3.1. Synthesis and structural characterization
A series of PAN-
b-PMMA diblock copolymers were synthesized by ATRP using a common PAN macroinitiator, following a procedure that assures good control over the relative block lengths [
15,
17]. BC compositions were selected to obtain different phase-separated morphologies, i.e., spherical, cylindrical, and lamellar structures with PAN as the minority block [
18], for BC10/90, BC26/74, and BC43/57, respectively. Polymeric precursor characteristics are collected in
Table 1. Due to the high value of the Flory-Huggins interaction parameters in the PAN-PMMA system, high molecular weight values are unnecessary to take advantage of BC peculiarities. A molecular weight of the PAN block of just 1.3 kDa is enough to induce phase separation [
9,
16], thus allowing to obtain morphologies with nanodomains as small as possible to be transformed in nanostructured carbons.
Carbons with N- and O-containing groups were synthesized in a tubular oven by treating BC powders under O
2 flow to a slow heating, 1 K min
-1, up to 553 K, followed by a 1 h stabilization and further heating up to 1073 K, 5 K min
-1, under N
2 flow, followed by 0.5 h stabilization and natural cooling down to room temperature. It is well-known that the first step stabilizes the structures formed by PAN cyclization, also introducing O-containing groups (the simplified mechanism is recalled in Scheme S1, in the supporting information [
19]). The complete PMMA decomposition, essentially by unzipping [
20], occurs during the second heating at higher temperatures, which also induces microporosity in the expected graphitic carbon [
10]. S-containing carbons were synthesized from 1/1 weight ratio of BC to sulphur mixtures through the same thermal treatment used for sulphur-free carbons but carried out under N
2 flow to avoid easy sulphur oxidation and burning [
10]. An illustrative chemical structure of the S-doped carbons is shown in Scheme S2 [
21].
To quantify the pyrolysis behavior, the BCs and the corresponding homopolymeric components were analyzed by TGA under conditions that resemble those used for the large-scale pyrolysis realized in the tubular oven. Char yields are reported in
Table 2 (the two series are named by adding the letter O or S after the acronym of the BC precursor, indicating when obtained through a stabilization under oxygen or in the presence of sulphur, respectively). As expected [
8,
16], PMMA content does not affect the carbon yield, which is proportional to the PAN content, with values ranging from ca. 6% for both BC10/90-O and BC10/90-S to 24% for BC43/57-O. The partially graphitic nature of the carbons was confirmed by Raman spectroscopy. As already reported for the carbons prepared from PAN homopolymer [
22], the spectra show the typical peaks centered at 1350 cm
-1 (the so-called D band) and 1593 cm
-1 (G band) associated with the breathing of C hexagons at the edges or the defects of the carbon structure, and to the in-plane vibration of
sp2 carbon of free of defects graphitic structures, respectively (see, as examples, the spectra of BC10/90-O and BC10/90-S in
Figure S1) [
10]. The intensity ratio of the G band to the D band reflected the graphitization degree. It was estimated in the range of 1.05-1.15 for all the carbons, in line with the values obtained for carbons from PAN precursors [
8,
22,
23].
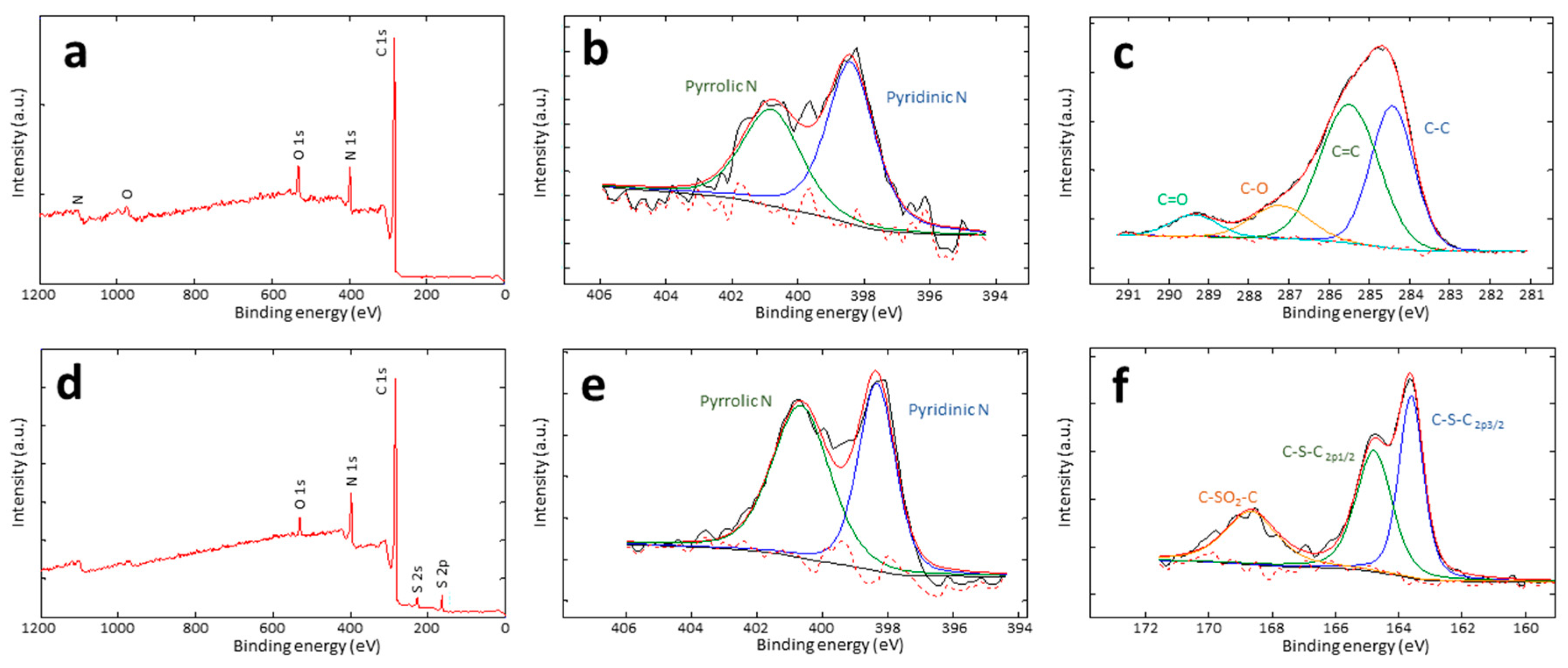
The effective incorporation of heteroatoms within the carbon network, namely nitrogen from the PAN precursor, oxygen through oxidative stabilization, and sulphur by direct addition and the specific types of functional groups were studied with XPS (
Figure 1 and
Table 3). N-containing structures consist of pyridinic (N 1s deconvoluted peak centered at 398.4 eV) and pyrrolic (at 400.8 eV) structures, eventually with small amounts of pyridine oxide groups (403.0 eV). The pyrrolic to pyridinic nitrogen ratio is 0.8-1.1 for both the BC-O and BC-S series. Concerning the bonds involving carbon in the BC-O series, the C 1s signal shows 4 deconvoluted peaks. Apart from the obvious peaks at 284.4 and 285.5 eV corresponding to C=C and C-H bonds, the two minor peaks at 287.2 and 289.4 eV can be attributed to carbons bonded to oxygen through a single or a double bond in ether-like (C-O) or carbonyls (C=O), respectively. In addition, the signal of S 2p of BC-S carbons can be fitted into three peaks. The smallest, centered at 168.6 eV, corresponding to < 10% of the total sulphur content in all the series, can be assigned to oxidized sulphur and is consistent with C-S(O)
2-C sulphone bridges. The two bigger peaks at 163.6 and 164.8 eV are due to the S 2p
1/2 and S2p
3/2 of C-S-C sulphide bridges, respectively [
24].
We observed that the composition of carbons almost coincides with each other within the two series, with the content of C, N, and O of approximately 81, 13, and 6 wt.% for the BC-O carbons, and C, N, O, and S of 80, 12, 6 and 3 wt.% for the BC-S series, i.e., with a 19-21 % total heteroatom content. As these values are also very similar to those already determined for carbons synthesized from PAN homopolymer as a precursor [
22], likely, the presence of PMMA blocks with different lengths in the copolymers does not influence the chemical processes involved in the formation of the pyrolytic carbons. A similar behavior was observed for PAN diblock copolymers with poly(butyl acrylate) [
11].
