Submitted:
27 December 2023
Posted:
28 December 2023
You are already at the latest version
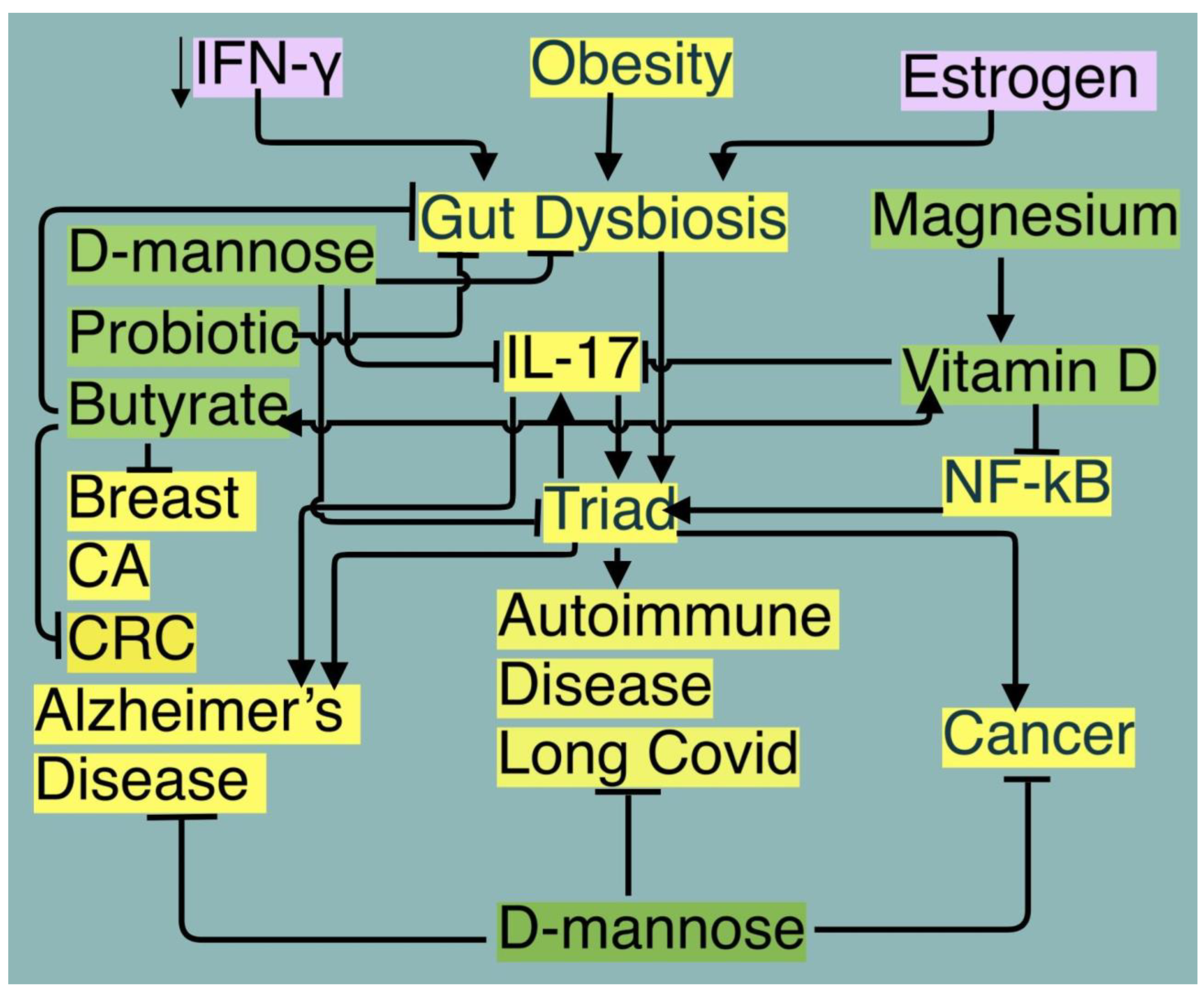
Abstract
Keywords:
Introduction
Conclusions
References
- COVID-19 Added to the list of Autoimmune Diseases https://www.autoimmuneregistry.org/long-covid-announcement.
- Altmann DM, Whettlock EM, Liu S, Arachchillage DJ, Boyton RJ. The immunology of long COVID. Nat Rev Immunol. 2023 Oct;23(10):618-634. [CrossRef]
- Peng K, Li X, Yang D, Chan SCW, Zhou J, Wan EYF; et al. Risk of autoimmune diseases following COVID-19 and the potential protective effect from vaccination: A population-based cohort study. EClinicalMedicine. 2023 Aug 16;63:102154. [CrossRef]
- Lim SH, Ju HJ, Han JH; et al. Autoimmune and Autoinflammatory Connective Tissue Disorders Following COVID-19. JAMA Netw Open. 2023;6(10):e2336120. [CrossRef]
- Herzum A, Micalizzi C, Molle MF, Parodi A. New-onset vitiligo following COVID-19 disease. Skin Health Dis. 2022 Jan 28;2(1):e86. [CrossRef]
- Schmidt AF, Rubin A, Milgraum D, Wassef C. Vitiligo following COVID-19: A case report and review of pathophysiology. JAAD Case Rep. 2022 Apr;22:47-49. [CrossRef]
- Dhanalakshmi, M., Sruthi, D., Jinuraj, K.R. et al. Mannose: A potential saccharide candidate in disease management. Med Chem Res 32, 391–408 (2023). [CrossRef]
- Chandra S, Sisodia SS, Vassar RJ. The gut microbiome in Alzheimer's disease: What we know and what remains to be explored. Mol Neurodegener. 2023 Feb 1;18(1):9. [CrossRef]
- Romanenko M, Kholin V, Koliada A, Vaiserman A. Nutrition, Gut Microbiota, and Alzheimer's Disease. Front Psychiatry. 2021 Aug 5;12:712673. [CrossRef]
- Deng Q, Luo Y, Chang C, Wu H, Ding Y, Xiao R. The Emerging Epigenetic Role of CD8+T Cells in Autoimmune Diseases: A Systematic Review. Front Immunol. 2019 Apr 18;10:856. [CrossRef]
- Fonseca, D.L., Filgueiras, I.S., Marques, A.H., Vojdani, E., Halpert, G., Ostrinski, Y.; et al. (2022). SARS-CoV-2 infection induces the production of autoantibodies in severe COVID-19 patients in an age-dependent manner. [CrossRef]
- Mousa WK, Chehadeh F, Husband S. Microbial dysbiosis in the gut drives systemic autoimmune diseases. Front Immunol. 2022 Oct 20;13:906258. [CrossRef]
- Kinashi Y, Hase K. Partners in Leaky Gut Syndrome: Intestinal Dysbiosis and Autoimmunity. Front Immunol. 2021 Apr 22;12:673708. [CrossRef]
- Fasano, A. Leaky gut and autoimmune diseases. Clin Rev Allergy Immunol. 2012 Feb;42(1):71-8. [CrossRef]
- Bogariu AM, Dumitrascu DL. Digestive involvement in the Long-COVID syndrome. Med Pharm Rep. 2022 Jan;95(1):5-10. [CrossRef]
- Zhang D, Zhou Y, Ma Y, Chen P, Tang J, Yang B; et al. Gut Microbiota Dysbiosis Correlates With Long COVID-19 at One-Year After Discharge. J Korean Med Sci. 2023 Apr 17;38(15):e120. [CrossRef]
- Fadlallah S, Bitar ER, Hussein H, Jallad MA, Matar GM, Rahal EA. The interplay between Epstein-Barr virus DNA and gut microbiota in the development of arthritis in a mouse model. Microbiol Spectr. 2023 Aug 24;11(5):e0204223. [CrossRef]
