Submitted:
16 December 2023
Posted:
26 December 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
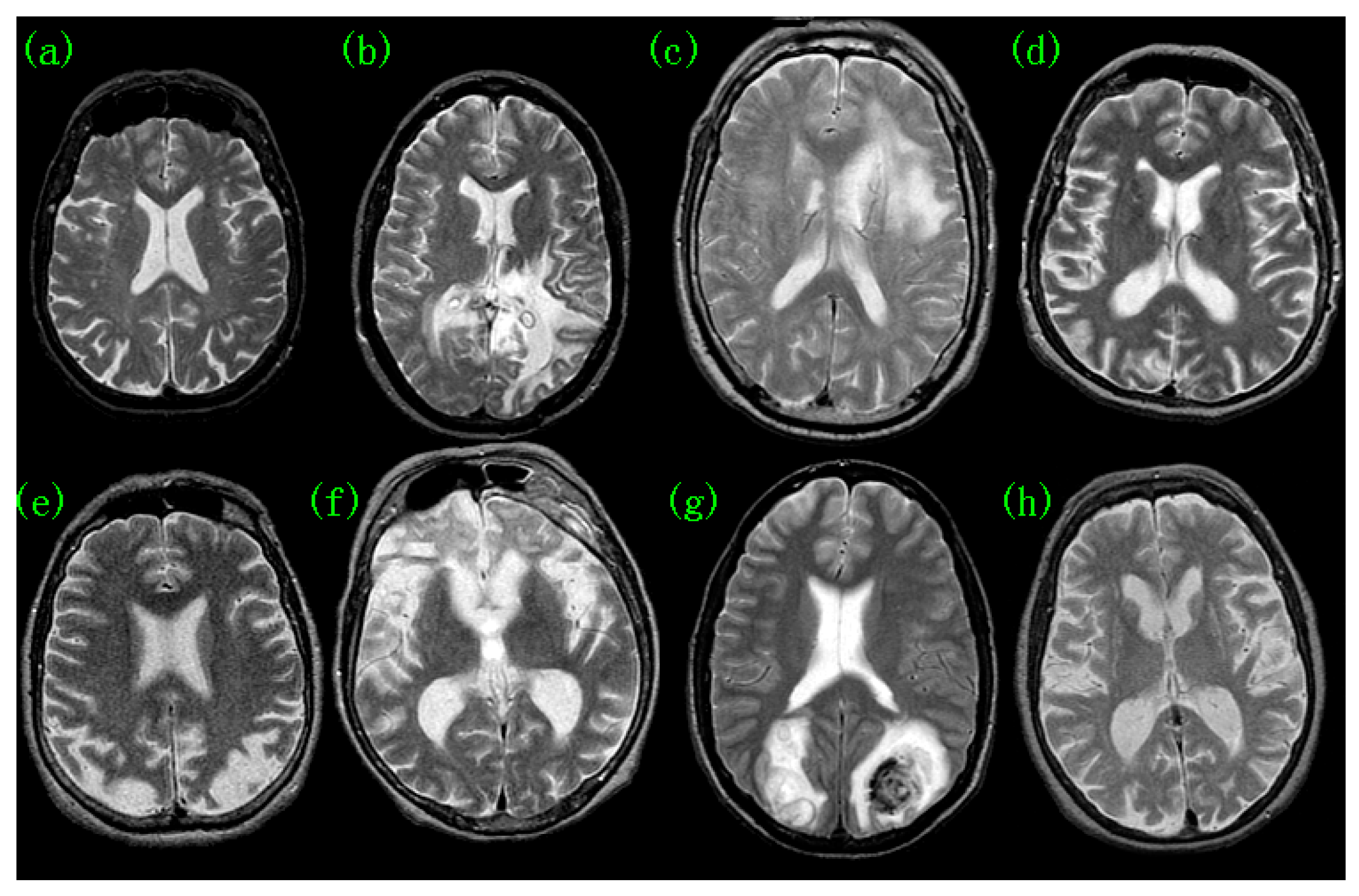
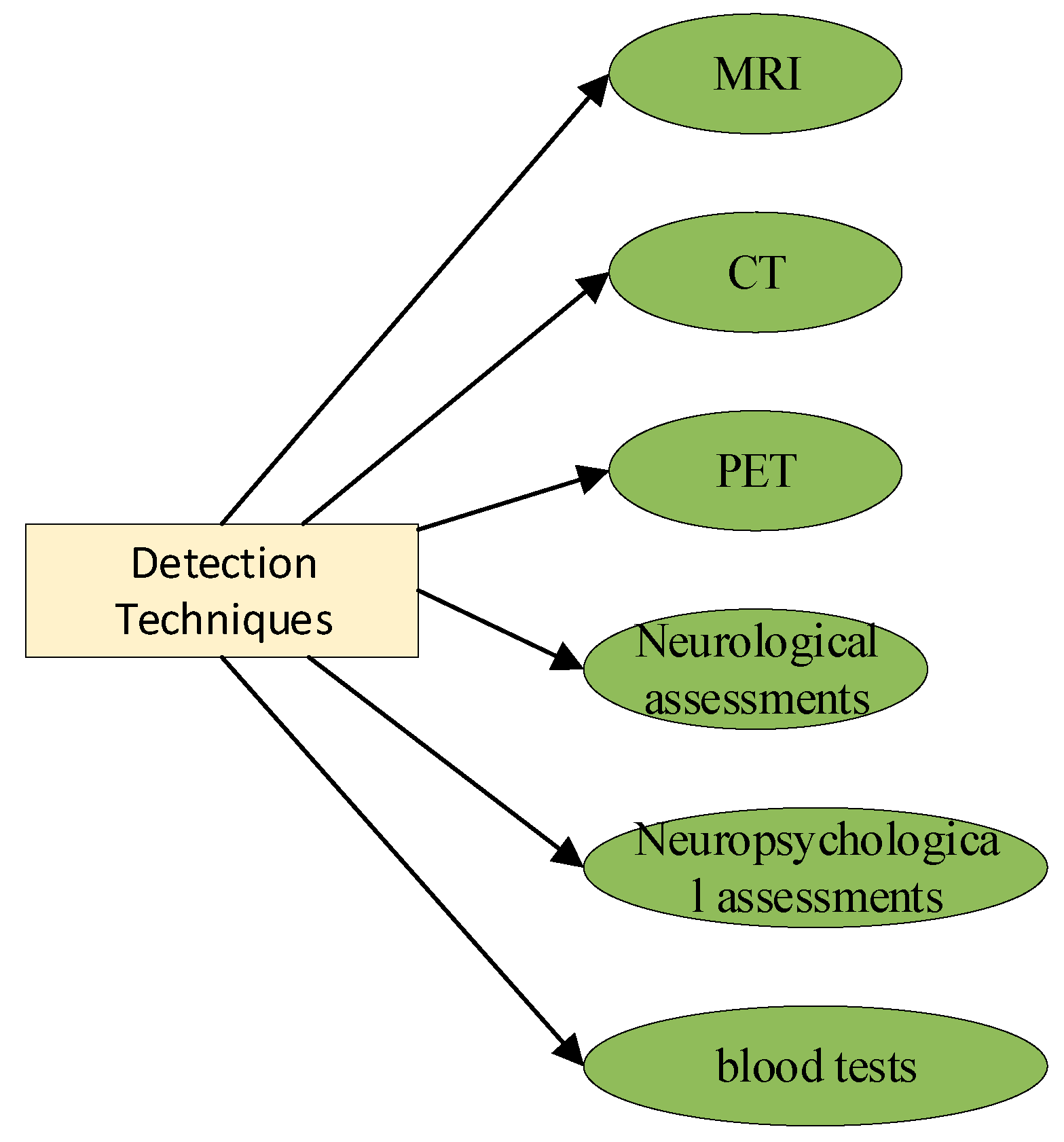
2. Pathological Brain Detection
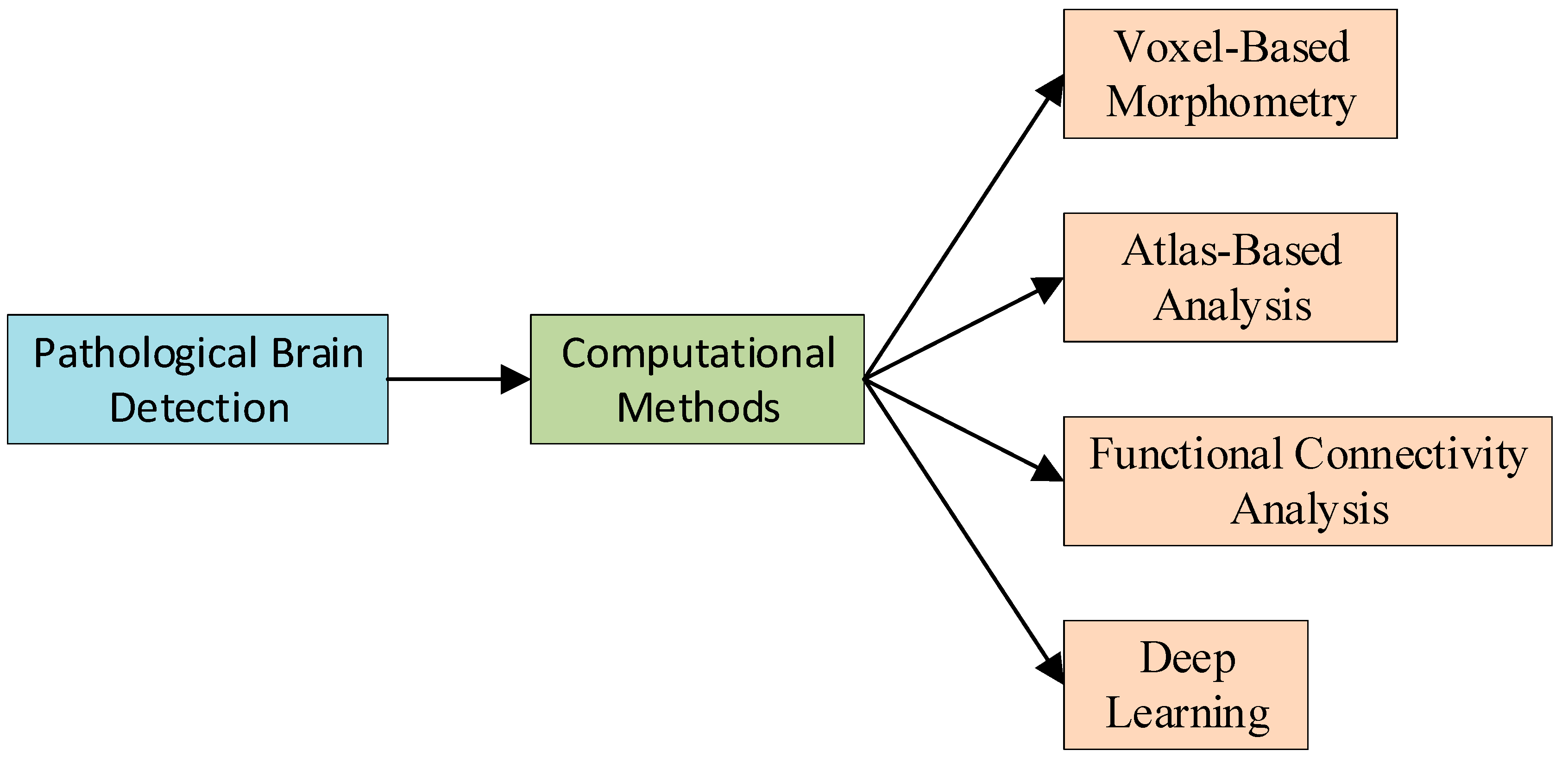
3. Common Computational Methods for PBD
3.1. Voxel-Based Morphometry
3.2. Atlas-Based Analysis
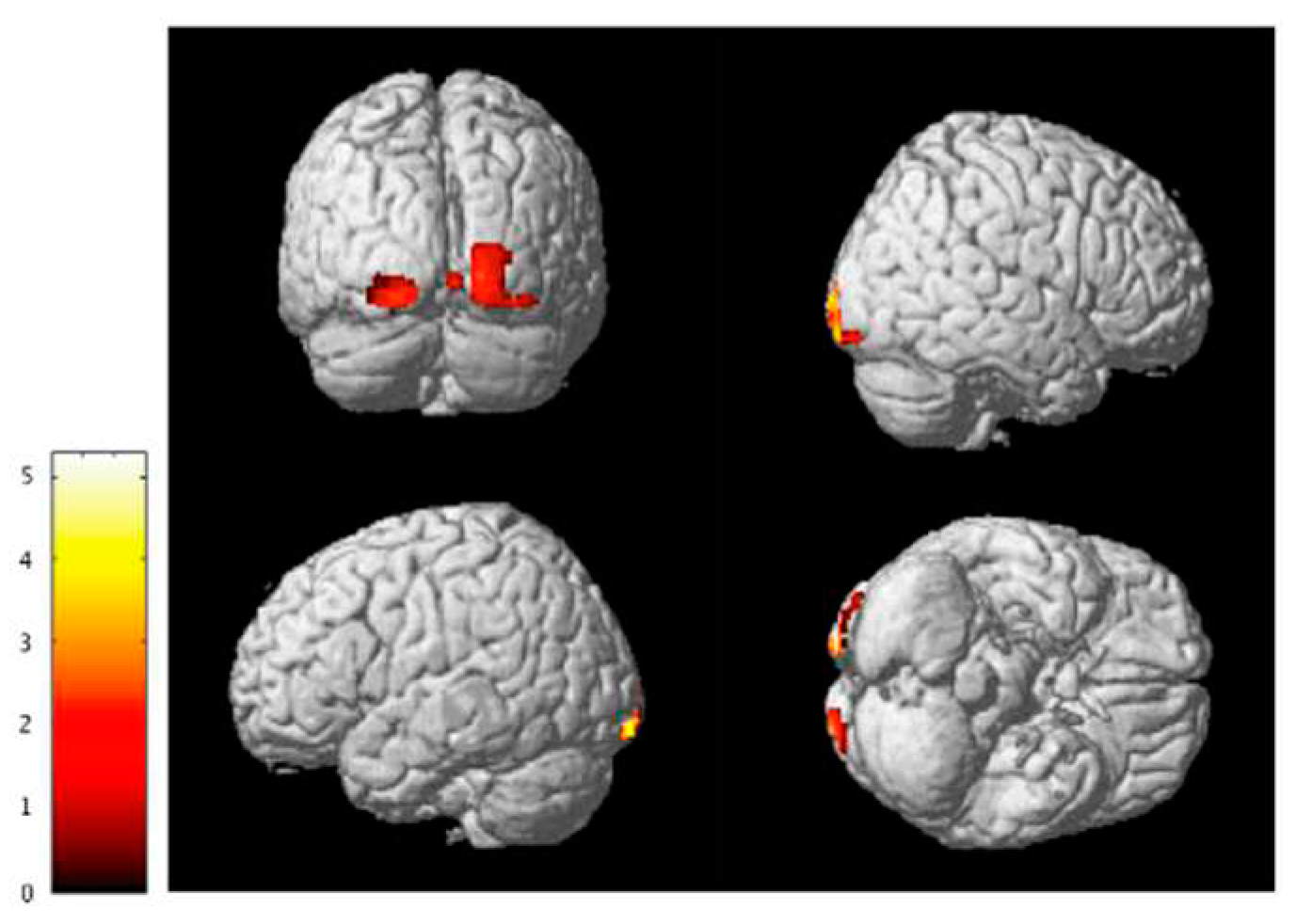
3.3. Functional Connectivity Analysis
3.4. Deep Learning
4. Discussions
5. Conclusion
Acknowledgments
References
- Fang, L.; Liu, Q.; Meyer, E.; Welle, A.; Huang, W.; Scheller, A.; Kirchhoff, F.; Bai, X. A subset of OPCs do not express Olig2 during development which can be increased in the adult by brain injuries and complex motor learning. Glia 2022, 71, 415–430. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Diano, M.; Battaglia, S. Editorial: Insights into structural and functional organization of the brain: evidence from neuroimaging and non-invasive brain stimulation techniques. Front. Psychiatry 2023, 14, 1225755. [Google Scholar] [CrossRef]
- Perens, J.; Salinas, C.G.; Roostalu, U.; Skytte, J.L.; Gundlach, C.; Hecksher-Sørensen, J.; Dahl, A.B.; Dyrby, T.B. Multimodal 3D Mouse Brain Atlas Framework with the Skull-Derived Coordinate System. Neuroinformatics 2023, 21, 269–286. [Google Scholar] [CrossRef]
- Zhang, Y.; Dong, Z.; Wu, L.; Wang, S. A hybrid method for MRI brain image classification. Expert Syst. Appl. 2011, 38, 10049–10053. [Google Scholar] [CrossRef]
- Aguilar-Ruiz, J.S.; Bifet, A.; Gama, J. Data Stream Analytics. Analytics 2023, 2, 346–349. [Google Scholar] [CrossRef]
- Albustanji, R.N.; Elmanaseer, S.; Alkhatib, A.A.A. Robotics: Five Senses plus One—An Overview. Robotics 2023, 12, 68. [Google Scholar] [CrossRef]
- Chapman, A.D.; Selhorst, S.; LaComb, J.; LeDantec-Boswell, A.; Wohl, T.R.; Adhicary, S.; Nielsen, C.M. Endothelial Rbpj Is Required for Cerebellar Morphogenesis and Motor Control in the Early Postnatal Mouse Brain. Cerebellum 2022, 22, 613–627. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wu, L. An mr brain images classifier via principal component analysis and kernel support vector machine. Prog. Electromagn. Res. 2012, 130, 369–388. [Google Scholar] [CrossRef]
