1. Introduction
Acute myocardial infarction is a major cause of mortality and heart failure development worldwide. Timely myocardial reperfusion, which remains the only treatment of choice up to date, can paradoxically exacerbate myocardial injury and cardiomyocyte death, known as ischemia/reperfusion (I/R) injury [
1]. I/R injury is a complex process during which physiological mechanisms in cardiomyocytes are unable to maintain homeostasis. The mechanism of I/R injury is multifactorial and involves the contribution of divergent pathways, with the reperfusion-induced oxidative stress being one of the main players [
2]. Rapid restoration of oxygen at the onset of reperfusion overwhelms the mitochondria, leading to redox balance deregulation and massive reactive oxygen species (ROS) production, lipid peroxidation and formation of toxic products which react with and inactivate subcellular components and further aggravate oxidative damage and overall cardiomyocyte dysfunction [
1,
3,
4]. The intrinsic defense mechanisms in place, in cardiac myocytes as in most cell types, to counteract oxidative stress and diminish the buildup of toxic radicals, consist of enzymatic antioxidants such as superoxide dismutase (SOD), catalase (CAT), glutathione peroxidases (GPxs) and thioredoxin (Trx) as well as the non-enzymatic antioxidants including glutathione (GSH). GSH may directly react with different free radicals, thus being the first cellular defense line against oxygen reactive species or acting as a co-factor of antioxidant enzymes [
5,
6,
7].
Pharmacological approaches that seek to ameliorate the effect of oxidative stress either enhancing antioxidant mechanisms or reducing ROS production have shown some promising results and are considered a potentially useful strategy in the management of I/R injury [
2,
8,
9,
10]. In addition to exogenous antioxidants, therapies targeting endogenous antioxidant systems, i.e., indirect antioxidants, have become of particular interest in treatment or prevention of cardiovascular diseases. Endogenous antioxidant systems in the heart and/or in circulation could be activated by various exogenous molecules, and some of them have shown cardioprotective potential in clinical settings as well. In particular, the use of compounds found in everyday used natural products and food as potential cardioprotective factors is of great interest [
6,
11].
In the present study, we used camphene, a monoterpene that can be found in various plants such as carrots, pepper, dill, fennel, nutmeg, thyme and cannabis. Camphene is used as an additive in foods and fragrances, and it is included in essential oils as well [
12]. It has been recognized to have antibiotic anti-fungal [
13,
14,
15], anti-inflammatory and analgesic properties [
16,
17], while it is also used as expectorant [
18] and antinociceptive [
19]. Furthermore, camphene has been reported to exhibit a hypolipidemic action [
12,
20] while it increases the expression of apolipoprotein ApoA1 protein levels in a dose-dependent manner [
21], resulting in increased formation of high-density lipoprotein (HDL) molecules [
20]. Although camphene has been shown to have antioxidant properties
in vitro [
22], data on the antioxidant effect of camphene in animal or cell systems are very limited. A recent report demonstrated the reduction of the starvation-induced ROS generation in L6 skeletal muscle cells in the presence of camphene [
23]; however, the mechanisms underlying this effect are unclear.
The aim of the current research is to characterize the potential cardioprotective effect of camphene administration in terms of infarct size reduction after myocardial I/R, and to investigate the underlying mechanism of action.
2. Materials and Methods
2.1. Animals
Adult (age 2-3 months old) Wistar rats were maintained in plexiglass cages in a controlled environment, namely temperature 22 ± 1° C, humidity 55 ± 2 %, with a 12 h day/ night circle, in a quiet and well-ventilated animal house. 2-4 animals were housed in each cage, with free access to food and water. All animals were acclimated to the researchers that handled them and/ or were involved in the euthanasia. All protocols applied were in accordance with the ethical regulations for the use of laboratory animals of the Aristotele University of Thessaloniki (13/16588), the Greek legislation on laboratory animals ("Guidelines for the care and use of laboratory animals", published by the Greek government 160/1991), and the regulations of the European Union (86/609).
2.2. Administration of camphene and experimental procedures for I/R in isolated hearts
Animals were randomly assigned to 3 groups. Control group and I/R group animals were given intraperitoneal (i. p.) bolus of 1 ml of Tween 80 (10%) while I/R-camphene group animals were administered a single dose, 30 μg/ g of body weight of camphene (Sigma Aldrich, Darmstadt, Germany) dissolved in Tween 80 (10%), 24h before any further intervention. Dosage for camphene was based on previously published studies [
24].
Animals were anesthetized with i.p. administration of ketamine/xylazine at 100 mg/kg and 10 mg/kg respectively (Merck, KGaA, Darmstadt, Germany) and they were injected with heparin (300 IU/ kg) through the femoral vein to prevent blood coagulation and the formation of clots in the cavities of the heart. Hearts were excised and placed immediately in ice- cold Krebs-Heinseleit buffer (KHB) (25 mM NaHCO3, 118.5 mM NaCl, 4.7 mM KCl, 1.2 mM MgSO4, 1.2 mM KH2PO4, 2.5 mM CaCl2 and 10 mM glucose, pH 7.4). Hearts were cannulated through the aorta and perfused in a Langendorff mode at a constant perfusion pressure of 70 mm Hg at 37οC with KHB gassed with 95% O2 – 5% CO2. Hearts were stabilized for 15 min prior to any interventions. Hearts from the animals assigned to the control group, underwent only the stabilization process, while hearts from the I/R groups (I//R and I/R-camphene) were subjected to 30 min of global ischemia, by clamping the aortic inflow, followed by either 40 min reperfusion, for molecular analysis, or 120 min reperfusion for infarct size determination.
2.3. Infarct size (IS) determination
Infarct size was determined using the 2,3,5-triphenyltetrazolium chloride (TTC) method. At the end of reperfusion, hearts were placed at – 20°C for 30 min, cut transversely into 6 slices of 2 mm thickness and incubated with 1% TTC in 0.1M phosphate buffer, pH 7.4, for 30 min at 37°C. Hearts were preserved overnight in PBS buffer (137 mM NaCl, 2.7 mM KCl, 10 mM Na
2HPO
4, 1.8 mM KH
2PO
4) containing 4% formaldehyde. The infarct size (IS) area was delineated and determined by a computerized planimetric method [
8]. IS was expressed as a percentage of the area at risk, which in the model of global ischemia represents the whole area of the left ventricle.