- Paray BA, Albeshr MF, Jan AT, Rather IA. Leaky Gut and Autoimmunity: An Intricate Balance in Individuals Health and the Diseased State. International Journal of Molecular Sciences. 2020; 21(24):9770. [CrossRef]
- Xu Wang, Shuangshaung Wu, Linman Li & Zhimin Yan (26 Oct 2023): Candida albicans overgrowth disrupts the gut microbiota in mice bearing oral cancer, Mycology. [CrossRef]
- Stojanov S, Berlec A, Štrukelj B. The Influence of Probiotics on the Firmicutes/Bacteroidetes Ratio in the Treatment of Obesity and Inflammatory Bowel disease. Microorganisms. 2020 Nov 1;8(11):1715. [CrossRef]
- Singh V, Lee G, Son H, Koh H, Kim ES, Unno T, Shin JH. Butyrate producers, "The Sentinel of Gut": Their intestinal significance with and beyond butyrate, and prospective use as microbial therapeutics. Front Microbiol. 2023 Jan 12;13:1103836. [CrossRef]
- Liu Q, Mak JWY, Su Q, Yeoh YK, Lui GC, Ng SSS; et al. Gut microbiota dynamics in a prospective cohort of patients with post-acute COVID-19 syndrome. Gut. 2022 Mar;71(3):544-552. [CrossRef]
- Zhang, F., Lau, R.I., Liu, Q. et al. Gut microbiota in COVID-19: Key microbial changes, potential mechanisms and clinical applications. Nat Rev Gastroenterol Hepatol 20, 323–337 (2023). [CrossRef]
- Brzychcy K, Dróżdż I, Skoczylas S, Płoszaj T, Sobolewska-Sztychny D, Skibińska M; et al. Gut microbiota in alopecia areata. Postepy Dermatol Alergol. 2022 Dec;39(6):1162-1170. [CrossRef]
- Carrington AE, Maloh J, Nong Y, Agbai ON, Bodemer AA, Sivamani RK. The Gut and Skin Microbiome in Alopecia: Associations and Interventions. J Clin Aesthet Dermatol. 2023 Oct;16(10):59-64 http://www.ncbi.nlm.nih.gov/pmc/articles/pmc10617895/.
- Sikora M, Stec A, Chrabaszcz M, Knot A, Waskiel-Burnat A, Rakowska A; et al. Gut Microbiome in Psoriasis: An Updated Review. Pathogens. 2020 Jun 12;9(6):463. [CrossRef]
- Myers B, Brownstone N, Reddy V, Chan S, Thibodeaux Q, Truong A; et al. The gut microbiome in psoriasis and psoriatic arthritis. Best Pract Res Clin Rheumatol. 2019 Dec;33(6):101494. [CrossRef]
- Olejniczak-Staruch I, Ciążyńska M, Sobolewska-Sztychny D, Narbutt J, Skibińska M, Lesiak A. Alterations of the Skin and Gut Microbiome in Psoriasis and Psoriatic Arthritis. Int J Mol Sci. 2021 Apr 13;22(8):3998. [CrossRef]
- Bzioueche H, Simonyté Sjödin K, West CE, Khemis A, Rocchi S, Passeron T; et al. Analysis of Matched Skin and Gut Microbiome of Patients with Vitiligo Reveals Deep Skin Dysbiosis: Link with Mitochondrial and Immune Changes. J Invest Dermatol. 2021 Sep;141(9):2280-2290. [CrossRef]
- Luan, M., Niu, M., Yang, P. et al. Metagenomic sequencing reveals altered gut microbial compositions and gene functions in patients with non-segmental vitiligo. BMC Microbiol 23, 265 (2023). [CrossRef]
- Wang Y, Xia X, Zhou X, Zhan T, Dai Q, Zhang Y; et al. Association of gut microbiome and metabolites with onset and treatment response of patients with pemphigus vulgaris. Front Immunol. 2023 Apr 14;14:1114586. https://doiorg/10.3389/fimmu.2023.1114586.