- Mikkelsen, A.C.D.; Thomsen, K.L.; Mookerjee, R.P.; Hadjihambi, A. The role of brain inflammation and abnormal brain oxygen homeostasis in the development of hepatic encephalopathy. Metab. Brain Dis. 2022, 38, 1707–1716. [Google Scholar] [CrossRef]
- Alonge, K.M.; Porte, D.; Schwartz, M.W. Distinct Roles for Brain and Pancreas in Basal and Postprandial Glucose Homeostasis. Diabetes 2023, 72, 547–556. [Google Scholar] [CrossRef]
- Ranjan, A.K.; Gulati, A. Controls of Central and Peripheral Blood Pressure and Hemorrhagic/Hypovolemic Shock. J. Clin. Med. 2023, 12, 1108. [Google Scholar] [CrossRef]
- Sorek, G.; Shaklai, S.; Gagnon, I.; Schneider, K.; Chevignard, M.; Stern, N.; Fadida, Y.; Kalderon, L.; Katz-Leurer, M. Impact of Subarachnoid Hemorrhage on the Cardiac Autonomic Function During Rehabilitation in Children After Severe Traumatic Brain Injury. Neurotrauma Rep. 2023, 4, 458–462. [Google Scholar] [CrossRef]
- Kirk, C.; Childs, C. Combat Sports as a Model for Measuring the Effects of Repeated Head Impacts on Autonomic Brain Function: A Brief Report of Pilot Data. Vision 2023, 7, 39. [Google Scholar] [CrossRef]
- Mut-Arbona, P.; Huang, L.; Baranyi, M.; Tod, P.; Iring, A.; Calzaferri, F.; Ríos, C.d.L.; Sperlágh, B. Dual Role of the P2X7 Receptor in Dendritic Outgrowth during Physiological and Pathological Brain Development. J. Neurosci. 2023, 43, 1125–1142. [Google Scholar] [CrossRef]
- Jin, M.; Cai, S.-Q. Mechanisms Underlying Brain Aging Under Normal and Pathological Conditions. Neurosci. Bull. 2022, 39, 303–314. [Google Scholar] [CrossRef]
- Parajuli, B.; Koizumi, S. Strategies for Manipulating Microglia to Determine Their Role in the Healthy and Diseased Brain. Neurochem. Res. 2022, 48, 1066–1076. [Google Scholar] [CrossRef]
