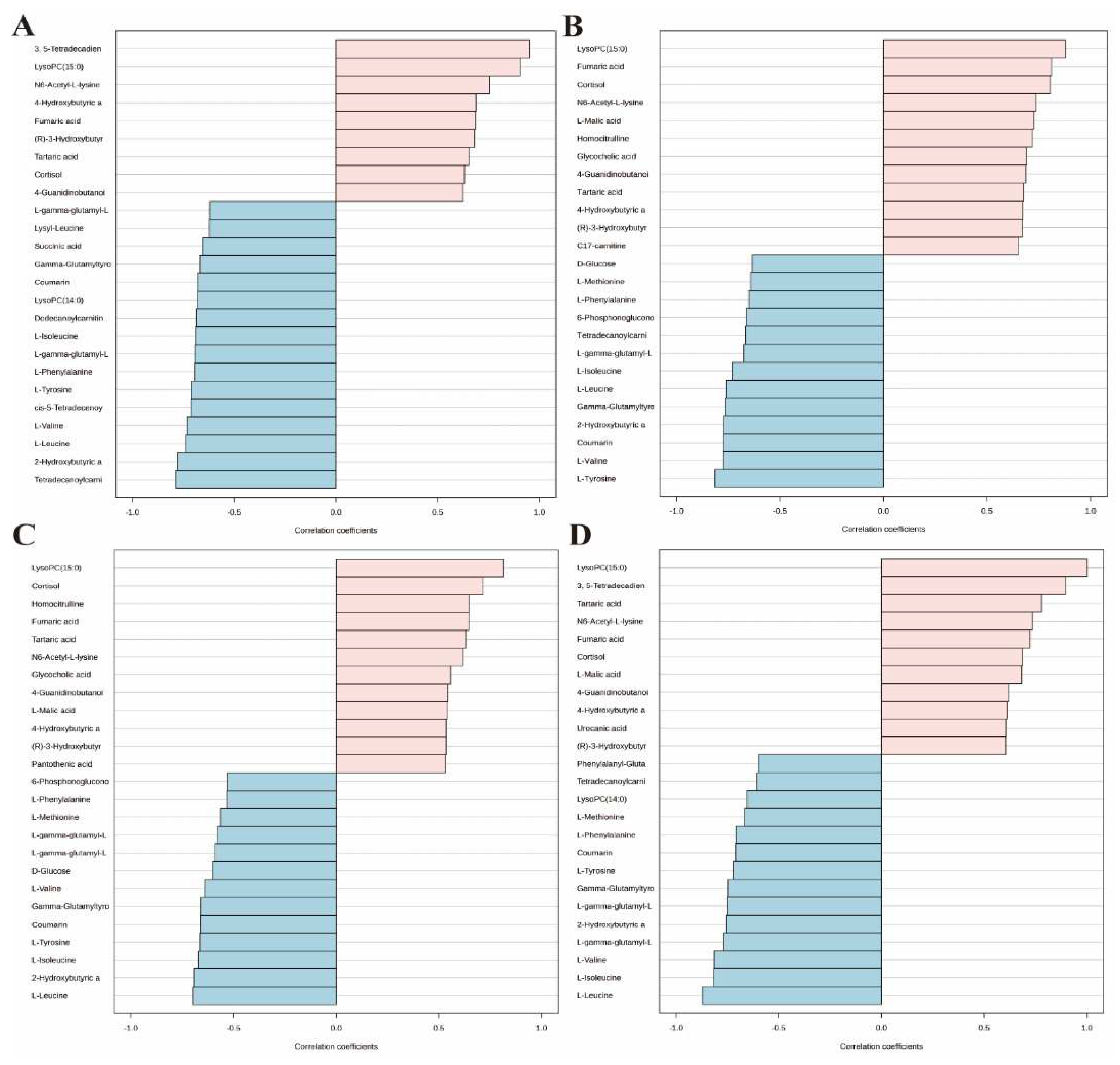
3.4. Subgroup analysis of diverse metabolites
3.5. Exploration of Metabolite Expression Patterns
3.6. Validation of Typical Biomarkers
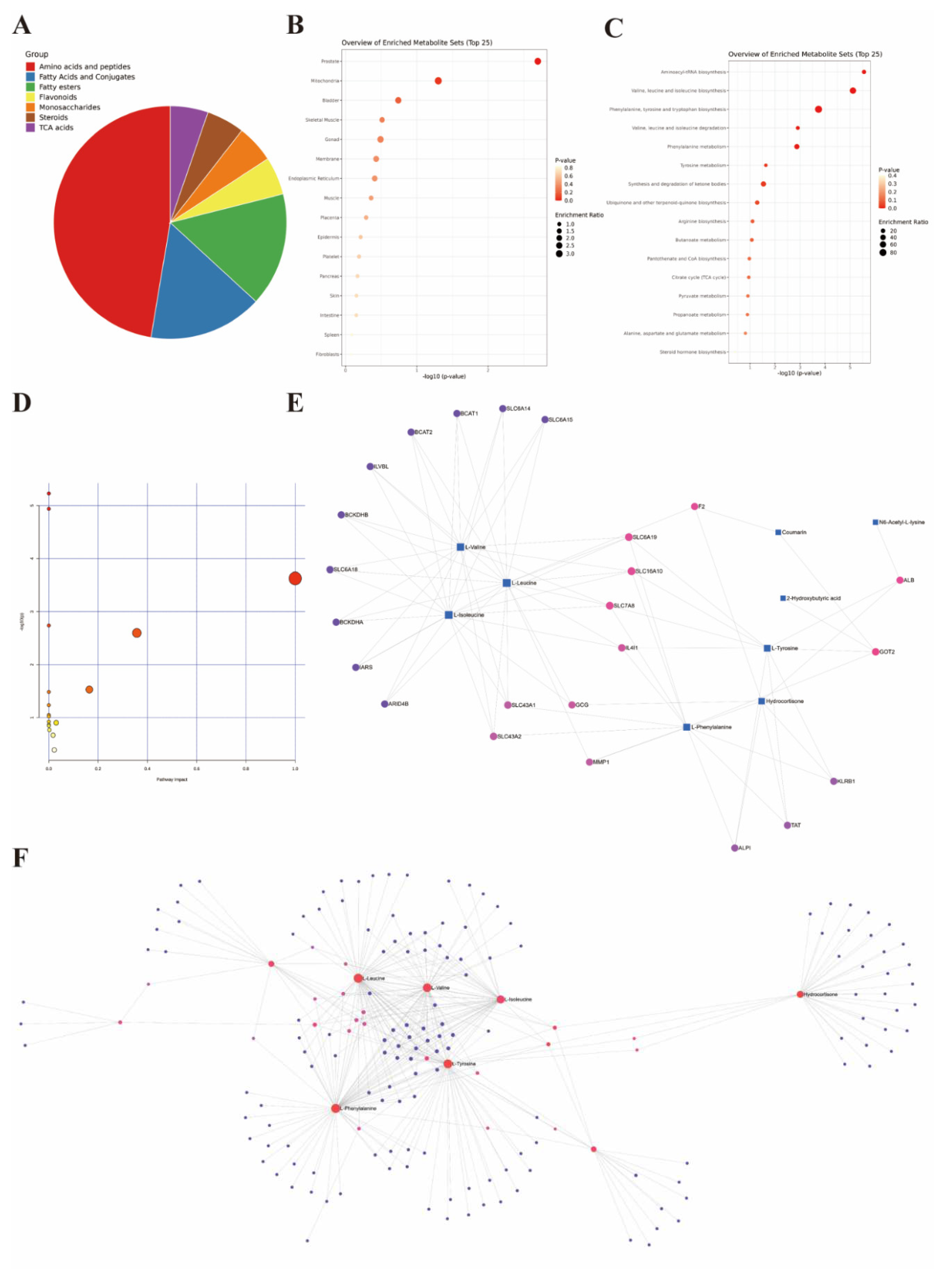
3.7. Comprehensive Analysis of Differential Metabolites
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Clemens, J.Q.; Erickson, D.R.; Varela, N.P.; Lai, H.H. Diagnosis and Treatment of Interstitial Cystitis/Bladder Pain Syndrome. The Journal of urology 2022, 208, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Neugent, M.L.; Kumar, A.; Hulyalkar, N.V.; Lutz, K.C.; Nguyen, V.H.; Fuentes, J.L.; Zhang, C.; Nguyen, A.; Sharon, B.M.; Kuprasertkul, A.; et al. Recurrent urinary tract infection and estrogen shape the taxonomic ecology and function of the postmenopausal urogenital microbiome. Cell reports. Medicine 2022, 3, 100753. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.M. Stress Incontinence in Women. The New England journal of medicine 2021, 384, 2428–2436. [Google Scholar] [CrossRef]
- Crandall, C.J.; Mehta, J.M.; Manson, J.E. Management of Menopausal Symptoms: A Review. Jama 2023, 329, 405–420. [Google Scholar] [CrossRef] [PubMed]
- Anger, J.T.; Bixler, B.R.; Holmes, R.S.; Lee, U.J.; Santiago-Lastra, Y.; Selph, S.S. Updates to Recurrent Uncomplicated Urinary Tract Infections in Women: AUA/CUA/SUFU Guideline. The Journal of urology 2022, 208, 536–541. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Liu, X.D.; Wang, J.W.; Meng, L.F.; Zhang, Y.G.; Wang, J.Y. The sphingosine-1-phosphate/RhoA/Rho associated kinases/myosin light chain pathway in detrusor of female rats is down-regulated in response to ovariectomy. Chinese medical journal 2020, 133, 1203–1210. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.H.; Chueh, K.S.; Juan, T.J.; Mao, J.W.; Lin, R.J.; Lee, Y.C.; Shen, M.C.; Sun, T.W.; Lin, H.Y.; Juan, Y.S. Effects of Therapeutic Platelet-Rich Plasma on Overactive Bladder via Modulating Hyaluronan Synthesis in Ovariectomized Rat. International journal of molecular sciences 2023, 24. [Google Scholar] [CrossRef] [PubMed]
- Kadekawa, K.; Kawamorita, N.; Shimizu, T.; Kurobe, M.; Turnbull, P.S.; Chandra, S.; Kambara, T.; Barton, J.C.; Russell, A.J.; Yoshimura, N. Effects of a selective androgen receptor modulator (SARM), GSK2849466A, on stress urinary incontinence and bladder activity in rats with ovariectomy-induced oestrogen deficiency. BJU international 2020, 125, 911–919. [Google Scholar] [CrossRef] [PubMed]