- Liu X, van Beek N, Cepic A, Andreani NA, Chung CJ, Hermes BM; et al. The gut microbiome in bullous pemphigoid: Implications of the gut-skin axis for disease susceptibility. Front Immunol. 2023 Nov 10;14:1212551. [CrossRef]
- Stochmal, A., Waśkiel-Burnat, A., Chrostowska, S. et al. Adiponectin as a novel biomarker of disease severity in alopecia areata. Sci Rep 11, 13809 (2021). [CrossRef]
- Barros G, Duran P, Vera I, Bermúdez V. Exploring the Links between Obesity and Psoriasis: A Comprehensive Review. Int J Mol Sci. 2022 Jul 6;23(14):7499. [CrossRef]
- Tanacan E, Atakan N. Higher incidence of metabolic syndrome components in vitiligo patients: A prospective cross-sectional study. An Bras Dermatol. 2020 Mar-Apr;95(2):165-172. [CrossRef]
- Sudre CH, Murray B, Varsavsky T, Graham MS, Penfold RS, Bowyer RC; et al. Attributes and predictors of long COVID. Nat Med. 2021 Apr;27(4):626-631. [CrossRef]
- Wang T, He C. Pro-inflammatory cytokines: The link between obesity and osteoarthritis. Cytokine Growth Factor Rev. 2018 Dec;44:38-50. [CrossRef]
- Coppola S, Avagliano C, Calignano A, Berni Canani R. The Protective Role of Butyrate against Obesity and Obesity-Related Diseases. Molecules. 2021 Jan 28;26(3):682. [CrossRef]
- Kwiat VR, Reis G, Valera IC, Parvatiyar K, Parvatiyar MS. Autoimmunity as a sequela to obesity and systemic inflammation. Front Physiol. 2022 Nov 21;13:887702. [CrossRef]
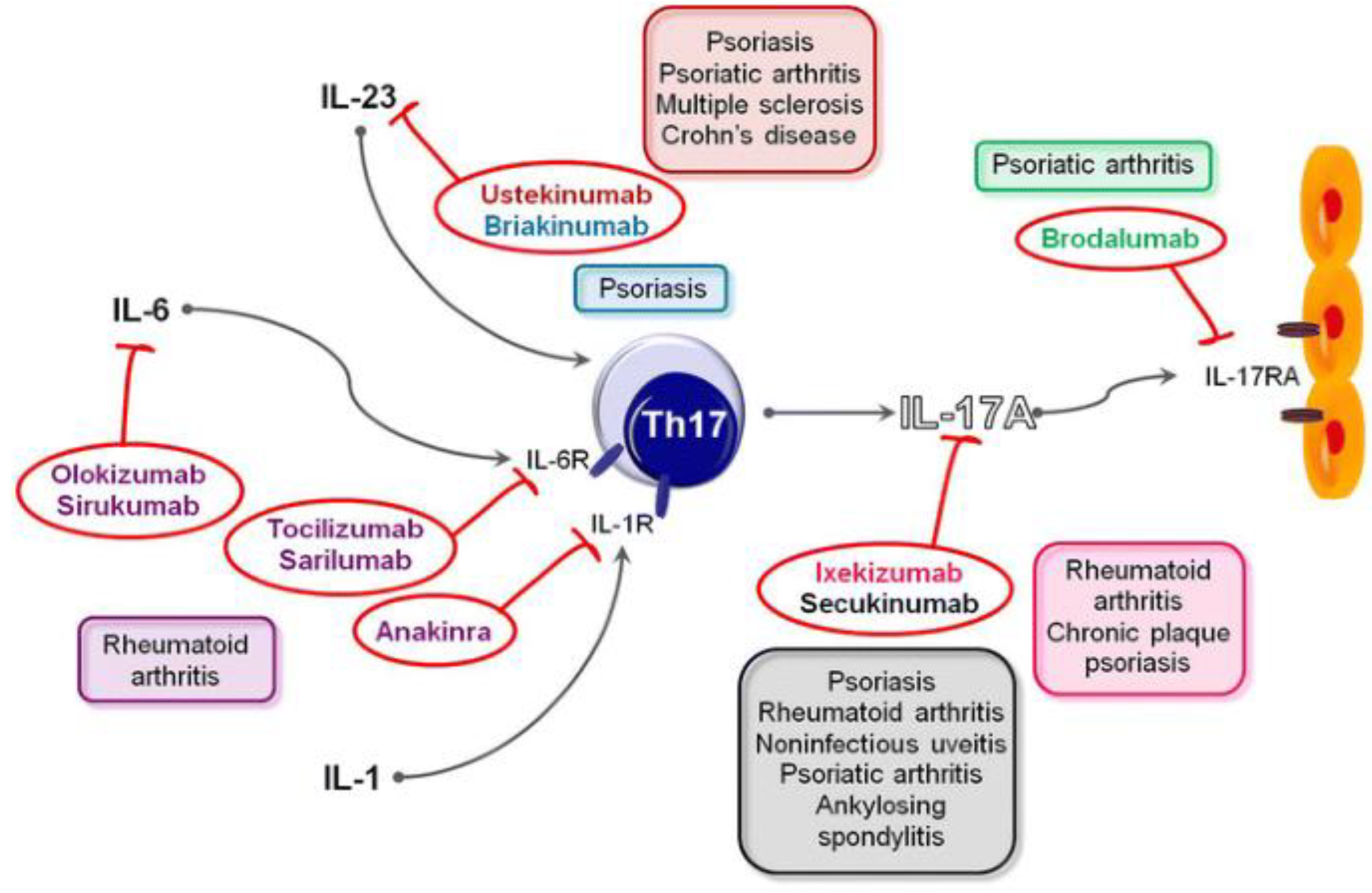
- Jung SM, Kim WU. Targeted Immunotherapy for Autoimmune Disease. Immune Netw. 2022 Feb;22(1):e9. [CrossRef]
- Pyrillou K, Burzynski LC, Clarke MCH. Alternative Pathways of IL-1 Activation, and Its Role in Health and Disease. Front Immunol. 2020 Dec 18;11:613170. [CrossRef]
- Neil A. Turner, Romana S. Mughal, Philip Warburton, David J. O'Regan, Stephen G. Ball, Karen E. Porter, Mechanism of TNFα-induced IL-1α, IL-1β and IL-6 expression in human cardiac fibroblasts: Effects of statins and thiazolidinediones, Cardiovascular Research, 76(1):81-90 October 2007. [CrossRef]
- Katz Y, Nadiv O, Beer Y. Interleukin-17 enhances tumor necrosis factor alpha-induced synthesis of interleukins 1,6, and 8 in skin and synovial fibroblasts: A possible role as a "fine-tuning cytokine" in inflammation processes. Arthritis Rheum. 2001 Sep;44(9):2176-84. [CrossRef]
- Jang DI, Lee AH, Shin HY, Song HR, Park JH, Kang TB; et al. The Role of Tumor Necrosis Factor Alpha (TNF-α) in Autoimmune Disease and Current TNF-α Inhibitors in Therapeutics. Int J Mol Sci. 2021 Mar 8;22(5):2719. [CrossRef]
- Chesler DA, Reiss CS. The role of IFN-gamma in immune responses to viral infections of the central nervous system. Cytokine Growth Factor Rev. 2002 Dec;13(6):441-54. [CrossRef]
- IFNγ guides the suppressive activity of Treg cells during viral infection.Nat Immunol 24, 751–752 (2023). [CrossRef]
- Ranta K, Nieminen K, Saariaho T, Kortekangas-Savolainen O, Kumpula EK, Kosonen J; et al. Evaluation of fungal extracts to determine immunomodulatory properties. J Investig Allergol Clin Immunol. 2013;23(4):226-33 https://pubmed.ncbi.nlm.nih.gov/23964551/.