2.4. LDH determination
Perfusate during the first 20 minutes of reperfusion of the heart was collected and was used to measure the activity of the enzyme Lactate Dehydrogenase (LDH) that was released from cells with damaged cell membranes [
25]. The measurement of the enzyme activity was based on the conversion of NADH at 340 nm (extinction coefficient ε=6220 M
-1cm
-1). The final concentration of the reagents in the reaction mixture were 0.2 M Tris-HCl pH 7.3, 1 mM sodium pyruvate, 0.132 mM nicotinamide adenine dinucleotide hydrogen (NADH) and appropriate amount of sample. The rate of conversion of NADH to NAD+ per min, which is proportional to the activity of LDH, was recorded for 5 minutes. LDH enzyme activity was expressed in U/ gr of heart tissue weight.
2.5. Determination of Protein Carbonyls
Protein carbonylation was determined by 2,4-dinitrophenyl (DNP) hydrazine (2,4-DNPH) derivatization [
26]. Briefly, frozen tissue samples were lysed with 50 mM sodium phosphate buffer pH 6.7 and centrifuged (11,000 g, 10 min, 4
o C). Part of the supernatant was kept for determination of total protein concentration. Samples were reacted with 10 mM of 2,4-DNPH in 2 M HCl for 1 h, deproteinized with 20% TCA and pellets were redissolved in 6 M guanidine hydrochloride. The absorbance was measured at 360 nm and carbonyl content was calculated, using the molar absorption coefficient of 22,000 M
-1cm
-1 relative to protein concentration. Values were expressed as nmol/ mg of protein.
2.6. Glutathione determination
GSH levels were estimated using GSH/GSSG recycling method [
27]. Briefly, heart samples were lysed with sulfosalicylic acid 0.6%, Triton-X 0.1%, 0.1M potassium phosphate buffer, 5mM EDTA, pH 7.5 and centrifuged (8000 g, 10 min, 4
οC). Total glutathione was measured using GSH reductase–DTNB recycling assay and the rate of color developed was measured at 412 nm. GSSG was measured by incubating a portion of the lysate with 2-vinyl pyridine for 1 h at room temperature prior to recycling assay. Both GSH and GSSG concentrations were calculated using standard curves and expressed in nmol/mg of total protein GSH was determined by subtracting GSSG content from total glutathione content.
2.7. Determination of antioxidant enzymes activity
Heart samples were homogenized (1:10 w/v) in 50 mM sodium phosphate, pH 7.4, sonicated for 3x15 sec and centrifuged (11000g, 10 min, 4°C). The supernatant was kept on ice for the measurement of enzymatic activities.
2.7.1. Catalase (CAT) activity assay
The determination of catalase activity was based on H
2O
2 conversion which was monitored spectrophotometrically at 240 nm (extinction coefficient ε=0.03941 mM
-1cm
-1) [
28]. The assay mixture contained 50 mM sodium phosphate buffer, pH 7.4, 30 mM H
2O
2 and tissue extract. The rate of the reaction per minute was recorded for 5 minutes, and the enzyme activity was expressed in U/mg of protein.
2.7.2. Superoxide dismutase (Mn-SOD) activity assay
Mn-SOD activity was determined by the rate of nitroblue tetrazolium (NBT) reduction at 550 nm in a reaction medium containing 50 mM phosphate buffer pH 8.0, 0.1 mM EDTA, 1 mM potassium cyanide, 0.056 mM NBT, 0.1 mM xanthine, and 8 mU xanthine oxidase [
29]. A standard curve with known amounts of SOD was used to calculate enzymatic activity from the % inhibition of NBT reduction in the samples. Enzyme activity was expressed as U/µg protein.
2.7.3. Glutathione peroxidase (GPx) activity assay
The determination of glutathione peroxidase (GPx) activity was based on the reduction of cumene hydroperoxide coupled with the reduction of GSSG by glutathione reductase. The assay mixture contained 50 mM potassium phosphate pH 7.0, 0.5 mM EDTA, 1 mM GSH, 0.1 Unit of GR, 0.15 mM NADPH, 0.2 mM of cumene hydroperoxide and tissue extract. The rate of conversion of NADPH to NADP+ was monitored spectrophotometrically at 340 nm (extinction coefficient ε=6200 M-1cm-1). Activity of the enzyme was expressed as U/mg of protein.
2.7.4. Glutathione reductase (GR) activity assay
Glutathione reductase was assayed in a mixture containing 50 mM sodium phosphate buffer pH 7.4, 2 mM EDTA, 1 mM GSSG, 0.15 mM NADPH and tissue extract. The rate of conversion of NADPH to NADP+ was monitored spectrophotometrically at 340 nm (extinction coefficient ε=6200 M-1cm-1). GR activity was expressed as mU/mg of protein [
30].
2.8. Statistical analysis
The statistical processing of the results was performed using Instat software, Graph Pad (San Diego, SA). All data were expressed as mean ± standard error (S.E.M) and n refers to individual measurements. To examine statistically significant differences, an analysis of variance (ANOVA) was performed. Unpaired t-test and Tukey test were used to compare samples between groups, respectively. A difference was considered statistically significant when p<0.05.