- Bonilla-Becerra, S.M.; de Oliveira, M.G.; Calmasini, F.B.; Rojas-Moscoso, J.A.; Zanesco, A.; Antunes, E. Micturition dysfunction in four-month old ovariectomized rats: Effects of testosterone replacement. Life sciences 2017, 179, 120–129. [Google Scholar] [CrossRef]
- Blum, A.; Wang, P.; Zenklusen, J.C. SnapShot: TCGA-Analyzed Tumors. Cell 2018, 173, 530. [Google Scholar] [CrossRef]
- Whiteaker, J.R.; Halusa, G.N.; Hoofnagle, A.N.; Sharma, V.; MacLean, B.; Yan, P.; Wrobel, J.A.; Kennedy, J.; Mani, D.R.; Zimmerman, L.J.; et al. CPTAC Assay Portal: a repository of targeted proteomic assays. Nature methods 2014, 11, 703–704. [Google Scholar] [CrossRef] [PubMed]
- Fahy, E.; Sud, M.; Cotter, D.; Subramaniam, S. LIPID MAPS online tools for lipid research. Nucleic acids research 2007, 35, W606–612. [Google Scholar] [CrossRef] [PubMed]
- Su, F.; Zhang, W.; Meng, L.; Zhang, W.; Liu, X.; Liu, X.; Chen, M.; Zhang, Y.; Xiao, F. Multimodal Single-Cell Analyses Outline the Immune Microenvironment and Therapeutic Effectors of Interstitial Cystitis/Bladder Pain Syndrome. Advanced science (Weinheim, Baden-Wurttemberg, Germany) 2022, 9, e2106063. [Google Scholar] [CrossRef]
- Zhang, W.; Qiao, X.; Xie, T.; Cai, W.; Zhang, X.; Chen, C.; Zhang, Y. Multi-Omics Approach Reveals Redox Homeostasis Reprogramming in Early-Stage Clear Cell Renal Cell Carcinoma. Antioxidants (Basel, Switzerland) 2022, 12. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Liu, X.; Wang, J.; Wang, X.; Zhang, Y. Immunogenic Cell Death Associated Molecular Patterns and the Dual Role of IL17RA in Interstitial Cystitis/Bladder Pain Syndrome. Biomolecules 2023, 13. [Google Scholar] [CrossRef]
- Pang, Z.; Chong, J.; Zhou, G.; de Lima Morais, D.A.; Chang, L.; Barrette, M.; Gauthier, C.; Jacques, P.; Li, S.; Xia, J. MetaboAnalyst 5.0: narrowing the gap between raw spectra and functional insights. Nucleic acids research 2021, 49, W388–w396. [Google Scholar] [CrossRef] [PubMed]
- Dieterle, F.; Ross, A.; Schlotterbeck, G.; Senn, H. Probabilistic quotient normalization as robust method to account for dilution of complex biological mixtures. Application in 1H NMR metabonomics. Analytical chemistry 2006, 78, 4281–4290. [Google Scholar] [CrossRef]
