Submitted:
19 December 2023
Posted:
26 December 2023
You are already at the latest version
Abstract
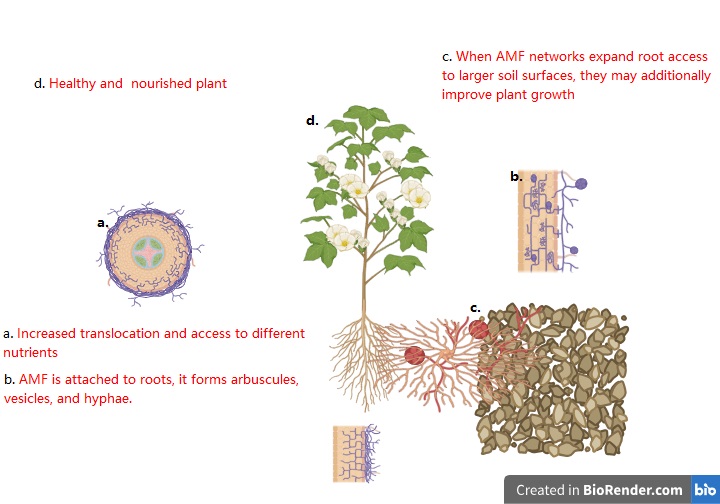
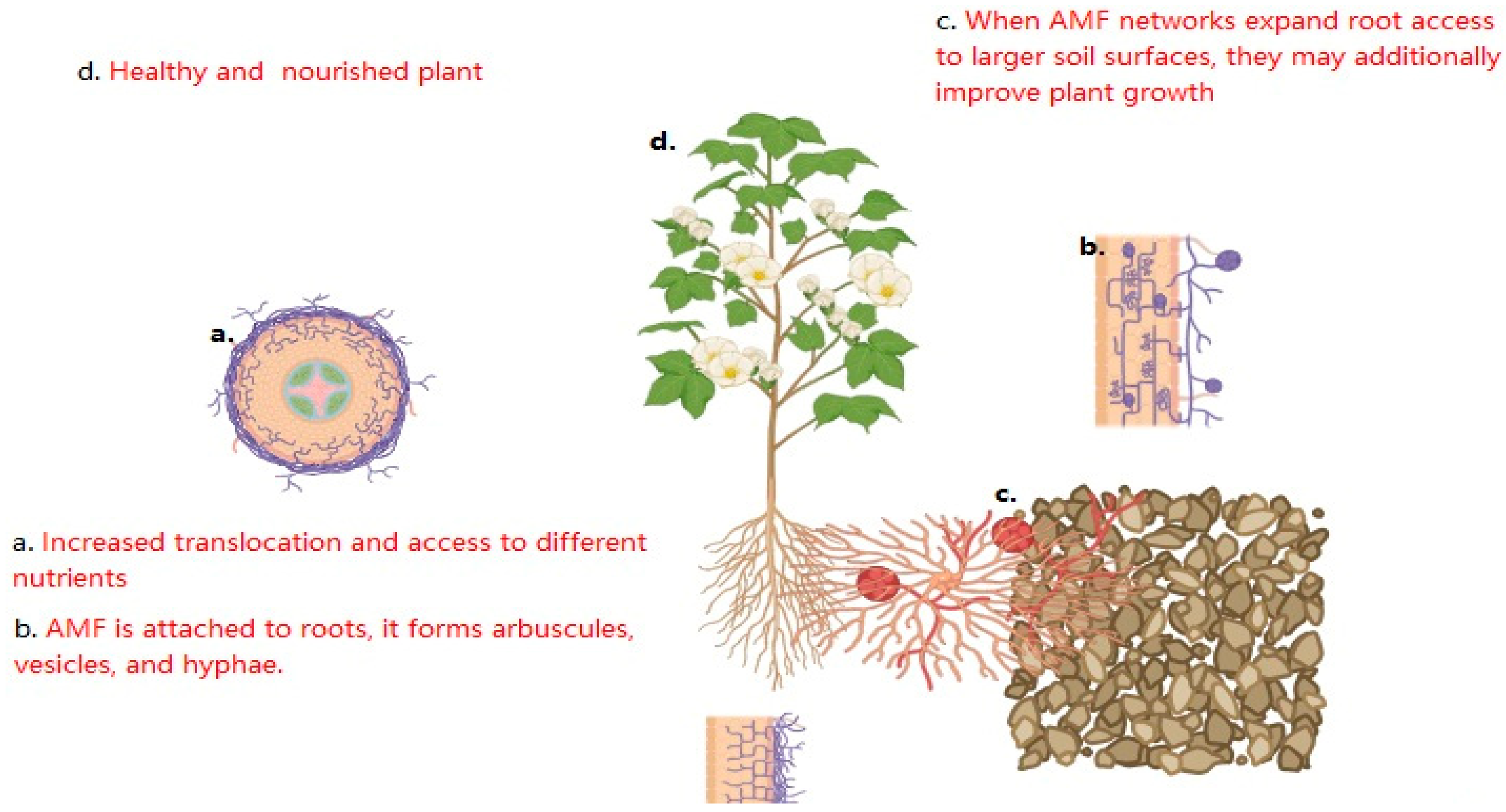
Keywords:
1.0. Introduction
2.0. History of Arbuscular Mycorrhizal Fungi
2.1. The process of Mycorrhizae Colonization in Plants
2.2. The traits of AMF Synmbiosis
3.0. AMF AND PLANT DEVELOPMENT
3.1. AMF and Mineral Nutrient Utilization
3.2. AMF use Bio-fertilizers
4.0. STRESS DUE TO BOTH ABIOTIC AND BIOTIC FACTORS.
4.1. Drought
4.2. Salinity
4.3. High and low temperatures
4.4. Heavy Metals
4.5. AMF and Combined Abiotic Stresses
5.0. AMF AND PLANT GROWTH
5.1. Germination
5.2. AMF and Plant Yield
5.3. Fight pathogens
5.4. Photosynthesis
5.5. Water use efficiency
5.6. Soil Enzymatic activity
6.0. Future prospects and conclusions
Author Contributions
Funding
Conflict of Interest Statement
References
- Abdel Latef, A. A., Chaoxing, H. (2011a). Effect of arbuscular mycorrhizal fungi on growth, mineral nutrition, antioxidant enzymes activity and fruit yield of tomato grown under salinity stress. Sci. Hort. 127, 228–233. [CrossRef]
- Abdel Latef, A. A., Chaoxing, H. (2011b). Arbuscular mycorrhizal influence on growth, photosynthetic pigments, osmotic adjustment and oxidative stress in tomato plants subjected to low temperature stress. Acta Physiol. Plant. 33, 1217–1225. [CrossRef]
- Abdel Latef, A. A., Chaoxing, H. J. (2014)a. Does the inoculation with Glomus mosseae improve salt tolerance in pepper plants? Plant Grow. Regul. 33, 644–653. [CrossRef]
- Aguilera, P., Pablo, C., Fernando, B., Fritz, O. (2014). Diversity of arbuscular mycorrhizal fungi associated with Triticum aestivum L. plants growing in an andosol with high aluminum level. Agri. Eco. Environ. 186, 178–184. [CrossRef]
- Ahanger, M. A., Alyemeni, M. N., Wijaya, L., Alamri, S. A., Alam, P., Ashraf, M., et al. (2018). Potential of exogenously sourced kinetin in protecting Solanum lycopersicum from NaCl-induced oxidative stress through up-regulation of the antioxidant system, ascorbate–glutathione cycle and glyoxalase system. PLoS One 13 (9), e0202–e0175. [CrossRef]
- Ahanger, M. A., Agarwal, R. M. (2017). Potassium up-regulates antioxidant metabolism and alleviates growth inhibition under water and osmotic stress in wheat (Triticum aestivum L.). Protoplasma 254 (4), 1471–1486. [CrossRef]
- Ahanger, M. A., Tittal, M., Mir, R. A., Agarwal, R. M. (2017a). Alleviation of water and osmotic stress-induced changes in nitrogen metabolizing enzymes in Triticum aestivum L. cultivars by potassium. Protoplasma 254 (5), 1953–1963. [CrossRef]
- Ahanger, M. A., Tomar, N. S., Tittal, M., Argal, S., Agarwal, R. M. (2017b). Plant growth under water/salt stress: ROS production; antioxidants and significance of added potassium under such conditions. Physiol. Mol. Biol. Plants. 23 (4), 731–744. [CrossRef]
- Ahanger, M. A., Tyagi, S. R., Wani, M. R., Ahmad, P. (2014). “Drought tolerance: role of organic osmolytes, growth regulators, and mineral nutrients,” in Physiological mechanisms and adaptation strategies in plants under changing environment, vol. 1 . Eds. Ahmad, P., Wani, MR (New York, NY: Springer), 25–55. [CrossRef]
- Ait-El-Mokhtar, M., Laouane, R. B., Anli, M., Boutasknit, A., Wahbi, S., Meddich, A. (2019). Use of mycorrhizal fungi in improving tolerance of the date palm (Phoenix dactylifera L.) seedlings to salt stress. Sci. Hori. 253, 429–438. [CrossRef]
- Al-Hmoud, G., Al-Momany, A. (2017). Effect of four mycorrhizal products on squash plant growth and its effect on physiological plant elements. Adv. Crop. Sci. Tech. 5, 260. [CrossRef]
- Ali, N., Masood, S., Mukhtar, T., Kamran, M. A., Rafique, M., Munis, M. F. H., et al. (2015). Differential effects of cadmium and chromium on growth, photosynthetic activity, and metal uptake of Linum usitatissimum in association with Glomus intraradices. Environ. Monitor. Assess. 187 (6), 311. [CrossRef]
- Al-Karaki, G., Mcmichael, B., Zak, J. (2004). Field response of wheat to arbuscular mycorrhizal fungi and drought stress. Mychorrhiza 14, 263–269. [CrossRef]
- Alqarawi, A. A., Abd-Allah, E. F., Hashem, A. (2014a). Alleviation of salt-induced adverse impact via mycorrhizal fungi in Ephedra aphylla Forssk. J. Plant. Interact. 9 (1), 802–810. [CrossRef]
- Amiri, R., Ali, N., Nematollah, E., Mohammad, R. S. (2017). Nutritional status, essential oil changes and water-use efficiency of rose geranium in response to arbuscular mycorrhizal fungi and water deficiency stress. Symbiosis 73, 15–25. [CrossRef]
- Amiri, R., Nikbakht, A., Etemadi, N. (2015). Alleviation of drought stress on rose geranium Pelargonium graveolen L Herit. in terms of antioxidant activity and secondary metabolites by mycorrhizal inoculation. Sci. Hort. 197, 373–380. [CrossRef]
- Arriagada C., Manquel D., Cornejo P., Soto J. , Sampedro I., Ocampo J. (2012). Effects of the co-inoculation with saprobe and mycorrhizal fungi on Vaccinium corymbosum growth and some soil enzymatic activities. Journal of Soil Science and Plant Nutrition. 12: 287-298. http://dx.doi.org/10.4067/S0718-95162012000200008. [CrossRef]
- Audet, P. (2014). “Arbuscular mycorrhizal fungi and metal phytoremediation: ecophysiological complementarity in relation to environmental stress,” in Emerging technologies and management of crop stress tolerance. Eds. Ahmad, P., Rasool, S. (San Diego: Academic Press), 133–160. [CrossRef]
- Augé, R. M., Toler, H. D., Saxton, A. M. (2014). Arbuscular mycorrhizal symbiosis and osmotic adjustment in response to NaCl stress: a meta-analysis. Front. Plant. Sci. 5, 562. [CrossRef]
- Bagheri, V., Shamshiri, M. H., Shirani, H., Roosta, H. (2012). Nutrient uptake and distribution in mycorrhizal pistachio seedlings under drought stress. J. Agric. Sci. Technol. 14, 1591–1604. [CrossRef]
- Balliu, A., Sallaku, G., Rewald, B. (2015). AMF Inoculation enhances growth and improves the nutrient uptake rates of transplanted, salt-stressed tomato seedlings. Sustainability 7, 15967–15981. [CrossRef]
- Barrow, C. J. (2012). Biochar potential for countering land degradation and for improving agriculture. App. Geogr. 34, 21–28. [CrossRef]
- Bárzana, G., Aroca, R., Ruiz-Lozano, J. M. (2015). Localized and nonlocalized effects of arbuscular mycorrhizal symbiosis on accumulation of osmolytes and aquaporins and on antioxidant systems in maize plants subjected to total or partial root drying. Plant Cell Environ. 38, 1613–1627. [CrossRef]
- Baslam, M., Garmendia, I., Goicoechea, N. (2011). Arbuscular mycorrhizal fungi (AMF) improved growth and nutritional quality of greenhouse grown lettuce. J. Agric. Food Chem. 59, 5504–C5515. [CrossRef]
- Bati, C. B., Santilli, E., Lombardo, L. (2015). Effect of arbuscular mycorrhizal fungi on growth and on micronutrient and macronutrient uptake and allocation in olive plantlets growing under high total Mn levels. Mycorrhiza 25 (2), 97–108. [CrossRef]
- Battini, F., Grønlund, M., Agnolucci, M., Giovannetti, M., Jakobsen, I. (2017). Facilitation of phosphorus uptake in maize plants by mycorrhizosphere bacteria. Sci. Rep. 7, 4686. [CrossRef]
- Bauddh, K., Singh, R. P. (2012). Growth: tolerance efficiency and phytoremediation potential of Ricinus communis (L.) and Brassica juncea (L.) in salinity and drought affected cadmium contaminated soil. Ecotoxicol. Environ. Saf. 85, 13–22. [CrossRef]
- Bhantana, P., Rana, M.S., Sun, Xc. et al. Arbuscular mycorrhizal fungi and its major role in plant growth, zinc nutrition, phosphorous regulation and phytoremediation. Symbiosis 84, 19–37 (2021). [CrossRef]
- Birhane, E., Sterck, F., Fetene, M., Bongers, F., Kuyper, T. (2012). Arbuscular mycorrhizal fungi enhance photosynthesis, water use efficiency, and growth of frankincense seedlings under pulsed water availability conditions. Oecologia 169, 895–904. [CrossRef]
- Bona, E., Cantamessa, S., Massa, N., Manassero, P., Marsano, F., Copetta, A., et al. (2017). Arbuscular mycorrhizal fungi and plant growth-promoting pseudomonads improve yield, quality and nutritional value of tomato: a field study. Mycorrhiza 27, 1–C11. [CrossRef]
- Borde, M., Dudhane, M., Jite, P. K. (2010). AM fungi influences the photosynthetic activity, growth and antioxidant enzymes in Allium sativum L. under salinity condition. Not. Sci. Biol. 2, 64–71. [CrossRef]
- Bowles, T. M., Barrios-Masias, F. H., Carlisle, E. A., Cavagnaro, T. R., Jackson, L. E. (2016). Effects of arbuscular mycorrhizae on tomato yield, nutrient uptake, water relations, and soil carbon dynamics under deficit irrigation in field conditions. Sci. Total Environ. 566, 1223–1234. [CrossRef]
- Birhane, E., Sterck, F.J., Fetene, M. et al. Arbuscular mycorrhizal fungi enhance photosynthesis, water use efficiency, and growth of frankincense seedlings under pulsed water availability conditions. Oecologia 169, 895–904 (2012). [CrossRef]
- Binti T. et al. Important Role of Mycorrhiza for Seed Germination and Growth of Dendrobium Orchids. Journal of Tropical Biodiversity and Biotechnology, [S.l.], v. 6, n. 2, p. jtbb60805, june 2021. ISSN 2540-9581. Available at: <https://jurnal.ugm.ac.id/jtbb/article/view/60805>. Date accessed: 29 june 2023. /*doi:http://dx.doi.org/10.22146/jtbb.60805. */ doi:. [CrossRef]
- 35. Bucking H, Liepold E, Ambilwade P (2012) The role of the mycorrhizal symbiosis in nutrient uptake of plants and the regulatory mechanisms underlying these transport processes. World â€TM s largest Science, Technology and Medicine Open Access book publisher. Intech Open.