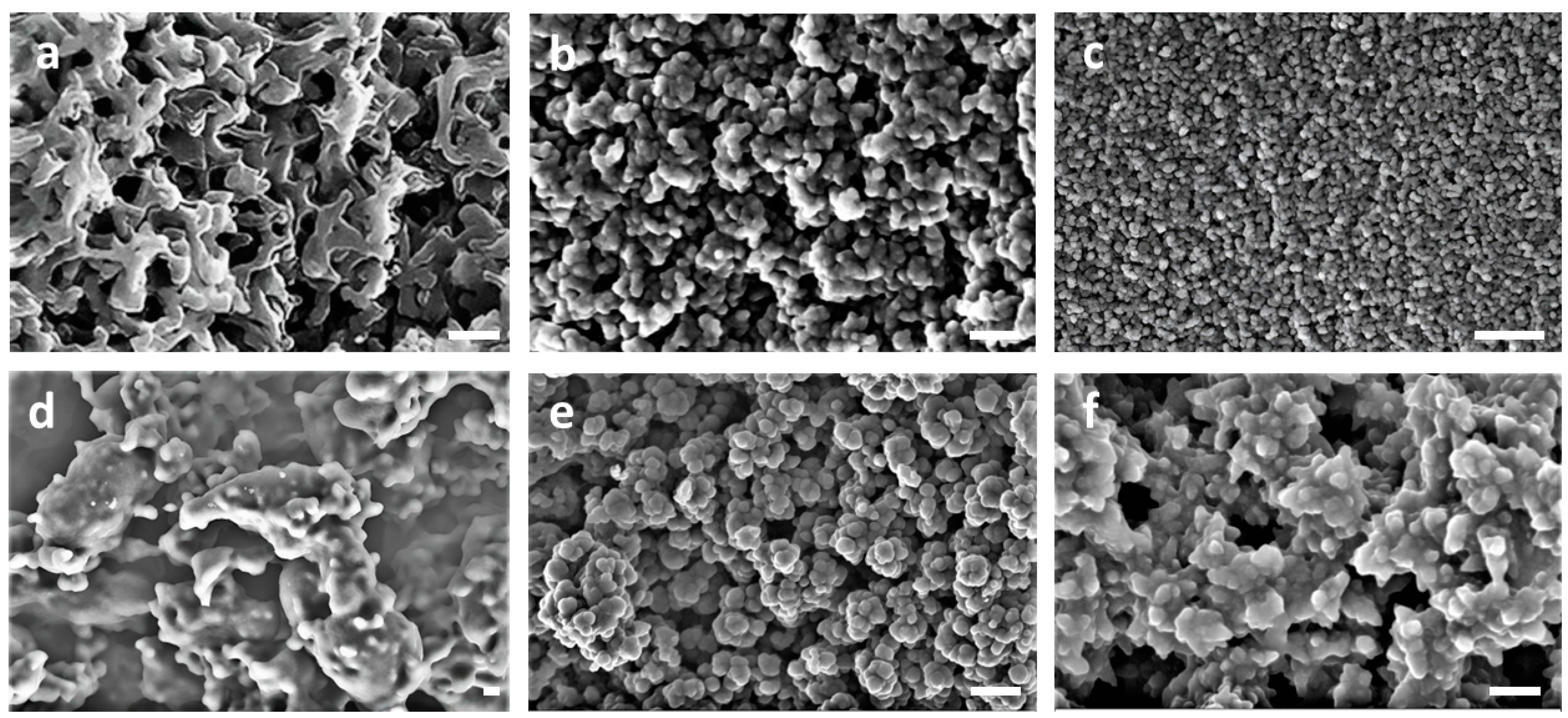
A direct visualization of the surface of carbons by SEM revealed their nanostructuration. The comparison of the BC-O carbons in
Figure 2a–c with the more homogeneous appearance of carbon from PAN homopolymer (PAN-O) in
Figure 2d confirms the role of PMMA as a sacrificial block to induce some porosity, at least in the mesoscale. In addition, an increase in the amount of PAN, i.e., in its volume fraction φ
PAN, entails the formation of less defined pores. It is also clear that the BC-O carbon structures do not resemble the morphologies expected from conventional diblock copolymer self-assembling. Only in the case of BC10/90-O and BC26/74-O is a partially bicontinuous carbon morphology visible, although predictable only for BCs with a higher φ
PAN. This apparent incongruity may be explained as follows, referencing the hypothesis proposed by Liu and co-workers [
25]. Self-assembly of BCs into thermodynamically equilibrated morphologies may be reached through thermal annealing at a temperature above both the glass transition temperature,
Tg, and the possible melting temperature,
Tm, of all the BC components [
26]. In the case of PAN-
b-PMMA, the
Tg of both blocks is in the range 363-373 K, whereas the
Tm of PAN at 573-593 K [
17] also corresponds to a temperature at which the polymer starts to decompose. Consequently, at the temperature of the stabilization step in oxygen, i.e., 553K, the cyclization-oxidation of PAN fixes the BC morphology in a metastable assembling before reaching the thermodynamically stable phase-separation. The structure resulting from complete PMMA decomposition in the second step of carbonization depends on BC composition, and carbons with degenerated bicontinuous structures cannot be expected from compositions, potentially leading to spherical or lamellar morphology. Only BCs with φ
PAN in the approximate range of 0.10-0.30, potentially forming cylindrical or gyroid morphologies, seem good candidates for synthesizing carbons with interconnected pores [
27,
28].
The carbons synthesized in the presence of sulphur were very similar to each other and to S-doped carbon from PAN homopolymer, PAN-S (
see some representative SEM images in
Figure 2e,f). The condensed globular aggregates appear composed of large-sized spherical shapes with dimensions in the range of 50-100 nm. This evidence indicates the lower efficacy of sulphur with respect to oxygen to stabilize the developing morphologies. Metastable phases are not fixed as in the presence of oxygen and carbon structures evolve further by a complete PMMA volatilization to a common pattern, regardless of the BC composition.
As a final consideration, SEM analysis also gives a first rough indication of part of the carbon mesoporosity (conventionally between 2 and 50 nm). Its quantification, as well as that of micropores (< 2 nm), are usually carried out by adsorption/desorption experiments with nitrogen and CO2, respectively.
3.2. Surface area and pore size
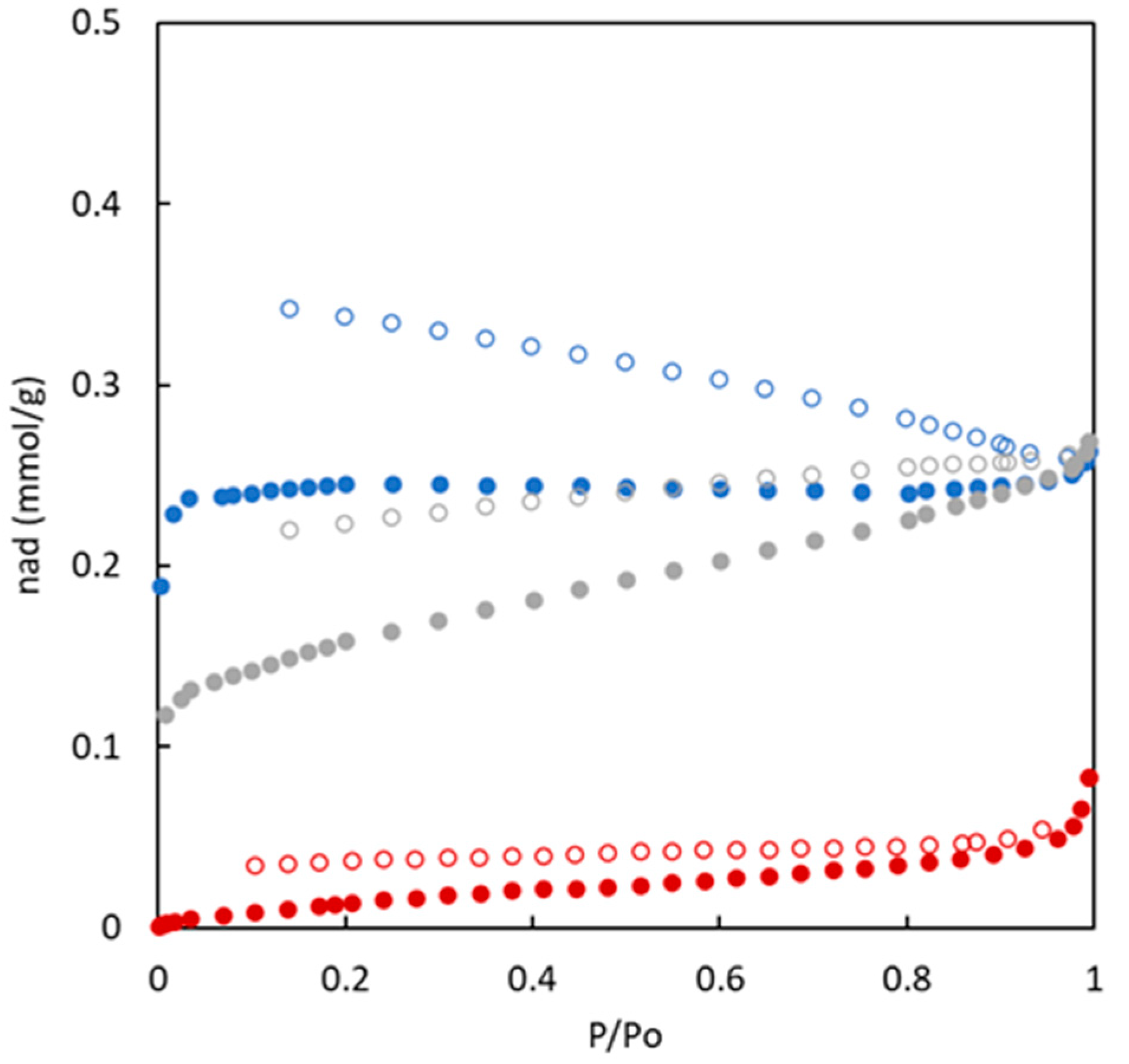
Nitrogen adsorption/desorption isotherms at 77 K of the carbons synthesized without (BC-O series) and with (BC-S series) the use of sulphur are shown
Figure 3 and
Figure 4, allowing us to obtain information about their porous structure. Certain differences are observed in the shape and magnitude of nitrogen adsorption isotherms.