- Delsing CE, Gresnigt MS, Leentjens J, Preijers F, Frager FA, Kox M; et al. Interferon-gamma as adjunctive immunotherapy for invasive fungal infections: A case series. BMC Infect Dis. 2014 Mar 26;14:166. [CrossRef]
- Abdel-Hamed EF, Ibrahim MN, Mostafa NE, Moawad HSF, Elgammal NE, Darwiesh EM; et al. Role of interferon gamma in SARS-CoV-2-positive patients with parasitic infections. Gut Pathog. 2021 May 4;13(1):29. [CrossRef]
- Vijayaraghava A, K R. Alteration of Interferon Gamma (IFN-γ) in Human Plasma with Graded Physical Activity. J Clin Diagn Res. 2014 Jun;8(6):BC05-7. [CrossRef]
- Hsieh, HS., Gong, YN., Chi, CY. et al. Gut microbiome profiles and associated metabolic pathways in patients of adult-onset immunodeficiency with anti-interferon-gamma autoantibodies. Sci Rep12, 9126 (2022). [CrossRef]
- Yue R, Wei X, Zhao J, Zhou Z, Zhong W. Essential Role of IFN-γ in Regulating Gut Antimicrobial Peptides and Microbiota to Protect Against Alcohol-Induced Bacterial Translocation and Hepatic Inflammation in Mice. Front Physiol. 2021 Jan 18;11:629141. [CrossRef]
- Gadotti AC, de Castro Deus M, Telles JP, Wind R, Goes M, Garcia Charello Ossoski R; et al. IFN-γ is an independent risk factor associated with mortality in patients with moderate and severe COVID-19 infection. Virus Res. 2020 Nov;289:198171. [CrossRef]
- Takahashi, T.; et al. “Sex differences in immune responses that underlie COVID-19 disease outcomes” Nature 588, 315–320 (2020). [CrossRef]
- Pujantell M, Altfeld M. “Consequences of sex differences in Type I IFN responses for the regulation of antiviral immunity” Front Immunol. 2022 Sep 16;13:986840. [CrossRef]
- Kumwenda P, Cottier F, Hendry AC, Kneafsey D, Keevan B, Gallagher H; et al. Estrogen promotes innate immune evasion of Candida albicans through inactivation of the alternative complement system. Cell Rep. 2022 Jan 4;38(1):110183. [CrossRef]
- Zaongo SD, Ouyang J, Isnard S, Zhou X, Harypursat V, Cui H; et al. Candida albicans can foster gut dysbiosis and systemic inflammation during HIV infection. Gut Microbes. 2023 Jan-Dec;15(1):2167171. [CrossRef]
- Faraj SS, Jalal PJ. IL1β, IL-6, and TNF-α cytokines cooperate to modulate a complicated medical condition among COVID-19 patients: Case-control study. Ann Med Surg (Lond). 2023 Apr 26;85(6):2291-2297. [CrossRef]
- Schultheiß C, Willscher E, Paschold L, Gottschick C, Klee B, Henkes SS; et al. The IL-1β, IL-6, and TNF cytokine triad is associated with post-acute sequelae of COVID-19. Cell Rep Med. 2022 Jun 21;3(6):100663. [CrossRef]
- Low RN, Low RJ, Akrami A. A review of cytokine-based pathophysiology of Long COVID symptoms. Front Med (Lausanne). 2023 Mar 31;10:1011936. [CrossRef]
- Möller, B., Villiger, P.M. Inhibition of IL-1, IL-6, and TNF-α in immune-mediated inflammatory diseases. Springer Semin Immun 27, 391–408 (2006). [CrossRef]
- Mikos H, Mikos M, Rabska-Pietrzak B, Niedziela M. The clinical role of serum concentrations of selected cytokines: IL-1β, TNF-α and IL-6 in diagnosis of autoimmune thyroid disease (AITD) in children. Autoimmunity. 2014 Nov;47(7):466-72. [CrossRef]
- Liu Y, Gao Y, Lin T. Expression of interleukin-1 (IL-1), IL-6, and tumor necrosis factor-α (TNF-α) in non-small cell lung cancer and its relationship with the occurrence and prognosis of cancer pain. Ann Palliat Med. 2021 Dec;10(12):12759-12766. [CrossRef]
- Yoshida N, Ikemoto S, Narita K, Sugimura K, Wada S, Yasumoto R; et al. Interleukin-6, tumour necrosis factor alpha and interleukin-1beta in patients with renal cell carcinoma. Br J Cancer. 2002 May 6;86(9):1396-400. [CrossRef]
- Ishijima T, Nakajima K. Inflammatory cytokines TNFα, IL-1β, and IL-6 are induced in endotoxin- stimulated microglia through different signaling cascades. Sci Prog. 2021 Oct;104(4):368504211054985. [CrossRef]
- Kim, M., Park, H.E., Lee, SH. et al. Increased risk of Alzheimer’s disease in patients with psoriasis: A nationwide population-based cohort study.Sci Rep 10, 6454 (2020). [CrossRef]
- Li CY, Tai YH, Dai YX, Chang YT, Bai YM, Tsai SJ; et al. Association of Alopecia Areata and the Risk of Dementia: A Nationwide Cohort Study. J Clin Psychiatry. 2021 Oct 26;82(6):21m13931. [CrossRef]
- Chang TH, Tai YH, Dai YX, Chang YT, Chen TJ, Chen MH. Association between vitiligo and subsequent risk of dementia: A population-based cohort study. J Dermatol. 2021 Jan;48(1):28-33. [CrossRef]
- Si ZZ, Zou CJ, Mei X, Li XF, Luo H, Shen Y; et al. Targeting neuroinflammation in Alzheimer's disease: From mechanisms to clinical applications. Neural Regen Res. 2023 Apr;18(4):708-715. [CrossRef]