- Bulgarelli R. G., Marcos F. C. C., Ribeiro R. V., López de Andrade S. A. (2017). Mycorrhizae enhance nitrogen fixation and photosynthesis in phosphorus-starved soybean (Glycine max L. Merrill), Environmental and Experimental Botany pp 140 : 26-33. [CrossRef]
- Bucher, M. (2007). Functional biology of plant phosphate uptake at root and mycorrhizae interfaces. New Phytol. 173, 11–26. [CrossRef]
- Bucking, H., Kafle, A. (2015). Role of arbuscular mycorrhizal fungi in the nitrogen uptake of plants: current knowledge and research gaps. Agronomy 5, 587–612. [CrossRef]
- Bunn, R., Lekberg, Y., Zabinski, C. (2009). Arbuscular mycorrhizal fungi ameliorate temperature stress in thermophilic plants. Ecology 90 (5), 1378–1388. [CrossRef]
- Calvo-Polanco, M., Sanchez-Romera, B., Aroca, R., Asins, M. J., Declerck, S., Dodd, I. C., et al. (2016). Exploring the use of recombinant inbred lines in combination with beneficial microbial inoculants (AM fungus and PGPR) to improve drought stress tolerance in tomato. Environ. Exp. Bot. 131, 47–57. [CrossRef]
- Castellanos-Morales, V., Villegas, J., Wendelin, S., Vierheiling, H., Eder, R., Cardenas-Navarro, R. (2010). Root colonization by the arbuscular mycorrhizal fungus Glomus intraradices alters the quality of strawberry fruit (Fragaria ananassa Duch.) at different nitrogen levels. J. Sci. Food Agric. 90, 1774–1782. [CrossRef]
- Cekic, F. O., Unyayar, S., Ortas, I. (2012). Effects of arbuscular mycorrhizal inoculation on biochemical parameters in capsicum annuum grown under long term salt stress. Turk. J. Bot. 36, 63–72. [CrossRef]
- Chandrasekaran, M., Chanratana, M., Kim, K., Seshadri, S., Sa, T. (2019). Impact of arbuscular mycorrhizal fungi on photosynthesis, water status, and gas exchange of plants under salt stress—a meta-analysis. Front. Plant Sci. 10, 457. [CrossRef]
- Chen, S., Jin, W., Liu, A., Zhang, S., Liu, D., Wang, F., et al. (2013). Arbuscular mycorrhizal fungi (AMF) increase growth and secondary metabolism in cucumber subjected to low temperature stress. Sci. Hort. 160, 222–229. [CrossRef]
- Chen, S., Zhao, H., Zou, C., Li, Y., Chen, Y., Wang, Z., et al. (2017). Combined Inoculation with multiple arbuscular mycorrhizal fungi improves growth, nutrient uptake and photosynthesis in cucumber seedlings. Front. Microbiol. 8, 25–16. [CrossRef]
- Clark, R. B., Zeto, S. K. (2000). Mineral acquisition by arbuscular mycorrhizal plants. J. Plant Nutr. 23, 867–902. [CrossRef]
- De Andrade, S. A. L., Domingues, A. P., Mazzafera, P. (2015). Photosynthesis is induced in rice plants that associate with arbuscular mycorrhizal fungi and are grown under arsenate and arsenite stress. Chemosphere 134, 141–149. [CrossRef]
- Dong, Y., Zhu, Y. G., Smith, F. A., Wang, Y., Chen, B. (2008). Arbuscular mycorrhiza enhanced arsenic resistance of both white clover Trifolium repens L. and ryegrass Lolium perenne L. plants in an arsenic-contaminated soil. Environ. Pollut. 155, 174–181. [CrossRef]
- Duan, X., Neuman, D. S., Reiber, J. M., Green, C. D., Arnold M., Saxton, A. M., et al. (1996). Mycorrhizal influence on hydraulic and hormonal factors implicated in the control of stomatal conductance during drought. J. Exp. Bot. 47 (303), 1541–1550. [CrossRef]
- Duc, N. H., Csintalan, Z., Posta, K. (2018). Arbuscular mycorrhizal fungi mitigate negative effects of combined drought and heat stress on tomato plants. Plant Physiol. Biochem. 132, 297–307. [CrossRef]
- Elhindi, K. M., El-Din, S. A., Elgorban, A. M. (2017). The impact of arbuscular mycorrhizal fungi in mitigating salt-induced adverse effects in sweet basil (Ocimum basilicum L.). Saudi J. Biol. Sci. 24, 170–179. [CrossRef]
- EL-Nashar, Y. I. (2017). Response of snapdragon Antirrhinum majus L. to blended water irrigation and arbuscular mycorrhizal fungi inoculation: uptake of minerals and leaf water relations. Photosynthetica 55 (2), 201–209. [CrossRef]
- El-Sawah AM, El-Keblawy A, Ali DFI, Ibrahim HM, El-Sheikh MA, Sharma A, Alhaj Hamoud Y, Shaghaleh H, Brestic M, Skalicky M, et al. Arbuscular Mycorrhizal Fungi and Plant Growth-Promoting Rhizobacteria Enhance Soil Key Enzymes, Plant Growth, Seed Yield, and Qualitative Attributes of Guar. Agriculture. 2021; 11(3):194. [CrossRef]
- Evelin, H., Giri, B., Kapoor, R. (2012). Contribution of Glomus intraradices inoculation to nutrient acquisition and mitigation of ionic imbalance in NaCl-stressed Trigonella foenum-graecum. Mycorrhiza 22, 203–217. [CrossRef]
- Evelin, H., Kapoor, R., Giri, B. (2009). Arbuscular mycorrhizal fungi in alleviation of salt stress: a review. Ann. Bot. 104, 1263–1281. [CrossRef]
- Gamalero, E., Lingua, G., Berta, G., Glick, B. R. (2009). Beneficial role of plant growth promoting bacteria and arbuscular mycorrhizal fungi on plant responses to heavy metal stress. Can. J. Microbiol. 55, 501–514. 245. [CrossRef]
- Garcés-Ruiz, M., Calonne-Salmon, M., Plouznikoff, K., Misson, C., Navarrete-Mier, M., Cranenbrouck, S., et al. (2017). Dynamics of short-term phosphorus uptake by intact mycorrhizal and non-mycorrhizal maize plants grown in a circulatory semi-hydroponic cultivation system. Front. Plant Sci. 8, 1471. [CrossRef]
- Garg, N., Chandel, S. (2012). Role of arbuscular mycorrhizal (AM) fungi on growth, cadmium uptake, osmolyte, and phytochelatin synthesis in Cajanus cajan (L.) Millsp. under NaCl and Cd stresses. J. Plant Growth Regul. 31 (3), 292–308. [CrossRef]
- Gao, X., Guo, H., Zhang, Q. et al. Arbuscular mycorrhizal fungi (AMF) enhanced the growth, yield, fiber quality and phosphorus regulation in upland cotton (Gossypium hirsutum L.). Sci Rep 10, 2084 (2020). [CrossRef]
- Garcia-Garrido JM, Tribak M, Rejon-Palomares A, Ocampo JA, Garcia-Romera I. Hydrolytic enzymes and ability of arbuscular mycorrhizal fungi to colonize roots. J Exp Bot. 2000 Aug;51(349):1443-8. PMID: 10944158.