- Rodkin, S.; Nwosu, C.; Sannikov, A.; Raevskaya, M.; Tushev, A.; Vasilieva, I.; Gasanov, M. The Role of Hydrogen Sulfide in Regulation of Cell Death following Neurotrauma and Related Neurodegenerative and Psychiatric Diseases. Int. J. Mol. Sci. 2023, 24, 10742. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wang, C.; Chen, J.; Lan, Y.; Zhang, W.; Kang, Z.; Zheng, Y.; Zhang, R.; Yu, J.; Li, W. Signaling pathways in brain tumors and therapeutic interventions. Signal Transduct. Target. Ther. 2023, 8, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Villadiego, J., et al., Full protection from sars-cov-2 brain infection and damage in susceptible transgenic mice conferred by mva-cov2-s vaccine candidate. Nature Neuroscience 2023, 26, 226–238. [CrossRef] [PubMed]
- Babiloni, A.H.; Baril, A.-A.; Charlebois-Plante, C.; Jodoin, M.; Sanchez, E.; De Baets, L.; Arbour, C.; Lavigne, G.J.; Gosselin, N.; De Beaumont, L. The Putative Role of Neuroinflammation in the Interaction between Traumatic Brain Injuries, Sleep, Pain and Other Neuropsychiatric Outcomes: A State-of-the-Art Review. J. Clin. Med. 2023, 12, 1793. [Google Scholar] [CrossRef] [PubMed]
- Johnson, N.H., et al., Inflammasome activation in traumatic brain injury and alzheimer's disease. Translational Research, 2023; 254, 1–12. [CrossRef]
- Alnaaim, S.A.; Al-Kuraishy, H.M.; Alexiou, A.; Papadakis, M.; Saad, H.M.; Batiha, G.E.-S. Role of Brain Liver X Receptor in Parkinson’s Disease: Hidden Treasure and Emerging Opportunities. Mol. Neurobiol. 2023, 61, 341–357. [Google Scholar] [CrossRef]
- Jahan, A.B.; Tanev, K. Neurobiological Mechanisms Of Depression Following Traumatic Brain Injury. Brain Inj. 2022, 37, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Townsend, L.; Pillinger, T.; Selvaggi, P.; Veronese, M.; Turkheimer, F.; Howes, O. Brain glucose metabolism in schizophrenia: a systematic review and meta-analysis of 18FDG-PET studies in schizophrenia. Psychol. Med. 2022, 53, 4880–4897. [Google Scholar] [CrossRef] [PubMed]
