1. Introduction to NIRS Imaging
Near-infrared spectroscopy (NIRS) has become a powerful tool in diverse biomedical imaging applications, offering valuable insights into biological tissues and processes [
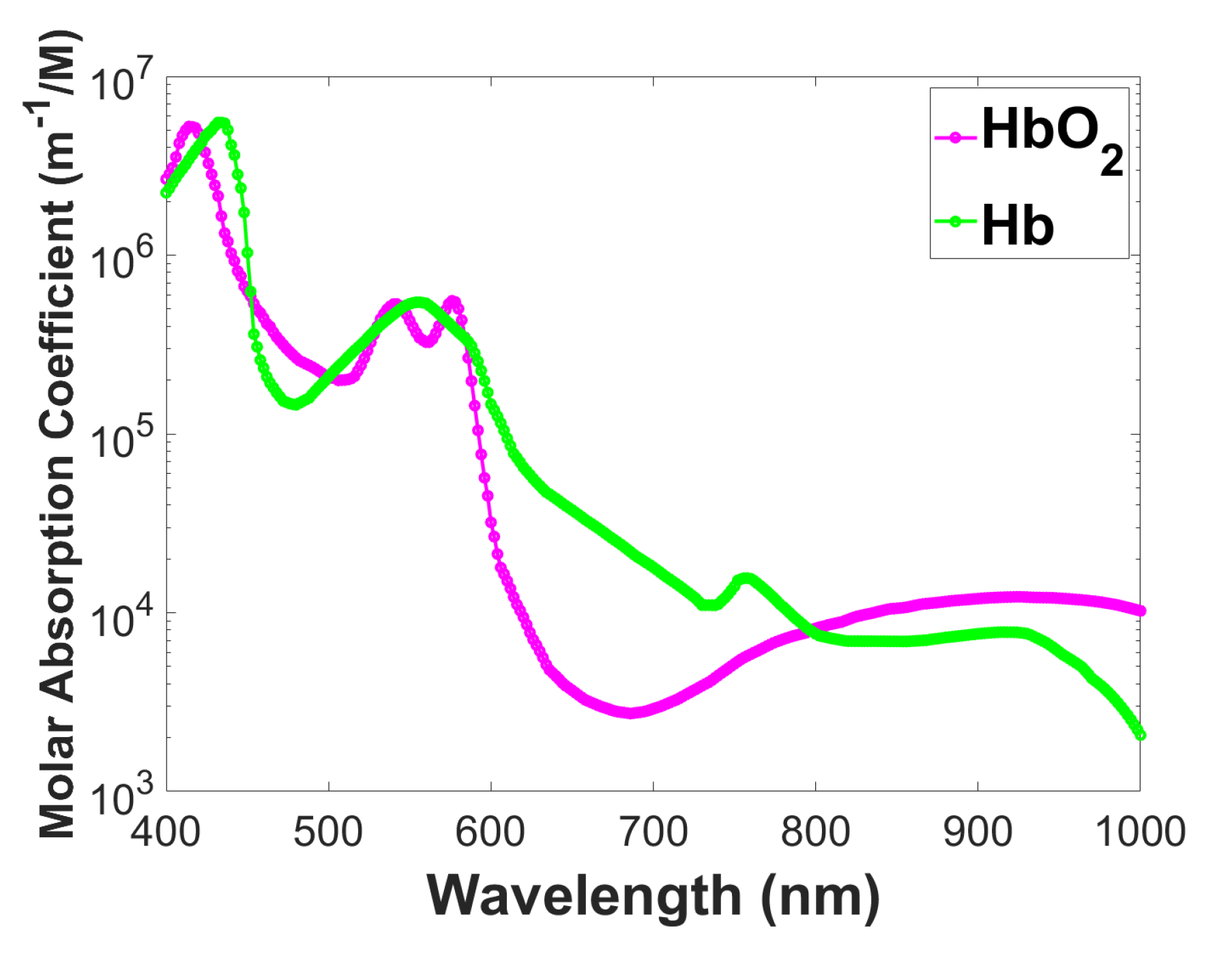
1]. One of its primary applications lies in functional brain imaging, where NIRS enables non-invasive monitoring of cerebral oxygenation and hemodynamics. By measuring changes in NIR light absorption, it provides real-time information about brain activity, making it particularly useful for studying cognitive functions and neurovascular coupling. Additionally, NIRS plays a pivotal role in cardiovascular imaging, allowing for the assessment of tissue oxygenation in the heart and peripheral vascular regions. The technique also proves valuable in detecting ischemic conditions and monitoring cardiac surgery interventions. Moreover, NIRS-based diffuse optical tomography is used in oncology, contributing to tumor early detection and characterization. By exploiting the distinct absorption spectra of various chromophores as shown in
Figure 1, NIRS facilitates the assessment of tissue composition and vascularity. This assists in tumor localization and differentiation. Furthermore, NIRS exhibits promise in musculoskeletal imaging, providing insights into oxygenation levels in skeletal muscles during exercise and rehabilitation. As a non-ionizing and portable imaging modality, NIRS offers advantages in point-of-care settings and continuous monitoring [
2,
3,
4].