- Gavito, M. E., Olsson, P. A., Rouhier, H., Medinapeñafiel, A., Jakobsen, I., Bago, A. (2005). Temperature constraints on the growth and functioning of root organ cultures with arbuscular mycorrhizal fungi. New Phytol. 168, 179–188. [CrossRef]
- Gholamhoseini, M., Ghalavand, A., Dolatabadian, A., Jamshidi, E., Khodaei-Joghan, A. (2013). Effects of arbuscular mycorrhizal inoculation on growth, yield, nutrient uptake and irrigation water productivity of sunflowers grown under drought stress. Agric. Water Manag. 117, 106–114. [CrossRef]
- Gianinazzi, S., Golotte, A., Binet, M. N., Van Tuinen, D., Redecker, D., Wipf, D. (2010). Agroecology: the key role of arbuscular mycorrhizas in ecosystem services. Mycorrhiza 20, 519–530. [CrossRef]
- Gomez-Bellot, M. J., Ortuño, M. F., Nortes, P. A., Vicente-Sánchez, J., Bañón, S., Sánchez Blanco, M. J. (2015). Mycorrhizal euonymus plants and reclaimed water: biomass, water status and nutritional responses. Sci. Hort. 186, 61–69. [CrossRef]
- Gutjahr, C., Paszkowski, U. (2013). Multiple control levels of root system remodeling in arbuscular mycorrhizal symbiosis. Front. Plant Sci. 4, 204. [CrossRef]
- Hameed, A., Dilfuza, E., Abd-Allah, E. F., Hashem, A., Kumar, A., Ahmad, P. (2014). “Salinity stress and arbuscular mycorrhizal symbiosis in plants,” in Use of microbes for the alleviation of soil stresses, vol. 1. Ed. Miransari, M. (NY: Springer Science+Business Media), 139–159. [CrossRef]
- Hammer, E. C., Nasr, H., Pallon, J., Olsson, P. A., Wallander, H. (2011). Elemental composition of arbuscular mycorrhizal fungi at high salinity. Mycorrhiza 21 (2), 117–129. [CrossRef]
- Hart, M., Ehret, D. L., Krumbein, A., Leung, C., Murch, S., Turi, C., et al. (2015). Inoculation with arbuscular mycorrhizal fungi improves the nutritional value of tomatoes. Mycorrhiza 25, 359–376. [CrossRef]
- Hasanuzzaman, M., Gill, S. S., Fujita, M. (2013). “Physiological role of nitric oxide in plants grown under adverse environmental conditions,” in Plant acclimation to environmental stress. Eds. Tuteja, N., Gill, S. S. (NY: Springer Science+Business Media), 269–322. [CrossRef]
- Hashem, A., Abd_Allah, E. F., Alqarawi, A. A., Aldubise, A., Egamberdieva, D. (2015). Arbuscular mycorrhizal fungi enhances salinity tolerance of Panicum turgidum Forssk by altering photosynthetic and antioxidant pathways. J. Plant Interact. 10 (1), 230–242. [CrossRef]
- Hashem, A., Alqarawi, A. A., Radhakrishnan, R., Al-Arjani, A. F., Aldehaish, H. A., Egamberdieva, D., et al. (2018). Arbuscular mycorrhizal fungi regulate the oxidative system, hormones and ionic equilibrium to trigger salt stress tolerance in Cucumis sativus L. Saudi J. Biol. Sci. 25 (6), 1102–1114. [CrossRef]
- He, F., Sheng, M., Tang, M. (2017). Effects of Rhizophagus irregularis on photosynthesis and antioxidative enzymatic system in Robinia pseudoacacia L. under drought Stress. Front. Plant Sci. 8, 183. [CrossRef]
- Hijri, M. (2016). Analysis of a large dataset form field mycorrhizal inoculation trials on potato showed highly significant increase in yield. Mycorrhiza 2, 209–214. [CrossRef]
- Impa, S. M., Nadaradjan, S., Jagadish, S. V. K. (2012). “Drought stress induced reactive oxygen species and anti-oxidants in plants,” in Abiotic stress responses in plants: metabolism, productivity and sustainability. Eds. Ahmad, P., Prasad, M. N. V. (LLC: Springer Science+ Business Media), 131–147. [CrossRef]
- Janouškova, M., Pavlíková, D. (2010). Cadmium immobilization in the rhizosphere of arbuscular mycorrhizal plants by the fungal extraradical mycelium. Plant Soil 332, 511–520. [CrossRef]
- Jiang, Y. N., Wang, W. X., Xie, Q. J., Liu, N., Liu, L. X., Wang, D. P., et al. (2017). Plants transfer lipids to sustain colonization by mutualistic mycorrhizal and parasitic fungi. Science 356, 1172–1175. [CrossRef]
- Jixiang, L., Yingnan, W., Shengnan, S., Chunsheng, M., Xiufeng, Y. (2017). Effects of arbuscular mycorrhizal fungi on the growth, photosynthesis and photosynthetic pigments of Leymus chinensis seedlings under salt-alkali stress and nitrogen deposition. Sci. Total Environ. 576, 234–241. [CrossRef]
- Kanwal, S., Bano, A., Malik, R. N. (2015). Effects of arbuscular mycorrhizal fungi on metals uptake, physiological and biochemical response of Medicago sativa L. with increasing Zn and Cd concentrations in soil. Am. J. Plant Sci. 6, 2906–2923. [CrossRef]
- Kapoor, R., Evelin, H., Mathur, P., Giri, B. (2013). “Arbuscular mycorrhiza: approaches for abiotic stress tolerance in crop plants for sustainable agriculture,” in Plant acclimation to environmental stress. Eds. Tuteja, N., Gill, S. S. (LLC: Springer Science+Business Media), 359–401. [CrossRef]
- Kayama, M., Yamanaka, T. (2014). Growth characteristics of ectomycorrhizal seedlings of Quercus glauca, Quercus salicina, and Castanopsis cuspidata planted on acidic soil. Trees 28, 569–583. [CrossRef]