3. Results
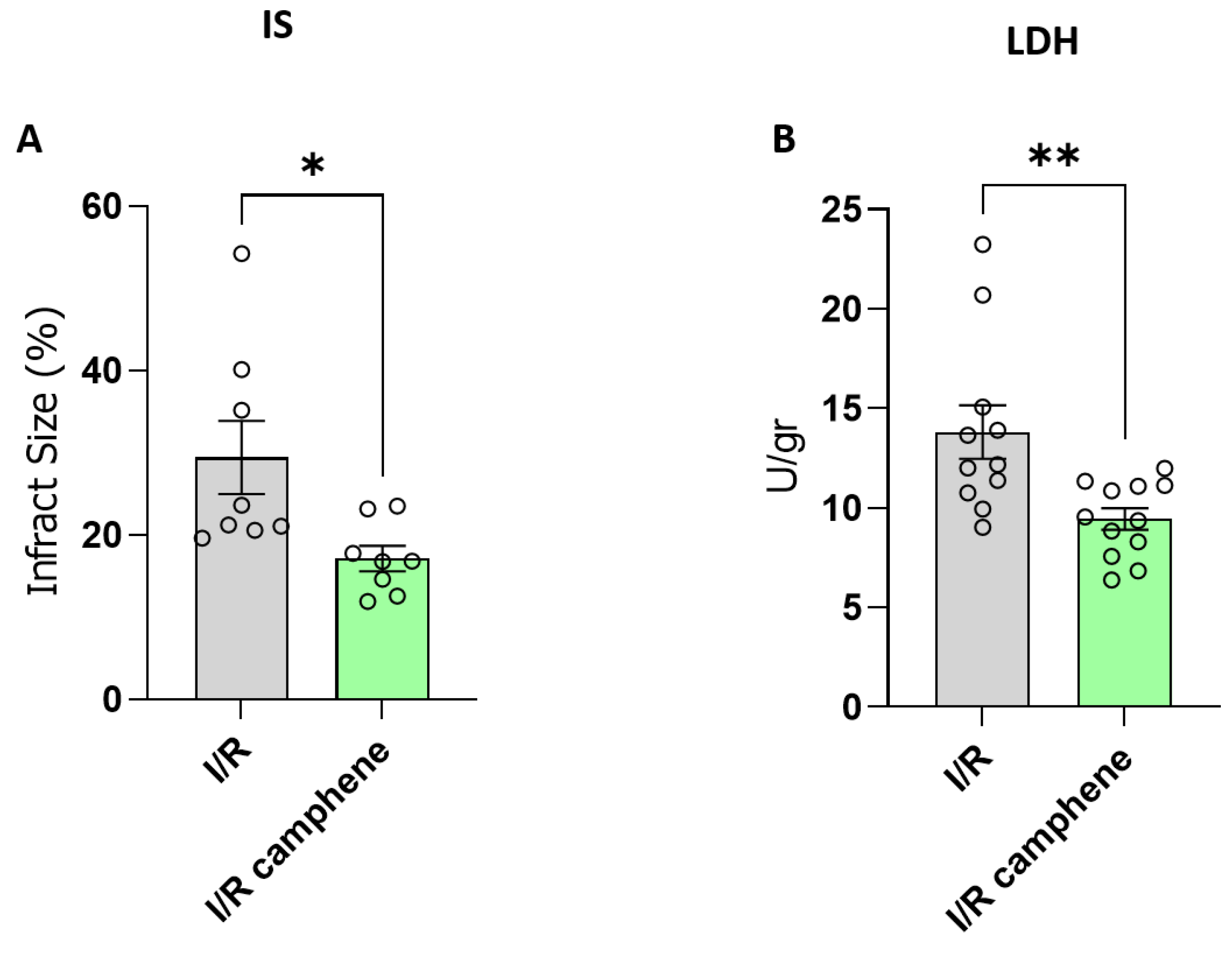
3.1. Camphene pretreatment reduces infarct size and ameliorates myocardial I/R injury
To explore the protective role of camphene in response to I/R injury, rat hearts were subjected to ex vivo I/R. Administration of camphene resulted in a significant reduction of infarct size compared with hearts isolated from animals that did not receive any treatment, specifically 29.45 % ± 4.44 in I/R hearts and 17.14 % ± 1.53 in I/R-camphene (
Figure 1A). This result was also accompanied by a decreased release of LDH in the effluent of hearts from animals pretreated with camphene compared with the hearts from non-treated animals, showing that the induction of cell death due to I/R in rat hearts is reduced in the presence of camphene pretreatment (
Figure 1B). Specifically, LDH activity was 13.81 ± 1.33 U/gr in the effluent from I/R hearts, while it was 9.44 ± 0.54 U/gr in the effluent of I/R hearts from animals pretreated with camphene.