Over the past century, optical medical imaging progress has significantly facilitated high-density data acquisition in modern medicine. This advancement offers increased precision, reduced susceptibility to errors, and a more extensive array of source-detector combinations and wavelengths than earlier methods. The term “light” encompasses wavelengths spanning the infrared, visible, and ultraviolet ranges. Since Maxwell’s elucidation of light as electromagnetic wave propagation in the 1800s, the scientific community has explored, measured, and documented a diverse array of physical processes and properties within the human body using optical imaging techniques.
NIRS diffuse optical tomography (NIRS-DOT) uses spectroscopy and tomographic method. NIRS-DOT key strength lies in its ability to non-invasively probe biological tissues with depth and detail [
5,
6]. By leveraging near-infrared light, NIRS-DOT enables the assessment of internal tissue composition and functional characteristics, offering a comprehensive view of anatomical structures and physiological processes. This imaging modality is highly effective in the investigation of diseases such as breast cancer [
7], where its capacity to discern optical properties within tissues aids in tumor early detection and characterization. The combination of multiple light sources and detectors, coupled with sophisticated image reconstruction algorithms [
8], allows NIRS-DOT to provide 3D images quickly. Noteworthy advancements include circular probes for continuous spectroscopic imaging. In addition, GPUs for accelerated image reconstruction facilitate real-time imaging capabilities. The ongoing efforts to enhance NIRS-DOT instruments by reducing costs through the integration of LEDs and photodetectors, and the development of specialized instruments for high-speed tissue imaging, underscore its potential as a versatile and impactful tool in medical diagnostics.
2. NIRS in Brain Imaging
Simple imaging methods based on NIRS include functional near-infrared spectroscopy (fNIRS). In fNIRS channel-wise measurements instead of fully tomographic image reconstruction occur [
9,
10]. The inherent transparency of biological tissues to NIR light allows for penetration into the brain, enabling real-time assessment of cerebral oxygenation levels and hemodynamic changes. The technique leverages spectroscopy principles, utilizing multiple wavelengths of light to quantify the concentration of chromophores in the tissue. In NIRS, light sources emitting near-infrared wavelengths are directed onto the scalp, and the resulting transmitted or reflected light is detected by optoelectronic sensors strategically placed over the head. Recorded signals undergo sophisticated signal processing to extract relevant physiological information. Method such as the Modified Beer-Lambert Law, are employed for estimation of hemoglobin concentrations changes and, consequently, oxygen saturation levels in cerebral tissue. Researchers are actively refining NIRS methodologies to improve spatial resolution, depth sensitivity, and specificity in detecting changes in regional cerebral oxygenation. Recent studies have explored multi-modal approaches, integrating NIRS with other neuroimaging techniques like functional magnetic resonance imaging (fMRI) [
11] and electroencephalography (EEG) [
9] to provide a more comprehensive understanding of brain function. NIRS’ technical nuances make it a valuable tool in neuroimaging, offering real-time insights into cerebral hemodynamics and oxygenation, with implications for research in neuroscience, cognitive science, and clinical applications such as brain-computer interfaces and monitoring cerebral auto-regulation in critically ill patients.
fNIRS is widely used for brain imaging, for example, to measure mental workload [
12,
13,
14,
15], measure mental stress [
16], and neurofeedback [
17]. It’s relatively simpler to design an fNIRS system [
18,
19,
20] than DOT. A patch for brain imaging was also shown [
18,
20]. However, signal quality depends on the design of the light sources and detectors, known as optodes [
21]. There is also a considerable amount of research into portable fNIRS systems [
2,
22]. An fNIRS system was also developed using Internet-of-Things technology [
23,
24,
23]. Furthermore, machine learning can be applied to fNIRS in real-time to enable the automatic classification of brain function [
14,
25,
26,
27].