- Kubikova, E., Moore, J. L., Ownlew, B. H., Mullen, M. D., Augé, R. M. (2001). Mycorrhizal impact on osmotic adjustment in Ocimum basilicum during a lethal drying episode. J. Plant Physiol. 158, 1227–1230. [CrossRef]
- Lehmann, A., Rillig, M. C. (2015). Arbuscular mycorrhizal contribution to copper, manganese and iron nutrient concentrations in crops—a meta-analysis. Soil Biol. Biochem. 81, 147–158. [CrossRef]
- Lehmann, A., Veresoglou, S. D., Leifheit, E. F., Rillig, M. C. (2014). Arbuscular mycorrhizal influence on zinc nutrition in crop plants: a meta-analysis. Soil Biol. Biochem. 69, 123–131. [CrossRef]
- Li, H., Chen, X. W., Wong, M. H. (2015). Arbuscular mycorrhizal fungi reduced the ratios of inorganic/organic arsenic in rice grains. Chemosphere 145, 224–230. [CrossRef]
- Li, H., Luo, N., Zhang, L. J., Zhao, H. M., Li, Y. W., Cai, Q. Y., et al. (2016a). Do arbuscular mycorrhizal fungi affect cadmium uptake kinetics, subcellular distribution and chemical forms in rice? Sci. Total Environ. 571, 1183–1190. [CrossRef]
- Li, J., Meng, B., Chai, H., Yang, X., Song, W., Li, S., et al. (2019). Arbuscular mycorrhizal fungi alleviate drought stress in C3 (Leymus chinensis) and C4 (Hemarthria altissima) grasses via altering antioxidant enzyme activities and photosynthesis. Front. Plant Sci. 10, 499. [CrossRef]
- Li, X., Zeng, R., Liao, H. (2016b). Improving crop nutrient efficiency through root architecture modifications. J. Integr. Plant Biol. 58, 193–202. [CrossRef]
- Liu, C., Ravnskov, S., Liu, F., Rubæk, G. H., Andersen, M. N. (2018). Arbuscular mycorrhizal fungi alleviate abiotic stresses in potato plants caused by low phosphorus and deficit irrigation/partial root-zone drying. J. Agric. Sci. 156, 46–58. [CrossRef]
- Liu, X., Song, Q., Tang, Y., Li, W., Xu, J., Wu, J., et al. (2013). Human health risk assessment of heavy metals in soil–vegetable system: a multi-medium analysis. Sci. Total. Environ. 463–464, 530–540. [CrossRef]
- Ludwig-Müller, J. (2010). “Hormonal responses in host plants triggered by arbuscular mycorrhizal fungi,” in Arbuscular mycorrhizas: Physiology and function. Eds. Koltai, H., Kapulnik, Y. (Dordrecht: Springer), 169–190. [CrossRef]
- Luginbuehl, L. H., Menard, G. N., Kurup, S., Van Erp, H., Radhakrishnan, G. V., Breakspear, A., et al. (2017). Fatty acids in arbuscular mycorrhizal fungi are synthesized by the host plant. Science 356, 1175–1178. [CrossRef]
- 92. Martin CA, Stutz JC (2004). Interactive effects of temperature and arbuscular mycorrhizal fungi on growth, P uptake and root respiration of Capsicum annuum L. Mycorrhiza. 14:241-4. [CrossRef] [PubMed]
- Maya, M. A., Matsubara, Y. (2013). Influence of arbuscular mycorrhiza on the growth and antioxidative activity in Cyclamen under heat stress. Mycorrhiza 23 (5), 381–390. [CrossRef]
- Mena-Violante, H. G., Ocampo-Jimenez, O., Dendooven, L., Martinez-Soto, G., Gonzalez-Castafeda, J., Davies, F. T., et al. (2006). Arbuscular mycorrhizal fungi enhance fruit growth and quality of chile ancho Capsicum annuum L. cv San Luis plants exposed to drought. Mycorrhiza 16, 261–267. [CrossRef]
- Miransari, M. (2017). “Arbuscular mycorrhizal fungi and heavy metal tolerance in plants,” in Arbuscular mycorrhizas and stress tolerance of plants. Ed. Wu, Q. S. (Singapore: Springer Nature), 174–161. [CrossRef]
- Mitra, D., Navendra, U., Panneerselvam, U., Ansuman, S., Ganeshamurthy, A. N., Divya, J. (2019). Role of mycorrhiza and its associated bacteria on plant growth promotion and nutrient management in sustainable agriculture. Int. J. Life Sci. Appl. Sci. 1, 1–10.
- 97. Mingsen Qin, Qi Zhang, Jianbin Pan, Shengjing Jiang, Yongjun Liu, Ali Bahadur, Zhenling Peng, Yue Yang, Huyuan Feng (2019). Hydrolytic enzymes and ability of arbuscular mycorrhizal fungi to colonize roots. European Journal of Soil Science. 71: 84-92. [CrossRef]
- Moghadam, H. R. T. (2016). Application of super absorbent polymer and ascorbic acid to mitigate deleterious effects of cadmium in wheat. Pesqui. Agropecu. Trop. 6 (1), 9–18. [CrossRef]
- Moradtalab, N., Roghieh, H., Nasser, A., Tobias, E. H., Günter, N. (2019). Silicon and the association with an arbuscular-mycorrhizal fungus (Rhizophagus clarus) mitigate the adverse effects of drought stress on strawberry. Agronomy 9, 41. [CrossRef]
- Morte, A., Lovisolo, C., Schubert, A. (2000). Effect of drought stress on growth and water relations of the mycorrhizal association Helianthemum almeriense–Terfezia claveryi. Mycorrhiza 10, 115–119. [CrossRef]
- Mohammadi K., Shiva K. ,Yousef S., Gholamreza H. (2011). A Review: Beneficial Effects of the Mycorrhizal Fungi for Plant Growth. J. Appl. Environ. Biol. Sci., 1:310-319.