- Basu, S.; Duren, W.; Evans, C.R.; Burant, C.F.; Michailidis, G.; Karnovsky, A. Sparse network modeling and metscape-based visualization methods for the analysis of large-scale metabolomics data. Bioinformatics 2017, 33, 1545–1553. [Google Scholar] [CrossRef]
- Farhadi, Z.; Khaksari, M.; Azizian, H.; Dabiri, S.; Fallah, H.; Nozari, M. Aging is associated with loss of beneficial effects of estrogen on leptin responsiveness in mice fed high fat diet: Role of estrogen receptor α and cytokines. Mechanisms of ageing and development 2020, 186, 111198. [Google Scholar] [CrossRef]
- Shetty, A.; Suresh, P.S. A synergy of estradiol with leptin modulates the long non-coding RNA NEAT1/ mmu-miR-204-5p/IGF1 axis in the uterus of high-fat-diet-induced obese ovariectomized mice. The Journal of steroid biochemistry and molecular biology 2021, 209, 105843. [Google Scholar] [CrossRef]
- Lee, K.C. Changes of muscarinic receptors and connexin-43 expression as a mechanism of overactive bladder in ovariectomized rats. World journal of urology 2015, 33, 1875–1879. [Google Scholar] [CrossRef] [PubMed]
- Rehfuss, A.; Schuler, C.; Maxemous, C.; Leggett, R.E.; Levin, R.M. Cyclical estrogen and free radical damage to the rabbit urinary bladder. International urogynecology journal 2010, 21, 489–494. [Google Scholar] [CrossRef]
- Tanidir, Y.; Ercan, F.; Tarcan, T. Exogenous testosterone and estrogen affect bladder tissue contractility and histomorphology differently in rat ovariectomy model. The journal of sexual medicine 2011, 8, 1626–1637. [Google Scholar] [CrossRef]
- Lee, Y.L.; Lin, K.L.; Wu, B.N.; Chuang, S.M.; Wu, W.J.; Lee, Y.C.; Ho, W.T.; Juan, Y.S. Epigallocatechin-3-gallate alleviates bladder overactivity in a rat model with metabolic syndrome and ovarian hormone deficiency through mitochondria apoptosis pathways. Scientific reports 2018, 8, 5358. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.L.; Lu, J.H.; Chueh, K.S.; Juan, T.J.; Wu, B.N.; Chuang, S.M.; Lee, Y.C.; Shen, M.C.; Long, C.Y.; Juan, Y.S. Low-Intensity Extracorporeal Shock Wave Therapy Promotes Bladder Regeneration and Improves Overactive Bladder Induced by Ovarian Hormone Deficiency from Rat Animal Model to Human Clinical Trial. International journal of molecular sciences 2021, 22. [Google Scholar] [CrossRef] [PubMed]