2.1. NIRS Time-series Signal Processing
Due to the complexity of fNIRS data, advanced signal processing techniques to extract meaningful information about brain function are required [
28]. Preprocessing steps involve addressing various sources of noise, such as motion artifacts and physiological interferences. These sources can significantly impact fNIRS signal quality. Signal quality is further enhanced through spatial filtering methods, such as adaptive filtering and principal component analysis. This is to mitigate contamination from superficial tissues and improve the specificity of recorded signals for cortical regions. The raw fNIRS data, comprising changes in oxyhemoglobin (HbO) and deoxyhemoglobin (Hb) concentrations, undergo statistical analyses to identify task-related activations. General linear models (GLMs) and machine learning algorithms are commonly employed for this purpose [
29]. GLMs allow for the modeling of the relationship between the observed fNIRS signals and the experimental paradigm. This enables the identification of brain regions involved in specific tasks. Machine learning approaches, including support vector machines and neural networks, leverage the high-dimensional nature of fNIRS data to classify different cognitive states or tasks.
Temporal and spectral analyses play a crucial role in characterizing brain function dynamics using fNIRS. Temporal analyses involve the assessment of hemodynamic response functions, allowing researchers to investigate neural activations’ timing and duration. Spectral analyses, such as wavelet transform and Fourier analysis, provide insights into the frequency domain of fNIRS signals. This facilitates the identification of oscillatory brain activity associated with different cognitive processes. Moreover, connectivity analyses in fNIRS signal processing focus on exploring functional connectivity patterns between different brain regions [
30]. Methods such as seed-based correlation analysis and graph theory are applied to elucidate the underlying networks involved in specific cognitive tasks or resting-state conditions. These approaches provide a comprehensive understanding of how different brain regions interact and contribute to cognitive processes. Research in fNIRS signal processing continually evolves, with a focus on addressing challenges such as data fusion from multiple channels, artifact removal, and real-time processing for applications like brain-computer interfaces. As fNIRS continues to gain traction in neuroscience and clinical research, advancements in signal processing techniques will undoubtedly play a pivotal role in unlocking the full potential of this versatile neuroimaging modality.
3. NIRS in Breast Cancer Imaging
In 2019, the United States witnessed a staggering toll of over 45,000 female deaths caused by breast cancer alone. This underscores its status as one of the most common cancers among women [
31,
32]. Across urban populations, breast cancer emerges as the foremost cancer diagnosis among women, constituting 20% of all recorded cancer cases in women’s cancer registers. While the majority of breast cancer occurrences manifest in women aged 50 and above, a noteworthy 32% of diagnoses pertain to those under 50. While invasive cases predominantly arise after the age of 50, a rising trend is discernible among women below this age threshold.
Remarkably, about 10% of newly diagnosed breast cancer cases in the U.S. target women younger than 45 years old. Various factors contribute to an elevated risk of breast cancer. These factors include early menarche, late onset of menopause, first full pregnancy after 31, familial history of premenopausal breast cancer, and personal experiences with breast cancer or benign proliferative breast disease. These nuanced risk factors emphasize the complexity and multifaceted nature of breast cancer etiology. They urge heightened awareness, early detection, and proactive health measures for women across diverse age groups [
33].