- Xiao P, Hu Z, Lang J, Pan T, Mertens RT, Zhang H; et al. Mannose metabolism normalizes gut homeostasis by blocking the TNF-α-mediated proinflammatory circuit. Cell Mol Immunol. 2023 Feb;20(2):119-130. [CrossRef]
- Torretta S, Scagliola A, Ricci L, Mainini F, Di Marco S, Cuccovillo I; et al. D-mannose suppresses macrophage IL-1β production. Nat Commun. 2020 Dec 11;11(1):6343. [CrossRef]
- Guo L, Hou Y, Song L, Zhu S, Lin F, Bai Y. D-Mannose Enhanced Immunomodulation of Periodontal Ligament Stem Cells via Inhibiting IL-6 Secretion. Stem Cells Int. 2018 Sep 9;2018:7168231. [CrossRef]
- Li M, Cheng H, Tian D, Yang L, Du X, Pan Y; et al. D-Mannose Suppresses γδ T Cells and Alleviates Murine Psoriasis. Front Immunol. 2022 Feb 28;13:840755. [CrossRef]
- Wang K, Chen W, Zhang Z, Deng Y, Lian JQ, Du P; et al. CD147-spike protein is a novel route for SARS-CoV-2 infection to host cells. Signal Transduct Target Ther. 2020 Dec 4;5(1):283. [CrossRef]
- Chambers, P.W. (2021) Basigin Binds Spike S on SARS-CoV2. Open Access Library Journal, 8: e8064. [CrossRef]
- Geng JJ, Tang J, Yang XM, Chen R, Zhang Y, Zhang K, Miao JL, Chen ZN, Zhu P. Targeting CD147 for T to NK Lineage Reprogramming and Tumor Therapy. EBioMedicine. 2017 Jun;20:98-108. [CrossRef]
- Wo J, Zhang F, Li Z, Sun C, Zhang W, Sun G. The Role of Gamma-Delta T Cells in Diseases of the Central Nervous System. Front Immunol. 2020 Oct 23;11:580304. [CrossRef]
- Gauthier T, Chen W. IFN-γ and TGF-β, Crucial Players in Immune Responses: A Tribute to Howard Young. J Interferon Cytokine Res. 2022 Dec;42(12):643-654. [CrossRef]
- Regal-McDonald K, Patel RP. Selective Recruitment of Monocyte Subsets by Endothelial N-Glycans. Am J Pathol. 2020 May;190(5):947-957. [CrossRef]
- Inês Alves et al. Host-derived mannose glycans trigger a pathogenic γδ T cell/IL-17a axis in autoimmunity.Sci. Transl. Med.15,eabo1930(2023). [CrossRef]
- Hagan Y, Gonzalez A, Kasler A, Macedo M, Rincon A, Jameson J, Increased proliferation of epidermal gamma delta T cells and expression of the transmembrane protein, BST2, in Alopecia areata. J Immunol 1 May 2022; 208 (1_Supplement): 55.19. [CrossRef]
- Curio S, Edwards SC, Suzuki T, McGovern J, Triulzi C, Yoshida N; et al. NKG2D signaling regulates IL-17A-producing γδT cells in mice to promote cancer progression. Discov Immunol. 2022 May 10;1(1):kyac002. [CrossRef]
- Plaza-Rojas L, Guevara-Patiño JA. The Role of the NKG2D in Vitiligo. Front Immunol. 2021 Feb 26;12:624131. [CrossRef]
- Swank Z, Senussi Y, Manickas-Hill Z, Yu XG, Li JZ, Alter G, et al., Persistent Circulating Severe Acute Respiratory Syndrome Coronavirus 2 Spike Is Associated With Post-acute Coronavirus Disease 2019 Sequelae, Clinical Infectious Diseases, 76(3):e487–e490 1 February 2023. [CrossRef]
- Dong, L., Xie, J., Wang, Y. et al. Mannose ameliorates experimental colitis by protecting intestinal barrier integrity. Nat Commun 13, 4804 (2022). [CrossRef]
- Zhang W, Cheng H, Gui Y, Zhan Q, Li S, Qiao W; et al. Mannose Treatment: A Promising Novel Strategy to Suppress Inflammation. Front Immunol. 2021 Sep 27;12:756920. [CrossRef]
- Shi YB, Yin D. A good sugar, d-mannose, suppresses autoimmune diabetes. Cell Biosci. 2017 Sep 25;7:48. [CrossRef]
- Wang H, Teng X, Abboud G, Li W, Ye S, Morel L. D-mannose ameliorates autoimmune phenotypes in mouse models of lupus. BMC Immunol. 2021 Jan 5;22(1):1. [CrossRef]
- Roth W, Zadeh K, Vekariya R, Ge Y, Mohamadzadeh M. Tryptophan Metabolism and Gut-Brain Homeostasis. Int J Mol Sci. 2021 Mar 15;22(6):2973. [CrossRef]
- Hodgkinson K, El Abbar F, Dobranowski P, Manoogian J, Butcher J, Figeys D; et al. Butyrate's role in human health and the current progress towards its clinical application to treat gastrointestinal disease. Clin Nutr. 2023 Feb;42(2):61-75. [CrossRef]
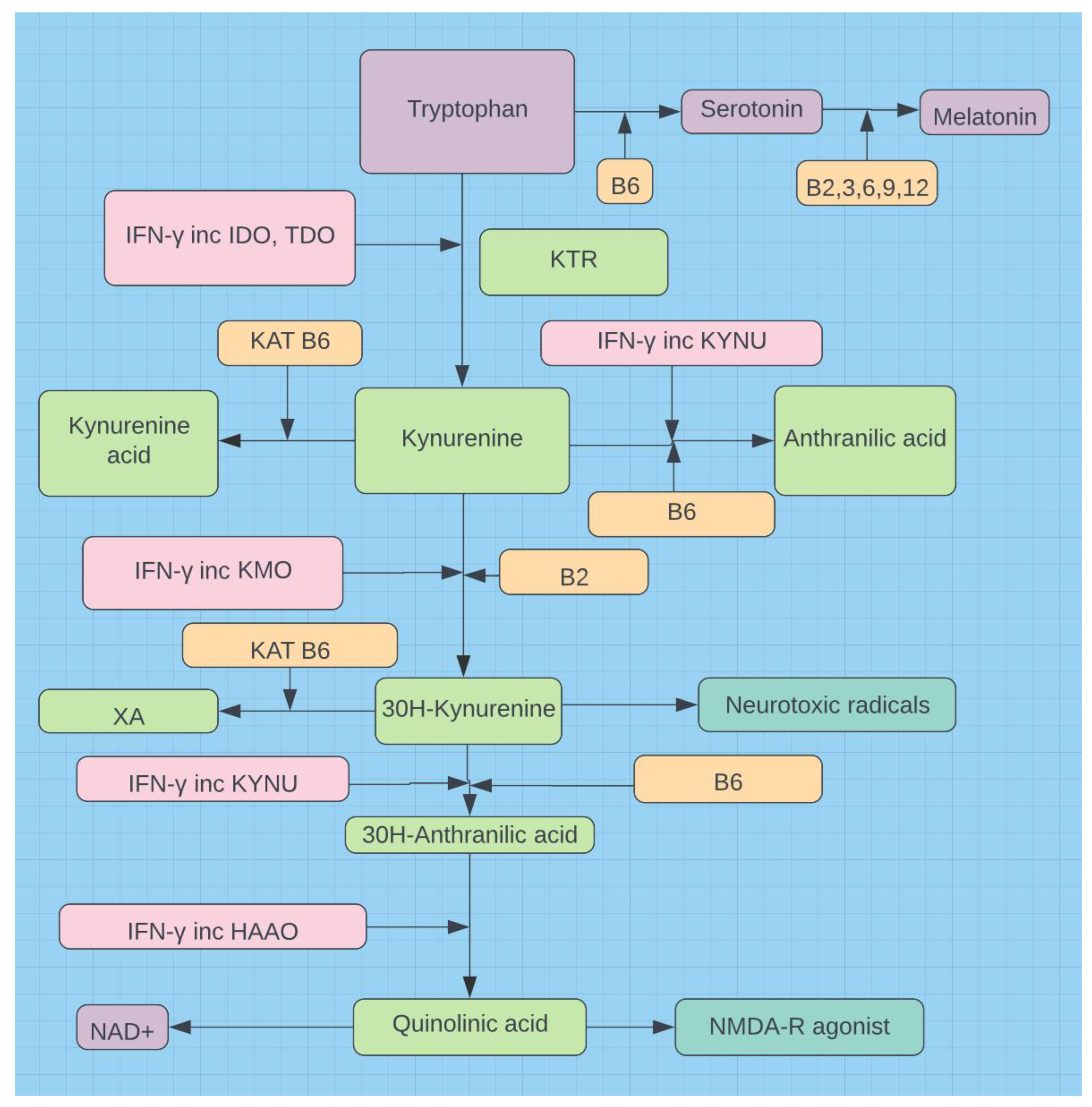
- Eroğlu İ, Eroğlu BÇ, Güven GS. Altered tryptophan absorption and metabolism could underlie long-term symptoms in survivors of coronavirus disease 2019 (COVID-19). Nutrition. 2021 Oct;90:111308. [CrossRef]
- Almulla AF, Supasitthumrong T, Tunvirachaisakul C, Algon AAA, Al-Hakeim HK, Maes M. The tryptophan catabolite or kynurenine pathway in COVID-19 and critical COVID-19: A systematic review and meta-analysis. BMC Infect Dis. 2022 Jul 15;22(1):615. [CrossRef]
- Brown J, Robusto B, Morel L. Intestinal Dysbiosis and Tryptophan Metabolism in Autoimmunity. Front Immunol. 2020 Aug 4;11:1741. [CrossRef]
- Wu D, Zhu Y. Role of kynurenine in promoting the generation of exhausted CD8+ T cells in colorectal cancer. Am J Transl Res. 2021 Mar 15;13(3):1535-1547 https://pubmed.ncbi.nlm.nih.gov/33841677/.