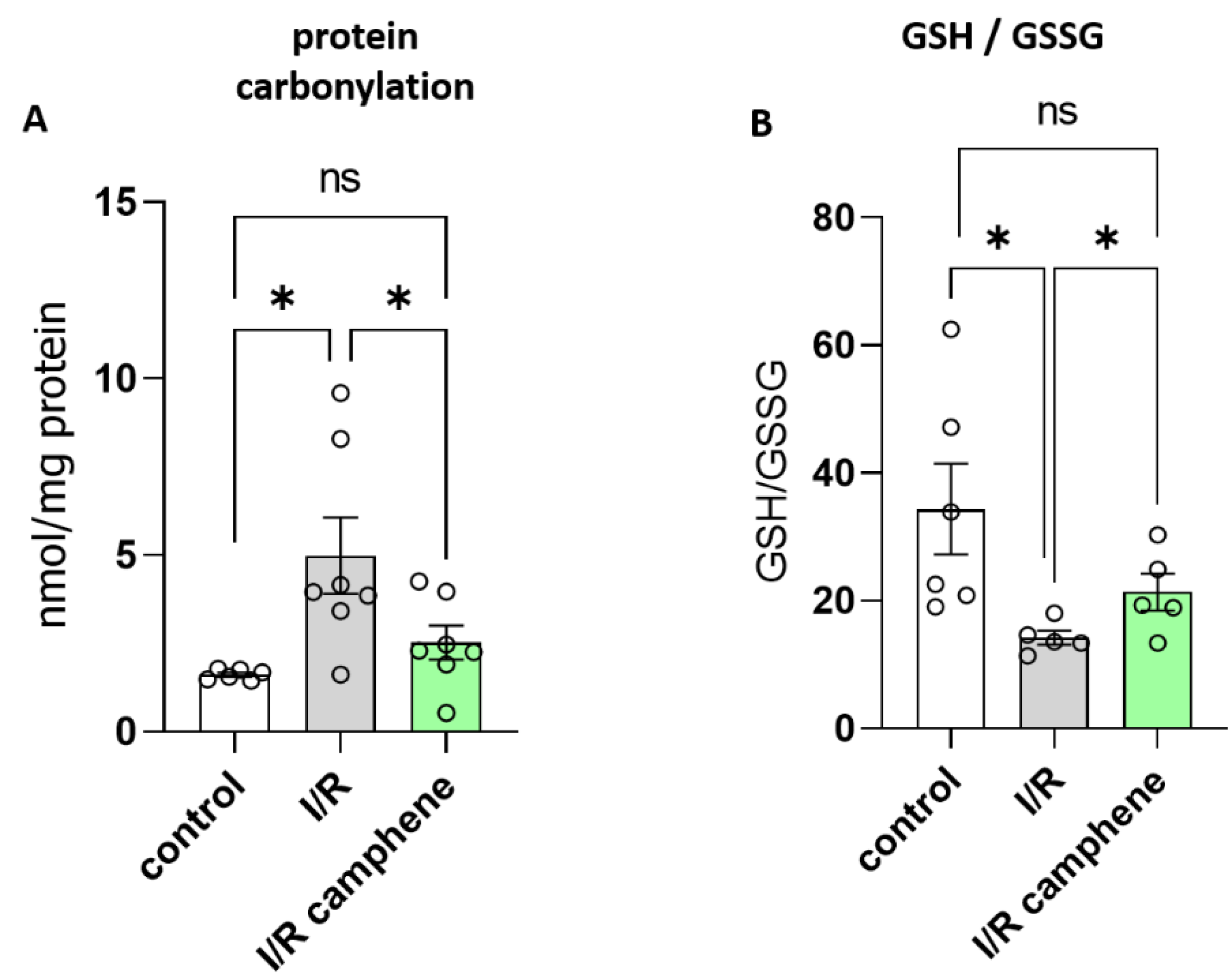
3.2. Camphene attenuates the I/R- induced oxidative stress
It is well established that oxidative stress is a major contributing factor in I/R injury. In order to assess the effect of camphene on the level of oxidative stress following myocardial I/R, protein carbonylation levels, as well as the GSH/GSSG ratio were evaluated. Carbonylation is a post- translational protein modification due to oxidative stress, while GSH system is the primary defense mechanism against ROS, with the ratio between reduced GSH and the oxidized form GSSG being used as an index of the redox status of cells [
5]. As expected, I/R increased the levels of protein carbonyls as a response to oxidative stress from 1.61 ± 0.57 nmol/mg of protein in control hearts to 4.98 ± 1.07 nmol/mg of protein in I/R hearts (
Figure 2A). This effect was attenuated in the hearts from animals treated with camphene prior to I/R, namely protein carbonyls were 2.52 ± 0.47 nmol/mg of total protein, which is significantly lower than those determined in hearts from non-treated animals (
Figure 2A). The beneficial effect of camphene on I/R-induced oxidative stress was confirmed when GSH/GSSG ratio was determined. The decreased GSH/GSSG ratio observed in the hearts of non-treated animals following I/R was 14.21 ± 1.09, significantly lower than the ratio in control hearts, which was 34.33 ± 7.1. GSH/GSSG ration was partially restored to 21.35 ± 2.89 in the hearts of camphene treated animals (
Figure 2B).
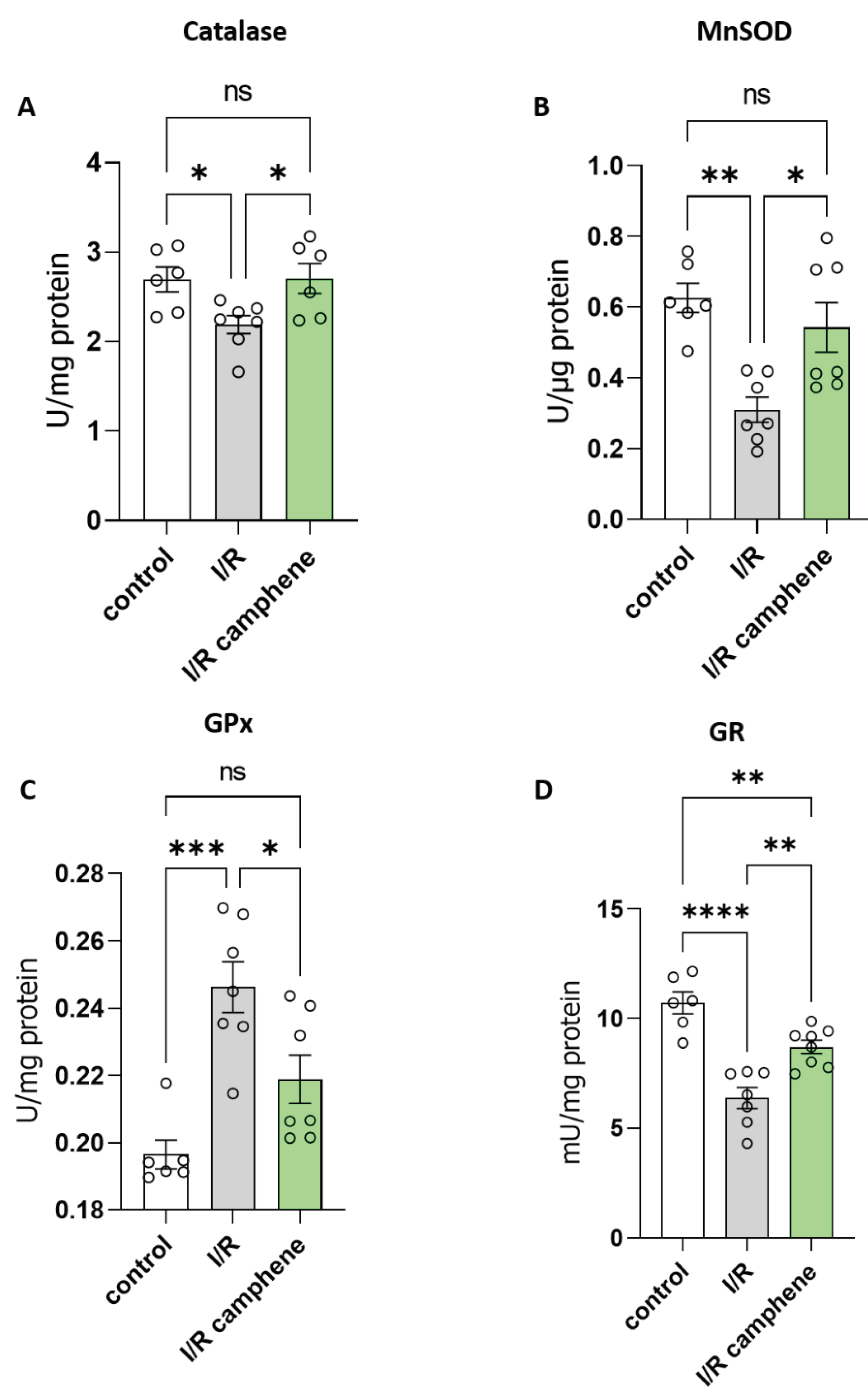
3.3. Camphene pretreatment activates antioxidant mechanisms in the heart
To identify the mechanisms underlying the protective role of camphene against oxidative stress induced by I/R, the activity of enzymes related to cellular antioxidant defense was evaluated. The results point to an activation of antioxidant intracellular mechanisms in the presence of camphene. Specifically, the enzymatic activity of catalase (CAT), the enzyme that catalyzes the conversion of H
2O
2 into H
2O and O
2 in cells, was decreased in I/R while this effect was attenuated in the presence of camphene (
Figure 3A). Furthermore, the activity of Mn-SOD, the enzyme that catalyzes the conversion of superoxide radicals to H
2O
2 and H
2O, was reduced by I/R in non-treated animals compared with control hearts, while this downregulation was prevented in hearts from animals treated with camphene prior to the I/R. Specifically, Mn-SOD activity was 0.62 ± 0.04 U/μg in control hearts, 0.32 ± 0.03 U/μg in I/R hearts and 0.48 ± 0.05 U/μg in I/R-camphene hearts (
Figure 3B). Additionally, glutathione peroxidase (GPx) activity was evaluated, since GPx is a cytoplasmic enzyme that catalyzes the conversion of H
2O
2 to H
2O, or the conversion of peroxides to the corresponding alcohols, with the simultaneous oxidation of the reduced form of glutathione GSH, converting it to GSSG. GPx activity was elevated in the hearts that underwent I/R compared to control hearts, while animal pretreatment with camphene led to attenuation of the I/R observed effect. Namely, GPx activity was 0.19 ± 0.004 U/mg, 0.24 ± 0.007 U/mg and 0.21 ± 0.007 in control, I/R and I/R-camphene hearts, respectively (