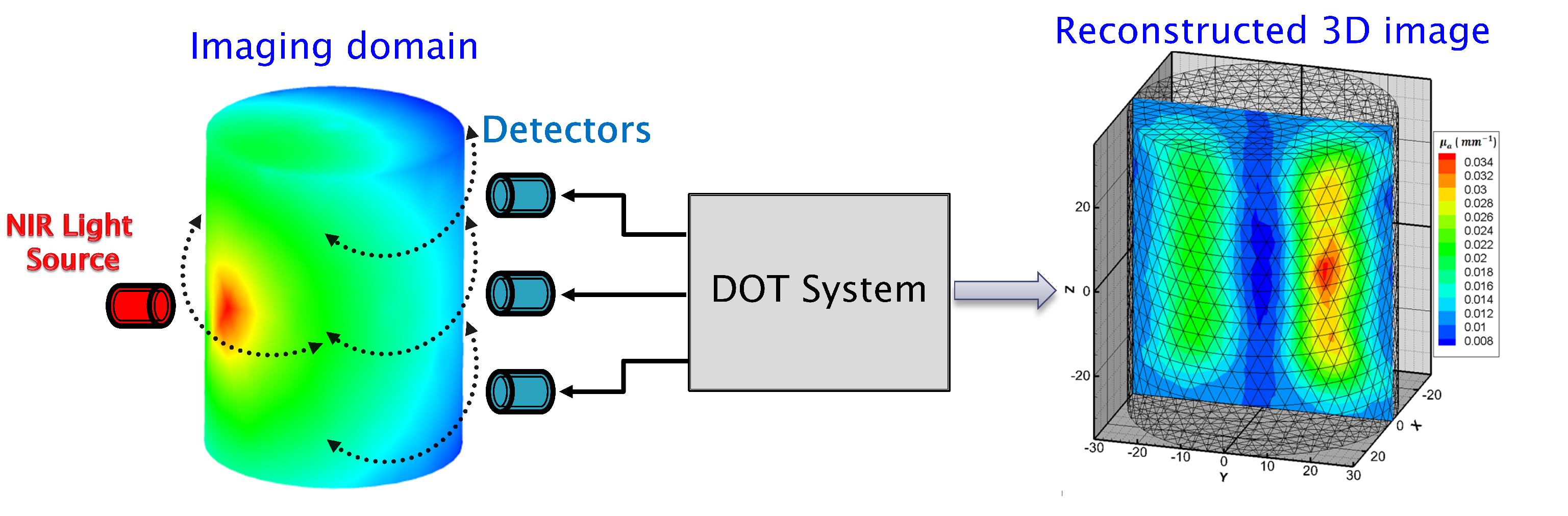
Diffuse optical tomography (DOT) imaging relies on the interaction of near-infrared light with biological tissues, providing valuable information on the distribution of optical properties for example absorption and scattering as shown in
Figure 2 [
34,
35]. Current research in DOT is focused on enhancing its spatial resolution, depth penetration, and quantitative accuracy to make it a more robust tool for clinical applications. Advanced reconstruction algorithms and computational models are being developed to address the inherent challenges associated with light scattering in biological tissues, aiming to improve the reconstruction of three-dimensional images with higher fidelity.
Moreover, the integration of DOT with other imaging modalities, such as magnetic resonance imaging (MRI) and computed tomography (CT), is a key area of investigation. This multimodal approach can synergistically combine the strengths of each imaging technique, providing complementary structural and functional information. Additionally, efforts are being made to optimize hardware components, including light sources and detectors, to enhance DOT systems’ sensitivity and specificity.
Researchers are conducting clinical studies, such as breast cancer detection, brain imaging, and functional monitoring of vital organs. Clinical trials are underway to validate the effectiveness of DOT in real-world scenarios and establish its reliability for routine medical use. Noteworthy studies in this field include the work of Gibson et al., who have contributed significantly to the development of advanced reconstruction algorithms for DOT . Additionally, the research conducted by Zhang et al. has explored the integration of DOT with other imaging modalities for improved breast cancer detection.
3.1. NIRS Image Reconstruction
Diffuse optical tomography (DOT) image reconstruction is a fundamental aspect of the imaging pipeline that involves the conversion of acquired data into meaningful three-dimensional representations of biological tissues’ internal optical properties. Given the inherent complexity of light propagation through tissues and the diffuse nature of the measurements, sophisticated algorithms are imperative for accurate reconstruction. The primary challenge lies in the ill-posed nature of the image reconstruction inverse problem, where multiple potential distributions of optical parameters can lead to the same set of measurements. Various mathematical frameworks, including iterative and analytical methods, are employed to tackle this problem. Iterative reconstruction algorithms, such as the popular gradient-based optimization techniques, aim to minimize the discrepancy between the measured and predicted data iteratively. Regularization techniques, such as Tikhonov regularization, are often incorporated to ensure stability and prevent overfitting in the presence of noise. Additionally, Bayesian methods, utilizing prior knowledge about the expected distributions of optical properties, play a crucial role in enhancing the robustness and accuracy of the reconstruction process.
The incorporation of anatomical information into DOT image reconstruction is a key area of research. Hybrid imaging approaches that integrate structural information from other modalities, such as X-ray computed tomography (CT) or magnetic resonance imaging (MRI), aid in refining the spatial localization and boundary definition of the optical targets. This multimodal fusion enhances the overall accuracy of the reconstructed images and addresses DOT limitations in resolving structural details. Advancements in regularization techniques also contribute significantly to improving the spatial resolution of reconstructed DOT images. Nonlinear regularization methods, such as total variation regularization, are increasingly employed to preserve edges and enhance image quality. Moreover, the integration of advanced forward models that incorporate anatomical and physiological priors, along with spatially varying optical properties, adds complexity to the reconstruction process but yields more accurate and anatomically faithful results.