- Navarro, J. M., Perez-Tornero, O., Morte, A. (2014). Alleviation of salt stress in citrus seedlings inoculated with arbuscular mycorrhizal fungi depends on the root stock salt tolerance. J. Plant Physiol. 171 (1), 76–85. [CrossRef]
- Nell, M., Wawrosch, C., Steinkellner, S., Vierheilig, H., Kopp, B., Lössl, A. (2010). Root colonization by symbiotic arbuscular mycorrhizal fungi increases sesquiterpenic acid concentrations in Valeriana officinalis L. Planta Med. 76, 393–398. [CrossRef]
- Nouri, E., Breuillinsessoms, F., Feller, U., Reinhardt, D. (2015). Phosphorus and nitrogen regulate arbuscular mycorrhizal symbiosis in Petunia hybrid. PLoS One 9, e90–841. [CrossRef]
- Orfanoudakis, M., Wheeler, C. T., Hooker, J. E. (2010). Both the arbuscular mycorrhizal fungus Gigaspora rosea and Frankia increase root system branching and reduce root hair frequency in Alnus glutinosa. Mycorrhiza 20, 117–126. [CrossRef]
- Orine D, Defossez E, Vergara F,Uthe H, van Dam NM, Rasmann S. Arbuscular mycorrhizal fungi prevent the negative effect of drought and modulatethe growth-defence trade-off in tomato plants. J Sustain Agric Environ. 2022;1:177–190. [CrossRef]
- OrtasI. (2010). Effect of mycorrhiza application on plant growth and nutrient uptake in cucumber production under field conditions. Spanish Journal of Agricultural Research, 8(S1), 116-122. [CrossRef]
- Ortas, I. (2012). The effect of mycorrhizal fungal inoculation on plant yield, nutrient uptake and inoculation effectiveness under long-term field conditions. Field Crops Res. 125, 35–48. [CrossRef]
- Ouziad, F., Hildebrandt, U., Schmelzer, E., Bothe, H. (2005). Differential gene expressions in arbuscular mycorrhizal-colonized tomato grown under heavy metal stress. J. Plant Physiol. 162, 634–649. [CrossRef]
- Paterson, E., Sim, A., Davidson, J., Daniell, T. J. (2016). Arbuscular mycorrhizal hyphae promote priming of native soil organic matter mineralization. Plant Soil. 408, 243–C254. [CrossRef]
- Plassard, C., Dell, B. (2010). Phosphorus nutrition of mycorrhizal trees. Tree Physiol. 30, 1129–1139. [CrossRef]
- Prasad, R., Bhola, D., Akdi, K., Cruz, C., Sairam, K. V. S. S., Tuteja, N., et al. (2017). Introduction to mycorrhiza: historical development,” in Mycorrhiza. Eds. Varma, A., Prasad, R., Tuteja, N. (Cham: Springer), 1–7. [CrossRef]
- Pringle, A., Bever, J. D., Gardes, M., Parrent, J. L., Rillig, M. C., Klironomos, J. N. (2009). Mycorrhizal symbioses and plant invasions. Ann. Rev. Ecol. Evol. Syst. 40, 699–715. [CrossRef]
- Punamiya, P., Datta, R., Sarkar, D., Barber, S., Patel, M., Da, P. (2010). Symbiotic role of Glomus mosseae in phytoextraction of lead in vetiver grass Chrysopogon zizanioides L. J. Hazard. Mater. 177, 465–474. [CrossRef]
- Rani, B. (2016) Effect of arbuscular mycorrhiza fungi on biochemical parameters in wheat Triticum aestivum L. under drought conditions. Doctoral dissertation, CCSHAU, Hisar.
- Rodriguez, R. J., Henson, J., Van Volkenburgh, E., Hoy, M., Wright, L., Beckwith, F., et al. (2008). Stress tolerance in plants via habitat-adapted symbiosis. Int. Soc. Microb. Ecol. 2, 404–416. [CrossRef]
- Ronsheim M. L. "The Effect of Mycorrhizae on Plant Growth and Reproduction Varies with Soil Phosphorus and Developmental Stage," The American Midland Naturalist 167(1), 28-39, (1 January 2012). [CrossRef]
- Rouphael, Y., Franken, P., Schneider, C., Schwarz, D., Giovannetti, M., Agnolucci, M. (2015). Arbuscular mycorrhizal fungi act as bio-stimulants in horticultural crops. Sci. Hort. 196, 91–108. [CrossRef]
- Ruiz-Lozano, J. M. (2003). Arbuscular mycorrhizal symbiosis and alleviation of osmotic stress. Mycorrhiza 13, 309–317. [CrossRef]
- Ruiz-Lozano, J. M., Aroca, R., Zamarreño, Á.M., Molina, S., Andreo-Jiménez, B., Porcel, R., et al. (2015). Arbuscular mycorrhizal symbiosis induces strigolactone biosynthesis under drought and improves drought tolerance in lettuce and tomato. Plant Cell Environ. 39 (2), 441–452. [CrossRef]
- Ruiz-Sánchez, M., Aroca, R., Muñoz, Y., Polón, R., Ruiz-Lozano, J. M. (2010). The arbuscular mycorrhizal symbiosis enhances the photosynthetic efficiency and the antioxidative response of rice plants subjected to drought stress. J. Plant Physiol. 167, 862–869. [CrossRef]
- Sabia, E., Claps, S., Morone, G., Bruno, A., Sepe, L., Aleandri, R. (2015). Field inoculation of arbuscular mycorrhiza on maize (Zea mays L.) under low inputs: preliminary study on quantitative and qualitative aspects. Italian J. Agron. 10, 30–33. [CrossRef]
- Sadhana, B. (2014). Arbuscular mycorrhizal fungi (AMF) as a biofertilizers—a review. Int. J. Curr. Microbiol. App. Sci. 3 (4), 384–400.
- Salam, E. A., Alatar, A., El-Sheikh, M. A. (2017). Inoculation with arbuscular mycorrhizal fungi alleviates harmful effects of drought stress on damask rose. Saudi J. Biol. Sci. 25 (8), 1772–1780. [CrossRef]
- Sameera A. Alghamdi (2019). Influence of mycorrhizal fungi on seed germination and growth in terrestrial and epiphytic orchids. Saudi Journal of Biological Sciences. 26: 495-502, . [CrossRef]
- Santander, C., Sanhueza, M., Olave, J., Borie, F., Valentine, C., Cornejo, P. (2019). Arbuscular mycorrhizal colonization promotes the tolerance to salt stress in lettuce plants through an efficient modification of ionic balance. J. Soil Sci. Plant Nutr. 19 (2), 321–331. [CrossRef]
- Sbrana, C., Avio, L., Giovannetti, M. (2014). Beneficial mycorrhizal symbionts affecting the production of health-promoting phytochemicals. Electrophoresis 35, 1535–1546. [CrossRef]
- Selosse, M. A., Strullu-Derrien, C., Martin, F. M., Kamoun, S., Kenrick, P. (2015). Plants, fungi and oomycetes: a 400-million years affair that shapes the biosphere. New Phytol. 206, 501–506. [CrossRef]
- Sharma, S., Prasad, R., Varma, A., Sharma, A. K. (2017). Glycoprotein associated with Funneliformis coronatum, Gigaspora margarita and Acaulospora scrobiculata suppress the plant pathogens in vitro. Asian J. Plant Pathol. 11 (4), 192–202. [CrossRef]
- Shen, H., Christie, P., Li, X. (2006). Uptake of zinc, cadmium and phosphorus by arbuscular mycorrhizal maize (Zea mays, L.) from a low available phosphorus calcareous soil spiked with zinc and cadmium. Environ. Geochem. Health 28, 111. [CrossRef]
- Sheng, M., Tang, M., Zhang, F., Huang, Y. (2011). Influence of arbuscular mycorrhiza on organic solutes in maize leaves under salt stress. Mycorrhiza 21, 423–430. [CrossRef]
- Smith, S. E., Read, D. J. (1997). Mycorrhizal symbiosis. San Diego: Academic Press, 607.
- Smith, S., Read, D. (2008). Mycorrhiza symbiosis, 3rd Ed. San Diego, CA: Academic Press.
- Smith, S. E., Smith, F. A., Jakobsen, I. (2003). Mycorrhizal fungi can dominate phosphate supply to plants irrespective of growth responses. Plant Physiol. 133, 16–20. [CrossRef]
- Souza, L. A., Andrade, S. A. L., Souza, S. C. R., Schiavinato, M. A. (2012). Evaluation of mycorrhizal influence on the development and phytoremediation potential of Canavalia gladiata in Pb contaminated soils. Int. J. Phytorem. 15, 465–476. [CrossRef]
- 136. Sun X F, Su Y Y, Zhang Y, et al. Diversity of arbuscular mycorrhizal fungal spore communities and its relations to plants under increased temperature and precipitation in a natural grassland. Chin Sci Bull, 2013, 58: 41094119, . [CrossRef]
- Takács, T., Vörös, I. (2003). Effect of metal non-adapted arbuscular mycorrhizal fungi on Cd, Ni and Zn uptake by ryegrass. Acta Agron. Hung. 51, 347–354.