Figure 3C). Glutathione reductase (GR) is localized in the cytoplasm of cells and catalyzes the conversion of the oxidized form of glutathione (GSSG) to its reduced form (GSH), using the coenzyme NADPH as an electron donor. When GR activity was assessed, it was found elevated in I/R hearts compared with control hearts. However, camphene pretreatment reduced the I/R observed effect, namely from 5.18 ±1.46 U/μg in I/R hearts to 2.52 ± 0.47 U/μg in I/R-camphene hearts (
Figure 3D).
4. Discussion
Timely and effective reperfusion of the ischemic myocardium is the only available treatment of choice up to date. However, instead of providing a salvation, reperfusion can paradoxically intensify injury, known as I/R injury, inducing cardiac arrhythmias, enhancing metabolic defects, and producing structural damage to cardiomyocytes in the heart [
31,
32]. Several mechanisms have been shown to contribute to I/R, namely the development of oxidative stress and the occurrence of intracellular Ca
2+-overload, as well as myocardial inflammation and alterations in cardiac metabolism [
32,
33]. However, it is becoming apparent that oxidative stress is the most critical pathogenetic factor; the production of a large amount of ROS during I/R directly or indirectly affects cardiac function, causes cardiomyocyte dysfunction, and promotes cell damage [
34,
35]. In the present work, we demonstrated that camphene, as a natural product, showed a cardioprotective role in response to myocardial I/R injury as evidenced by the limitation of infarct size and amelioration of cell damage in the hearts of animals pretreated with camphene. Camphene has a therapeutic effect through the upregulation of antioxidant defense mechanisms and consequently attenuation of oxidative stress and cell damage.
Under physiological conditions, a critical balance exists between ROS production and the endogenous antioxidant system maintaining redox homeostasis. However, under pathological stimuli, including I/R, the excess production of ROS and/or the inability of the antioxidant defense mechanisms to counteract them lead to the opening of the mitochondrial permeability transition pore, lipid peroxidation, and DNA damage, thereby causing cellular dysfunction and death [
3,
32]. Thus, strategies that prevent or alleviate oxidative stress response are being developed for the treatment of myocardial I/R damage and several studies have reported beneficial effects of antioxidants rendering resistance to the heart against I/R injury. In particular, finding safe and effective drugs derived from natural products is an active research line in this direction. Accumulating evidence suggests that the active components of various medicinal plants, such as flavonoids and phenolics, can protect myocardial cells by regulating oxidative stress and have a good therapeutic effect on I/R injury [
11,
36,
37]. Our study demonstrated that augmented LDH activity and the increased infarct size elicited by I/R damage, were significantly mitigated (
Figure 1) in the rats treated with camphene, indicating that camphene attenuated the I/R-induced myocardial injury.