Researchers are actively exploring innovative strategies, such as machine learning-based approaches, to enhance the efficiency and accuracy of DOT image reconstruction. Convolutional neural networks and deep learning architectures are being applied to learn complex mappings between measured data and underlying optical property distributions, bypassing the need for explicit mathematical models. DOT image reconstruction emphasizes the continuous refinement of algorithms and methodologies to unlock the full potential of this non-invasive imaging modality for biomedical applications. As research in DOT progresses, the synergy of computational techniques and experimental advancements holds the key to overcoming challenges and furthering the clinical utility of diffuse optical tomography in medical imaging.
4. NIRS in Breast Cancer Imaging
Diffuse optical tomography (DOT) is an optical imaging approach applicable to the examination of soft tissues such as breast tissues. Employing near-infrared (NIR) light, DOT facilitates the visualization of internal anatomical structures and provides functional insights into tissue. The fundamental mechanism of DOT involves the measurement of NIR light transmission or reflection from tissues, enabling the determination of their optical characteristics within the tissue. This system incorporates a model-based image reconstruction methodology, allowing for the computation of concentrations of hemoglobin, water, and lipids within the tissues. Given the deployment of multiple light sources and detectors in DOT instrumentation, the implementation of multiplexing techniques is imperative [
36]. These techniques are essential for rapid alternation of light and detectors, ensuring high-speed performance, and for system calibration to rectify potential measurement inaccuracies [
37]. The intricate interplay of these components underscores the sophistication of DOT technology, emphasizing the need for meticulous calibration and technological precision in order to extract accurate and meaningful information from optical imaging of breast tissues. Certain DOT instruments enable the utilization of circular probes to conduct uninterrupted spectroscopic imaging, as documented in [
6].
5. Challenges and Future Scope
The application of diffuse optical tomography (DOT) presents a significant challenge in estimating the internal optical properties of tissue based on measurements acquired at the tissue boundary. This challenge arises from the highly scattered nature of near-infrared (NIR) light within the tissue, rendering the estimation problem, also known as the inverse problem, which is nonlinear, ill-posed, and occasionally underdetermined [
34]. Despite these complexities, there exist algorithms designed for the rapid reconstruction of 3D DOT images [
38]. Recent efforts focused on leveraging Graphics Processing Units (GPUs) to enhance the speed of image reconstruction [
39]. Real-time imaging capabilities of DOT have been demonstrated using GPUs [
40], and innovations such as a specialized instrument for high-speed tissue imaging of specific regions have been put forward [
41,
42].
Despite the considerable size of DOT instruments and the slow data collection process, ongoing research work aims to lower instrument costs by incorporating Light-Emitting Diodes (LEDs) and photodetectors [
43,
44,
45]. Furthermore, DOT is being explored as a point-of-care imaging system [
46], with educational applications to teach students about optical medical imaging systems [
47]. Addressing challenges during data collection, a practical method has been proposed, involving the measurement and subtraction of superficial noise from the signal of interest, proving effective in enhancing data accuracy [
48]. Notably, current research in this field is rapidly expanding, revealing the potential of NIR light in medical imaging for early-stage breast cancer detection and brain function imaging.
6. Discussion and Conclusion
In conclusion, the technical landscape of Near-Infrared Spectroscopy (NIRS) for medical imaging, with a specific focus on brain and breast applications, reflects a dynamic interplay between advancements, challenges, and future prospects. The ongoing trend of integrating NIRS with complementary modalities like functional magnetic resonance imaging (fMRI) and electroencephalography (EEG) enhances the depth and specificity of information obtained. However, challenges related to spatial resolution and the impact of extracerebral tissues underscore the need for continued refinement, with promising avenues in signal processing techniques and multi-modal fusion.
In breast imaging, NIRS showcases potential as a non-invasive method for early breast cancer detection. Current trends highlight advancements in spectral tomography and synergistic integration with established imaging techniques like mammography. Limited spatial resolution and tissue heterogeneity require innovative solutions, including multi-wavelength and frequency-domain NIRS. Ongoing clinical trials are crucial for validating NIRS as a diagnostic tool in breast imaging.