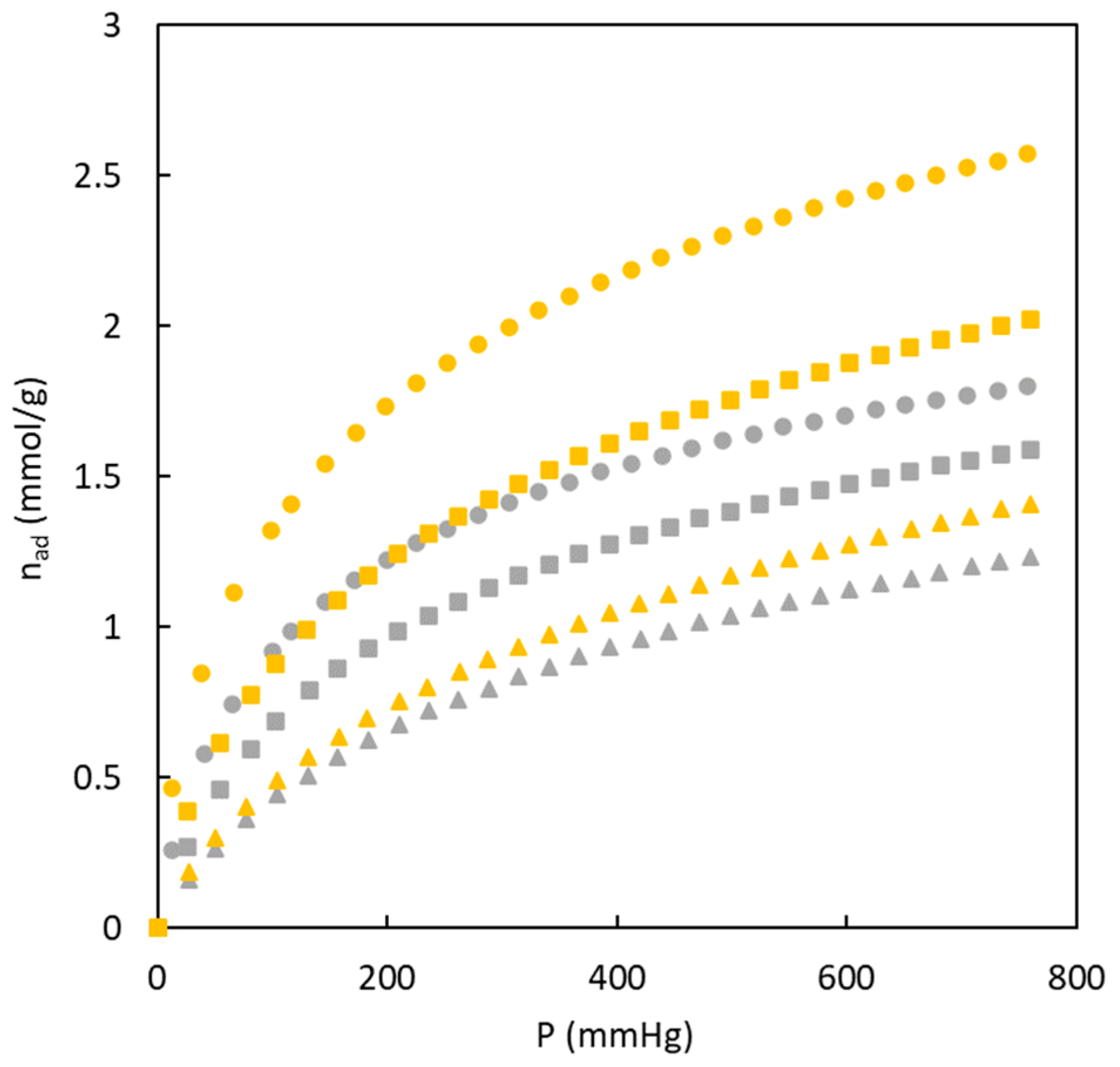
The experimental data corresponding to materials fabricated in the absence of sulphur showed that the amount of adsorbed nitrogen is low, and the shape of the isotherms for this type of materials are included in type I [
29]. Isotherm type I corresponds to materials with a microporous structure reaching a constant value when the relative pressure increases. The desorption part of the isotherm does not agree with the adsorption data due to the low adsorption amount. Concerning the effect of precursor composition, an increase in the MMA amount causes an increase in adsorbed nitrogen.
The analysis of the nitrogen adsorption/desorption isotherms for materials obtained in the presence of sulphur (see
Figure 4) indicate important differences in comparison with the materials without sulphur. On the one hand, the amount of nitrogen adsorbed in the surface of the materials is higher for sulphur-doped materials in the entire relative pressure range. All these materials reach a first plateau close to 5 mmol g
-1. At high relative pressure, adsorbed nitrogen is mainly enhanced for the BC10/90-S material. In addition, for these materials a hysteresis loop is observed for all sulphur-doped materials.
Considering the IUPAC classification of adsorption/desorption isotherms, the experimental data included in
Figure 4 allows to include all isotherms in the type IV. This type of shape of nitrogen adsorption isotherm at 77 K is characteristic of solids with an important mesoporosity. This type of solids and pore size (higher than microporous) tend to generate nitrogen multilayers when relative pressure increases (increasing the amount of adsorbed nitrogen). The first part of the isotherms at low relative pressure refers to the formation of the monolayer. In addition, the presence of a hysteresis loop is related to the nitrogen condensation in the mesopores.
The experimental data corresponding to adsorption/desorption isotherms of N
2 at 77 K can be used to analyze the porous structure of solids. Specifically, surface area and information about pore size have been included in
Table 2. The calculated data show that the materials in the absence of sulphur reach very low surface area values (below 20 m
2 g
-1). On the other hand, using sulphur as a doping agent allows us to reach materials with a similar surface area (between 369 and 372 m
2 g
-1). The presence of sulphur atoms during carbon production accounts for an increase in surface area, which agrees with previous studies [
30]. The influence of the BC composition in the precursor on the surface area value is evident only in the materials synthesized without sulphur. An increase in PMMA sacrificial block accounts for increased surface area caused by this polymer's removal during carbon production. The results corresponding to carbons with sulphur do not show the influence of polymers' composition. The overall behavior generally shows the following trend: BC10/90-S > BC26/74-S > BC10/90-O > BC26/74-O > BC43/57-O.
Nitrogen adsorption isotherms also allow us to calculate the porous volume of each pore type. Analyzing the materials in the absence of sulphur, very different types of pores are generated. For instance, a low PMMA content in the BC precursor appeared to generate only mesopores in the material, while a larger presence enhanced the generation of micropores. Though a lower presence of PMMA increases the pore size, from an overall point of view, an important decrease in the porous structure is observed.
The opposite behavior (in relation to the percentage of microporosity) was observed for sulphur-doped carbons. However, the presence of sulphur does not allow for reaching a high microporosity percentage because the presence of this type of atom tends to increase pore diameter [
30]. In the same way as previously commented for materials synthesized in the absence of sulphur, a decrease in the amount of the sacrificial PMMA block composition tends to decrease the porous structure. However, the presence of sulphur increases the porous volume in all cases.
All these results agree with the fact that the pore size in BC10/90-S is larger than in the other materials; further, it favors the formation of multilayers at high relative pressures, as observed in
Figure 4. In the case of BC26/74-S, it shows a similar behavior at relative pressures lower than 0.7. However, the higher presence of microporosity avoids the sharp increase in nitrogen adsorption at high relative pressures.
These experimental results and the differences observed depending on the copolymer composition allow us to conclude that the tuning of PMMA amounts promotes the synthesis of materials with a different degree of pore size and volumes. A comparison of present experimental data with those obtained for carbons from PAN homopolymers [
22] indicates that the use of BC precursors only increases the surface area generated for supramicropores and mesopores (determined with N
2 at 77 K) in sulphur-doped carbons and a slight decrease is observed for the other materials.
Several studies [
31,
32] have concluded that the values for different parameters determined using the nitrogen adsorption experimental data at 77 K need to be revised to characterize carbons' porous structure due to diffusion issues in micropores. For this reason, additional experimental data were obtained by replacing the adsorbate; in particular, carbon dioxide adsorption isotherms at 273 K were recorded for all the investigated materials. These previous studies concluded that this type of experimental data allows us to analyze microporosity deeply, specifically ultramicroporosity (pore diameter below 0.7 nm), where nitrogen molecules present diffusion limitations.
