Submitted:
23 December 2023
Posted:
25 December 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Experimental section
2.1. Materials
2.2. Synthesis of functionalized bromine-naphthalene-based polyaminal networks (PAN-BrNA)
2.3. Synthesis of functionalized hydroxyl-naphthalene-based polyaminal networks (PAN-HNA)
2.4. Instrumentation
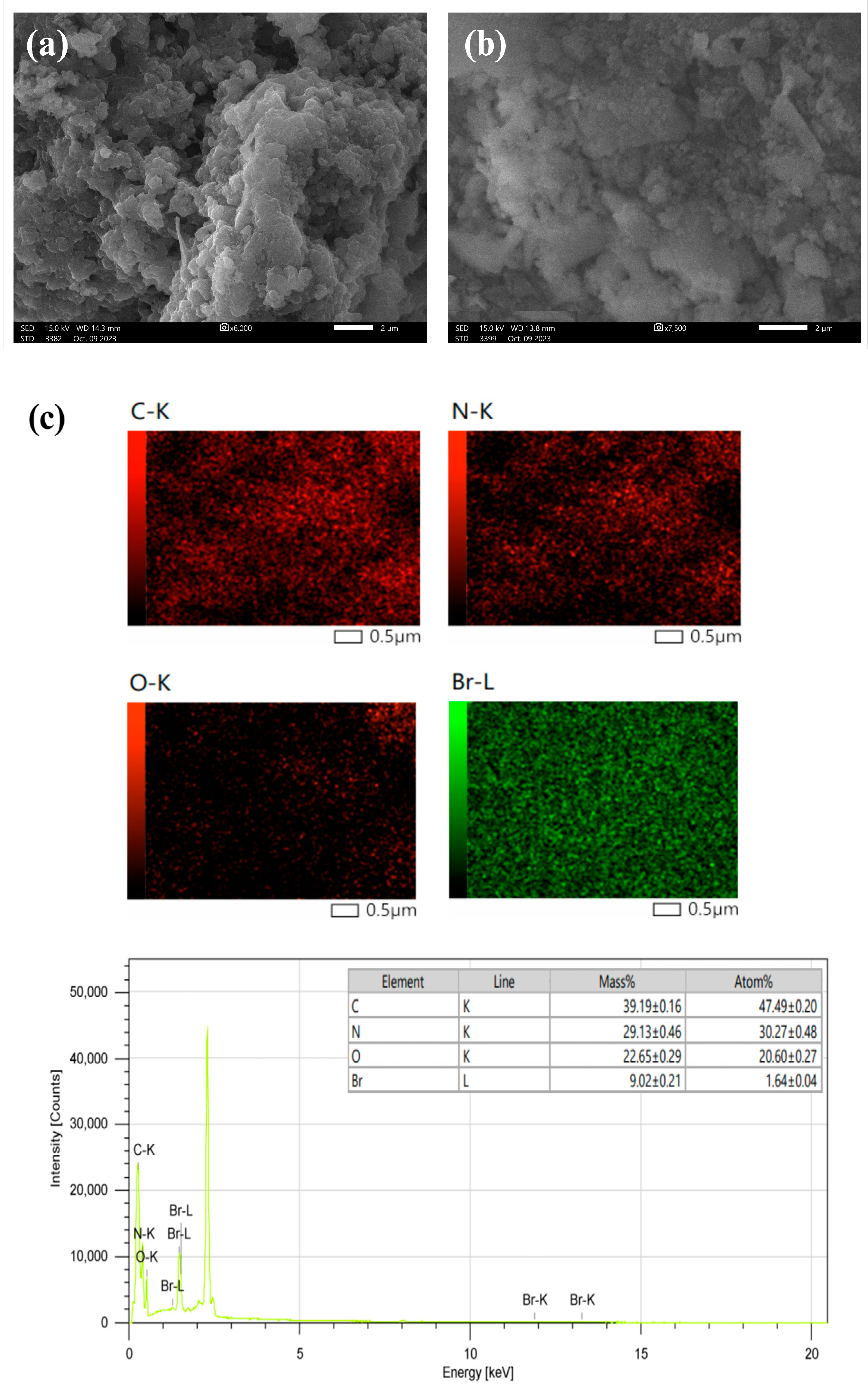
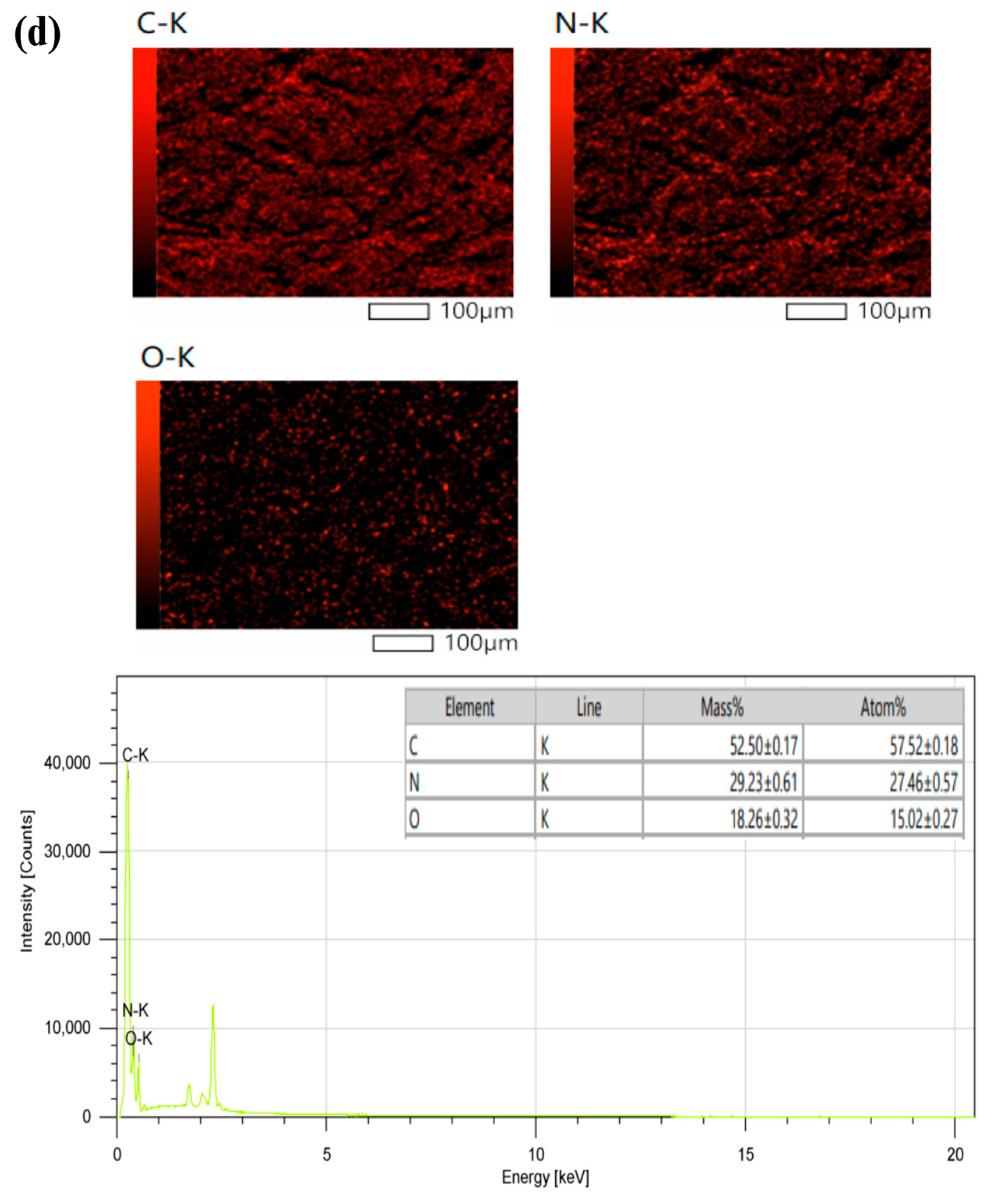
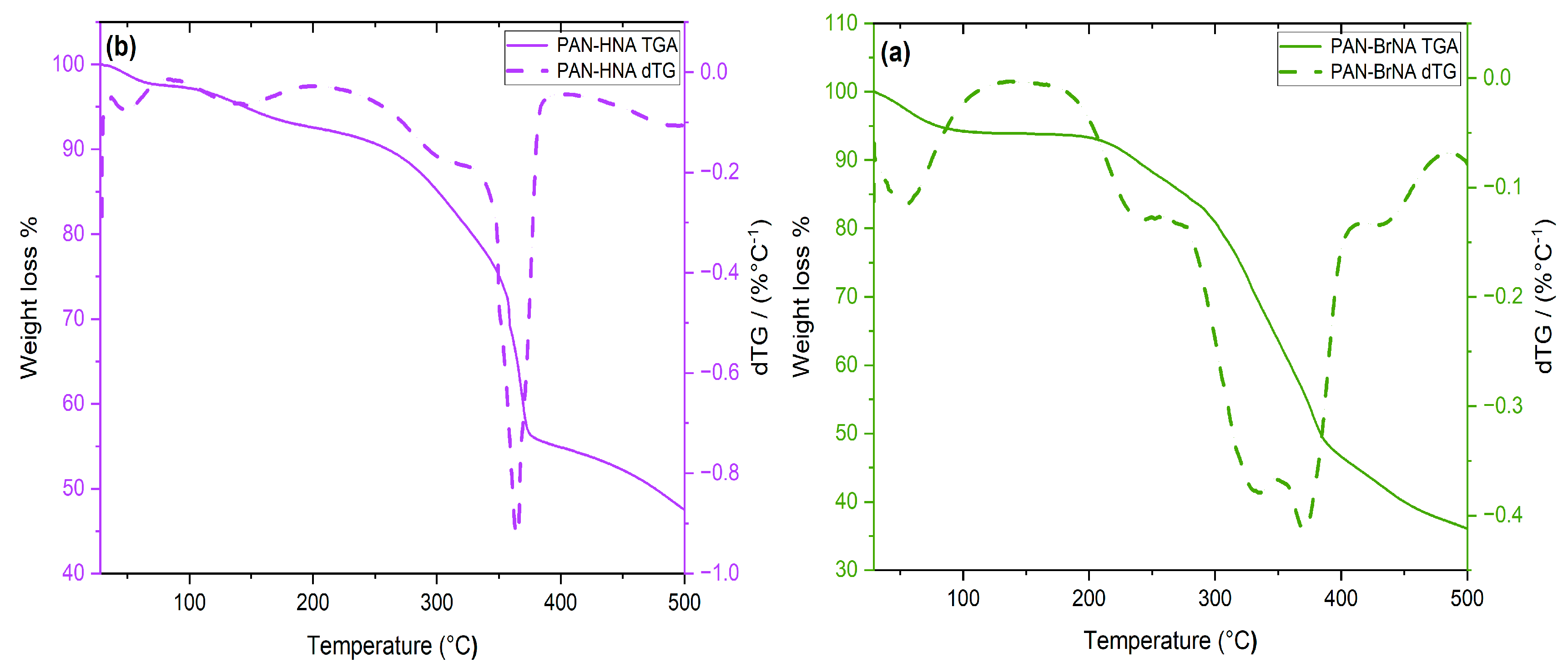
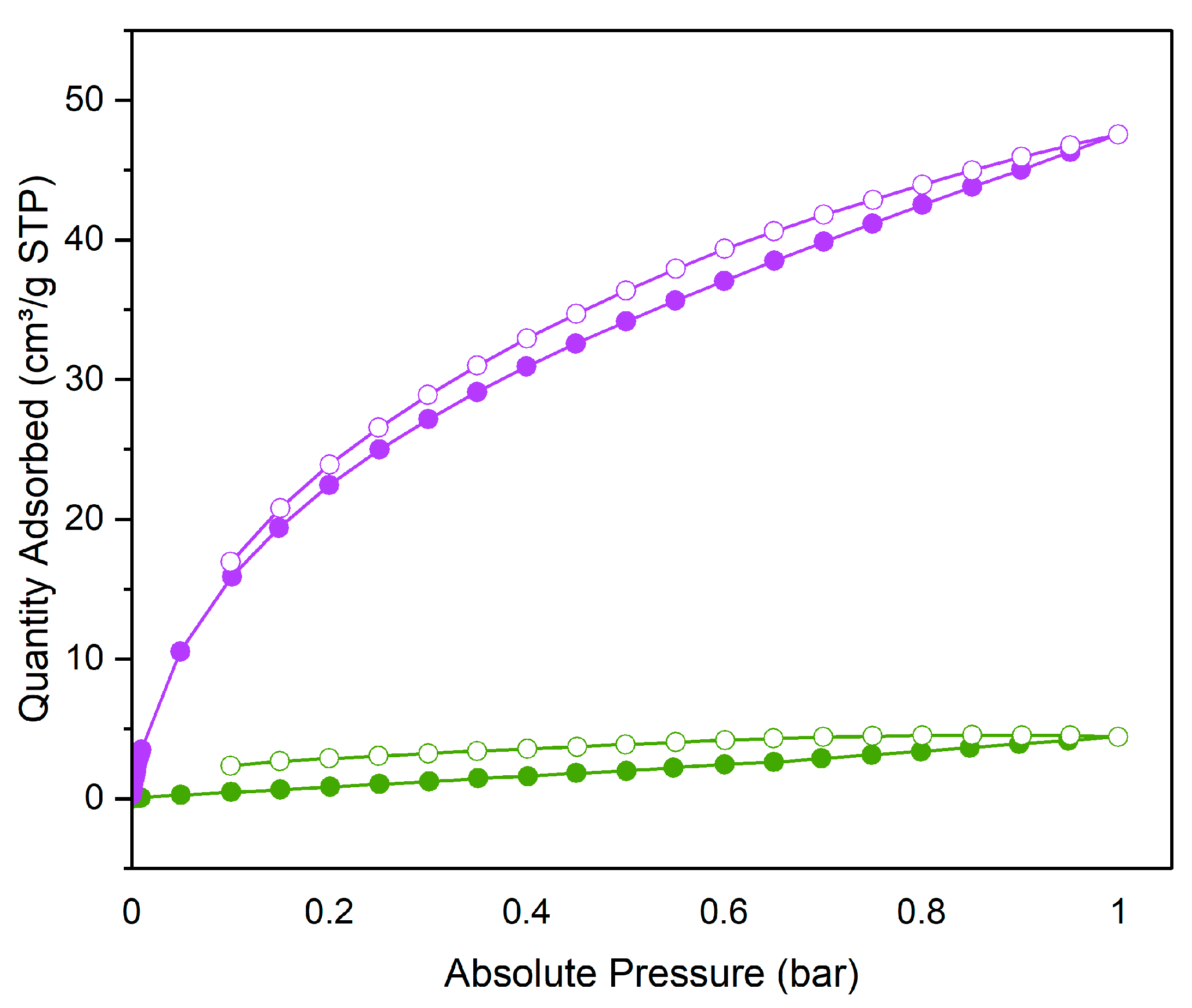
3. Results and discussion
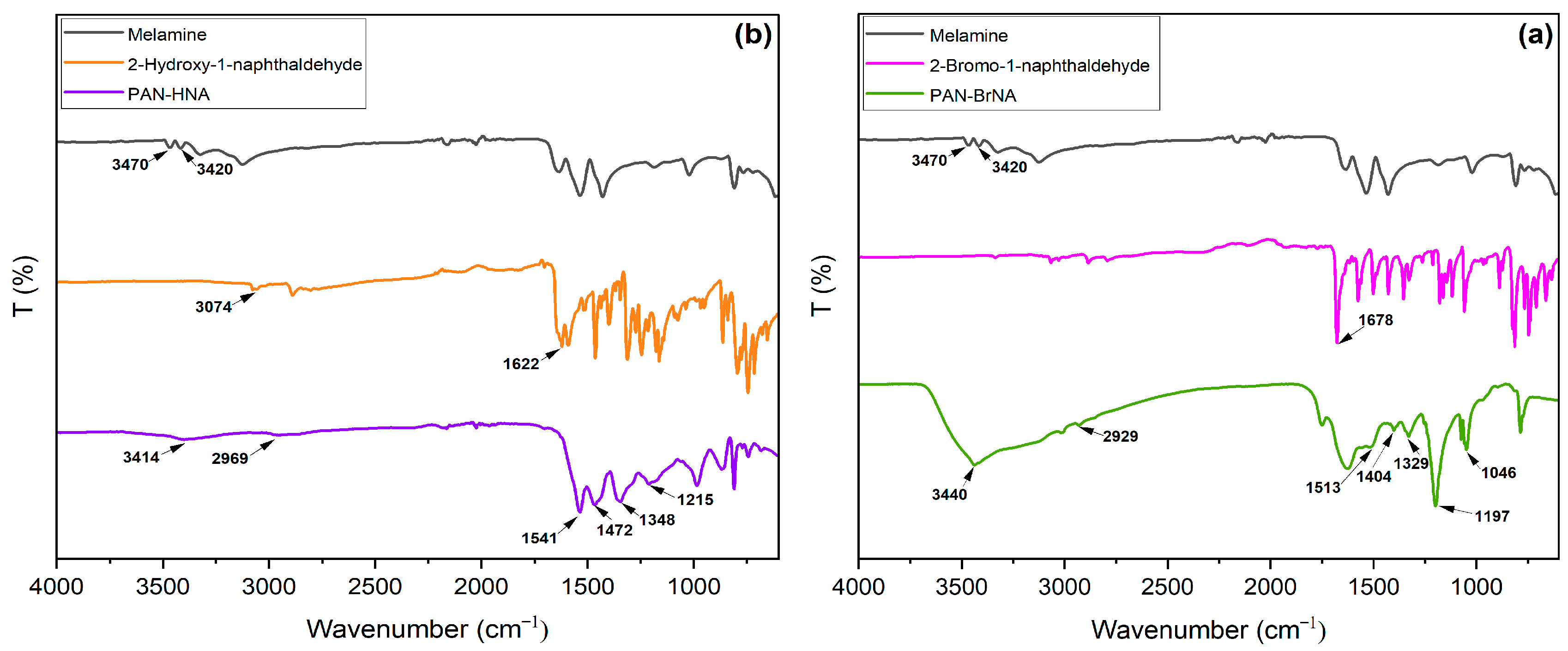
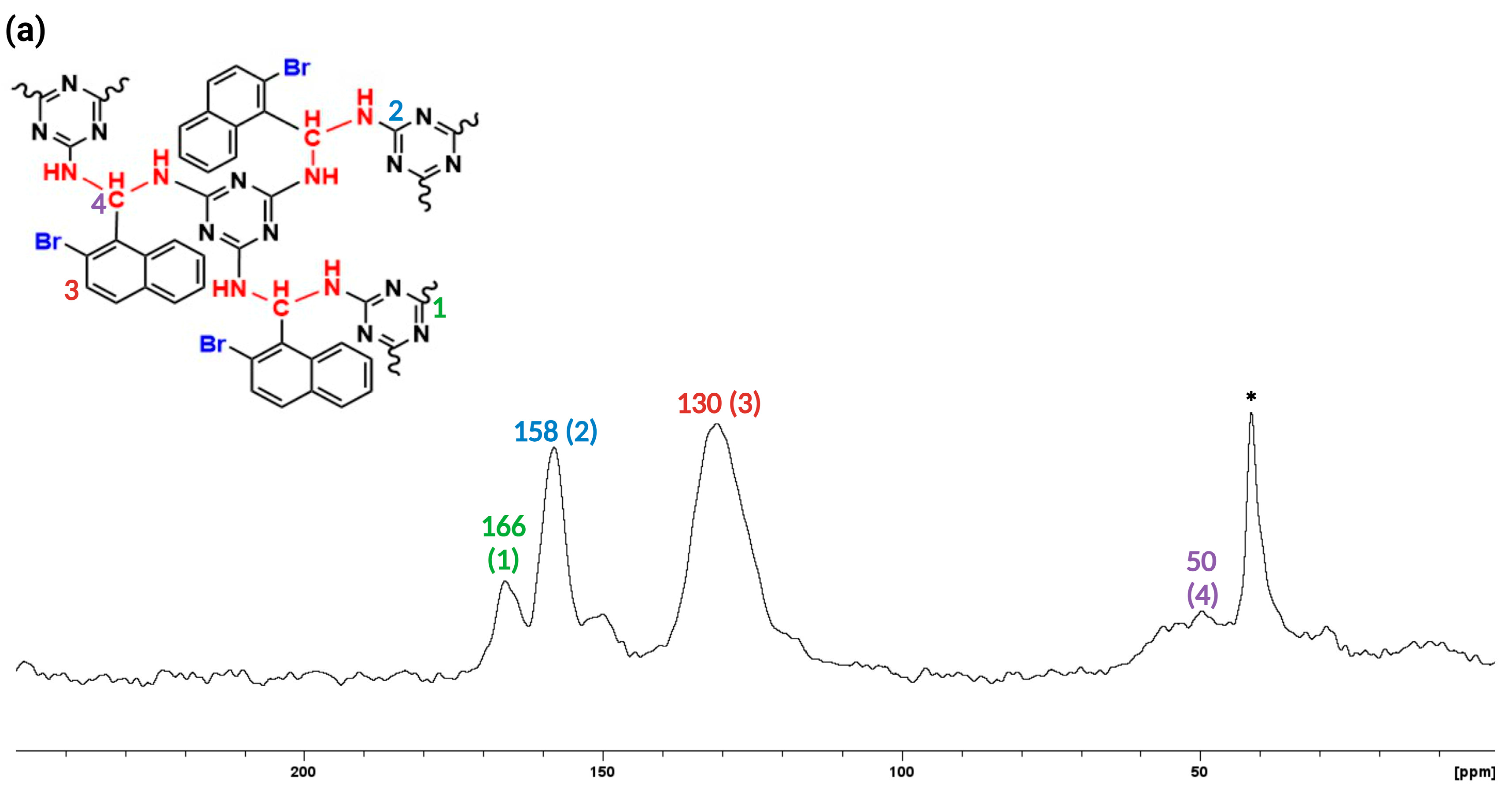
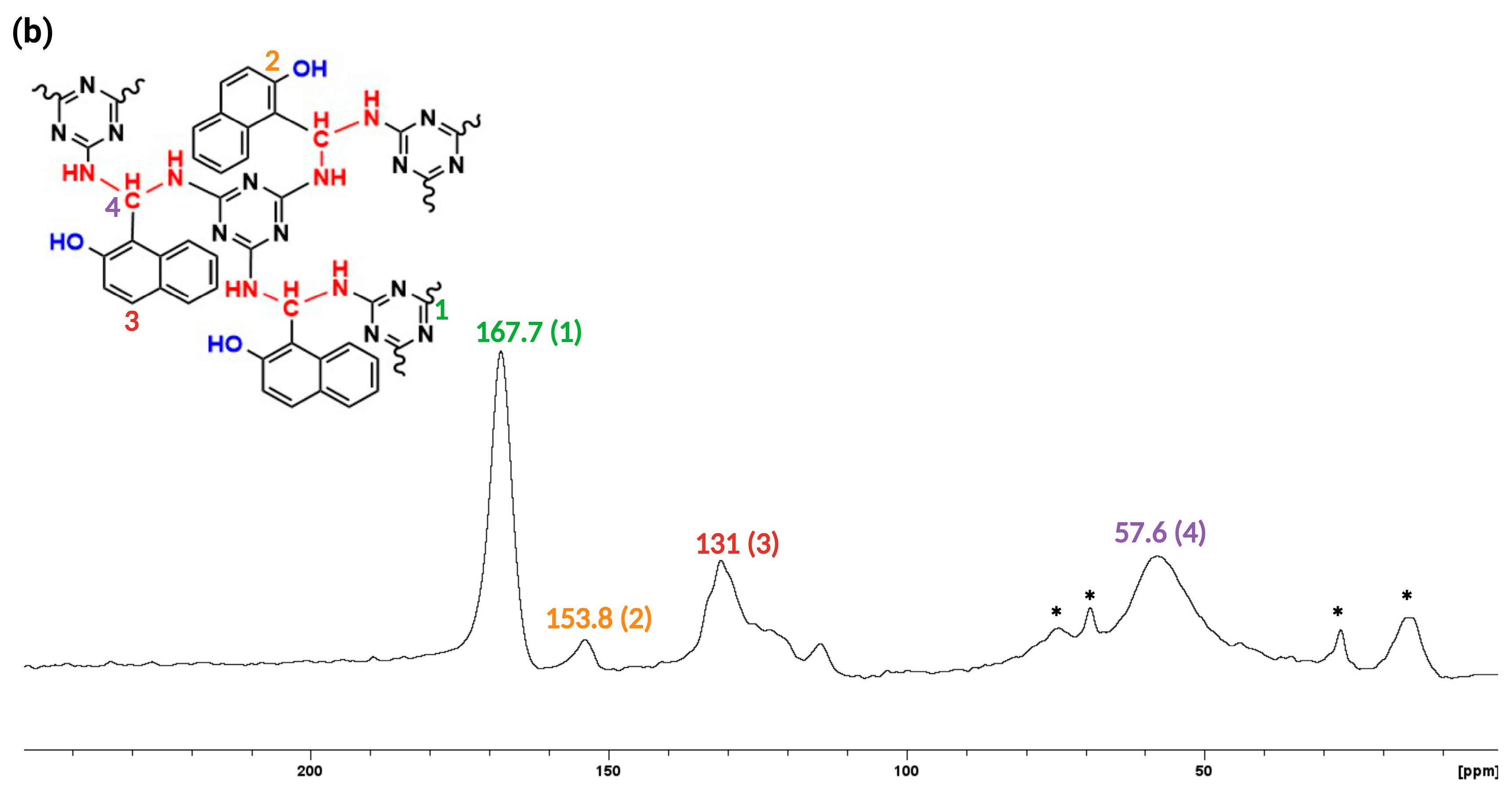
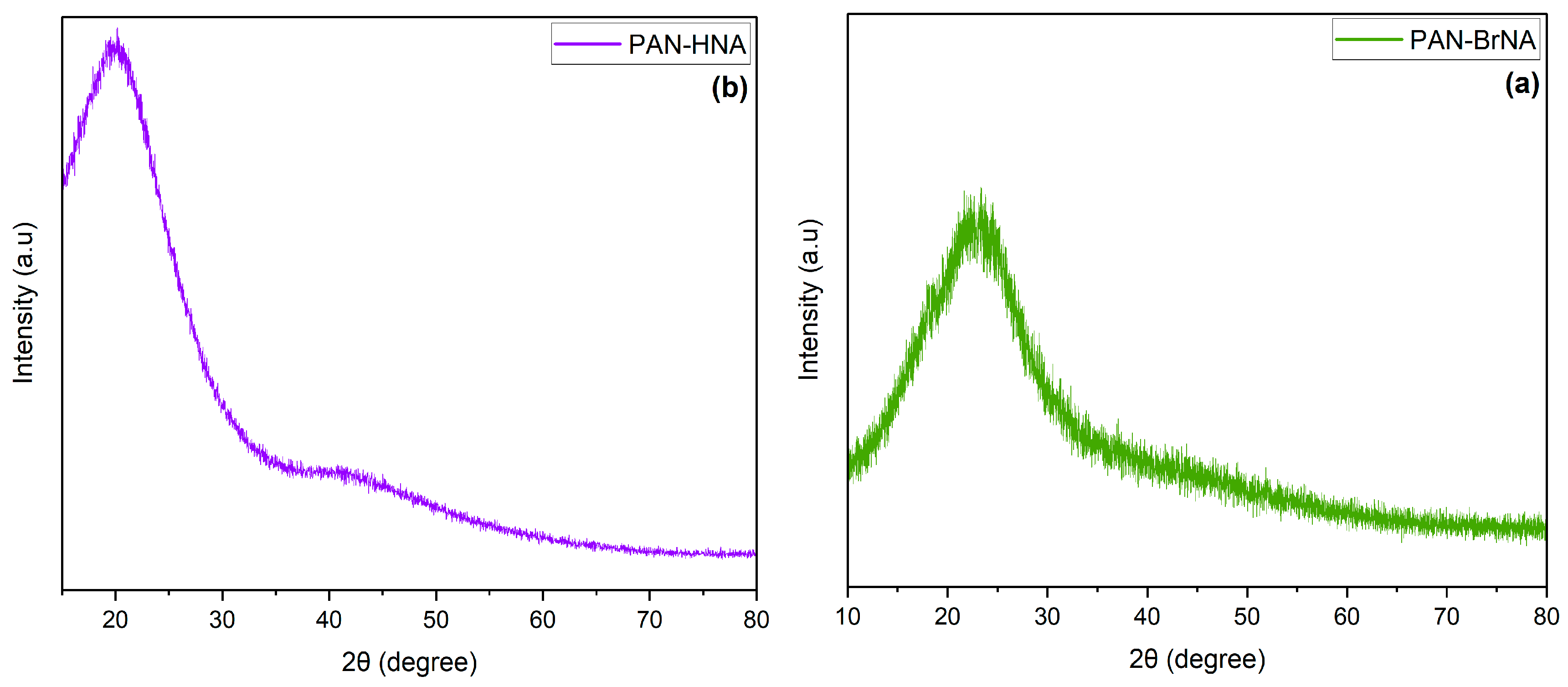
3.1. Synthesis and Characterization

3.2. CO2 adsorption
4. Conclusions
References
- A Jaleel et al., ‘Hydrogenation of CO2 to formates on ruthenium(III) coordinated on melamine polymer network’, J. CO2 Util., vol. 35, pp. 245–255, Jan. 2020. [CrossRef]