The paper concludes by outlining the future scope of NIRS in medical imaging. In brain imaging, the integration of advanced signal processing techniques, such as machine learning algorithms, holds promise for improving spatial localization and decoding cognitive states. For breast imaging, the exploration of molecular-specific contrast agents and advancements in hardware technology aim
to overcome existing limitations and facilitate early-stage tumor detection. As NIRS continues to evolve, addressing technical challenges and leveraging synergies with other imaging modalities,
its integration into routine clinical workflows for brain and breast imaging appears increasingly viable. This paper contributes to the ongoing discourse, providing a technical overview of the current landscape, challenges, and future prospects of Near-Infrared Spectroscopy in the dynamic field of
medical imaging.
References
- Heise, H.M. Medical Applications of NIR Spectroscopy. Near-Infrared Spectroscopy: Theory, Spectral Analysis, Instrumentation, and Applications. [CrossRef]
- Saikia, M.; Besio, W.; Mankodiya, K. WearLight: Toward a Wearable, Configurable Functional NIR Spectroscopy System for Noninvasive Neuroimaging. IEEE Transactions on Biomedical Circuits and Systems 2019, 13. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Luo, S.; Yang, D.; Zhang, S. Applications of smartphone-based near-infrared (NIR) imaging, measurement, and spectroscopy technologies to point-of-care (POC) diagnostics. Journal of Zhejiang University. Science. B 2021, 22, 171. [Google Scholar] [CrossRef] [PubMed]
- Saikia, M.J.; Kanhirodan, R. Development of handheld near-infrared spectroscopic medical imaging system. Biophotonics Congress: Optics in the Life Sciences Congress 2019 (BODA,BRAIN,NTM,OMA,OMP) (2019), paper DS1A.6, 2019. [Google Scholar] [CrossRef]
- Vasudevan, S.; Forghani, F.; Campbell, C.; Bedford, S.; O’Sullivan, T.D. Method for Quantitative Broadband Diffuse Optical Spectroscopy of Tumor-Like Inclusions. Applied Sciences 2020, Vol. 10, Page 1419 2020, 10, 1419. [Google Scholar] [CrossRef]
- Saikia, M.J. A Spectroscopic Diffuse Optical Tomography System for the Continuous 3-D Functional Imaging of Tissue - A Phantom Study. IEEE Transactions on Instrumentation and Measurement 2021, 70. [Google Scholar] [CrossRef]
- Papadoliopoulou, M.; Matiatou, M.; Koutsoumpos, S.; Mulita, F.; Giannios, P.; Margaris, I.; Moutzouris, K.; Arkadopoulos, N.; Michalopoulos, N.V. Optical Imaging in Human Lymph Node Specimens for Detecting Breast Cancer Metastases: A Review. Cancers 2023, Vol. 15, Page 5438 2023, 15, 5438. [Google Scholar] [CrossRef] [PubMed]
- Okawa, S.; Hoshi, Y. A Review of Image Reconstruction Algorithms for Diffuse Optical Tomography. Applied Sciences 2023, Vol. 13, Page 5016 2023, 13, 5016. [Google Scholar] [CrossRef]
- Chen, J.; Xia, Y.; Zhou, X.; Rosas, E.V.; Thomas, A.; Loureiro, R.; Cooper, R.J.; Carlson, T.; Zhao, H. fNIRS-EEG BCIs for Motor Rehabilitation: A Review. Bioengineering 2023, Vol. 10, Page 1393 2023, 10, 1393. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Machado, A.; Chowdhury, R.A.; Spilkin, A.; Vincent, T.; Ümit Aydin. ; Pellegrino, G.; Lina, J.M.; Grova, C. Diffuse optical reconstructions of functional near infrared spectroscopy data using maximum entropy on the mean. Scientific Reports 2022 12:1 2022, 12, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Revealing the spatiotemporal requirements for accurate subject identification with resting-state functional connectivity: a simultaneous fNIRS-fMRI study.