- Lionetto L, Ulivieri M, Capi M, De Bernardini D, Fazio F, Petrucca A; et al. Increased kynurenine-to-tryptophan ratio in the serum of patients infected with SARS-CoV2: An observational cohort study. Biochim Biophys Acta Mol Basis Dis. 2021 Mar 1;1867(3):166042. [CrossRef]
- Fernandes BS, Inam ME, Enduru N, Quevedo J, Zhao Z. The kynurenine pathway in Alzheimer's disease: A meta-analysis of central and peripheral levels. Braz J Psychiatry. 2023 May-Jun;45(3):286-297. [CrossRef]
- Gouasmi R, Ferraro-Peyret C, Nancey S, Coste I, Renno T, Chaveroux C; et al. The Kynurenine Pathway and Cancer: Why Keep It Simple When You Can Make It Complicated. Cancers (Basel). 2022 Jun 4;14(11):2793. [CrossRef]
- Zhang Y, Tao Y, Gu Y, Ma Q. Butyrate facilitates immune clearance of colorectal cancer cells by suppressing STAT1-mediated PD-L1 expression. Clinics (Sao Paulo). 2023 Nov 4;78:100303. [CrossRef]
- Schoeller A, Karki K, Jayaraman A, Chapkin RS, Safe S. Short chain fatty acids exhibit selective estrogen receptor downregulator (SERD) activity in breast cancer. Am J Cancer Res. 2022 Jul 15;12(7):3422-3436. https://pubmed.ncbi.nlm.nih.gov/35968335/.
- Bombardi C, Grandis A, Pivac N, Sagud M, Lucas G, Chagraoui A; et al. Serotonin modulation of hippocampal functions: From anatomy to neurotherapeutics. Prog Brain Res. 2021;261:83-158. [CrossRef]
- Pisa D, Alonso R, Rábano A, Rodal I, Carrasco L. Different Brain Regions are Infected with Fungi in Alzheimer's Disease. Sci Rep. 2015 Oct 15;5:15015. [CrossRef]
- Mayr A, Hinterberger G, Dierich MP, Lass-Flörl C. Interaction of serotonin with Candida albicans selectively attenuates fungal virulence in vitro. Int J Antimicrob Agents. 2005 Oct;26(4):335-7. [CrossRef]
- Nguyen LN, Lopes LC, Cordero RJ, Nosanchuk JD. Sodium butyrate inhibits pathogenic yeast growth and enhances the functions of macrophages. J Antimicrob Chemother. 2011 Nov;66(11):2573-80. [CrossRef]
- Wu, Y., Du, S., Johnson, J.L. et al. Microglia and amyloid precursor protein coordinate control of transient Candida cerebritis with memory deficits. Nat Commun 10, 58 (2019). [CrossRef]
- Wu Y, Du S, Bimler LH, Mauk KE, Lortal L, Kichik N; et al. Toll-like receptor 4 and CD11b expressed on microglia coordinate eradication of Candida albicans cerebral mycosis. Cell Rep. 2023 Oct 31;42(10):113240. [CrossRef]
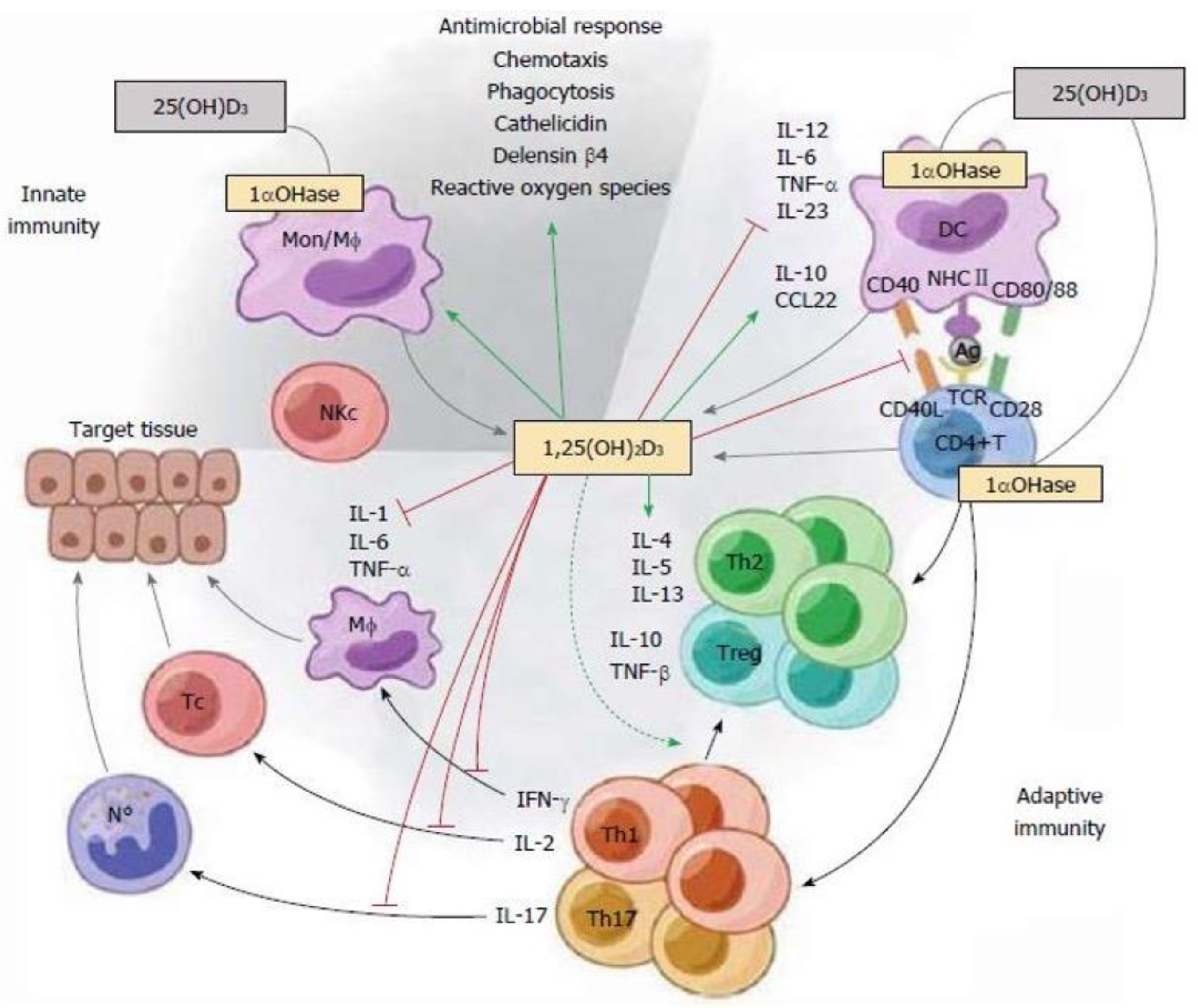
- Moreno-Torres M, Guzmán C, Petrov PD, Jover R. Valproate and Short-Chain Fatty Acids Activate Transcription of the Human Vitamin D Receptor Gene through a Proximal GC-Rich DNA Region Containing Two Putative Sp1 Binding Sites. Nutrients. 2022; 14(13):2673. [CrossRef]
- Singh P, Rawat A, Alwakeel M, Sharif E, Al Khodor S. The potential role of vitamin D supplementation as a gut microbiota modifier in healthy individuals. Sci Rep. 2020 Dec 10;10(1):21641. [CrossRef]