- Anand, M.; Wang, C.; French, J.; Isaacson-Schmid, M.; Wall, L.L.; Mysorekar, I.U. Estrogen affects the glycosaminoglycan layer of the murine bladder. Female pelvic medicine & reconstructive surgery 2012, 18, 148–152. [Google Scholar] [CrossRef]
- Acevedo-Alvarez, M.; Yeh, J.; Alvarez-Lugo, L.; Lu, M.; Sukumar, N.; Hill, W.G.; Chai, T.C. Mouse urothelial genes associated with voiding behavior changes after ovariectomy and bladder lipopolysaccharide exposure. Neurourology and urodynamics 2018, 37, 2398–2405. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, H.; Yao, J.L.; Chaux, A.; Zheng, Y.; Hsu, I.; Izumi, K.; Chang, C.; Messing, E.M.; Netto, G.J.; Yeh, S. Expression of androgen and oestrogen receptors and its prognostic significance in urothelial neoplasm of the urinary bladder. BJU international 2012, 109, 1716–1726. [Google Scholar] [CrossRef] [PubMed]
- Reuter, S.E.; Evans, A.M. Carnitine and acylcarnitines: pharmacokinetic, pharmacological and clinical aspects. Clinical pharmacokinetics 2012, 51, 553–572. [Google Scholar] [CrossRef]
- Guo, M.; Cao, X.; Ji, D.; Xiong, H.; Zhang, T.; Wu, Y.; Suo, L.; Pan, M.; Brugger, D.; Chen, Y.; et al. Gut Microbiota and Acylcarnitine Metabolites Connect the Beneficial Association between Estrogen and Lipid Metabolism Disorders in Ovariectomized Mice. Microbiology spectrum 2023, 11, e0014923. [Google Scholar] [CrossRef]
- Zhao, S.; Feng, X.F.; Huang, T.; Luo, H.H.; Chen, J.X.; Zeng, J.; Gu, M.; Li, J.; Sun, X.Y.; Sun, D.; et al. The Association Between Acylcarnitine Metabolites and Cardiovascular Disease in Chinese Patients With Type 2 Diabetes Mellitus. Frontiers in endocrinology 2020, 11, 212. [Google Scholar] [CrossRef]
- Kang, M.; Yoo, H.J.; Kim, M.; Kim, M.; Lee, J.H. Metabolomics identifies increases in the acylcarnitine profiles in the plasma of overweight subjects in response to mild weight loss: a randomized, controlled design study. Lipids in health and disease 2018, 17, 237. [Google Scholar] [CrossRef]
- Mezzullo, M.; Gambineri, A.; Di Dalmazi, G.; Fazzini, A.; Magagnoli, M.; Baccini, M.; Vicennati, V.; Pelusi, C.; Pagotto, U.; Fanelli, F. Steroid reference intervals in women: influence of menopause, age and metabolism. European journal of endocrinology 2021, 184, 395–407. [Google Scholar] [CrossRef]