- Saikia, M.J.; Besio, W.G.; Mankodiya, K. The Validation of a Portable Functional NIRS System for Assessing Mental Workload. Sensors 2021, 21, 3810. [Google Scholar] [CrossRef] [PubMed]
- Ali, J.; Cody, J.; Maldonado, Y.; Ramakrishna, H. Near-Infrared Spectroscopy (NIRS) for Cerebral and Tissue Oximetry: Analysis of Evolving Applications. Journal of Cardiothoracic and Vascular Anesthesia 2022, 36, 2758–2766. [Google Scholar] [CrossRef] [PubMed]
- Saikia, M.J.; Kuanar, S.; Borthakur, D.; Vinti, M.; Tendhar, T. A machine learning approach to classify working memory load from optical neuroimaging data. 2021, p. 69. [CrossRef]
- Saikia, M.J. K-means Clustering Machine Learning Approach Reveals Groups of Homogeneous Individuals with Unique Brain Activation, Task, and Performance Dynamics using fNIRS. IEEE Transactions on Neural Systems and Rehabilitation Engineering 2023. [Google Scholar] [CrossRef] [PubMed]
- Ghouse, A.; Candia-Rivera, D.; Valenza, G. Multivariate Pattern Analysis of Entropy estimates in Fast- and Slow-Wave Functional Near Infrared Spectroscopy: A Preliminary Cognitive Stress study. Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society, EMBS. [CrossRef]
- Flanagan, K. .; Saikia, M.J.; Saggio, G.; Flanagan, K.; Saikia, M.J. Consumer-Grade Electroencephalogram and Functional Near-Infrared Spectroscopy Neurofeedback Technologies for Mental Health and Wellbeing. Sensors 2023, Vol. 23, Page 8482 2023, 23, 8482. [Google Scholar] [CrossRef] [PubMed]
- Abtahi, M.; Cay, G.; Saikia, M.J.; Mankodiya, K. Designing and testing a wearable, wireless fNIRS patch. Institute of Electrical and Electronics Engineers Inc., 2016, Vol. 2016-Octob, pp. 6298–6301. [CrossRef]
- Tsow, F.; Kumar, A.; Hosseini, S.H.; Bowden, A. A low-cost, wearable, do-it-yourself functional near-infrared spectroscopy (DIY-fNIRS) headband. HardwareX 2021, 10, e00204. [Google Scholar] [CrossRef] [PubMed]
- Saikia, M.J.; Mankodiya, K. A Wireless fNIRS Patch with Short-Channel Regression to Improve Detection of Hemodynamic Response of Brain. Institute of Electrical and Electronics Engineers Inc., 2018, pp. 90–96. [CrossRef]
- Saikia, M.J.; Mankodiya, K. 3D-printed human-centered design of fNIRS optode for the portable neuroimaging. [CrossRef]
- Momtahen, S.; Shokoufi, M.; Ramaseshan, R.; Golnaraghi, F. Near-Infrared Handheld Probe and Imaging System for Breast Tumor Localization. IEEE Canadian Journal of Electrical and Computer Engineering 2023, 46, 246–255. [Google Scholar] [CrossRef]
- Saikia, M.J.; Cay, G.; Gyllinsky, J.V.; Mankodiya, K. A Configurable Wireless Optical Brain Monitor Based on Internet-of-Things Services. 3rd International Conference on Electrical, Electronics, Communication, Computer Technologies and Optimization Techniques, ICEECCOT 2018. [CrossRef]
- Saikia, M.J. Internet of things-based functional near-infrared spectroscopy headband for mental workload assessment. SPIE, 2021, Vol. 11629, pp. 143–150. [CrossRef]
- Khan, M.A.; Asadi, H.; Hoang, T.; Lim, C.P.; Nahavandi, S. Measuring Cognitive Load: Leveraging fNIRS and Machine Learning for Classification of Workload Levels. Communications in Computer and Information Science 2024, 1963 CCIS, 313–325. [Google Scholar] [CrossRef]
- Cao, J.; Garro, E.M.; Zhao, Y. EEG/fNIRS Based Workload Classification Using Functional Brain Connectivity and Machine Learning. Sensors 2022, 22, 7623. [Google Scholar] [CrossRef] [PubMed]
- Saikia, M.J.; Brunyé, T.T. K-means clustering for unsupervised participant grouping from fNIRS brain signal in working memory task. SPIE, 2021, Vol. 11629, pp. 159–164. [CrossRef]
- Patashov, D.; Menahem, Y.; Gurevitch, G.; Kameda, Y.; Goldstein, D.; Balberg, M. fNIRS: Non-stationary preprocessing methods. Biomedical Signal Processing and Control 2023, 79, 104110. [Google Scholar] [CrossRef]
- Gao, Y.; Jia, B.; Houston, M.; Zhang, Y. Hybrid EEG-fNIRS Brain Computer Interface Based on Common Spatial Pattern by Using EEG-Informed General Linear Model. IEEE Transactions on Instrumentation and Measurement 2023, 72. [Google Scholar] [CrossRef]
- de Oliveira Franco, Á.; de Oliveira Venturini, G.; da Silveira Alves, C.F.; Alves, R.L.; Vicuña, P.; Ramalho, L.; Tomedi, R.; Bruck, S.M.; Torres, I.L.; Fregni, F.; Caumo, W. Functional connectivity response to acute pain assessed by fNIRS is associated with BDNF genotype in fibromyalgia: an exploratory study. Scientific Reports 2022 12:1 2022, 12, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Giaquinto, A.N.; Sung, H.; Miller, K.D.; Kramer, J.L.; Newman, L.A.; Minihan, A.; Jemal, A.; Siegel, R.L. Breast Cancer Statistics, 2022. CA: A Cancer Journal for Clinicians 2022, 72, 524–541. [Google Scholar] [CrossRef] [PubMed]
- Basic Information About Breast Cancer | CDC.