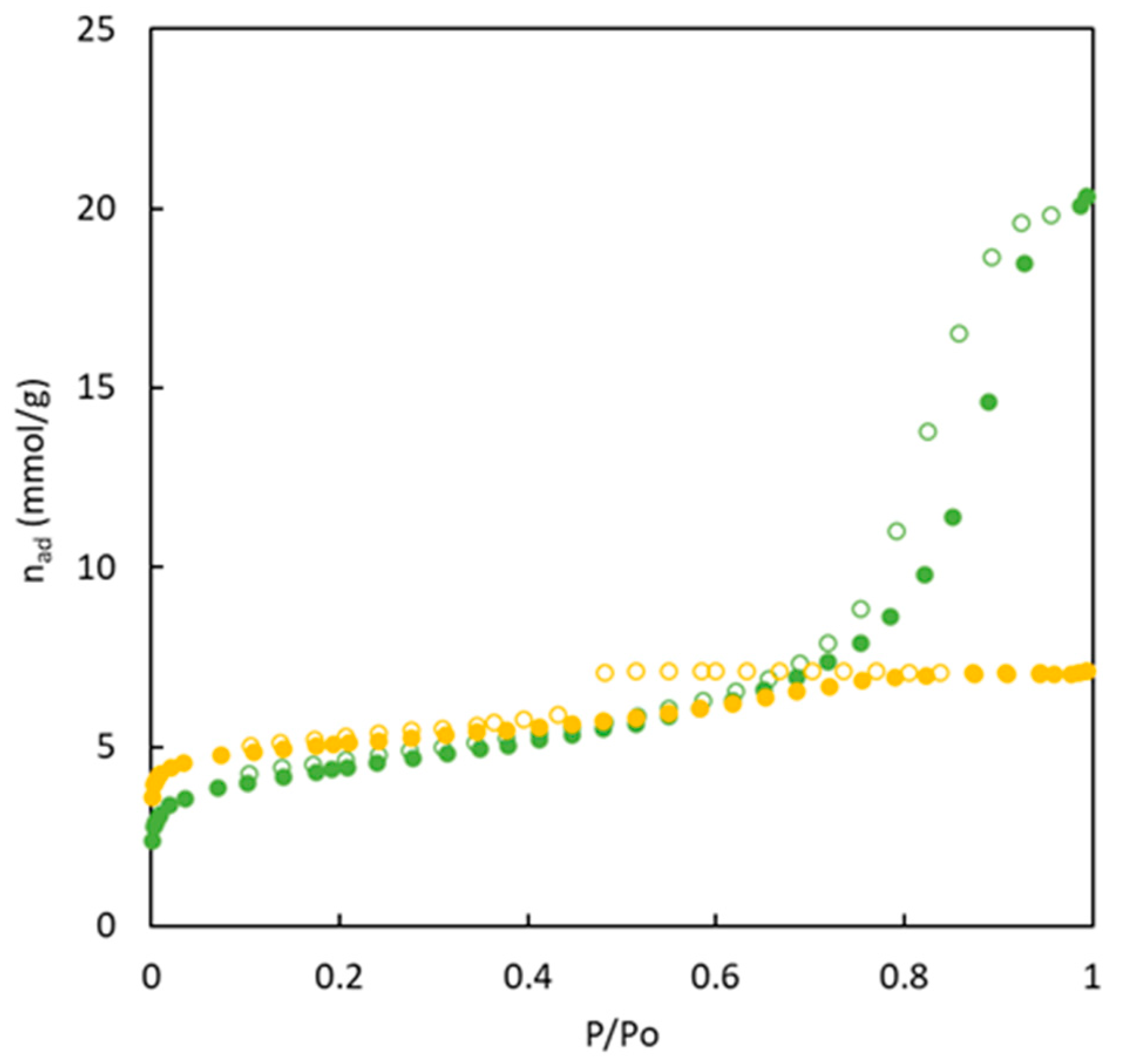
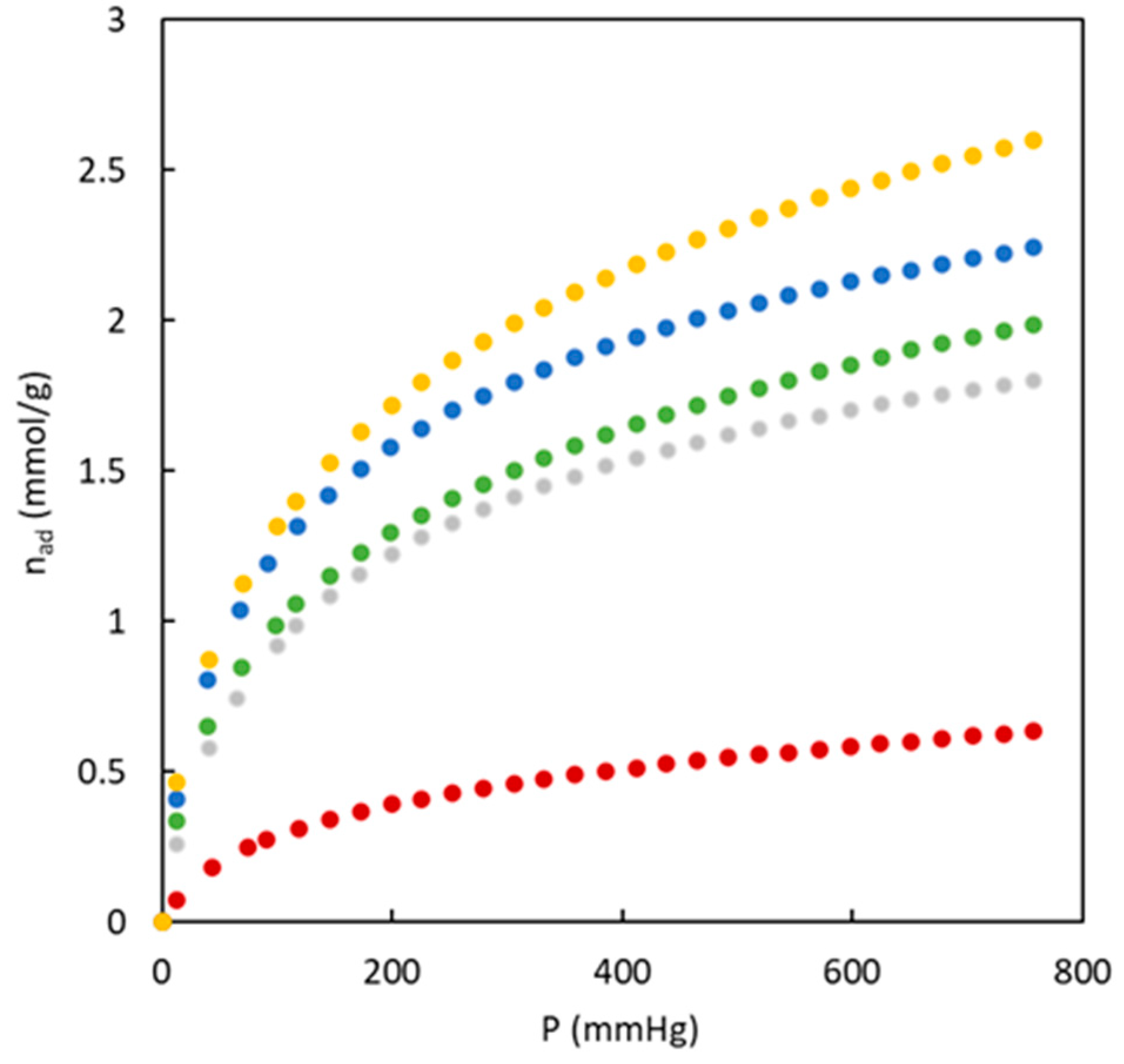
Figure 5 shows the experimental data corresponding to CO
2 adsorption isotherms at 273 K for the different carbons synthesized in the present work. An important difference is observed when the experimental adsorption isotherms in
Figure 5 are compared with previously discussed data of nitrogen adsorption isotherms at 77 K because the differences between materials with and without sulphur are low. BC43/57-O shows the highest difference compared to the others, with a low value of adsorbed CO
2. This behavior agrees with the microporosity percentage (
Table 2) that showed the absence of this type of pores. The calculated surface area value with CO
2 adsorption isotherm (77.6 m
2 g
-1) indicated that this material possesses an ultramicropore structure not detected by the N
2 adsorption isotherm. The observed trend with the surface area due to ultramicroporosity is: BC26/74-S > BC10/90-O > BC26/74-S > BC26/74-O > BC43/57-O. This trend does not agree with the microporosity percentage as this latter is determined with N
2 adsorption at 77 K.
In general, the joint analysis of surface area determined with N
2 at 77 K and CO
2 at 273 K indicates that surface area due to ultramicroporosity (pore size lower than 0.7 nm) is similar for the main part of carbons with or without the use of sulphur. The main difference is observed in the surface area generated by pores below 0.7 nm.