A range of endogenous antioxidants, primarily enzymatic antioxidants (catalase -CAT, superoxide dismutase-SOD, glutathione peroxidase-GPx, glutathione reductase-GR, thioredoxin reductase-TrxR, etc.), are present in the cells to maintain redox homeostasis. Glutathione in its reduced state (GSH) is the most common non-protein thiol in animal cells and it is considered to be one of the most powerful endogenous antioxidant systems in the cardiovascular system due to its key contribution to scavenging overreactive oxygen species (ROS). The scavenging activity of GSH maintains thiol groups of enzymes and other proteins in their reduced state, thus preventing cell membrane lipid peroxidation and limiting cardiomyocytes loss [
38,
39]. The ratio GSH/GSSG is largely considered as a marker of the cellular redox state, since it is affected by oxidative stress and in some cases even as a quantitative marker of the severity of the disease. A decrease in the GSH/GSSG levels has been described in animal models of I/R [
40] and this was corroborated in our study. However, camphene treatment significantly attenuated this effect, increasing the GSH/GSSG ratio in heart tissues after I/R (
Figure 2B). Furthermore, the reduction of oxidative stress in the presence of camphene was confirmed when the levels of protein carbonylation, also extensively used as a marker for cardiac oxidative stress [
41], were determined. Treatment of animals with camphene reversed the reduction of myocardial protein carbonyls observed in I/R (
Figure 2A).
Enzymatic antioxidants such as CAT, SOD, GPx and GR, which are also crucial components of the cellular defense mechanisms against oxidative stress, are significantly impacted by I/R. However, controversies exist, with some studies demonstrating increased activities during reperfusion as a protective response, while others report decreased activities due to oxidative inactivation [
42,
43,
44]. It seems that the impact of I/R on endogenous antioxidants can vary depending on factors such as the duration and severity of ischemia, the extent of reperfusion injury, and the cellular context [
44]. I/R depressed the activities of CAT, Mn-SOD, and GR while camphene treatment reversed this effect (
Figure 3A, B and D). On the other hand, GPx activity was increased in I/R hearts with camphene treatment again attenuating the effect (
Figure 3C). Besides the duration of ischemia and/or reperfusion [
43,
44], GPx activity can differ according to the cellular content of lipid peroxides or even the form of the enzyme, being selenium dependent or independent [
45].
Overall, the results of the present study demonstrate that a single dose of camphene prior to the induction of I/R can attenuate the oxidative stress- induced damage in myocardium. Camphene modulates the activities of endogenous antioxidant enzymes contributing to the maintenance of cellular redox homeostasis. Further research and a better understanding of the mechanism of camphene may provide a novel strategy for preventing myocardial reperfusion injury.
5. Conclusions
Camphene effectively protects cardiomyocytes against I/R injury activating endogenous antioxidants mechanisms and maintaining redox balance. Recently, considerable progress has been achieved in treatments designed to reduce oxidative stress during myocardial I/R in vitro and in vivo. In particular, targeted enhancement of the GSH system in the myocardium has been considered as a potential therapeutic strategy to prevent myocardial injury. In this respect, camphene may be a promising plant-derived natural compound with therapeutic potential in ischemic heart disease, especially in high-risk populations. However, one of the greatest challenges is to transform the efficacy of preclinical animal research into clinical practice.
Author Contributions
Conceptualization AL; methodology and formal analysis RS, MA and CR; writing-original draft preparation RS, MA and AL; writing-review and editing RS, MA, CR and AL; supervision AL All authors have read and agreed to the published version of the manuscript
Funding
This research was partly funded by the postgraduate program “Applications in Biology”, of the School of Biology, Aristotle University of Thessaloniki.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Committee on the Ethics of Animal Experiments of the Directorate of Veterinary Services of Prefecture of Thessaloniki (protocol code 177474(710) 27/04/2021).
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Yellon DM, Hausenloy DJ. Myocardial reperfusion injury. N Engl J Med. 2007; 357(11):1121-35. [CrossRef]
- Andreadou I., Daiber A., Baxter G.F., Brizzi M.F., Di Lisa F., Kaludercic N., Lazou A., Varga Z.V., Zuurbier C.J., Schulz R., Ferdinandy P. Influence of cardiometabolic comorbidities on myocardial function, infarction, and cardioprotection: Role of cardiac redox signaling. Free Radical Biology and Medicine, 2021; 166, 33-52. [CrossRef]
- Egea J, Fabregat I, Frapart YM, Ghezzi P, Görlach A, Kietzmann T, Kubaichuk K, Knaus UG, Lopez MG, Olaso-Gonzalez G, et al. European contribution to the study of ROS: A summary of the findings and pro-spects for the future from the COST action BM1203 (EU-ROS). Redox Biol. 2017; 13:94-162. doi:10.1016/j.redox.2017.05.007. Epub 2017 May 18. Erratum in: Redox Biol. 2018 Apr;14 :694-696. PMID: 28577489; [CrossRef]
- Davidson SM, Adameová A, Barile L, Cabrera-Fuentes HA, Lazou A, Pagliaro P, Stensløkken KO, Garcia-Dorado D; EU-CARDIOPROTECTION COST Action (CA16225). Mitochondrial and mitochondrial-independent pathways of myocardial cell death during ischaemia and reperfusion injury. J Cell Mol Med. 2020; 24(7):3795-3806. [CrossRef]
- Pizzorno J. Glutathione! Integr Med (Encinitas). 2014; 13(1):8-12. PMID: 26770075.