- Kalleinen, N.; Polo-Kantola, P.; Irjala, K.; Porkka-Heiskanen, T.; Vahlberg, T.; Virkki, A.; Polo, O. 24-hour serum levels of growth hormone, prolactin, and cortisol in pre- and postmenopausal women: the effect of combined estrogen and progestin treatment. The Journal of clinical endocrinology and metabolism 2008, 93, 1655–1661. [Google Scholar] [CrossRef]
- Cohn, A.Y.; Grant, L.K.; Nathan, M.D.; Wiley, A.; Abramson, M.; Harder, J.A.; Crawford, S.; Klerman, E.B.; Scheer, F.; Kaiser, U.B.; et al. Effects of Sleep Fragmentation and Estradiol Decline on Cortisol in a Human Experimental Model of Menopause. The Journal of clinical endocrinology and metabolism 2023, 108, e1347–e1357. [Google Scholar] [CrossRef] [PubMed]
- Chihara, I.; Negoro, H.; Kono, J.; Nagumo, Y.; Tsuchiya, H.; Kojo, K.; Shiga, M.; Tanaka, K.; Kandori, S.; Mathis, B.J.; et al. Glucocorticoids coordinate the bladder peripheral clock and diurnal micturition pattern in mice. Communications biology 2023, 6, 81. [Google Scholar] [CrossRef]
- Knezevic, E.; Nenic, K.; Milanovic, V.; Knezevic, N.N. The Role of Cortisol in Chronic Stress, Neurodegenerative Diseases, and Psychological Disorders. Cells 2023, 12. [Google Scholar] [CrossRef]
- Perrin-Cocon, L.; Agaugué, S.; Coutant, F.; Saint-Mézard, P.; Guironnet-Paquet, A.; Nicolas, J.F.; André, P.; Lotteau, V. Lysophosphatidylcholine is a natural adjuvant that initiates cellular immune responses. Vaccine 2006, 24, 1254–1263. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Jia, H.M.; Cui, F.X.; Yang, Y.; Zhao, Y.; Yang, M.H.; Zou, Z.M. The Effect of Chinese Herbal Medicine Formula mKG on Allergic Asthma by Regulating Lung and Plasma Metabolic Alternations. International journal of molecular sciences 2017, 18. [Google Scholar] [CrossRef]
- Kosinska, M.K.; Liebisch, G.; Lochnit, G.; Wilhelm, J.; Klein, H.; Kaesser, U.; Lasczkowski, G.; Rickert, M.; Schmitz, G.; Steinmeyer, J. A lipidomic study of phospholipid classes and species in human synovial fluid. Arthritis and rheumatism 2013, 65, 2323–2333. [Google Scholar] [CrossRef]