- Y. Hu et al., ‘New-Generation Anion-Pillared Metal–Organic Frameworks with Customized Cages for Highly Efficient CO2 Capture’, Adv. Funct. Mater., vol. 33, no. 14, p. 2213915, Apr. 2023. [CrossRef]
- F. E. C. Othman, N. Yusof, and A. F. Ismail, ‘Activated-Carbon Nanofibers/Graphene Nanocomposites and Their Adsorption Performance Towards Carbon Dioxide,’ Chem. Eng. Technol., vol. 43, no. 10, pp. 2023–2030, Oct. 2020. [CrossRef]
- M. Cavallo, M. Dosa, N. G. Porcaro, F. Bonino, M. Piumetti, and V. Crocellà, ‘Shaped natural and synthetic zeolites for CO2 capture in a wide temperature range’, J. CO2 Util., vol. 67, p. 102335, Jan. 2023. [CrossRef]
- S. Yuan et al., ‘Triazine-functionalized highly ordered hierarchically porous organic polymer with high CO2 uptake capacity and catalytic activity for microwave-assisted Knoevenagel condensation reaction’, Colloids Surf. Physicochem. Eng. Asp., vol. 607, p. 125475, Dec. 2020. [CrossRef]
- K. S. Song, P. W. Fritz, and A. Coskun, ‘Porous organic polymers for CO2 capture, separation and conversion’, Chem. Soc. Rev., vol. 51, no. 23, pp. 9831–9852, 2022. [CrossRef]
- W. Wang, M. Zhou, and D. Yuan, ‘Carbon dioxide capture in amorphous porous organic polymers,’ J. Mater. Chem. A, vol. 5, no. 4, pp. 1334–1347, 2017. [CrossRef]
- G. Li, B. Zhang, and Z. Wang, ‘Facile Synthesis of Fluorinated Microporous Polyaminals for Adsorption of Carbon Dioxide and Selectivities over Nitrogen and Methane,’ Macromolecules, vol. 49, no. 7, pp. 2575–2581, Apr. 2016. [CrossRef]
- Z. Xiang et al., ‘Systematic Tuning and Multifunctionalization of Covalent Organic Polymers for Enhanced Carbon Capture,’ J. Am. Chem. Soc., vol. 137, no. 41, pp. 13301–13307, Oct. 2015. [CrossRef]