- Barteková M., Adameová A, Görbe A., Ferenczyová K., Pecháňová O., Lazou A., Dhalla N.S., Ferdinandy P., Giricz Z. Natural and synthetic antioxidants targeting cardiac oxidative stress and redox signaling in car-diometabolic diseases, Free Radic Biol Med. 2021; 169:446-477. [CrossRef]
- Matuz-Mares D., Riveros-Rosas H., Vilchis-Landeros M.M., Vázquez-Meza H. Glutathione Participation in the Prevention of Cardiovascular Diseases. Antioxidants 2021; 10, 1220. [CrossRef]
- Papatheodorou I., Galatou E., Panagiotidis G.-D., Ravingerová T., Lazou A. Cardioprotective Effects of PPARβ/δ Activation against Ischemia/Reperfusion Injury in Rat Heart Are Associated with ALDH2 Upreg-ulation, Amelioration of Oxidative Stress and Preservation of Mitochondrial Energy Production. Int. J. Mol. Sci. 2021; 22, 6399. [CrossRef]
- Papatheodorou I., Makrecka-Kuka M., Kuka J., Liepinsh E., Dambrova M., Lazou A. Pharmacological acti-vation of PPARβ/δ preserves mitochondrial respiratory function in ischemia/reperfusion via stimulation of fatty acid oxidation-linked respiration and PGC-1α/NRF-1 signaling. Front Endocrinol (Lausanne). 2022, 15;13:941822. [CrossRef]
- Barlaka E., Galatou E., Mellidis K., Ravingerova T., Lazou A. Role of Pleiotropic Properties of Peroxisome Proliferator-Activated Receptors in the Heart: Focus on the Nonmetabolic Effects in Cardiac Protection. Cardiovasc Ther. 2016; 34(1):37-48. [CrossRef]
- Tsoumani M, Georgoulis A, Nikolaou PE, Kostopoulos IV, Dermintzoglou T, Papatheodorou I, Zoga A, Efen-takis P, Konstantinou M, Gikas E, Kostomitsopoulos N, Papapetropoulos A, Lazou A, Skaltsounis AL, Hausenloy DJ, Tsitsilonis O, Tseti I, Di Lisa F, Iliodromitis EK, Andreadou I. Acute administration of the ol-ive constituent, oleuropein, combined with ischemic postconditioning increases myocardial protection by modulating oxidative defense. Free Radic Biol Med. 2021; 166:18-32. [CrossRef]
- Verschueren K. fourth ed. Handbook of Environmental Data on Organic Chemicals. John Wiley and Sons, New York. 2001;1–2:419.
- de Carvalho HC, Ieque AL, Valverde TL, Baldin VP, Meneguello JE, Campanerut-Sá PAZ, Vandresen F, Ghi-raldi Lopes LD, Passos Souza MR, Santos NCS, Dias Siqueira VL, Caleffi-Ferracioli KR, Lima Scodro RB, Cardoso RF. Activity of (-)-Camphene Derivatives Against Mycobacterium tuberculosis in Acidic pH. Med Chem. 2021; 17(5):485-492. [CrossRef]
- de Freitas BC, Queiroz PA, Baldin VP, do Amaral PH, Rodrigues LL, Vandresen F, R Caleffi-Ferracioli K, de L Scodro RB, Cardoso RF, Siqueira VL. (-)-Camphene-based derivatives as potential antibacterial agents against Staphylococcus aureus and Enterococcus spp. Future Microbiol. 2020; 15:1527-1534. [CrossRef]
- Yamaguchi M.U., Barbosa da Silva A.P., Ueda-Nakamura T., Dias Filho B.P., Conceição da Silva C., Naka-mura C.V. Effects of a Thiosemicarbazide Camphene Derivative on Trichophyton mentagrophytes. Molecules 2009, 14, 1796-1807. [CrossRef]
- Gadotti VM, Huang S, Zamponi GW. The terpenes camphene and alpha-bisabolol inhibit inflammatory and neuropathic pain via Cav3.2 T-type calcium channels. Mol Brain. 2021; 14(1):166. [CrossRef]
- Lin CT, Chen CJ, Lin TY, Tung JC, Wang SY. Anti-inflammation activity of fruit essential oil from Cin-namomum insularimontanum Hayata. Bioresour Technol. 2008; 99(18):8783-7. [CrossRef]
- Boyd EM, Sheppard P. Nutmeg Oil and Camphene as Inhaled Expectorants. Arch Otolaryngol. 1970; 92(4):372–378. [CrossRef]
- Quintans-Júnior L., Moreira J.C. F., Pasquali M. A. B., Rabie S. M. S., Pires A. S., Schröder R., Rabelo T. K., Santos J.P. A., Lima P. S. S., Cavalcanti S.C. H, Araújo A.A. S., Quintans J.S. S., Gelain D.P. Antinociceptive Activity and Redox Profile of the Monoterpenes (+)-Camphene, p-Cymene, and Geranyl Acetate in Experi-mental Models. International Scholarly Research Notices, 2013, Article ID 459530, 11 pages, 2013. [CrossRef]
- Vallianou I, Hadzopoulou-Cladaras M. Camphene, a plant-derieved monoterpene, exerts its hypolipidemic action by affecting SREBP-1 and MTP expression. PLoS ONE. 2016; 11(1):e0147117.
- Chroni A, Liu T, Gorshkova I, Kan HY, Uehara Y, Von Eckardstein A, Zannis VI. The central helices of ApoA- I can promote ATP-binding cassette transporter A1 (ABCA1)-mediated lipid efflux. Amino acid res-idues 220–231 of the wild-type ApoAI are required for lipid efflux in vitro and high density lipoprotein formation in vivo. The Journal of biological chemistry. 2003; 278(9):6719–6730.
- Yang L, Liu H, Xia D, Wang S. Antioxidant Properties of Camphene-Based Thiosemicarbazones: Experi-mental and Theoretical Evaluation. Molecules. 2020; 25(5):1192. [CrossRef]
- Suji B, Jisu K, Byung M. Camphene Attenuates Skeletal Muscle Atrophy by Regulating Oxidative Stress and Lipid Metabolism in Rats. Nutrients 2020; 12, 3731.