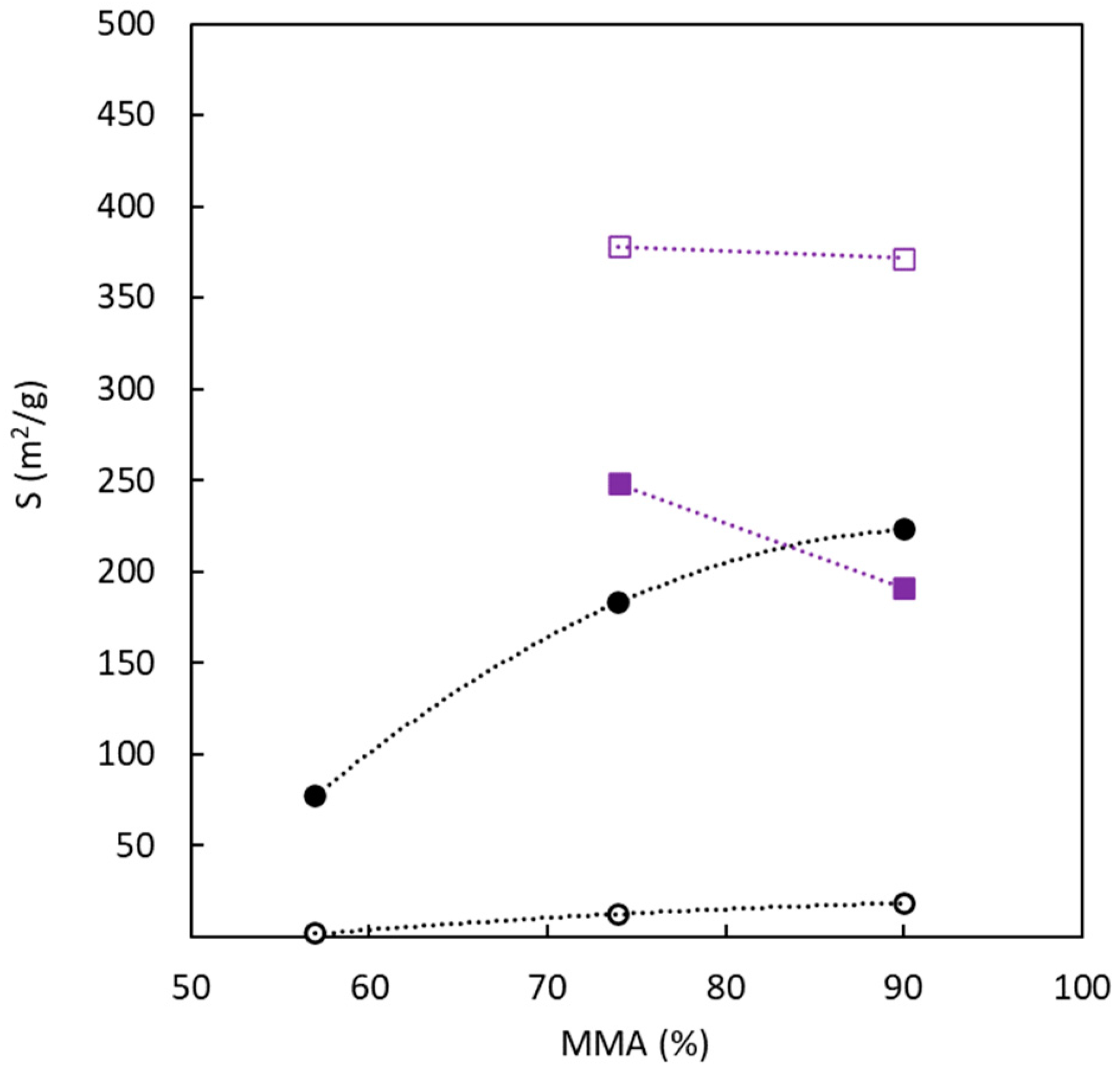
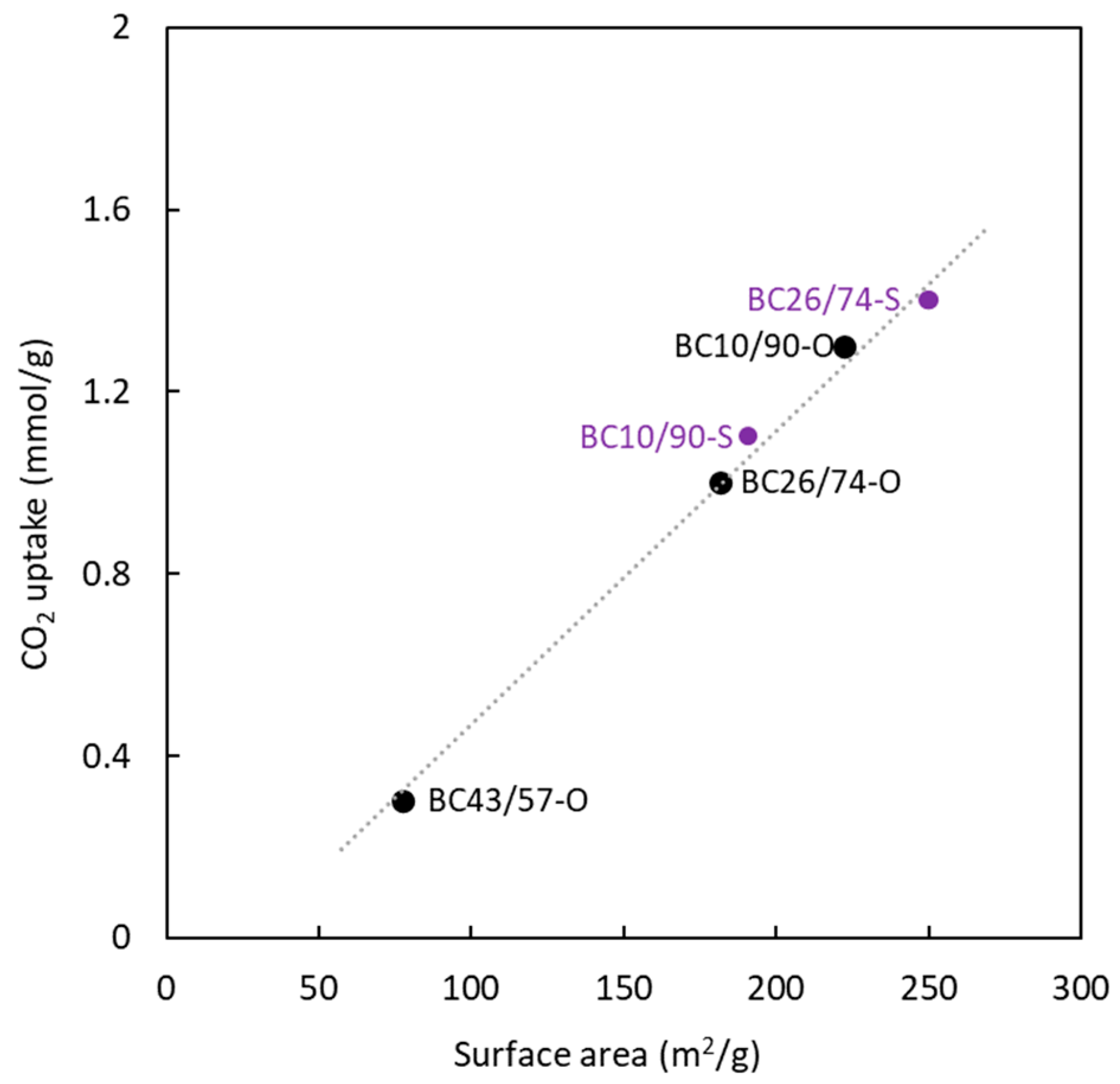
Figure 6 analyses the influence of BC composition (in terms of MMA molar amount, expressed as a percentage) in the precursor on the surface area determined for both adsorbates. The surface area determined with N
2 at 77 K shows a very important influence of the sulphur presence and a low contribution of the copolymer composition. On the other hand, in the presence of sulphur, the MMA molar content in the precursor significantly affects the surface area values determined from CO
2 adsorption at 273 K. In the absence of sulphur, an increase in the amount of PMMA tends to increase the ultramicroporosity. The opposite behavior is observed for sulphur-doped carbons. Concerning the surface area's magnitude, using an MMA percentage higher than 70 % allows for reaching similar values, notwithstanding the use of sulphur in the production process. Taking into account the previously discussed results, it is possible to conclude that the copolymer composition has a higher influence on the ultramicroporous structure and the surface area generated by this type of pores.
Table 2 also allows us to evaluate the role of medium pore size on the surface area, which decreases with the increase in pore size.
For the influence of S-doping on the porosity > 0.7 nm, it has already been presumed by Fletcher et al. [
33] that the formation of S-containing groups during graphitization preferably occurs on thermodynamically more stable surface termination sites of graphitic structures (Scheme S2). Because of their bigger dimensions concerning carbon atoms, sulphur atoms tend to protrude from the graphite layers, increasing the interlayer distance and the size of the crystallites, therefore developing additional microporosity (mainly related to supramicropores 0.7 nm < D
p < 2 nm). A further hypothesis to support the higher values of the porosity may be given to the presence of the larger and polarizable
d orbitals in the sulphur, where the lone pair of electrons may interact with the oxygen to strengthen adsorption of the CO
2 used to get the specific measurement [
30]. These previous conclusions are in agreement with the experimental results (
Table 2) because carbons with sulphur reach similar values of ultramicroporosity but in addition they reach an enhancement in supramicroporosity (determined with N
2 at 77 K) in comparison with carbons without sulphur.
The comparison of present results with the characteristics of carbons generated from PAN homopolymers [
22] allows us concluding that the largest influence is related to the porous structure with D
p > 0.7 nm and mainly in S-dopped material that enhances this type of porosity (supramicropores and mesopores). These conclusions agree with those concerning the role of a sacrificial component in this type of material that tries to increase mesoporosity [
9].
The suitability of the carbons prepared in the present work for separating gas streams by adsorption was assessed; in particular, a gas stream with a mixture of CO
2 and N
2 corresponding to a post-combustion stream was considered. To carry out this type of evaluation, CO
2 and N
2 isotherms at 273 K were compared; an example of the obtained experimental results is shown in
Figure 7.