- Marra, S.; Ferru-Clément, R.; Breuil, V.; Delaunay, A.; Christin, M.; Friend, V.; Sebille, S.; Cognard, C.; Ferreira, T.; Roux, C.; et al. Non-acidic activation of pain-related Acid-Sensing Ion Channel 3 by lipids. The EMBO journal 2016, 35, 414–428. [Google Scholar] [CrossRef] [PubMed]
- Zhai, G.; Pelletier, J.P.; Liu, M.; Aitken, D.; Randell, E.; Rahman, P.; Jones, G.; Martel-Pelletier, J. Activation of The Phosphatidylcholine to Lysophosphatidylcholine Pathway Is Associated with Osteoarthritis Knee Cartilage Volume Loss Over Time. Scientific reports 2019, 9, 9648. [Google Scholar] [CrossRef] [PubMed]
- Jurczak, A.; Delay, L.; Barbier, J.; Simon, N.; Krock, E.; Sandor, K.; Agalave, N.M.; Rudjito, R.; Wigerblad, G.; Rogóż, K.; et al. Antibody-induced pain-like behavior and bone erosion: links to subclinical inflammation, osteoclast activity, and acid-sensing ion channel 3-dependent sensitization. Pain 2022, 163, 1542–1559. [Google Scholar] [CrossRef] [PubMed]
- Rimola, V.; Hahnefeld, L.; Zhao, J.; Jiang, C.; Angioni, C.; Schreiber, Y.; Osthues, T.; Pierre, S.; Geisslinger, G.; Ji, R.R.; et al. Lysophospholipids Contribute to Oxaliplatin-Induced Acute Peripheral Pain. The Journal of neuroscience : the official journal of the Society for Neuroscience 2020, 40, 9519–9532. [Google Scholar] [CrossRef] [PubMed]
- Gentry, C.; Stoakley, N.; Andersson, D.A.; Bevan, S. The roles of iPLA2, TRPM8 and TRPA1 in chemically induced cold hypersensitivity. Molecular pain 2010, 6, 4. [Google Scholar] [CrossRef] [PubMed]
- Handa, R.K.; Buckalew, V.M., Jr. Effect of lysophosphatidylcholine on renal hemodynamics and excretory function in anesthetized rats. Life sciences 1992, 51, 1571–1575. [Google Scholar] [CrossRef] [PubMed]
- Connelly, M.A.; Wolak-Dinsmore, J.; Dullaart, R.P.F. Branched Chain Amino Acids Are Associated with Insulin Resistance Independent of Leptin and Adiponectin in Subjects with Varying Degrees of Glucose Tolerance. Metabolic syndrome and related disorders 2017, 15, 183–186. [Google Scholar] [CrossRef]
- Batch, B.C.; Shah, S.H.; Newgard, C.B.; Turer, C.B.; Haynes, C.; Bain, J.R.; Muehlbauer, M.; Patel, M.J.; Stevens, R.D.; Appel, L.J.; et al. Branched chain amino acids are novel biomarkers for discrimination of metabolic wellness. Metabolism: clinical and experimental 2013, 62, 961–969. [Google Scholar] [CrossRef]