- Constantinides, C.; Han, L.K.M.; Alloza, C.; Antonucci, L.A.; Arango, C.; Ayesa-Arriola, R.; Banaj, N.; Bertolino, A.; Borgwardt, S.; Bruggemann, J.; et al. Brain ageing in schizophrenia: evidence from 26 international cohorts via the ENIGMA Schizophrenia consortium. Mol. Psychiatry 2022, 28, 1201–1209. [Google Scholar] [CrossRef]
- Liu, F.-T.; Lu, J.-Y.; Li, X.-Y.; Liang, X.-N.; Jiao, F.-Y.; Ge, J.-J.; Wu, P.; Li, G.; Shen, B.; Wu, B.; et al. 18F-Florzolotau PET imaging captures the distribution patterns and regional vulnerability of tau pathology in progressive supranuclear palsy. Eur. J. Nucl. Med. 2023, 50, 1395–1405. [Google Scholar] [CrossRef]
- Hiono, T.; Kobayashi, D.; Kobayashi, A.; Suzuki, T.; Satake, Y.; Harada, R.; Matsuno, K.; Sashika, M.; Ban, H.; Kobayashi, M.; et al. Virological, pathological, and glycovirological investigations of an Ezo red fox and a tanuki naturally infected with H5N1 high pathogenicity avian influenza viruses in Hokkaido, Japan. Virology 2023, 578, 35–44. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, L.; Wang, S. Magnetic resonance brain image classification by an improved artificial bee colony algorithm. Prog. Electromagn. Res. 2011, 116, 65–79. [Google Scholar] [CrossRef]
- Billot, B.; Magdamo, C.; Cheng, Y.; Arnold, S.E.; Das, S.; Iglesias, J.E. Robust machine learning segmentation for large-scale analysis of heterogeneous clinical brain MRI datasets. Proc. Natl. Acad. Sci. 2023, 120. [Google Scholar] [CrossRef]
- Lagares, A.; Castaño-Leon, A.M.; Richard, M.; Tsitsopoulos, P.P.; Morales, J.; Mihai, P.; Pavlov, V.; Mejan, O.; de la Cruz, J.; Payen, J.F.; et al. Variability in the indication of brain CT scan after mild traumatic brain injury. A transnational survey. Eur. J. Trauma Emerg. Surg. 2022, 49, 1189–1198. [Google Scholar] [CrossRef]
- Ward, J., et al., Brain pet imaging: Frontotemporal dementia. PET clinics 2023, 18, 123–133. [CrossRef]