- R. Dawson, D. J. Adams, and A. I. Cooper, ‘Chemical tuning of CO2 sorption in robust nanoporous organic polymers’, Chem. Sci., vol. 2, no. 6, p. 1173, 2011. [CrossRef]
- B. Zhang, J. Yan, G. Li, and Z. Wang, ‘Carboxyl-, Hydroxyl-, and Nitro-Functionalized Porous Polyaminals for Highly Selective CO2 Capture’, ACS Appl. Polym. Mater., vol. 1, no. 6, pp. 1524–1531, Jun. 2019. [CrossRef]
- P. M. C. Matias, D. Murtinho, and A. J. M. Valente, ‘Triazine-Based Porous Organic Polymers: Synthesis and Application in Dye Adsorption and Catalysis,’ Polymers, vol. 15, no. 8, p. 1815, Apr. 2023. [CrossRef]
- B. Zhang, J. Yan, Y. Shang, and Z. Wang, ‘Synthesis of Fluorescent Micro- and Mesoporous Polyaminals for Detection of Toxic Pesticides,’ Macromolecules, vol. 51, no. 5, pp. 1769–1776, Mar. 2018. [CrossRef]
- S. Zappia et al., ‘Microporous Polymelamine Framework Functionalized with Re(I) Tricarbonyl Complexes for CO2 Absorption and Reduction’, Polymers, vol. 14, no. 24, p. 5472, Dec. 2022. [CrossRef]
- H. Li, C. Li, J. Chen, L. Liu, and Q. Yang, ‘Synthesis of a Pyridine-Zinc-Based Porous Organic Polymer for the Co-catalyst-Free Cycloaddition of Epoxides,’ Chem. - Asian J., vol. 12, no. 10, pp. 1095–1103, May 2017. 20 May. [CrossRef]
- Z. Li, Y. Zhi, Y. Ni, H. Su, Y. Miao, and S. Shan, ‘Novel melamine-based porous organic materials as metal-free catalysts for copolymerization of SO2 with epoxide’, Polymer, vol. 217, p. 123434, Mar. 2021. [CrossRef]
- R. A. El-Ghazawy et al., ‘Preparation and characterization of melamine-based porous Schiff base polymer networks for hydrogen storage,’ J. Polym. Res., vol. 21, no. 6, p. 480, Jun. 2014. [CrossRef]