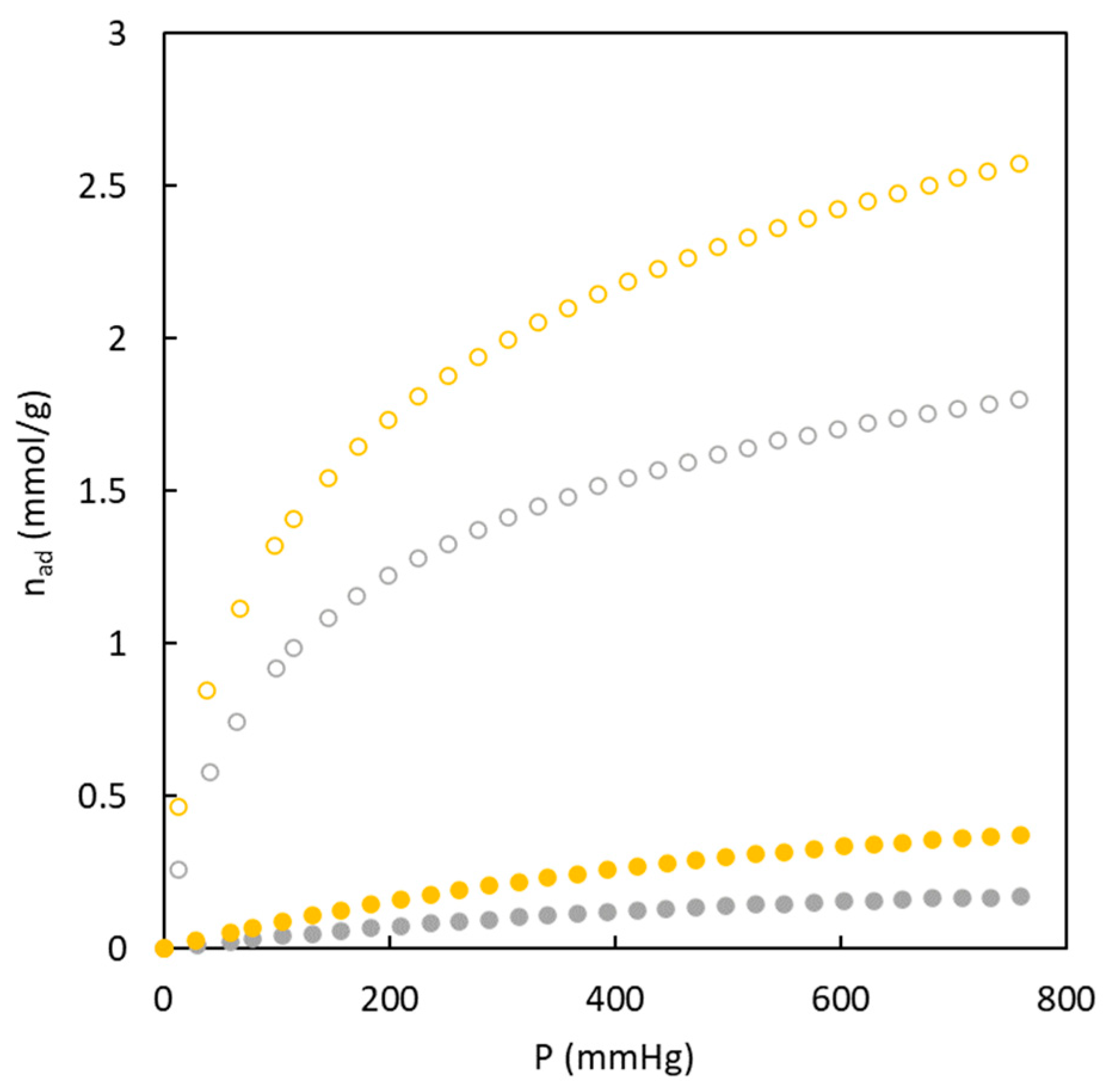
These experimental data show that the carbons have a higher affinity for CO
2 molecules, reaching values ten times higher than N
2 adsorption. This behavior is interesting to be used as adsorbent to reach a suitable separation.
Figure 7 also shows the role of sulphur on the adsorption isotherms of both gases. It can be seen that the presence of sulphur enhances the adsorption of both gases. On the one hand, the greater nitrogen adsorption may be related to the high surface area previously determined (368.6 m
2 g
-1). In comparison, the higher carbon dioxide adsorption may be produced by the joined effect of higher ultramicropore surface area (249.9 m
2 g
-1) and the affinity of this gas for sulphur, which agrees with previous studies [
22].
The experimental data of adsorption isotherms for both gases employed in the present work were used to calculate the apparent selectivity value using equation 1: this way, it was possible to compare the suitability of these carbons for nitrogen purification from a mixture containing 15 mol.% CO
2 and 85 mol.% N
2, which usually identifies a post-combustion stream composition.
nCO2 and nN2 are the adsorbed amounts of the two gases, and PCO2 and PN2 correspond to the partial pressure of carbon dioxide and nitrogen, respectively.
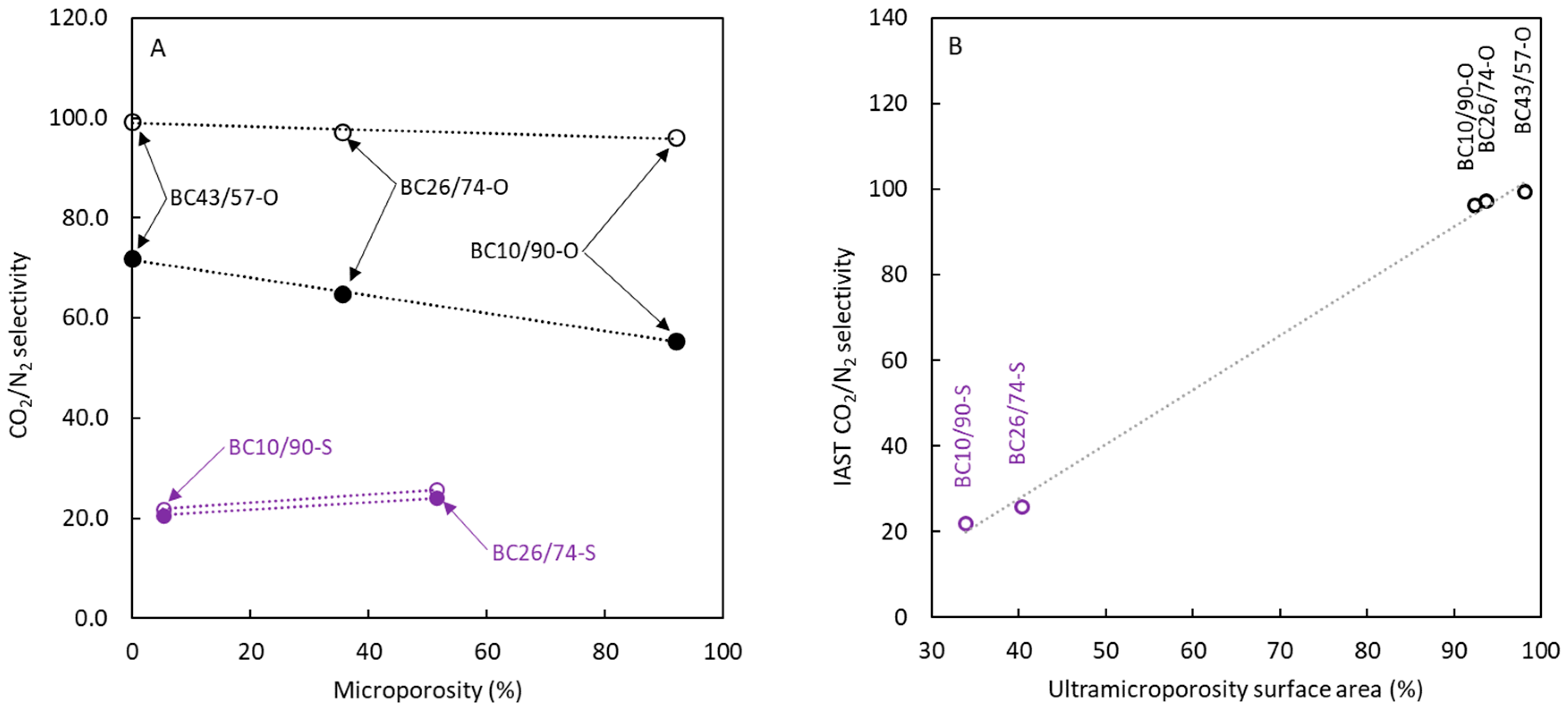
Figure 8a shows the calculated values of CO
2/N
2 apparent selectivity
versus each carbon's microporosity percentage. The analysis of the influence of this solid characteristic on CO
2 adsorption (and selectivity in this case) can be interesting because several studies [
32,
34,
35] have indicated its possible influence on adsorption selectivity when different gases are employed.
Figure 8A shows that the influence of mesoporosity has a low effect, probably due to the absence of ultramicroporosity presence. The most important result observed for apparent selectivity data is the important enhancement in CO
2 selectivity with the absence of sulphur in carbons that can be related to the negligible surface area corresponding to supramicropores and mesopores (surface area determined with N
2 at 77 K) and the main presence of ultramicropores. Several authors considered that the estimation of CO
2 selectivity can be improved using the ideal adsorption solution theory (IAST) [
36] because this thermodynamic analysis allows taking into account a certain degree of competition between molecules for the active sites in the solid surface. This theory analyses the gas-solid adsorption equilibrium based on vapor-liquid equilibrium concepts [
37,
38].
The calculated CO
2 selectivity data using IAST are also included in
Figure 8 (empty symbols). The trends about the influence of microporosity on IAST selectivity that were obtained are similar to those previously discussed for apparent selectivity. Concerning the magnitude of this parameter, IAST allows us to estimate values of CO
2 selectivity higher than those corresponding to apparent selectivity. This difference agrees with the effect of competitive adsorption between considered gases. The increase in IAST values for sulphur-doped carbons is negligible, but the difference is high for non-doped materials, approaching values close to 100. Specifically for non-doped carbons, IAST calculations indicate that a purity in adsorbed molecules close to 95% CO
2 can be achieved.