- Zhang, Y.-D.; Zhang, Z.; Zhang, X.; Wang, S.-H. MIDCAN: A multiple input deep convolutional attention network for Covid-19 diagnosis based on chest CT and chest X-ray. Pattern Recognit. Lett. 2021, 150, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Yang, J., Preclinical diagnosis of magnetic resonance (mr) brain images via discrete wavelet packet transform with tsallis entropy and generalized eigenvalue proximal support vector machine (gepsvm). Entropy 2015, 17, 1795. [CrossRef]
- Groechel, R.C.; Tripodis, Y.; Alosco, M.L.; Mez, J.; Qiu, W.Q.; Goldstein, L.; Budson, A.E.; Kowall, N.W.; Shaw, L.M.; Weiner, M.; et al. Biomarkers of Alzheimer’s disease in Black and/or African American Alzheimer’s Disease Neuroimaging Initiative (ADNI) participants. Neurobiol. Aging 2023, 131, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Saint-Jalmes, M.; Fedyashov, V.; Beck, D.; Baldwin, T.; Faux, N.G.; Bourgeat, P.; Fripp, J.; Masters, C.L.; Goudey, B. Disease progression modelling of Alzheimer’s disease using probabilistic principal components analysis. NeuroImage 2023, 278, 120279. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhou, J.; Guo, M.; Cheng, S.; Chen, Y.; Jiang, N.; Li, X.; Hu, S.; Tian, Z.; Li, Z.; et al. A systematic review and meta-analysis of voxel-based morphometric studies of migraine. J. Neurol. 2022, 270, 152–170. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Bartholomay, K.L.; Marzelli, M.J.; Miller, J.G.; Bruno, J.L.; A Lightbody, A.; Reiss, A.L. Neuroanatomical Profile of Young Females with Fragile X Syndrome: A Voxel-Based Morphometry Analysis. Cereb. Cortex 2021, 32, 2310–2320. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Zheng, S.; Yang, Z.; Wu, Y.; Qiu, J.; Cheng, Y.; Lin, P.; Lin, Y.; Guan, J.; Mikulis, D.J.; et al. Voxel-based morphometry and a deep learning model for the diagnosis of early Alzheimer’s disease based on cerebral gray matter changes. Cereb. Cortex 2022, 33, 754–763. [Google Scholar] [CrossRef]