- Vallianou I, Peroulis N, Pantazis P, Hadzopoulou-Cladaras M. Camphene, a plant-derived monoterpene, reduces plasma cholesterol and triglycerides in hyperlipidemic rats independently of HMG-CoA reductase activity. PLoS One. 2011; 6(11):e20516. [CrossRef]
- Burd JF, Usategui-Gomez M. Immunochemical studies on lactate dehydrogenase. Biochim Biophys Acta. 1973; 310(1):238-47. [CrossRef]
- Colombo G, Clerici M, Garavaglia ME, Giustarini D, Rossi R, Milzani A, Dalle-Donne I. A step-by-step pro-tocol for assaying protein carbonylation in biological samples. J Chromatogr B Analyt Technol Biomed Life Sci. 2016; 1019:178-90. [CrossRef]
- Rahman I, Kode A, Biswas SK. Assay for quantitative determination of glutathione and glutathione disul-fide levels using enzymatic recycling method. Nat Protoc. 2006; 1(6):3159-65. [CrossRef]
- Cohen G, Dembiec D, Marcus J. Measurement of catalase activity in tissue extracts. Anal Biochem. 1970; 34:30-8. [CrossRef]
- Beauchamp C, Fridovich I. Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem. 1971; 44(1):276-87. [CrossRef]
- Carlberg I, Mannervik B. Glutathione reductase. Methods Enzymol. 1985; 113:484-90. [CrossRef]
- Dhalla N.S., Elmoselhi A.B., Hata T., Makino N. Status of myocardial antioxidants in ischemia–reperfusion injury, Cardiovascular Research, 2000; 47, 3, 446–456. [CrossRef]
- Hausenloy D.J., Yellon D.M. Myocardial ischemia-reperfusion injury: a neglected therapeutic target. J Clin Invest. 2013; 123(1):92-100. [CrossRef]
- Davidson S.M., Ferdinandy P., Andreadou I., Bøtker H.E., Heusch G., Ibáñez B., Ovize M., Schulz R., Yellon D.M., Hausenloy D.J., Garcia-Dorado D. CARDIOPROTECTION COST Action (CA16225). Multitarget Strategies to Reduce Myocardial Ischemia/Reperfusion Injury: JACC Review Topic of the Week. J Am Coll Cardiol. 2019; 73(1):89-99. [CrossRef]
- Xiang M., Lu Y., Xin L., Gao J., Shang C., Jiang Z., Lin H., Fang X., Qu Y., Wang Y., Shen Z., Zhao M., Cui X. Role of Oxidative Stress in Reperfusion following Myocardial Ischemia and Its Treatments. Oxid Med Cell Longev. 2021; 2021:6614009. [CrossRef]
- Bradic J, Zivkovic V, Srejovic I, Jakovljevic V, Petkovic A, Turnic TN, Jeremic J, Jeremic N, Mitrovic S, Sobot T, Ponorac N, Ravic M, Tomovic M. Protective Effects of Galium verum L. Extract against Cardiac Ischemia/Reperfusion Injury in Spontaneously Hypertensive Rats. Oxid Med Cell Longev. 2019;2019:4235405. [CrossRef]
- Draginic N, Milosavljevic I, Andjic M, Jeremic J, Nikolic M, Sretenovic J, Kocovic A, Srejovic I, Zivkovic V, Bolevich S, Bolevich S, Curcic S, Jakovljevic V. Short-Term Administration of Lemon Balm Extract Ameliorates Myocardial Ischemia/Reperfusion Injury: Focus on Oxidative Stress. Pharmaceuticals (Basel). 2022 Jul 8;15(7):840. [CrossRef]
- Chang X., Zhang T., Zhang W., Zhao Z., Sun J.. Natural Drugs as a Treatment Strategy for Cardiovascular Disease through the Regulation of Oxidative Stress. Hindawi Oxidative Medicine and Cellular Longevity 2020, Article ID 5430407, 20 pages. [CrossRef]
- Tan M., Yin Y., Ma X., Zhang J., Pan W., Tan M., Zhao Y., Yang T., Jiang T., Li H. Glutathione system en-hancement for cardiac protection: pharmacological options against oxidative stress and ferroptosis. Cell Death Dis 2023; 14, 131. [CrossRef]
- Daiber A., Andreadou I., Oelze M., Davidson S. M., Hausenloy D.J. Discovery of new therapeutic redox tar-gets for cardioprotection against ischemia/reperfusion injury and heart failure. Free Radical Biology and Medicine, 2021; 163, 325-343. ISSN 0891-5849. [CrossRef]
- Bertero E., Maack C. Ins and Outs of Glutathione in Cardiac Ischemia/Reperfusion Injury. J Circulation Re-search 2023; 133, 10, 877-879 doi:10.1161/CIRCRESAHA.123.323715%U https://www.ahajournals.org/doi/abs/10.1161/CIRCRESAHA.123.32371535. [CrossRef]
- Fedorova M, Griesser E, Vemula V, Weber D, Ni Z, Hoffmann R. Protein and lipid carbonylation in cellular model of nitrosative stress: mass spectrometry, biochemistry and microscopy study. Free Radic Biol Med. 2014; 75 Suppl 1:S15. [CrossRef]
- Haramaki N, Stewart DB, Aggarwal S, Ikeda H, Reznick AZ, Packer L. Networking antioxidants in the iso-lated rat heart are selectively depleted by ischemia-reperfusion. Free Radic Biol Med. 1998; 25(3):329-39. [CrossRef]
- Leichtweis, S., Ji, L.L. Glutathione deficiency intensifies ischaemia-reperfusion induced cardiac dysfunction and oxidative stress. Acta Physiologica Scandinavica 2001; 172: 1-10. [CrossRef]
- Marczin N., El-Habashi N., Hoare G. S., Bundy R. E., Yacoub M. Antioxidants in myocardial ischemia–reperfusion injury: therapeutic potential and basic mechanisms, Archives of Biochemistry and Biophysics, 2003; 420, 2,222-236, ISSN 0003-9861. [CrossRef]
- Aceto A., Mezzetti A., Di Ilio C., Calafiore A. M., De Cesare D., Bosco G., Acciai N., Cappelletti L., Federici G., Cuccurullo F. Effect of Ischaemia-Reperfusion on Glutathione Peroxidase, Glutathione Reductase and Glutathione Transferase Activities in Human Heart Protected by Hypothermic Cardioplegia, Free Radical Research Communications, 1990; 8:2, 85-91. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).