- Talaat, N. B., Shawky, B. T. (2014). Protective effects of arbuscular mycorrhizal fungi on wheat (Triticum aestivum L.) plants exposed to salinity. Environ. Exp. Bot. 98, 20–31. [CrossRef]
- Thirkell, T. J., Charters, M. D., Elliott, A. J., Sait, S. M., Field, K. J. (2017). Are mycorrhizal fungi our sustainable saviours considerations for achieving food security. J. Ecol. 105, 921–929. [CrossRef]
- Turrini, A., Bedini, A., Loor, M. B., Santini, G., Sbrana, C., Giovannetti, M., et al. (2018). Local diversity of native arbuscular mycorrhizal symbionts differentially affects growth and nutrition of three crop plant species. Biol. Fertil. Soils 54, 203–217. [CrossRef]
- Van der Heijden, M. G., Martin, F. M., Selosse, M. A., Sanders, I. R. (2015). Mycorrhizal ecology and evolution: the past, the present, and the future. New Phytol. 205, 1406–1423. [CrossRef]
- Wagg, C., Barendregt, C., Jansa, J., Heijden, M. G. A. (2015). Complementarity in both plant and mycorrhizal fungal communities are not necessarily increased by diversity in the other. J. Ecol. 103, 1233–1244. [CrossRef]
- Wahid, A., Gelani, S., Ashraf, M., Foolad, M. R. (2007). Heat tolerance in plants: an overview. Environ. Exp. Bot. 61, 199–223. [CrossRef]
- Wang, Y., Jing, H., Gao, Y. (2012). Arbuscular mycorrhizal colonization alters subcellular distribution and chemical forms of cadmium in Medicago sativa L. and resists cadmium toxicity. PLoS One 7, 3161–3164. [CrossRef]
- Wang, Y., Wang, M., Li, Y., Wu, A., Huang, J. (2018). Effects of arbuscular mycorrhizal fungi on growth and nitrogen uptake of Chrysanthemum morifolium under salt stress. PLoS One 13 (4), e0196408. [CrossRef]
- Wu, Z., McGrouther, K., Huang, J., Wu, P., Wu, W., Wang, H. (2014). Decomposition and the contribution of glomalin-related soil protein (GRSP) in heavy metal sequestration: field experiment. Soil Biol. Biochem. 68, 283–290. [CrossRef]
- Yang, S., Li, F., Malhi, S. S., Wang, P., Dongrang, S., Wang, J. (2004). Long term fertilization effects on crop yield and nitrate nitrogen accumulation in soil in Northwestern China. Agron. J. 96, 1039–1049. [CrossRef]
- Yang X, Mariotte P, Guo J, Hautier Y, Zhang T. Suppression of arbuscular mycorrhizal fungi decreases the temporal stability of community productivity under elevated temperature and nitrogen addition in a temperate meadow. Sci Total Environ. 2021 Mar 25;762:143137. [CrossRef] [PubMed]
- Yin, N., Zhang, Z., Wang, L., Qian, K. (2016). Variations in organic carbon, aggregation, and enzyme activities of gangue-fly ash-reconstructed soils with sludge and arbuscular mycorrhizal fungi during 6-year reclamation. Envi. Sci. Pollut. Res. 23 (17), 17840–17849. [CrossRef]
- Yooyongwech, S., Phaukinsang, N., Cha-Um, S., Supaibulwatana, K. (2013). Arbuscular mycorrhiza improved growth performance in Macadamia tetraphylla L. grown under water deficit stress involves soluble sugar and proline accumulation. Plant Growth Regul. 69, 285–293. [CrossRef]
- Yooyongwech, S., Samphumphuang, T., Tisarum, R., Theerawitaya, C., Chaum, S. (2016). Arbuscular mycorrhizal fungi (AMF) improved water deficit tolerance in two different sweet potato genotypes involves osmotic adjustments via soluble sugar and free proline. Sci Hort. 198, 107–117. [CrossRef]
- Yost, R. S., Fox, R. L. (1982). Influence of mycorrhizae on the mineral contents of cowpea and soybean grown in an oxisol. Agron. J. 74 (3), 475–481. [CrossRef]
- Yousaf, B., Liu, G., Wang, R., Imtiaz, M., Zia-ur-Rehman, M., Munir, M. A. M., et al. (2016). Bioavailability evaluation, uptake of heavy metals and potential health risks via dietary exposure in urban-industrial areas. Environ. Sci. Pollut. Res. 23, 22443–22453. [CrossRef]
- Zeng, L., JianFu, L., JianFu, L., MingYuan, W. (2014). Effects of arbuscular mycorrhizal (AM) fungi on citrus quality under nature conditions. Southwest China J. Agric. Sci. 27, 2101–2105. [CrossRef]
- Zhang, F., Jia-Dong, H. E., Qiu-Dan, N. I., Qiang-Sheng, W. U., Zou, Y. N. (2018a). Enhancement of drought tolerance in trifoliate orange by mycorrhiza: changes in root sucrose and proline metabolisms. Not. Bot. Horti. Agrobot. Cluj-Napoca 46, 270. [CrossRef]
- Zhang, T., Hub, Y., Zhang, K., Tian, C., Gu, J. (2018b). Arbuscular mycorrhizal fungi improve plant growth of Ricinus communis by altering photosynthetic properties and increasing pigments under drought and salt stress. Ind. Crop. Prod. 117, 13–19. [CrossRef]
- .
- 7878.