The calculated values using IAST indicate no differences between selectivity values for non-doped materials. This finding can be attributed to the very low surface area associated with large micropores and mesopores; further, the effect on IAST selectivity caused by the presence of ultramicroporosity is similar for all non-doped materials though the CO2 adsorption could be different.
To better elucidate the influence of low-size pores upon selectivity, plot B is included in
Figure 8. The results in this plot support the previous discussion, highlighting a clear enhancement of selectivity with the surface area contributed by the low-size pores, specifically corresponding to ultramicroporosity.
Considering that CO
2 uptake and selectivity are important parameters to assess the suitability of adsorbents for industrial applications,
Table 2 also includes information about the amount of CO
2 adsorbed at 15 kPa. The experimental data corresponding to this parameter show its direct relation (see
Figure 9) with the surface area corresponding to ultramicroporosity (determined with CO
2 at 273 K). However, this relation was not observed for large micropores and mesopores (determined with N
2 at 77 K). Therefore, it is possible to conclude that ultramicroporosity has the most important role in the CO
2 separation process by adsorption. The CO
2 uptake and selectivity results indicated that BC 10/90-O carbon possesses suitable characteristics for CO
2 separation from a typical post-combustion gas stream.
Table 4 reviews CO
2 uptake and selectivity obtained for different carbonaceous materials from different precursors. On such basis, it may be observed that the carbons produced with an activation step tend to generate a high carbon dioxide uptake but with relatively low selectivity values. Only materials fabricated from graphene oxide and carbon nanofibers can reach interesting values for both parameters. A negative issue of these materials is related to the complex synthesis procedure in comparison with other materials.
In general, the most promising carbon, i.e., BC10/90-O, synthesized in the present work, shows interesting CO2 uptake and selectivity values. It is worth noting that the materials used in the present work have not been activated; for this reason, the surface area is low, and activated carbons can reach higher values of CO2 uptake. On the other hand, the activation procedure is an energy-intensive process that increases both the cost of the adsorbent and the carbon footprint.
This work also analyzes the influence of temperature on CO
2 adsorption isotherms.
Figure 10 shows some examples of the gas adsorption experimental results for this type of analysis. In all cases, a temperature increase favors the decrease in the amount of CO
2 adsorbed, which agrees with the existence of physical adsorption. In order to use these materials for gas separation operation (specifically for CO
2 separation) by adsorption, chemical adsorption must be avoided to reduce the cost associated with sorbent regeneration.
The experimental data displayed in
Figure 10 also highlight that the effect of temperature on the amount of CO
2 adsorbed is different for both materials (BC 26/74-O and BC 26/74-S), and this difference could affect the energy involved in the adsorption process.
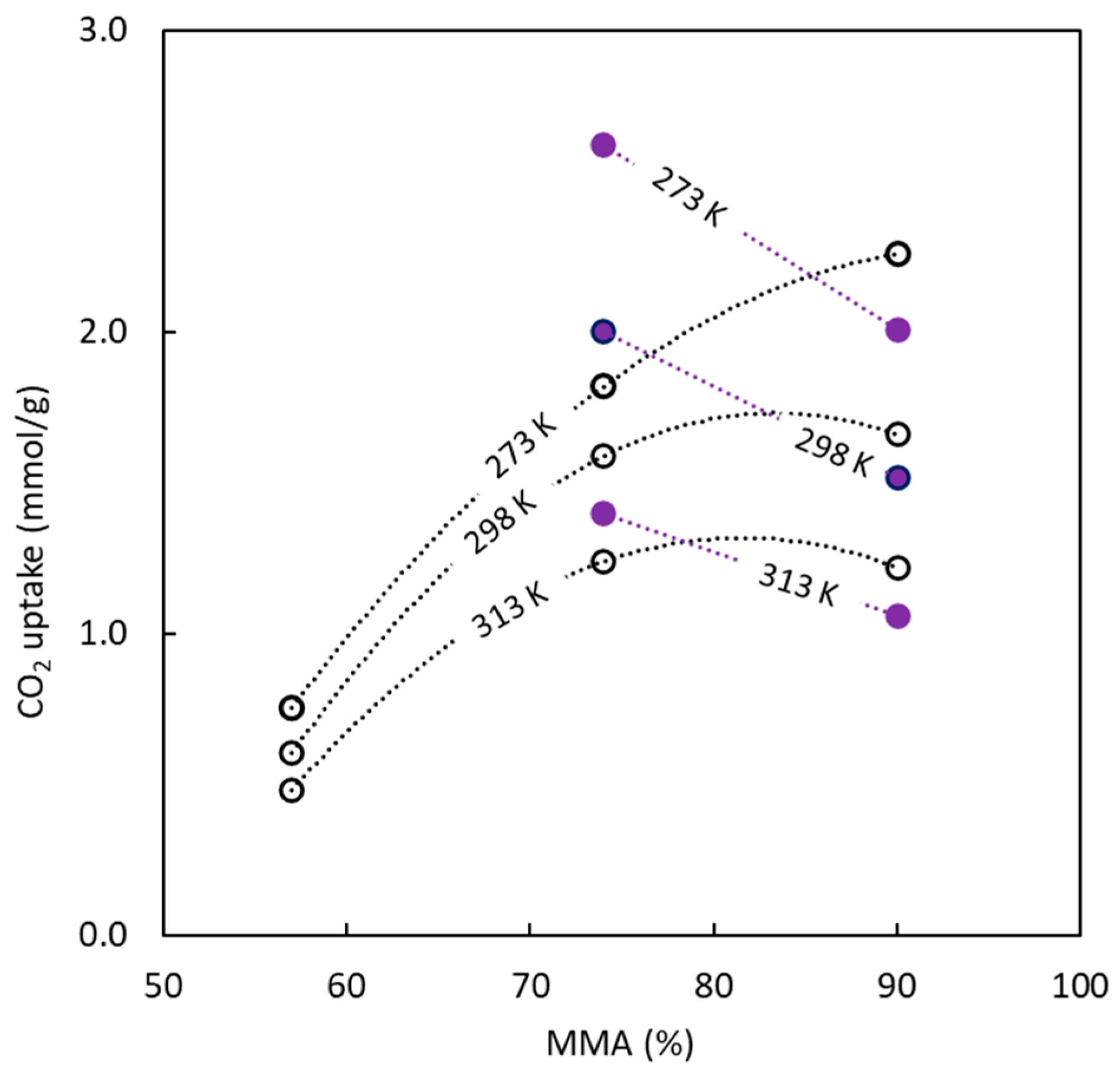
In relation to the influence of copolymer composition upon CO
2 uptake,
Figure 11 tries to explain the effect of temperature and copolymer composition. For all adsorbents, an increase in temperature causes a decrease in the amount of CO
2 adsorbed, but the effect caused by BC composition shows different behaviors. The sulphur-doped carbons show a decrease in the CO
2 uptake values with MMA concentration, unlike the materials synthesized without sulphur, which show the opposite behavior. These trends are influenced by the pore size that sacrificial PMMA generates. For materials produced in the presence of sulphur, an increase in MMA percentage in the precursor increases the pore size while lowering the CO
2 uptakes (previously discussed). The opposite effect is observed for carbons obtained without sulphur.
The experimental data corresponding to CO
2 adsorption isotherms were exploited to analyze the behavior of some widely used equations (Langmuir, Freundlich, and Toth) due to their simplicity and because they have shown good results.
Table 5 displays the fitting parameters corresponding to each model and the sum of square errors for the different series of experimental data.