- di Filippo L, Frara S, Nannipieri F, Cotellessa A, Locatelli M, Rovere Querini P; et al. Low Vitamin D Levels Are Associated With Long COVID Syndrome in COVID-19 Survivors. J Clin Endocrinol Metab. 2023 Sep 18;108(10):e1106-e1116. [CrossRef]
- Yang CY, Leung PS, Adamopoulos IE, Gershwin ME. The implication of vitamin D and autoimmunity: A comprehensive review. Clin Rev Allergy Immunol. 2013 Oct;45(2):217-26. [CrossRef]
- Murdaca G, Tonacci A, Negrini S, Greco M, Borro M, Puppo F; et al. Emerging role of vitamin D in autoimmune diseases: An update on evidence and therapeutic implications. Autoimmun Rev. 2019 Sep;18(9):102350. [CrossRef]
- Mazanova A, Shymanskyi I, Lisakovska O, Labudzynskyi D, Khomenko A; et al. The link between vitamin D status and NF-κB-associated renal dysfunction in experimental diabetes mellitus. Biochim Biophys Acta Gen Subj. 2022 Jul;1866(7):130136. [CrossRef]
- Tabarkiewicz, J., Pogoda, K., Karczmarczyk, A. et al. The Role of IL-17 and Th17 Lymphocytes in Autoimmune Diseases. Arch. Immunol. Ther. Exp. 63, 435–449 (2015). [CrossRef]
- Saberi B, Dadabhai AS, Nanavati J, Wang L, Shinohara RT, Mullin GE. Vitamin D levels do not predict the stage of hepatic fibrosis in patients with non-alcoholic fatty liver disease: A PRISMA compliant systematic review and meta-analysis of pooled data. World J Hepatol. 2018 Jan 27;10(1):142-154. [CrossRef]
- Trapani V, Rosanoff A, Baniasadi S, Barbagallo M, Castiglioni S, Guerrero-Romero F; et al. The relevance of magnesium homeostasis in COVID-19. Eur J Nutr. 2022 Mar;61(2):625-636. [CrossRef]
- Ashique S, Kumar S, Hussain A, Mishra N, Garg A, Gowda BHJ; et al. A narrative review on the role of magnesium in immune regulation, inflammation, infectious diseases, and cancer. J Health Popul Nutr. 2023 Jul 27;42(1):74. [CrossRef]
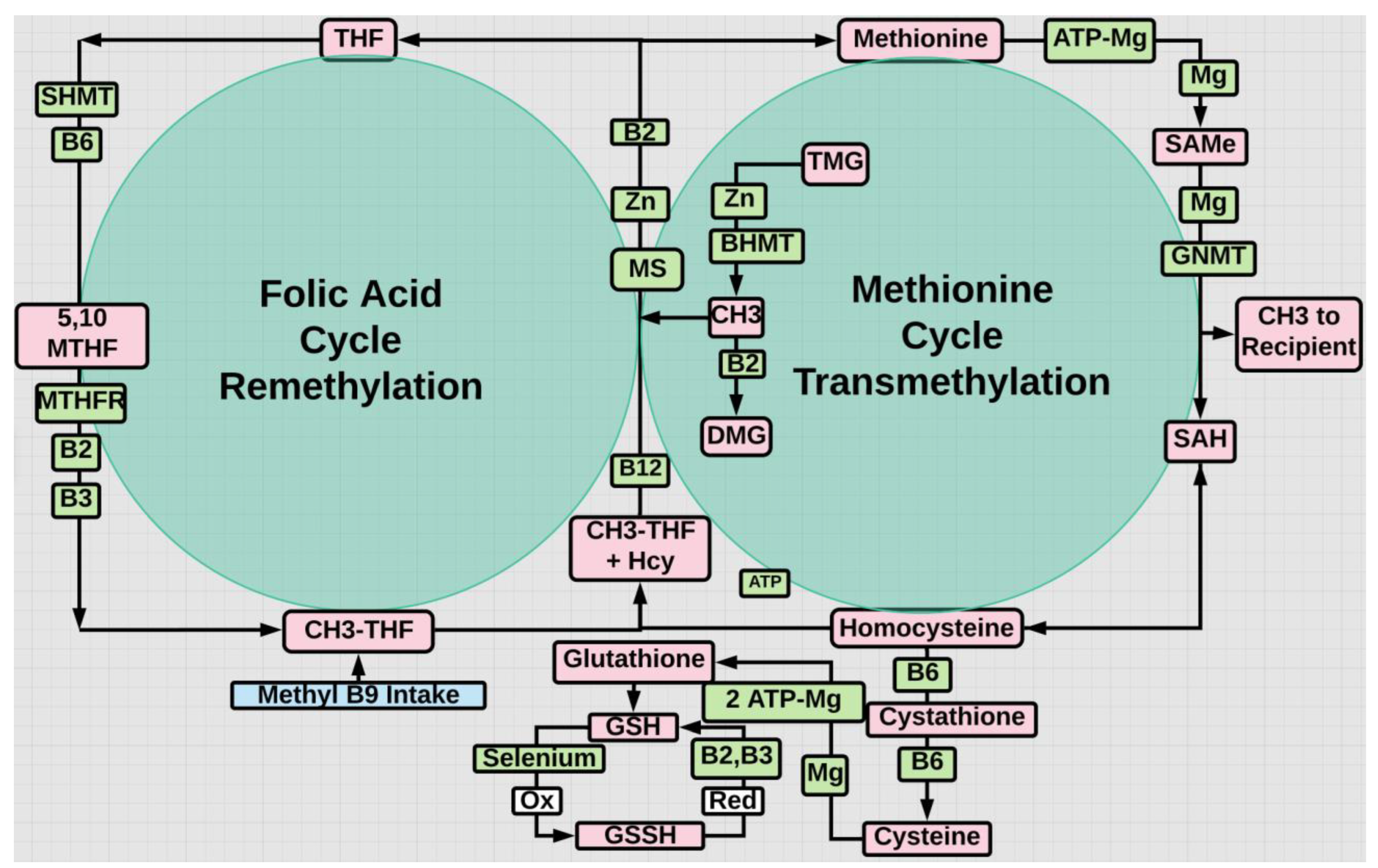
- Ballestar, E., Sawalha, A.H. & Lu, Q. Clinical value of DNA methylation markers in autoimmune rheumatic diseases. Nat Rev Rheumatol 16, 514–524 (2020). [CrossRef]
- Wardowska A. m6A RNA Methylation in Systemic Autoimmune Diseases-A New Target for Epigenetic-Based Therapy? Pharmaceuticals (Basel). 2021 Mar 5;14(3):218. [CrossRef]
- Li J, Li L, Wang Y, Huang G, Li X, Xie Z; et al. Insights Into the Role of DNA Methylation in Immune Cell Development and Autoimmune Disease. Front Cell Dev Biol. 2021 Nov 1;9:757318. [CrossRef]
- Lazzerini PE, Capecchi PL, Selvi E, Lorenzini S, Bisogno S, Galeazzi M; et al. Hyperhomocysteinemia, inflammation and autoimmunity. Autoimmun Rev. 2007 Aug;6(7):503-9. [CrossRef]
- Hu X, Wang JB, Zhao Y, Fang Y, Chen C, Ge M; et al. Homocysteine as a trigger and potential therapeutic target for autoimmune diseases. Autoimmun Rev. 2023 Sep;22(9):103389. [CrossRef]