- Sato, R.; Kudo, K.; Udo, N.; Matsushima, M.; Yabe, I.; Yamaguchi, A.; Tha, K.K.; Sasaki, M.; Harada, M.; Matsukawa, N.; et al. A diagnostic index based on quantitative susceptibility mapping and voxel-based morphometry may improve early diagnosis of Alzheimer’s disease. Eur. Radiol. 2022, 32, 4479–4488. [Google Scholar] [CrossRef]
- Zhou, X.; Wu, R.; Zeng, Y.; Qi, Z.; Ferraro, S.; Xu, L.; Zheng, X.; Li, J.; Fu, M.; Yao, S.; et al. Choice of Voxel-based Morphometry processing pipeline drives variability in the location of neuroanatomical brain markers. Commun. Biol. 2022, 5, 1–12. [Google Scholar] [CrossRef]
- Schregel, K.; Heinz, L.; Hunger, J.; Pan, C.; Bode, J.; Fischer, M.; Sturm, V.; Venkataramani, V.; Karimian-Jazi, K.; Agardy, D.A.; et al. A Cellular Ground Truth to Develop MRI Signatures in Glioma Models by Correlative Light Sheet Microscopy and Atlas-Based Coregistration. J. Neurosci. 2023, 43, 5574–5587. [Google Scholar] [CrossRef]
- Jyothi, P.; Singh, A.R. Deep learning models and traditional automated techniques for brain tumor segmentation in MRI: a review. Artif. Intell. Rev. 2022, 56, 2923–2969. [Google Scholar] [CrossRef]
- Grandjean, J.; Desrosiers-Gregoire, G.; Anckaerts, C.; Angeles-Valdez, D.; Ayad, F.; Barrière, D.A.; Blockx, I.; Bortel, A.; Broadwater, M.; Cardoso, B.M.; et al. A consensus protocol for functional connectivity analysis in the rat brain. Nat. Neurosci. 2023, 26, 673–681. [Google Scholar] [CrossRef] [PubMed]
- Ardesch, D.J.; Libedinsky, I.; Scholtens, L.H.; Wei, Y.; Heuvel, M.P.v.D. Convergence of Brain Transcriptomic and Neuroimaging Patterns in Schizophrenia, Bipolar Disorder, Autism Spectrum Disorder, and Major Depressive Disorder. Biol. Psychiatry: Cogn. Neurosci. Neuroimaging 2023, 8, 630–639. [Google Scholar] [CrossRef]
- Seguin, C.; Sporns, O.; Zalesky, A. Brain network communication: concepts, models and applications. Nat. Rev. Neurosci. 2023, 24, 557–574. [Google Scholar] [CrossRef] [PubMed]
- Sharifani, K., et al., Machine learning and deep learning: A review of methods and applications. World Information Technology and Engineering Journal 2023, 10, 3897–3904.