- Magkos, F.; Bradley, D.; Schweitzer, G.G.; Finck, B.N.; Eagon, J.C.; Ilkayeva, O.; Newgard, C.B.; Klein, S. Effect of Roux-en-Y gastric bypass and laparoscopic adjustable gastric banding on branched-chain amino acid metabolism. Diabetes 2013, 62, 2757–2761. [Google Scholar] [CrossRef]
- Zheng, Y.; Ceglarek, U.; Huang, T.; Li, L.; Rood, J.; Ryan, D.H.; Bray, G.A.; Sacks, F.M.; Schwarzfuchs, D.; Thiery, J.; et al. Weight-loss diets and 2-y changes in circulating amino acids in 2 randomized intervention trials. The American journal of clinical nutrition 2016, 103, 505–511. [Google Scholar] [CrossRef]
- Fontana, L.; Cummings, N.E.; Arriola Apelo, S.I.; Neuman, J.C.; Kasza, I.; Schmidt, B.A.; Cava, E.; Spelta, F.; Tosti, V.; Syed, F.A.; et al. Decreased Consumption of Branched-Chain Amino Acids Improves Metabolic Health. Cell reports 2016, 16, 520–530. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Richardson, N.E.; Green, C.L.; Spicer, A.B.; Murphy, M.E.; Flores, V.; Jang, C.; Kasza, I.; Nikodemova, M.; Wakai, M.H.; et al. The adverse metabolic effects of branched-chain amino acids are mediated by isoleucine and valine. Cell metabolism 2021, 33, 905–922.e906. [Google Scholar] [CrossRef] [PubMed]
- Auro, K.; Joensuu, A.; Fischer, K.; Kettunen, J.; Salo, P.; Mattsson, H.; Niironen, M.; Kaprio, J.; Eriksson, J.G.; Lehtimäki, T.; et al. A metabolic view on menopause and ageing. Nature communications 2014, 5, 4708. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; De Hoedt, A.; Wiggins, E.; Haywood, K.; Jin, P.; Greenwood, B.; Narain, N.R.; Tolstikov, V.; Bussberg, V.; Barbour, K.E.; et al. Diagnostic Utility of Serum and Urinary Metabolite Analysis in Patients with Interstitial Cystitis/Painful Bladder Syndrome. Urology 2021, 157, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Arnold, S.; Victor, M.B.; Beyer, C. Estrogen and the regulation of mitochondrial structure and function in the brain. The Journal of steroid biochemistry and molecular biology 2012, 131, 2–9. [Google Scholar] [CrossRef]
- Zhao, W.; Hou, Y.; Song, X.; Wang, L.; Zhang, F.; Zhang, H.; Yu, H.; Zhou, Y. Estrogen Deficiency Induces Mitochondrial Damage Prior to Emergence of Cognitive Deficits in a Postmenopausal Mouse Model. Frontiers in aging neuroscience 2021, 13, 713819. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