- Lu M, Peng K, Song L, Luo L, Liang P, Liang Y. Association between Genetic Polymorphisms in Methylenetetrahydrofolate Reductase and Risk of Autoimmune Diseases: A Systematic Review and Meta-Analysis. Dis Markers. 2022 May 31;2022:4568145. [CrossRef]
- Kalkan G, Yigit S, Karakuş N, Ateş O, Bozkurt N, Ozdemir A; et al. Methylenetetrahydrofolate reductase C677T mutation in patients with alopecia areata in Turkish population. Gene. 2013 Nov 1;530(1):109-12. [CrossRef]
- Izmirli M, Sen BB, Rifaioglu E, Gogebakan B, Aldemir O, Sen T, Ekiz O, Alptekin D. Methylenetetrahydrofolate reductase (MTHFR) C677T polymorphism in psoriasis in southern Turkey. An Bras Dermatol. 2016 Sep-Oct;91(5):611-613. [CrossRef]
- Zhang HZ, Wu JH, Huang Q, Yang Q, Sima Q, Chen KY, Li ZR, He GH. Associations of methylenetetrahydrofolate reductase gene (MTHFR) rs1801131 and rs1801133 polymorphisms with susceptibility to vitiligo: A meta-analysis. J Cosmet Dermatol. 2021 Jul;20(7):2359-2368. [CrossRef]
- Lin X, Meng X, Song Z. Homocysteine and psoriasis. Biosci Rep. 2019 Nov 29;39(11):BSR20190867. [CrossRef]
- Jadeja SD, Mansuri MS, Singh M, Patel H, Marfatia YS, Begum R. Association of elevated homocysteine levels and Methylenetetrahydrofolate reductase (MTHFR) 1298 A > C polymorphism with Vitiligo susceptibility in Gujarat. J Dermatol Sci. 2018 May;90(2):112-122. [CrossRef]
- Dutta, T., Mitra, S., Saha, A. et al. A comprehensive meta-analysis and prioritization study to identify vitiligo associated coding and non-coding SNV candidates using web-based bioinformatics tools. Sci Rep 12, 14543 (2022). [CrossRef]
- Malhotra K, Madke B. An Updated Review on Current Treatment of Alopecia Areata and Newer Therapeutic Options. Int J Trichology. 2023 Jan-Feb;15(1):3-12. https://pubmed.ncbi.nlm.nih.gov/37305188/.
- Landeck L, Sabat R, Ghoreschi K, Man XY, Fuhrmeister K, Gonzalez-Martinez E; et al. Immunotherapy in psoriasis. Immunotherapy. 2021 May;13(7):605-619. [CrossRef]
- Faraj S, Kemp EH, Gawkrodger DJ. Patho-immunological mechanisms of vitiligo: The role of the innate and adaptive immunities and environmental stress factors. Clin Exp Immunol. 2022 Jan 28;207(1):27-43. [CrossRef]
- Thompson JM, Park MK, Qureshi AA, Cho E. Race and Alopecia Areata amongst US Women. J Investig Dermatol Symp Proc. 2018 Jan;19(1):S47-S50. [CrossRef]
- Nicholas MN, Chan AR, Hessami-Booshehri M. Psoriasis in patients of color: Differences in morphology, clinical presentation, and treatment. Cutis. 2020 Aug;106(2S):7-10;E10. [CrossRef]
- Dégboé B, Atadokpèdé F, Saka B, Adégbidi H, Koudoukpo C, Yédomon H; et al. Vitiligo on black skin: Epidemiological and clinical aspects in dermatology, Cotonou (Benin). Int J Dermatol. 2017 Jan;56(1):92-96. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