- Aslani, S.; Jacob, J. Utilisation of deep learning for COVID-19 diagnosis. Clin. Radiol. 2023, 78, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.-T.; Hu, B.; Chen, T.-Q.; Chen, Z.-H.; Shang, Y.-X.; Li, Y.-T.; Wang, R.; Wang, W. Internet addiction-induced brain structure and function alterations: a systematic review and meta-analysis of voxel-based morphometry and resting-state functional connectivity studies. Brain Imaging Behav. 2023, 17, 329–342. [Google Scholar] [CrossRef] [PubMed]
- Xin, M.; Qu, Y.; Peng, X.; Zhu, D.; Cheng, S. A systematic review and meta-analysis of voxel-based morphometric studies of fibromyalgia. Front. Neurosci. 2023, 17, 1164145. [Google Scholar] [CrossRef]
- Cheng, H.; Teng, J.; Jia, L.; Xu, L.; Yang, F.; Li, H.; Ling, C.; Liu, W.; Li, J.; Li, Y.; et al. Association between morphologic features of intracranial distal arteries and brain atrophy indexes in cerebral small vessel disease: a voxel-based morphometry study. Front. Neurol. 2023, 14, 1198402. [Google Scholar] [CrossRef]
- Liu, J.; Lei, Y.; Diao, Y.; Lu, Y.; Teng, X.; Chen, Q.; Liu, L.; Zhong, J. Altered whole-brain gray matter volume in form-deprivation myopia rats based on voxel-based morphometry: A pilot study. Front. Neurosci. 2023, 17. [Google Scholar] [CrossRef]
- Mosch, B., et al., Brain morphometric changes in fibromyalgia and the impact of psychometric and clinical factors: A volumetric and diffusion-tensor imaging study. Arthritis Research & Therapy, 2023; 25, 81. [CrossRef]
- Tomoda, A.; Polcari, A.; Anderson, C.M.; Teicher, M.H. Reduced Visual Cortex Gray Matter Volume and Thickness in Young Adults Who Witnessed Domestic Violence during Childhood. PLOS ONE 2012, 7, e52528. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, S.; Xin, H.; Feng, M.; Zhang, Q.; Sui, C.; Guo, L.; Liang, C.; Wen, H. Disrupted Gray Matter Networks Associated with Cognitive Dysfunction in Cerebral Small Vessel Disease. Brain Sci. 2023, 13, 1359. [Google Scholar] [CrossRef]
- Warren, S.L., et al., Functional magnetic resonance imaging, deep learning, and alzheimer's disease: A systematic review. Journal of Neuroimaging 2023, 33, 5–18. [CrossRef]
- Zhao, H.; Cai, H.; Mo, F.; Lu, Y.; Yao, S.; Yu, Y.; Zhu, J. Genetic mechanisms underlying brain functional homotopy: a combined transcriptome and resting-state functional MRI study. Cereb. Cortex 2022, 33, 3387–3400. [Google Scholar] [CrossRef]
- Xie, X.; Feng, M.; Rong, Y.; Hu, J.; Zhou, W.; Li, Y.; Liao, H.; Shi, L.; He, H.; Tong, Q.; et al. Whole brain atlas-based diffusion kurtosis imaging parameters for the evaluation of multiple cognitive-related brain microstructure injuries after radiotherapy in lung cancer patients with brain metastasis. Quant. Imaging Med. Surg. 2023, 13, 5321–5332. [Google Scholar] [CrossRef]
- Liu, C.-F.; Leigh, R.; Johnson, B.; Urrutia, V.; Hsu, J.; Xu, X.; Li, X.; Mori, S.; Hillis, A.E.; Faria, A.V. A large public dataset of annotated clinical MRIs and metadata of patients with acute stroke. Sci. Data 2023, 10, 1–14. [Google Scholar] [CrossRef]
- Voruz, P., et al., Brain functional connectivity alterations associated with neuropsychological performance 6–9 months following sars-cov-2 infection. Human Brain Mapping 2023, 44, 1629–1646. [CrossRef] [PubMed]
- A Moreau, C.; Kumar, K.; Harvey, A.; Huguet, G.; Urchs, S.G.W.; Schultz, L.M.; Sharmarke, H.; Jizi, K.; Martin, C.-O.; Younis, N.; et al. Brain functional connectivity mirrors genetic pleiotropy in psychiatric conditions. Brain 2022, 146, 1686–1696. [Google Scholar] [CrossRef]
- Voigt, K.; Liang, E.X.; Misic, B.; Ward, P.G.D.; Egan, G.F.; Jamadar, S.D. Metabolic and functional connectivity provide unique and complementary insights into cognition-connectome relationships. Cereb. Cortex 2022, 33, 1476–1488. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, S.; Zhang, Y. Artificial intelligence for visually impaired. Displays 2023, 77. [Google Scholar] [CrossRef]