- 159. Zhu X, Song F, Xu H. Influence of arbuscular mycorrhiza on lipid peroxidation and antioxidant enzyme activity of maize plants under temperature stress. Mycorrhiza. 2010 Jun;20(5):325-32. [CrossRef] [PubMed]
- Zhu, X., Song, F., Liu, S., Liu, T., Zhou, X. (2012). Arbuscular mycorrhizae improve photosynthesis and water status of Zea mays L. under drought stress. Plant Soil Environ. 58, 186–191. [CrossRef]
- Zhu, X. C., Song, F. B., Xu, H. W. (2010a). Arbuscular mycorrhizae improve low temperature stress in maize via alterations in host water status and photosynthesis. Plant Soil. 331, 129–137. [CrossRef]
- Zhu, X. C., Song, F. B., Liu, S. Q., Liu, F. L. (2016). Arbuscular mycorrhiza improve growth, nitrogen uptake, and nitrogen use efficiency in wheat grown under elevated CO2. Mycorrhiza 26, 133–140. [CrossRef]
- Zhu, X. C., Song, F. B., Xu, H. W. (2010b). Effects of arbuscular mycorrhizal fungi on photosynthetic characteristics of maize under low temperature stress. Acta Ecol. Sin. 21, 470–475. [CrossRef]
- Zou, Y. N., Srivastava, A. K., Wu, Q. S. (2016). Glomalin: a potential soil conditioner for perennial fruits. Int. J. Agric. Biol. 18, 293–297. [CrossRef]
- Zou, YN., Qin, QY., Ma, WY. et al. Metabolomics reveals arbuscular mycorrhizal fungi-mediated tolerance of walnut to soil drought. BMC Plant Biol 23, 118 (2023). [CrossRef]
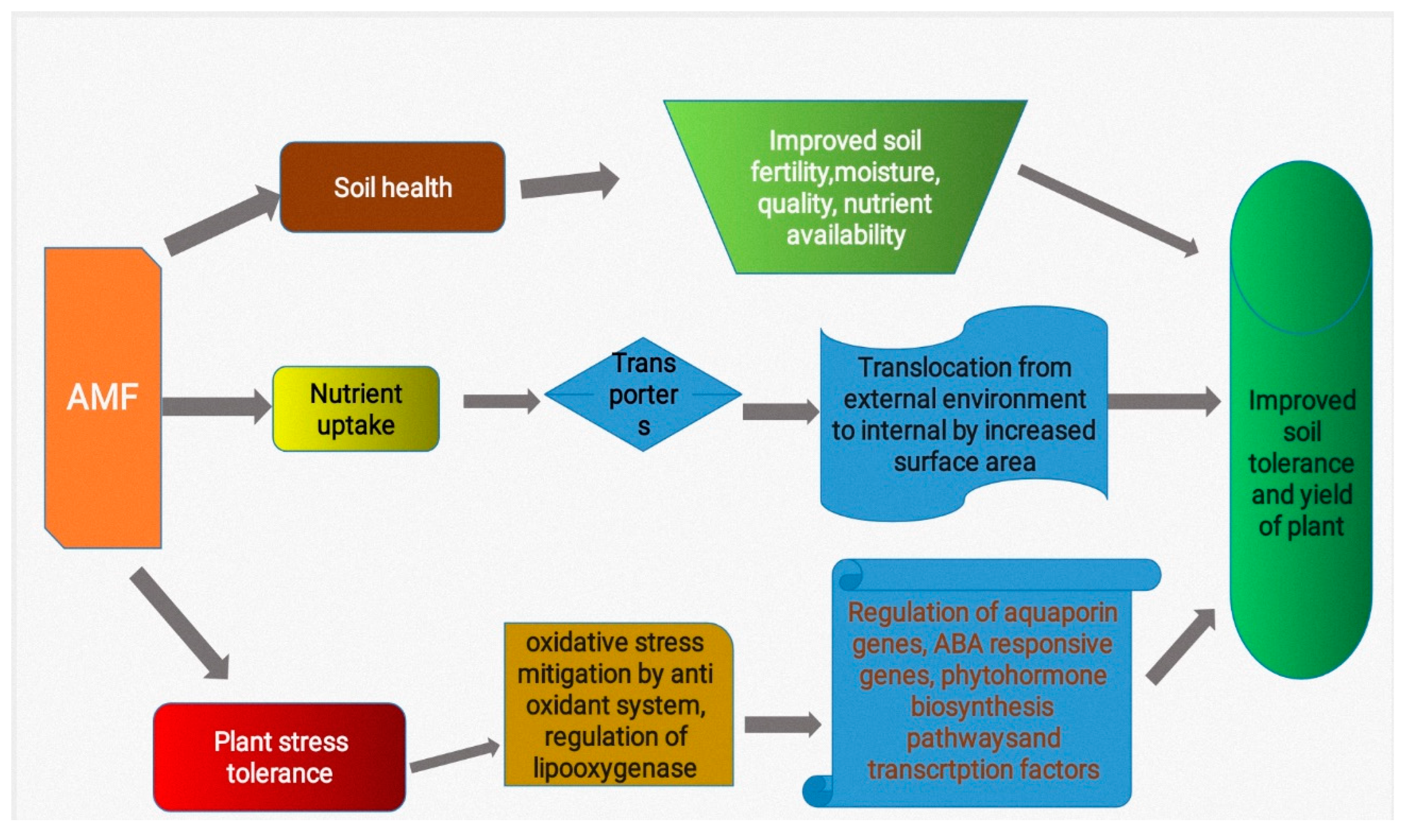
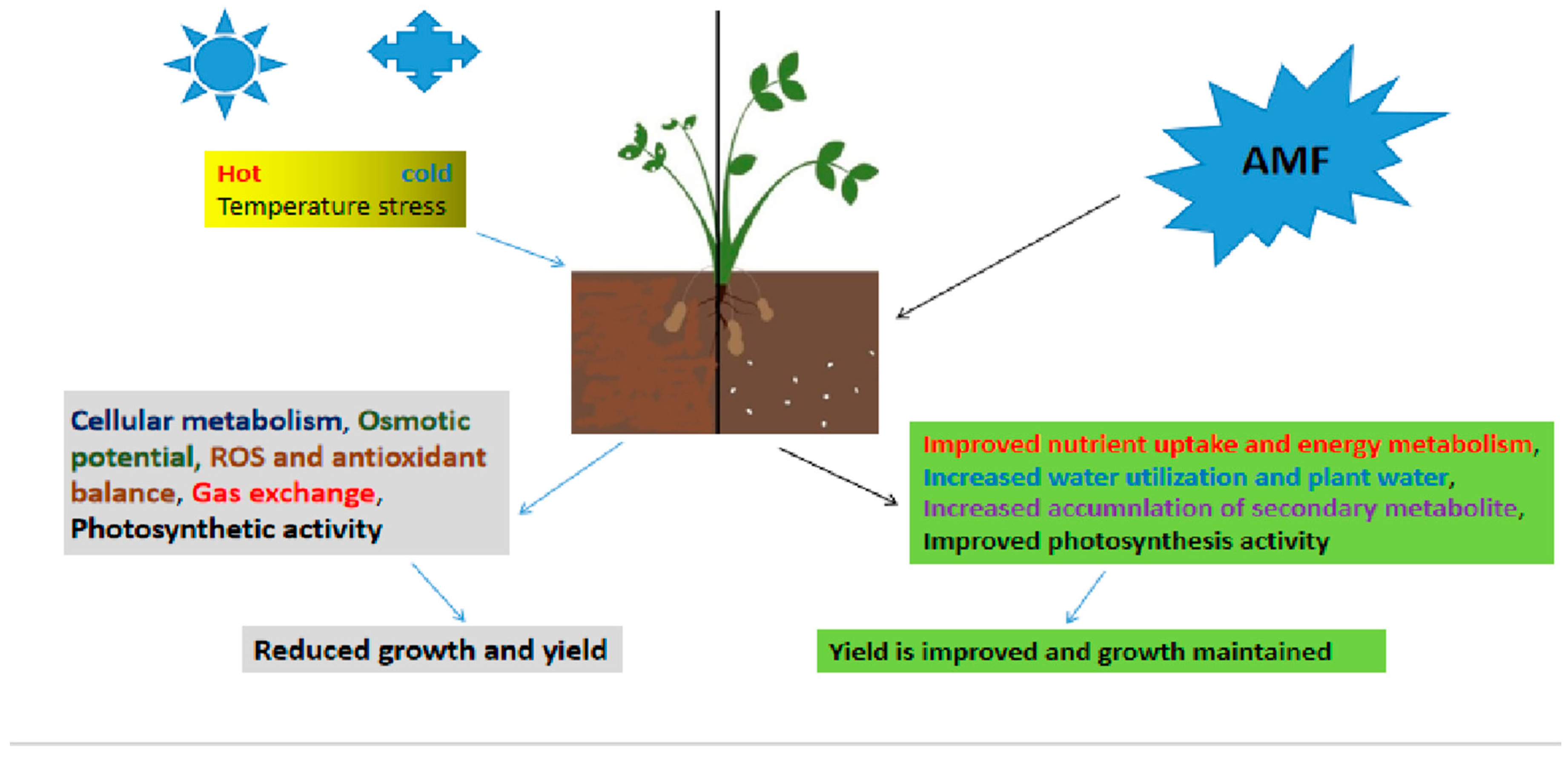
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