- C. Liu et al., ‘One-pot synthesis of nitrogen-rich aminal- and triazine-based hierarchical porous organic polymers with highly efficient iodine adsorption,’ Polymer, vol. 194, p. 122401, Apr. 2020. [CrossRef]
- R. Sandín, M. González-Lucas, P. A. Sobarzo, C. A. Terraza, and E. M. Maya, ‘Microwave-assisted melamine-based polyaminals and their application for metal cations adsorption,’ Eur. Polym. J., vol. 155, p. 110562, Jul. 2021. [CrossRef]
- K. Yuan et al., ‘Facile synthesis and study of functional porous organic polyaminals with ultrahigh adsorption capacities and fast removal rate for rhodamine B dye,’ Microporous Mesoporous Mater., vol. 344, p. 112234, Oct. 2022. [CrossRef]
- P. A. Sobarzo, A. Tundidor, E. S. Sanz-Perez, C. A. Terraza, and E. M. Maya, ‘Effect of thiophene, furan moieties and zinc ions on melamine-based porous polyaminals properties and catalytic activity on CO2 cycloaddition reaction’, Eur. Polym. J., vol. 177, p. 111444, Aug. 2022. [CrossRef]
- R. Bardestani, G. S. Patience, and S. Kaliaguine, ‘Experimental methods in chemical engineering: specific surface area and pore size distribution measurements—BET, BJH, and DFT,’ Can. J. Chem. Eng., vol. 97, no. 11, pp. 2781–2791, Nov. 2019. [CrossRef]
- A. O. Mousa, M. G. Mohamed, C.-H. Chuang, and S.-W. Kuo, ‘Carbonized Aminal-Linked Porous Organic Polymers Containing Pyrene and Triazine Units for Gas Uptake and Energy Storage,’ Polymers, vol. 15, no. 8, p. 1891, Apr. 2023. [CrossRef]
- E. S. Sanz-Pérez, L. Rodríguez-Jardón, A. Arencibia, R. Sanz, M. Iglesias, and E. M. Maya, ‘Bromine pre-functionalized porous polyphenylenes: New platforms for one-step grafting and applications in reversible CO2 capture’, J. CO2 Util., vol. 30, pp. 183–192, Mar. 2019. [CrossRef]