- Boyd, N.F.; Jensen, H.M.; Cooke, G.; Han, H.L.; Lockwood, G.A. Mammographic densities and the prevalence and incidence of histological types of benign breast disease. European Journal of Cancer Prevention 2000, 9, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Arridge, S.R. Optical tomography in medical imaging. Inverse Problems 1999, 15, R41. [Google Scholar] [CrossRef]
- Saikia, M.J. Design and development of a functional diffuse optical tomography probe for real-time 3D imaging of tissue. SPIE 2021, 11639, 213–218. [Google Scholar]
- Poorna, R.; Kanhirodan, R.; Saikia, M.J. Square-waves for frequency multiplexing for fully parallel 3D diffuse optical tomography measurement. SPIE, 2021, Vol. 11639, pp. 219–226. [CrossRef]
- Saikia, M.J. An embedded system based digital onboard hardware calibration for low-cost functional diffuse optical tomography system. SPIE, 2021, Vol. 11632, pp. 1–8. [CrossRef]
- Saikia, M.J.; Kanhirodan, R.; Vasu, R.M. High-speed GPU-based fully three-dimensional diffuse optical tomographic system. International Journal of Biomedical Imaging 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Saikia, M.J.; Kanhirodan, R. High performance single and multi-GPU acceleration for Diffuse Optical Tomography. Institute of Electrical and Electronics Engineers Inc., 2014, pp. 1320–1323. [CrossRef]
- Saikia, M.J.; Rajan, K.; Vasu, R.M. 3-D GPU based real time Diffuse Optical Tomographic system. Souvenir of the 2014 IEEE International Advance Computing Conference, IACC 2014, 1099. [Google Scholar] [CrossRef]
- Saikia, M.J.; Kanhirodan, R. Development of DOT system for ROI scanning. Optical Society of America (OSA), 2014, p. T3A.4. [CrossRef]
- Saikia, M.J.; Kanhirodan, R. Region-of-interest diffuse optical tomography system. Review of Scientific Instruments 2016, 87, 013701. [Google Scholar] [CrossRef] [PubMed]
- Yuan, G.; Alqasemi, U.; Chen, A.; Yang, Y.; Zhu, Q. Light-emitting diode-based multiwavelength diffuse optical tomography system guided by ultrasound. 2014, 19, 126003. doi:10.1117/1.JBO.19.12.126003. [CrossRef]
- Zhang, X. Instrumentation in Diffuse Optical Imaging. Photonics 2014, Vol. 1, Pages 9-32 2014, 1, 9–32. [Google Scholar] [CrossRef] [PubMed]
- Saikia, M.; Manjappa, R.; Kanhirodan, R. A cost-effective LED and photodetector based fast direct 3D diffuse optical imaging system. 2017, Vol. 10412. [CrossRef]
- Saikia, M.J.; Mankodiya, K.; Kanhirodan, R. A point-of-care handheld region-of-interest (ROI) 3D functional diffuse optical tomography (fDOT) system. [CrossRef]
- Saikia, M.J.; Kanhirodan, R. A tabletop Diffuse Optical Tomographic (DOT) experimental demonstration system. SPIE, 2019, Vol. 10869, p. 11. [CrossRef]
- Saikia, M.J.; Manjappa, R.; Mankodiya, K.; Kanhirodan, R. Depth sensitivity improvement of region-of-interest diffuse optical tomography from superficial signal regression. OSA - The Optical Society, 2018, Vol. Part F99-C, p. CM3E.5. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).