- Wang, J.; Satapathy, S.C.; Wang, S.; Zhang, Y. LCCNN: a Lightweight Customized CNN-Based Distance Education App for COVID-19 Recognition. Mob. Networks Appl. 2023, 1–16. [Google Scholar] [CrossRef]
- Indira, D.; Goddu, J.; Indraja, B.; Challa, V.M.L.; Manasa, B. A review on fruit recognition and feature evaluation using CNN. Mater. Today: Proc. 2021, 80, 3438–3443. [Google Scholar] [CrossRef]
- Zhang, Y.-D.; Wang, S.-H.; Yang, X.-J.; Dong, Z.-C.; Liu, G.; Phillips, P.; Yuan, T.-F. Pathological brain detection in MRI scanning by wavelet packet Tsallis entropy and fuzzy support vector machine. SpringerPlus 2015, 4, 1–16. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, J.; Wang, S.; Dong, Z.; Phillips, P. Pathological brain detection in MRI scanning via Hu moment invariants and machine learning. J. Exp. Theor. Artif. Intell. 2016, 29, 299–312. [Google Scholar] [CrossRef]
- Eide, S., et al., Interleukin-6 as a marker of huntington's disease progression: Systematic review and meta-analysis. Brain, Behavior, & Immunity - Health 2023, 30, 100635. [CrossRef]
- Choudhary, G.; Fränti, P. Predicting onset of disease progression using temporal disease occurrence networks. Int. J. Med Informatics 2023, 175, 105068. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, S.; Ji, G. A Rule-Based Model for Bankruptcy Prediction Based on an Improved Genetic Ant Colony Algorithm. Math. Probl. Eng. 2013, 2013, 1–10. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, X.; Lu, S.; Wang, H.; Phillips, P.; Wang, S. Smart detection on abnormal breasts in digital mammography based on contrast-limited adaptive histogram equalization and chaotic adaptive real-coded biogeography-based optimization. Simulation 2016, 92, 873–885. [Google Scholar] [CrossRef]
- Wiem, T.; Ali, D. Deep second generation wavelet autoencoders based on curvelet pooling for brain pathology classification. Biomed. Signal Process. Control. 2023, 83. [Google Scholar] [CrossRef]
- Rubinski, A., et al., Polygenic effect on tau pathology progression in alzheimer's disease. Annals of Neurology 2023, 93, 819–829. [CrossRef]
- van der Flier, W.M., et al., Towards a future where alzheimer’s disease pathology is stopped before the onset of dementia. Nature Aging 2023, 3, 494–505. [CrossRef] [PubMed]
- Yon-Hernández, J.A.; Wojcik, D.Z.; García-García, L.; Magán-Maganto, M.; Franco-Martín, M.; Canal-Bedia, R. Neuropsychological profile of executive functions in autism spectrum disorder and schizophrenia spectrum disorders: a comparative group study in adults. Eur. Arch. Psychiatry Clin. Neurosci. 2022, 273, 719–730. [Google Scholar] [CrossRef] [PubMed]
- A Cormie, M.; Kaya, B.; E Hadjis, G.; Mouseli, P.; Moayedi, M. Insula-cingulate structural and functional connectivity: an ultra-high field MRI study. Cereb. Cortex 2023, 33, 9787–9801. [Google Scholar] [CrossRef] [PubMed]
- Deutsch, A.; Palmer, L.; McMullen, T.; Le, T.; Toth, M.; Marino, M.; Vaughan, M.; Schwartz, C.; Edwards, A. A Postacute Care Function Process Quality Measure for the Collection of Standardized Self-Care and Mobility Data: Development, Implementation, and Quality Measure Scores. Arch. Phys. Med. Rehabilitation 2022, 103, 1061–1069. [Google Scholar] [CrossRef]
- Gokdemir, Y.; Eralp, E.E.; Ergenekon, A.P.; Yegit, C.Y.; Yanaz, M.; Mursaloğlu, H.; Uzunoglu, B.; Kocamaz, D.; Tastan, G.; Coskun, O.K.; et al. Implementation of standardized cystic fibrosis care algorithm to improve the center data-quality improvement project international collaboration. J. Cyst. Fibros. 2023, 22, 710–714. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).