- M. Ibrahim, N. Tashkandi, N. Hadjichristidis, and N. S. Alkayal, ‘Synthesis of Naphthalene-Based Polyaminal-Linked Porous Polymers for Highly Effective Uptake of CO2 and Heavy Metals’, Polymers, vol. 14, no. 6, p. 1136, Mar. 2022. [CrossRef]
- F. Tang, J. Hou, K. Liang, J. Huang, and Y. Liu, ‘Melamine-Based Metal-Chelating Porous Organic Polymers for Efficient CO2 Capture and Conversion’, Eur. J. Inorg. Chem., vol. 2018, no. 37, pp. 4175–4180, Oct. 2018. [CrossRef]
- N. Zhang, B. Zou, G.-P. Yang, B. Yu, and C.-W. Hu, ‘Melamine-based mesoporous organic polymers as metal-Free heterogeneous catalyst: Effect of hydroxyl on CO2 capture and conversion’, J. CO2 Util., vol. 22, pp. 9–14, Dec. 2017. [CrossRef]
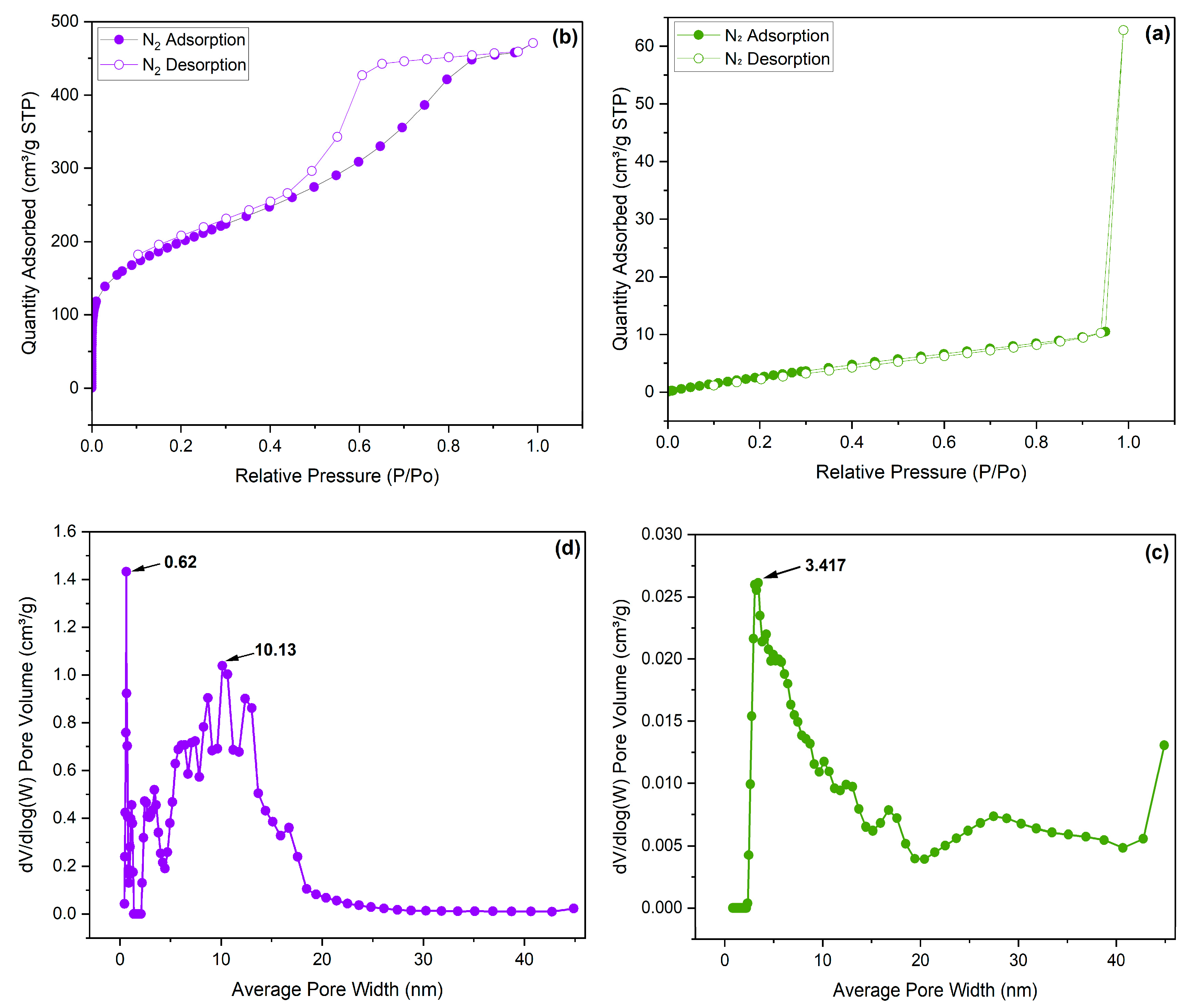
| Sample | SBET (m2/g) | Smicro (m2/g) | Vmicro (cm3/g) | Vmicro (cm3/g) |
|---|---|---|---|---|
| PAN-BrNA | 16.699 | 0 | 0 | 0 |
| PAN-HNA | 716.757 | 107.789 | 0.04257 | 0.04257 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