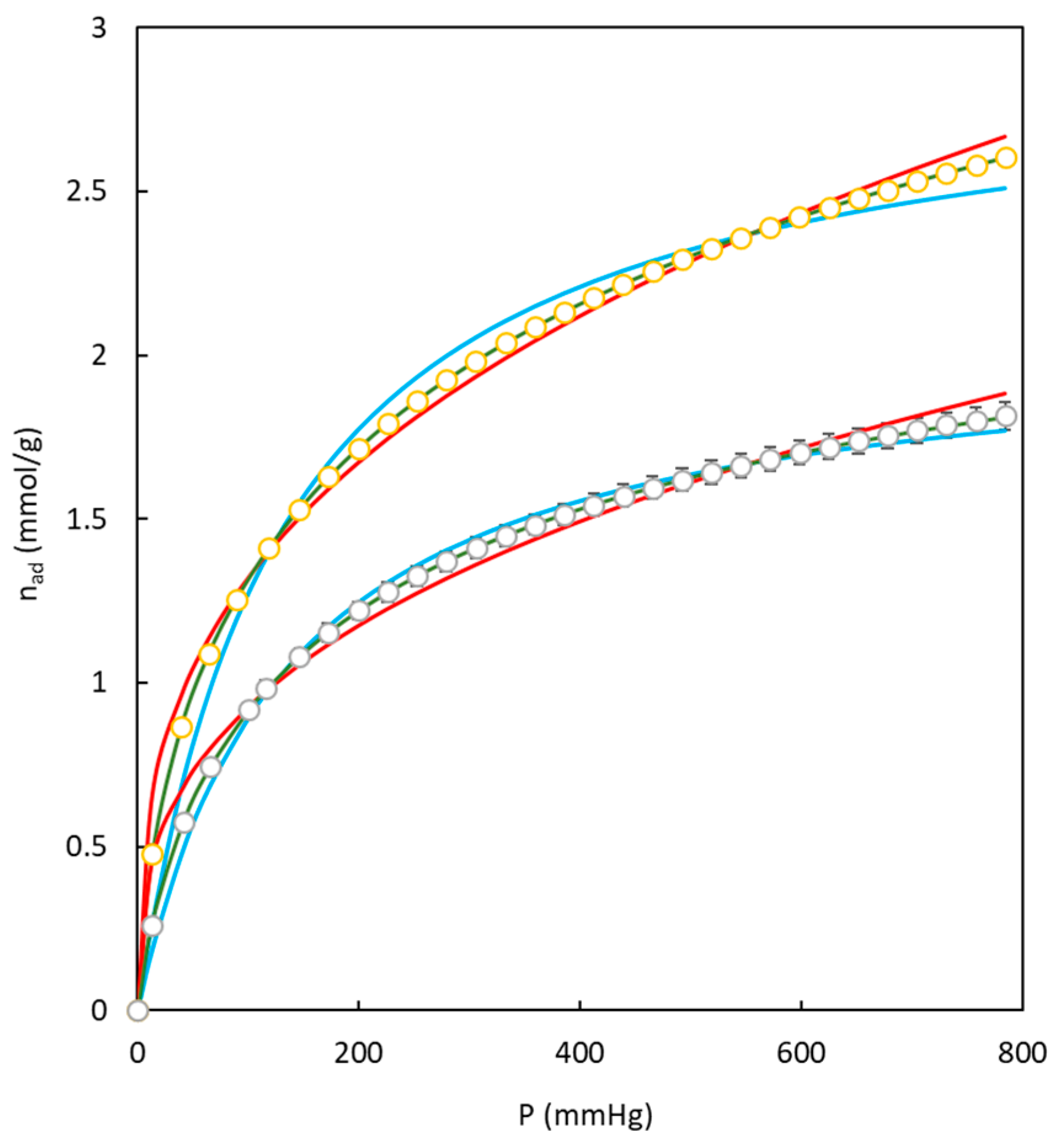
Figure 12 compares the behavior of each model to fit the experimental data of adsorption isotherms corresponding to two different carbons employed in the present work.
Figure 12 and
Table 5 demonstrate that the Toth model better fits the experimental data, while Langmuir and Freundlich models reach higher deviations.
Toth model includes in its equation the t parameter that allows us to evaluate the homogeneity degree of a carbon surface [
48]. If
t takes values close than 1, the Toth model is transformed on the equation developed by Langmuir. On this basis, it is possible to conclude that the material surface possesses homogeneous characteristics. The carbons synthesized in the present work showed t values far from 1 for CO
2 adsorption. For this reason, an important degree of surface heterogeneity can be present in these materials. The sulphur-doped carbons showed
t values far from 1. These results agree with the previously analyzed data that show an increase in the functional groups with the presence of sulphur atoms in the carbon structure [
22].
The experimental data corresponding to CO
2 adsorption isotherms at different temperatures were used to calculate the heat of adsorption. The Clausius-Clapeyron equation (equation 2) has been used, assuming that this parameter is not dependent on temperature [
49].
where Q
st (kJ mol
-1) is the heat of adsorption, R is the ideal gas constant (8.314 J mol
-1 K
-1), θ is the CO
2 surface coverage at a pressure P (Pa) and temperature T (K).
To determine the heat of adsorption at different CO
2 uptakes, suitable modeling of the influence of pressure on the amount of CO
2 adsorbed is needed. The Toth model was chosen based on the results shown in
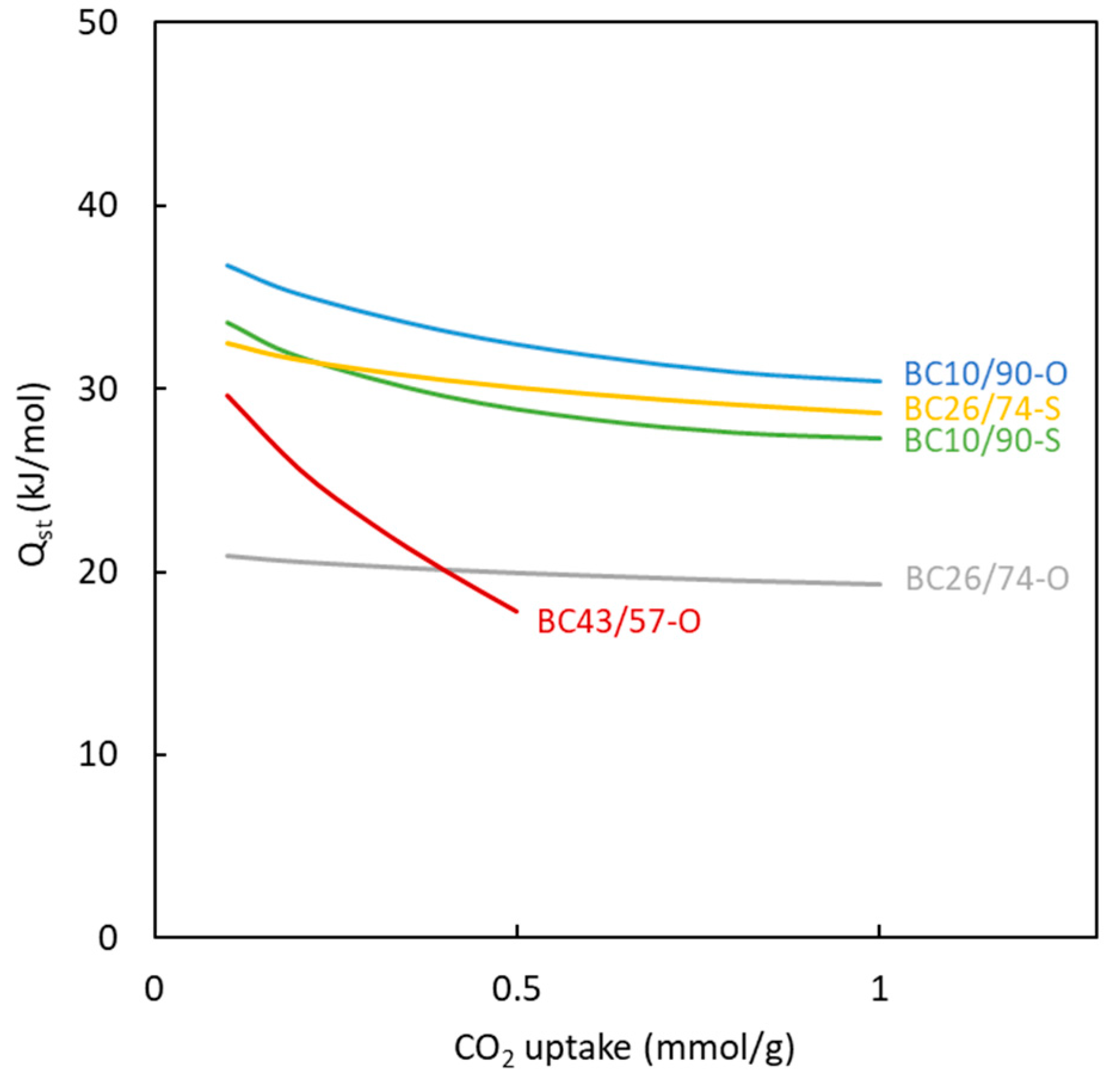
Table 5. The values of heat of adsorption calculated for the different materials and at different degrees of surface coverage are shown in
Figure 13. These data allow us to conclude that the magnitude of the heat involved in the adsorption of CO
2 molecules for all the carbons is 20-35 kJ mol
-1. The magnitude of this parameter allows us to confirm that the adsorption type is physical, in agreement with the same conclusion reached when the influence of temperature on the amount of CO
2 adsorbed was investigated.
Considering the influence of the surface coverage by the adsorbed CO
2, a decrease in the heat of adsorption value is observed for all materials. This type of behavior has commonly been observed [
50,
51] for carbons. It is attributed to the energetic heterogeneity of the surface of the carbons. At low CO
2 coverage (i.e., low amount of CO
2 adsorbed onto the surface), the molecules are mainly adsorbed in the ultramicropores, showing the most intense interactions between adsorbate and adsorbent. This behavior again agrees with the values of the t parameter of the Toth model (far from 1).
A comparison between the calculated results corresponding to the different synthesized carbons shows similar behavior for most of them. BC10/90-O carbon previously exhibited an important microporosity degree that can be attributed to the high heat of adsorption. On the other hand, the lowest heat of adsorption value (BC26/74-O) is reached for carbon with a low degree of microporosity. BC26/74-O carbon shows a high influence of adsorbed CO2 on the heat of adsorption. This finding is due to the presence of only ultramicroporosity in the solid, which contributes to large interactions between adsorbate and adsorbent.
All the sulphur-doped carbons show similar adsorption behaviors. BC10/90-S carbon shows a low amount of microporosity, but the heat of adsorption is similar to most of the carbons. This behavior can be due to the presence of functional groups in all the doped carbons generated by sulphur, which increase the intensity of chemical interactions with CO2 and are not affected by both the surface coverage and the microporosity degree. This behavior agrees with the previous discussion about the value of the t parameter of the Toth model for sulphur-doped materials.